 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
As a processed product of traditional Chinese medicine Curcumae Radix, Curcumae Radix Carbonisata (CRC) has been widely used in the treatment of liver diseases in ancient medical books. In this study, novel carbon dots (CDs) extending from 1.0 to 4.5 nm were separated from fluid extricates of CRC. Meanwhile, a liver fibrosis model induced by carbon tetrachloride (CCl4) was utilized to determine the inhibitory effects of CRC-CDs against liver fibrosis. The results exhibited the CRC-CDs with a quantum yield of 1.34% have a significant inhibitory effect on CCl4-induced liver fibrosis, as demonstrated by improving hepatocyte degeneration and necrosis, inflammatory cell infiltration and fibrotic tissue hyperplasia, downregulating the levels of alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), total bile acid (TBA), triglyceride (TG), tumour necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-1β in the serum, upregulating the contents of superoxide dismutase (SOD), reduced glutathione (GSH), and downregulating the concentration of malondialdehyde (MDA), which lays an important foundation for the development of CRC-CDs as a novel drug for the treatment of liver fibrosis, and provide a certain experimental basis for the clinical application of CRC-CDs in the future.
Graphical Abstract
Introduction
As a kind of common diseases worldwide, liver fibrosis caused by chronic liver damage is mainly composed of many factors, including alcohol, non-alcoholic fatty liver disease, viral hepatitis, autoimmune hepatitis, cholestasis, liver disease [Citation1]. The generation of fibrotic responses in the liver induces the accumulation of extracellular matrix (ECM) components, which in turn leads to fibrous scarring [Citation2]. Meanwhile, the presence of fibrous scars can cause the loss of liver cells and normal liver dysfunction, and even cause cirrhosis or liver cancer [Citation3].
However, since liver fibrosis is a reversible process, effectively delaying, alleviating, and reversing liver fibrosis has become an important factor in preventing liver cirrhosis and liver cancer [Citation4]. At present, no effective drug for the treatment of liver fibrosis has been approved for marketing. The existing drugs for the treatment of liver fibrosis, such as N-acetylcysteine, corticosteroids, and interferon, have problems such as high toxicity and many adverse reactions after long-term use of the drugs [Citation5,Citation6]. Therefore, there is an urgent need for a safer and lower-cost drug to treat liver fibrosis. In this regard, the research on the hepatoprotective activity of nanoparticles in the field of nanomaterials has attracted the attention of many scholars.
Carbon dots (CDs), a novel type of carbon-based nanomaterials, have attracted widespread attention in many fields, including phototherapy [Citation7], bioimaging [Citation8], drug delivery and cancer therapy [Citation9,Citation10], owing to their good biocompatibility [Citation11], predominant photoluminescence [Citation12], and excellent water dispersibility [Citation13]. Due to their noteworthy preferences, the natural activities of CDs in medication, including hemostasis [Citation14], immune regulation [Citation15], anti-inflammatory [Citation16], hypoglycaemic [Citation17], anti-tumour [Citation18], bacteriostatic [Citation19], anti-viral [Citation20], have been affirmed by different scientific experiments, which gives a modern era of drugs for the revelation of viable control or treatment of certain diseases.
As a traditional Chinese medication broadly utilized in China, Curcumae Radix (CR), the dried tuberous root of Curcuma wenyujin Y. H. Chen et C. Ling, Curcuma Longa L., Curcuma kwangsiensis S. G. Lee et C. F. Liang or Curcuma phaeocaulis Val, is used to treat diseases related to the liver meridian after processing. Curcumae Radix Carbonisata (CRC), the charcoal prepared product of CR, is mostly used in the treatment of hepatitis in the ancient medical classics. Despite numerous clinical reports on the efficacy of CRC, its material basis remains unclear.
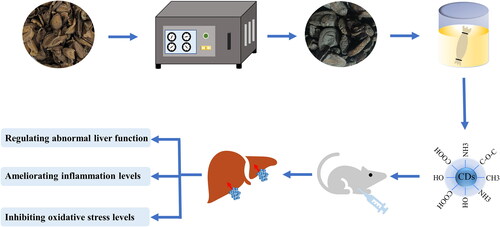
Herein, a novel CDs (named CRC-CDs) prepared by one-step pyrolysis method using CRC as sole carbon sources, and their inhibitory effects on carbon tetrachloride (CCl4)-induced liver fibrosis have been studied for the first time. The underlying mechanism was explored by observing the macroscopic images and pathological changes of liver tissue, analyzing the changes of ALT, AST, TBIL, DBIL, TBA and TG in the serum, determining the levels of inflammatory factors (TNF-α, IL-6, IL-1β) and oxidative stress indicators (SOD, GSH, MDA).
Materials and methods
Materials
CR was purchased from Beijing Qiancao Co., Ltd (Beijing, China), and CRC was prepared in our laboratory. Dialysis membranes (molecular weight cut-off was 1000 Da) were purchased from Beijing Ruida Henghui Technology Development Co., Ltd. (Beijing, China). Dulbecco’s Modified Eagle Medium (DMEM) and foetal bovine serum (FBS) were purchased from Corning Co., Ltd. (New York, USA). SOD, GSH and MDA kits were purchased from Nanjing Jiancheng Bioengineering Institute of China (Nanjing, China). Cell Counting Kit-8 (CCK-8), TNF-α, IL-6 and IL-1β were brought from Cloud-Clone Corp (Wuhan, China). Antibodies against IKKα, p-IκBα, p65 and GAPDH were supplied by affinity (California, USA) or Cell Signalling technology (Boston, USA). The analytical grade chemicals and reagents were purchased from China National Pharmaceutical Industry Corporation Ltd (Beijing, China). All the experiments were performed utilizing deionized water (DW).
Animals
All the animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals endorsed by the Committee of Ethics of Animal Experimentation of the Beijing University of Chinese Medicine. Male C57BL/6J mice (weighing 20.0 ± 2.0 g) were acquired from SiPeiFu Biotechnology Co., Ltd. (Beijing, China) and housed under the following conditions: temperature, (24.0 ± 1.0)°C; relative humidity, 55–65%, and a 12 h light/dark cycle, with ad libitum access to food and water.
