?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease, affecting almost 1% of world population. Although the exact cause of RA is not known but the complex interaction between inflammatory mediators like tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2) and nitric oxide (NO) is accountable for cartilage destruction in joints. Gold is used for arthritis treatment since long without knowing its mechanism of action. Hence, the present study was designed to assess antiarthritic activity of nanogold (AuNGs) in collagen-induced arthritic (CIA) rat model by virtue of decreasing inflammatory mediators and oxidative stress. After induction CIA rats were treated with AuNGs in phosphate buffer at a dose of 20 μg/kg body weight for 20 days and found a significant decrease in the level of inflammatory mediators like TNF-α, IL-1β, COX-2 and transcription factor NF-kB (Nuclear factor-kB), which was found to be elevated in CIA rats. Additionally imbalance in oxidant and antioxidant status were determined and perceived that AuNGs remarkably attenuates the imbalance in level of antioxidant and oxidant near to normal. In consistent to biochemical results, mRNA expression of NF-kB, TNF-α, COX-2, and iNOS were also up-regulated in CIA rats, which were considerably down regulated by AuNGs treatment. These findings were positively correlated with the histological results of joints, displayed reduced inflammation and bone erosion in treated group. This study demonstrates the ability of AuNGs to ameliorate production of inflammatory mediators and oxidative stress in CIA rats.
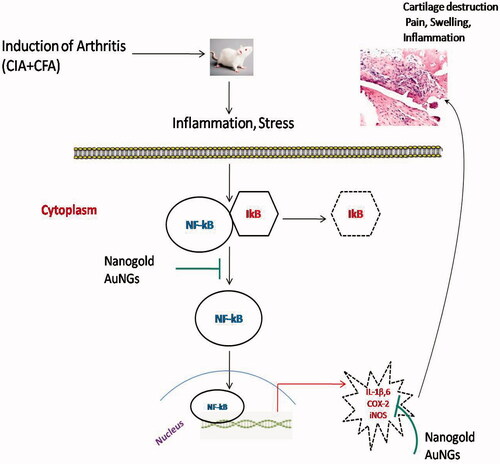
Induction of arthritis in rats showed increased inflammation, which activate the transcription factor NF-kB through activation of of IkB kinases (IKK) and ubiquination/proteosome degradation of IKB and transportation of activated NF-kB from cytoplasm to nucleus. In nucleus activated NF-kB bind to the promoter region of target gene and up regulate the production of pro-inflammatory cytokines, COX-2 and other inflammatory mediators that leads to cartilage destruction. AuNGs inhibit the activation of NF-kB and other inflammatory mediators and attenuate inflammation and cartilage destruction. COX-2: cyclooxygenase-2; IKK: IkB kinases; IKB: I Kappa B; IL-1β: interleukin-6; IL-6: interleukin-6; iNOS: inducible nitric oxide synthase; NF-kB: nuclear transcription factor kappa B; ROS: reactive oxygen species; TNF-α: tumour necrosis factor-alpha.
Graphical Abstract
Introduction
Rheumatoid arthritis (RA) is an autoimmune and systemic inflammatory disease characterized by infiltration of various inflammatory cells in the synovial lining of peripheral joints followed by pronounced tumour like pannus formation due to activation and proliferation of synovium leading to chronic inflammation, oedema, pain and finally joint destruction in patients with established RA. These activated synoviocytes (resident macrophages of joint) responsible for release of variety of toxic inflammatory mediators such as tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and reactive oxygen species/reactive nitrogen species (ROS/RNS). ROS/RNS are free radicals with unpaired electron derived from molecular oxygen or nitrogen and are toxic to cellular components such as proteins, membrane lipids and DNA, result in significant damage to cell structures, and cumulate into a situation known as oxidative stress. In RA, these inflammatory mediators and ROS/RNS are chief regulator of inflammation and joints destruction [Citation1–3]. These cytokines synergistically act and induce expression of matrix metalloproteinases (MMPs) from extra cellular matrix fragments and activated synoviocytes, which is a hall marker of arthritis [Citation4]. Expression of these noxious inflammatory mediators and MMPs is regulated by transcription factor NF-kB [Citation5,Citation6].
NF-kB is a transcription factor, regulate expression of many genes involved in inflammatory responses and their activity is strongly modulated by various stimuli such as pro-inflammatory cytokines, UV light and oxidative stress [Citation7]. Therefore, down-regulation of pro-inflammatory cytokines and NF-kB might be a suitable treatment strategy for RA. Till date there is no permanent cure for RA is available. The current treatment modalities only provide symptomatic relief from pain and inflammation.
