?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cisplatin is widely used in cancer treatment, but the application is limited due to toxicities and its acquired resistance. In this study, we delivered cisplatin to prostate cancer cells by linking the platinum prodrug Pt(IV) to melanin-like nanoparticles (MeNPs), a promising photothermal therapeutic agent with excellent biocompatibility. As expected, the Pt(IV)-MeNPs exhibited brilliant synergic photothermal-chemotherapy upon near-infrared reflection exposure. Compared with free cisplatin, Pt(IV)-MeNPs displayed highly effective antitumour activity both in vitro and in vivo.
Introduction
Cisplatin is a kind of common chemotherapy drugs based on platinum group elements. Cisplatin has been widely used in tumour treatment, including testicular cancer, ovarian cancer, breast cancer, bladder cancer and prostate cancer [Citation1–3]. Together with the analogues carboplatin and oxaliplatin, cisplatin is applied in the first-line treatment of malignant tumours. The anti-tumour effect of platinum-based drugs mainly depends on binding with DNA to form DNA adducts [Citation4,Citation5]. Although the efficacy of platinum compounds in anti-tumour activity has been universally recognized, their application is still facing some restrictions, such as some serious side effects due to binding to the essential amino acids in the plasma. Several novel platinum compounds have also been synthesized to further promote the anti-tumour activities [Citation6,Citation7]. The platinum prodrug Pt(IV) shows a number of advantages over the conventional Pt(II) compounds. The pharmacological properties of platinum are also slightly modified after Pt(II) oxidation, such as lipophilicity, dynamic stability and reduction potential [Citation8]. As a prodrug, the Pt(IV) compound is required to be reduced in the surrounding environment first by bio-reductive agents such as ascorbic acid (AsA) or glutathione (GSH) before releasing the cytotoxic Pt(II) fragments [Citation9,Citation10].
With the continuous development of nanotechnology, the enhanced permeability and retention (EPR) effect of nanoparticles is widely used in anti-tumour research, which could reduce the drug accumulation in normal tissues and increase the tumour-specific targeting action [Citation11–15]. Recently, nanomaterials with photothermal therapeutic (PTT) effect, such as gold nanoparticles, palladium nanosheets, graphene and carbon nanotubes, have been applied as functional drug carriers for the combination of chemotherapy and photothermal therapy [Citation16–18]. These nanocarriers not only reduce the required concentration of chemotherapy drugs but also regulate drug release via near-infrared (NIR) irradiation to achieve the synergistic activity of chemotherapy and photothermal therapy [Citation19–22]. Melanin is a popular photosensitizer that has strong absorptivity in the NIR region and ∼40% photothermal conversion efficiency. Furthermore, unlike other metal or carbon materials, melanin nanoparticles (MeNPs) present high biocompatibility and low cytotoxicity. They are able to significantly reduce the inflammatory response [Citation23,Citation24]. The structure of MeNPs makes it easy to react with other chemical groups, in order to obtain more unique properties by surface modification [Citation25,Citation26].
In this report, we presented the conjugate combining cisplatin prodrug Pt(IV) and MeNPs. MeNPs were synthesized as a functional nanocarrier and photothermal agent, connecting the cisplatin prodrug Pt(IV)-COOH which could be reduced and release cytotoxic Pt(II) under a reductive environment like in tumour. The drug delivery platform performed synergic highly efficient photothermal therapy and chemotherapy properties for treating prostate cancer in vitro and in vivo, upon NIR exposure and reductive agent based drug release.
Methods
Materials
Dopamine hydrochloride, N-hydroxysuccinimide (NHS), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), succinic anhydride and cis-diamineplatinum(II) dichloride were purchased from Sigma-Aldrich (St. Louis, MO). 4-arm polyethylene glycol (PEG) amine (Mw = 2000) was purchased from JenKem Technology (Plano, TX).
Synthesis of NH2-MeNPs
MeNPs were synthesized according to a previous protocol [Citation21]. Briefly, 3 ml ammonia aqueous solution (30%), 40 ml ethanol and 90 ml water were mixed under stirring overnight, in which 500 mg dopamine hydrochloride was added. MeNPs were collected by centrifugation and washed with water for three times. To obtain NH2 modified MeNPs, excessive 4-arm PEG amine was dissolved and mixed with MeNPs in Tris buffer solution (10 mM, pH = 8.5). After vigorous stirring for 12 h, NH2 modified MeNPs were purified and washed with Amicon® Ultra Centrifugal Filters (MWCO: 100 kD, EMD Millipore, Darmstadt, Germany), then dispersed in deionized water and stored under 4 °C for future experiments.