Preparation of CRC-CDs
CR-CDs were synthesized through a one-step pyrolysis method and taking the CR as only biomass precursor. To begin with, 100 g dried CR was placed in crucible, covered by a layer of aluminium foil and the lid to ensure good leak proofness. Then, the CR was carbonized using the muffle furnace (TL0612, Beijing Zhong Ke Aobo Technology Co., Ltd., China) at 350 °C for 1 h to collect the CRC. Next, 50 g CRC was ground into fine powder and boiled with DW twice in the water bath at 100 °C for 1h each time. Subsequently, the decoction liquid filtered out with 0.22 μm organic microporous membrane was concentrated to 50 ml. Finally, the CRC-CDs solution was dialysed and collected through a 1000 Da molecular weight cut-off dialysis membrane for 7 days. The obtained CRC-CDs were stored at 4 °C until further use. The preparation process of CRC-CDs is shown in .
Characterization of CRC‑CDs
The morphology, microstructure and distribution state of CRC-CDs were revealed by transmission electron microscopy (TEM, Tecnai G220, FEI Company, USA) with an accelerating voltage of 100 kV, while the lattice spacing of CRC-CDs and other details were uncovered using a HRTRM (JEN-1230, Japan Electron Optics Laboratory, Japan). X-ray diffractometer (XRD, Bruker AXS, Karlsruhe, Germany) was performed with Cu K-alpha radiation. The spectral properties of the CDs were recorded by the ultraviolet spectrophotometer (UV-Vis) (CECIL, Cambridge, UK) and by the fluorescence (FL) spectrophotometer (F-4500, Tokyo, Japan). The surface composition of the sample was analyzed using Fourier transform infra-red (FTIR) spectroscopy (Thermo Fisher, Fremont, California, USA) and X-ray photoelectron spectroscopy (XPS) (ESCALAB 250Xi, Thermo Fisher Scientific, USA).
Cytotoxicity assay of CRC-CDs
The CCK-8 experiment was performed to distinguish the cytotoxicity of CRC-CDs to RAW 264.7 mouse macrophage. Cells were cultured in DMEM medium containing 20% foetal bovine serum in a humidified 5% CO2 atmosphere at 37 °C. Subsequently, the three cells were seeded in a 96-well plate at a density of 1 × 105 cells per 100 μL/well and incubated for 24 h, respectively. Then, different concentrations of CRC-CDs (2000, 1000, 500, 250, 125, 62.5, 31.25, 15.63 μg/mL) were added to the designated wells for 24 h and the control cells were treated with DMEM medium. After these plates were washed thrice with PBS, 10 μL CCK-8 was added for an additional 4 h of incubation. Additionally, a microplate reader (Biotek, Vermont, USA) was utilised to record the absorbance of each well. Finally, the cell viability (%) calculation formula is as follows:
where Ae, Ab and Ac represent the absorbance of the experimental, blank and control groups, respectively, at 450 nm.
Models of liver fibrosis and drug treatment
The liver fibrosis model was established as previously reported [Citation21]. A total of 49 C57BL/6J male mice were randomly divided into seven groups (n = 7 in each): control group, CCl4 group, positive (silybin, 100 mg/kg) + CCl4 group, CRC-CDs (high[H]: 9.33 mg/kg, medium[M]: 4.67 mg/kg, low[L]: 2.34 mg/kg) + CCl4 group and high-dose CRC-CDs group (9.33 mg/kg). Except for the control group and the high-dose CRC-CDs group, the mice in the other groups were intraperitoneally injected with 10% CCl4 olive oil solution (CCl4 and olive oil solution according to the volume ratio of 1:9 preparation mixed evenly, 5 ml/kg) to induce liver fibrosis, twice a week, with an interval of 3 d for 8 consecutive weeks. In addition, silybin and CRC-CDs are administered simultaneously with CCl4. Mice in the control group and the high-dose CRC-CDs group were intraperitoneally injected with an equal volume of olive oil solution. At the same time, except the control group and the CCl4 group were given the same amount of normal saline, the other groups were given the corresponding dose of drugs by intragastric administration, once a day, for 8 consecutive weeks.
Sample collection
During the experiment, the weight of mice was recorded every day, and the changes of weight of mice were analyzed. After the last administration, the mice in each group were weighed after fasting for 12 h. Meanwhile, the mice were sacrificed at 12 h after the last administration. Mice were anaesthetized by intraperitoneal injection of 4% chloral hydrate (0.40 g/kg). Mouse blood was collected from the venous sinus retro-orbitally into serum tubes immediately after the mice are anaesthetized. Then, the supernatant was obtained by centrifugation at 750 × g for 10 min. For each mouse, the volume of eyeballs blood collected is 0.75 ml. Mice after blood collection were euthanized via cervical dislocation under anaesthesia. After the mice were sacrificed, the liver tissue was removed and weighed respectively to calculate the liver index.
Histopathological study
After observation and photography, portion of the liver tissue was rapidly placed in 4% paraformaldehyde solution, dehydrated, implanted in paraffin, and stained with hematoxylin-eosin (H&E) staining, Masson staining, and Sirius red staining. Structural changes and collagen fibre deposition in the liver tissues of each group were observed under a microscope at magnifications of 100×, and photographed for analysis.
Biochemical analysis
The changes in alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), bilirubin (DBIL), total bile acid (TBA) and triglyceride (TG) in the sera were determined using an automatic biochemical analyzer (XI-800, Sismecon Co., Ltd, Japan).
Detection of inflammatory factors
The remaining portion serum of mice was utilized to detect the levels of inflammatory cytokines (TNF-α, IL-6 and IL-1β) by ELISA according to the instruction manuals.
Measurement of oxidative stress levels
The liver tissue samples were weighed and processed with 9 times volume of pre-cooling PBS solution to make 10% liver tissue homogenate. Then, the supernatant was obtained by centrifugation at 750 × g for 10 min to detect the levels of MDA, SOD, and GSH according to the manufacturer’s instructions.
Statistical analysis
All data from experiment were carried out utilizing IBM SPSS (version 25.0, Chicago, IL, USA). The values were represented as means ± standard deviation. Multiple comparisons were performed using a one-way analysis of variance (ANOVA) or two-way ANOVA followed by the least-significant difference test or Tukey post hoc test. p < 0.05 and p < 0.01 were considered statistically significant.
