Abstract
Aims
Our study aimed to investigate the expression and prognostic role of homeobox C6 (HOXC6) in prostate cancer (PCa).
Methods
Relative expression of HOXC6 at mRNA and protein levels in tissues and cell lines of PCa were measured using quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analysis, respectively. Association between HOXC6 expression and clinical factors was analyzed by Chi-square test. HOXC6 effects on the proliferation, invasion and metastasis of PCa cells were severally examined through CCK-8 and transwell assays.
Results
Relative expressions of HOXC6 at mRNA and protein levels were obviously higher in both PCa tissues and cells than in adjacent non-cancerous tissues and normal human prostate epithelial cells (p < .05). Chi-square test demonstrated that high expression of HOXC6 was significantly associated with PSA concentration, Gleason score and TNM stage (p < .05). The down-regulation of HOCX6 remarkably inhibited the proliferation, migration and invasion of PCa cells. Kaplan-Meier analysis showed that patients with high HOXC6 expression had shorter overall survival than those with low HOXC6 expression (log rank test, p < .001).
Conclusion
Up-regulated HOXC6, in PCa patients, could not only participate in the progression of PCa but also function as an independent prognostic marker for the cancer.
Introduction
Prostate cancer (PCa), an epitheliogenic malignant tumor in prostate, is the second most common malignancy and one of the major causes for global cancer-related deaths among men, seeing about 29,000 cases in 2013 all over the world [Citation1–5]. The cancer shows no obvious symptoms at early stage, which increases the risk of death among the patients [Citation6,Citation7]. PCa is one of the most common cancers in Occident, especially in America, its incidence rate in Asian countries is relatively low. However, the evidence has demonstrated that the incidence of PCa displays an increasing trend, especially in Asian countries [Citation8], which may result from accelerated aging, irrational diet structure, hormone abuse and so on. At present, most PCa patients can receive radical prostatectomy [Citation9]. However, approximately 30% of the PCa patients will suffer recurrence after surgery and most of them will develop distant or local metastasis [Citation10,Citation11]. Currently, challenge facing scholars in PCa field is to accurately predict the outcomes of the patients. Therefore, it is necessary to find some markers to improve prognosis prediction for PCa patients.
Homeobox (HOX) genes are involved in the formation of specific organs during embryogenesis and fetal development [Citation12,Citation13]. HOXC6 is a member of the HOX family and locates on human chromosome 12q13.3 [Citation14]. It is reported that HOXC6 participates in milk production and mammary gland development [Citation15,Citation16]. Moreover, aberrant expression of HOXC6 has been reported in various cancers, such as head and neck squamous cell carcinoma, gastrointestinal cancer, breast cancer, leukemia and osteosarcoma [Citation15,Citation17–20]. In earlier researches, HOXC6 showed raising tendency in PCa and was involved in the cancer development [Citation21,Citation22]. However, its prognostic value in PCa is never known.
In the present study, we detected the expression of HOXC6 in PCa tissues and cell lines. Meanwhile, we analyzed its relationship with clinical factors among PCa cases and evaluated its influence on the proliferation, migration and invasion of PCa cells. Besides, we assessed the value of HOXC6 in the prognosis of PCa as well.
Materials and methods
Patients and specimens
A total of 126 pathologically diagnosed PCa patients at The Second Medical Center, Chinese PLA General Hospital were enrolled in our study, who underwent surgical resection. All of the patients received no preoperative chemotherapy or radiotherapy before sampling. This study was approved by the Ethics Committee of the hospital. Written informed consents were obtained from all participants in advance.
One hundred twenty six PCa tissues and 55 adjacent non-cancerous ones were collected and frozen in liquid nitrogen, immediately. Then all tissue samples were stored at −80 °C for later use. Clinical variables of all patients were recorded in a database at diagnosis. A 5-year follow-up was conducted every 3 months. Patients who died from unexpected events or other diseases were excluded from our study.
Cell lines and cell culture
Human PCa cell line LNCaP and normal human prostate epithelial cells RWPE-1 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in DMEM medium (Gibco®Life Technologies, Carlsbad, CA, USA) supplemented with 100 U/ml penicillin sodium, 10% fetal bovine serum (FBS) and 100 mg/ml streptomycin sulfate in an atmosphere of 5% CO2 at 37 °C.
Cell transfection
Human LNCaP cells were transfected by HOXC6-specific siRNA (si-HOXC6) and negative control siRNA (si-NC) using Lipofectamine 2000 transfection reagent (Life Technologies, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocols.