Synthesis of Pt(IV)-OH, Pt(IV)-COOH and Pt(IV)-MeNP
Pt(IV)-OH and Pt(IV)-COOH were prepared as previously described [Citation10]. The 1H NMR of Pt(IV)-COOH in DMSO-d6 are shown in Supplementary Figure S1. Pt(IV)-MeNPs were synthesized as depicted in Scheme 1(b). Briefly, EDC·HCl (0.191 g, 1 mmol) and NHS (0.115 g, 1 mmol) dissolved in de-ionized water under stirring. Exceeded Pt(IV)-COOH was added to the aqueous solution. After the mixture became clear, 0.5 g NH2-MeNP in 100 ml water was added and the reaction mixture was kept stirring at room temperature for 24 h then centrifuged and washed. Inductively coupled plasma mass spectrometer (ICP-MS, Optima 5300DV, PE, Waltham, MA) was performed to determine the Pt content.
Characterization
Transmission electron microscopy (TEM) images were obtained on a JEM 2011 transmission electron microscope (JEOL, Japan). The size, polydispersity index (PDI) and zeta potential were measured by dynamic light scattering (DLS) with NanoBrook 90Plus PALS (Brookhaven Ins. Co., Holtsville, NY). The UV–Vis spectra of NH2-MeNP was recorded by using a UV–Vis spectrometer (Cary60, Agilent Technologies, Palo Alto, CA).
Temperature measurements during laser irradiation
Pt(IV)-MeNPs were dispersed in phosphate buffered solution (PBS). Then Pt(IV)-MeNPs and cisplatin solutions were irradiated by an 808 nm continuous-wave diode laser at 0.5 W/cm2, respectively. Temperature was measured every 5 s with the thermometer probe. PBS was applied as the control.
The photothermal conversion efficiency was calculated as follows.
η was defined as the photothermal conversion efficiency, calculated with EquationEquation (1)(1)
(1) described by Roper [Citation27]:
(1)
(1)
where h was the heat transfer coefficient, S was the surface area of the container, ΔTmax was the temperature increase of the nanoparticles at the maximal steady-state temperature, Qs was the heat associated with the NIR light absorbance of the solvent, I was the incident laser density and A808 was the absorbance of the nanoparticles at 808 nm. The value of hS was derived according to EquationEquation (2)
(2)
(2) :
(2)
(2)
where τ was the sample system time constant, ms was the mass of solvent, Cs was the mass and the heat capacity of the solvent. Based on the EquationEquations (1)
(1)
(1) and Equation(2)
(2)
(2) , the photothermal conversion efficiency (η) was calculated.
In vitro drug release
Dialysis method was applied to detect the in vitro drug release of platinum. Pt(IV)-MeNPs were dispersed in 3 ml deionized water with dialysis bags (MWCO: 3500), which were incubated in 0.1 mM AsA or PBS solution at 37 °C. A 0.5 W/cm2 808 nm continuous-wave diode laser was used to investigate the drug release with or without laser irradiation. One millimetre of supernatant was taken out at a selected time and replaced with 1 ml fresh buffer solution. Pt concentration was measured by ICP-MS. Cumulative amount of the released drug was calculated to evaluate the drug release behaviour.
Cytotoxicity assay
Prostate cancer cells of PC3, DU145 and LnCap were seeded in 96-well plates at a density of 5 × 103 cells per well, incubated at 37 °C with 5% CO2 for 12 h. The culture media were replaced of 100 μl of media with 10% fetal bovine serum containing equivalent concentrations of PBS, free cisplatin, Pt(IV)-COOH, Pt(IV)-MeNPs. The max drug content in all the groups was kept at 32 µM of cisplatin. After 48 h of incubation, CCK-8 assay was used to detect the amount of viable cells. In consideration of the PTT efficacy, PC3, DU145 and LnCap cells were seeded at a density of 5 × 103 cells per well in 96-well plates and incubated at 37 °C with 5% CO2. The culture media were replaced of 100 μl media with or without 10% fetal bovine serum containing Pt(IV)-MeNPs with concentrations of 5 µM in platinum. After 12 h incubation, the cells were irradiated with a 0.5 W/cm2 808 nm laser for 15 min. After another 36 h incubation, the viability of cells was detected by a CCK-8 assay.
