?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to evaluate the efficiency of using a natural substance, curcumin, encapsulated in CD44-targeting hyaluronate–polylactide (HA-PLA) nanoparticles (NPs) for the modulation of macrophage polarity from the pro-inflammatory M1 to anti-inflammatory M2 phenotype. For this purpose, the characterization of the NPs was monitored using 1HNMR, FTIR, DLS and FE-SEM. The effects of curcumin-encapsulated HA-PLA NPs on the viability of LPS/IFN-γ stimulated peritoneal macrophages were determined using MTT assay. The cellular uptake of free curcumin and nano-formulated curcumin was assessed using confocal microscopy. Also, the expression levels of iNOS-2 (M1 marker), Arg-1 (M2 marker) and also pro-inflammatory cytokines were measured by real-time PCR. Data showed that the nano-formulated curcumin with spherical shape, an average diameter of 102.5 nm and high cellular uptake was significantly less toxic to peritoneal macrophages. Furthermore, the nano-formulated curcumin effectively indicated a reduction in iNOS-2 and an increase in Arg-1 levels than free curcumin. The change in macrophage phenotype by curcumin-encapsulated HA-PLA NPs could suppress the inflammation in LPS/IFN-γ stimulated macrophages as evidenced by a major reduction in pro-inflammatory cytokines. Conclusively, the results suggested that the curcumin formulation with CD44-targeting HA-PLA NPs might be a promising platform for the treatment of inflammatory diseases.
Introduction
Besides their role in host defence, macrophages protect tissue homeostasis and modulate inflammatory responses [Citation1]. Macrophages are extremely plastic and versatile cells, which are preserved in a range of pro-inflammatory M1 state or anti-inflammatory M2 state in response to the local environmental cues [Citation2]. M1 phenotypes are induced by inflammatory stimuli such as Th1 cytokine interferon-γ (IFN-γ) and toll-like receptor (TLR) ligands like lipopolysaccharide (LPS). These macrophages enhance secretion of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6 and production of nitrogen species and reactive oxygen to guarantee effective microbial eradication; and upregulate CD86, CD80 and MHC-I/II expression levels [Citation3]. However, a continuous activation of M1 phenotype may trigger collateral tissue injure and chronic inflammation [Citation4]. Conversely, in the wound-healing process or in the presence of diverse non-inflammatory factors, such as IL-4, IL-10 and IL-13 macrophages switch to a M2 phenotype and secrete high amounts of anti-inflammatory cytokines such as IL-4, IL-10, TGF-β for tissue restoration and re-modeling. Enhanced Arg-1 activity and up-regulated surface expression of macrophage mannose receptor (MMR) are occurred in the anti-inflammatory and remodelling effects of M2 macrophages as well [Citation5].
It has been demonstrated that macrophages exist mostly in M1 state triggering tissue damage in chronic immune-mediated inflammation damages and autoimmune disorders [Citation6]. Hence, a strategy that can shift macrophage type from an M1 to M2 phenotype may be a hopeful approach for the treatment of chronic inflammatory diseases.
Previous findings indicated that curcumin be able to not only directly stimulate the polarization of macrophages to M2 phenotype, but also induce switching of macrophages from M1 to M2 phenotype [Citation7–9]. Curcumin, as a major bioactive constituent in turmeric (Curcuma longa), has been described as a brilliant molecule among lots of naturally occurring substances for powerful anti-inflammatory, antioxidant, antidiabetic and anticancer activities [Citation10–15]. However, some fundamental limiting parameters of therapeutic purposes of curcumin are its highly instability in physiological pH and extremely low water solubility [Citation16–19]. Lots of pre-clinical and clinical reports show a poor bioavailability of curcumin in biological systems [Citation20].
Among numerous strategies that have been proposed to improve different hydrophobic compounds bioavailability, nanomedicine appears to be a highly feasible approach to enhance the effectiveness of the compounds [Citation21–25]. Herein, we proposed applying a Nanoparticle (NP) delivery system based on natural anionic polysaccharide hyaluronic acid (HA) that is encapsulated with curcumin for efficient re-polarization of peritoneal macrophages in vitro. HA-based NPs have presented as promising nano delivery systems owing to the non-toxic, biocompatible, biodegradable and non-immunogenic properties of HA. Importantly, HA has been described to specifically bind to CD44 receptors overexpressed on surface of macrophages [Citation26,Citation27].
In this study, curcumin was encapsulated into synthetized HA-PLA conjugates and evaluated their efficiency to modulate functional polarity of peritoneal macrophages towards anti-inflammatory M2 phenotypes as a novel platform for targeted natural compound therapy of inflammatory diseases.
Materials and methods
Materials
Sodium hyaluronate (HA, 20,000 Da) was purchased from Lifecore Biomedical Co. (Chaska, MN). Adipic acid dihydrazide (ADH), coumarin-6, dimethyl sulphoxide (DMSO), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), thioacetamide (TAA), lipopolysaccharide (LPS), interferon gamma (IFN-γ) and poly (d,l)-lactide were obtained from Sigma-Aldrich Co. (St. Louis, MO). N-Hydroxysuccinimide (NHS) and N,N′-dicyclohexylcarbodiimide (DCC) were obtained from Fluka Ltd. (Buchs, Switzerland). All other reagents were of the highest grade commercially available.