Disease modifying anti-rheumatic drugs (DMARDs) are non-steroidal anti-inflammatory drugs (NSAIDs) are current approaches for RA treatment. Methotrexate (MTX) is most commonly used DMARDs, often approved as a first line drug for the treatment of RA. MTX is a folate analogue intended to inhibit dihydrofolate reductase and obstructs with DNA and RNA synthesis requisite for cell proliferation [Citation8]. In RA it inhibits, synthesis and proliferation of the lymphocytes and other cells accountable for inflammation in the joint [Citation9]. Though use of MTX/DMARDs or NSAIDs is effective for only a very short period of time, long-term application causes numerous life threatening side effects such as gastric ulcer stomatitis and immunosuppression [Citation10,Citation11]. Therefore, researchers are trying to screen new natural or manmade biological components which inhibit inflammation with lower toxicity and higher efficiency.
Nowadays, nanotechnology paying much attention because of its vast application in energy management, information technology, electronics and medicines [Citation12]. Materials of nanoscale level are synthesized by précised engineering with controlled physicochemical properties, possess increased surface to volume ratio and high proportion of surface exposed atoms [Citation13]. Discovery of therapeutic inorganic nanoparticles (NPs) brings a profound impact in medical sciences. However, very less study is known to report about their use in the treatment of rheumatic diseases [Citation14,Citation15]. AuNGs have been explored extensively in biomedical applications since long and its area of use is extremely wide [Citation16–18]. In particular, it encompasses clinical chemistry, genomics, immunoanalysis [Citation19], proteineomics [Citation20], detection and photothermolysis of microorganisms and cancer cells [Citation21–23]. Furthermore, it also used in the targeted delivery of drugs, DNA and antigens, optical bioimaging and the scrutinizing of cells and tissues using modern techniques [Citation24–26]. In general, it can be said that AuNGs could be used in nearly all medical applications such as prevention, diagnostics, therapy and hygiene. Chrysotherapy is a treatment of arthritis by using gold compound and beneficial effect is likely attributable to the local release of gold ions that can be traced to the surrounding connective tissues. Gold could be used to build a new class of anti-arthritic drugs with high affectivity and minimum side effects. Though, AuNGs are used in treatment of various diseases, there are limited scientific documentation of AuNGs are available in relation to RA treatment [Citation27,Citation28]. Hence, the present study was designed to investigate the ameliorating effect of AuNGs on CIA induced rat, a model that mimics the clinical and histological features of human RA.
Materials and methods
Tri-sodium citrate dihydrate (Na3C6O7·2H2O), Chloroauric acid (HAuCl4), Complete Freund’s Adjuvant (CFA), Collagen type II (CII), 1-chloro-2,4-dinitrobenzene (CDNB), Thiobarbituric acid (TBA) and Trichloroacetic acid (TCA) were obtained from Sigma-Aldrich (St. Louis, MO). Trizol reagent was purchased from Invitrogen (Carlsbad, CA). Maxima SYBR green/ROX qPCR master mix (2×) and Verso cDNA synthesis kit were obtained from Thermo Scientific (Waltham, MA). All other chemicals and reagents used were of analytical grade.
Synthesis of AuNGs
AuNGs were synthesized according to the procedure described by McFarland et al. [Citation29] with some modifications. In short, HAuCl4 (1 mM) was mixed with 20 ml of double distilled water and stirred at 80 °C, afterward 1% tri-sodium citrate dihydrate was added. Solution colour was changed from transparent to first purple followed by reddish and after 10 min deep red color. This shows progress of AuNGs formation which further undergo for characterization.
Characterization
To determine size, AuNGs were evaluated by JEOL JEM-2100 F transmission electron microscope (JEOL, Japan) at an accelerating voltage of 200 kV. TEM samples were prepared by coating of NPs on carbon-coated copper grids at room temperature. A drop of homogeneous suspension of AuNGs after sonication was placed, air-dried and imaged. Size distribution of AuNGs was further explored by dynamic light scattering (DLS) spectrometer at 830 nm, using DynaPro-TC-04 DLS (Wyatt Technology, Santa Barbara, CA) equipped with a temperature controlled microsampler. The mean hydrodynamic radius (Rh) and polydispersity were assessed by the Stokes–Einstein equation:
where Rh is hydrodynamic radius, k is Boltzman's constant, T is absolute temperature, η is viscosity of solvent and D is translational diffusion coefficient. X-ray diffraction (XRD) pattern of AuNGs was recorded using a Rigaku Miniflex X-ray diffractometer with Cu Kα radiation (λ = 1.54060 Å) in 2θ ranging from 20° to 80°.
Test animals
Male Wistar albino rats were obtained from the central animal house facility of the institute (UCMS & GTB Hospital, Delhi, India). Animals were allowed to accustom for one week prior to initiation of experiment. They were randomly divided into four groups and maintained under standard conditions (light:dark 14:10 h, ambient temperature 22 ± 2 °C, humidity 40–45% and free access to food and water). All animals received humane care in compliance with animal ethic guidelines of the committee. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC Approval No. IAEC/2012–03: Dated 1 May 2012). Animals were kept under close observation for food and water intake, body weight, activity etc. until the experiment was over.