In vitro cellular uptake
PC3 cells were seeded in 6-well plates at a density of 106 cells per well, incubated at 37 °C with 5% CO2 for 12 h. The cells were treated with cisplatin, Pt(IV)-COOH and Pt(IV)-MeNPs with the final platinum concentration in the culture medium regulated to 5 μM and then incubated at 37 °C for 4 h. As previously described [Citation28], to remove surface-bound drugs, cells were washed three times with ice-cold PBS, incubated with 1.5 ml of 0.15 M sodium chloride (pH 3.0 was adjusted by acetic acid) for 3 min at 4 °C, then rinsed with 2 ml of cold PBS, harvested by scraping in ice-cold PBS, centrifuged. Thereafter, the cells were lysed by adding 200 μl cell lysis buffer (Promega lysis buffer) and then the cell lysis solution was frozen at −20 °C for 20 min and thawed at room temperature. 100 μl of the cell lysis solution for each sample was used directly to measure the Pt content by ICP-MS. The other 100 μl of the cell lysis solution was used to determine the protein content in each cell sample by using bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). The platinum content was expressed as nano-grams of Pt per milligram of total proteins.
Mitochondrial membrane potential (MMP) analysis
PC3 cells were seeded into black 96-well microplates. After 24 h, drugs (cisplatin, Pt(IV)-COOH and Pt(IV)-MeNPs at the concentration of platinum in 10 μM) were added to the wells for 1, 4, 8 or 24 h, respectively. Cells treated with MeNPs and without treatment were used as controls. At each end of drug treatment, the medium was removed and the cells were incubated at 37 °C for 1 h with 5 mg/l of JC-1 (5,50,6,60-tetrachloro-1,10,3,30-tetraethyl-imidacarbocyanine iodide), then washed twice with PBS and placed in fresh medium without serum. Images were collected after 4 h incubation and viewed by Confocal fluorescence imaging (Leica TCS SP5) at excitation wavelength of 488 nm and emission of 530 nm for green and at excitation wavelength of 540 nm and emission of 590 nm for red. The fluorescence of both green fluorescence (JC-1 monomers) and red fluorescence (JC-1 aggregates) were measured at each endpoint with Micro Plate Reader (BioTek, Winooski, VT). Data were presented as the ratio of green to red signals (520 nm/590 nm).
PC3 xenograft tumour model
The tumour model was constructed by subcutaneous injection with 200 µl of PC3 cell suspension (a mixture of RPMI-1640 medium and Matrigel in 1:1 volume ratio) with a density 1 × 107 cells/ml into the back region of healthy male Balb/c nude mice. When the volume of the PC3 tumour xenograft reached ∼100 mm3, the mice were used for the following in vivo experiments.
Blood circulation and biodistribution
Healthy female Balb/C mice were intravenously injected with 200 μl Pt(IV)-MeNP solution. Blood circulation was measured by drawing 20 μl of blood from one side of orbital venous plexus at various time points post-intravenously injection. The percentage of Pt was calculated by taking into consideration that blood constitutes 9% of body weight [Citation29]. The Pt content in blood was determined by ICP-MS.
In order to investigate the biodistribution, randomized groups of three nude mice bearing PC3 xenografts with an average tumour volume of 100 mm3 received a single dose of 10 µmol/kg in platinum of either cisplatin or Pt(IV)-MeNPs (40 mg/kg) at time zero. Twenty-four hours after the intravenous injection, organs and tumours were harvested. Tissue samples required prior digestion with concentrated nitric acid. Gentle heating was required to complete the digestion. Following evaporation to near dryness, the digests were taken up in 1 M hydrochloric acid and again heated to near dryness to remove excess nitric acid. After repeating this last stage with 0.1 M hydrochloric acid (2 ml), the digests were dissolved in 1 ml of 0.1 M hydrochloric acid for Pt analysis. Pt content was determined by ICP-MS.
