Abstract
In recent years, the application of vaccines shows limitations, including the high number of vaccine administrations and the fear of safety and effectiveness. In this regard, advanced vaccine products have been developed, like the combined vaccines, or are under development, such as nucleic acid vaccines (DNA and RNA), polymer-based vaccines, etc. Moreover, the possible use of traditional, like aluminium hydroxide and aluminium phosphate, or innovative adjuvants, like monophosphoryl lipid A, polysaccharides and nanoparticulate system, may further increase vaccine effectiveness. This review article focuses on the combined vaccines, which, especially when they are associated with adjuvants, reduce the dosing frequency, and prolong the duration of action, thus providing better vaccine coverage. Marketed preparations, like Typhim Vi, Peda typh and Boostrix showed better vaccine coverage for diseases like typhoid, tetanus, diphtheria and acellular pertussis. The future aspect for the development of combined vaccines will protect not only against infectious diseases but likely even against various infectious conditions, like pneumonia, meningococcal infection and respiratory syncytial virus infection.
Introduction
Vaccines stimulate immune system by antigens consisting of either, live or killed micro-organisms, or their subunits, of protein or polysaccharidic structure. Immunity is developed by mimicking either a bacterial or viral infection, is not responsible for causing illness but of inducing antibody production. Vaccines thus protect from some deadly and serious diseases, like tetanus, smallpox, yellow fever, diphtheria, measles, whooping cough and polio. Once the immunostimulation is diminished, the cells in the body will retain memory T-cells and B-lymphocytes which are mainly involved in the cell-mediated antigen response [Citation1]. The main objective of combined vaccines is to protect the body from multiple diseases at a time [Citation2]. Advantages of combined vaccines are to minimize the frequency of injections, thus reducing trauma to infants and improving vaccine coverage, to reduce the vaccination cost and to facilitate the incorporation of new vaccines in the immunization schedule.
DTaP (diphtheria, tetanus, acellular pertussis vaccine) vaccine
The spores of anaerobic gram-positive bacteria, Clostridium tetani, are the causative agent of tetanus and are usually found in a wound [Citation3]. Muscle spasm and contraction, fever, jaw stiffness, neck stiffness, sweating and rapid heart rate are the symptoms of tetanus. The infection can also affect the autonomic nervous system and cause seizures. Gram-positive encapsulated bacillus, Corynebacterium diphtheriae, is the causative agent diphtheria which generally spread through human respiratory droplets. Systemic toxicity, polyneuropathy, pharyngeal infection and myocarditis are caused by toxigenic strains of C. diphtheriae whereas cutaneous infection is caused by non-toxigenic strains. B. pertussis is a gram-negative bacillus which causes pertussis (also known as whooping cough). Coughing is accompanied by some other problems like weight loss, syncope and subconjunctival haemorrhages. The paediatric population needs administration of both tetanus and diphtheria toxoids and therefore, are available in combination form. The cell-free purified toxins of C. diphtheria and C. tetani are used to produce toxoids. The toxins are converted into toxoid with the help of formaldehyde and for the purpose of immunogenicity, aluminium salt is added. In general, a paediatric formulation of diphtheria-tetanus toxoid (DT) contains an amount of diphtheria toxoid which is 2–3-fold the amount of tetanus toxoid, but the adult formulation is inverted, with half, or less than half, the amount of diphtheria compared to tetanus toxoid [Citation4]. Boostrix and Infanrix by GSK and Daptacel by Sanofi Pasteur are some of the paediatric formulations marketed in the United States and India. The lifetime immunization with a booster vaccine is recommended after a series of vaccination in childhood by the World Health Organization [Citation5].
DTaP-IPV-Hib vaccine
DTaP-IPV-Hib vaccine is a DTaP-based pentavalent vaccine which contains the combination of tetanus and diphtheria toxoids, acellular pertussis adsorbed, inactivated poliovirus and Haemophilus b conjugate (tetanus toxoid conjugate) vaccine (DTaP-IPV/Hib). The first marketed preparation approved in the US, based on DTaP includes both type b Haemophilus influenzae (Hib) and poliovirus antigen. The vaccine formulation consists of a liquid form of IPV and DTaP along with a lyophilized form of Hib, which is then reconstituted prior to administration [Citation6]. No significant interaction was observed between DTaP and Hib components of vaccines when used in combination. The vaccine is administered via intramuscular route in four-dose series and the first dose is given at the age of 2 months and the remaining three doses are administered at 4, 6 and 15–18 months of age at a dose of 0.5 mL per administration. The combined vaccine increases immunogenicity against all diseases including Hib and the adverse event profile is the same as the vaccines administered separately and is well tolerated. Mild fever, drowsiness, redness and irritation at the site of action are the side effects associated with this vaccine [Citation7].
DTaP-IPV-Hep B-Hib vaccine
DTaP-IPV-Hep B-Hib is a hexavalent combined vaccines and consists of diphtheria with two acellular pertussis antigens (PT and PHA) and tetanus toxoids, Hib and inactivated poliovirus vaccine (IPV-types 1, 2 and 3). The action of vaccine includes the stimulation of active immunity through endogenously produced antibodies to fight against diphtheria, tetanus, hepatitis B, pertussis, Hib and poliovirus. The vaccine delivers primary as well as booster immunization against major childhood diseases simultaneously. Prior vaccination no requirement of reconstitution as the formulation is available in the fully liquid form, hence the administration is facilitated and also decreases the risk of medication error. The vaccine is recommended to be used both for primary vaccination (three doses at least 4 weeks apart from 6 weeks of age) as well as for booster vaccination till 2 years of age. Hexaxim manufactured by Sanofi Pasteur (Lyon, France) is a liquid suspension intended to be used as primary immunization for infants and booster immunization up to 24 months of age by intramuscular route [Citation8]. The vaccination schedule is between 6 and 14 weeks or 2 and 6 months of age.
