Abstract
In the present work, PEG4000 as a hydrophilic polymer was conjugated to the surface of graphene oxide (GO) for effective drug loading and targeting release of doxorubicin. The synthesized nanohybrid was characterized with scanning electron microscope (SEM), X-ray diffraction spectroscopy (XRD) and Fourier-transformed infrared spectroscopy (FTIR). Doxorubicin as an anticancer drug was immobilized on the nanohybrid surface, and the release profile at two diverse pH besides the MTT assay was investigated. The IC50 value for the nanohybrid obtained was 0.31 μg/mL. In this work, PEG4000 as a biocompatible polymer grafted on the GO surface to increase the biodispersibility of the drug carrier in a biological environment, but it is the first report of PEG 4000-GO nanohybrid-based drug carrier.
Introduction
Graphene, a unique member of carbon-based nanomaterials, with a honeycomb lattice of carbon atoms discovered in 2004, possesses outstanding properties such as conjugated planar structure, great superficial area, extraordinary mechanical and chemical stability, excellent conductivity and respectable biocompatibility, and provide unique physical and chemical properties [Citation1,Citation2]. These properties facilitate the design of advanced drug nanocarrier to deliver a broad range of therapeutics [Citation3–6]. In spite of GO solubility in aqueous media, but charge screening effect, makes GO flakes aggregated [Citation7]. In this regard, in the past few years, the functionalization of graphene surface has been considerably studied to improve its biological properties [Citation8–11]. However, before the application of graphene and GO-based drug delivery agent, it is vital to address the following issues [Citation12,Citation13]. The required modifications to enhance drug-loading capacity [Citation14] and to reduce its toxicity in addition to rising their biocompatibility are a decisive consideration before their clinical application. The last but not the least step is to fabricate a smart system to discharge its drug at the specific site [Citation15,Citation16]. The atom doping technique or reaction via oxygen-based functional groups is common covalent functionalization methods [Citation17,Citation18]. On the contrary, the Van der Waals forces and the electrostatic binding are dominant in the non-covalent functionalization methods [Citation17,Citation19]. The non-covalent technique for the surface amendment of carbon-based nanomaterials is extensively explored on CNTs [Citation20,Citation21]. Freshly, biodegradable polymers such as gelatin, chitosan, albumin, poly(lactic acid) (PLA), poly(lactic-co-glycolic) acid (PLGA) have been used to modify GO surface mainly due to their continuous release behaviour [Citation22,Citation23]. Among the stabilizing agents, polyethylene glycol (PEG) is the most studied one [Citation24,Citation25]. Since PEG can be connected with various anticancer drugs and their derivatives (camptothecin, doxorubicin, paclitaxel), it could avoid the drug excretion by kidney and degradation by the biological environment [Citation26,Citation27]. The aim of this work was to functionalize GO surface covalently with PEG 4000 to build a biocompatible drug carrier for loading DOX. The release profile at two different pH was investigated. The MTT assay for GO and GO-PEG 4000 against murine mammary tumour cancer cells (A549) was studied, and the good biocompatibility results were obtained compared to other polymers suggesting the proposed system as a potential drug delivery agent.
Experimental section
Chemical materials
Graphite was purchased from Sigma-Aldrich, and it was used without further purification. Several analytical grades of polyethylene glycols (PEG), H2SO498%, H2O230wt%, NaNO3, sodium sulphate (Na2SO4), KMnO4 were obtained from Merck Co. Ltd, Germany. Thionyl chloride (SOCl2), was purchased from Sigma-Aldrich (Saint Louis, MO). Hydrochloric acid (HCl), nitric acid (HNO3), anhydrous tetrahydrofuran (THF) and anhydrous N,N-dimethylformamide (DMF) were purchased from Fisher Scientific (Pittsburgh). THF and DMF were dried over CaH2 before use. Doxorubicin hydrochloride (DOX) was purchased from EBEWE Pharma Co. Ltd. A dialysis chamber for the drug release was purchased from Sigma-Aldrich (Saint Louis, MO).
