Abstract
MicroRNAs (miRNAs) have had a revolutionary impact on cancer research over the recent years. They emerge as important players in tumourigenesis, leading to a paradigm shift in oncology. Ovarian cancer is the leading cause of death among gynaecologic malignancies. Therefore, there is a strong need for prognostic and predictive markers for early diagnosis which helps optimize and personalize treatment. Asymptomatically, ovarian cancer is often diagnosed at advanced and incurable stages. Efficient targeting and sustained release of miRNAs/anti-miRNAs using nanoparticles conjugated with antibodies and/or peptides could reduce the required therapeutic dosage while minimizing systemic and cellular toxicity. Given miRNAs importance in clinical oncology, here we focus on the development of miRNA nanoformulations to achieve enhanced cellular uptake, bioavailability and accumulation at the tumour site. Although many obstacles need to be overcome, miRNA therapy could be a powerful tool for ovarian cancer prevention and treatment. In this review, we discuss about the emerging roles of miRNAs in various aspects of ovarian cancer.
Introduction
Nanotheranostic agents are applied in different types, such as gold, silver and magnetic nanoparticles (NPs), along with nanoshells and nanocages that can be conjugated to biological and conventional therapeutics (drugs, ligands and antibodies) to enhance delivery rate and efficiency of therapy. Nanotheranostics are now being widely used for targeted drug/aptamers delivery, as well as diagnostic imaging of the various stages of diseases (by MRI, CT scan, ultrasound etc) [Citation1].
Cancer is one of the most common causes of death worldwide, and has become a major public health challenge [Citation2]. Epithelial ovarian cancer (EOC) is the second most common gynaecological cancer with a 5-year survival rate of only 30% in advanced stages [Citation3,Citation4]. Worldwide ovarian cancer metastatized into extra-abdominal regions is the second most encountered gynaecologic cancer [Citation5]. A 5-year survival rate for individuals diagnosed with advanced cervical, colorectal, kidney or pancreatic cancer is better than ovarian cancer [Citation6]. EOC is a life-threatening disease determined by late-stage presentation and high pathological and molecular heterogeneity [Citation7]. The standard treatment for EOC is aggressive primary surgery followed by platinum-based chemotherapy [Citation8]. Ovarian cancer is an important leading cause of death due to gynaecologic malignancies among women in developed countries [Citation9]. High-grade serous ovarian cancer (HGSOC) accounts for 70–80% of ovarian cancer deaths [Citation10]. Accumulating evidence has revealed that microRNAs (miRNAs or miRs) are extensively involved in cancer progression and suppression by regulating thousands of cancer-associated genes [Citation11]. Circulating cell-free miRNAs have been shown to have the potential to be considered as new biomarkers in cancer diagnosis, predict prognosis and response to therapy [Citation12].
MiRNAs, biogenesis and functions
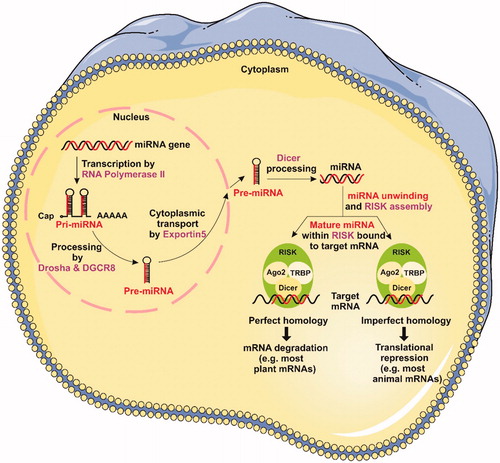
The identification of miRNAs and their mechanism of action was originally made in the nematode Caenorhabditis elegans [Citation13]. The first miRNA was discovered in 1993, but additional insights for miRNAs mode of action required simultaneously published work by Ruvkun's team [Citation13]. Under a standard nomenclature system, names are assigned to experimentally confirmed miRNAs before publication [Citation14,Citation15]. The prefix “miR” is followed by a dash and a number, the latter often indicating order of naming. For example, miR-124 was named and likely discovered prior to miR-456. A capitalized “miR-” refers to the mature form of the miRNA, while the non-capitalized “mir-” refers to the pre-miRNA and the pri-miRNA, and “MIR” refers to the gene that encodes them [Citation16].
