 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Adjuvants play an essential role in the induction of immunity against leishmaniasis. In this study, monophosphoryl lipid A (MPL) and imiquimod (IMQ) were used as TLR ligands adjuvants to enhance immunogenicity and rate of protection against leishmaniasis. Nanoliposomes containing soluble Leishmania antigens (SLA) and adjuvants were consisted of DSPC, DSPG and Chol prepared by using lipid film method followed by bath sonication. The size of nanoliposomes was around 95 nm and their zeta potential was negative. BALB/c mice were immunized by liposomal formulations of lip/SLA, lip/MPL/SLA, lip/IMQ/SLA, lip/MPL/IMQ/SLA, lip/SLA + lip/IMQ, lip/SLA + lip/MPL, lip/SLA + lip/MPL/IMQ and five controls of SLA, lip/MPL, lip/IMQ, lip/MPL/IMQ and buffer by subcutaneously (SC) injections, three times in 2 weeks intervals. The synergic effect of two adjuvants when they are used in one formulation showed significantly (p < .001) smaller footpad swelling and the lowest parasite burden in lymph node and foot after the challenge. IgG2a in these groups showed the higher titre compared to control groups, which is compatible with the high IFN-γ production and lowest IL-4. Taken together the results indicated that co-delivery of MPL and IMQ adjuvants and antigen in nanoliposome carrier could be an appropriate delivery system to induce cellular immunity pathway against leishmaniasis.
Introduction
Leishmaniasis is a neglected disease caused by different species of Leishmania. Cutaneous leishmaniasis (CL) is not typically a life-threatening infection, but causes a disfiguring stigma. According to World Health Organization (WHO), over one billion people live in endemic areas at risk of infection. One million cases of CL reported over the last five years, and there are over 20,000 death of visceral leishmaniasis (VCL) annually [Citation1]. There is no available vaccine against any form of leishmaniasis, but there are many evidences which indicate the possibility of developing an effective vaccine against leishmaniasis. Being immunized after recovery of CL is an important reason for this belief [Citation2,Citation3].
Sandfly collects Leishmania parasite during the blood meal. The parasite in the gut environment multiplies and transmits during the next bite into a susceptible individual. In case of zoonotic form of the disease, an animal reservoir involves during transmission [Citation4]. Clinical manifestation of leishmaniasis includes CL, mucocutaneous (MCL) and VCL which the visceral form is mortal [Citation5]. Vaccination against Leishmania dates back to early 20th century, which the virulent forms of the parasite utilized for leishmanization. This method was not safe as 5–10% developed severe disease, and it was used during the Iran–Iraq War [Citation6,Citation7].
Protection against leishmaniasis depends upon induction of Th1 type of immune response [Citation8]. Survival and replication of the parasite depend on the target cell: macrophages. These cells produce nitric oxide (NO) and other oxidative intermediates [Citation9].
The type of CD4 + helper T cell (Th) subset plays an important role in resistance or susceptibility to Leishmania major infection [Citation10]. In resistant mice, which are infected with L. major, producing IFN γ by Th1 promotes healing pathway that is dependent on activated effector macrophages generating NO. In contrast, in susceptible mice activated Th2 and producing IL-4 and IL-10 led to deactivation of macrophages and inhibited intracellular parasite killing. The key factor that determines resistance or susceptibility to L. major in mice is the production of IL-12. It may be considered that the proliferation and differentiation of memory CD4 + T into IFNγ producing cells is related to the IL-12 [Citation10,Citation11].
Soluble Leishmania antigen (SLA), which is a crude antigen, as a first-generation vaccine could be provided without any high tech facilities [Citation3,Citation12,Citation13]. SLA plus appropriate adjuvants could stimulate immune response to control infection [Citation13–17].
To enhance immune response with smaller doses of antigen, adjuvants are used [Citation18]. The first adjuvant compound, which has been used in the past, was aluminium salts. They form a depot which releases antigen slowly; some efficient adjuvants interact with immune cell receptors directly [Citation19]. Adjuvants determine the type of immune response to activate. There are two classes of adjuvants: i) delivery systems and ii) immune potentiator [Citation18].
Liposomes are synthetic lipid vesicles, which play two roles in vaccine delivery system: protect antigen from degradation and deliver the cargo to the immune cells. Liposome electric charge, size, number of lipid layer and lipid composition affect the liposome potency in activating immune system [Citation18,Citation20–23]. Depending on the chemical properties, hydrophilic compounds entrapped within the liposome and lipophilic compounds intercalated into the lipid bilayer [Citation19,Citation24]. Liposomes are uni- or multi-lamellar vesicles, which the size varies from 25 nm to few µm [Citation25].