Measles, mumps and rubella (MMR) and measles, mumps, rubella and varicella (MMRV)
Vaccines
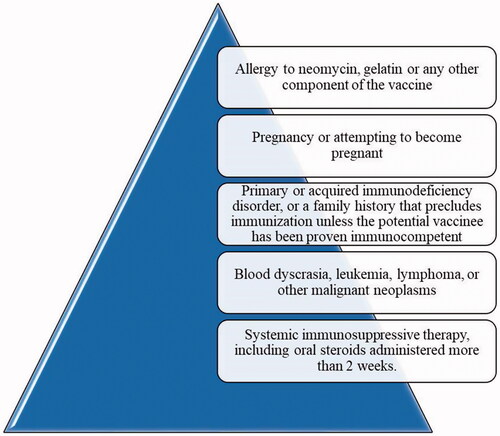
MMR are viral diseases associated with significant morbidity and fatality rate in children. Patients suffering from measles may suffer from encephalitis and few patients sustain permanent brain damage. Meningitis and inflammation of testicles are some of the severe forms of mumps. In the 1970s, the expanded immunization spectrum was observed against MMR diseases by the combined form of live attenuated MMR vaccine which provided less number of doses and also easy administration [Citation9]. The Centers for Disease Control and Prevention (CDC) recommended that children should receive their doses of vaccine in two specific age ranges: one dose in the few months after the first birthday, and the second dose in-between the ages of 4 and 6 years. The MMR vaccine manufactured by Cadila Healthcare Limited (Ahmedabad, India) is a live and freeze-dried product which consists of RA 27/3 rubella strain, Hoshino mumps strain and Edmonston-Zagreb measles strain of microorganisms. MMRV is a combined vaccine of MMRV which functions as an alternative to separate vaccines of varicella-zoster virus (VZV) and MMR vaccine because it possesses a similar vaccination schedule and safety profile. Two marketed MMRV vaccines are: Priorix-Tetra by GlaxoSmithKline Biologicals (Rixensart, Belgium) and Pro-Quad by Merck & Co. Inc. (West Point, PA). The development of combined vaccines is based on the individual vaccine performance and schedule [Citation10]. It is understood from the study that the second dose of this vaccine showed more immunogenic response when administered after 4–6 months interval from the first dose. The first dose is given between 9 and 24 month-old children. The vaccine is contraindicated in the conditions reported in [Citation11].
Polysaccharide vaccines
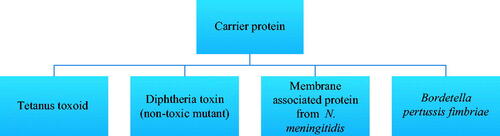
Polysaccharide vaccines are inactivated sub-units of long chains of sugar molecules which generally show a T-cell independent response. The response of antibodies in vaccines increases with age in a stepwise manner and in infants, B-cell responses are poor to T-cell independent polysaccharide antigens. The factors that implicate this process involve the restricted conversion of IgM to IgG2 antibodies with disruption in complement-mediated activities which results in disorganization of peripheral areas of the spleen [Citation12]. On repeated administrations, this vaccine is not able to induce a strong immune response. Polysaccharide vaccines are available for typhoid, meningococcal and pneumococcal diseases [Citation13]. The direct stimulation of B-cells by polysaccharide antigens results in poor antibody response in <2-year children, for a limited capacity to switch to IgG2 antibody subclass. Hence it is necessary to transform the T-independent polysaccharide antigens into T-dependent protein-polysaccharide antigens, in order to stimulate B-cells not directly but through the T-helper cells. This may be obtained by the conjugation of polysaccharide to one largely known proteic antigen, such as either tetanus or diphtheria toxoid, towards which all new-borns are vaccinated, thus they are able to mount an efficient anti-protein helper response which results cross-reactive with the polysaccharide moiety, as demonstrated over and over again and recently indirectly confirmed [Citation14]. The immunogenicity of polysaccharides can be enhanced by chemical conjugation of proteins which are used as a carrier in vaccine formulation and it evokes antisaccharide antibody response which is T-cell-dependent [Citation15]. These conjugate vaccines are immunogenic in infants and produce long-term protection against the disease [Citation16]. When the polysaccharide vaccine compares with protein conjugate vaccine, it demonstrates that the conjugate vaccine requires 2–3 doses for infants and one dose for an adult while polysaccharide vaccine requires one dose. No further booster dose is required for the polysaccharide conjugate vaccine, whereas polysaccharide vaccine requires a booster in every 3–5 years. Special precautions are taken in case of polysaccharide vaccines as these preparations are contraindicated for children under 2 years (for example, meningococcal groups A, B and C polysaccharides–protein conjugate vaccine). This vaccine provides protection against diseases caused by serogroups A, B and C. Carrier proteins used in conjunction with a polysaccharide vaccine are shown in .
