 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Psoriasis is a commonly encountered chronic dermatological disease, presenting with inflammatory symptoms in patients. Systemic treatment of psoriasis is associated with several adverse effects, therefore the development of a customized topical treatment modality for psoriasis would be an interesting alternative to systemic delivery. The therapeutic modality explored in this article was the comparative treatment of psoriatic patients using nanoparticulated methotrexate in the form of jojoba oil-based microemulsion with or without fractional erbium YAG laser. Assessment parameters included follow-up photography for up to 8 weeks of treatment, estimation of the psoriasis severity [TES (thickness, erythema, scales)] score, and histopathological skin evaluation. The prepared methotrexate microemulsion was clinically beneficial and safe in treatment of psoriasis vulgaris. The concomitant use of the fractional laser provided improvement in the psoriatic plaques within shorter time duration (3 weeks compared to 8 weeks of treatment), presenting an alternative topical treatment modality for psoriasis vulgaris.
Introduction
Psoriasis is a chronic dermatological disease that occurs in a significant percent of the population [Citation1,Citation2]. It can occur at any age with prevalence in young adulthood [Citation3], affecting the skin and sometimes the nails and joints of patients [Citation4]. Several therapies are available for the topical treatment of psoriasis, however, they do not provide an adequate response owing to the inadequate percutaneous absorption and poor patient compliance caused by greasiness and stickiness of some formulations, and some patients even remain untreated [Citation5].
Methotrexate (MTX) was US Food and Drug Administration (FDA) approved for the treatment of psoriasis, and is currently recommended for practically all forms of moderate or severe psoriasis, including psoriatic arthritis [Citation6]. MTX competitively inhibits dihydrofolate reductase enzyme which converts dihydrofolate to tetrahydrofolate (fully reduced folic acid); the latter being a necessary cofactor in the synthesis of DNA. Upon inhibition of the DNA synthesis, MTX restricts hyperplasia of the epithelium, triggers programmed death of activated T cells implicated in psoriasis, and prevents neutrophilic chemotaxis [Citation7]. MTX also decreases the production of some proinflammatory cytokines. MTX also exerts partially reversible inhibition downstream on thymidylate synthetase, inhibiting cell division in the S-phase [Citation8]. Furthermore, MTX exhibits an anti-inflammatory action in psoriasis, since it causes the accumulation of adenosine which upon binding to the A2A receptor in endothelial cells, it inhibits apoptosis, chemotaxis of neutrophils and release of inflammatory mediators [Citation9].
Because systemic MTX is associated with several adverse effects, topical MTX has become a more-interesting alternative [Citation10]. However, the topical action of MTX is challenging, since it suffers limited percutaneous diffusivity owing to its aqueous solubility, its ionization at physiological pH, and unfavourable lipid/water coefficient [Citation11,Citation12]. A remedy to this problem might be the co-utilization of advanced physical enhancement techniques such as fractional erbium: YAG (yttrium aluminium garnet) laser in addition to a suitable customized nano-based delivery system for enhancing the topical percutanous penetration of MTX for treatment of psoriasis.
Fractional lasers were used in the treatment of dermatological diseases. They ablate the skin in the form of fractions and divide laser beams to microbeams [Citation13], creating minute ablation channels in the skin which provide shunts for topically administered drugs, especially those of large molecular weight [Citation14].
Many nanocarriers were promising as topical delivery systems [Citation15–21]. Microemulsions are among the most promising nanocarriers, being composed of aqueous and oily phases in addition to surfactants and cosurfactants [Citation22]. In our study, we chose microemulsion as a topical nanocarrier for MTX, and jojoba wax was selected as the oily phase of the microemulsion since it exhibits moisturizing and anti-inflammatory effects which are beneficial in psoriasis treatment. In a study that was previously reported by our research group with the same microemulsion ingredients, we were able to obtain high skin deposition to achieve topical rather than transdermal treatment of psoriasis [Citation23], in addition to other reports highlighting the established topical penetration enhancement of this microemulsion [Citation24].
Therefore, the aim of the current study was to contrast the clinical efficacy of the topically applied MTX microemulsion preparation based on jojoba oil in comparison with the concomitant application of fractional erbium:YAG laser with the MTX microemulsion as a treatment modality for plaque psoriasis.
Materials and methods
Materials
Jojoba oil was purchased from the Egyptian Natural Oil Company, Cairo, Egypt. Tween-80 and Span-85 were purchased from Al-Gomhoreya Pharmaceutical Company, Al America, Egypt. MTX solution was purchased from Mylan Pharmaceutical Inc., Greensboro, NC. Lidocaine HCl injection was purchased from Hospira Inc., Nashville, TN.