Cell counting kit-8 (CCK-8) assay
The growth of PCa cells were assessed using CCK-8 counting kit. After transfection, the cells were cultivated in 96-well plates for 0, 12 h, 24 h, 48 h and 60 h, respectively. Then, they were incubated for 4 h after adding CCK-8. Absorbance was measured adopting a wavelength of 450 nm.
Transwell assay
The effects of HOXC6 on the migration and invasion of PCa cells were detected with transwell chamber inserts. Cells cultivated in DMEM without FBS were seeded onto the upper chamber with a concentration of 50,000 cells/well. FBS was added into the lower chamber as an attractant. After incubation for 4 h, cells moved to the bottom surface were fixed with methanol and stained with crystal violet. Finally, the invasive or migratory cells were counted in seven random viral fields with a microscope. For invasion assay, membrane in the upper chamber was pre-coated with Matrigel. These assays were carried out in triplicate.
QRT-PCR analysis
Total RNA was extracted from both tissue samples and cell lines using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the instructions. Then the RNA was reversely transcribed to synthesize cDNA using SuperScript First Strand cDNA System (Invitrogen). Next, RT-PCR amplification was conducted with the 7500 Real-Time PCR systems (Applied Biosystems, Carlsbad, CA, USA). GAPDH was taken as an internal control. Relative expression of HOXC6 mRNA was calculated using −2ΔΔCT method. Each sample was in triplicate.
Western blot analysis
Total protein was severally isolated from tissue samples of patients with PCa and PCa cell line. Then the proteins were separated through SDS-PAGE gels and brands were transferred onto PVDF membranes. After being blocked by 5% non-fat milk, the membranes were subsequently incubated with primary antibody at 4 °C overnight. Secondary antibody anti-HOXC6 mouse monoclonal antibody (Novus Biologicals, LLC, Colorado, USA) was added and incubated at room temperature. For detection, ECL kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, U.K.) was used according to the manufacturer's instructions.
Statistical analysis
All data were shown as mean ± SD. Statistical analyses were carried out using SPSS 19.0 (SPSS Inc., Chicago, IL, USA), GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA),Sigmaplot 12.5 (Systat Software Inc., San Jose, USA). Differences between two groups were analyzed via the student’s t-test. The relationship between HOXC6 expression and clinical profiles was analyzed through Chi-square test. Survival curves were plotted using Kaplan–Meier analysis to estimate overall survival of the PCa patients. Clinical significance of HOXC6 was evaluated applying Cox regression analysis. Results with p < .05 were considered to be statistically significant.
Results
Upregulated expression of HOXC6 in PCa tissues and cells
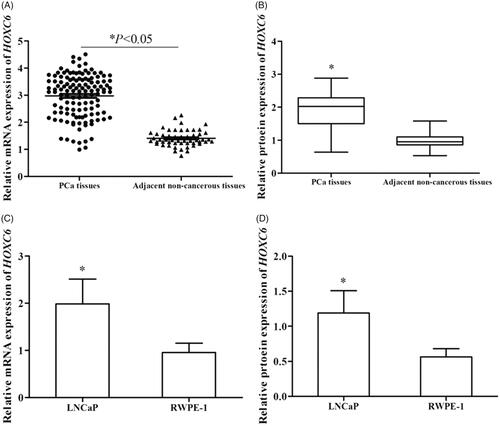
We examined the relative expression of HOXC6 at mRNA and protein levels in both PCa tissues and cell lines via qRT-PCR and Western blot analysis, respectively. The expression of HOXC6 was significantly higher in PCa tissues than in adjacent non-cancerous ones at both mRNA and protein levels (, p < .05). Meanwhile, the same trend was found in PCa cells and normal human prostate epithelial cells RWPE-1 at mRNA and protein levels (, p < .05).
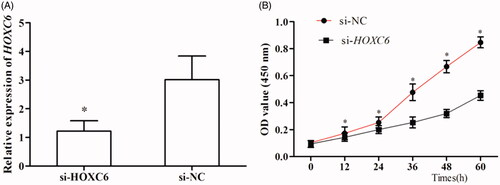
Figure 1. Relative expression of HOXC6 was determined using qRT-PCR and Western blot analysis. Compared with adjacent non-cancerous tissues, relative expression of HOCX6 was significantly up-regulated in PCa tissues at both mRNA (A) and protein (B) levels (p < .05). Relative expression of HOXC6 was also distinctively higher in cell line LNCaP than in normal human prostate epithelial cells RWPE-1 at both mRNA (C) and protein (D) levels (p < .05).