Methods
Synthesis of HA–ADH
The synthesis of HA–ADH followed the protocol of Park et al. [Citation28] and Luo et al. [Citation29] and Briefly, ADH (100 mg, 250 μmol) was dissolved in ddH2O (20 ml) to prepare HA solution of 5 mg ml−1. 10 mmol of ADH was added to the HA solution and dissolved completely by stirring for 20 min, followed by adjusting pH to 4.8 using 1.0 N HCl. Then, an equal amount of ethanol (50 vol.%) was added to the HA–ADH solution. After mixing the ethanol/HA–ADH solution for 30 min, EDC (1 mmol) was added to the mixed solution and the pH of the solution was maintained at 4.8. The reaction was stopped in 2 h by increasing the pH of the mixture to 7.0 with NaOH. Finally, the solution was dialyzed using pre-washed dialysis membrane tube (MWCO of 7000) and then lyophilized for 3 days.
Synthesis of HA–ADH–PLA
HA–ADH–PLA was synthesized according the protocol of Park et al. [Citation28]. In brief, PLA (200 mg), DCC (3.1 mg) and NHS (1.73 mg) were dissolved in 5 ml of DMSO. Then, 5 mg of HA–ADH was dissolved in 5 ml DMSO and mixed and stirred with the activated PLA solution for 24 h. The resultant solution of HA-PLA was dialyzed and freeze-dried for 3 days. The synthetized HA-ADH-PLA were characterized using 1 H-NMR and FTIR.
Fabrication of curcumin-encapsulated HA–PLA NPs
About 0.2 mg of curcumin and 15 mg of HA–ADH–PLA were dissolved completely in 10 ml of acetone. The mixture was added dropwise into ddH2O (40 ml), ultra-sonicated in 10 ml of aqueous solution with 1% PVA and subsequently stirred overnight at room temperature in the dark place. After vacuum evaporation of the solvent, the NPs were resuspended in ddH2O and filtered via a 0.22-μM pore size filter. NP suspensions were stored in 4 °C until use.
Measurement of particle size and zeta potential
The NPs size (diameter, nm), and surface charge (zeta potential, mV) were determined using a Dynamic Light Scattering Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK). Field emission scanning electron microscopy (FE-SEM) system (Hitachi S-4800 FE-SEM) was applied to determine the shape and surface morphology of prepared NPs.
Analysis of drug encapsulation and drug loading
Following fabrication of curcumin-encapsulated HA–PLA NPs, the supernatant was isolated and compared with the total amount of curcumin to determine the curcumin-loading efficiency of the NPs. The amount of non-entrapped curcumin in aqueous phase was determined at wavelengths 450 nm (λ-max of curcumin using a Lambda 950 Visible-UV spectrophotometer (PerkineElmer Fremont, CA)). The percent of curcumin encapsulated on the NPs (EE) (EquationEquation (1)(1)
(1) and drug loading (DL) (EquationEquation (2)
(2)
(2) were measured using the following formula:
(1)
(1)
(2)
(2)
Analysis of releasing profile analysis
In vitro drug release profile of curcumin from drug-loaded HA–PLA NPs were performed using the dialysis method at pH 4.4 (acetate buffer) and 7.4 (phosphate buffer). Briefly, 20–30 mg of curcumin-encapsulated HA–PLA NPs were dispersed in acetate buffer or phosphate buffer (5 ml) and transferred to dialysis membrane tubing (MW cut-off:3500) placed in 30 ml of acetate buffer or phosphate buffer with stirring at 120 rpm at 37 °C. At selected time intervals, the environmental buffer solution was replaced with the same volume of buffer, and the concentration of the released curcumin in the removed buffer was calculated applying a calibration curve at the wavelength where curcumin showed their maximum absorbance (450 nm), quantitatively monitored using Lambda 950 Visible-UV spectrophotometer (PerkinElmer Fremont, CA). Then, the accumulative ratios of the released curcumin were determined as a function of time. All of the operations were performed in triplicate.
Isolation of C57BL/6 mouse peritoneal macrophages
All animal studies were performed according to an approved protocol by Tabriz University of Medical Sciences Animal Ethics Committee. C57BL/6 mice (6–8 weeks old, weighting 20 ± 5 g) were purchased from rom the Animal Breeding Facility Centre (ABFC) of Pasteur Institute, Karaj, Iran. Briefly, 5 ml of 3% (w/v) sterile Brewer-thioglycollate medium was injected into the peritoneal cavity of each mouse to recruit macrophages to peritoneal cavity. 5 days after injection, the mice were euthanized and peritoneal fluid was collected from peritoneal cavity using cold PBS (with 3% FCS). The cell pellets were attained after centrifugation at 1500 rpm for 8 min. The cells will be incubated in RPMI1640 supplemented with 10% FBS and 1% penicillin/streptomycin for 2 h and washed twice to eliminate non-adherent cells. The cells were cultured for cytotoxicity, polarization and anti-inflammatory assays.