Preparation and characterization of the microemulsions
The microemulsions were prepared using the water dilution method [Citation22]. The selected microemulsion composition consisted of 40% Jojoba oil, 45% Tween-80 and Span-85 at a ratio of 3:1 as surfactant and co surfactant, respectively and 15% water. The chosen concentration was based on preliminary studies conducted which delineated that these ratios provided the best skin deposition based on factorial skin modelling [Citation23,Citation24]. The prepared microemulsions contained 0.1% MTX. The particle size and the polydispersity index (PDI) of the obtained microemulsion were measured using the Zetasizer device (nanoZS; Malvern, Worcestershire, UK).
Patients
This clinical study was conducted following the approval of the research ethics committee for experimental and clinical studies at the National Research Centre, Cairo University, Egypt (approval number 14017) with the principles outlined in the Declaration of Helsinki for human subject experimentation being followed. Informed consent was obtained from the patients, and the privacy rights were continuously observed.
Thirty patients with plaque psoriasis presenting to the Dermatology Outpatient Clinic of the National Research Centre were enrolled in this study. The diagnosis of plaque psoriasis was based on the clinical assessment and the histopathological features.
Inclusion criteria were psoriatic patients who haven’t received any systemic anti-psoriatic treatment for at least 3 months or topical antipsoriatic treatment for 2 weeks before starting the study. Exclusion criteria were patients having cardiovascular, renal or liver diseases. Patients with photosensitive or psychological disorders or immunodeficient patients were also excluded from the study.
All patients were well informed verbally about the objective of the study and the drug-related possible side effects. All participating patients provided written consent for participation, and were subjected to full history taking; personal history (age, gender and smoking habits), present history (onset, course, duration and precipitating factors) and past history (autoimmune diseases, previous treatment modalities and the response to such modalities) in addition to family history.
Clinical and dermatological examinations were conducted for each patient. The severity of psoriasis was quantitatively evaluated using the PASI score (Psoriasis Area and Severity Index) according to Fredrikson and Pettersson [Citation25], which involved the assessment of erythema (E), infiltration (I), desquamation (D) and body surface area involvement (A) over four body regions [head (h), trunk (t), upper (u) and lower (l) extremities]. PASI score is calculated by the following formula (EquationEquation (1)(1)
(1) ):
(1)
(1)
Patients were photographed before treatment. In addition, laboratory tests including complete blood count, urine analysis, liver function, and serum creatinine tests were done before the treatment was started, as well as throughout the treatment course. A skin biopsy was obtained by a 3 mm punch under local lidocaine anaesthesia from each psoriatic plaque before starting the treatment.
Clinical procedure
For each patient, we studied the effect of the topical MTX microemulsion alone over a selected psoriatic plaque on the left side of the body (left plaque) and the effect of the combined MTX microemulsion and fractional erbium:YAG laser over a similar psoriatic plaque [as regard anatomical site, size and TES (thickness, erythema, scales) score] on the right side of the body (right plaque).
For each patient, two plaques were treated in matched anatomical areas (e.g. one on each elbow). Each patient was instructed to apply a very thin film of 0.5 ml of the MTX microemulsion formulation over both psoriatic plaques three times weekly for a total treatment course of 8 weeks. Patients were asked to report any irritation or allergic contact sensitization during the treatment course, or any delayed reactions that might occur after completing the course. Weekly follow-up with the patients was conducted in the clinic, in which one of the two plaques was treated with fractional erbium: YAG laser, and the other was left as a control. In this study we used the fractional erbium:YAG laser (Fotona, LLC, Farmers Branch, TX) with a wavelength of 2.940 nm and an articulated arm was used to deliver the laser beam onto the skin. A spot diameter of 7 mm was used to achieve fluence of 3 J/cm2, and the laser hand piece was located 2 cm from the skin surface to minimize the ablation effect on stratum corneum by this laser.
Assessment tools
The following tools were employed to evaluate the treatment responses:
Photography
High-resolution digital photographs of each psoriatic plaque were obtained before treatment and with each visit till the end of the treatment course.
Psoriasis severity (TES) score
We used a physician-based, four-point scoring system in which the thickness, erythema, and scale within each plaque was rated from 0 (none) to 3 (severe) to evaluate the changes in the severity of individual psoriatic plaques before treatment and with each visit till the end of the treatment course [Citation26]. The TES score is the sum of the scores given to each individual parameter (thickness, erythema and scale).