The conjugated vaccine includes adjuvants like aluminium salt in the formulation not more than 1.25 mg per single dose [Citation17]. The quantity of free polysaccharide bound to protein in vaccines affects the immunological reaction. An excess amount of free polysaccharide may lead to increase immunological response to group C vaccine conjugate. The polysaccharides structure, selection of carrier protein, the extent of conjugation, the level of cross-linking and the existence of aluminium salt are some of the characteristics of conjugated vaccine which affect immune response. This preparation is a freeze-dried product of a particular polysaccharide antigen from Neisseria meningitidis group A, C, Y and W135 routed by subcutaneous injection. The diluent used in the formulation is pyrogen-free sterile distilled water. Thimerosal is used as a preservative which prevents bacterial and fungal growth in the formulation whereas lactose is added as a stabilizer in the range of 2.5–5 mg [Citation18].
Meningococcal polysaccharide vaccine
Meningitis is caused by encapsulated bacteria Neisseria meningitidis (meningococcus) which includes inflammation of meninges surrounding the brain and spinal cord. There are 13 serogroups of meningococcal meningitis, out of which A, B, C, W135, X and Y are the most common causes of meningococcal disease in humans. The invasive meningococcal disease is prevalent in infants, immuno-compromised individuals and young adults living in the close vicinity [Citation16].
Vaccines against groups A, C, Y and W135 meningococcal disease
Most polysaccharide vaccines are weak immunogenic in new-borns and fail to provide the immunological response in the individual. The repeated administration leads to the immune hypo-responsiveness with group C polysaccharide vaccine. A major drawback of meningococcal vaccine is its lack of immunogenicity in <2-year children and repeated administration of vaccine diminishes its immunogenic potency. Immunity may last for 5 years, but in some cases, it may last for 1 year. Hyporesponsiveness is the primary concern in this case and also known as immune tolerance. It is the phenomenon occurs when a person is unable to show the immune response to same or a higher dose of booster vaccine as compared to primary vaccine [Citation19].
Polysaccharide vaccine for typhoid fever
S. typhi causes acute and life-threatening disease, typhoid fever, transmits through contaminated food, water and poor sanitation. Currently available vaccines are reported below [Citation20]:
Purified Vi polysaccharide parenteral vaccine.
Oral attenuated Ty2la (live oral vaccine).
Purified Vi polysaccharide conjugated tetanus toxoid as a parenteral vaccine.
For the production of Vi vaccine, fermentation of S. typhi bacteria is carried out. The currently available Vi capsular polysaccharide-based vaccine is a linear alpha 1–4 linked polygalacturonic acid (PGA) in which C2 and C3 position of galacturonic acid are N-acetylated and O-acetylated, respectively. The potency and immunogenicity of vaccine can be determined by the extent of O-acetylation at C3 and molecular weight. The study suggests that the removal of O-acetyl group decreases the immunogenicity [Citation21]. There is no booster response after revaccination. It is not suggested for infants and children below 2 years age [Citation22]. The available Vi vaccines are given in the form of subcutaneous or intramuscular injection which generally contains 25 µg of the polysaccharide. The purified polysaccharide vaccine stimulates B-cell responses and provides Vi antibody similar to other polysaccharide vaccines. This results in depletion of B-cell without generation of new B-cells. As there is no immunological memory, the serum antibody level attained after the first dose cannot be further boosted by revaccination. Marketed preparations of polysaccharide vaccines for typhoid fever are given in .
Table 1. Marketed preparations of polysaccharide vaccines for typhoid.
Polysaccharide vaccine for pneumonia
Streptococcus pneumonia (SP) is the most common cause of pneumonia which is associated with an acute infection of lung parenchyma [Citation23]. Vaccine prevents a specific type of lung infection and the occurrence of pneumonia. Conjugated and unconjugated pneumonia polysaccharide vaccine can significantly reduce pneumonia. 23-valent pneumococcal polysaccharide vaccine (PPSV23) was used in adults to fight against pneumonia caused by the 23 capsular serotypes present in the vaccine. In children less than 2 years, this vaccine is not sufficiently efficacious and hence 7-valent pneumococcal conjugate vaccine (PSV7) was developed containing seven serotypes from PPSV23. The 13-valent pneumococcal conjugate vaccine (PCV13) contains seven serotypes from PCV7 and five serotypes from PPSV23 with one additional serotype which is neither present in PPSV23 nor in PCV7. The types of polysaccharide vaccine used for pneumonia are given in .
Table 2. Types of polysaccharide vaccine for pneumonia.
The PPSV23 vaccine is administered intramuscularly or subcutaneously in the deltoid muscle with a single dose of 0.5 mL and multi-dose of 5 mL. Fatigue, headache and myalgia are the common side effects of the vaccine and sometimes swelling at the site of injection can also be observed. Dose of 0.5 mL contains 25 µg of polysaccharide for every 23 serotypes included in the formulation with 0.25% phenol added as a preservative. Patients with the highest risk of pneumococcal infection are subjected to polysaccharide vaccines. The capsular polysaccharide vaccine is composed of non-conjugated pneumococcal polysaccharide vaccine. Serotype-specific antibodies which are responsible for opsonization, phagocytosis are enhanced by pneumococcal capsular polysaccharide vaccine due to its highly antigenic nature. The non-conjugated pneumococcal polysaccharide vaccines show T-cell non-dependent immune action, but it directly activates B-cells and, in analogy with other plain polysaccharide vaccines, are scarcely immunogenic in <2-year old children. This vaccine lacks booster effect and does not establish immune memory. Very small effect on the nasopharyngeal carriage is elicited because mucosal immunity is not induced by the vaccine. The pneumococcal conjugate vaccine consists of bacterial capsular polysaccharide and each polysaccharide combines with a protein carrier molecule. This innovative approach brings forth the effective vaccination for prevention of invasive pneumococcal diseases. Seven-valent PCV contains inactivated diphtheria toxoid as a carrier protein which helps to induce immunity for all seven serotypes. Unlike the 23-valent non-conjugated vaccine, the conjugated vaccines build up immune memory by activating B-cell and T-cell mediated response. Hence this vaccine can be used in children below 2 years age. The vaccine imparts the adaptive characteristics and shows booster response as well. The colonizing ability of pneumococci of vaccine serotypes is eradicated by asymptomatic carriers. This activity of asymptomatic carriers is due to mucosal immunity of vaccine.