In this study, two adjuvants were used in liposome formulation, imiquimod (IMQ) (TLR7 ligand) and MPL (TLR4 ligand), which trigger Toll-like receptors to activate immune system [Citation26,Citation27]. MPL is a portion of Salmonella minnesota lipopolysaccharide in which the (R)-3-hydroxytetradecanoyl group and 1-phosphate have been omitted by hydrolysis. MPL stimulate immune system through innate immune response by TLR4 receptor [Citation28,Citation29]. IMQ is a synthetic small-molecule compound in the imidazoquinoline family, which is FDA approved, it is used in dermatology lesion as an immunopotentiator agent. It could stimulate innate immune system through cytokine induction [Citation30]. IMQ is the agonist of TLR7 and has immunomodulatory activity. Innate immune cells could be activated through Toll-like receptor agonists [Citation31,Citation32]. Pathogen-associated molecular patterns are recognized through receptors of innate immune system. TLRs include the main class of cell surface PRP’s, which are presented on dendritic cells and macrophages. TLR7 commonly recognize single strand RNA (of viruses) and lead Th1-type cytokine secretion [Citation31]. These TLRs agonists activate innate immune cells by TLR/MyD88/NF-kB and IFN regulatory factor 3/7 pathways [Citation32]. Antigen, which was used, is SLA. Eight liposomal formulations were designed to investigate the effect of antigen and adjuvant in liposome formulation, and thirteen groups containing 10 mice designed to study the experimental vaccine.
Materials and methods
Soluble Leishmania antigen preparation
Leishmania major MRHO/IR/75/ER, Iran vaccine strain pellet was obtained from three litre of parasite medium received from the Vaccinology and Molecular Parasitology Department of Razi Vaccine and Serum Research Institute, Iran.
SLA preparation was carried out using the protocol developed by Scott et al., with minor modifications [Citation33]. The parasite washed four times using PBS buffer (rpm: 4500, 20 min, RT). The pellet dissolved in 10 mM HEPES buffer (pH 7.5) solution in10% sucrose (HS buffer). The number of promastigotes was adjusted to 1.2 × 109/ml in HS buffer solution containing enzyme inhibitor cocktail, 50 µl/ml (Sigma, St. Louis, MO). The parasites were then lysed by freeze-thaw method followed by probe sonication in an ice bath. The supernatant of the centrifuged lysate parasites was collected, dialyzed against HS buffer solution and sterilized by passage through a 0.22 µ membrane and stored at −70 °C until use. The protein concentration of the SLA was determined using Lowry protocol [Citation34].
Liposome preparation and characterization
DSPC and DSPG were purchased from Lipoid (Ludwigshafen, Germany). Cholesterol, chloroform and MPL provided from Sigma and IMQ from Tocris (Bristol, UK). HPLC grade methanol and DMSO were purchased from Scharlau (Barcelona, Spain).
Liposomes containing DSPC:DSPG:Chol (molar ratio15:2:3, 40 mM) were prepared by dissolving the lipids in chloroform in a round-bottom flask. The solvent was then removed using rotary evaporator (IKA, Staufen, Germany) resulting in a thin lipid film deposited on the wall of flask. The lipid film was then freeze-dried (Christ, Bonn, Germany) overnight to ensure total removal of the solvent [Citation56]. The lipid film in control groups hydrated with sterile buffer (HS buffer containing 10% sucrose), and in vaccinated groups hydrated with SLA. Liposomes containing IMQ prepared as explained before with the ratio of DSPC:DSPG:Chol:IMQ (15:2:3:1.25), according to the formulation they hydrated in buffer or SLA solution. Liposomes containing MPL prepared at a ratio of DSPC:DSPG:Chol:MPL (23.7:3.2:2.31:0.25 mg/ml). Due to the control or vaccinated group they hydrated in buffer or SLA solution. The multilamellar vesicles (MLVs) obtained were then converted to unilamellar ones using bath type sonicator (Branson 5510, Marshall Scientific, Hampton, NH) at 45 °C for 30 min under argon.
The particle size of liposomes was measured using Dynamic Light Scattering Instrument (Nano-ZS, Malvern Instruments, Malvern, UK). The zeta potential was determined on the same machine using the zeta potential mode as the average of 20 measurements [Citation57]. Then, the encapsulation efficiency of liposomes was calculated for SLA and IMQ using following equation:
For this purpose, a sample of fabricated liposomes isolated before dialysis and another sample isolated after the following procedures for dialysis. The liposomes containing SLA or IMQ dialyzed against HS buffer for 2.5 d, exchanging buffer each 8 h. The concentration of SLA in the liposomes was determined using BCA protein assay kit [Citation35]. The concentration of IMQ was determined by spectrophotometry at 318 nm using a UV–visible spectrophotometer in triplicate using a calibration curve of IMQ. DMSO/water (9/1 V/V) was used to dissolve and dilute the liposomes.
Immunization groups of BALB/c mice
A total of 130 female BALB/c mice, 6–8 weeks old, were purchased from Pasteur Institute (Tehran, Iran). The mice were maintained in animal house of Center for Research and Training in Skin Disease and Leprosy (Tehran University of Medical Sciences, Tehran, Iran) and fed with tap water and laboratory pellet chow. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the (Tehran University of Medical Sciences) (IACUC protocol: [http://research.tums.ac.ir/informatics/77/Animal%20Research.doc]).