Result
Characterization of CR-CDs
The TEM image () shows that the CRC-CDs were spherical-like particles without obvious aggregation. The particle size distribution of CRC-CDs was concentrated range of 1.0 − 4.5 nm (), which was concluded by examining actual measurements of more than 100 particles using ImageJ software. As shown in , the HRTEM image of the CRC-CDs indicated that the lattice distance of RRR-CDs was 0.216 nm, which was near to the value of the crystal plane of graphite [Citation22]. The spatial distribution of atoms in CRC-CDs was further analyzed by XRD. A typical amorphous diffraction peak (2θ = 23.371°) can be seen in , from which it can be inferred that CRC-NCS is composed of highly amorphous carbon structure and crystal.
Figure 2. Morphological characterisations of CRC-CDs. (A) Transmission electron microscopy (TEM) images of CRC-CDs displaying ultra-small particles. (B) Particle size distribution histogram of CRC-CDs. (C) High-resolution TEM image of CRC-CDs and lattice spacing of CRC-CDs (in the Middle). (D) X-ray diffraction pattern (E) Ultraviolet-visible spectrum. (F) Fluorescence spectra. (G) Fluorescence spectra of FP-CDs with different excitation wavelengths. (H) Fourier transform infra-red spectrum.

The UV-Vis spectra of CRC-CDs () displayed a weak absorption peak around 260 nm, which may be caused by the π–π* electronic transition of the conjugated C=C bonds and aromatic sp2 domains [Citation23]. The optical properties of CRC-CDs were further investigated utilizing FL spectrophotometer. As illustrated in , CRC-CDs exhibited the maximum emission at 450 nm while excited at 341 nm, the quantum yield of CRC-CDs was calculated to be 5.34% using quinine sulphate as a reference. As the excitation wavelength increased from 300 to 390 nm (), the maximum emission wavelength of CRC-CDs had a red-shift and its fluorescence intensity appeared a trend of expanding to begin with and after that diminishing, indicating that CRC-CDs had excitation-emission wavelength dependence. The reason for this phenomenon may be related to the different particle sizes of nanoparticles and the different distribution of surface energy traps [Citation24].
The surface chemical characteristics of the CRC-CDs were analyzed by FTIR spectroscopy (). The strong characteristic absorption peak at 3448 cm−1 may be the stretching vibration peak of O–H and N–H bonds. The absorption peaks at 2918 and 2850 cm−1 belonged to the stretching vibration peaks of –CH3 and –CH2, respectively. In addition, the absorption peak of 1635 cm−1 was associated with the stretching vibration peak of –C=O bonds, while the peak at 1384 cm−1 belonged to –C–N bonds. The peak at 1107 cm−1 was related to C–O–C bonds. The results showed that the surface of CRC-CDs contained amino, carbonyl, hydroxyl groups, which made CRC-CDs have good solubility and biological activity.
The element composition and surface chemical bonds in detail of CRC-CDs were determined by using XPS. Furthermore, three apparent element peaks in XPS spectra () were observed clearly at 284.81, 399.84 and 531.67 eV, indicating that the CRC-CDs were mainly composed of C (67.43%), N (4.36%), O (27.67%) and small amount of S (0.54%). The C1s spectrum () was divided into three peaks at 284.48, 286.02 and 287.83 eV, which were consistent with C–C/C=C, C–O, C=O/C=N [Citation25]. Two peaks at 530.80 and 532.27 eV in the O 1s () spectrum were attributed to the presence of C–O and C=O, respectively [Citation26]. The N1s spectrum () was separated into two peaks at 399.21 and 400.14 eV, which confirmed the existence of C–N and N–H bonds [Citation27]. The XPS experimental results are basically consistent with the FTIR characterization, which indicated that FP-CDs contain various groups such as carboxyl, hydroxyl, and amino groups.
Cytotoxicity detection
The results of CCK-8 experiments () showed that CRC-CDs could promote the proliferation of RAW264.7 cells in the dose range of 250–2000 μg/mL. However, CRC-CDs could inhibit the viability of RAW264.7 cells to a certain extent at the administration concentration of 15.63–250 μg/mL. This provides a potential reference for the clinical application of nanomedicine in the future.
Effects of CRC-CDs on weight changes in liver fibrosis mice
As illustrated in , at the third week, the weight of mice in the CCl4 group was significantly lower than that in the control group and the silybin + CCl4 group (p < 0.01). At the fourth week, except for the above two groups, the weight of mice in the CCl4 group was significantly lower than that in the CRC-CDs low-dose + CCl4 group (p < 0.01). With the development of the experiment, at the fifth week, except for the above groups, the weight of mice in the CCl4 group was significantly lower than that in the CRC-CDs high-dose + CCl4 group (p < 0.01). At the sixth week, except for the above groups, the weight of mice in the CCl4 group was lower than that in the CRC-CDs medium dose + CCl4 group (p < 0.05). With the progress of the experiment, at the eighth week, the body weight of the mice in each of the above-mentioned administration groups was significantly higher than that in the CCl4 group (p < 0.01). The experimental results showed that the body weight of liver fibrosis mice increased significantly after the intervention of CRC-CDs.
Figure 5. Effects of CRC-CDs on weight changes in mice with carbon tetrachloride-induced liver fibrosis. Mice were assigned into seven groups, namely normal control group (NC), CCl4 group (CCl4), silybin + CCl4 group (silybin + CCl4), high dose of CRC-CDs + CCl4 group (CRC-CDs-H + CCl4, 9.33 mg/kg), medium dose of CRC-CDs + CCl4 group (CRC-CDs-M + CCl4, 4.67 mg/kg), low dose of CRC-CDs + CCl4 group (CRC-CDs-L + CCl4, 2.34 mg/kg) and high dose of CRC-CDs group (CRC-CDs-H, 9.33 mg/kg). data were expressed as means ± standard deviation. ##p < 0.01 vs. normal control group, *p < 0.05 and **p < 0.01 vs. CCl4 group.

Effects of CRC-CDs on liver weight and liver index in liver fibrosis mice
Liver index is a pathological index to measure the degree of liver damage, which can directly reflect the degree of liver damage in experimental animals. As shown in ), compared with the control group, the liver weight and liver index of the mice in the CCl4 group were significantly increased (p < 0.01). In sharp contrast, compared with CCl4 group, liver weight and liver index of mice in Silybin + CCl4 group and CRC-CDs high-dose, medium-dose, and low-dose + CCl4 groups were decreased to a certain extent (p < 0.05). In addition, compared with the control group, there was no significant difference in the liver weight and liver index of the mice in the CRC-CDs high-dose group, indicating that CRC-CDs had no significant effect on the liver mass and liver index of the normal mice.