A miRNA is a small non-coding endogenous RNA molecule (containing about 22 nucleotides) found in plants, animals and some viruses, which has functions in mRNA silencing and post-transcriptional regulation of gene expression [Citation17]. MiRNAs directly interact with partially complementary sites located in the 3′ untranslated region (3′UTR) of target mRNAs and repress their translation [Citation18]. More than 60% of all mRNAs are estimated to contain miRNA target sites at their 3′UTR region, suggesting a tight regulation as well as their involvement in normal cellular homeostasis and in diseased states [Citation19]. To date, 940 human miRNAs have been identified, which are annotated and catalogued in a searchable Web-based miRNA database known as miRbase [Citation20]. Out of 232 mammalian miRNAs analyzed, 40% existed within introns of coding RNA, 10% within introns of noncoding RNA (ncRNA) and 13% within exons of ncRNA. In addition, a small portion of miRNAs exists within exons (mainly 3′UTR) of coding “host” genes, some of which are expressed from the same transcriptional unit. The remaining miRNAs are intergenic, with mostly undefined transcriptional units as of yet. For that purpose, computational tools were developed for prediction of miRNA core promoters [Citation21].
The pre-miRNAs are assembled in large protein complexes known as RNA-inducing silencing complex (RISC or miRISC). One of the known proteins that form RISC is RNA-specific endonuclease Dicer, which is involved in the processing of pre-miRNA into the mature form [Citation22]. Each RISC or miRNA ribonucleoprotein complex) miRNP (contains a member of the Argonaute (Ago) protein family; the Ago protein probably binds directly to the mRNA in these complexes, although there is no evidence for this. The Ago protein has four isoforms which only Ago2, also known as “slicer,” has the capacity to cleave the target mRNA [Citation23] ().
Identifying the mRNA targets of miRNAs
Many methods have been proposed to identify miRNAs targets [Citation24]. Initially experimental methods have focused on the effects of miRNA–target interactions at levels that range from broad phenotypes to changes in the frequency of mRNAs and proteins. More recently, methods to directly capture Ago-bound RNAs have become available () [Citation25].
miRNA and cancer
MiRNAs are generally involved in post-transcriptional gene regulation. They are highly conserved among a wide range of species [Citation26]. MiRNAs are key regulators of cellular pathways, and they appear to play an important role in tumourigenesis [Citation27]. Several studies have documented the implication of miRNAs in nearly every carcinogenesis process, including tumour development, apoptosis, invasion and metastasis, as well as anticancer drug resistance [Citation28]. Currently, it is well known that miRNAs can be upregulated or downregulated in various human cancers. Overexpressed miRNAs may have functions as oncogenes by downregulating tumour suppressor genes, whereas the downregulated miRNAs may act as tumour suppressor genes by negatively regulating oncogenes [Citation29]. Important insights into the functions of miRNAs in cancer have been provided through the demonstration involved in known oncogenic pathways. Human RAS oncogene family (H-, K- and N-RAS) contains binding sites for the let-7 family of miRNAs in their 3′UTR [Citation30]. miR-221 from complex samples is demonstrated in the total RNA isolated from human prostate cancer cells and xenografts [Citation31]. A combination of upregulated miR-155 and downregulated miR-141 is found to result in a 97% overall correct classification of the matched malignant and non-malignant tissue samples [Citation32]. By calculating the differences between delta cycle thresholds (Ct) of miR-503 and miR-511, adrenocortical carcinomas are significantly distinguished from benign adenomas with high sensitivity and specificity. These miRNAs may be considered as helpful biomarkers for the diagnosis of adrenocortical malignancy [Citation33].