Histopathological evaluation
A biopsy was obtained by a 3 mm punch under local lidocaine anaesthesia from each psoriatic plaque after completion of the treatment course to evaluate the skin pathological changes. Formalin-fixed skin fragments were embedded in paraffin and stained with hematoxylin and eosin, and examined with the use of light microscope (Model LeicaQuin LM 3000, Germany).
Statistical analysis
All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL) v.16 for Microsoft Windows. Continuous variables were tested for normality by the Shapiro–Wilk normality test. Values are presented as mean ± standard deviation, or in the case of non-normally distributed data as median and inter-quartile range.
For comparison between paired parameters (before and after treatment), paired t-test or the Wilcoxon matched-pairs signed-rank test were used according to data normality. For comparison between independent groups, the chi-squared test was used to compare percentages between different groups of patients. Normally distributed data were analyzed using independent samples t-test. Non-normally distributed data were analyzed using the Mann–Whitney U-test. p values <.05 were considered statistically significant.
Results
Preparation and characterization of the microemulsion
The MTX microemulsion was successfully prepared using the water dilution method, with a particle size 56 ± 3 nm, and a PDI value of 0.31 ± 0.02.
Patients
The mean PASI score for the patients before treatment was 8.587 ± 5.081, indicating that most of the patients suffered from mild psoriasis () [Citation15]. The MTX formulation was tolerable by all 30 patients and they all completed the treatment course of 8 weeks. No changes in liver function tests or blood count test were observed in all patients.
Table 1. Clinical data of the patients.
Effect of MTX formulation on the selected psoriatic plaques
According to and , there was a statistically significant reduction of the TES score of the psoriatic plaque after 3 and 8 weeks, respectively from the start of the treatment course in both the left and right sides (p < .05), with significant progress in treatment from 3 weeks to 8 weeks in both sides. Upon comparing the TES score between the right and left sides after 3 weeks, the right side treated with the microemulsion/laser combination displayed significantly lower TES score compared to the left side treated with the microemulsion formulation only (p < .05). Similar results were obtained after 8 weeks of treatment, in which the right side treated with the microemulsion/laser combination displayed a significantly lower TES score compared to the left side treated with the microemulsion formulation only (p < .05).
Figure 1. Assessment of the left elbow psoriatic lesion treated with MTX microemulsion formulation only along the treatment course for patient 1: (A) before treatment, (B) after 3 weeks of treatment and (C) after 8 weeks of treatment.
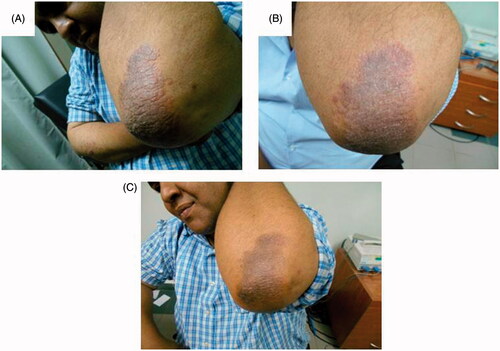
Figure 2. Assessment of the right elbow psoriatic lesion treated with MTX microemulsion formulation/fractional erbium:YAG laser along the treatment course for patient 1: (A) before treatment, (B) after 3 weeks of treatment and (C) after 8 weeks of treatment.
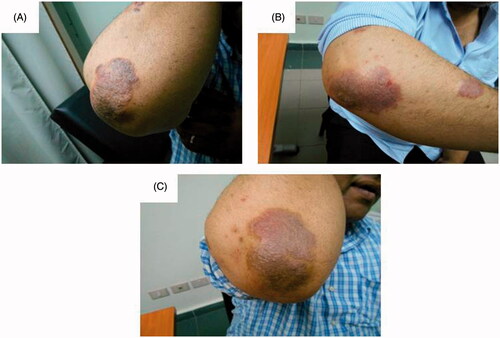
Figure 3. Assessment of the left knee psoriatic lesion treated with MTX microemulsion formulation only along the treatment course for patient 2: (A) before treatment, (B) after 3 weeks of treatment and (C) after 8 weeks of treatment.