Figure 6. Effects of CRC-CDs on liver weight (A) and liver index (B) in mice with carbon tetrachloride-induced liver fibrosis. Mice were assigned into seven groups, namely normal control group (NC), CCl4 group (CCl4), silybin + CCl4 group (silybin + CCl4), high dose of CRC-CDs + CCl4 group (CRC-CDs-H + CCl4, 9.33 mg/kg), medium dose of CRC-CDs + CCl4 group (CRC-CDs-M + CCl4, 4.67 mg/kg), low dose of CRC-CDs + CCl4 group (CRC-CDs-L + CCl4, 2.34 mg/kg) and high dose of CRC-CDs group (CRC-CDs-H, 9.33 mg/kg). data were expressed as means ± standard deviation. #p < 0.05 and ##p < 0.01 vs. normal control group, *p < 0.05 and **p < 0.01 vs. CCl4 group.

Effect of CRC-CDs on liver morphology and pathological tissue of liver fibrosis mice
The observation results of the appearance and morphology of the liver tissue of the mice in each group were shown in . The liver of the mice in the control group had a smooth surface, ruddy colour, and sharp edges. However, there were granular substances on the surface of the livers of mice in the CCl4 group, with dull colour and dull edges, which indicated that the modelling was successful. In contrast, the distribution of granular matter on the liver surface of mice in silybin + CCl4 group and different doses of CRC-CDs + CCl4 group was significantly improved compared with CCl4 group.
Figure 7. Effects of CRC-CDs on the appearance and pathological changes of liver tissue in mice with carbon tetrachloride-induced liver fibrosis. (A) Appearance of liver tissue, (B) HE staining (× 100), (C) Masson staining (× 100), (D) Sirius red staining (× 100). Mice were assigned into seven groups, namely normal control group (NC), CCl4 group (CCl4), silybin + CCl4 group (silybin + CCl4), high dose of CRC-CDs + CCl4 group (CRC-CDs-H + CCl4), medium dose of CRC-CDs + CCl4 group (CRC-CDs-M + CCl4), low dose of CRC- CDs + CCl4 group (CRC-CDs-L + CCl4) and high dose of CRC-CDs group (CRC-CDs-H).

The results of HE staining (), Masson staining () and Sirius red staining () showed that the hepatocytes in the control group were intact and evenly arranged radially with the central vein as the centre. The hepatic lobule structure was clear, and there was no collagen fibre hyperplasia around the liver tissue. In sharp contrast, the liver tissue of the mice in the CCl4 group was severely damaged, with disordered arrangement of liver cells, massive steatosis and necrosis of liver cells, and obvious infiltration of inflammatory cells. The hepatic lobule structure was severely damaged, and hyperplastic collagen fibres were visible in the portal area of liver tissue and around the central vein, which indicated that the model of liver fibrosis was successful. Compared with CCl4 group, the degree of liver tissue injury in silybin + CCl4 group and CRC-CDs high, medium, and low dose + CCl4 groups were significantly reduced, and the phenomenon of hepatocyte degeneration and necrosis, inflammatory cell infiltration and fibrotic tissue hyperplasia were also significantly improved. In addition, compared with the control group, the liver morphology, and pathological sections of mice in the high-dose CRC-CDs group showed no obvious abnormalities, indicating that CRC-CDs had no effect on the liver of normal mice.
Effects of CRC-CDs on liver function indexes in serum of liver fibrosis mice
The activities of ALT and AST in serum can accurately reflect the degree of liver injury. As illustrated in , compared with control group (ALT: 28.57 ± 2.99 U/L, AST: 126.31 ± 13.83 U/L), the levels of ALT (50.71 ± 3.20 U/L) and AST (170.60 ± 17.60 U/L) in model group were significantly increased (p < 0.01), which presented that a liver fibrosis model induced by CCl4 was successfully established. Conversely, the contents of ALT in the silybin + CCl4 group (30.43 ± 2.64 U/L) and CRC-CDs + CCl4 groups (H: 30.86 ± 2.85 U/L, M: 30.14 ± 2.67 U/L, L: 31.29 ± 2.06 U/L) were significantly reduced in comparison to the model group (p < 0.01). compared with model group, the levels of AST in the silybin + CCl4 group (147.10 ± 6.80 U/L) and CRC-CDs + CCl4 groups (H: 146.17 ± 8.98 U/L, M: 145.43 ± 27.61 U/L, L: 147.80 ± 18.00 U/L) were significantly decreased (p < 0.05).
Figure 8. Effects of CRC-CDs on the contents of (A) ALT, (B) AST, (C) TBIL, (D) DBIL, (E) TBA, and (F) TG in serum of mice with carbon tetrachloride-induced liver fibrosis. Mice were assigned into seven groups, namely normal control group (NC), CCl4 group (CCl4), silybin + CCl4 group (silybin + CCl4), high dose of CRC-CDs + CCl4 group (CRC-CDs-H + CCl4, 9.33 mg/kg), medium dose of CRC-CDs + CCl4 group (CRC-CDs-M + CCl4, 4.67 mg/kg), low dose of CRC-CDs + CCl4 group (CRC-CDs-L + CCl4, 2.34 mg/kg) and high dose of CRC-CDs group (CRC-CDs-H, 9.33 mg/kg). data were expressed as means ± standard deviation (SD). ##p < 0.01 vs. normal control group, *p < 0.05 and **p < 0.01 vs. CCl4 group.

Serum biochemical indexes TBIL and DBIL are of great significance in judging liver function damage. Different from the control group (TBIL: 1.21 ± 0.52 μmol/L, DBIL: 0.42 ± 0.12 μmol/L), the TBIL (2.32 ± 0.24 μmol/L) and DBIL (0.61 ± 0.13 μmol/L) levels of the model group () also increased (p < 0.01). Meanwhile, the levels of TBIL and DBIL in the silybin + CCl4 group (TBIL: 1.55 ± 0.22 μmol/L, DBIL: 0.44 ± 0.09 μmol/L), the high dose of CRC-CDs (TBIL: 1.54 ± 0.44 μmol/L, DBIL: 0.47 ± 0.06 μmol/L), the medium dose of CRC-CDs (TBIL: 1.53 ± 0.39 μmol/L, DBIL: 0.45 ± 0.05 μmol/L) and the low dose of CRC-CDs (TBIL: 1.53 ± 0.43 μmol/L, DBIL: 0.46 ± 0.05 μmol/L) was significantly descending in comparison to that of the model group (p < 0.01).