MiR-221 and miR-222 are examples of miRNAs that act as oncogenes. They exert oncogenic functions by targeting and inhibiting the expression of the tumour suppressor gene p27Kip [Citation34]. There is also evidence of possible role of miRNAs in p53-induced cell death. For example, p53-mediated upregulation of miR-34 is known to induce cell death in C. elegans as well as in mammalian cells. Many other miRNAs other than miR-34 family members are now known to be regulated by p53, viz., miR-194, miR-207, miR-107, miR-215, miR-192, miR-16–1, miR-143, miR-145 and miR-216 [Citation35]. It has been shown that p53 transcriptionally induces miR-34 expression, and this induction is important in p53-mediated apoptosis of cancer cells [Citation36,Citation37]. In the study of chronic lymphocytic leukemia (CLL), low expression of miR-29 b, miR-29c, miR-181 family, and miR-223 was found to be strongly associated with disease progression in CLL cases harboring 17p deletion, whereas high expression of miR-181a in those harboring trisomy 12 suggested more aggressive disease. These biomarkers may be clinically useful to assess the tumour behavior in CLL [Citation38]. It provided evidence that the relative concentration of miR-155 in serum significantly discriminated primary breast cancer patients from healthy women. Within the primary breast cancer cohort, patients at advanced tumour stages had significantly higher miR-34a than patients at early tumour stages. In the metastatic patients, miR-10b, miR-34a and miR-155 were correlated with the presence of overt metastases. miR10b is down-regulated in breast tumour tissue compared with normal breast tissue [Citation39].
miRNAs and ovarian cancer
Numerous studies have shown that dysregulation of miRNAs is involved in a wide variety of human diseases including ovarian cancer [Citation40]. The Cancer Genome Atlas (TCGA) project analyzed mRNA expression, miRNA expression, promoter methylation, and DNA copy number in a total of 489 HGSOCs and showed that ovarian cancer could be separated into 3 miRNA subtypes [Citation41] miR-200, a family of tumour suppressor miRNAs, consisting of miR-141, miR-200a, miR-200 b, miR-200c and miR-429, is significantly involved in the inhibition of epithelial-to-mesenchymal transition (EMT), repression of cancer stem cell self-renewal and differentiation, modulation of cell division and apoptosis, and reversal of chemoresistance [Citation42]. Hence, the miR-200 family miRNAs may act as master regulators in ovarian cancer by targeting some cancer-related genes [Citation43]. High expression of miR-200c may predict improved survival in women with ovarian cancer [Citation44].
Recent evidence indicates that miRNAs play important roles in various pathways related to anticancer drug resistance, e.g. influencing the response to the conventional chemo-agents cisplatin and microtubule-targeting drugs [Citation2,Citation45]. In ovarian cancer, 11 miRNAs were upregulated (miR-16, miR-20a, miR-21, miR-23a, miR-23b, miR-27a, miR-93, miR-141, miR-200a, miR-200b and miR-200c) and 12 miRNAs were downregulated (miR-10b, miR-26a, miR-29a, miR-99a, miR-100, miR-125a, miR-125b, miR-143, miR-145, miR-199a, miR-214 and let-7b) [Citation46].
TCGA project has analyzed mRNA/miRNA expression, promoter methylation and DNA copy number in a total of 489 high-grade serous ovarian adenocarcinomas [Citation47] and reported that high-grade serous ovarian cancer was characterized by TP53 mutations in almost every tumour (96%). In addition, there was a low but statistically significant prevalence of recurrent somatic mutations in 8 other genes including NF1, BRCA1, BRCA2, RB1 and CDK12. They also showed that ovarian cancers could be separated into 4 transcriptional subtypes, 3 miRNA subtypes and 4 promoter methylation subtypes. Integrated genomic analysis revealed a miRNA-regulatory network that defined a robust integrated mesenchymal subtype associated with poor survival in 459 cases of serous ovarian cancer and 560 cases independent of cohort data [Citation48]. Eight key miRNAs (miR-25, miR-29c, miR-101, miR-128, miR-141, miR-182, miR-200a and miR-506) were identified and predicted to regulate 89% of the targets in this network. Recently, Davidson et al. summarized the clinical and diagnostic roles of miRNAs in ovarian carcinoma in their review of approximately 100 publications [Citation49]. In addition, various miRNAs have also been identified as potential prognostic indicators, and promise utility in future practice. Mentioned studies are summarized in . A panel of 74 overexpressed and 49 underexpressed miRNAs in ovarian cancer was compared to ovarian surface epithelium (OSE) which was identified in another study, as well as histotype-specific signatures [Citation58]. A panel of 42 miRNAs differentiating ovarian cancer from OSE was found by Shahab et al., of which 33 and 9 miRNAs were overexpressed and under-expressed in ovarian cancer, respectively. The authors found only infrequently negative association between miRNAs and their targets [Citation59].
Table 1. Circulation miRNAs as potential diagnostic biomarker for ovarian cancer.