Preparation of graphene oxide
Hummers and Offeman modified method was employed to synthesis GO from natural graphite powder [Citation28]. Briefly, graphite (0.5 g), NaNO3 (0.5 g) and 23 mL of concentrated H2SO4 were mixed and stirred in an ice bath for 1 h, followed by slow addition of 1.5 g KMnO4 into the solution and stirring at 35 °C for 1 day. To the above mixture, 23 mL of deionized water was added slowly to prevent the reaction temperature exceeds from 98 °C and stirred for 30 min. In the end, 5 mL of H2O2 (30%) and 70 mL of deionized water were added to the mixture. The reaction mixture was filtered off by gravity filtration, and the product was washed with HCl solution (3%) and deionized water consecutively. These processes were repeated several times along with centrifugation. It was tried to use the freeze-drying method in order to prevent GO aggregation.
Preparation of acyl chloride-functionalized graphene oxide
GO powder (1 g) was poured into 20 mL of DMF and sonicated for 1 h. Then, 60 mL of thionyl chloride (0.82 mol) was added and reaction proceeds at 80 °C for three days to convert carboxyl group (COOH) on the GO surface to acyl chloride (GO-Cl). The product was centrifuged and washed with anhydrous THF and dried [Citation29].
Preparation of PEG 4000 – functionalized GO
GO-Cl (200 mg) obtained from the previous step was added into DMF (10 mL) and sonicated for 30 min to exfoliate the individual sheets and to form a stable (GO-Cl)/DMF solution. To this solution, 5 mL of pyridine and 2 g of PEG 4000 were added. The temperature of the mixture was kept at 120 °C and stirred for 3 days. The reaction mixture was then poured into ethanol, and filtered through a Buchner funnel under reduced pressure. Finally, the resulting powder was dried at 60 °C [Citation30,Citation31].
Characterization
Fourier-transformed infrared (FT-IR) spectroscopy study was conducted through KBr pellet technique with a Bruker Tensor 27 in the 4,000–400 cm−1 range at a resolution of 0.5 cm−1. UV-Vis spectrophotometer (Shimadzu 1700) was employed to record UV-Vis spectra. XRD (powder X-ray diffraction) patterns were obtained by Siemens diffractometer with Cu-Kα radiation at 35 kV in the scan range of 2θ from 4 to 40°. Scanning electron microscopy (SEM) was performed using an Texas operating at 15 kV.
Cytotoxicity measurements
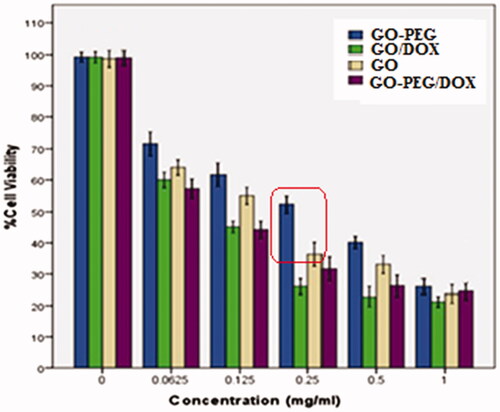
The in vitro cytotoxicity of drug carriers (GO and GO-PEG) deprived of drug and similarly GO-DOX and GO-PEG/DOX against adenocarcinomic human alveolar basal epithelial cells (A549) were studied through MTT assay. In brief, 5,000 cells/well were seeded into 96-well culture plates and incubated for 24 h at 37 °C. Cell incubation was carried out by the GO, GO-PEG, GO/DOX and GO-PEG/DOX at concentrations of 0–1 μg/L for 72 h. Afterwards, the cells were washed with 1X PBS and treated with 20 μL MTT reagent, followed by incubation for 2 h until the manifestation of purple colour that signifies the metabolization of MTT; 200 μL DMSO was added in each well to make formazan salt soluble. Then, the absorbance of the solution was measured at 570 nm.
