?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Radiotherapy (RT) is a highly valuable method in cancer therapy, but its therapeutic efficacy is limited by its side effects and tumour radiation resistance. The resistance is mainly induced by hypoxia in the tumour microenvironment (TME). As a nano-oxygen carrier, Haemoglobin-based oxygen carriers (HBOCs) administration is a promising strategy to alleviate tumour hypoxia which may remodel TME to ameliorate radiation resistance and enable RT more effective. In this study, we administered fractionated RT combined with HBOC to treat Miapaca-2 cell and Hela cell xenografts on nude mice. The study found that HBOC relieved hypoxic environment and down-regulate expression of hypoxia-inducible factor-1α (Hif-1α) both in regular (100 mm3) and large (360/400 mm3) tumours. The proliferation and metastasis of tumour tissue also decreased after HBOC application. Nevertheless, in vivo RT combined with HBOC performed more effectively to suppress tumour growth in large tumours than in regular tumours. This is due to more severe hypoxic regions exist in the large solid tumours compared to the regular counterparts, and HBOC administration may be more effective in alleviating hypoxia in large tumours. Thus, HBOC sensitization therapy is more suitable for large solid tumours.
Introduction
Radiotherapy plays a crucial part in the treatment of malignancies. Previous studies have shown that 70% of malignant tumour patients need radiotherapy at different phases of disease progression [Citation1]. For advanced or recurrence malignant tumours, radiotherapy is also one of the most effective treatment measures to alleviate the clinical symptoms, prolong the survival and improve the quality of life of patients [Citation2]. Despite its wide use, RT is not effective for all tumours, and its clinical use is compromised so far by limited efficacy and side effects [Citation3]. The vascular, stromal, and immunological changes in TME induced by hypoxia lead to the promotion of radioresistance and tumour recurrence [Citation4,Citation5]. Hypoxia is a common feature of solid tumours. It results from the insufficient oxygen supply brought by rapid proliferation and expansion of tumour mass, and it is considered as one of the most critical causes of radiotherapy failure [Citation6].
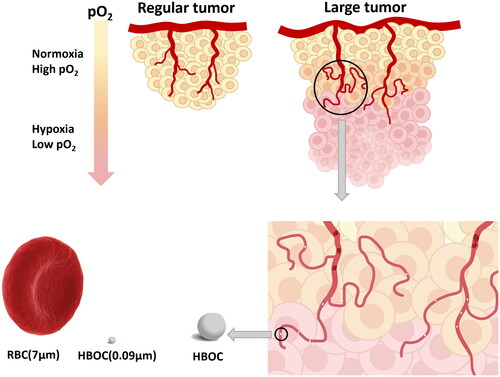
To solve this problem, numerous therapeutic strategies have been developed to overcome hypoxia-induced radioresistance in the past six decades, including improving tumour oxygenation by hyperbaric oxygen, oxygen-mimetic radiosensitizers, and hypoxia-selective cytotoxins [Citation7–9]. The use of HBOCs is one of the most promising treatments to raise the intra-tumoral oxygen level. HBOCs usually involve chemically cross-linked acellular haemoglobin and are capable of transporting oxygen [Citation10]. It is a kind of nanoscale particle (about 30 nm) which could enter into the disordered blood vessels and penetrate deep into solid tumours that red blood cells (RBCs) couldn’t easily access [Citation11,Citation12].
It is reported that radiotherapy or chemotherapy combined with HBOCs therapy increased tissue oxygenation in tumour [Citation13,Citation14]. Currently several research groups have applied HBOCs to chemoradiotherapy sensitization of oncology. And most studies demonstrated that increasing tumour oxygenation can inhibit tumour growth [Citation15]. While there were still some researches showing that HBOCs cannot inhibit tumour growth, these studies have not obtained a consistent effect on HBOCs [Citation13,Citation16]. Numerous reasons could lead to the above different experimental results, such as different types of tumours and HBOC administration methods, different fractionated radiotherapy schemes, etc.