MiRNA expression profiles in high grade serous ovarian cancers
Paola et al. showed that miR-1246 was significantly up-regulated in the serum of patients with HGSOC compared to healthy individuals [Citation60]. Eun Ji Nam et al. analyzed the miRNA expression profiles of 20 serous ovarian cancer tissues using a two-color system. The up-regulated miRNAs (n = 11) included miR-200a, miR-200b, miR-200c, miR-20a, miR-21, miR-23a, miR-23b, miR-27a, miR-141, miR-16 and miR-93, and the down-regulated miRNAs (n = 12) were miR-214, miR-26a, miR-29a, let-7b, miR-100, miR-10b, miR-125a, miR-125b, miR-143, miR-145, miR-199a-AS and miR-99a. Of these miRNAs, the most frequently up-regulated miRNA was miR-21 (17 of 20 cases), and the most frequently down-regulated miRNA was miR-125b [Citation61].
In other reports that compared miRNA expression in ovarian cancers and normal ovarian tissues [Citation17–19], five miRNAs were down-regulated (miR-140–3p, miR-143–5p, miR-34b-5p, miR-34c-5p and miR-145) and three were up-regulated (miR-96, miR-15b and miR-16) [Citation62,Citation63]. Downregulation of miR-34a/b/c, miR449b, miR-503 and miR-507 has been observed in late stage and high grade ovarian cancers [Citation64,Citation65]. Petrillo et al. showed that the expression levels of some miRNAs were associated with prognosis. They observed that miR-181a-5p, miR-199a-5p and miR-199a-3p were more expressed in tumours of patients with PFI <6 months, with residual tumour >1 cm, and their expression levels were associated to patients’ survival [Citation66].
Clinical relevance of cell-free miRNAs in ovarian cancer
About 20 studies have explored the diagnostic miRNAs in ovarian cancer. Taylor et al. showed the elevation of miR-21, miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-205 and miR-214 in ovarian cancer patients. These miRNAs were over-expressed even in patients with early stages of ovarian cancer. The miRNA signatures from exosomes were equivalent from the originating tumour cells, indicating that circulating miRNA profiles precisely reflected the tumour profiles [Citation67]. Following this study, a variety of reports have confirmed the diagnostic potential of circulating miRNAs in body fluids such as serum, plasma, whole blood and urine [Citation68–70] as summarized in .
miRNAs in ovarian cancer progression; nano and ovarian cancer
miRNAs are also used for prognosis and therapy in cancer [Citation71]. The clinical application of miRNAs has rapidly matured from aspirational to now exploiting its diagnostic and therapeutic potential. We refer the reader to the comprehensive reviews describing the mechanisms of miRNAs action [Citation72]. The study demonstrated that in vivo nano-liposomal delivery of miR-15a and miR-16 decreased tumour growth in preclinical chemo-resistant orthotopic ovarian cancer mouse model in support of combination therapies [Citation73].
Over the past decade, nanotechnology has received remarkable attention for cancer therapy. At the molecular level, treatment with AuNPs has been shown to alter the profiles of a series of secretory cytokines [Citation74]. AuNPs can efficiently mute single gene expression, quite similarly like a traditional siRNA; but alternatively, they can also be used in targeting and silencing siRNA (exogenous) and miRNA (endogenous) [Citation75]. Dendrimer-grafted nano conjugated with gadolinium, which possesses a positively-charged nanosurface, can be used to interact with the negatively charged Let–7g miRNA (MIRLET7G, a putative RNAi agent), thereby forming Gd-NGO/Let–7g complexes, that demonstrated anti-neoplastic properties. Gd-NGO has also been shown to effectively carry the anthracycline anticancer drug epirubicin (EPI). Conjugation of MIRLET7G and EPI with Gd-NGO (Gd-NGO/Let–7g/EPI) enhanced the inhibition of cancer (glioblastoma) cell growth in comparison with the single conjugates (Gd-NGO/Let–7g and Gd-NGO/EPI) [Citation76].