Combination therapy
PC3 tumour-bearing male Balb/C nude mice were randomly divided into four groups (n = 4) and intravenously injected with (i and ii) PBS, (iii) cisplatin, (iv and v) Pt(IV)-MeNPs at 10 µmol platinum per kg mouse weight on day 1. The mice of group ii (treated with PBS) and v [treated with Pt(IV)-MeNPs] received laser treatment for 10 min (808 nm laser, 0.3 W/cm2) 12 h after injection. All the mice were sacrificed on day 15. The tumour growth and body weight were monitored every 3 days (day 0, 3, 6, 9, 12, 15) by measuring perpendicular diameters using a caliper. Tumour volume was calculated as follows:
where W and L are the shortest and longest diameters, respectively. Serum was collected for biochemical studies after monitoring period. Serial sections of various formalin-fixed organs and excised tumours embedded in paraffin were stained with haematoxylin and eosin (H&E) and analysed at 40 × magnification.
Results and discussion
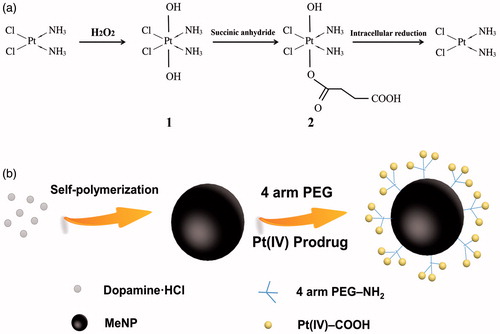
Scheme 1(a) presented the synthesis process of Pt(IV)-COOH compound. Cytotoxic cisplatin would be released after reduction of Pt(IV) prodrug under reductive environment. Scheme 1(b) displayed the fabrication process of Pt(IV)-MeNPs. MeNPs was prepared by dopamine oxidation and self-polymerization [Citation30]. Previous study reported the Michael addition and Schiff base reaction could functionalize MeNPs in Tris buffer [Citation31]. We chose 4-arm PEG amine instead of traditional amine-PEG in order to attach more Pt to the platform, along with better biocompatibility and longer circulation time [Citation32]. Additionally, Pt(IV)-COOH was afforded by reaction with Pt(IV)-OH and succinic anhydride in a molar ratio of 1:1. The Pt(IV)-COOH was further attached to NH2-MeNPs via pendant amine groups. To play the role of antitumour activity, synthesized Pt(IV)-MeNPs could release MeNPs and cisplatin when they were internalized by cancer cells where the intracellular environment was reductive or acidic. The platinum content in Pt(IV)-MeNPs was 4.2% in weight determined by ICP-MS. The diameter of NH2-MeNPs and Pt(IV)-MeNPs were 86.1, 73.7 nm, respectively, measured by dynamic DLS (). The TEM image of NH2-MeNPs was shown in . The UV–vis absorption spectra of NH2-MeNP solutions were displayed in , which showed great absorption in the NIR region. As the result, the NPs exhibited excellent photothermal effects with laser at 808 nm. Therefore, we monitored the temperature change of our synthesized NH2-MeNP solution under NIR irradiation. The temperature increased about 15 °C with the concentration of 1.0 mg/ml after irradiation by 808 nm laser at the power intensity of 0.5 w/cm2 and the temperature change was significantly associated with concentration (). Photothermal conversion efficiency of Pt(IV)-MeNP solution was 29.6%, presenting a higher value than most traditional PTT agents.
Figure 1. (a) Size distribution determined by DLS of NH2-MeNPs and Pt(IV)-MeNPs. (b) TEM image of NH2-MeNPs. (c) UV–vis absorption spectra of synthesized NH2-MeNPs. (d) Photothermal heating curves of synthesized NH2-MeNP solution with varied concentrations irradiated under laser exposure at a power density of 0.5 W/cm2.