At an early stage, our research group has administered HBOC in combination with chemotherapy and radiotherapy to a variety of xenograft tumours on nude mice. In the course of the experiment, we found that HBOC was not effective for all tumours [Citation17,Citation18]. We speculated that the phase (size) of solid tumours was the most predominant factor for previous inconsistent results. It is generally acknowledged that the deeper inside the solid tumour, the more severe the hypoxic condition is [Citation19]. Compared with the regular solid tumours, the larger tumours are more anoxic and need molecules that can penetrate the deeper part of the tumour for oxygen supply (). As mentioned above, RBC could hardly access interior of tumour tissue with vascular disorders to deliver O2 while HBOC could permeate deeply into tumour interstitium to supply O2. We reviewed the relevant literature and found that currently there are no researches investigating the effects of HBOC on solid tumours in different phases. In this study, we used different volume of tumours to simulate different phases of solid tumours. We verified this hypothesis by using two kinds of animal models. Additionally, this research also aims to further explore the underlying mechanism of how HBOC enhances radiotherapy in tumour-bearing mice.
Materials and methods
Reagents and instruments
HBOC used in this study was acquired by Redpharm (Beijing) Biotechnology Co. Ltd. (Beijing, China). The product was kept at 2–30 °C until use. The concentration of HBOC (200 kDa) solution was maintained at 6.5 g/dL which owns a relatively lower O2 affinity (P50 = 40 mmHg). Small animal PXI irradiator (X-RAD 320, PRECISION, USA) was used for mice irradiation. The CC3 (Servicebio, GB11532), Hif-1α (BIOSS, bs-0737R), Ki67 (Abcam, ab16667), CD31 (Abcam, ab281583) antibodies were used for immunohistochemical staining. β-actin antibody, anti-mouse IgG, anti-rabbit IgG, BCA protein assay kit, RIPA lysis buffer, and protease inhibitor cocktail were acquired from Beyotime Institute of Biotechnology (Jiangsu, China). Hif-1α antibody used in Western Blot was purchased from Proteintech Group, Inc. (Wuhan, China). The hypoxic cell probe Pimonidazole hydrochloride (Hypoxyprobe™ Green kit) was purchased from Hypoxyprobe (Burlington, MA, USA). The DHE dye liquor used for ROS colouration was acquired from Sigma-Aldrich (USA). Dulbecco’s modified Eagle’s medium (DMEM) and penicillin/streptomycin (100 U/mL) were purchased from Cytiva Company (USA), fetal bovine serum (FBS) was purchased from Biological Industries company (Israel). 0.25% trypsin-EDTA was acquired from Biosharp (Beijing, China). All other chemicals and solvents were of analytical grade.
Cell culture
Miapaca-2 cell line and Hela cell line were purchased from Procell Life Science & Technology Co. Ltd. (Wuhan, China). The cell line was maintained and propagated, respectively in DMEM (Miapaca-2 cell) and MEM (Hela cell) supplemented with 10% FBS and 1% 100 U/ml penicillin/streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. Adherent cells were passaged with 0.25% trypsin-EDTA when they reached 80–90% confluency.
Establishment of xenograft tumour model
Female athymic BALB/c nude mice, 5 weeks old, weighing 16 ± 1 g were used for this study. At the start of the study, mice were inoculated subcutaneously with 5 × 106 Miapaca-2 cells suspended in 0.2 ml DMEM into the right armpit of body. The experiment was initiated when the tumours, respectively reached a volume of ∼100 mm3 (regular) and 360 mm3 (large). For Hela cell tumour-bearing model, 2 × 106 Hela cells suspended in 0.2 ml DMEM containing 50% Matrigel Matrix (Corning, USA) were injected subcutaneously into the right flank of mice. When the tumour volume reached ∼100 mm3, the tumour was peeled off from the mice and then cut into small pieces about 1 mm3. Then the pellets were inoculated subcutaneously into the right flank of nude mice with an inoculum needle. The nude mice bearing Hela cell tumour began receiving treatment once tumour volume reached ∼100 mm3 (regular) and 400 mm3 (large). All the mice were maintained in specific pathogen-free (SPF) conditions in accordance with state guidelines for humane treatment under 12/12 h light/dark cycles. The experimental mice received food and water ad libitum. All experimental protocols were approved by the Ethics Committee of the Institution of Blood Transfusion, Chinese Academy of Medical Sciences.