Tris (bipyridine) ruthenium (II) chloride [Ru(bpy)3] + 2-encapsulated SiNPs, in conjugation with miR-21 molecular beacon (miR-21-MB) and AS1411 aptamer, is a potential cancer theranostic agent (bu-S-AS/MB nanocomposite). miR-21 is involved in a number of pathological conditions, including cardiovascular diseases, pulmonary disorders and cancer [Citation77]. The bu-S-AS/MB nanocomposite was observed to inhibit miR-21 and induce apoptosis by caspase activation. This phenomenon can be simultaneously monitored by virtue of the molecular beacon [Citation78]. A recent study showed a gold NP delivery system for anti-miR-21 to be an excellent platform to target and silence miR-21 in ovarian cancer cells, inhibiting the sphere-forming capacity of tumour-initiating cells. miR-155 is downregulated in ovarian tumour-associated dendritic cells (DCs) and is essential for optimal antigen presentation and activation of T cells by DCs [Citation79]. PEI-based nanocomplexes were used to deliver miR-155 to tumour-associated DCs, which increased the expression of miR-155 in vitro and resulted in increased anti-tumour immunity, thus, increased survival of the mice (by 65%). miR-124 was downregulated in ovarian cancer and acted as a tumour suppressor by targeting proteins such as myc while increasing the expression of p27, subsequently leading to cell cycle arrest at G1 phase because of the loss of phospho-Rb and decreased expression of the myc protein. Transfection of miR-124 in an ovarian cancer cell line reduced the invasive and migration capability of ovarian cancer cells and increased their sensitivity to etoposide by two fold. While miR-124 was restored in ovarian cancer xenograft tumours using 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) NPs, it resulted in a significant decrease in tumour weight alone and in combination with etoposide [Citation80]. Using TCGA, Nishimura et al. identified miR-520d-3p as an independent prognostic marker for EOC, and showed that miR-520d-3p functioned as a tumour suppressor, upstream of the EPHA2 gene. They investigated the effect of dual targeting of EPHA2 with nano-liposomes loaded with miR-520d-3p and EphA2-siRNA which showed synergistic anti-tumour efficiency and greater therapeutic efficacy in vivo than either monotherapy alone [Citation81]. It has been demonstrated that miR-214 represses p53 in ovarian cancer cells and knockdown of miR- 214 increases sensitivity to cisplatin and doxorubicin. Another study showed that TWIST1 (a highly conserved transcription factor) regulates the two pathways IKKb/NF-jB and PTWN/AKT through the expression of the miR199a-2/214 cluster [Citation82]. Yang et al. correspondingly showed miR-214 and miR-199 to be altered in ovarian cancer, and that miR-214 significantly induced cell survival and cisplatin resistance through targeting the PTEN/AKT pathway. Latest, Zheng et al. found that miR-214 disturbs DNA damage responses through downregulation of RNF8 (a protein that plays a key role in DNA damage response) and causes chromosomal instability in ovarian cancer [Citation83].
Conclusion
Many studies now report miRNA dysregulation as a driver of ovarian cancer disease progression. The benefit of miRNA profiling will help us to obtain more accurate diagnoses and even better prognoses. In addition, for clinical use, sensitivity and specificity of miRNA in each disease need to be further investigated. Identifying the miRNAs that are able to affect cancer would be a good start and would aid in improving the targeting and development of new drugs.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Mody VV, Siwale R, Singh A, et al. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2:282.
- Kanakkanthara A, Miller JH. MicroRNAs: novel mediators of resistance to microtubule-targeting agents. Cancer Treat Rev. 2013;39:161–170.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10.
- Didžiapetrienė J, Bublevič J, Smailytė G, et al. Significance of blood serum catalase activity and malondialdehyde level for survival prognosis of ovarian cancer patients. Medicina (Kaunas). 2014;50:204–208.
- Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–1016.
- Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet. 2014;384:1376–1388.
- Vaughan S, Coward JI, Bast RC, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725.
- Ye G, Fu G, Cui S, et al. MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: implications for chemoresistance. J Cell Sci. 2011;124:359–368.
- Bowtell DD, Böhm S, Ahmed AA, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668–679.
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287.
- Schwarzenbach H, Nishida N, Calin GA, et al. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145–156.
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854.
- Ambros V, Bartel B, Bartel DP, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–279.
- Griffiths-Jones S, Grocock RJ, Van Dongen S, et al. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Suppl 1):D140–D1D4.
- Wright MW, Bruford EA. Naming ‘junk’: human non-protein coding RNA (ncRNA) gene nomenclature. Human Genomics. 2011;5:1.
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355.
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110.
- Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887.