Induction of arthritis
Arthritis in male Wistar rats was induced by immunization with Collagen type II (CII), emulsified with Complete Freund's Adjuvant (CFA) as described earlier by Campo et al. [Citation30]. Briefly, collagen was dissolved in 0.1 M acetic acid (2 mg/ml) by gently stirring overnight at 4 °C and emulsified with CFA. Each rat was immunized at base of tail with 0.2 ml (200 μg) emulsion by subcutaneous injection and booster dose was given 14 days after immunization to stabilize disease symptoms.
Assessment of arthritic index (AI) and footpad thickness
Arthritis development was monitored by a blinded independent observer without knowledge of the treatment protocol with a macroscopic scoring system of four limbs ranging from 0 to 15 (1 point for each swollen or red toe, 1 point for mid-foot digit or knuckle and 5 points for a swollen ankle). The scores of four paws were added and a total score of 60 for each rat were considered as RA induction [Citation31]. Clinical severity was also assessed by measuring footpad thickness using a dial-gauge caliper.
Grouping of animals
Animals were randomly divided into four groups (6 animals each) and treatments were started on the 25th day for 20 days. An overview of treatment is shown in .
Figure 1. Schematic representation of the experimental model. Time course study of arthritis induction and treatment with reference drug MTX and AuNGs in collagen-induced arthritic rats.
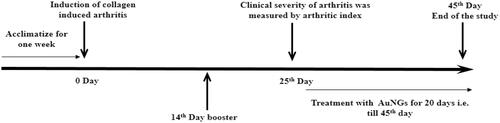
Group I – Control (non-induced + normal saline).
Group II – Induced + Normal Saline
Group III – Induced + Treated with MTX 0.25 mg/kg body weight i.p. once in a week
Group IV – Induced + Treated with AuNGs 20 μg/kg body weight i.p. daily for 20 days
*Dose was selected on the basis of primlinary study in our laboratory.
Collection of samples
After completion of treatment, rats were fasted overnight and blood sample was collected by retro-orbital vein puncture for biochemical studies. Serum was separated and used for estimation of lipid peroxidation, reduced glutathione, cytokines, NF-kB and cyclooxygenase-2 (COX-2).
Assessment of oxidative stress parameters
Lipid peroxidation (LPO) was determined in serum as described previously by Satoh [Citation32]. Briefly, 0.5 ml serum was first precipitated with 20% TCA and formed precipitate then suspended in 0.05 N sulphuric acid and TBA (0.07% in 1 M sodium sulfate). This solution was incubated for half an hour in boiling water bath, resulted in MDA-TBA adduct formation which further extracted with butanol. LPO was measured spectrophotometrically at 532 nm and results were expressed as nM/ml.
Reduced glutathione content (GSH) was estimation in whole blood according to the procedure described by Tietze [Citation33]. In short, reaction mixture (1 ml) contained glutathione reductase (1 unit), NADPH [0.2 µM/ml in 0.01 M/0.005 M phosphate EDTA buffer (pH 7.5)] and 25 µl of hemolysate. A chromophoric product was formed after addition of DTNB (Ellman's reagent), measured at 412 nm. The result was expressed as μM/ml of blood.
Estimation of cytokine levels
Level of cytokines TNF-α and IL-1β were determined in serum by standard ELISA kits (Pierce Biotechnology, Rockford, IL) as per manufacturer’s instruction using microplate reader (Thermo Scientific, Waltham, MA).
Estimation of NF-kB and COX-2 levels
The NF-kB and COX-2 levels were measured in serum by standard ELISA kits (Cusa Biotech, Hubei Province, China and MyBiosource, CArespectively) using microplate reader.
RNA extraction and quantitative real time PCR of rat joints
To compare expression of mRNA in each group, mRNA levels of TNF-α, NF-kB, COX-2 and inducible nitric oxide synthase (iNOS) were analyzed in joint tissue of rats using qPCR. Briefly, after euthanization of rats, synovial tissues were removed as described by Hyc et al. [Citation34] and kept overnight in Trizol for isolation of RNA according to the manufacturer’s protocols. Extracted RNA was dissolved in nuclease free water and concentration was determined by using NanoDrop (Thermo Scientific, Wilmington, DE). Purity of RNA was calculated by taking ration of absorbance at 260/280 nm and 260/230 nm. RNA integrity was examined by agarose gel electrophoresis.
For each experiment, 1 μg of RNA from individual joints (non pooling strategy used) of six rats, each treatment group was used for amplification and generation of single strand cDNA using Verso cDNA Synthesis Kit (Thermo Scientific, Waltham, MA) which further used to quantify mRNA levels of NF-kB, TNF-α, COX-2 and iNOS using qPCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primer sequence for NF-kB, TNF-α, COX-2, iNOS and GAPDH is shown in .