The increase of serum TBA is proportional to the degree of liver tissue damage. As shown in , compared with the control group (3.27 ± 1.18 μmol/L), the serum TBA content of mice in CCl4 group (5.08 ± 1.51 μmol/L) was significantly increased (p < 0.01). The serum TBA content in silybin + CCl4 group (3.39 ± 0.87 μmol/L) and CRC-CDS groups (H: 3.62 ± 0.75 μmol/L, M: 3.43 ± 1.02 μmol/L, L: 3.49 ± 0.94 μmol/L) were significantly lower than that of the CCl4 group (p < 0.05). The levels of TG in serum and liver are related to lipid metabolism, and elevated TG levels reflect lipid metabolism disorders. Compared with the control group (0.73 ± 0.14 mmol/L), the content of TG () in CCl4 group (1.12 ± 0.06 mmol/L) was significantly increased (p < 0.01). The level of TG in silybin + CCl4 group (0.76 ± 0.08 mmol/L) and CRC-CDs high, medium, and low dose + CCl4 groups (H: 0.77 ± 0.04 mmol/L, M: 0.75 ± 0.07 mmol/L, L: 0.78 ± 0.08 mmol/L) were significantly decreased in comparison to the model group (p < 0.01). In addition, compared with the blank control group, there was no significant difference in the serum levels of the above indexes in the CRC-CDs high-dose group, indicating that CRC-CDs had no significant effect on the liver function of normal mice. In addition, compared with the control group, there was no significant difference in the contents of the above indexes in the CRC-CDs high-dose group, indicating that CRC-CDs had no significant effect on the liver function of normal mice.
Effects of CRC-CDs on serum levels of TNF-α, IL-6 and IL-1β in mice with liver fibrosis
After certain injury, hepatocytes release many inflammatory factors, such as TNF-α, IL-6, IL-1β, which can further induce the occurrence and development of liver fibrosis. As shown in , the concentration of TNF-α, IL-6 and IL-1β in the model group (TNF-α: 83.25 ± 16.70 pg/mL, IL-6: 100.53 ± 7.63 pg/mL, IL-1β: 92.19 ± 8.74 pg/mL) was significantly upregulated (p < 0.01) in comparison to that of the control group (TNF-α: 43.99 ± 12.02 pg/mL, IL-6: 47.97 ± 2.31 pg/mL, IL-1β: 41.60 ± 4.06 pg/mL). Additionally, compared with that in the model group, the levels of TNF-α, IL-6 and IL-1β in the silybin + CCl4 group (TNF-α: 51.04 ± 7.58 pg/mL, IL-6: 54.37 ± 2.25 pg/mL, IL-1β: 47.91 ± 6.60 pg/mL), the high-dose (TNF-α: 53.11 ± 6.45 pg/mL, IL-6: 59.05 ± 3.42 pg/mL, IL-1β: 46.88 ± 3.49 pg/mL), medium-dose (TNF-α: 52.34 ± 5.91 pg/mL, IL-6: 60.34 ± 2.78 pg/mL, IL-1β: 44.59 ± 3.17 pg/mL) and low-dose (TNF-α: 57.11 ± 6.82 pg/mL, IL-6: 61.64 ± 1.39 pg/mL, IL-1β: 45.78 ± 4.55 pg/mL) CRC-CDs groups produced a significant reduction (p < 0.01). In addition, there were no significant differences in the above indicators between the blank group and the high-dose CRC-CDs group, indicating that the levels of TNF-α, IL-6 and IL-1β in the serum of normal mice were not significantly affected by CRC-CDs.
Figure 9. Effects of CRC-CDs on the contents of (A) TNF-α, (B) IL-6 and (C) IL-1β in serum of mice with carbon tetrachloride-induced liver fibrosis. Mice were assigned into seven groups, namely normal control group (NC), CCl4 group (CCl4), silybin + CCl4 group (silybin + CCl4), high dose of CRC-CDs + CCl4 group (CRC-CDs-H + CCl4, 9.33 mg/kg), medium dose of CRC-CDs + CCl4 group (CRC-CDs-M + CCl4, 4.67 mg/kg), low dose of CRC-CDs + CCl4 group (CRC-CDs-L + CCl4, 2.34 mg/kg) and high dose of CRC-CDs group (CRC-CDs-H, 9.33 mg/kg). data were expressed as means ± standard deviation (SD). ##p < 0.01 vs. normal control group, **p < 0.01 vs. CCl4 group.

Effect of CRC-CDs on the levels of SOD, GSH and MDA in liver tissue homogenate of mice with liver fibrosis
Changes in oxidative stress indicators were assessed by measuring the antioxidant levels (SOD and GSH) and the amount of damage (MDA) in the liver tissue (). The levels of SOD and GSH () in the model group (SOD: 174.73 ± 44.77 U/mg prot, GSH: 7.60 ± 1.86 μmol/g prot, p < 0.01) was significantly descending in comparison to that of the control group (SOD: 288.65 ± 69.66 U/mg prot, GSH: 17.25 ± 3.61 μmol/g prot). Meanwhile, the level of MDA () in the model group (4.98 ± 0.65 nmol/mg port, p < 0.01) was significantly higher than that in the control group (2.13 ± 0.50 nmol/mg port). Compared with model group, the contents of SOD (silybin: 242.15 ± 30.46 U/mg prot, CRC-CDs-H: 230.52 ± 37.42 U/mg prot, CRC-CDs-M: 259.76 ± 26.03 U/mg prot, CRC-CDs-L: 254.87 ± 62.44 U/mg prot) and GSH (silybin: 12.93 ± 2.06 μmol/g prot, CRC-CDs-H: 10.90 ± 2.17 μmol/g prot, CRC-CDs-M: 11.82 ± 3.84 μmol/g prot, CRC-CDs-L: 11.22 ± 2.48 μmol/g prot) in silybin + CCl4 group and CRC-CDs groups showed an upward trend (p < 0.01), whereas the level of MDA was markedly decreased in in silybin + CCl4 group (2.31 ± 0.28 nmol/mg prot, p < 0.01) and CRC-CDs groups (H: 2.69 ± 0.99 nmol/mg prot, M: 2.43 ± 0.76 nmol/mg prot, L: 2.41 ± 0.58 nmol/mg prot, p < 0.01). The experimental results show that CRC-CDs can alleviate the liver damage caused by excessive oxidative stress by improving the body’s antioxidant capacity. In addition, the above indexes were not significantly different between the control group and the high-dose CRC-CDs group, indicating that CRC-CDs had no significant effect on the contents of SOD, GSH and MDA in the liver tissue of normal mice.