Patient suffering from HIV (human immunodeficiency virus), alcoholics and diabetic patients are less respondent to polysaccharide vaccine. The reason for the poor response of the patient is diminished or absent antibodies response [Citation24,Citation25].
Novel approaches in vaccine delivery
Flu vaccine admixture of mannan and flu antigen
The vaccine is composed of a carbohydrate polymer comprising mannose and influenza virus (flu) antigens in admixture. The flu antigens are obtained from either human or animal influenza virus-like avian flu or equine flu. The flu antigen is generally taken from the whole inactivated influenza virus. Mannose is a carbohydrate polymer, consists of mannan and more preferably oxidized mannan. Mannan is a polysaccharide which enhances the formation of some antibodies like IgG1, IgG2a and IgA in serum and IgA in mucosal sites and in the lung. Oxidized mannan is a non-toxic and an effective adjuvant combined with antigens for vaccination against infections which occur via mucosal membrane [Citation26]. In the survey regarding the adjuvant effect of mannan in the vaccine, it is mentioned that no significant immunization was produced by mannan when used in a small quantity. Hence the study was carried out using varying levels of mannan concentration [Citation27]. The intra-nasal administration of admixture showed that inactivated H1N1 mixed with mannan induced higher serum IgG and respiratory-tract IgA than H1N1 alone. The action is mannan dose-dependent and IgG1 and IgG2 were induced by the vaccination and it induces humoral and cellular immunity.
The adjuvants
In the formulation of vaccines, adjuvants are considered as the main part which enhances or modifies the immune response of vaccine in the body. By incorporating adjuvant, the efficacy of weak antigens can be enhanced. The adjuvants are heterogeneous and large and are divided into two categories: (a) immunostimulants and (b) adjuvants as a delivery system/carriers. The action of immunostimulants is due to the interaction with receptors like TLR and others while the action of adjuvants as a delivery system includes various mechanisms to produce immune response depending on the characteristic of adjuvant and antigen. Other factors for the choice of adjuvants are the type of antigen and immune response, the age of the patient and route of administration. Currently available human vaccine adjuvants are aluminium compounds, oil-in-water emulsion like MF59, AS03, virosomes, monophosphoryl lipid A (MPL), etc. [Citation28].
Aluminium adjuvants and immuno-potentiation
In the formulation of vaccine for infectious diseases and immunotherapy, aluminium adjuvants have been used alone for over 70 years in humans and are still the most usually employed. The aluminium adjuvants enhance the immune response against antigens and toxic proteins. Nowadays, this adjuvant is widely used in the preparation of human vaccines like hepatitis B, pertussis, anthrax, diphtheria and H. influenzae. Aluminium adjuvant is also used for allergic diseases and is tested for diabetes mellitus immunotherapy. The response generated by aluminium adjuvants is mainly antibody-mediated and activates innate immune cells that directly produce signals for activation of lymphocytes. There are two types of aluminium adjuvants mainly used in vaccine preparation: aluminium hydroxide adjuvant (AH) and aluminium phosphate adjuvant (AP). AP and AH show different physicochemical composition and attribute significantly in formulation with antigens. AH shows a typical crystalline structure with chemical formula Al(O)OH and is made up of needle-shaped nanoparticles with size (4 nm × 2 nm × 10 nm) and a large surface area of 508–512 m2/g. These needle-shaped nanoparticles aggregate to form a shape in the size range of 16–18 µm. AH in neutral pH shows a positive surface charge with 11.4 points of zero charge (PZC) which is mainly responsible for the positive surface charge. Another aluminium adjuvant is AP which readily gets dissolved into the body after injection, shows chemical formula Al(OH)x(PO4)y and PZC around 4.4–5.5 which signifies the negative surface charge in neutral pH. Hydroxyl and phosphate ratio of surface changes according to the formulation and manufacturing conditions. Electrostatic interaction between adjuvants and vaccine antigen is affected by opposite surface charge. CD4T cells direct antibody-mediated protection which is induced by aluminium containing vaccines [Citation29]. It is also an inducer of Th2 associated immune response. The proposed study states that aluminium activates the component of inflammation complex NALP3 which is involved in the initiation of some pro-inflammatory cytokines like IL1β [Citation30].
Polycaprolactone admixture with aluminium adjuvant nanoparticles
The admixture of polycaprolactone with aluminium adjuvant in tetanus vaccine shows improved efficacy and action as compared to conventional TT vaccine. The formulation shows immunopotentiation effect by simultaneous formation of Th2, i.e. humoral as well as cell-mediated Th1 action. The formulation shows ≥80% adsorption and retains 93–95% of antigen in admixture. The report of histopathology and serum biochemistry shows the decreased level of toxicity as compared to conventional vaccine [Citation31].