- Griffiths‐Jones S. The microRNA registry. Nucleic Acids Res. 2004;32(Suppl 1):D109–DD11.
- Zhou X, Ruan J, Wang G, et al. Characterization and identification of microRNA core promoters in four model species. PLoS Comput Biol. 2007;3:e37.
- Hurteau GJ, Carlson JA, Spivack SD, et al. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res. 2007;67:7972–7976.
- Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349.
- Thomson DW, Bracken CP, Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–6853.
- Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821.
- Akbarzadeh A, Kafshdooz L, Razban Z, et al. An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif Cells Nanomed Biotechnol. 2018;46:263–267.
- Fu L-l, Wen X, Bao J-k, et al. MicroRNA-modulated autophagic signaling networks in cancer. Int J Biochem Cell Biol. 2012;44:733–736.
- Cho WC. Role of miRNAs in lung cancer. Expert Rev Mol Diagn. 2009;9:773–776.
- Mehrdad S, Masoud F, Nasim A, et al. Magnetic carbon nanotubes: preparation, physical properties, and applications in biomedicine. Artif Cells Nanomed Biotechnol. 2018 [cited 2017 Oct 18]; [17 p.]. DOI:10.1080/21691401.2017.1389746
- Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647.
- Siva AC, Nelson LJ, Fleischer CL, et al. Molecular assays for the detection of microRNAs in prostate cancer. Mol Cancer. 2009;8:1.
- Jung M, Mollenkopf HJ, Grimm C, et al. MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J Cell Mol Med. 2009;13:3918–3928.
- Tömböl Z, Szabó PM, Molnár V, et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr Relat Cancer. 2009;16:895–906.
- Panahi Y, Farshbaf M, Mohammadhosseini M, et al. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif Cells Nanomed Biotechnol. 2017;45:788–799.
- Kumar A, Chandna S. Evidence for a radiation-responsive ‘p53 gateway’ contributing significantly to the radioresistance of lepidopteran insect cells. Sci Rep. 2018;8:2.
- Chang T-C, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752.
- Raver-Shapira N, Marciano E, Meiri E, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743.
- Visone R, Rassenti LZ, Veronese A, et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood. 2009;114:3872–3879.
- Ebrahimi E, Akbarzadeh A, Abbasi E, et al. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol. 2015;44:290–297.
- Kinose Y, Sawada K, Nakamura K, et al. The role of microRNAs in ovarian cancer. BioMed Res Int. 2014;2014:249393.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404.
- Feng X, Wang Z, Fillmore R, et al. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173.
- Hu X, Macdonald DM, Huettner PC, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464.
- Shi C, Zhang Z. The prognostic value of the miR‐200 family in ovarian cancer: a meta‐analysis. Acta Obstet Gynecol Scand. 2016;95:505–512.
- Giovannetti E, Erozenci A, Smit J, et al. Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Crit Rev Oncol Hematol. 2012;81:103–122.
- Vang S, Wu H-T, Fischer A, et al. Identification of ovarian cancer metastatic miRNAs. PloS One. 2013;8:e58226.
- Network CGAR. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615.
- Tabrizi MHN, Davaran S, Entezami AA. Synthesis of diclofenac polymeric prodrugs and their hydrolysis reactivity. Iran Polym J. 1996;5:243–251.
- Davidson B, Tropé CG, Reich R. The clinical and diagnostic role of microRNAs in ovarian carcinoma. Gynecol Oncol. 2014;133:640–646.
- Nakamura K, Sawada K, Yoshimura A, et al. Clinical relevance of circulating cell-free microRNAs in ovarian cancer. Mol Cancer. 2016;15:48.
- Davaran S, Rashidi MR, Pourabbas B, et al. Adriamycin release from poly(lactide-coglycolide)-polyethylene glycol nanoparticles: synthesis, and in vitro characterization. Int J Nanomed. 2006;1:535–539.
- Xu Y-Z, Xi Q-H, Ge W-L, et al. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac J Cancer Prev. 2013;14:1057–1060.
- Hong F, Li Y, Xu Y, et al. Prognostic significance of serum microRNA-221 expression in human epithelial ovarian cancer. J Int Med Res. 2013;41:64–71.
- Shapira I, Oswald M, Lovecchio J, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110:976–983.