Figure 10. Effects of CRC-CDs on the content of (A) SOD, (B) GSH, and (C) MDA in liver tissue homogenate of mice with carbon tetrachloride-induced liver fibrosis. Mice were assigned into seven groups, namely normal control group (NC), CCl4 group (CCl4), silybin + CCl4 group (silybin + CCl4), high dose of CRC-CDs + CCl4 group (CRC-CDs-H + CCl4, 9.33 mg/kg), medium dose of CRC-CDs + CCl4 group (CRC-CDs-M + CCl4, 4.67 mg/kg), low dose of CRC-CDs + CCl4 group (CRC-CDs-L + CCl4, 2.34 mg/kg) and high dose of CRC-CDs group (CRC-CDs-H, 9.33 mg/kg). data were expressed as means ± standard deviation (SD). #p < 0.05 and ##p < 0.01 vs. normal control group, *p < 0.05 and **p < 0.01 vs. CCl4 group.

Discussion
As a newly added carbon-based nanomaterial, CDs has been widely used in biomedical fields such as cell imaging, biosensing and targeted drug delivery due to its advantages of good biocompatibility, low toxicity, wide source of raw materials and low cost, and has attracted continuous attention from many researchers at home and abroad [Citation28–30]. Of note, Chinese herbal medicine is rich in a variety of active ingredients that make them a direct route to heteroatom [Citation31]. Based on these advantages, CDs with novel biological activity prepared from Chinese herbal medicine as precursor carbon source are worth developing to further expand their application in the field of biological activity.
Charcoal drugs, the processed products of Chinese herbal medicine, have extensive pharmacological effects after high temperature carbonization. However, the material basis is still unclear and controversial. High temperature carbonization is a key step in the processing of charcoal drugs, which is like the bottom-up pyrolysis of CDs. In our previous work, it was found that CDs are the active components for the different pharmacodynamic effects of charcoal drugs, and their biological activities were preliminarily proved. We have reported that CDs derived from Junci Medulla Carbonisata [Citation14] and Pollen Typhae Carbonisata [Citation32] have promising haemostatic bioactivity; CDs derived from Phellodendri Chinensis Cortex Carbonisata [Citation15] can regulate M1/M2 macrophage polarization to exert antipsoriatic effects; CDs derived from Crinis Carbonisatus [Citation33] have neuroprotective effect; CDs derived from Radix Sophorae Flavescentis Carbonisata [Citation34] have a role in the treatment of alcoholic gastric ulcers.
Liver fibrosis is a common consequence of the liver’s healing response to chronic injury and is characterized by excessive deposition of ECM [Citation35]. The main mediator of fibrosis is usually hepatic stellate cells, which can be activated and transdifferentiated into myofibroblasts in response to hepatic inflammatory response and cytokine stimulation, inducing secretion of ECM proteins [Citation36]. Over time, liver fibrosis will gradually develop into irreversible cirrhosis or even liver cancer. Therefore, it is expected to reverse or prevent the occurrence and development of liver fibrosis by eliminating pathogenic factors from various ways, improving the degree of liver injury, inhibiting inflammatory response, and degrading ECM. However, currently the drugs on the market for the treatment of liver fibrosis have some problems, such as not obvious clinical efficacy and many side effects. Therefore, it is urgent to develop a potential safe and effective anti-liver fibrosis drug. Since the characteristics and pathological responses of CCl4-induced hepatic fibrosis are partially like those of human hepatic fibrosis, it is the most used chemical in animal models of hepatic fibrosis [Citation37].
Based on the revelation of previous studies, with CR as the only biomass carbon source, we synthesized green-friendly CRC-CDs by one-step high-temperature pyrolysis method. XPS and FTIR spectra were used to confirm that CRC-CDs had abundant hydrophilic groups on the surface, such as hydroxyl, amino, and carboxyl groups, corresponding to good water solubility. At the same time, the CCK-8 experiment demonstrated the low toxicity advantage of CRC-CDs. Then we further explore the inner biological activity.
Liver damage will lead to changes in liver index, which can be calculated to preliminarily judge the nature and degree of visceral organ lesions [Citation38]. In this study, the liver index of mice in the CCl4 group was significantly increased, whereas the intervention of CRC-CDs effectively alleviated the increase of liver index in mice with liver fibrosis, indicating that CRC-CDs had a significant improvement effect on liver lesions caused by liver fibrosis. In addition, it can be seen from the liver pathological sections that the liver tissue of mice in the CCl4 group was severely damaged, and obvious hyperplastic collagen fibres were seen in the portal area of liver tissue and around the central vein. After treatment with CRC-CDs, the inflammatory lesions and degeneration necrosis of hepatocytes were significantly improved, and the proliferation of collagen fibres was alleviated, indicating that CRC-CDs had an obvious inhibitory effect on liver fibrosis. Further analysis found that the levels of ALT, AST, TBA, TBIL, DBIL and TG in the serum of mice in the CCl4 group were significantly increased, indicating that the liver of the mice was severely damaged. After treatment with CRC-CDs, the contents of above indexes in serum decreased significantly, indicating that CRC-CDs has the role of protecting liver and anti-liver fibrosis. This result was consistent with the pathological observation.
Inflammatory reaction plays a crucial role in liver fibrosis. Inflammatory cytokines such as TNF-α, IL-6, and IL-1β are involved in and promote the process of liver fibrosis, which are used to characterize the degree of liver fibrosis. As a cytokine released by mononuclear macrophages during CCl4-induced liver fibrosis, TNF-α can stimulate mesangial cells to express cytokines such as adhesion molecules and vasoactive peptides, and induce the proliferation of fibroblasts and promote the deposition of collagen fibres [Citation39]. Il-1β and IL-6 are also important proinflammatory cytokines in the body, which can induce the synthesis of acute phase response proteins in hepatocytes and mediate hepatocyte injury. In this study, the levels of TNF-α, IL-6 and IL-1β in serum of mice in the CCl4 group were significantly increased, which could be reversed after treatment with CRC-CDs, suggesting that CRC-CDs could inhibit the secretion of inflammatory factors such as TNF-α, IL-6 and IL-1β to improve the degree of liver fibrosis.