Emulsion-based vaccine adjuvants
Squalene-based MF59 is an oil-in-water emulsion to increase haemagglutination inhibition (HI) titer and cross-potentiation. Another squalene-based oil-in-water adjuvant is AS03 which was widely used in H1N1 vaccine and flu vaccines. MF59 initiates local immunostimulatory action at the site of injection [Citation32], which induces up-regulation of cytokines and immunity genes responsible for promoting CD11b + and MHCII + cells. Apart from this, it enhances dendritic cell antigen uptake [Citation33].
MPL
MPL is an adjuvant which mainly affects the TLR pathway required for host adaptive and innate immunity [Citation34]. MPL acts as a TLR agonist and stimulates an immune response and can be used in combination with aluminium salt known as AS04. Currently, this combination is used in the vaccine for human papillomavirus and hepatitis B [Citation35,Citation36].
Nanomaterials as vaccine carriers
Injection is the most preferred delivery system but is associated with some disadvantages. Hence to overcome the limitations of this delivery system, mucosal delivery is developed which stimulate systemic as well as mucosal immunity. Mucosal vaccines perform [Citation37] the following functions:
a. Inhibit the entry of pathogens in the body.
b. Inactivate pathogens.
Mucosal vaccine delivery has shown advantages for ease of administration and no risk of transmission of infection. But this vaccine delivery showed limitation due to less efficiency in the delivery of potentially active epitopes, sometimes limited humoral and cell-mediated response and insufficient use of adjuvants.
In nanoparticle-based mucosal vaccines, the adjuvant can overcome some limitations of the conventional delivery system. The sustained release delivery system provides better and enhanced cell-mediated, humoral and mucosal immune response and also prevents degradation of loaded antigens. Organic and inorganic nanomaterials can be used in the formulation where inorganic materials show limitations like the inability to target cell, the absence of immunogenicity and very low degradation in vivo whereas organic materials are comparatively safe, less toxic and present no carcinogenic and immunogenic effects. Polymer-based nanomaterials as a delivery carrier show advantages like good biocompatibility, large surface area, a non-immunogenic, non-viral vector, easy to load drug and target appropriate cell, tissue or organ [Citation38].
Polymeric nanomaterials as vaccine adjuvants
Apart from aluminium and its adjuvants, polymeric nanomaterials manufactured from renewable nanomaterials can be used as a beneficial adjuvant for vaccine preparation. Micro- and nano-polymers are used as vaccines or drug carrier system in which particle size and constituents can be modified. Polymerization is the process by which drug or vaccine antigens are covered. By using chemical bonding, nanoparticles are bound to the drug or antigens by adsorption. The burst release of antigens from nanoparticles can be controlled and better mucosal, humoral and cellular response can be obtained. From the study including chitosan coated PLGA nanoparticles, it was observed that it is a safe delivery system for NDV DNA vaccine [Citation39].
Liposomal vaccine delivery system
First, Allison and Gregoriadis studied the immune response of the vaccine combined with liposomes [Citation40]. The plasticity and versatile characteristics of liposomes revealed them a key delivery system for vaccines. The composition and formulation of liposomes depend upon the characteristic properties of lipid composition, surface charge, size and position of adjuvant. The chemical nature of drug (hydrophilic and lipophilic) represents the location of a compound either inside aqueous space or lipid bilayer of liposome structure. Adsorption or chemical linking techniques are used to attach the antigens to the surface of liposome [Citation41]. The combination of various adjuvants and antigens can form a liposomal vaccine. A cationic liposome pDNA vaccine of encapsulated ovalbumin (OVA) with pDNA (antigen) showed an enhanced retention time with large vesicle size. Poor immune response and enhanced lymphatic drainage are observed when polyethylene glycol is added to coat the liposomes [Citation42]. The PEGylated dimethyl dioctadecyl ammonium (DDA)/trehalose dibehenate (TDB)-based liposomes decrease the depot effect and also alter the immunological response. The small size liposomes induce TH2 activation whereas large liposomes induce TH1 action with increased INF- γ and IgG-2a/IgG1 ratio [Citation43]. The admixture of vaccine like OVA with positive, negative or neutral liposomes results into different antibody response.
Liposomal DNA vaccines
Amidi et al. anticipated that the liposomes are artificial microbes which produce specific antigenic response hence can be used as a vaccine delivery system [Citation44]. The admixture of plasmid DNA encoding hsp65 (heat shock protein) in the form of liposome induces a protective immune response and reduces fungal infection and treat the pulmonary fungal infection, paracoccidiomycosis [Citation45]. The vaccine contains a bacterial transcription and translation system along with a gene encoding β-galactosidase or a luciferase–nucleoprotein (NP) fusion epitope in the form of liposomes for a higher antigenic response.
Cationic liposome adjuvant vaccines
Cationic liposomes are commonly used as a carrier for cell transfection and act as an adjuvant. Incorporation of cationic lipids forms the bilayer of liposomes but sometimes additional lipids are required. The adsorption of positive charge liposome on the negatively charged cell surface is increased due to the high surface charge density. Cationic liposomes penetrate the cell membrane and depending upon formulation type and size of liposome it activates different cellular pathways [Citation46].
Some examples of cationic liposome adjuvant are:
CAF01
CLDC1-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl) imidazolinium-chloride and cholesterol and
Cationic lipid nucleic acid complexes (CLNCs) and the ectodomain (E1ecto) of WEEV
Immunopotentiation of reconstituted influenza virosomes (IRIVs)
The small unilamellar vesicles (SUVs) of IRIVs contain spike projections of the influenza surface glycoproteins HA and neuraminidase which activates MHC-I and MHC-II and induce B- and T-cell responses [Citation47]. The excellent tolerability and immunogenicity are shown by virosome-based influenza vaccine Inflexal V (Crucell NV, Leiden, Netherlands) in immunocompromised patients. Another virosome-based vaccine of hepatitis A consists of inactivated hepatitis A virus (Epaxal by Crucell NV, Leiden, Netherlands) and shows excellent tolerability and high immunogenicity for 9–12 years after vaccination [Citation48].