Table 1. Sequence of primers used in quantitative real-time PCR.
Real-time PCR was performed on Qiagen Thermal Cycler (Rotor-Gene Q 5plex HRM System, Qiagen). Amplificatory reactions were achieved according to the manufacturer's protocol using Maxima SYBR Green/ROX qPCR Master Mix (2×) kit (Thermo Scientific, Waltham, MA). In brief, qPCR was executed with 25 µl of total reaction mixture, comprising 1–2 μl ≤500 ng cDNA, 1 μl 0.9 μM primer (forward and reverse) and 12.5 μl of maxima SYBR Green master mix and nuclease free water. Initial denaturation was carried out at 95 °C for 5 min followed by 40 cycles of 3-step PCR (denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s). PCR products were analyzed by measuring increased fluorescence due to binding of SYBR Green I Dye to dsDNA and quantified by using threshold cycle (Ct). The relative fold change in expression of NF-kB, TNF-α, iNOS mRNA was calculated by the 2−ΔΔCt method as described by Schmittgen et al. [Citation40]. The results were normalized to the mRNA expression level of GAPDH in each sample.
Histological studies
Joint tissues were taken for histological examination from CO2 euthanized animals, fixed in 10% formalin buffer and then decalcified by 10% EDTA solution. After decalcification, samples were processed, cut into 3–4 µm thick sections and then stained with haematoxylin and eosin staining. Any changes were evaluated on the basis of histological parameters like inflammation, pannus formation and erosion of cartilage using 0–4 point scale. Normal =0, inflammatory cell infiltration = 1, synovium hyperplasia = 2, pannus formation = 3 and severe cartilage and bone erosion = 4.
Statistical analysis
All values were expressed as mean ± SD. Data were analyzed by a one-way ANOVA using SPSS version 16 (SPSS, Chicago, IL) statistical program and individual comparisons of treatment were gained by Tukey’s test for multiple comparisons. A difference of p < .05 was considered to be statistically significant.
Results
Characterization of AuNGs
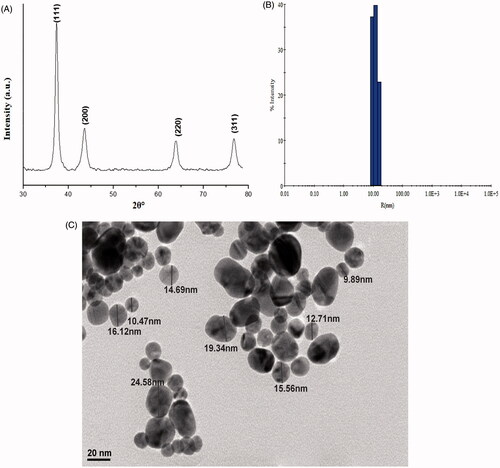
shows XRD pattern of AuNGs. The main diffraction peaks 2θ appears at 38°, 44.25°, 64.5° and 77.5° in which Bragg’s law correspond to the planes are (111), (200), (220) and (311), respectively. These peaks are the characteristics and in good agreement with the standard XRD peaks of AuNGs as reported in previous studies (JCPDS-040784) [Citation41,Citation42]. DLS measurements were performed to further investigate size and polydispersity of AuNGs (). The hydrodynamic radii of AuNGs was increased slightly due aggregation in the medium and it was recorded ∼20 nm. The shape and size of AuNGs was further examined by TEM as shown in . TEM images of AuNGs clearly show isolated particles with ∼15 nm in size. The average diameter of AuNGs was calculated by measuring several particles in random fields of TEM view demonstrated that most of the NPs were spherical in shape.
Figure 2. Characterization of AuNGs. (A) X-ray diffraction pattern of AuNGs was recorded at room temperature using Rigaku Miniflex X-ray diffractometer with Cu-Kα radiation (λ = 1.54060 Å) in 2θ ranging from 20° to 80°; (B) DLS measured the hydrodynamic radii of AuNGs. Samples of AuNGs were prepared in 20 mM phosphate buffer, pH 7.0 and sonicated well before measurements; (C) TEM images of AuNGs was recorded with a JEOL JEM-2100F transmission electron microscope with an accelerating voltage of 200 kV.