Cytotoxicity assay
Cytotoxicity of curcumin-encapsulated HA–PLA NPs was evaluated at 24 h using the MTT (3- [4, 5-dimethylthiazol-2yl]-2, 5-diphenyl tetrazolium bromide). It is based on the capacity of mitochondrial succinate dehydrogenase in living cells to decrease the yellow water-soluble tetrazolium salt into an insoluble, coloured formazan complex which is measured spectrophotometrically. First, the peritoneal macrophages were stimulated with 100 ng/mL of LPS plus 100 ng/mL of IFN-γ for 12 h to display M1 phenotype. Then, 2000 cells per were seeded into 96-well plates and kept for 24 h in the incubator to promote cell attachment. Then, cells were treated with different dosing level of free curcumin and curcumin-encapsulated HA–PLA NPs (1–30 μM). MTT assay at 24 h after treatment was performed to evaluate the cytotoxic effect of the NPs. The cells were incubated for 4 h with 0.5 mg/mL of MTT and the content of the wells was removed and 25 μL of Sorensen’s glycine buffer and 200 μL of pure DMSO were added to it. The number of living cells following the respective treatments was quantified by measuring the absorbance of formazan, at 570 nm using Beckmann Coulter ELISA plate reader (BioTek Power Wave XS) with a reference wavelength of 630 nm. All experiments were repeated three times.
In vitro uptake of curcumin NPs into peritoneal macrophages
Peritoneal macrophages were exposed to free curcumin and curcumin-encapsulated HA–PLA NPs for 24 h with the concentration of 15 μM. Cells were washed thrice with PBS and continued to stain with DAPI for 20 min and then washed thrice with PBS. Cell images were taken using laser scanning confocal microscope (LSCM).
Quantitative real-time PCR assay
The in vitro efficiency of curcumin-encapsulated HA–PLA NPs to modulate macrophage polarization from M1 to M2 phenotype and their anti-inflammatory effects was evaluated by determining the change in the expression levels of M1 marker (iNOS2) and M2 marker (Arg1), and pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 in peritoneal macrophages after treatment with different concentration of free curcumin and nano curcumin NPs. 200,000 cells per well of the peritoneal macrophages were stimulated with LPS (100 ng/mL) plus IFN-γ (100 ng/mL) in six-well plates to display M1 phenotype by 12-h incubation. Subsequently, the M1 macrophages were treated with three concentration of free curcumin and nano curcumin for 24 h. After drug exposure time, total RNA was extracted by the Trizol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Purity and quantity of total RNA were verified using NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Roskilde, Denmark). Additionally, the integrity of RNA samples was confirmed using agarose gel electrophoresis on 1.0% agarose gel containing GelRed™ (Biotium, Inc., Hayward, CA). cDNA was synthesized by 2-step RT-PCR kit (Thermo Fisher, K1622) according to the instructions of the manufacturer. Next, the expression level of genes was determined by quantitative real-time PCR technique. Specific primers and Hot Taq EvaGreen qPCR Mix were used following the instructions of the manufacturer. The program for real-time PCR consisted of an initial denaturation step at 95 °C for 5 min and 38 cycles of denaturation (95 °C for 10 s), annealing (53 °C for 35 s), and extension (72 °C for 20 s). Finally, amplicons were experienced with melting curve analysis of 65–95 °C. Changes that happened in expression amounts of genes between the control and the cells that treated with curcumin normalized to β-Actin mRNA amounts, calculated with the 2−ΔΔCT method.
Statistical analysis
All experiments were performed in three independent tests. Results were reported as mean ± SD. All statistical analyses using the software GraphPad Prism (version 6.01, GraphPad Software, La Jolla, CA, USA) were conducted. Compare data using a tow-way ANOVA was performed. (p < .05 shows the statistical significance).
Results and discussion
Nanomedicine approach appears an effective strategy for enhancing the efficiency of curcumin in therapeutic applications [Citation30,Citation31]. Using a variety of nanoformulation such as polymeric NPs, gold/magnetic NPs, liposomes, micelles and dendrimers for the encapsulation of curcumin has been demonstrated successful in improving its effectiveness in treating various diseases, especially different types of cancers and inflammatory disorders and importantly, some of these nanoformulated curcumin could be translated after preclinical experiments and human clinical trials [Citation10,Citation32,Citation33].
In the present study, we applied HA–PLA NPs for the encapsulation of curcumin to repolarize peritoneal macrophages in vitro. HA, as a naturally occurring polysaccharide, comprised of repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid has a strong interaction with its two main surface receptors including CD44 and RHAMM (hyaluronan-mediated motility receptor) [Citation34]. It is unique among glycosaminoglycans (GAGs) and one of the main constituents of the extra cellular matrix (ECM) that influences considerably to proliferation and migration of cells [Citation35]. The high expression of CD44 on the membrane of peritoneal macrophages convince the potential targeting of these cells for HA-based NPs delivery system. PLA is a synthetic FDA approved biodegradable polymer which is extensively applied for encapsulation of therapeutic agents in studies of drug delivery due to its very low toxicity, excellent biocompatibility and biomechanical properties [Citation36].