Thirteen (13) groups, 10 mice per group, were SC immunized three times at two weeks intervals with the following vaccines: SLA, Lip/SLA, Lip/MPL/SLA, Lip/IMQ/SLA, Lip/MPL/IMQ/SLA, Lip/SLA + Lip/IMQ, Lip/SLA + Lip/MPL, Lip/SLA + Lip/MPL/IMQ and HS buffer, empty Lip, Lip/MPL, Lip/IMQ, and Lip/MPL/IMQ as control groups. Since liposomal carriers are the basis of the main work, only a group of non-liposomal antigens (SLA) is considered and the antigen/adjuvant group, as previously employed, are not considered in this study. Due to the formulation, each injection contains 25 µg MPL, 50 µg SLA and 50 µg of IMQ. Two weeks after the last booster blood sampling for IgG isotyping performed, and three mice in each group were sacrificed and the spleen removed for cytokine assay.
Challenge with L. major promastigotes
Two weeks after the last booster, different group of mice (seven per group) was challenged SC in the left footpad with L. major promastigotes (1.5 × 106 in 50 µl volume) harvested at stationary phase. Lesion development was recorded in each mouse by measurement of footpad swelling (or thickness) with a metric caliper (Asimeto, Hong Kong, China). Grading of lesion size was done for 7–8 weeks by subtracting the thickness of the uninfected contralateral footpad from that of the infected one [Citation36].
Quantitative parasite burden after challenge
The number of viable L. major parasites was estimated in the lymph nodes and infected footpad of mice using limiting dilution assay as described previously [Citation20,Citation37]. Briefly, the mice were sacrificed at week 7 post-challenge; the lymph node and infected footpad tissues were aseptically removed. The tissues homogenized in 2 ml RPMI 1640 supplemented with 10% v/v heat-inactivated FCS (Eurobio, Scandinavia, France), 2 mM glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin sulphate (RPMI-FCS). The homogenates for each mouse were diluted with the same media in eight serial 10-fold dilutions and cultured in triplicate using flat-bottom-96 well plates (Nunc AS, Roskilde, Denmark). The diluted samples were incubated at 25 ± 1 °C for 7–10 d. The plates containing a solid layer of rabbit blood agar were used to culture and detect promastigotes. The positive and negative wells (presence and absence of motile parasite, respectively) were detected using an invert microscope (Olympus, Tokyo, Japan). The number of viable parasites per tissue weight was calculated using the following formula:
Antibody isotyping assay
Blood samples were collected from the mice before the L. major challenge. The sera were titrated to assess the level of anti-SLA total IgG, IgG1 and IgG2a antibodies using ELISA method. Briefly, 96-well microtiter plates were coated with 100 µl/well of 0.5 µg/ml SLA provided in PBS, incubated overnight at 4 °C. Plates were washed with PBST (PBS containing 0/05% tween 20 and blocked by adding 250 µl per well of 2.5% of bovine serum albumin in PBST and incubated at 37 °C for 1:30 h. Serum samples were diluted to 1:500 in PBST and applied to the plates and incubated at 37 °C for 1:15 h. After washing with PBST, the plates were treated with HRP-rabbit anti-mouse IgG isotype according to the manufacturer’s instructions (Southern Biotech, Birmingham, AL), optical density (OD) was read at 450 nm (BIOTEK ELX800, BioTek Instruments Inc., Winooski, VT).
In vitro spleen cells response (cytokine assay)
Cytokine assay performed for three mice of each group before and after the challenge. For this purpose, three mice in each group were sacrificed two weeks after the last booster at the same time of challenge experiment and three mice seven weeks after the challenge were sacrificed. The spleens were aseptically removed. Mononuclear cells were isolated. The cells were washed and re-suspended in complete medium (RPMI 1640-FCS) and seeded at 2 × 106/ml in 96-well flat-bottom plates (Nunc AS, Roskilde, Denmark). The spleen cells were stimulated in vitro with either two concentration of SLA (10 and 5 µg/ml) or medium alone, as negative control and ConA, as positive control. They were incubated at 37 °C with 5% CO2 for 72 h. The culture supernatants were collected and the level of IL-4 and IFN-γ were titrated using ELISA method according to the manufacturer’s instructions (Mabtech, Nacka Strand, Sweden).
Statistical analysis
One-way ANOVA was used to compare the means of the groups’ data. In the case of significant F value, Tukey’s multiple comparison test was carried out as a post-test to compare the means in different groups of mice. p < .05 was considered to be statistically significant.
Results
SLA analysis
The protein concentration of SLA was determined by Lowry method which was 9 mg/ml.
Liposome characterization
The average size of eight formulations was approximately 95 nm in diameter (). The polydispersity index (PDI) of all formulations measured and the average was 0.2, which indicates fair homogeneity of formulations (). The zeta potential of the liposomal preparations was negative () due to the presence of negatively charged phospholipid DSPG in the formulations. Encapsulation efficiency percentage of SLA and IMQ in prepared formulations was quantified. It is shown in .
Figure 1. (a). Size of eight formulations which is characterized by Malvern zatasizer. (b) Poly dispersity index of eight formulations characterized by Malvern zetasizer. (c) Zeta potential of eight formulations which is characterized by Malvern zetasizer.