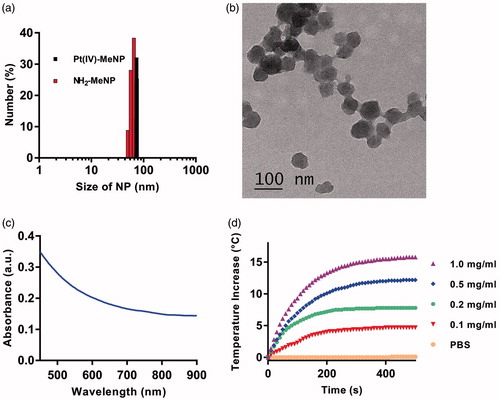
To evaluate the efficiency of Pt(IV)-MeNPs as a potential drug platform for drug delivery, cisplatin release upon chemical reduction was investigated. Pt(IV)-MeNPs were dispersed into PBS buffer with or without 0.1 mM AsA as the bio-reductant for a period of 48 h. After centrifuge at a specific time point, Pt(II) species content in the supernatant was detected by ICP-MS. As shown in , few amount of Pt(II) species were detected when lack of chemical reduction. Significant difference was found between the groups with or without AsA treating. For the AsA treated group, 60% of Pt(II) was released within 8 h and it exceeded 80% after 48 h monitoring. AsA acted as a reductive agent. It was able to donate electrons to various enzymatic and a few non-enzymatic reactions. Since the release media could simulate intracellular conditions, Pt(IV)-MeNPs were expected to undergo similar release process in cancer cells. Therefore, low-toxic Pt(IV) prodrugs converted to high-toxic cisplatin(II) along with the release of Pt(II), which enhanced the antitumour efficacy.
Figure 2. (a) Pt(II) release profiles of Pt(IV)-MeNPs in PBS (pH = 7.4) with or without 0.1 mM AsA solution. (b) Chemical reduction of Pt(II) from the Pt(IV)-MeNP in PBS buffer (pH 7.4) by 0.1 mM AsA with or without NIR laser irradiation (808 nm, 0.5 W/cm2, 15, 30 and 120 min, respectively). *p < .05 and **p < .01 was calculated by Student's t-test.
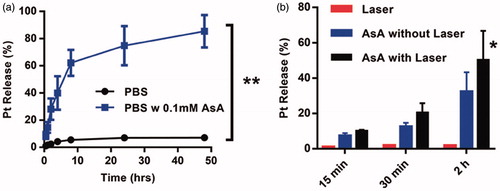
Additionally, we investigated the relationship between photothermal effect and drug release upon chemical reduction. A significant increase of Pt(II) release was achieved by 2 h NIR irradiation in the presence of 0.1 mM AsA, whereas the change of Pt(II) release could not be detected in the absence of AsA under 0.5 W/cm2 laser irradiation, indicating that NIR laser was not able to promote the chemical reduction separately ().
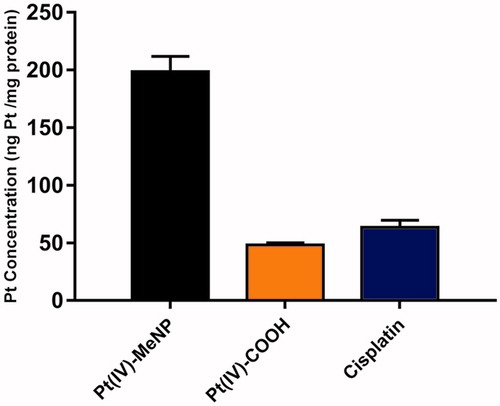
The in vitro cellular cytotoxicities of Pt(IV)-MeNPs, Pt(IV)-COOH prodrug, MeNPs and free cisplatin were measured by CCK-8 assay on three prostate cell lines: PC3, LnCap and DU145. As shown in ), no obvious toxicity was found for MeNPs without NIR during our analysed concentration range after 48 h incubation with the cells, demonstrating excellent biocompatibility of MeNPs as a non-toxic material for drug delivery systems. On the other side, Pt(IV)-MeNPs, Pt(IV)-COOH prodrug and free cisplatin all displayed certain inhibition effects with the increase in concentrations. Half inhibitory concentration (IC50) was calculated and displayed in Supplementary Table S1. According to the result, IC50 of Pt(IV)-MeNPs was slightly higher than that of free cisplatin, which was attributed to the +4 valence of the central Pt ions. Meanwhile, for Pt(IV)-COOH, its Pt atom was in +4 valence and thus it showed much less cytotoxicity than cisplatin. In addition, Pt(IV)-MeNPs exhibited increased cytotoxicity compared to Pt(IV)-COOH prodrug due to higher cell uptake of the nanoparticles. We tested the intracellular uptake of cisplatin, Pt(IV)-COOH and Pt(IV)-MeNPs, respectively. Results showed the uptake in 4 h by PC3 cells of Pt(IV)-MeNPs (198.5 ng Pt/mg protein) was nearly four times higher than both Pt(IV)-COOH (48.2 ng Pt/mg protein) and cisplatin (63.4 ng Pt/mg protein), in accordance with the fact that nanoparticles were internalized via endocytosis more efficiently than small molecular via simple diffusion (). To further identify the PTT efficiency of Pt(IV)-MeNPs, PC3, LnCap and DU145 cell lines were incubated with or without Pt(IV)-MeNPs at a concentration of 5 µM in Pt. Cells were irradiated by NIR at a power density of 0.5 w/cm2 for 15 min after 12 h drug treatment. After another 36 h incubation, CCK-8 assay showed that the photothermal killing efficacies were notable in all three cell lines compared to those without NIR. The cell viability reduced almost 2/3 treated with Pt(IV)-MeNPs and NIR irradiation, confirming the synergic effective photothermal-chemotherapy antitumour activity in vitro ().