Effect of AuNGs on Arthritic index and foot pad swelling
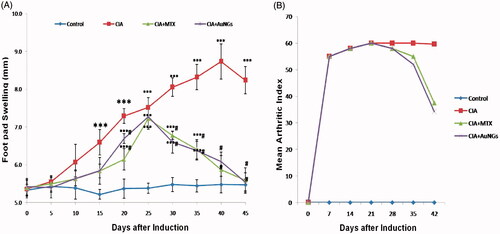
Arthritis index () and foot pad thickness () both represents severity of disease and are used to evaluate the efficacy of treatment. CIA rats displayed an increased in AI and foot pad thickness as evidenced by inflammation in joints, erythema and oedema in paws and ankles. The extent of inflammation and erythema, oedema of foot pad was decreased (p < .05) in AuNGs treated rats as compared to the CIA rats. Changes in arthritic score and paw oedema volume were positively correlated.
Effect of AuNGs on inflammatory mediators
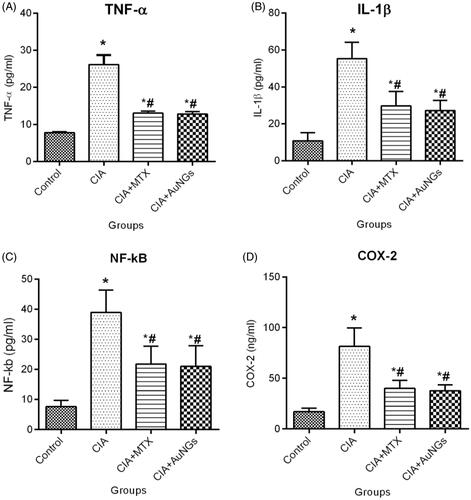
In order to determine ameliorating effects of AuNGs on the production of pro-inflammatory cytokines, responsible for joint inflammation and cartilage destruction, we measured serum levels of TNF-α () and IL-1β () in CIA rats. Significant increment in serum levels of pro-inflammatory cytokines, TNF-α and IL-1β was detected in CIA rats as shown in . AuNGs treatment resulted a substantial reduction in the level of proinflammatory cytokines TNF-α (51.21%) and IL-1β (50.76%) as compared to the induced rats (p < .05) and these results were comparable to that of the reference drug (MTX)-treated rats.
Effect of AuNGs on NF-kB and COX-2 level
The level of NF-kB and COX-2 activities in CIA-induced rats was considerably higher as compared to control (). Reduction in the activity of NF-kB (47.42%) and COX-2 (53.80%) was perceived in AuNGs-treated rats and the results were comparable to MTX-treated drugs.
Effect of AuNGs on CIA induced oxidative damages
To assess effects of AuNGs on CIA-induced oxidative tissue damage, MDA and GSH levels were measured in the serum of CIA rats. Increased oxidative stress causes oxidation of membrane lipids and cellular damage. Elevated, MDA levels are a good indicator of lipid peroxidation. It was observed that MDA levels were increased significantly in the serum of CIA rats, however, AuNGs treatment suppressed MDA levels (p < .05) (). The reduced GSH levels in whole blood of CIA rats were also recovered after treatment with AuNGs. These finding showed that the administration of AuNGs suppresses CIA-induced oxidative damages and these results were comparable to the MTX group ().
Effect of AuNGs on NF-kB, TNF-α, COX-2 and iNOS mRNA expression
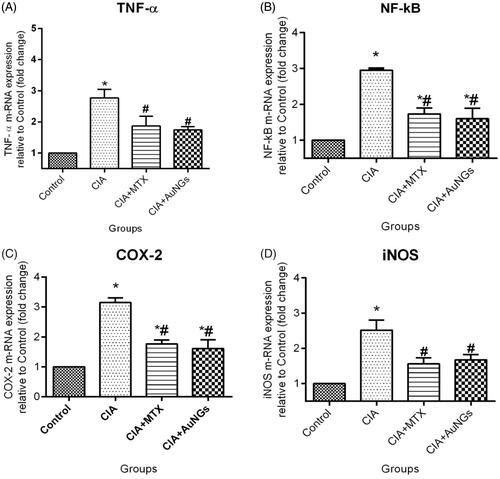
To determine inhibitory mechanism of AuNGs on ROS/RNS and other inflammatory mediators in CIA rats, we examined m-RNA expression levels of TNF-α (), NF-kB (), COX-2 () and iNOS () by qPCR. In CIA rats, expression level of TNF-α, NF-kB, COX-2 and iNOS was significantly increased in comparison to healthy control rats. However, after treatment with AuNGs these up-regulations were considerably inhibited (). Treatment with both reference drug MTX and AuNGs, expression of these inflammatory mediators decreases significantly in comparison to CIA rats (p < .05). These results indicate that reductions in expression of TNF-α, NF-kB, COX-2 and iNOS at transcriptional levels contributed to the inhibitory effect of AuNGs on ROS and inflammatory cytokines production.
Figure 6. Effect of AuNGs on mRNA expression (fold change) in joints of CIA rats, TNF-α (A) NF-kB (B) COX-2 (C) iNOS (D). *Significantly different from control (p < .05); #significantly different from induced group (p < .05). Data are expressed as mean ± SD (n = 6) and analyzed by one-way ANOVA followed by Tukey's multiple range test.