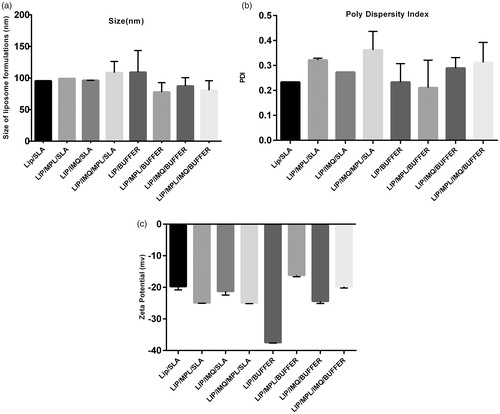
Table 1. Encapsulation efficiency of SLA and IMQ.
Challenge results
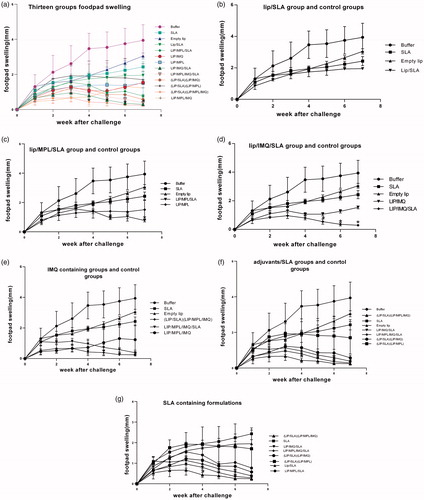
To investigate the effects of immunization on protection of mice against leishmaniasis, the immunized mice were challenged with L. major promastigotes and lesion development was monitored by measurement of footpad thickness weekly. Each vaccinated group compared with control groups separately in ). SLA containing formulations compared in . The lesion swelling after four weeks shows significance between the immunized mice and buffer group and after eight weeks the lesion swelling in groups of mice immunized with lip/MPL/IMQ/SLA, lip/SLA + lip/MPL/IMQ, lip/MPL/SLA, lip/IMQ/SLA and lip/SLA + lip/IMQ was significantly (p < .0001) smaller than other groups compared to control groups. The footpad thickness was measured for 7 weeks. Each point represents the average increase in footpad thickness ± SEM in each group.
Figure 2. Footpad swelling in BALB/c mice immunized SC, three times in two week intervals with Buffer, SLA, empty Lip, Lip / SLA, Lip/MPL, Lip/IMQ, Lip/MPL/SLA, Lip/IMQ/SLA, Lip/MPL/IMQ/SLA, Lip/SLA + Lip/IMQ, Lip/SLA + Lip/MPL, Lip/SLA + Lip/MPL/IMQ, and Lip/MPL/IMQ after challenge with 106 L. major promastigotes in left hind footpads. The footpad thickness was measured weekly for 7 weeks. Each point represents the average increase in footpad thickness ± SEM (n = 6). *p < .05 when the immunized mice compared with mice received buffer. To estimate better the footpad swelling is shown in seven graph; (a) all thirteen groups are compared together (b) Lip/SLA group compared with control groups (c) Lip/MPL/SLA group compared with control groups (d) Lip/IMQ/SLA group compared with control groups (e) liposome containing IMQ groups compared with control groups (f) adjuvants/SLA groups compared with control groups and (g) SLA containing groups comparing together.
Parasite burden
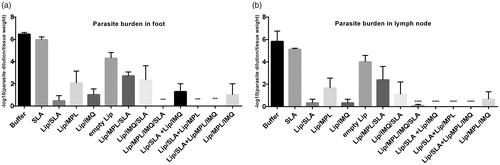
The number of viable L. major parasites was determined in the subiliac lymph nodes and footpad of different groups of mice at week seven post inoculation (). As noted, the group of mice inoculated with lip/MPL/IMQ/SLA, lip/SLA + lip/MPL, lip/SLA + lip/MPL/IMQ, lip/SLA + lip/IMQ showed almost no parasites in the foot and lymph nodes compared with the control group received HS buffer (p < .0001, respectively). There was no significant difference between the mice received buffer and mice immunized with SLA.
Figure 3. Foot and lymph node parasite burden in BALB/c mice immunized SC, three times in two week intervals with buffer, SLA, empty Lip, Lip / SLA, Lip/MPL, Lip/IMQ, Lip/MPL/SLA, Lip/IMQ/SLA Lip/MPL/IMQ/SLA, Lip/SLA + Lip/IMQ, Lip/SLA + Lip/MPL, Lip/SLA + Lip/MPL/IMQ, and Lip/MPL/IMQ after challenge with L. major promastigotes. A limiting dilution analysis was performed 7 weeks after challenge on the cells isolated from footpad and lymph node of individual mice and cultured in triplicate in eight serial dilutions. The number of viable parasite per tissue was determined by the following formula: −log10 (parasite dilution/tissue weight). The bar represent the average score ± SEM (n = 2). ****p < .0001, ***p < .001 when the immunized mice compared with mice received buffer. (a) Represents parasite burden in foot and (b) represents parasite burden in lymph node.