Figure 3. In vitro antitumour activity against prostate cancer cells. The cytotoxicity of Pt(IV)-MeNPs, Pt(IV)-COOH, Cisplatin and MeNPs on PC3 (a), DU145 (b) and LnCap (c) cells. (d) The photothermal effect of prostate cancer cells treated by Pt(IV)-MeNPs with NIR, Pt(IV)-MeNPs without NIR or PBS with NIR, respectively. The power density of irradiation was 0.5 W/cm2 for 15 min.
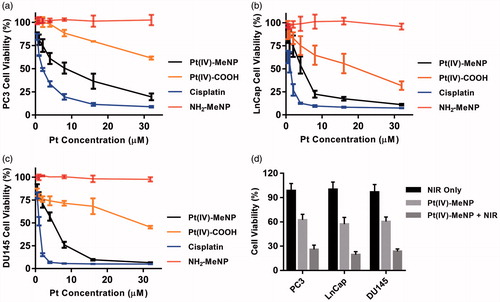
Figure 4. Cellular uptake of various drugs by PC3 cells at 4 h determined by ICP-MS. The initial platinum concentration of all drugs was 5 μM. Cellular uptake was expressed in the unit of (ng Pt/mg proteins).
Moreover, one of the antitumour actions of Pt(IV)-MeNPs is to cause depolarization of the mitochondrial membrane. JC-1 is a fluorescent dye that detects the depolarization of mitochondrial membranes. By staining PC3 cells with JC-1 and measuring its red (aggregates) to green (monomers) fluorescence intensity ratio, Pt(IV)-MeNPs ranked the lowest indicating it induced most mitochondrial membrane depolarization, possibly due to the high intracellular uptake (Supplementary Figure S2).
To investigate the in vivo blood circulation, Pt(IV)-MeNPs were injected into healthy Balb/C mice intravenously. As shown in , Pt(IV)-MeNPs prolonged the circulation time. There was still 10.17% platinum rest in blood circulation 24 h after injection measured by ICP-MS. To investigate the biodistribution of Pt(IV)-MeNPs in vivo, we injected intravenously cisplatin and Pt(IV)-MeNPs solution with same Pt concentration, respectively. Main organs were picked up 24 h after injection. Results displayed that the Pt(IV)-MeNPs accumulated in kidney, liver and tumour. However, free cisplatin mainly accumulated in kidney when no obvious cisplatin was found in tumour (). This phenomenon demonstrated that nanoparticles could accumulate in tumour via the EPR effect. In addition, the total accumulation amount in vivo of Pt(IV)-MeNPs was much higher than free cisplatin, which also proved that the better cell uptake ability of Pt(IV)-MeNPs.
Figure 5. In vivo chemo-photothermal therapy treatment of tumour by using Pt(IV)-MeNPs. (a) The blood circulation of Pt(IV)-MeNPs. (b) The biodistribution of the tumour and main organs of PC3 xenograft tumour-bearing nude mice sacrificed at 24 h post-injection of Pt(IV)-MeNPs or cisplatin at 24 h post-injection. (c) The tumour volume evolution of mice in different groups during the therapeutic period. (d) Photographs of the excised tumours of mice after sacrifice. (e) Curves of tumour temperature increase after NIR irradiation for the groups treated with Pt(IV)-MeNPs + NIR or NIR only. (f) The body weight changes of mice during the therapeutic period. *p < .05 and **p < .01 were calculated by Student’s t-test.