Effect of AuNGs on histopathology of joint tissue
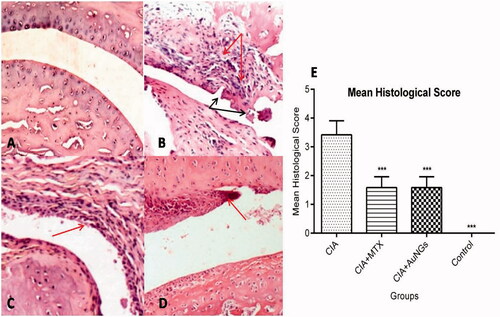
Persistent with the biochemical and molecular alterations, histological findings of joint tissues, using H&E, revealed a massive infiltration of inflammatory cells, pannus formation and destruction of articular cartilage in CIA rats (). AuNGs treatment ameliorated the changes at histological level significantly accompanied by a reduction in bone/cartilage damage and cell influx.
Figure 7. Histological examination of swollen paw. Normal architecture of knee joints in control rats (A), massive influx of inflammatory cells (Red arrow) and cartilage destruction (Black arrow) in CIA rats (B), show significant less influx of inflammatory cells and inflammation in MTX (C) and AuNGs treated rats (D). Mean histological score is shown in (E). ***p < .05.
Discussion
The autoimmune nature of RA and toxic effect of allopathic drugs has changed treatment strategy of RA in recent years. Ability of AuNGs in treating autoimmune diseases has augmented the curiosity of investigators to explore anti-inflammatory and anti-oxidant activity of AuNGs in CIA rats [Citation43,Citation44]. CIA is the most studied model to know about the mechanism underlying pathogenesis involved in human disease and is frequently used to assess pharmacological activity of new anti-arthritic therapeutics [Citation45,Citation46]. Present study was designed to evaluate ameliorating effect of AuNGs as a therapeutic agent for the treatment of experimental model of RA, and the data which we obtained show that they could be positively used for this purpose.
The antiangiogenic effect of AuNGs has been well documented but their mechanism underlying anti-inflammatory effect are still not very well understood [Citation47]. This study established that AuNGs significantly inhibit effects of inflammation by suppressing a key inflammatory pathway related to NF-kB, COX-2, iNOS and pro-inflammatory cytokines production. Inflammation is a kind of defense mechanism of body which occurs due to a reaction of injury or infections. However, undue or constant inflammation may cause several pathological conditions such as RA [Citation48,Citation49]. Since RA is autoimmune disease and exact cause of the disease is unclear. While, various studies have reported that uncontrolled production of pro-inflammatory cytokines and a disruption in the regulation of cytokine signaling pathway in RA patient. Increased level of inflammatory mediators is hall mark of arthritis [Citation50]. Therefore, inhibiting the overproduction of inflammatory cytokines might be an important treatment strategy to prevent or suppress RA. Pro-inflammatory cytokines, TNF-α and IL-1β have attracted more attention as they can be localized to the infected tissue. TNF-α drives early joint inflammation and facilitates inflammatory cell infiltration in the synovium by promoting adhesion of neutrophils and lymphocytes to endothelial cells [Citation51]. In addition, it also induces production of IL-1β, IL-6 and macrophage chemo-tactic protein-1 (MCP-1). TNF-α together with IL-1β activate synoviocytes and enhance production of metalloproteinase (MMPs) resulting in cartilage destruction [Citation52]. Elevated level of TNF-α, IL-1β is found in the serum and tissue of RA patients and is used as a marker of arthritic severity in both clinical and laboratory animals. Our findings showed that AuNGs inhibits synthesis of pro-inflammatory cytokines represent the importance of AuNGs as an anti-inflammatory compound (). The reduced production of inflammatory cytokines by AuNGs resulted in suppression of NF-kB (could inhibit NF-kB nucleus translocation through a reduction in Ikβ phosphorylation), the key transcription factor responsible for cytokines production under pathological conditions. This decrease in NF-kB activation may also causes suppression in COX-2 level. COX-2 is a key enzyme in the conversion of arachidonic acid of cell membrane to prostaglandin 2 (PGE2), an inflammatory mediator. In this study, we found that AuNGs suppressed COX-2 enzyme activity significantly in CIA treated rats (). This may in part be responsible for some anti-inflammatory properties of AuNGs.