Association between HOXC6 expression and clinical factors of patients with PCa
The correlation between HOXC6 expression and clinical characteristics was examined to see whether it was involved in the development of PCa (). Accordingly, high HOXC6 expression was significantly related to the PSA level (p = .026), Gleason score (p = .034) and TNM stage (p = .017). However, other clinical parameters such as age (p = .369), diabetes (p = .134) and hematuria (p = .182) had no obvious influence on the expression of HOXC6.
Table 1. Relationship between HOXC6 expression and clinical characteristics of patients with PCa.
HOXC6 down-regulation inhibiting the proliferation of PCa cells
We studied the role of HOXC6 in the proliferation of PCa cells via CCK-8 assay. After transfected by si-HOXC6, HOXC6 showed obviously down-regulated expression compared to those transfected with si-NC (, p < .05). Then, the proliferation of cells transfected with si-HOXC6 was found to be significantly slower than those transfected with si-NC, indicating decreased expression of HOXC6 remarkably suppressed the proliferation of PCa cells (, p < .05).
The knockdown of HOXC6 suppressing the migration and invasion of PCa cells
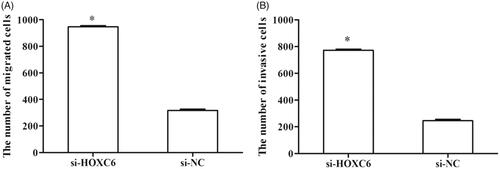
Transwell assay was performed to investigate the effects of HOXC6 on the migration and invasion of PCa cells. Cell migration assay showed that a migratory number of si-HOXC6-transfected cells was remarkably decreased compared with si-NC-transfected cells (, p < .05). Moreover, cell invasion assay demonstrated that an invasive number of si-HOCX6-transfected cells was smaller than that of si-NC-transfected cells (, p < .05).
Prognostic value of HOXC6 in PCa
To investigate the prognostic value of HOXC6 in PCa, we performed a 5-year follow-up. Based on data during this course, we conducted Kaplan–Meier analysis. According to the analysis, patients with high HOXC6 expression had shorter overall survival than those with low expression (log rank test, p < .001, ). Then, univariate and multivariate analyses manifested that high HOXC6 expression (HR = 5.951, 95CI% = 2.831–12.509, p = .000) and hematuria (HR = 2.022, 95CI% = 1.253–3.264, p = .004) were significantly correlated with the prognosis of PCa (), both of which might be independent prognostic biomarkers for the cancer.
Figure 4. Kaplan–Meier analysis was carried out to investigate the relationship of HOXC6 expression with overall survival of PCa patients. The curves showed that patients with low HOXC6 expression lived longer than those with high HOXC6 expression (log rank test, p < .001).
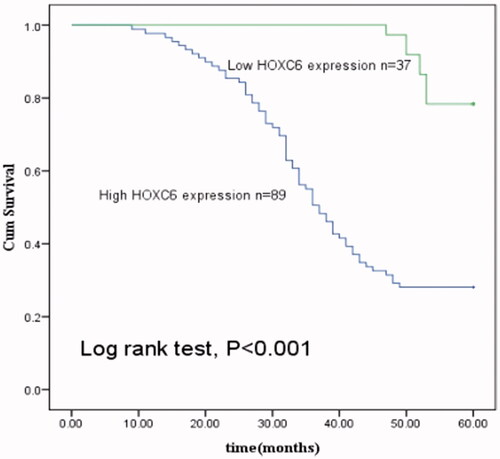
Table 2. Univariate and multivariate analyses adjusting clinical factors to estimate prognostic value of HOXC6 in PCa, using cox regression analysis.
Discussion
PCa, a clinically heterogeneous disease, is highly affected by hormonal and genetic factors, which can influence the tumor behavior as well as clinical managements and outcomes of the patients [Citation23–25]. Currently, prostate-specific antigen (PSA) is the gold standard predicting postoperative recurrence of PCa. Besides, tumor grade, tumor stage, Gleason score and PSA levels have all been used in PCa diagnosis or prognosis in multiple studies, whose accuracy are limited [Citation26,Citation27]. Besides, some other genetic biomarkers have also been investigated in PCa such as lncRNA MALAT-1, AQP5 and so on [Citation28,Citation29]. However, they only possess low accuracy.