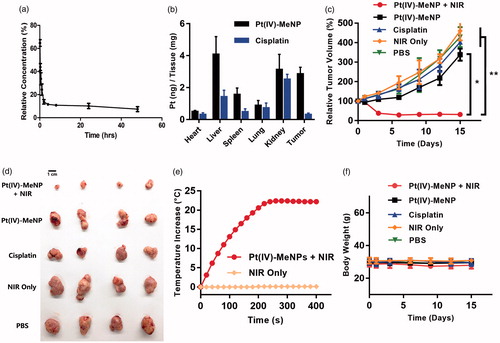
The antitumour activity of Pt(IV)-MeNPs was evaluated in nude mice bearing PC3 tumours. The mice were divided into five groups randomly when the tumour volume grew to approximate 100 mm3 then administered with (i) PBS, (ii) PBS + NIR, (iii) free cisplatin, (iv) Pt(IV)-MeNPs and (v) Pt(IV)-MeNPs + NIR, respectively. The mice were sacrificed after 15 days monitoring. The tumours were collected and displayed in . showed tumour growth curves of each group. Similar curves were obtained in the PBS, PBS + NIR and free cisplatin groups. Tumour volumes increased quickly with time, indicating free cisplatin or NIR only could not inhibit the tumour growth. Pt(IV)-MeNPs without NIR presented a better antitumour effect than free cisplatin. Tumour growth of group (iv) was inhibited at the beginning of monitoring period but the tumour volume grew up again about 6 days after injection. The Pt(IV)-MeNPs with the laser irradiation group showed the best therapeutic antitumour effect. The tumour range expanded and skin temperature rose about 1-day post-NIR irradiation due to the photothermal effect. After laser treatment in group (v), the skin surrounding tumour location got dark and redness. The tumour surface became hot as the result of photothermal effect. Curves of tumour temperature increase after NIR irradiation for the group (ii) and group (v) were recorded. The temperature increased quickly after NIR irradiation for the group treated with Pt(IV)-MeNPs but there was no temperature increase in the group (ii) (). Additionally, no obvious changes in the body weight of the mice in each group during our monitoring period (). Post-mortem histopathology of the heart, liver, spleen, lungs and kidneys did not show obvious morphological differences between groups. Vacuolated and necrotic tumour cells were observed in the Pt(IV)-MeNPs with NIR group, indicating a strong antitumour activity (Supplementary Figure S3). No abnormal liver and kidney functions with the serum biochemical analysis were found during the study (Supplementary Figure S4). Our results proved the tissue and blood biocompatibility and excellent antitumour activity of Pt(IV)-MeNPs. No serious event was found during the monitoring period, except the skin scar after irradiation which could be self-repaired over time.
Conclusions
In summary, we developed a MeNP based drug delivery platform with synergic highly efficient photothermal therapy and chemotherapy properties. MeNPs were designed as the functional nanocarrier and photothermal agents. Cisplatin, as the antitumour chemotherapy drug, was linked to MeNPs in the form of Pt(IV) prodrug. This platform displayed excellent photothermal conversion efficiency. Pt(IV)-MeNPs with laser irradiation presented brilliant combined chemo-photothermal antitumour activity both in vitro and in vivo. No serious adverse events were observed due to the great biocompatibility of the synthesized NPs. Therefore, Pt(IV)-MeNPs have a great potential as a safe, effective and promising drug delivery platform for cancer therapy. It is promising to be as a wonderful candidate drug for cancer treatment in the future.
Supplemental_material-revised.docx
Download MS Word (3.9 MB)Disclosure statement
All authors declare that they have no conflicts of interests.
Additional information
Funding
References
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584.
- Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075–2094.
- Eatemadi A, Darabi M, Afraidooni L, et al. Comparison, synthesis and evaluation of anticancer drug-loaded polymeric nanoparticles on breast cancer cell lines. Artif Cells Nanomed Biotechnol. 2016;44:1008–1017.
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320.
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498.