In vivo tumour suppression experiments
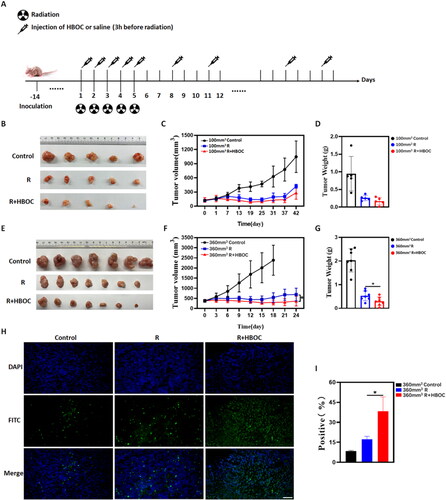
The whole experiment had abided by Institutional Guidelines and Protocols. For the Miapaca-2 cell model, when the tumour volume reached ∼100 and 360 mm3, mice were respectively divided into two groups (100 and 360 mm3). The above two groups were randomly assigned into three independent subgroups to receive treatment. The subgroups were as follows: control, irradiation-only (R), and irradiation-HBOC combination (R + HBOC). As shown in , the local irradiation was administered at a dose of 3 Gy for five consecutive days. Additionally, 600 mg/kg HBOC was administered on R + HBOC group through the tail vein 3 h before X-ray irradiation (R group was injected with saline solution). After 5 days of radiotherapy, the HBOC or saline injection was maintained three times a week for 5 weeks (100 mm3) or 3 weeks (360 mm3).
Figure 2. Tumour suppression experiment of Miacapa-2 cell model. (A) Schematic illustration of experimental scheme for the Miacapa-2 cell tumour model. (B) The photographs of tumours, (C) tumour volume curves of mice treated with various treatments, and (D) tumour weight of mice in different groups at the end of experiment of 100 mm3 group (n = 5). (E) The photographs of tumours, (F) tumour volume curves of mice treated with various treatments, and (G) tumour weights of mice in different groups at the end of experiment of 360 mm3 group (n = 7). (H) Representative fluorescence images and (I) AOD of tumour slices (360 mm3) stained by TUNEL (n = 3). Scale bar = 50 μm. Data between two groups was compared by students t-test. *p < 0.05.
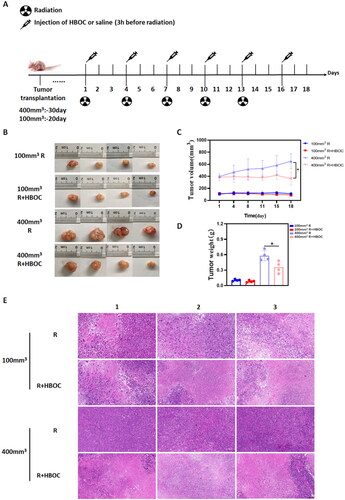
For the Hela cell tumour model, we did not consider the control group (without any treatment) in the experimental scheme for the purpose of minimizing the number of experimental animals. When the tumour volume reached ∼100 and 400 mm3, 16 mice were assigned into four groups: 100 mm3 irradiation-only (100 mm3 R), 100 mm3 irradiation + HBOC (100 mm3 R + HBOC), 400 mm3 irradiation-only (400 mm3 R), 400 mm3 irradiation + HBOC (400 mm3 R + HBOC). Radiation of 3 Gy was administered on the above four groups every 3 days, five times in total (). The volume of the xenograft was measured with a caliper every 3 days. On the last day of treatment, the tumours were removed and weighed. The tumour volume was calculated with the following formula:
(1)
(1)
Figure 3. Tumour suppression experiment of Hela cell tumour model. (A) Schematic illustration of experiment scheme of Hela cell tumour model. (B) The photographs of tumours, (C) tumour volume curves, and (D) tumour weight of mice in different groups at the end of experiment (n = 4). (E) Representative images of histological slices from different groups (n = 3). Scale bar = 100 μm. Data between two groups was compared by student’s t-test. *p < 0.05.
Immunohistochemistry (IHC)
On the last day of therapy, tumours were removed from the mice and fixed in 10% formalin, and embedded in paraffin for IHC analysis. The tissues were cut into slices of 3 μm. Then deparaffinize and rehydrate the slices with a graded series of ethanol following the standard protocol. Subsequently, the sections were stained with antibodies targeting Hif-1α (1:200), CD31 (1:2000), ki67 (1:100), and CC3 (1:600). The expression of the above indicators was quantified by densitometric analysis using Image-Pro Plus 6.0 software. Firstly, the three random fields of vision of every sample were captured by CaseViewer. Then the brown portion in these visions was taken as the positive signal of all samples. The average optical density (AOD) value was calculated by EquationEquation (2)(2)
(2) :
(2)
(2)
IOD: integrated optical density
AREA: the pixel area of each photograph.