Future prospects
Nucleic acid vaccines
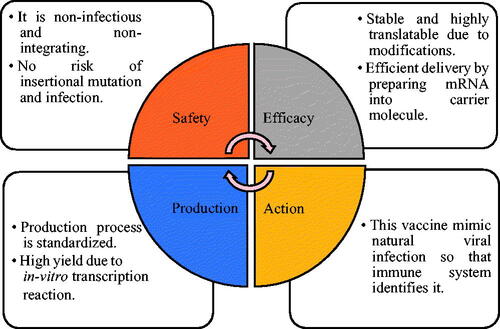
Nucleic acid-based therapies are the new and advanced method to increase the therapeutic action against diseases [Citation49]. Nucleic acid vaccine (DNA and mRNA) is used to deliver a particular sequence which contains a code for proteins of the pathogen causing various diseases and immune system to recognize these proteins as antigens. By adjusting the antigens to be delivered, nucleic acid vaccines maintain the balance in humoral and cellular protection. This nucleic acid vaccine is unique to treat pathogens difficult to invade and the key advantages are shown in . The mRNA used in conventional vaccine contains the antigen and 5′ and 3′ untranslated region. In recent mRNA vaccines, two types of RNA are used: (a) non-replicating RNA and (b) self-amplifying virally derived RNA. Viral replication machinery which amplifies intracellular RNA and protein expression and antigen are present in self-amplifying RNA.
Approaches and features of mRNA vaccine
Two approaches in the delivery of mRNA vaccine are used by (a) inserting mRNA into dendritic cell ex vivo and (b) injecting mRNA directly with or without a carrier. The process of delivery of mRNA into the dendritic cell is followed by the reinfusion of these transfected cells in the recipient. Due to such cell-mediated immune response, mRNA vaccines are effectively used in cancer therapy. In case of direct injection approach, naked mRNA is injected which targets antigen-presenting cells like macrophages, dendritic cells, Langerhans cells and B-lymphocytes. To facilitate the prolonged antigen expression in vivo, non-toxic and efficient RNA carriers have been developed by considering faster approach and cell-specific delivery. The addition of adjuvants immunomodulate the action of vaccines efficiently and increase the potency of the vaccine. Intrinsic immunogenicity or ability to evoke immune modulator protein of mRNA is beneficial for the use of traditional as well as novel adjuvants. For example, self-replicating RNA vaccine shows increased immunogenicity and action when RNA is incorporated in MF59-based nanoemulsion which is cationic in nature wherein MF59 is an adjuvant developed by Novartis. Another example is TriMix which is a combination of mRNA with immune activating proteins like CD70, CD40 ligand and TLR4 (Toll-like receptor 4). This combination increases the immunogenicity of naked and non-purified mRNA in cancer vaccines to further result in increased dendritic cell maturation and cytotoxic T-lymphocyte response. mRNA-carrier complex size and type of carrier also affect cytokine action, e.g. RNActive (CureVac AG) shows the adjuvant dependent activity which includes protamine complex with RNA. Protamine is a polycationic peptide and acts through TLR7 (Toll-like receptor 7) signalling.
mRNA vaccines in infectious diseases
Two types of mRNA vaccines can be used in case of infectious disease: (a) self-amplifying (replicon RNA vaccine) and (b) non-replicating mRNA vaccine. The latter type of vaccine can be further divided as ex vivo loading of dendritic cells, and direct in vivo injection into the anatomical site.
Self-amplifying mRNA (SAmRNA) vaccine
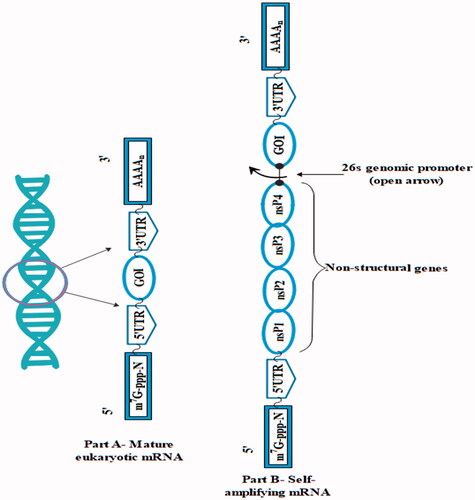
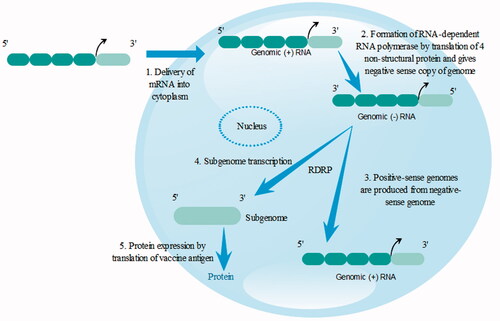
The self-amplifying vaccines are larger as compared to non-amplifying mRNA with size approximately 9–10 kb and contain basic elements of mRNA with a 3′UTR, 5′UTR, a poly (A) tail of variable length and a cap. depicts the structure of mature eukaryotic mRNA and SAmRNA from alphavirus.