Results and discussion
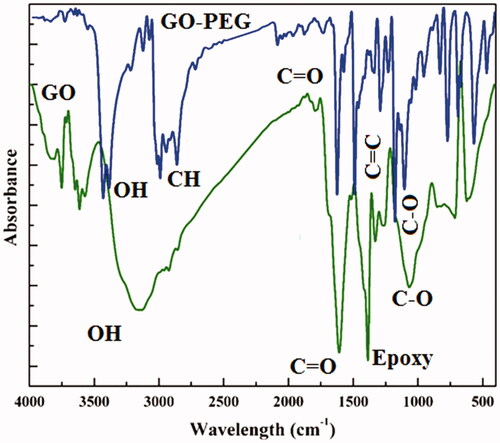
shows the FT-IR spectrums of GO and GO-PEG 4000. In the GO sample (green line), the peaks at 3152, 1608, 1386 and 1174 cm−1 are related to OH stretching, Carbonyl, epoxy and C-O group vibrations respectively approve the successful oxidation process [Citation32], while in the case of GO-PEG 4000 (blue line) the peak OH in GO shifted from 3152 cm−1 to 3450 cm−1and also the appearance of C-H group bands in 2919 cm−1 approved the successful attachment of PEG to GO surface and its edges. The presence of carbonyl groups, C = C bonds, C-O at 1621, 1497, 1207 cm−1 was also identified [Citation33]. These data demonstrated the successful synthesis of GO-PEG 4000.
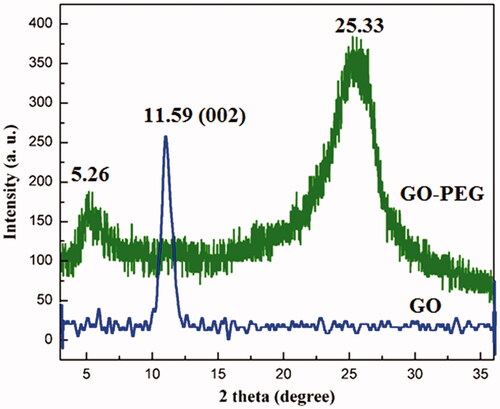
The effect of the functionalization with PEG4000 on the hybrid crystal was investigated by X-ray diffraction method. In , the XRD patterns of GO and GO-PEG4000 are shown individually. In the GO spectrum (blue line), the appearance of a peak at 2θ = 11.59°that is related to 002 diffractions of GO flakes approves the successful oxidation step and the formation of GO flakes with a d-spacing value of 7.629 nm, while in the GO-PEG 4000, two peaks at 2θ = 5.26 and 25.33° are a result of functionalization, the first peak is related to GO indicating after functionalization of the d-spacing value increased to 16.7872 nm [Citation33].
The wide peak at 2θ = 25.33° proposes that the sample is in an uneven state and being stacked in different directions, suggesting that after functionalization of GO surface, GO-PEG4000 sheets have been exfoliated successfully.
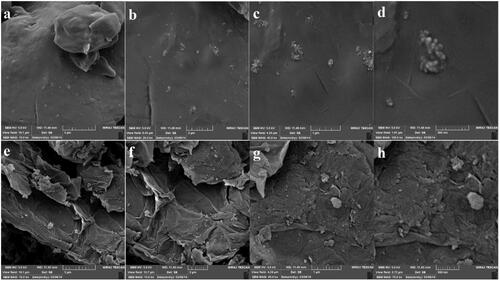
As shows, the SEM technique was used to study the morphology of the hybrid. In the GO sample (images e–h), the GO flakes on the Desigual surface were identified, while in the GO-PEG 4000 (images a–d), it seems that a layer of PEG 4000 polymer cover the GO surface and a smooth surface was detected. In other words, PEG 4000 conjugate on graphene sheets has been exfoliated successfully.
Since GO aggregates in a physiological medium, fundamental research focused on modifying GO surface by PEG or other biocompatible polymers; however, to our knowledge, there is not any report using PEG 4000 for modifying GO surface. In this regard to investigate the drug delivery properties of the prepared hybrid, the drug loading and release profile along the MTT assay were studied. The drug loading capacity of the GO-PEG 4000 with DOX as a model drug calculated was 87% through the following procedure; at first 0.1 gr of GO-PEG 4000 was immersed in 10 mL of 100 ppm stock solution of DOX, and finally, the volume was adjusted to 50 mL. After 72 h, the amount of loaded drug was calculated through a calibration curve. The crumpled accumulated pieces on GO nanosheets are believed to be PEG 4000.