In vitro cytokine production by splenocytes
The supernatant of cultured splenocytes restimulated in vitro before the challenge with SLA to analyse the level of IFN-γ and IL-4, cytokine markers of Th1 and Th2 immune responses, respectively. As shown in , the level of IFN-γ in lip/MPL/IMQ/SLA, lip/SLA + lip/IMQ, lip/IMQ/SLA and lip/SLA + lip/MPL/IMQ groups was significantly high in comparison to buffer group. The ratio of IFNγ/IL4 is shown in . IFN-γ in following groups in comparison with buffer group had significantly higher titre (p < .0001) lip/IMQ/SLA, lip/MPL/IMQ/SLA, lip/SLA + lip/IMQ, lip/SLA + lip/MPL/IMQ. The significance of the difference between the lip/IMQ and lip/MPL/SLA groups with the buffer group was p < .001. IL4 in the same groups with the highest titre of IFN-γ had the low titre (. Cytokine assay after the challenge is shown in , and the IFNγ/IL4 ratio is shown in . The results imply the high titre of IFNγ versus IL4.
Figure 4. Cytokine levels in different groups of immunized mice at week 2 after the last booster injections. Mononuclear splenocytes were cultured in the presence of SLA (10 μg/ml) and the released IFN-γ (a) or IL4 (b) in the culture supernatants were detected using ELISA method results are shown as the mean ± SEM (n = 3). ****p < .0001, ***p < .001.
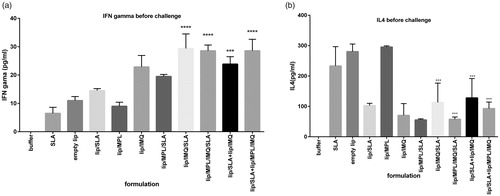
Figure 5. Cytokine levels in different groups of immunized mice 7 weeks after the challenge with L. major promastigotes. Mononuclear splenocytes were cultured in the presence of SLA (10 μg/ml) and the released IFN-γ (a) or IL4 (b) in the culture supernatants were detected using ELISA method. Results are shown as the mean ± SEM (n = 3). ****p < .0001.
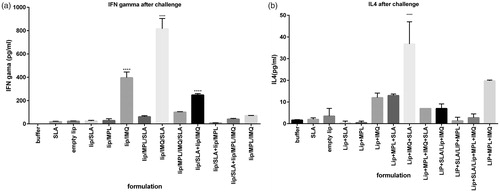
Table 2. IFN-γ/IL-4 Ratio as a marker of cell immunity activation calculated in all groups before the challenge with L. major promastigotes.
Table 3. IFN-γ/IL-4 Ratio as a marker of cell immunity activation calculated in all groups after the challenge with L. major promastigotes.
Antibody response
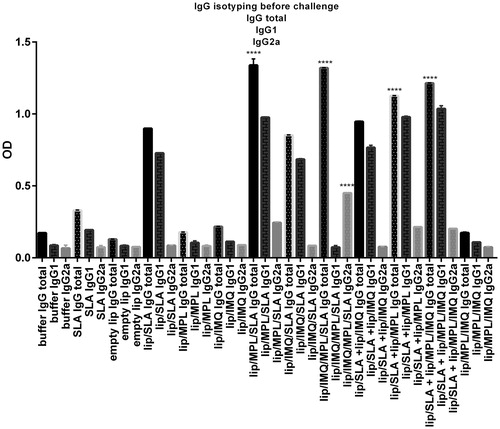
To assess the type of immune response generated in different groups of mice, serum levels of anti-SLA specific total IgG, IgG1 or IgG2a antibodies were evaluated before the challenge. The result of IgG isotyping before challenge is shown in . The immunized mice, which has SLA in their formulation, showed significantly (p < .0001) higher level of total IgG antibody titre compared with the group received buffer. The highest titre of the total IgG in following formulations determined: lip/MPL/SLA (1.33), lip/IMQ/MPL/SLA (1.32), lip/SLA + lip/MPL/IMQ (1.21), lip/SLA + lip/MPL (1.12), lip/SLA + lip/IMQ (0.94), lip/SLA (0.88), lip/IMQ/SLA (0.85). Moreover, the level of IgG1 antibody in the same group of mice was significantly higher than buffer group. In case of IgG2a, the sera of mice immunized with lip/MPL/SLA, lip/IMQ/MPL/SLA, lip/SLA + lip/MPL, and lip/SLA + lip/MPL/IMQ showed significant difference with the group received buffer. Interestingly, the highest ratio of IgG2a/IgG1 antibodies titre was seen in the sera of mice immunized with lip/MPL/IMQ/SLA (6.1) compared with HS buffer (0.77).
Figure 6. Levels of anti-SLA-specific total IgG,IgG1,IgG2a in pooled sera of different group of BALB/c mice immunized SC, three times in 2 week intervals, with buffer, SLA, empty Lip, Lip / SLA, Lip/MPL, Lip/IMQ, Lip/MPL/SLA, Lip/IMQ/SLA Lip/MPL/IMQ/SLA, Lip/SLA + Lip/IMQ, Lip/SLA + Lip/MPL, Lip/SLA + Lip/MPL/IMQ, and Lip/MPL/IMQ. Blood samples were collected from the mice 2 weeks after the last booster. The SLA-specific total IgG, IgG1 and IgG2a were assessed using ELISA method. The assays were performed in triplicate at 1/500 serum dilution which achieved after checkerboard planning. Values are the mean ± SD. ****p < .0001, ***p < .001 when the immunized mice compared with other groups.