Oxidative stress is one of the main pathological mechanisms in the progression of liver fibrosis, and its elevated level is a hallmark of the existence and development of liver fibrosis [Citation40]. After being metabolized by the cytochrome P450 enzyme in the liver, CCl4 will generate derived free radicals, which can irreversibly bind to biological macromolecules such as proteins and lipids and trigger lipid peroxidation, thereby damaging the structure and function of hepatocytes and further leading to liver fibrosis [Citation41]. Antioxidant enzymes such as SOD and GSH are scavengers of free radicals in the liver and are responsible for the first line of defence against endogenous oxidative damage. Antioxidative enzymes such as SOD and GSH are responsible for the first line of defence against endogenous oxidative damage [Citation42]. In this study, CRC-CDS can significantly down-regulate the content of MDA in mice with CCl4-induced liver fibrosis, and contribute to the recovery of SOD and GSH activities, which indicates that CRC-CDS can reduce the oxidative stress response in the process of liver fibrosis by improving the body’s antioxidant capacity. In terms of experimental results, CRC-CDs had a certain inhibitory effect on CCl4-induced liver fibrosis mouse models, among which the treatment effect was best at medium doses. The reason for this phenomenon may be that the CRC-CDs of nanoscale particles are unstable colloidal solutions, and in the case of high concentrations, the precipitation phenomenon will occur, which will reduce the efficacy.
Conclusion
In summary, CRC-CDs with a diameter ranging from 1.0-4.5 nm mainly exhibited inhibitory effects against liver fibrosis induced by CCl4 by diminishing the abnormal increase of liver function index including ALT, AST, TBIL, DBIL, TBA and TG, alleviating the levels of inflammation cytokines, and reducing the levels of oxidative stress, which lays an important foundation for the development of CRC-CDs as a new drug for the treatment of liver fibrosis, and provide a certain experimental basis for the clinical application of CRC-CDs in the future.
Ethical approval
The animal protocols were approved by the Committee of Ethics of Animal Experimentation of the Beijing University of Chinese Medicine (BUCM-4-2021052802-2044).
Author contributions
YZ and HQ designed the study. YZ, HK and YL performed the research. YZ, HK and YZ analyzed the data. YZ wrote the manuscript, which was revised by YL. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. YZ and HQ confirm the authenticity of all the raw data.
Disclosure statement
No potential conflict of interest was reported by the author(s)
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875. doi: 10.3390/cells9040875.
- Khatun M, Ray RB. Mechanisms underlying hepatitis C virus-associated hepatic fibrosis. Cells. 2019;8(10):1249. doi: 10.3390/cells8101249.
- Li H. Advances in anti hepatic fibrotic therapy with traditional Chinese medicine herbal formula. J Ethnopharmacol. 2020;251:112442. doi: 10.1016/j.jep.2019.112442.
- Mu M, Zuo S, Wu RM, et al. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/smad signaling pathway. Drug Des Devel Ther. 2019;13:1819–4115. doi: 10.2147/DDDT.S186726.
- Wang P, Cui Y, Wang J, et al. Mesenchymal stem cells protect against acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res Ther. 2022;13(1):94. doi: 10.1186/s13287-022-02754-x.
- Biolato M, Bianco A, Lucchini M, et al. The disease-modifying therapies of relapsing-remitting multiple sclerosis and liver injury: a narrative review. CNS Drugs. 2021;35(8):861–880. doi: 10.1007/s40263-021-00842-9.
- Liu H, Lv X, Qian J, et al. Graphitic carbon nitride quantum dots embedded in carbon nanosheets for near-infrared imaging-guided combined photo-chemotherapy. ACS Nano. 2020;14(10):13304–13315. doi: 10.1021/acsnano.0c05143.
- Zhao N, Wang Y, Hou S, et al. Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Mikrochim Acta. 2020;187(6):351. doi: 10.1007/s00604-020-04328-1.
- Wang C, Guan W, Peng J, et al. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020;103:247–258. doi: 10.1016/j.actbio.2019.12.015.
- Tiron A, Stan CS, Luta G, et al. Manganese-doped N-hydroxyphthalimide-derived carbon dots-theranostics applications in experimental breast cancer models. Pharmaceutics. 2021;13(11):1982. doi: 10.3390/pharmaceutics13111982.
- Tejwan N, Saha SK, Das J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv Colloid Interface Sci. 2020;275:102046. doi: 10.1016/j.cis.2019.102046.
- Zhao P, Jin B, Zhang Q, et al. High-quality carbon nitride quantum dots on photoluminescence: effect of carbon sources. Langmuir. 2021;37(5):1760–1767. doi: 10.1021/acs.langmuir.0c02966.
- Cui F, Sun J, Ji J, et al. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J Hazard Mater. 2021;406:124330. doi: 10.1016/j.jhazmat.2020.124330.
- Cheng J, Zhang M, Sun Z, et al. Hemostatic and hepatoprotective bioactivity of Junci medulla carbonisata-derived carbon dots. Nanomedicine. 2019;14(4):431–446. doi: 10.2217/nnm-2018-0285.
- Zhang M, Cheng J, Hu J, et al. Green phellodendri chinensis cortex-based carbon dots for ameliorating imiquimod-induced psoriasis-like inflammation in mice. J Nanobiotechnology. 2021;19(1):105. doi: 10.1186/s12951-021-00847-y.
- Zhao Y, Zhang Y, Kong H, et al. Protective effects of carbon dots derived from armeniacae semen Amarum carbonisata against acute lung injury induced by lipopolysaccharides in rats. Int J Nanomedicine. 2022;17:1–14. doi: 10.2147/IJN.S338886.
- Sun Z, Lu F, Cheng J, et al. Hypoglycemic bioactivity of novel eco-friendly carbon dots derived from traditional Chinese medicine. J Biomed Nanotechnol. 2018;14(12):2146–2155. doi: 10.1166/jbn.2018.2653.
- Bajpai VK, Khan I, Shukla S, et al. Multifunctional N-P-doped carbon dots for regulation of apoptosis and autophagy in B16F10 melanoma cancer cells and in vitro imaging applications. Theranostics. 2020;10(17):7841–7856. doi: 10.7150/thno.42291.