Characterization of HA–ADH–PLA
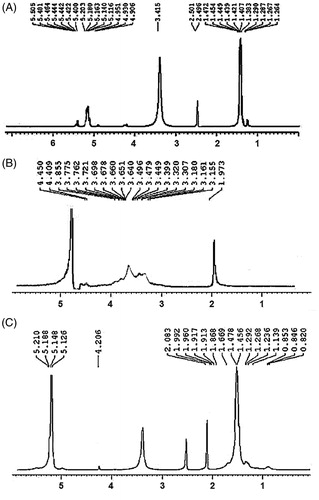
The physicochemical properties of HA–ADH–PLA were characterized using 1 H-NMR and FTIR. As shown in , the 1 H-NMR spectrum of the HA is δ 1.97 (s, 3 H, –NH–CO–CH3), according to the literature. shows the 1 H-NMR spectrum of PLA where the multiplets located at δ 1.38, δ 1.45 (2d, –O–CO–CH(CH3)–O–), and δ 5.1 (m, –O–CO–CH(CH3)–), consistent with the one found in literature. 1 H-NMR spectrum of HA–ADH–PLA revealed a chemical shifted spectrum of δ 2.08 (s, 3 H, –NH–CO–CH3) for HA; and δ 1.45, δ 1.47 (2d, O–CO–CH(CH3)–O–), and δ 5.1 (m, –O–CO–CH(CH3)–O–) for PLA, which demonstrated the successful synthesis of HA–ADH–PLA conjugates ().
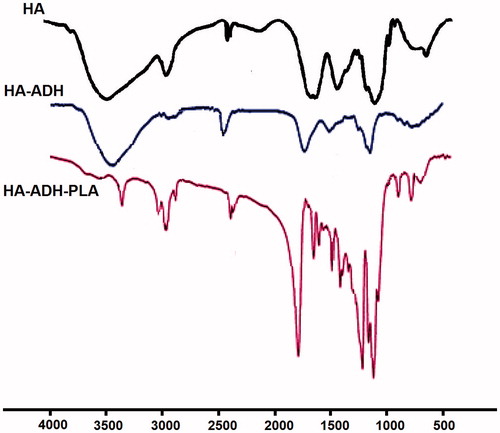
shows FT-IR spectra of HA, HA-ADH and HA-ADH-PLA. In the FTIR spectrum of HA, the absorption peaks at 3372 cm−1 and 1078 cm−1 was attributed to N–H and C–N stretching. The peaks at 1187 cm−1 and 1614 cm−1 were assigned to the C–O–C ester and amide of HA. Absorption bands at 1300 ∼ 1500 cm−1 related to three bands of C–H bending in CH3 groups of PLA and the strong absorption band at 1757 cm−1 was attributed to the C = O stretching vibrations of the ester carbonyl group of PLA. Also, FT-IR spectrum shows bands at 2800 ∼ 2900 cm−1 for stretching in the C-H of PLA. These peaks of FTIR were similar to found in literature [Citation37,Citation38]. Shifting the peaks of HA amide bond from 1614 to 1648 cm−1 generate HA–ADH amide bond, which demonstrated the bonding of HA to ADH. Furthermore, the appearance of the amide peak at 1623 cm−1 revealed the generation of a nHA–ADH–PLA amide bond. According to the 1HNMR and FTIR spectra, we confirmed the fabrication of HA–ADH–PLA successfully.
Characterization of curcumin-encapsulated HA–ADH–PLA NPs
The particle size data of curcumin-encapsulated HA–ADH–PLA NPs by DLS showed () an average size of 102.5 nm. These particles demonstrated zeta potential of −24.5 ± 2.2 mV, which suggested a good stability of curcumin-encapsulated HA–ADH–PLA NPs. FE-SEM () images show that the average size is around 86 nm with spherical morphology. From the FE-SEM images it is clear that the prepared NPs have a smooth surface with uniform and spherical in shape.
Figure 3. Dynamic light scattering (DLS) (A) and Field emission scanning electron microscopy (FE-SEM) (B) characterization of curcumin-encapsulated HA–PLA NPs.
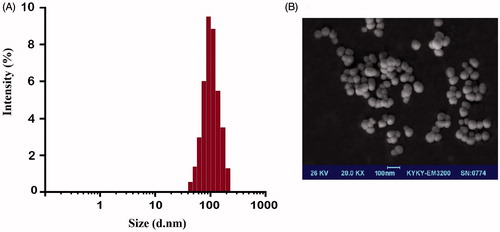
After encapsulation of curcumin into HA–ADH–PLA NPs, the size of particles was altered to 102.5 nm, dispersion of the particles was considerably improved. The modest deviation in diameter determined by DLS and SEM is ascribable to changes in the surface states of the samples under the assay conditions applied. Precisely, NPs were in a fully hydrated form when assessed using DLS, while they must be severely dehydrated for SEM characterization.
The entrapment efficiency and drug-loading efficiency of curcumin-encapsulated HA–ADH–PLA NPs system was found to be ≈ 88% and 3.7%. Usage of PVA, as a stabilizing agent, reduce drug leakage from NPs and improve drug-loading efficiency. Also, applying O/W (acetone/ddH2O) ratio 1:4 can increase drug-loading efficiency [Citation37].