Several previous studies displayed relation between pathogenesis of RA with oxidative stress in both experimental and in RA patients [Citation53]. Increased production of ROS along with reduction in antioxidant was detected in RA patients and involved as a mediator for cartilage destruction [Citation54,Citation55]. Therefore, we also assessed antioxidant property of AuNGs in the treated and untreated CIA rats and found that level of MDA (formed as a result of peroxidation of lipid), a well-established marker of oxidative stress, was significantly high in CIA rats, however, GSH concentration, which is believed as an important antioxidant accountable for removal of ROS was quite low, which were found to be restored to near normal values in the AuNGs treated rats (). These results in accordance to the previously reported data showing antioxidant activity of AuNGs [Citation56,Citation57]. This reduction in level of oxidative stress marker probably due to lesser production of key enzyme (iNOS) responsible for generation of ROS/RNS under pathological conditions. In consistence with biochemical parameters, qPCR analysis of NF-kB, TNF-α COX-2 and iNOS genes also showed that AuNGs down regulates mRNA expression of these genes in treated rats which were higher in CIA rats ().
Finally histopathlogical analysis of joint tissue of CIA rats was carried out to confirm ameliorating effect of AuNGs. Severe destruction of joint was observed in CIA rats with massive infiltrating inflammatory cells (). Treatment with AuNGs showed mild destruction of joint, probably due to inhibition of accumulation and infiltration of inflammatory cells like peripheral mononuclear cells in joint. Therefore, it is evident that AuNGs have an effective antiarthritic action as it minimizes most of the adverse effects of CIA.
Conclusions
The present study showed a novel approach for the treatment of arthritis using nanoscale gold particles. Our results suggest that AuNGs exhibited promising anti-inflammatory as well as antiarthritic activity and may be applicable to the modulation of various chronic inflammatory diseases.
| Abbreviations | ||
| COX-2 | = | cyclooxygenase-2 |
| IkB | = | I Kappa B |
| IKK | = | IkB kinases |
| IL-1β | = | interleukin-1β |
| iNOS | = | inducible nitric oxide synthase |
| NF-kB | = | nuclear transcription factor kappa B |
| ROS | = | reactive oxygen species |
| TNF-α | = | tumour necrosis factor-alpha |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916.
- Iwamoto T, Okamoto H, Toyama Y, et al. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275:4448–4455.
- Jones BA, Riegsecker S, Rahman A, et al. Role of ADAM-17, p38 MAPK, cathepsins, and the proteasome pathway in the synthesis and shedding of fractalkine/CX(3) CL1 in rheumatoid arthritis. Arthritis Rheum. 2013;65:2814–2825.
- Lark MW, Bayne EK, Flanagan J, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Investig. 1997;100:93–106.
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation . Cold Spring Harb Perspect Biol. 2009;1:a001651
- Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Sig Transduct Target Ther. 2017;2:17023.
- Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179.
- Seeger DR, Cosalich DDB, Smith JM, et al. Analogs of pteroylglutamic acid. II. 4-aminoderivatives. J Am Chem Soc. 1949;71:1297–1301.
- Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am J Med Sci. 1951;221:1762–1782.
- Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488.
- Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–712.
- Khan MJ, Husain Q. Influence of pH and temperature on the activity of SnO2-bound α-amylase: a genotoxicity assessment of SnO2 nanoparticles. Prep Biochem Biotechnol. 2014;44:558–571.
- Khan MJ, Qayyum S, Alam F, et al. Effect of tin oxide nanoparticle binding on the structure and activity of α-amylase from Bacillus amyloliquefaciens. Nanotechnology. 2011a;22:455708.
- Rao K, Aziz S, Roome T, et al. Gum acacia stabilized silver nanoparticles based nano-cargo for enhanced anti-arthritic potentials of hesperidin in adjuvant induced arthritic rats. Artif Cells Nanomed Biotechnol. 2018 [Jan 30]; [11 p.]. DOI:https://doi.org/10.1080/21691401.2018.1431653
- Warnasooriya N, Joud F, Bun P, et al. Imaging gold nanoparticles in living cell environments using heterodyne digital holographic microscopy. Opt Express. 2010;18:3264–3273.
- Sokolov K, Follen M, Aaron J, et al. Real-time vital optical imaging of precancer using antiepidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004.
- Tsai CY, Shiau AL, Cheng PC, et al. A biological strategy for fabrication of Au/EGFP nanoparticle conjugates retaining bioactivity. Nano Lett. 2004;4:1209–1212.
- Levy R, Thanh NT, Doty RC, et al. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc. 2004;126:10076–10084.
- Liu X, Dai Q, Austin L, et al. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J Am Chem Soc. 2008;130:2780–2782.
- Tang D, Yuan R, Chai Y. Biochemical and immunochemical characterization of the antigen-antibody reaction on a non-toxic biomimetic interface immobilized red blood cells of crucian carp and gold nanoparticles. Biosens Bioelectron. 2007;22:1116–1120.
- Bagheri S, Yasemi M, Safaie-Qamsari E, et al. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif Cells Nanomed Biotechnol. 2018 [Jan 26]; [10 p.]. DOI:https://doi.org/10.1080/21691401.2018.1430585
- El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834.