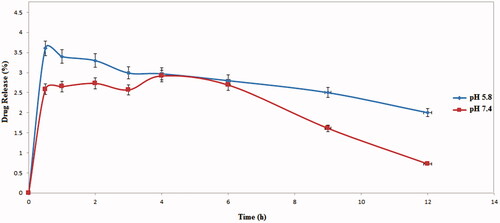
The release behaviour of the hybrid in two different pH (5.8 and 7.4) was studied. In pH 5.8, (acidic media) since there are two kinds of possible H-bonding between drug and carrier, more release occurred, while in the neutral pH (7.4), there are four kinds of H-bonding between drug and carrier, and hence, a negligible release was observed. The drug release profile was divided into three steps; in the first step, as shown in , a burst discharge occurred in the first 1 h of the release procedure and almost 2.5% and 3.5% of drug were released at pH 7.4 and 5.8. Such burst release is related to those drugs that were attached to the hybrid surface via hydrogen bonding. In the next step, a continued release was observed up to three hours. In this part of release profile, those drug molecules were discharged that were immobilized on the hybrid surface via π–π stacking. In the final part of release, due to decomposition of doxorubicin molecule under daylights, the release per cent began to decrease.
All the drug loading and release profile data suggest that it has high potential to be used as a drug carrier.
Following the importance of the hybrid nontoxicity, the MTT assay measurement was carried out. The IC50 values for GO, GO/DOX, GO-PEG and GO-PEG/DOX were 0.217, 0.88, 0.31 and 0.118 mg/mL individually. GO-PEG/DOX indicates higher biocompatibility than GO/DOX in high concentration most probably due to more released drug from the GO/DOX. Furthermore, both pure GO and GO-PEG indicated a concentration-dependent toxicity. It means that as the concentration increased the toxicity increased that is reported before. Indeed, as shown in , GO-PEG toxicity decreased noticeably compared to pure GO, it could originate from PEG that covers GO surface and reduces its aggregation in physiological media.
Conclusion
GO-PEG 4000 hybrid as a new biocompatible drug delivery system was designed and synthesized. The comprehensive characterization techniques approve the synthesis and functionalization of GO hybrid. The high drug loading per cent (81%) and continued pH-dependent release in two different pH was achieved. The hybrid shows more release in acidic pH. The MTT assay results showed that GO-PEG is more biocompatible than GO.
Acknowledgement
This work was supported by the University of Tabriz and RCPN. We thank our colleagues from the Chemistry Department of Tabriz University who provided insight and expertise that greatly assisted the research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aqel A, El-Nour KMA, Ammar RA, et al. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arabian J Chem. 2012;5:1–23.
- Gogotsi Y, Presser V. Carbon nanomaterials. Tylor Francis; 2013.
- Wang Y, Li Z, Wang J, et al. Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011;29:205–212.
- Abbasi E, Akbarzadeh A, Kouhi M, et al. Graphene: synthesis, bio-applications, and properties. Artif Cells Nanomed Biotechnol. 2016;44:150–156.
- Ma N, Liu J, He W, et al. Folic acid-grafted bovine serum albumin decorated graphene oxide: an efficient drug carrier for targeted cancer therapy. J Colloid Interface Sci. 2017;490:598–607.
- Ma N, Zhang B, Liu J, et al. Green fabricated reduced graphene oxide: evaluation of its application as nano-carrier for pH-sensitive drug delivery. Int J Pharm. 2015;496:984–992.
- Zhang L, Xia J, Zhao Q, et al. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6:537–544.
- Liu J, Tang J, Gooding JJ. Strategies for chemical modification of graphene and applications of chemically modified graphene. J Mater Chem. 2012;22:12435–12452.