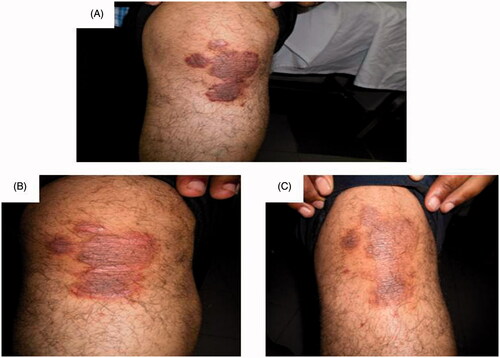
Figure 4. Assessment of the right knee psoriatic lesion treated with MTX microemulsion formulation/fractional erbium:YAG laser along the treatment course for patient 2: (A) before treatment, (B) after 3 weeks of treatment and (C) after 8 weeks of treatment.
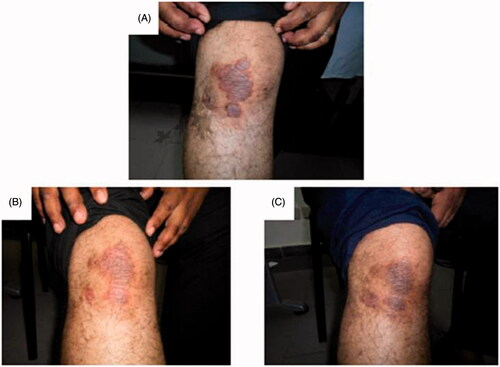
Table 2. TES score of psoriatic plaques before and after 3 and 8 weeks of the treatment.
The percent reduction of the TES score on the right side was statistically significantly higher than the percent reduction on the left side after 3 weeks of the treatment course, with a statistical insignificance after 8 weeks of treatment (p > .05) (). The cured lesions showed a decreased epidermal thickening ().
Figure 5. Patient with psoriatic plaques over the right elbow before treatment with microemulsion/laser combination (A,C) and after 8 weeks of treatment (B,D), showing a decreased epidermal thickness.
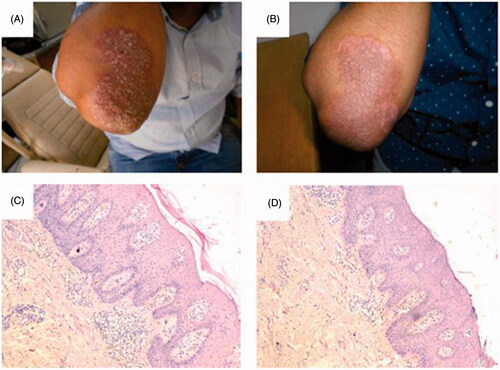
Table 3. Comparison between right and left psoriatic plaques regarding the percent change of TES score after 3 and 8 weeks of treatment course.
Discussion
From a dermatologist’s perspective, there are lots of expectations from nanotechnology. In the current article, we attempted the formulation of MTX in the form of soft colloidal particles i.e. microemulsion, which was known for its both topical and transdermal delivery enhancement of drugs. Furthermore, the fractional erbium laser is a very useful tool for obtaining good clinical outcomes in dermatology. Therefore, in the presented work, the combination of laser with the nanoformulation of MTX was compared to the mere use of MTX nanoformulation, in order to conclude whether this combination therapy would provide more usefulness in the treatment of plaque psoriasis. The clinical outcome in patients was first assessed after 3 weeks of treatment in order to delineate if the presence of laser would accelerate the response to treatment, and then after 8 weeks which is a commonly encountered treatment time course for psoriasis [Citation15,Citation24].
As delineated in the current study, topical MTX microemulsion prepared from jojoba oil significantly treated psoriatic plaques and exhibited no side effects. The enhanced MTX skin deposition encountered with the MTX microemulsion might be ascribed to the penetration enhancer influence of the used ingredients, and the small size of the microemulsion formulation.
The concomitant use of fractional erbium:YAG laser with the microemulsion formulation resulted in 24.4% more reduction in TES score compared to the mere use of microemulsion after 3 weeks, and a 6.72% more reduction in TES score compared to the mere use of microemulsion after 8 weeks, suggesting its synergistic effect for psoriasis treatment, and the acceleration of the clinical improvement of the psoriatic plaques within only about 3 weeks of treatment. This supports the findings of the previous studies declaring that certain lasers especially fractional lasers can augment transcutaneous drug delivery [Citation27], and could be ascribed to the creation of ablation channels within the skin, that provide more access pathways for topically applied delivery systems.
Upon examination of skin biopsies of patients, it was evident that a decrease in epidermal thickening occurred with patients, which was proportionate with the decrease of the indurations and scales of the psoriatic lesions, providing a microscopical proof of efficacy of the topical treatment modality.