TUNEL
The above 3 μm sections were used for terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) staining. The apoptosis cells in the tumours were measured by TUNEL using the Fluorescein (FITC) Tunel Kit (Servicebio, Wuhan, China), following the manufacturer’s protocol. Similar to the IHC analysis, the CaseViewer software was used to randomly capture three 400-fold pictures, trying to fill the entire field of view with tissue and keeping the background light consistent. The Image-Pro Plus 6.0 software was used to quantify the apoptotic cells. Taking the green fluorescence as a positive signal for apoptotic cells, and blue fluorescence as a signal for total cells. Calculate the apoptotic cell rate with EquationEquation (3)(3)
(3) :
(3)
(3)
Histological examination
The tumour tissue was immediately fixed with 10% neutralized formalin after removed from mice. The immobilized tissue was then dehydrated and embedded in a paraffin layer. Subsequently, the tissue was then cut into 3 μm slices, dehydrated again, and stained with haematoxylin as well as eosin. The sections were observed under an optical microscope.
Western blot
Western blotting was used to determine the level of Hif-1α in Hela cell tumour. The tissue samples were lysed in RIPA lysis buffer with a mixture of protease inhibitors (Beyotime, Shanghai, China). The extracted proteins were separated by 6% Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred into polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk followed by incubation with the following antibodies against Hif-1α and β-actin. Afterwards, a SuperSignal West Pico chemiluminescence ECL kit (Amersham Pharmacia Biotech, UK) was used to develop the PVDF film. The photographs of WB were captured through an imager (SH-compact523, Hangzhou Shenhua Technology Co., Ltd.) and the software named SHST Capture. Quantization of proteins was realized by ImageJ software (National Institutes of Health, Bethesda, MA, USA).
Tumour hypoxia
Hypoxyprobe™ Green Kit was used for the detection of hypoxic cells. Mice were injected with 60 mg/kg of pimonidazole hydrochloride intraperitoneally 0.5 h before tumour collection. Tissue required to be cryopreserved as quickly as possible after it was isolated from the body, avoiding the loss of nitro-reduction products. Then the Frozen tumour tissue sections were incubated with Hypoxyprobe Mab1 and biotin-conjugated F(ab′)2 successively. The sections were observed with a fluorescence microscope and the green light was taken as a positive signal for hypoxic cells. Hypoxia degree of each sample was quantitatively evaluated by CaseViewer software and Image-Pro Plus 6.0 software (same as TUNEL statistical analysis). EquationEquation (2)(2)
(2) is used to calculate the AOD value.
Reactive oxygen species (ROS)
Frozen sections of tumour tissue were rewarmed at room temperature. Spontaneous fluorescence quenching agent was added to the sections for 5 min and washed the sections with running water. Subsequently, drop ROS dye solution on the sections and incubate them in the dark at 37 °C for 30 min, washing with PBS for three times. The DAPI dye solution was then added and kept the sections in the dark at room temperature for 10 min, repeating the cleaning steps described above. The final operation was to seal them with an anti-fluorescence quencher and observed under a fluorescence microscope. The red light was taken as a positive signal for ROS. CaseViewer software and Image-Pro Plus 6.0 software were used to quantify the ROS content. EquationEquation (2)(2)
(2) is used to calculate the AOD value.
Statistical analysis
All results are presented as the mean ± standard deviation (SD). Statistical analysis was performed using the software GraphPad Prism 8.0.2. Data from different treatment groups was compared by One-way ANOVA and Student’s t-test, p-value < 0.05 was considered statistically significant.
Results
Antitumor efficacy of radiation-HBOC combination therapy in vivo
The changes in tumour size for mice are shown in and . For the Miapaca-2 cell xenograft, tumour growth rate in the control group was the fastest. Starting at day 6–8 of therapy, the average tumour volumes of R and R + HBOC groups were significantly smaller compared to the vehicle control (). Although the tumour volume in R + HBOC group was smaller than that in R group, there is no statistically difference between R + HBOC and R group in 100 mm3 tumour model (). Starting at day 18 of the whole experiment, the R + HBOC group had statistically smaller average tumour volume compared to the R group in 360 mm3 tumour model (). More apoptotic cells were induced in R + HBOC group examined by TUNEL in 360 mm3 tumour model (). The densitometric analysis of the raw data for TUNEL is shown in Table S1.