Many of the SAmRNA are obtained from the alphavirus genome and this SAmRNA contains ORF encoding four non-structural proteins 1, 2, 3, 4 (nsP1,2,3,4) and subgenomic promoter is present in additional RNA. mRNA is incapable of inducing infectious virus by replacing the genes encoding a viral structural protein with the genes of interest in the viral genome which delivers mRNA to the cytosol of the cell. The combination with the host cell's ribosome results to the formation of functional components of RNA-dependent RNA polymerase (RDRP) or viral genome replication apparatus: nsP1, nsP2, nsP3 and nsP4.
Features of SAmRNA vaccine include
Auto-replication
High degree of expression of encoded vaccines antigen in a host cell
Enhanced duration
Self-amplifying mRNA delivery is a complicated process and includes multiple steps as shown in . In this case, it must overcome RNase mediated degradation and clearance. After reaching to the target cell, it enters the cytoplasm by translocating through the cellular membrane. It engages translation machinery of host and starts translation of non-structural proteins. Cellular uptake can be greatly affected by hydrophilicity and negative charge of RNA. Electrostatic complexation with either cationic lipid/polymer or physical delivery using electroporation can avoid barrier and increase the uptake [Citation50]. The SAmRNA shows great potential to produce a large amount of antigen in the small amount of vaccine. One of the studies showed that 100 ng of RNA replicon vaccine encodes respiratory syncytial virus (RSV) fusion F when complexed as lipid nanoparticle generated strong T and B cell mediated response in mice. When 1 µg dose was given, it showed that it protects the immune system by acting against RSV infection in the cotton rat intranasal challenge system. The benefits of SAM vaccine are that they are self-generating particular adjuvants as dsRNA structures, replication intermediates and different themes that enhance their high potency [Citation51].
Conclusions
Combined vaccines show tremendous potential for protection from numerous infectious diseases. Toxicity study and efficacy testing of combined vaccine are the key issues in the development of such vaccines. Cost-effective vaccines can be developed based on the emulsion type, nanoparticulate system and polymeric particles. Combined vaccine approach may more and more be used in the future to minimize the frequency of injections, coverage of better vaccination, reduction in vaccine cost and compliance to a patient.
| Abbreviations | ||
| Ig | = | immunoglobulin |
| DTaP | = | diphtheria, tetanus and acellular pertussis |
| DT | = | diphtheria-tetanus toxoid |
| Hib | = | Haemophilus influenzae type B |
| IPV | = | inactivated poliovirus |
| MMR | = | measles, mumps and rubella |
| MMRV | = | measles, mumps and rubella and varicella |
| CDC | = | Centers for Disease Control and Prevention |
| IN | = | intranasal |
| PMID | = | N-phosphonomethyliminodiacetic acid |
| TNF-α | = | tumour necrosis factor-α |
| IL-12 | = | interleukin-12 |
| PPSV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| TLR7 | = | Toll-like receptor 7 |
| SAmRNA | = | self-amplifying mRNA |
| RDRP | = | RNA-dependent RNA polymerase |
| RSV | = | respiratory syncytial virus |
| MPL | = | monophosphoryl lipid A |
| AH | = | aluminium hydroxide |
| AP | = | aluminium phosphate |
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Vaccines [Internet]. World Health Organization; 2018; [cited 2018 Oct 15]. Available from: http://www.who.int/topics/vaccines/en/
- Skibinski D, Baudner B, Singh M, et al. Combination vaccines. J Glob Infect Dis. 2011;3:63.
- Gautret P, Wilder-Smith A. Vaccination against tetanus, diphtheria, pertussis, and poliomyelitis in adult travelers. Travel Med Infect Dis. 2010;8:155–160.
- Lee HJ, Choi JH. Tetanus–diphtheria–acellular pertussis vaccination for adults: an update. Clin Exp Vac. 2017;6:22–30.
- Vaccine Position Papers [Internet]. World Health Organization; 2018; [cited 2018 Oct 15]. Available from: http://www.who.int/immunization/documents/positionpapers/en/.Sanofi. Pasteur Inc. Diphtheria and tetanus toxoids and acellular pertussis adsorbed inactivated poliovirus and Haemophilus b conjugate (tetanus toxoid conjugate) vaccine (Pentacel): US product information. Available from: http://www.fda.gov/cber/label/pentacelLB.pdf
- Gold R, Barreto L, Ferro S, et al. Safety and immunogenicity of a fully liquid vaccine containing five-component pertussis-diphtheria-tetanus-inactivated poliomyelitis-Haemophilus influenza type B conjugate vaccines administered at two, four, six and 18 months of age. Can J Infect Dis Med Microbiol. 2007;18:241–248.
- Dhillon S, Keam SJ. DTaP-IPV/Hib vaccine (Pentacel)®. Paediatr Drugs. 2008;10:405–416.
- McCormack PL. DTaP-IPV-Hep B-Hib Vaccine (Hexaxim®): a review of its use in primary and booster vaccination®. Paediatr Drugs. 2013;15:59–70.
- Sood A, Mitra M, Joshi HA, et al. Immunogenicity and safety of a novel MMR vaccine (live, freeze-dried) containing the Edmonston-Zagreb measles strain, the Hoshino mumps strain, and the RA 27/3 rubella strain: results of a randomized, comparative, active-controlled phase III clinical trial. Hum Vac Immunother. 2017;13:1523–1530.
- Ma S, Li X, Xiong Y, et al. Combination measles–mumps–rubella–varicella vaccine in healthy children. Medicine. 2015;94:1–10.
- Marin M, Broder KR, Temte JL, et al. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1–12.
- Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11:54.