HOXC6 belongs to the HOX family, whose members are important regulators in cell differentiation and cellular proliferation during development [Citation19]. To our knowledge, altered expression of HOXC6 has been observed in various tumor types, such as leukemia and serous ovarian cancer [Citation30]. In the present study, we measured the expression of HOXC6 in PCa tissues and cells. Also, the results showed that compared with normal controls, PCa tissues and cells manifested relatively heightened HOXC6 expression at mRNA and protein levels, which was in accordance with findings from previous reports [Citation17,Citation31]. Hence, HOXC6 might act as an oncogene in PCa. Then, Chi-square test showed that high HOXC6 expression was related to PSA level, Gleason score and TNM stage, indicating HOXC6 might participate in the development and progression of PCa.
As we all know, HOXC6 plays important roles in the growth, invasion and metastasis of tumor cells. For instance, Ramachandran et al. claimed that the loss of HOXC6 in vitro significantly induced the apoptosis of PCa cells and HOXC6 could act as a promising therapeutic target in PCa [Citation22]. Larsen et al. reported genetic loss of HOXC6 resulted in the loss of endocrine cells derived from endoderm [Citation32]. Based on previous findings and our hypothesis, we carried out CCK-8 and transwell assays to detect the effects of HOXC6 on the proliferation, migration and invasion of PCa cells. All results demonstrated that the knockdown of HOXC6 in vitro significantly inhibited the progression of PCa cells, which further highlighted the relationship between HOXC6 expression and the tumorigenesis of PCa.
In previous studies, the prognostic value of HOXC6 has been reported in several cancers. According to Du et al., elevated expression level of HOXC6 in esophageal squamous cell carcinoma predicted unfavorable prognosis for the patients [Citation33]. Zhang et al. suggested that HOXC6 expression was increased in gastric cancer tissues and patients with high expression of HOXC6 had shorter survival time than those with low expression [Citation34]. In our study, Kaplan–Meier and Cox analyses were performed to evaluate the relationship between HOXC6 expression and the prognosis of PCa patients. Kaplan–Meier survival curves showed that patients with high HOXC6 expression faced a higher risk of death than those with low HOXC6 expression, indicating HOXC6 was linked to the cancer prognosis. Besides, Cox analysis revealed that HOXC6 was a prognostic factor for PCa. However, it is notable that in the report of Augs et al., HOXC6 had no relation with the prognosis of PCa patients undergoing radical prostatectomy [Citation21]. Such a difference might be due to dissimilarity in treatments, which needs more investigations to further illustrate. Since very few of the previous studies refer to the impact of HOXC6 on PCa prognosis, the results need to be verified.
In conclusion, the expression of HOXC6 is increased in PCa tissues and cells. High expression of HOXC6 was significantly correlated with PSA, Gleason score and TNM stage of the patients. The down-regulation of HOXC6 could significantly suppress cell growth via inhibiting their proliferation, migration and invasion. In addition, HOXC6 could be an independent biomarker for the prognosis of PCa patients. However, considering limited sample size and other unfavorable factors in our study, as well as unclear mechanism of PCa, further studies are needed in the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092.
- Soares N. d C P, Teodoro AJ, Oliveira FL, et al. Influence of lycopene on cell viability, cell cycle, and apoptosis of human prostate cancer and benign hyperplastic cells. Nutr Cancer. 2013;65:1076–1085.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
- Keane FK, Chen MH, Zhang D, et al. The likelihood of death from prostate cancer in men with favorable or unfavorable intermediate-risk disease. Cancer. 2014;120:1787–1793.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
- Reiter RJ, Tan DX, Manchester LC, et al. A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Invest. 2013;31:365–373.
- Kong HY, Byun J. Screening and characterization of a novel RNA aptamer that specifically binds to human prostatic acid phosphatase and human prostate cancer cells. Mol Cells. 2015;38:171–179.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Chao C, Chi M, Preciado M, et al. Methylation markers for prostate cancer prognosis: a systematic review. Cancer Causes Control. 2013;24:1615–1641.
- Daniunaite K, Jarmalaite S, Kalinauskaite N, et al. Prognostic value of RASSF1 promoter methylation in prostate cancer. J Urol. 2014;192:1849–1855.
- Lin YL, Xie PG, Wang L, et al. Aberrant methylation of protocadherin 17 and its clinical significance in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1376–1382.
- Huang L, Pu Y, Hepps D, et al. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology 2007;148:1235–1245.