Discussion
Despite remarkable endeavour for developing a prophylactic vaccine against leishmaniasis, currently there is not any permissible vaccine to prevent this disease [Citation38]. Cellular immunity response, which directs activation of macrophages and killing the parasite, is responsible for the host resistance. Innate and adaptive immunity is leading up effective activation of dendritic cells, macrophages and antigen-specific T cells [Citation39]. SLA is a mixture of Leishmania antigens purified from Leishmania parasite containing all its components [Citation40]. Therefore, in this experiment, SLA as a first-generation vaccine, MPL and IMQ as immunostimulatory adjuvants were co-entrapped in liposomes and used to immunize BALB/c mice. TLR4 is expressed on dendritic cells and macrophages and some non-immune cells [Citation28]. MPL is administered in human through hepatitis B and human papillomavirus vaccines [Citation29], and it has been used as adjuvant in many studies for Leishmania vaccine either [Citation30,Citation41,Citation42]. Triggering TLRs pathways simultaneously is used in YF17D vaccination which activates subsets of DCs [Citation31]. IMQ is used as a therapeutic agent for skin infection and diseases including in the treatment of human papilloma virus, basal cell carcinoma and actinic keratosis [Citation32,Citation43,Citation44]. The main goal of this study was to compare the adjuvanticity of each immunostimulatory factor when it is used alone or together in different liposomal formula with or without SLA in mice model. The vaccine injection route was subcutaneously in groin of mice where the popliteal and inguinal lymph node presents [Citation45]. IMQ has been used topically as an adjuvant in another study in agreement with the present research. ALM (autoclaved L. major) injected subcutaneously along with the topical IMQ at the site of injection that provided protection against Leishmania infection [Citation27].
Liposome composition has a great role in protecting the cargo from the body clearance [Citation46]. Phospholipids, which are used in liposome preparation, are 1,2-distearoyl-sn-glycero-3-phosphocholine(DSPC), 1,2-distearoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (DSPG) and cholesterol. DSPC and DSPG with high transition temperature (55 °C) increase the rigidity by lower fluidity and reduce the clearance by MPS [Citation21,Citation46,Citation47]. Cholesterol positioning in the phospholipid bilayer excess the distance between the choline head group and diminish the strength of hydrogen bonding and electrostatic interaction, which makes the membrane stable and prevent intrusion of water and molecules [Citation25,Citation30].
Synthetic phospholipids affect the charge of liposomes, according to the head groups [Citation48]. Because of the presence of neutral PC (DSPC) and negative PG(DSPG) head groups of phospholipids, the liposome charge has shown a negative charge, which could be influenced by adjuvants and antigen charges (). The average size of liposomal formulations was around 95 nm. Lymphatic capillary uptake depends on size, charge and hydrophobicity [Citation49]. The size of the vehicle is important for lymphatic drainage and uptaking through endocytosis by dendritic cells [Citation50]. Particle sizes between 10 and 100 nm removed by lymphatic vessels as a choice, particles larger than 100 nm remain at the site of injection as a reservoir, and particles smaller than 10 nm removed by blood vessels [Citation49]. Zeta potential of liposomal formulations was between ∼−16 mV (lip/MPL/buffer) and ∼−37 mV (liposome/buffer), which implies the impact of antigen and adjuvants charges on zeta potential of liposome formulations ().
There are some reports which included the optimal charge of macrophage interaction is negative charge [Citation20]. Moreover, the rate of protection and produced immune response were analysed and compared with control groups, which received buffer or antigen in soluble form. In this study based on footpad swelling immunization with SLA, empty liposome, lip/SLA, lip/MPL, lip/SLA + lip/MPL, lip/IMQ and lip/MPL/IMQ induced no protection in BALB/c mice. The ratio of IFNγ/IL4 as a marker of Th1 response before the challenge indicated the high level of IL4 production, which supports the footpad swelling except in lip/IMQ group. The wound swelling in lip/IMQ group had a steady state between 3 and 5 week and then it grew. This can be the result of IMQ stimulating factor, which did not work proprietary in the absence of antigen. According to the footpad swelling (), 7 weeks after the challenge in following formulations: lip/SLA + lip/MPL/IMQ, lip/IMQ/SLA, lip/MPL/IMQ/SLA, lip/MPL/SLA and lip/SLA + lip/IMQ, the curve growth trend show healing the swelling footpad in these groups, which is supported by IFNγ/IL4 ratio before the challenge. One of the effective strategies to increase expansion of T cells through the relevant PRP pathways is co-delivery of adjuvants and antigens [Citation51]. In other studies, the vaccine carrier, which has been used, was cubosomes or synthetic nanoparticles as a dual carrier for adjuvant and antigen delivery [Citation26,Citation51]. In this research using nanoliposome as a dual adjuvant and antigen carrier explain the high titre of IgG in the SLA containing groups in comparison with the control groups. In IgG isotyping assay, the highest ratio of IgG2a/IgG1antibody titre in the lip/MPL/IMQ/SLA and lip/SLA + lip/IMQ/MPL groups is shown in before the challenge, which is the sign of cellular immunity activation and the results are compatible with wound swelling and other findings. Cytokine assays after challenge indicated Th1 and Th2 response and partial protection in lip/IMQ/SLA group, although BALB/c mice immune response as a sensitive animal model could not support the results. Our findings are inconsistent with previous reports by Rostamian and Niknam [Citation52] and Cargnelutti et al. [Citation53]. Rostamian and Niknam had been used SLA, resiquimod (R848) and MPL for vaccination without liposomal carrier, their reports showed no protection effect of R848, and there was no synergy effect of adjuvants, they have shown the protection against Leishmania infection in SLA group [Citation52]. In Cargnelutti et al.’s study, they had a comparison between Montanide ISA 763 adjuvant and R848 which indicated the protection effect of Montanide and no protection effect of R848 [Citation53]. Lower concentration of adjuvant may affect the protection. Using nanoliposome carrier could be an effective cargo for slow release of vaccine. Protection effect of SLA is inconsistent with previous reports and this study [Citation15,Citation16,Citation33], which could be the result of injecting attenuated parasite during the challenges. Our studies confirm the results of previous studies [Citation27,Citation54], using liposome and MPL in formulating Leishmania vaccine has had a protection effect because of sustained activation of cellular immunity [Citation42].