The findings of our study demonstrate that MTX microemulsion prepared from jojoba oil was both safe and clinically promising in the treatment of psoriasis. The concomitant use of the fractional laser played a role in the enhancement of the delivery of the MTX preparation to provide more improvement in the psoriatic plaques within shorter time duration. The described regimen could be considered a possible topical treatment alternative for psoriasis with a clinical proof of concept, but needs more investigations on larger scale of patients.
| Abbreviations | ||
| MTX | = | methotrexate |
| YAG | = | yttrium aluminium garnet |
| PASI | = | Psoriasis Area and Severity Index |
| HPLC | = | high-performance liquid chromatography |
| TES score | = | thickness, erythema, scales |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665–1668.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Derm Rep. 2014;3:61–78.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64:18–23.
- Abdelgawad R, Nasr M, Hamza MY, et al. Topical and systemic dermal carriers for psoriasis. Int J Curr Pharm Res. 2016;8:4–9.
- Carretero G, Puig L, Dehesa L, et al. Guidelines on the use of methotrexate in psoriasis. Actas Dermosifiliogr. 2010;101:600–613.
- Elango T, Dayalan H, Gnanaraj P, et al. Impact of methotrexate on oxidative stress and apoptosis markers in psoriatic patients. Clin Exp Med. 2014;14:431–437.
- Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997;23:739–755.
- Festugato M. Adenosine: an endogenous mediator in the pathogenesis of psoriasis. An Bras Dermatol. 2015;90:862–867.
- Ali MF, Salah M, Rafea M, et al. Liposomal methotrexate hydrogel for treatment of localized psoriasis: preparation, characterization and laser targeting. Med Sci Monit. 2008; 14:166–174.
- Weinstein GD, McCullough JL, Olsen E. Topical methotrexate therapy for psoriasis. Arch Dermatol. 1989;125:227–230.
- Wong TW, Zhao YL, Sen A, et al. Pilot study of topical delivery of methotrexate by electroporation. Br J Dermatol. 2005;152:524–530.
- Forster B, Klein A, Szeimies RM, et al. Penetration enhancement of two topical 5-aminolaevulinic acid formulations for photodynamic therapy by erbium:YAG laser ablation of the stratum corneum: Continuous versus fractional ablation. Exp Dermatol. 2010;19:806–812.
- Paasch U, Haedersdal M. Laser systems for ablative fractional resurfacing. Expert Rev Med Devices. 2011;8:67–83.
- Abdelgawad R, Nasr M, Moftah NH, et al. Phospholipid membrane tubulation using ceramide doping “cerosomes”: characterization and clinical application in psoriasis treatment. Eur J Pharm Sci. 2017;101:258–268.
- Fadel M, Samy N, Nasr M, et al. Topical colloidal indocyanine green-mediated photodynamic therapy for treatment of basal cell carcinoma. Pharm Dev Technol. 2017;22:545–550.
- Bsieso EA, Nasr M, Moftah NH, et al. Could nanovesicles containing a penetration enhancer clinically improve the therapeutic outcome in skin fungal diseases? Nanomedicine. 2015;10:2017–2031.
- Bseiso EA, Nasr M, Sammour OA, et al. Novel nail penetration enhancer vesicles “nPEVs” for treatment of onychomycosis. Drug Deliv. 2016;23:2813–2819.
- Garg T, Rath G, Goyal AK. Nanotechnological approaches for the effective management of psoriasis. Artif Cells Nanomed Biotechnol. 2017;44:1371–1382.
- Parnami N, Garg T, Rath G, et al. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol. 2014;42:406–412.
- Agrawal U, Gupta M, Vyas SP. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif Cells Nanomed Biotechnol. 2015;43:33–39.
- Hathout RM, Nasr M. Transdermal delivery of betahistine hydrochloride using microemulsions: physical characterization, biophysical assessment, confocal imaging and permeation studies. Colloids Surf B Biointerfaces. 2013;110:254–260.
- Nasr M, Abdel-Hamid S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modelling. Drug Dev Ind Pharm. 2016;42:636–643.
- Nasr M, Abdel-Hamid S, Moftah NH, et al. Jojoba oil soft colloidal nanocarrier of a synthetic retinoid: preparation, characterization and clinical efficacy in psoriatic patients. Curr Drug Deliv. 2017;14:426–432.
- Fredriksson T, Pettersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica. 1978;157:238–244.
- Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51:563–569.
- Sklar LR, Burnett CT, Waibel JS, et al. Laser assisted drug delivery: a review of an evolving technology. Lasers Surg Med. 2014;46:249–262.