In vitro drug release studies were preformed through the dialysis method at pH 4.4 and 7.4 and the release pattern is shown in . The drug release profiles at pH 7.4 showed a burst release in the first 8 h followed by a controlled release of curcumin over a period of one week and about 33% of drug was released during 144 h. Curcumin that is adsorbed on to the NPs surface and drug entrapped close to the surface might be the cause for initial burst release, since the dissolution rate of the polymer close to the surface is high, the quantity of drug released will be also high. Also, the partial spontaneous release of curcumin-encapsulated HA–ADH–PLA NPs at pH 7.4 assisted these particles to decrease drug loss through circulation, which kept more drug to target cells.
Figure 4. Drug release profiles of curcumin-encapsulated HA–PLA NPs in PBS at pH 4.4 and 7.4. The data are presented as mean ± SD (n = 3).
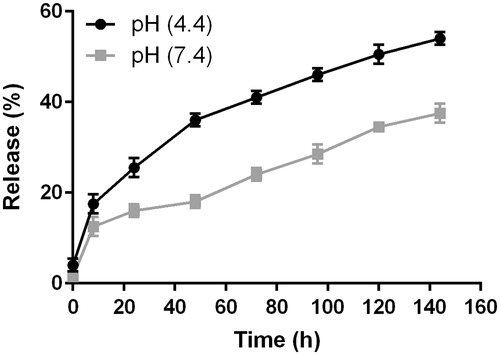
The cellular uptake of NPs most likely happens through endocytosis or potentially pinocytosis, in which lysosomal (pH 4–5) processing and the further release of drug may happen at low pH. Thus, it is essential to inspect the release pattern of curcumin-NPs in environment with acidic pH. In compared to the release profiles at pH 7.4, the drug releases at pH 4.4 were much quicker in the simulated acidic environment in the lysosome, with near to 50% of the total drug content being released within the first 120 h. These data suggested that curcumin-encapsulated HA–ADH–PLA NPs can function efficiently within target cells. These obtained data of in vitro drug release suggest that curcumin-encapsulated HA–ADH–PLA NPs might effectively act on repolarization of macrophages.
Cytotoxicity
Peritoneal macrophages are the most abundant cells amongst various immune cell populations exist in the mouse peritoneal cavity, which contribute to defend against infection in the peritoneal cavity usually by releasing inflammatory mediators. Several reports have proposed that peritoneal macrophages are capable to migrate and recruit to inflammatory sites [Citation26]. Thus, targeting peritoneal macrophages and modulating their phenotype without exerting cytotoxicity may have potential for treating inflammatory diseases.
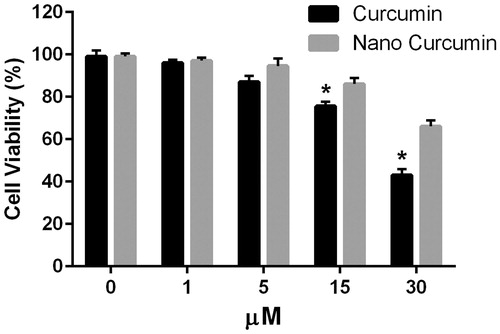
In order to examine the effects of curcumin-encapsulated HA–ADH–PLA NPs on viability of peritoneal macrophages, the cells were cultured for 24 h with or without different concentrations (1–30 μM) of curcumin-encapsulated HA–ADH–PLA NPs and free curcumin. As shown in , curcumin-encapsulated HA–ADH–PLA NPs had a minor effect on cell viability than free curcumin. After 24 h, there was no significant difference in the viability between the free curcumin and nano-encapsulated curcumin treatment at the initial concentration (1 and 5 μM). After treatment with 15 μM of free and nano-encapsulated curcumin, the viability was around 75% and 80%, respectively. Nano-encapsulated curcumin and free curcumin reduced cell viability of macrophages to about 65% and 40% at 30 μM, respectively.
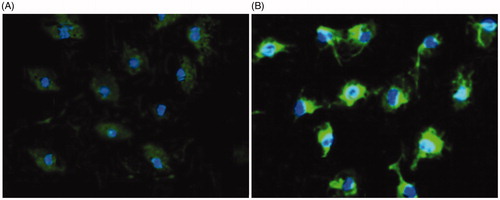
Figure 5. Cellular uptake of free curcumin (A) and nanoformulated curcumin (B) after 24 h of treatment using confocal microscopy (DAPI-blue light, curcumin-green light).
Generally, the cytotoxicity of free curcumin was significantly lowered by treatment of macrophages with curcumin-encapsulated HA–ADH–PLA NPs, suggesting that both cellular uptake of carriers and intracellular release of curcumin occur relatively slow without affecting cell viability. Notably, our data showed that curcumin-encapsulated HA–ADH–PLA NPs in 30 μM concentration had cytotoxic effects. Many studies indicate that safe and effective concentrations of curcumin range from 2.5 to 100 μM in macrophage cell lines.
According cell viability results, we applied curcumin at concentrations of 1, 5 and 15 μM incubated for 24 h in the subsequent experiment.