- Zhang JZ, Bonnitcha P, Wexselblatt E, et al. Facile preparation of mono-, di- and mixed-carboxylato platinum(IV) complexes for versatile anticancer prodrug design. Chem Eur J. 2013;19:1672–1676.
- Chin CF, Tian Q, Setyawati MI, et al. Tuning the activity of platinum(IV) anticancer complexes through asymmetric acylation. J Med Chem. 2012;55:7571–7582.
- Kumar A, Huo S, Zhang X, et al. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano. 2014;8:4205–4220.
- Graf N, Bielenberg DR, Kolishetti N, et al. alpha(V)beta(3) integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug. ACS Nano. 2012;6:4530–4539.
- Xiao H, Qi R, Liu S, et al. Biodegradable polymer - cisplatin(IV) conjugate as a pro-drug of cisplatin(II). Biomaterials. 2011;32:7732–7739.
- Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417.
- Sano K, Nakajima T, Choyke PL, et al. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano. 2013;7:717–724.
- Miao W, Shim G, Lee S, et al. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials. 2013;34:3402–3410.
- Song W, Tang Z, Zhang D, et al. Anti-tumor efficacy of c(RGDfK)-decorated polypeptide-based micelles co-loaded with docetaxel and cisplatin. Biomaterials. 2014;35:3005–3014.
- Portilho FL, Helal-Neto E, Cabezas SS, et al. Magnetic core mesoporous silica nanoparticles doped with dacarbazine and labelled with 99mTc for early and differential detection of metastatic melanoma by single photon emission computed tomography. Artif Cells Nanomed Biotechnol. 2018;27:1–8.
- Zhang W, Guo Z, Huang D, et al. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32:8555–8561.
- Mirrahimi M, Hosseini V, Kamrava SK, et al. Selective heat generation in cancer cells using a combination of 808 nm laser irradiation and the folate-conjugated Fe2O3@Au nanocomplex. Artif Cells Nanomed Biotechnol. 2018;1:1–13.
- Mohammadi Gazestani A, Khoei S, Khoee S, et al. In vivo evaluation of the combination effect of near-infrared laser and 5-fluorouracil-loaded PLGA-coated magnetite nanographene oxide. Artif Cells Nanomed Biotechnol. 2018;15:1–9.
- Zhang Z, Wang J, Chen C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv Mater. 2013;25:3869–3880.
- Li J, Lyv Z, Li Y, et al. A theranostic prodrug delivery system based on Pt(IV) conjugated nano-graphene oxide with synergistic effect to enhance the therapeutic efficacy of Pt drug. Biomaterials. 2015;51:12–21.
- Liu Y, Ai K, Liu J, et al. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater. 2013;25:1353–1359.
- Ghaznavi H, Hosseini-Nami S, Kamrava SK, et al. Folic acid conjugated PEG coated gold-iron oxide core-shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif Cells Nanomed Biotechnol. 2017; 10:1–11.
- Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114:5057–5115.
- Tong L, Zhao Y, Huff TB, et al. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater. 2007;19:3136–3141.
- Lin LS, Cong ZX, Cao JB, et al. Multifunctional Fe3O4@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano. 2014;8:3876–3883.
- Zeng Y, Zhang D, Wu M, et al. Lipid-AuNPs@PDA nanohybrid for MRI/CT imaging and photothermal therapy of hepatocellular carcinoma. ACS Appl Mater Interfaces. 2014;6:14266–14277.
- Roper DK, Ahn W, Hoepfner M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J Phys Chem C Nanomater Interfaces. 2007;111:3636–3641.
- Duong HT, Huynh VT, de Souza P, et al. Core-cross-linked micelles synthesized by clicking bifunctional Pt(IV) anticancer drugs to isocyanates. Biomacromolecules. 2010;11:2290–2299.
- Riches AC, Sharp JG, Thomas DB, et al. Blood volume determination in the mouse. J Physiol. 1973;228:279–284.
- Lee H, Rho J, Messersmith PB. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 2009;21:431–434.
- Liu X, Cao J, Li H, et al. Mussel-inspired polydopamine: a biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano. 2013;7:9384–9395.
- Hall MD, Amjadi S, Zhang M, et al. The mechanism of action of platinum(IV) complexes in ovarian cancer cell lines. J Inorg Biochem. 2004;98:1614–1624.