- Medley CD, Smith JE, Tang Z, et al. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem. 2008;80:1067–1072.
- Golchin K, Golchin J, Ghaderi S, et al. Gold nanoparticles applications: from artificial enzyme till drug delivery. Artif Cells Nanomed Biotechnol. 2018;46:250–254.
- Malik T, Chauhan G, Rath G, et al. Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif Cells Nanomed Biotechnol. 2017 [Dec 12]; [12 p.]. DOI:https://doi.org/10.1080/21691401.2017.1414054
- Daraee H, Eatemadi A, Abbasi E, et al. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:410–422.
- Gottlieb NL, Smith PM, Smith EM. Gold excretion correlated with clinical course during chrysotherapy in rheumatoid arthritis. Arthritis Rheum. 1972;15:582–592.
- Thakor AS, Jokerst J, Zavaleta C, et al. Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett. 2011;11:4029–4036.
- McFarland AD, Haynes CL, Mirkin CA, et al. Color my nanoworld. J Chem Educ. 2004;81:544A.
- Campo GM, Avenoso A, Campo S, et al. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003;5:R122–R131.
- Holmdahl R, Carlsen S, Mikulowska A, et al. Genetic analysis of murine models for rheumatoid arthritis. In: Adolpho KW, editor. Human genome methods. New York: CRC Press; 1998. p. 215–238.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43.
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522.
- Hyc A, Osiecka-Iwan A, Dziunycz P, et al. Preparation of rat synovial membrane for studies of cytokine secretion. Folia Histochem Cytobiol. 2007;45:57–60.
- Khan MS, Halagowder D, Devaraj SN. Methylated chrysin induces coordinated attenuation of the canonical Wnt and NF-kB signaling pathway and up-regulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem Biol Interact. 2011b;193:12–21.
- Adán N, Guzmán-Morales J, Ledesma-Colunga MG, et al. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. J Clin Investig. 2013;123:3902–3913.
- Zhang L, Sun T, Yu E, et al. TNF-α expression, not iNOS expression, is correlated with NF-κB activation in the spinal cord of rats following peripheral nerve injury. Afr J Biotechnol. 2011;10:6372–6380.
- Hu PJ, Yu J, Zeng ZR, et al. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195–200.
- Azizian M, Bathaie SZ, Ashrafi M, et al. Investigation of p53 and p27 expressions in the N-nitroso-N-methylureainduced breast cancer in female Wistar Albino rats. Physiol Pharmacol. 2014;18:337–346.
- Schmittgen TD, Zakrajsek BA, Mills AG, et al. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204.
- Sun X, Jiang X, Dong S, et al. One-step synthesis and size control of dendrimer-protected gold nanoparticles: a heat-treatment-based strategy. Macromol Rapid Commun. 2003;24:1024–1028.
- Hu J, Wang Z, Li J. Gold nanoparticles with special shapes: controlled synthesis, surface-enhanced Raman scattering, and the application in biodetection. Sensors. 2007;7:3299–3311.
- Tsai CY, Shiau AL, Chen SY, et al. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56:544–554.
- Brown CL, Bushell G, Whitehouse MW, et al. Nanogold-pharmaceutics. Gold Bull. 2007;40:245–250.
- Pandey S. Various techniques for the evaluation of anti arthritic activity in animal models. J Adv Pharm Technol Res. 2010;1:164–171.
- Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact. 2001;1:377–385.
- El-Ansary MR, Eldin TAS, Ali OMS, et al. Functionalized gold nanoparticles for inhibition of vascular endothelial growth factor in arthritic patients. J Nanomed Res. 2015;2:00036.
- Dinarello C. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112:321S–329S.
- Palladino M, Bahjat F, Theodorakis E, et al. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003;2:736–746.
- Isomaki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997;29:499–507.
- Szekanecz Z, Halloran MM, Volin MV, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:1266–1277.
- Rengel Y, Ospelt C, Gay S. Proteinases in the joint: clinical. Relevance of proteinases in joint destruction. Arthritis Res Ther. 2007;9:221.
- Umar S, Umar K, Sarwar AH, et al. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine. 2014;21:847–856.
- Sun Y, Zhao DL, Liu ZX, et al. Beneficial effect of 20-hydroxyecdysone exerted by modulating antioxidants and inflammatory cytokine levels in collagen-induced arthritis: a model for rheumatoid arthritis. Mol Med Rep. 2017;16:6162–6169.
- Taysi S, Polat F, Gul M, et al. Lipid peroxidation, some extracellular anti-oxidants, and anti-oxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol Int. 2002;21:200–204.
- Sul OJ, Kim JC, Kyung TW, et al. Gold nanoparticles inhibited the receptor activator of nuclear factor-κb ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci Biotechnol Biochem. 2010;74:2209–2213.
- Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327.