Cellular uptake of curcumin-encapsulated HA–ADH–PLA NPs
The potential of curcumin-encapsulated HA–ADH–PLA NPs as the system designed for treatment of inflammatory diseases was investigated through its uptake into peritoneal macrophages. The distribution of NPs inside cells was observed on fluorescent images gained by confocal fluorescence microscopy when curcumin was excited at wavelength of 488 nm (). In the case of peritoneal macrophages, the distinct difference in cellular uptake of free curcumin and nanoformulated curcumin through the fluorescence intensity was obviously gained. Poor green fluorescence intensity was detected for the case of free curcumin indicating the low cellular uptake of free curcumin into the peritoneal macrophages. In contrast, for curcumin-encapsulated HA–ADH–PLA NPs, strong green fluorescence intensity was detected representing high cellular uptake of curcumin nanoparticles into the peritoneal macrophages. nanoformulated curcumin mostly placed in the cytoplasm of macrophages in monolayer culture.
Figure 6. Effect of different concentrations of free curcumin and nanoformulated curcumin for 24 h on peritoneal macrophage viability. Macrophage viability was determined by MTT assay. Data were expressed as the mean ± SD of 3 independent experiments. *p < .05 compared to 15 and 30 μM nano-curcumin.
In vitro repolarization study
To investigate the efficiency of curcumin-encapsulated HA–ADH–PLA NPs to re-polarize peritoneal macrophages from M1 to M2 phenotypes, real-time PCR was applied to measure iNOS and Arg-1 gene expression levels in the M1 macrophages after treatment with different concentration of the nano-curcumin and free curcumin for 24 h ().
Figure 7. In vitro polarization study. iNOS-2 (M1 marker) and Arg-1 (M2 marker) in M1 peritoneal macrophages treated with free curcumin and curcumin-encapsulated HA–PLA NPs in different concentration for 24 h. *p < .05 compared to M1 macrophages that were attained by stimulating macrophages with LPS/IFN-γ, n = 3.
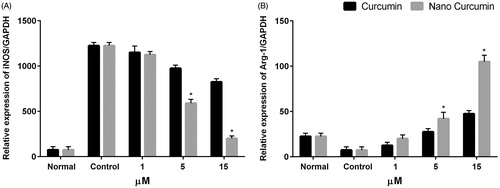
We used iNOS-2, as a M1 marker and Arg-1, as a M2 marker to evaluate re-polarization potential of nano curcumin in peritoneal macrophages. Macrophages can switch their polarity to M1 or M2 phenotype with characteristic expression profiles of surface markers as well as cytokines and chemokines secretion depending on the presence of stimuli in the local environment. iNOS-2 and Arg-1 were further established to define a simple system for indicating M1 and M2 macrophages [Citation4,Citation26]. LPS and IFN-γ stimulation enhanced iNOS level to 1300-fold, indicating the successfully polarization of peritoneal macrophages to M1 phenotype. Treatment of M1 macrophages with different concentration of curcumin and nano-curcumin significantly decreased iNOS level compared to untreated cells in dose-dependent manner (p < .05) (). It has been found that 5 μM and 15 μM of nano-curcumin could further decline iNOS level in compared to free curcumin. Treatment with 15 μM of curcumin and nano-curcumin significantly downregulated iNOS expression level from 1300-fold to about 850 and 200-fold, respectively. Furthermore, treatment of the M1 cells with free curcumin and nanocurcumin increased Arg-1 level compared to untreated and M1 cells (). As expected, nanocurcumin significantly enhanced the expression level of Arg-1 in compared to free curcumin especially at concentration of 15 μM (p < .05). Treatment with 15 μM of nanocurcumin increased Arg-1 expression level in M1 cells up to 50% compared to free curcumin. The results indicated that peritoneal macrophages were successfully re-polarized from M1 to M2 type by curcumin encapsulated in HA–ADH–PLA NPs.
In vitro anti-inflammatory effect of curcumin encapsulated in HA–ADH–PLA NPs
Following macrophage phenotype switching to the M2 type, we examined in vitro anti-inflammatory effect of curcumin-encapsulated HA–ADH–PLA NPs in peritoneal macrophages. The macrophages were treated with different concentration of free and nanocurcumin for 24 h before stimulation with LPS for 12 h. The LPS/IFN-γ stimulation upregulated TNF-α, IL-1β, and IL-6 in untreated macrophages to 190-fold, 310-fold and 245-fold, respectively, compared to control cells ().
Figure 8. In vitro anti-inflammatory study in peritoneal macrophages. expression of pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6 mRNA level at 24 h after treatment of macrophages with free curcumin and curcumin-encapsulated HA–PLA NPs, followed by stimulation with LPS/IFN-γ. qPCR was applied to measure mRNA levels. *p < .05 versus LPS/IFN-γ-treated group, n = 3.