For Hela cell xenograft, HBOC administration slowed tumour growth significantly in 400 mm3 tumour model (). The pathological sections indicated that more necrotic regions were observed in 400 mm3 R + HBOC group compared to 400 mm3 R group. And there were more nuclear swelled cancer cells and pathological mitosis in 400 mm3 R group (). While we only observed subtle pathological variation in 100 mm3 tumour model after HBOC administration. The pathological results suggested a more evident histological changes after HBOC administration in 400 mm3 group rather than 100 mm3 group.
Hypoxia alleviation effect of radiation-HBOC combination therapy
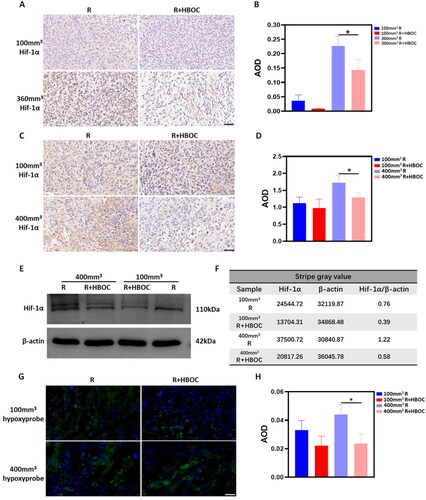
As a sensitive hypoxia indicator, the expression of Hif-1α reflects the degree of hypoxia inside the tumour tissue. According to the IHC results, HBOC administration did improve the oxygen supply inside the Miacapa-2 cell tumour (). The AOD statistical results in showed the expression of Hif-1α was evidently decreased after HBOC administration both in 100 and 360 mm3 tumour model. But only the difference in 360 mm3 tumour mice was statistically significant. The IHC results of Hif-1α expression trend in Hela cell tumour tissue was similar to Miacapa-2 cell xenograft (). The difference between the R group and R + HBOC group was no statistical significance in regular tumour model. The semi-quantitative results of WB was consistent with the above IHC trend of Hif-1α (): The expression of Hif-1α in the 100 mm3R + HBOC and 400 mm3 R + HBOC groups was lower than its corresponding groups (the raw images were shown in Figure S1). But its expression in 400 mm3 R + HBOC group decreased more significantly. The hypoxyprobe results showed the number of hypoxic cells in R + HBOC groups was less than that in R groups (). These experimental results universally suggested that HBOC decreased the number of hypoxic cells in both 100 and 400 mm3 tumour models, enhancing the oxygen supply. It can be proved that HBOC alleviated the anoxic status of tumour tissue primarily in large tumours. All the densitometric analyses mentioned above are shown in Tables S2–S4.
Figure 4. Hypoxia alleviation effect of HBOC in tumour-bearing mice. IHC staining of Hif-1α in (A) Miapaca-2 cell tumour model and (C) Hela cell tumour model. The AOD analysis of (B) Miapaca-2 cell tumour model and (D) Hela cell tumour model. (E) WB and (F) its stripe gray ratio of Hif-1α in the regular Hela tumour model (100 mm3) and large tumour model (400 mm3). (G) Immunofluorescence staining of hypoxyprobe and (H) AOD result in the Hela tumour model. Representative images of each group were shown. Scale bar = 50 μm. Data between two groups was compared by student’s t-test (n = 3). *p < 0.05.
HBOC reduced tumour metastasis and invasion in vivo
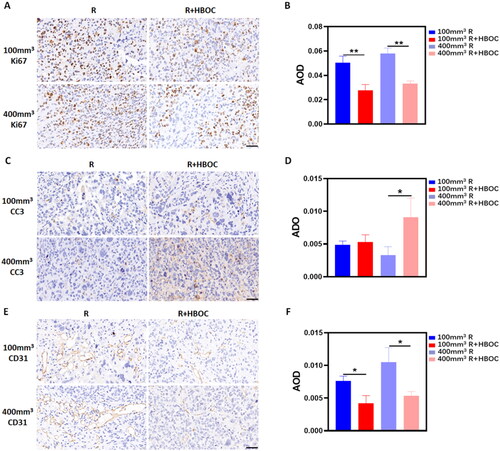
To investigate the metastasis and invasion of cancer cells in vivo, slices of Hela cell tumour tissue were stained with CC3, Ki67, and CD31 antibodies. As shown in , the Ki67 expression in R groups was slightly higher compared to R + HBOC groups. The densitometric analysis showed that the Ki67 expression decreased after HBOC administration (). Additionally, the CC3 expression increased in R + HBOC group compared with R group in 400 mm3 Hela cell tumour model (), and the difference was statistically significant (). The vascular endotheliogenesis marker of CD31 showed that its expression in R groups was higher than that in R + HBOC groups whether in 100 or 400 mm3 tumour models (). All the densitometric analyses mentioned above are presented in Tables S5–S7.