- Meng X, Joosse SA, Müller V, et al. Diagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patients. Br J Cancer. 2015;113:1358–1366.
- Liang H, Jiang Z, Xie G, et al. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumor Biol. 2015;36:5305–5313.
- Vaksman O, Tropé C, Davidson B, et al. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis. 2014;35:2113–2120.
- Wyman SK, Parkin RK, Mitchell PS, et al. Repertoire of microRNAs in epithelial ovarian cancer as determined by next generation sequencing of small RNA cDNA libraries. PloS One. 2009;4:e5311.
- Shahab SW, Matyunina LV, Mezencev R, et al. Evidence for the complexity of microRNA-mediated regulation in ovarian cancer: a systems approach. PLoS One. 2011;6:e22508.
- Todeschini P, Salviato E, Paracchini L, et al. Circulating miRNA landscape identifies miR-1246 as promising diagnostic biomarker in high-grade serous ovarian carcinoma: a validation across two independent cohorts. Cancer Lett. 2017;388:320–327.
- Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695.
- Vogt M, Munding J, Grüner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322.
- Lee C-H, Subramanian S, Beck AH, et al. MicroRNA profiling of BRCA1/2 mutation-carrying and non-mutation-carrying high-grade serous carcinomas of ovary. PloS One. 2009;4:e7314.
- Zhang L, Volinia S, Bonome T, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci. 2008;105:7004–7009.
- Davaran S, Rashidi MR, Khandaghi R, et al. Development of a novel prolonged-release nicotine transdermal patch. Pharmacol Res. 2005;51:233–237.
- Petrillo M, Zannoni G, Beltrame L, Martinelli E, DiFeo A, Paracchini L, et al. Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: a retrospective longitudinal analysis using matched tumor biopsies. Ann Oncol. 2016;27:625–634.
- Nasajpour A, Mandla S, Shree S, et al. “Nanostructured fibrous membranes with rose spike-like architecture”. Nano Lett. 2017;17:6235–6240. pp
- Zhou J, Gong G, Tan H, et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol Rep. 2015;33:2915–2923.
- Mostafavi E, Ataie A, Ahmadzadeh M, et al. Synthesis of nano-structured Bi1-xBaxFeO3 ceramics with enhanced magnetic and electrical properties. Mater Chem Phys. 2015;162:106–112.
- Kapetanakis N-I, Uzan C, Jimenez-Pailhes A-S, et al. Plasma miR-200b in ovarian carcinoma patients: distinct pattern of pre/post-treatment variation compared to CA-125 and potential for prediction of progression-free survival. Oncotarget. 2015;6:36815.
- Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122.
- Masliah-Planchon J, Garinet S, Pasmant E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget. 2016;7:38892.
- Herizchi R, Abbasi E, Milani M, et al. Current methods for synthesis of gold nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:596–602.
- Kafshdooz L, Kafshdooz T, Razban Z, et al. The application of gold nanoparticles as a promising therapeutic approach in breast and ovarian cancer. Artif Cells Nanomed Biotechnol. 2016;44:1222–1227.
- Conde J, Rosa J, Jesús M, et al. Gold-nanobeacons for simultaneous gene specific silencing and intracellular tracking of the silencing events. Biomaterials. 2013;34:2516–2523.
- Mostafavi E, Ataie A, Ahmadzadeh M. Characterization of nano-structured multiferroic bismuth ferrite produced via solid state reaction route. Adv Mater Res. 2014;829:683–687.
- Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–713.
- Li H, Mu Y, Lu J, et al. Target-cell-specific fluorescence silica nanoprobes for imaging and theranostics of cancer cells. Anal Chem. 2014;86:3602–3609.
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693.
- Seviour E, Sehgal V, Lu Y, et al. Functional proteomics identifies miRNAs to target a p27/Myc/phospho-Rb signature in breast and ovarian cancer. Oncogene. 2016;35:691–701.
- Nishimura M, Jung E-J, Shah MY, et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013;3:1302–1315.
- Prahm KP, Novotny GW, Høgdall C, Høgdall E. Current status on microRNAs as biomarkers for ovarian cancer. Apmis. 2016;124:337–355.
- Mahdian-shakib A, Dorostkar R, Tat M, et al. Differential role of microRNAs in prognosis, diagnosis, and therapy of ovarian cancer. Biomed Pharmacother. 2016;84:592–600.