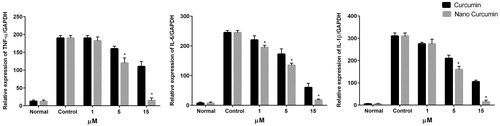
When peritoneal macrophages were treated with both free and nanocurcumin, TNF-α, IL-1β and IL-6 gene expression levels was significantly decreased in dose-dependent manner with more pronounced effect by curcumin encapsulated in HA–ADH–PLA NPs. Treatment with 15 μM of curcumin decreased TNF-α, IL-1β and IL-6 gene expression levels to 110-, 105- and 60-fold while the reduction in expression levels for nanocurcumin was 15-, 15.5- and 7.5-fold, respectively, indicating that 15 μM of curcumin encapsulated in HA–ADH–PLA NPs effectively suppressed the inflammation caused by IFN-γ/LPS in peritoneal macrophages in relative to curcumin.
Consistent our findings, numerous studies have revealed that curcumin could decrease the expression level of inflammatory cytokines, such as IL-1β, IL-6, TNF-α and reduce the production of nitric oxide (NO) [Citation7,Citation39]. Also, several reports have showed that curcumin can exert anti-inflammatory activity in IFN-γ/LPS-stimulated macrophages through various mechanisms. Curcumin sharply downregulates the LPS-induced expression of IL-6 in RAW264.7 macrophage cells by inhibiting the NF-κB signalling and suppressing Signal transducer and activator of transcription 1 (STAT1) phosphorylation [Citation40]. Furthermore, a curcumin derivative suppressed NO, TNF-α, and IL-1β expression by blocking the NF-κB/MAPK-signalling cascade in IFN-γ/LPS-treated RAW264.7 macrophages [Citation8].
Our results indicated efficiently modulation of peritoneal macrophages towards an anti-inflammatory phenotype by curcumin encapsulated in CD44-targeting NPs (HA-PLA) in comparison to free curcumin. However, the successful M2 macrophage re-polarization in an in vitro culture model system may not be comparable with an in vivo situation. So, in addition to investigation of curcumin-mediated macrophage re-polarization in other macrophages, in vivo model studies need to be establish for further validation of the efficiency of curcumin-encapsulated HA–ADH–PLA NPs in repolarization of macrophages.
Conclusions
In conclusion, the obtained results indicated that peritoneal macrophages were powerfully polarized towards anti-inflammatory phenotype by curcumin-encapsulated in HA-based NPs in vitro which may have potential for the efficiently treatment of various inflammatory diseases. curcumin was efficiently capsulated into HA–PLA NPs and in vitro treatment of the macrophages with curcumin-encapsulated HA–PLA NPs significantly reduced iNOS and upregulated Arg-1 expression levels in relative to free curcumin indicating the macrophage re-polarization from pro-inflammatory M1 to anti-inflammatory phenotype. Following phenotypic switching of macrophages, curcumin-encapsulated HA–PLA NPs reduced inflammation in peritoneal macrophages caused by LPS by decreasing expression level of pro-inflammatory cytokines TNF-α, IL-1β and IL-6. The results indicated efficiently delivery of curcumin to macrophages and modulation of macrophage phenotype by HA–PLA NPs for anti-inflammatory effect.
Acknowledgements
Authors would like to thank Department of Medical Biotechnology, Faculty of advanced Medical Sciences, Tabriz University of Medical Sciences and Australian Regenerative Medicine Institute of Monash University for supporting this project.
Disclosure statement
The authors declare that they have no competing interests.
References
- Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif Cells Nanomed Biotechnol. 2017;1–11. doi:10.1080/21691401.2017.1337022
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
- Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2015;5:230–238.
- Tran T-H, Krishnan S, Amiji MM, et al. MicroRNA-223 induced repolarization of peritoneal macrophages using CD44 targeting hyaluronic acid nanoparticles for anti-inflammatory effects. PLoS One. 2016;11:e0152024.
- Zamani R, Pilehvar-Soltanahmadi Y, Alizadeh E, et al. Macrophage repolarization using emu oil-based electrospun nanofibers: possible application in regenerative medicine. Artif Cells Nanomed Biotechnol. 2017;1–8. doi:10.1080/21691401.2017.1367689
- Liu Y-C, Zou X-B, Chai Y-F, et al. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529.
- Zhou Y, Zhang T, Wang X, et al. Curcumin modulates macrophage polarization through the inhibition of the toll-like receptor 4 expression and its signaling pathways. Cell Physiol Biochem. 2015;36:631–641.
- Chen F, Guo N, Cao G, et al. Molecular analysis of curcumin-induced polarization of murine RAW264. 7 macrophages. J Cardiovasc Pharmacol. 2014;63:544–552.
- Gao S, Liu N, Wang L, et al. Curcumin ameliorates experimental autoimmune myocarditis by inducing M2 macrophage polarization. Circulation. 2014;130(Suppl 2):A17425–A1742A.
- Montazeri M, Sadeghizadeh M, Pilehvar-Soltanahmadi Y, et al. Dendrosomal curcumin nanoformulation modulate apoptosis-related genes and protein expression in hepatocarcinoma cell lines. Int J Pharma. 2016;509:244–254.
- Alibakhshi A, Ranjbari J, Pilehvar-Soltanahmadi Y, et al. An update on phytochemicals in molecular target therapy of cancer: potential inhibitory effect on telomerase activity. CMC. 2016;23:2380–2393.
- Zarghami N, Sheervalilou R, Fattahi A, et al. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. MRMC. 2017;17:1.