In conclusion, we have used the synergic effect of two adjuvants as TLR ligands to activate the cellular immunity against Leishmania parasite. Applying nanoliposome vehicle for antigen delivery mimic the parasite figure and could be an alternative for traditional vaccines that use whole microbe with many side effects [Citation50]. Liposome itself has an adjuvanticity effect but not enough to protect against CL [Citation55]. Simultaneous use of nanoliposome carrier for separated delivery of antigen and adjuvant can be effective, but it did not make any significant difference with the groups, which deliver antigen and adjuvant in one liposomal carrier. Comparing the footpad swelling in separated graphs indicated that IMQ could be a good choice for Leishmania adjuvant vaccine, and synergic effect of two adjuvants combination shows the prophylactic efficacy of the formulation. Recently Cargnelutti et al.’s study showed partial protection effect of antigenic extracts of Leishmania associated with Montanide ISA 763 adjuvant against Leishmania amazonensis, which it is recommended to apply Montanide adjuvant in liposomal formulation against L. major in further studies in combination with IMQ to improve its efficacy [Citation53]. Nanoliposome entrapping different concentrations of IMQ is also recommended for further studies.
Disclosure statement
The authors declare no financial or commercial conflict of interest.
Additional information
Funding
References
- Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671.
- Khamesipour A. Therapeutic vaccines for leishmaniasis. Expert Opin Biol Ther. 2014;14:1641–1649.
- Noazin S, Khamesipour A, Moulton LH, et al. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis—a meta-analysis. Vaccine. 2009;27:4747–4753.
- Schlein Y, Jacobson RL, Messer G. Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proc Natl Acad Sci. 1992;89:9944–9948.
- Silveira F, Lainson R, De Castro Gomes C, et al. Immunopathogenic competences of Leishmania (V.) braziliensis and L.(L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31:423–431.
- Khamesipour A, Dowlati Y, Asilian A, et al. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23:3642–3648.
- Nadim A, Javadian E, Tahvildar-Bidruni G, et al. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1983;76:377–383.
- Sjölander A, Baldwin TM, Curtis JM, et al. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J Immunol. 1998;160:3949–3957.
- Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103.
- Locksley RM, Louis JA. Immunology of leishmaniasis. Curr Opin Immunol. 1992;4:413–418.
- Okwor I, Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res. 2008;41:123–136.
- Mendonça SC. Differences in immune responses against Leishmania induced by infection and by immunization with killed parasite antigen: implications for vaccine discovery. Parasites Vectors. 2016;9:492.
- Eskandari F, Talesh GA, Parooie M, et al. Immunoliposomes containing soluble Leishmania antigens (SLA) as a novel antigen delivery system in murine model of leishmaniasis. Exp Parasitol. 2014;146:78–86.
- Shargh VH, Jaafari MR, Khamesipour A, et al. Cationic liposomes containing soluble Leishmania antigens (SLA) plus CpG ODNs induce protection against murine model of leishmaniasis. Parasitol Res. 2012;111:105–114.
- Shargh VH, Jaafari MR, Khamesipour A, et al. Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine. 2012;30:3957–3964.
- Firouzmand H, Badiee A, Khamesipour A, et al. Induction of protection against leishmaniasis in susceptible BALB/c mice using simple DOTAP cationic nanoliposomes containing soluble Leishmania antigen (SLA). Acta Tropica. 2013;128:528–535.
- Rhee EG, Mendez S, Shah JA, et al. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J Exp Med. 2002;195:1565–1573.
- Mohan T, Verma P, Rao DN. Novel adjuvants & delivery vehicles for vaccines development: a road ahead. Indian J Med Res. 2013;138:779.
- Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2:159–182.
- Badiee A, Jaafari MR, Khamesipour A, et al. The role of liposome charge on immune response generated in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63). Exp Parasitol. 2009;121:362–369.