Figure 5. Tumour metastasis and invasion in vivo. (A) IHC staining of Ki67 and (B) AOD analysis in Hela tumour model. (C) IHC staining of CC3 and (D) AOD analysis in Hela tumour model. (E) IHC staining of CD31 and (F) AOD in analysis in Hela tumour model. Representative images of each group were shown. Scale bar = 50 μm. Data between two groups was compared by student’s t-test (n = 3). *p < 0.05. **p < 0.01.
ROS quantification in vivo
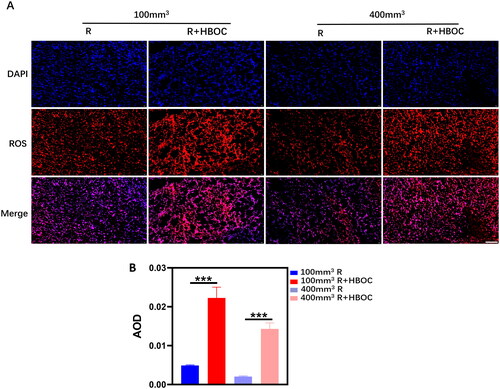
The blue DAPI represents the nucleus and the red signal represents ROS in tissue slices. HBOC resulted in a significantly increased expression of ROS both in 400 and 100 mm3 tumours (). The statistical results showed that the ROS content in group R of 400 mm3 tumours is statistically lower than that of 100 mm3 tumours (). The densitometric analyses mentioned above are presented in Table S8.
Discussion
RT could suppress the tumour growth which is one of the most common treatments for unresectable tumours [Citation20–22]. However, the severe complications associated with RT would reduce the quality of patients’ life and induce the risk of recurrence. The main issue of RT is that it fails to alleviate the problem of tumour hypoxia, leading to a series of subsequent events [Citation23,Citation24]. In this study, regular and large xenograft tumour models were used to simulate the early and advanced tumours. And simultaneously HBOC was applied to radiotherapy to enhance its sensitivity during the treatment process.
At the beginning of the whole study, we found that HBOC couldn’t augment the efficacy of RT apparently to restrain the growth in 100 mm3 Miacapa-2 cell tumour model (the regular tumour model). The experimental result was inconsistent with the most previous study () [Citation14,Citation25]. Afterwards, we still use the same fractionated RT therapy to treat 360 mm3 Miacapa-2 cell tumour mice (the larger model). Interestingly, the results differed from the 100 mm3 tumour model. As shown in , the tumour volume in group R + HBOC was obviously smaller than that in group R, and the difference between the two groups was statistically significant. The tumour growth curve in , tumour weight in and more apoptotic cells in group R + HBOC () correlatively confirmed that HBOC sensitization therapy was more effective in large tumours. The above experimental results indicated HBOC enhanced the sensitivity of the 360 mm3 tumour model more effectively compared to 100 mm3 tumour. We speculated that the primary reason for this result was the degree of hypoxia in solid tumours. With the progression of tumour, its volume and its interior become increasingly hypoxic. Later, we used the Hela cell xenograft to verify this conjecture. We found that HBOC did have a better ability to inhibit tumour growth on large tumours (). Tumour weight measured at the end of different treatments and images of the tumours confirmed this result again (). The pathological results suggested more obvious pathological changes between 400 mm3 R + HBOC and 400 mm3 R group (). This indicated that HBOC did exert more radiosensitization efficacy on large tumours and HBOC was probably more appropriate for large solid tumours.