- Montazeri M, Pilehvar-Soltanahmadi Y, Mohaghegh M, et al. Antiproliferative and apoptotic effect of dendrosomal curcumin nanoformulation in P53 mutant and wide-type cancer cell lines. ACAMC. 2017;17:662–673.
- Sadeghzadeh H, Pilehvar-Soltanahmadi Y, Akbarzadeh A, et al. The effects of nanoencapsulated curcumin-Fe3O4 on proliferation and hTERT gene expression in lung cancer cells. Anticancer Agents Med Chem. 2017;17:1363–1373.
- Pirmoradi S, Fathi E, Farahzadi R, et al. Curcumin affects adipose tissue-derived mesenchymal stem cell aging through TERT gene expression. Drug Res. 2017. doi:10.1055/s-0043-119635
- Rami A, Zarghami N. Comparison of inhibitory effect of curcumin nanoparticles and free curcumin in human telomerase reverse transcriptase gene expression in breast cancer. Adv Pharma Bull. 2013;3:127.
- Tabatabaei Mirakabad FS, Akbarzadeh A, Milani M, et al. A comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol. 2016;44:423–430.
- Badrzadeh F, Akbarzadeh A, Zarghami N, et al. Comparison between effects of free curcumin and curcumin loaded NIPAAm-MAA nanoparticles on telomerase and PinX1 gene expression in lung cancer cells. Asian Pac J Cancer Prev. 2014;15:8931–8936.
- Zeighamian V, Darabi M, Akbarzadeh A, et al. PNIPAAm-MAA nanoparticles as delivery vehicles for curcumin against MCF-7 breast cancer cells. Artif Cells Nanomed Biotechnol. 2016;44:735–742.
- Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artif Cells Nanomed Biotechnol. 2017;1–9. doi:10.1080/21691401.2017.1347879
- Mohammadian F, Pilehvar-Soltanahmadi Y, Mofarrah M, et al. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:1972–1978.
- Mohammadian F, Abhari A, Dariushnejad H, et al. Effects of chrysin-PLGA-PEG nanoparticles on proliferation and gene expression of miRNAs in gastric cancer cell line. Iran J Cancer Prev. 2016;9:1–8.
- Mohammadian F, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif Cells Nanomed Biotechnol. 2017;45:1201–1206.
- Amirsaadat S, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Silibinin-loaded magnetic nanoparticles inhibit hTERT gene expression and proliferation of lung cancer cells. Artif Cells Nanomed Biotechnol. 2017;45:1649–1656.
- Mohammadian F, Abhari A, Dariushnejad H, et al. Upregulation of Mir-34a in AGS gastric cancer cells by a PLGA-PEG-PLGA chrysin nano formulation. Asian Pac J Cancer Prev. 2015;16:8259–8263.
- Tran TH, Rastogi R, Shelke J, et al. Modulation of macrophage functional polarity towards anti-inflammatory phenotype with plasmid DNA delivery in CD44 targeting hyaluronic acid nanoparticles. Sci Rep. 2015;5:16632.
- Sharma M, Sahu K, Singh SP, et al. Wound healing activity of curcumin conjugated to hyaluronic acid: in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol. 2017;1–9.
- Park JK, Yeom J, Oh EJ, et al. Guided bone regeneration by poly(lactic-co-glycolic acid) grafted hyaluronic acid bi-layer films for periodontal barrier applications. Acta Biomater. 2009;5:3394–3403.
- Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release. 2000;69:169–184. doi:10.1080/21691401.2017.1358731
- Anganeh MT, Mirakabad FST, Izadi M, et al. The comparison between effects of free curcumin and curcumin loaded PLGA-PEG on telomerase and TRF1 expressions in calu-6 lung cancer cell line. Int J Biosci. 2014;4:134–145.
- Adahoun MA, Al-Akhras M-AH, Jaafar MS, et al. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif Cells Nanomed Biotechnol. 2017;45:98–107.
- Chaharband F, Kamalinia G, Atyabi F, et al. Formulation and in vitro evaluation of curcumin-lactoferrin conjugated nanostructures for cancerous cells. Artif Cells Nanomed Biotechnol. 2017;1–11. doi:10.1080/21691401.2017.1337020
- Liang H, Friedman JM, Nacharaju P. Fabrication of biodegradable PEG–PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif Cells Nanomed Biotechnol. 2017;45:297–304.
- Mero A, Campisi M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers. 2014;6:346–369.
- Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9:7081–7092.
- Raquez J-M, Habibi Y, Murariu M, et al. Polylactide (PLA)-based nanocomposites. Prog Polym Sci. 2013;38:1504–1542.
- Chen YN, Hsu SL, Liao MY, et al. Ameliorative effect of curcumin-encapsulated hyaluronic Acid–PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. IJERPH. 2016;14:11.
- Mason M, Vercruysse KP, Kirker KR, et al. Attachment of hyaluronic acid to polypropylene, polystyrene, and polytetrafluoroethylene. Biomaterials. 2000;21:31–36.
- Gao S, Zhou J, Liu N, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015;85:131–139.
- Kim S-J. Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells. J Periodontal Implant Sci. 2011;41:157–163.