- Klein D, Benchellal M, Kleff V, et al. Hox genes are involved in vascular wall-resident multipotent stem cell differentiation into smooth muscle cells. Sci Rep. 2013;3:2178.
- Hussain I, Bhan A, Ansari KI, et al. Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer. Biochim Biophys Acta. 2015;1849:697–708.
- Bodey B, Bodey B, Jr., Siegel SE, et al. Immunocytochemical detection of the homeobox B3, B4, and C6 gene products in breast carcinomas. Anticancer Res. 2000;20:3281–3286.
- Ansari KI, Hussain I, Shrestha B, et al. HOXC6 Is transcriptionally regulated via coordination of MLL histone methylase and estrogen receptor in an estrogen environment. J Mol Biol. 2011;411:334–349.
- Moon SM, Kim SA, Yoon JH, et al. HOXC6 is deregulated in human head and neck squamous cell carcinoma and modulates Bcl-2 expression. J Biol Chem. 2012;287:35678–35688.
- Fujiki K, Duerr EM, Kikuchi H, et al. Hoxc6 is overexpressed in gastrointestinal carcinoids and interacts with JunD to regulate tumor growth. Gastroenterology. 2008;135:907–916.
- Bodey B, Bodey B, Jr,., Siegel SE, et al. Homeobox B3, B4, and C6 gene product expression in osteosarcomas as detected by immunocytochemistry. Anticancer Res. 2000;20:2717–2721.
- Bijl J, van Oostveen JW, Kreike M, et al. Expression of HOXC4, HOXC5, and HOXC6 in human lymphoid cell lines, leukemias, and benign and malignant lymphoid tissue. Blood. 1996;87:1737–1745.
- Hamid AR, Hoogland AM, Smit F, et al. The role of HOXC6 in prostate cancer development. Prostate. 2015;75:1868–1876.
- Ramachandran S, Liu P, Young AN, et al. Loss of HOXC6 expression induces apoptosis in prostate cancer cells. Oncogene. 2005;24:188–198.
- Ibeawuchi C, Schmidt H, Voss R, et al. Exploring prostate cancer genome reveals simultaneous losses of PTEN, FAS and PAPSS2 in patients with PSA recurrence after radical prostatectomy. IJMS. 2015;16:3856–3869.
- Ronnau CG, Verhaegh GW, Luna-Velez MV, et al. Noncoding RNAs as novel biomarkers in prostate cancer. Biomed Res Int. 2014;2014:591703.
- Niu WB, Gui SL, Lin YL, et al. Promoter methylation of protocadherin8 is an independent prognostic factor for biochemical recurrence of early-stage prostate cancer. Med Sci Monit. 2014;20:2584–2589.
- Roberts MJ, Chow CW, Schirra HJ, et al. Diagnostic performance of expression of PCA3, Hepsin and miR biomarkers inejaculate in combination with serum PSA for the detection of prostate cancer. Prostate. 2015;75:539–549.
- Henriquez-Hernandez LA, Valenciano A, Foro-Arnalot P, et al. Single nucleotide polymorphisms in DNA repair genes as risk factors associated to prostate cancer progression. BMC Med Genet. 2014;15:143.
- Wang F, Ren S, Chen R, et al. Development and prospective multicenter evaluation of the long noncoding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancer. Oncotarget. 2014;5:11091–11102.
- Li J, Wang Z, Chong T, et al. Over-expression of a poor prognostic marker in prostate cancer: AQP5 promotes cells growth and local invasion. World J Surg Onc. 2014;12:284.
- Tait DL, Bahrani-Mostafavi Z, Vestal CG, et al. Downregulation of HOXC6 in serous ovarian cancer. Cancer Invest. 2015;33:303–311.
- Vinarskaja A, Yamanaka M, Ingenwerth M, et al. DNA methylation and the HOXC6 paradox in prostate cancer. Cancers (Basel). 2011;3:3714–3725.
- Larsen BM, Hrycaj SM, Newman M, et al. Mesenchymal Hox6 function is required for mouse pancreatic endocrine cell differentiation. Development. 2015;142:3859–3868.
- Du YB, Dong B, Shen LY, et al. The survival predictive significance of HOXC6 and HOXC8 in esophageal squamous cell carcinoma. J Surg Res. 2014;188:442–450.
- Zhang Q, Jin XS, Yang ZY, et al. Upregulated Hoxc6 expression is associated with poor survival in gastric cancer patients. Neoplasma. 2013;60:439–445.