- Badiee A, Jaafari MR, Khamesipour A, et al. Enhancement of immune response and protection in BALB/c mice immunized with liposomal recombinant major surface glycoprotein of Leishmania (rgp63): the role of bilayer composition. Colloids Surf B Biointerfaces. 2009;74:37–44.
- Badiee A, Khamesipour A, Samiei A, et al. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol. 2012;132:403–409.
- Bardania H, Tarvirdipour S, Dorkoosh F. Liposome-targeted delivery for highly potent drugs. Artif Cells Nanomed Biotechnol. 2017;45:1478–1489.
- Daraee H, Etemadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:381–391.
- Chibowski E, Szcześ A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption. 2016;22:755–765.
- Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547.
- Zhang WW, Matlashewski G. Immunization with a toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect Immun. 2008;76:3777–3783.
- Cluff CW. Monophosphoryl lipid a (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Berlin, Germany: Springer; 2009. p. 111–123.
- Das A, Ali N. Combining cationic liposomal delivery with MPL-TDM for cysteine protease cocktail vaccination against Leishmania donovani: evidence for antigen synergy and protection. PLoS Negl Trop Dis. 2014;8:e3091.
- Askarizadeh A, Jaafari MR, Khamesipour A, et al. Liposomal adjuvant development for leishmaniasis vaccines. Ther Adv Vaccines. 2017;5:85–101.
- Querec T, Bennouna S, Alkan S, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424.
- Lin P, Torres G, Tyring SK. Changing paradigms in dermatology: antivirals in dermatology. Clin Dermatol. 2003;21:426–446.
- Scott P, Pearce E, Natovitz P, et al. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–227.
- Wessel D, Flügge U. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143.
- Bainor A, Chang L, McQuade TJ, et al. Bicinchoninic acid (BCA) assay in low volume. Anal Biochem. 2011;410:310–312.
- Jaafari MR, Ghafarian A, Farrokh-Gisour A, et al. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine. 2006;24:5708–5717.
- Titus RG, Marchand M, Boon T, et al. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555.
- Alvar J, Croft SL, Kaye P, et al. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31:B244–B249.
- Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138.
- Bhowmick S, Ravindran R, Ali N. Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized Th1 response. Vaccine. 2007;25:6544–6556.
- Ravindran R, Maji M, Ali N. Vaccination with liposomal leishmanial antigens adjuvanted with monophosphoryl lipid–trehalose dicorynomycolate (MPL-TDM) confers long-term protection against visceral leishmaniasis through a human administrable route. Mol Pharmaceutics. 2011;9:59–70.
- Reed SG, Coler RN, Campos-Neto A. Development of a leishmaniasis vaccine: the importance of MPL. Exp Rev Vaccines. 2003;2:239–252.
- Sauder DN. Mechanism of action and emerging role of immune response modifier therapy in dermatologic conditions. J Cutan Med Surg. 2004;8:3–12.
- Urosevic M, Dummer R. Role of imiquimod in skin cancer treatment. Am J Clin Dermatol. 2004;5:453–458.
- Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods. 2008;332:170–174.
- Li J, Wang X, Zhang T, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10:81–98.
- Badiee A, Jaafari MR, Khamesipour A. Leishmania major: immune response in BALB/c mice immunized with stress-inducible protein 1 encapsulated in liposomes. Exp Parasitol. 2007;115:127–134.
- Bernasconi V, Norling K, Bally M, et al. Mucosal vaccine development based on liposome technology. J Immunol Res. 2016;2016:5482087.
- Bagby TR, Cai S, Duan S, et al. Impact of molecular weight on lymphatic drainage of a biopolymer-based imaging agent. Pharmaceutics. 2012;4:276–295.
- Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3:13.
- Rizwan S, McBurney W, Young K, et al. Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses. J Control Release. 2013;165:16–21.
- Rostamian M, Niknam HM. Evaluation of the adjuvant effect of agonists of toll-like receptor 4 and 7/8 in a vaccine against leishmaniasis in BALB/c mice. Mole Immunol. 2017;91:202–208.
- Cargnelutti DE, Salomón MC, Celedon V, et al. Immunization with antigenic extracts of Leishmania associated with Montanide ISA 763 adjuvant induces partial protection in BALB/c mice against Leishmania (Leishmania) amazonensis infection. J Microbiol Immunol Infect. 2016;49:24–32.
- Mazumder S, Maji M, Ali N. Potentiating effects of MPL on DSPC bearing cationic liposomes promote recombinant GP63 vaccine efficacy: high immunogenicity and protection. PLoS Negl Trop Dis. 2011;5:e1429.
- Mutiso JM, Macharia JC, Gicheru MM. A review of adjuvants for Leishmania vaccine candidates. J Biomed Res. 2010;24:16–25.
- Kirby C, Gregoriadis G. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Nat Biotechnol. 1984;2:979.
- Iman M, Huang Z, Szoka FC, et al. Characterization of the colloidal properties, in vitro antifungal activity, antileishmanial activity and toxicity in mice of a distigmasterylhemisuccinoyl-glycero-phosphocholine liposome-intercalated amphotericin B. Int J Pharm. 2011;408:163–172.