- Peeters CC, Lagerman PR, de Weers O, et al. Preparation of polysaccharide-conjugate vaccines. In: Vaccine protocols. Humana Press; 2003. p. 153–173.
- Caporuscio S, Ieraci R, Valesini G, et al. Anti-polysaccharide and anti-diphtheria protective antibodies after 13-valent pneumococcal conjugate vaccination in rheumatoid arthritis patients under immunosuppressive therapy. Clin Immunol. 2018;195:18–27.
- Bardotti A, Averani G, Berti F, et al. Size determination of bacterial capsular oligosaccharides used to prepare conjugate vaccines against Neisseria meningitides groups Y and W135. Vaccine. 2005;23:1887–1899.
- Ferlito C, Biselli R, Cattaruzza MS, et al. Immunogenicity of meningococcal polysaccharide ACWY vaccine in primary immunized or revaccinated adults. Clin Exp Immunol. 2018;194:361–370.
- Jodar L, Griffiths E, Feavers I. Scientific challenges for the quality control and production of group C meningococcal conjugate vaccines. Vaccine. 2004;22:1047–1053.
- Immunology and Vaccine-Preventable Diseases – Pink Book; [Internet]. Cdc.gov.; 2018; [cited 2018 Oct 15]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/prinvac.pdf
- Lesinski G, Julie Westerink M. Vaccines against polysaccharide antigens. CDTID. 2001;1:325–334, 225–334.
- Guzman CA, Borsutzky S, Griot-Wenk M, et al. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811.
- Kothari S, Kothari N, Lee E, et al. Development of an efficient and scalable method for processing and purification of Vi capsular polysaccharide. Proc Vaccinol. 2010;2:78–81.
- Szu SC, Taylor DN, Trofa AC, et al. Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccines. Infect Immun. 1994;62:4440–4444.
- Steel HC, Cockeran R, Anderson R, et al. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediat Inflamm. 2013;2013:1.
- Pitsiou GG, Kioumis IP. Pneumococcal vaccination in adults: does it really work? Respir Med. 2011;105:1776–1783.
- Daniels CC, Rogers PD, Shelton CM. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther. 2016;21:27–35.
- Pietersz GA, Esparon SE, Proudfoot O, inventors. MacFarlane Burnet Centre for Medical Res Ltd-Fairfield Hospital, assignee. Flu vaccine admixture of mannan and flu antigen. United States patent US 8,182,821. 2012 May 22.
- Stambas J, Pietersz G, McKenzie I, et al. Oxidised mannan as a novel adjuvant inducing mucosal IgA production. Vaccine. 2002;20:1068–1078.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597.
- Hogen Esch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2013;3:406.
- Bansal V, Kumar M, Bhardwaj A, et al. In vivo efficacy and toxicity evaluation of polycaprolactone nanoparticles and aluminum based admixture formulation as vaccine delivery system. Vaccine. 2015;33:5623–5632.
- Mbow ML, De Gregorio E, Valiante NM, et al. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416.
- Milanetti F, Germano V, Nisini R, et al. Safety and immunogenicity of co‐administered MF 59‐adjuvanted 2009 pandemic and plain 2009–10 seasonal influenza vaccines in rheumatoid arthritis patients on biologicals. Clin Exp Immunol. 2014;177:287–294.
- Vesikari T, Groth N, Karvonen A, et al. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second-year seasonal vaccination. Vaccine. 2009;27:6291–6295.
- Kaurav M, Madan J, Sudheesh M, et al. Combined adjuvant-delivery system for new generation vaccine antigens: alliance has its own advantage. Artif Cells Nanomed Biotechnol. 2018;1–14. DOI:10.1080/21691401.2018.1513941
- Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vac. 2009;8:1663–1679.
- Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin Biol Ther. 2008;8:235–247.
- Li L, Lin SL, Deng L, et al. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in black seabream Acanthopagrus schlegelii bleeker to protect from Vibrio parahaemolyticus. J Fish Dis. 2013;36:987–995.
- Han J, Zhao D, Li D, et al. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers. 2018;10:31.
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145.
- Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252.
- Carstens MG, Camps MG, Henriksen-Lacey M, et al. Effect of vesicle size on tissue localization and immunogenicity of liposomal DNA vaccines. Vaccine. 2011;29:4761–4770.
- Ribeiro AM, Souza ACO, Amaral AC, et al. Nanobiotechnological approaches to delivery of DNA vaccine against fungal infection. J Biomed Nanotechnol. 2013;9:221–230.
- Badiee A, Khamesipour A, Samiei A, et al. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol. 2012;132:403–409.
- Amidi M, Van Helden M, Tabataei N, et al. Induction of humoral and cellular immune responses by antigen expressing immunostimulatory liposomes. J Control Release. 2012;164:323–330.
- Christensen D, Henriksen-Lacey M, Kamath AT, et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release. 2012;160:468–476.
- Gluck R, Burri KG, Metcalfe I. Adjuvant and antigen delivery properties of virosomes. Curr Drug Deliv. 2005;2:395–400.
- Herzog C, Hartmann K, Künzi V, et al. Eleven years of Inflexal® V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381–4387.
- Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vac. 2014;2:159–182.
- Brito LA, Kommareddy S, Maione D, et al. Self-amplifying mRNA vaccines. In: Advances in genetics, vol. 89. Massachusetts: Academic Press; 2015. p. 179–233.
- Shende P, Patel C. siRNA: an alternative treatment for diabetes and associated conditions. J Drug Target. 2019;27:174–182.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261.