- Dreyer DR, Park S, Bielawski CW, et al. The chemistry of graphene oxide. Chem Soc Rev. 2010;39:228–240.
- Nejati-Koshki K, Mesgari M, Ebrahimi E, et al. Synthesis and in vitro study of cisplatin-loaded Fe3O4 nanoparticles modified with PLGA-PEG6000 copolymers in treatment of lung cancer. J Microencapsul. 2014;31:815–823.
- More MP, Patil MD, Pandey AP, et al. Fabrication and characterization of graphene-based hybrid nanocomposite: assessment of antibacterial potential and biomedical application. Artif Cells Nanomed Biotechnol. 2016;45:1–13.
- Gürel HH, Salmankurt B. Biological applications of graphene oxide. AIP Publishing; 2016.
- Liu J, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9:9243–9257.
- Ajami M, Davoodi SH, Habibey R, et al. Effect of DHA + EPA on oxidative stress and apoptosis induced by ischemia-reperfusion in rat kidneys. Fundam Clin Pharmacol. 2013;27:593–602.
- Deshpande A, Rhodes C, Shah N, et al. Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm. 1996;22:531–539.
- Heil RW, Jr, Kenknight BH. Controlled release drug delivery. Google Patents. 2001
- Georgakilas V, Otyepka M, Bourlinos AB, et al. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev. 2012;112:6156–6214.
- Pham TA, Kumar NA, Jeong YT. Covalent functionalization of graphene oxide with polyglycerol and their use as templates for anchoring magnetic nanoparticles. Synthetic Metals. 2010;160:2028–2036.
- Georgakilas V, Tiwari JN, Kemp KC, et al. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev. 2016;116:5464–5519.
- Ebrahimi E, Akbarzadeh A, Abbasi E, et al. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol. 2016;44:290–297.
- Herranz MÁ, Martín N. Noncovalent functionalization of carbon nanotubes. In: Dirk MG, Martin N, editors. Carbon nanotubes and related structures: synthesis, characterization, functionalization, and applications. Wiley‐VCH Verlag GmbH & Co. KGaA; 2010. p. 103–134.
- Pazoki-Toroudi H, Nassiri-Kashani M, Tabatabaie H, et al. Combination of azelaic acid 5% and erythromycin 2% in the treatment of acne vulgaris. Cutaneous Ocul Toxicol. 2010;30:286–291.
- Langer R, Chasin M. Biodegradable polymers as drug delivery systems. Informa Health Care. 1990.
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822.
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75:1–18.
- Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009;61:1177–1188.
- Sezgin Z, Yüksel N, Baykara T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur J Pharm Biopharm. 2006;64:261–268.
- Pazoki-Toroudi H, Nassiri-Kashani M, Tabatabaie H, et al. Combination of azelaic acid 5% and erythromycin 2% in the treatment of acne vulgaris. J Dermatolog Treat. 2010;21:212–216.
- Mei Q, Zhang K, Guan G, et al. Highly efficient photoluminescent graphene oxide with tunable surface properties. Chem Commun (Camb). 2010;46:7319–7321.
- Mansourpanah Y, Shahebrahimi H, Kolvari E. PEG-modified GO nanosheets, a desired additive to increase the rejection and antifouling characteristics of polyamide thin layer membranes. Chem Eng Res Design. 2015;104:530–540.
- Zhu H, Gao L, Jiang X, et al. Positively charged graphene oxide nanoparticle: precisely label the plasma membrane of live cell and sensitively monitor extracellular pH in situ. Chem Commun (Camb). 2014;50:3695–3698.
- Badrzadeh F, Akbarzadeh A, Zarghami N, et al. Comparison between effects of free curcumin and curcumin loaded NIPAAm-MAA nanoparticles on telomerase and PinX1 gene expression in lung cancer cells. Asian Pac J Cancer Prev. 2014;15:8931–8936.
- Wang C, Feng L, Yang H, et al. Graphene oxide stabilized polyethylene glycol for heat storage. Phys Chem Chem Phys. 2012;14:13233–13238.