- Wang H, Zhang M, Ma Y, et al. Selective inactivation of gram-negative bacteria by carbon dots derived from natural biomass: Artemisia argyi leaves. J Mater Chem B. 2020;8(13):2666–2672. doi: 10.1039/c9tb02735a.
- Tong T, Hu H, Zhou J, et al. Glycyrrhizic-Acid-Based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16(13):e1906206. doi: 10.1002/smll.201906206.
- Liu X, Liu W, Ding C, et al. Taxifolin, extracted from waste Larix olgensis roots, attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR and TGF-β1/smads signaling pathways. Drug Des Devel Ther. 2021;15:871–887. doi: 10.2147/DDDT.S281369.
- Xin Q, Shah H, Xie W, et al. Preparation of blue- and green-emissive nitrogen-doped graphene quantum dots from graphite and their application in bioimaging. Mater Sci Eng C Mater Biol Appl. 2021;119:111642. doi: 10.1016/j.msec.2020.111642.
- Jahanbakhshi M, Habibi B. A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: application to electroanalytical determination of H2O2 in fetal bovine serum. Biosens Bioelectron. 2016;81:143–150. doi: 10.1016/j.bios.2016.02.064.
- Wang T, Luo H, Jing X, et al. Synthesis of fluorescent carbon dots and their application in ascorbic acid detection. Molecules. 2021;26(5):1246. doi: 10.3390/molecules26051246.
- Manchala S, Gandamalla A, Vempuluru NR, et al. High potential and robust ternary LaFeO3/CdS/carbon quantum dots nanocomposite for photocatalytic H2 evolution under sunlight illumination. J Colloid Interface Sci. 2021;583:255–266. doi: 10.1016/j.jcis.2020.08.125.
- Chandrasekaran P, Arul V, Sethuraman MG. Ecofriendly synthesis of fluorescent nitrogen-doped carbon dots from Coccinia grandis and its efficient catalytic application in the reduction of methyl orange. J Fluoresc. 2020;30(1):103–112. doi: 10.1007/s10895-019-02474-1.
- Hoseini AA, Farhadi S, Zabardasti A, et al. A novel n-type CdS nanorods/p-type LaFeO3 heterojunction nanocomposite with enhanced visible-light photocatalytic performance. RSC Adv. 2019;9(42):24489–24504. doi: 10.1039/c9ra04265b.
- Mishra V, Patil A, Thakur S, et al. Carbon dots: emerging theranostic nanoarchitectures. Drug Discov Today. 2018;23(6):1219–1232. doi: 10.1016/j.drudis.2018.01.006.
- Sri S, Kumar R, Panda AK, et al. Highly biocompatible, fluorescence, and zwitterionic carbon dots as a novel approach for bioimaging applications in cancerous cells. ACS Appl Mater Interfaces. 2018;10(44):37835–37845. doi: 10.1021/acsami.8b13217.
- Luo WK, Zhang LL, Yang ZY, et al. Herbal medicine derived carbon dots: synthesis and applications in therapeutics, bioimaging and sensing. J Nanobiotechnology. 2021;19(1):320. doi: 10.1186/s12951-021-01072-3.
- Li D, Xu KY, Zhao WP, et al. Chinese medicinal herb-derived carbon dots for common diseases: efficacies and potential mechanisms. Front Pharmacol. 2022;13:815479. doi: 10.3389/fphar.2022.815479.
- Yan X, Zhao Y, Luo J, et al. Hemostatic bioactivity of novel pollen Typhae carbonisata-derived carbon quantum dots. J Nanobiotechnology. 2017;15(1):60. doi: 10.1186/s12951-017-0296-z.
- Zhang Y, Wang S, Lu F, et al. The neuroprotective effect of pretreatment with carbon dots from Crinis carbonisatus (carbonized human hair) against cerebral ischemia reperfusion injury. J Nanobiotechnology. 2021;19(1):257. doi: 10.1186/s12951-021-00908-2.
- Hu J, Luo J, Zhang M, et al. Protective effects of radix Sophorae flavescentis carbonisata-based carbon dots against ethanol-induced acute gastric ulcer in rats: anti-Inflammatory and antioxidant activities. Int J Nanomedicine. 2021;16:2461–2475. doi: 10.2147/IJN.S289515.
- Elpek GO. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol. 2014;20(23):7260–7276. doi: 10.3748/wjg.v20.i23.7260.
- Zhang CY, Yuan WG, He P, et al. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22(48):10512–10522. doi: 10.3748/wjg.v22.i48.10512.
- Shrestha N, Chand L, Han MK, et al. Glutamine inhibits CCl4 induced liver fibrosis in mice and TGF-β1 mediated epithelial-mesenchymal transition in mouse hepatocytes. Food Chem Toxicol. 2016;93:129–137. doi: 10.1016/j.fct.2016.04.024.
- Tanriverdi G, Kaya-Dagistanli F, Ayla S, et al. Resveratrol can prevent CCl4-induced liver injury by inhibiting notch signaling pathway. Histol Histopathol. 2016;31(7):769–784. doi: 10.14670/HH-11-720.
- Sun H, Chen G, Wen B, et al. Oligo-peptide I-C-F-6 inhibits hepatic stellate cell activation and ameliorates CCl4-induced liver fibrosis by suppressing NF-κB signaling and wnt/β-catenin signaling. J Pharmacol Sci. 2018;136(3):133–141. doi: 10.1016/j.jphs.2018.01.003.
- Yang JH, Kim SC, Kim KM, et al. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-β/smad signaling and relieving oxidative stress. Eur J Pharmacol. 2016;783:92–102. doi: 10.1016/j.ejphar.2016.04.042.
- Zhang X, Kuang G, Wan J, et al. Salidroside protects mice against CCl4-induced acute liver injury via down-regulating CYP2E1 expression and inhibiting NLRP3 inflammasome activation. Int Immunopharmacol. 2020;85:106662. doi: 10.1016/j.intimp.2020.106662.
- Liu W, Wang Z, Hou JG, et al. The liver protection effects of maltol, a flavoring agent, on carbon tetrachloride-Induced acute liver injury in mice via inhibiting apoptosis and inflammatory response. Molecules. 2018;23(9):2120. doi: 10.3390/molecules23092120.