To investigate whether HBOC can better alleviate hypoxia in large tumours, we measured the hypoxic cells and expression of Hif-1α in tumour tissue. As shown in , the downregulation of Hif-1α and reduction of hypoxic cells proved the capacity of HBOC in improving oxygen supply. In summary, HBOC is remarkably more effective in alleviating hypoxia in advanced solid tumours (400 mm3) in this experimental study. It may be the dominant factor why HBOC sensitization is more effective in advanced tumour. The high tumour interstitial fluid pressure (IFP) and vascular disorder in the interior of tumour are much severe in advanced tumours, leading the micron-sized RBC in vessel could scarcely enter into interstitium to transport oxygen [Citation26]. In 2022, Jiang found that only the smallest nanoparticles of the three sizes (35, 84, and 134 nm) could permeate into the inner part of a large solid tumour to alleviate the tumour hypoxia microenvironment [Citation27]. As mentioned before, HBOC is able to penetrate deep into regions with disordered vessels to supply oxygen where RBC cannot easily access. Correspondingly, it is more effective in alleviating hypoxia in large tumours, and RT in combination with HBOC may be appropriate for more anoxic tumours.
Generally, the solid tumour tissue is chronically in anoxic environment [Citation28]. As the tumour mass grows, there are more and more cells that are further away from the blood vessels. The interior of tumour which can’t obtain sufficient nutrient supply would develop a new vascular system. But usually, this new vascular system is disordered and lacks of normal physiological function. That makes tumour cells become more invasive and malignant to grab nutrients, adapting themselves to the hypoxic environment [Citation29,Citation30]. In this study, we observed that the expression of Ki67 and CC3 varied noticeably after HBOC administration (). The proliferative marker antigen Ki67 is usually considered as one of the indicators of tumour proliferation and the tumour metastasis suppressor gene CC3 is usually considered as one of the indicators of tumour proliferation and metastasis [Citation31,Citation32]. The decrease of Ki67 () as well as the increase of CC3 () suggested that HBOC inhibited the malignant growth and metastasis of tumours during RT. We attributed this phenomenon to alleviation of hypoxic microenvironment in solid tumour. In normal tissues, Hif-1α generally leads to apoptosis and necrosis, but in tumour tissue the increase of Hif-1α protein always activates downstream pathways, such as metabolic reprogramming, abnormal angiogenesis, metastasis, immortalization, etc. [Citation33]. As a common biomarker indicating angiogenesis, CD31 usually ascends in the malignantly proliferative tissue [Citation34,Citation35]. As mentioned above, the vascular disorder is also closely related to its anoxic state, and shows that tumour angiogenesis decreased after HBOC application. And the densitometric statistical analysis confirmed the result (). This suggests that HBOC could repress the malignant angiogenesis behaviour of tumours. Although TME renders tumour cells more invasive and malignantly proliferative [Citation36–38], the increased oxygen supply brought by HBOC may be able to remodel the TME inside the tumour through alleviating the hypoxic condition, improving the sensitivity of tumour cells to irradiation.
The previous studies found that ROS also contributed to sensitization of oxygen supply therapy while it is part of the explanation [Citation39]. The high-energy radiation produced by RT itself can damage DNA directly inside the nucleus or indirectly through the free radicals. Some of these free radicals come from water molecules and some from oxygen which is dissolved in the cytoplasm [Citation40,Citation41]. During the RT on mice, we observed that the ROS content in tumour tissue increased after HBOC exposure both in 100 or 400 mm3 Hela cell tumour model (). The improvement of oxygen content increased the source of ROS, provoking the high-energy radiation to produce more ROS. However, there was no statistical change in ROS production between 100 mm3 R + HBOC and 400 mm3 R + HBOC group. This demonstrated that the increment of ROS was not enough to make a difference in this research. It wasn’t the critical factor that led to a more effective sensitization of HBOC on large tumours.
As a kind of nanocarrier, HBOC can enter blood vessels deep inside the tumour to supply oxygen. The degree of hypoxia in early phase tumours is not as severe as in advanced tumours, therefore the sensitization effect of HBOC is not as obvious as advanced tumours. Based on the above experimental results, we consider that it is the oxygen supply that plays the most important role in inhibiting tumour growth. While the increased oxygen content may also stimulate radiation rays to produce more ROS to attack tumour cells, it wasn’t the main factor influencing the experimental results for solid tumours in different phases. Increased oxygen supply caused by HBOC reduces the expression of Hif-1α, regulated downstream signal pathways, such as proliferation and migration, eventually suppressed tumour growth and induced the relief of radiation resistance. In the future clinical radiotherapy, HBOC may enable radiotherapy to achieve the same therapeutic effects through dose reduction, diminishing the side effects on patients, and enhancing the clinical use of radiotherapy. In addition, HBOC may be more suitable for radiochemotherapy sensitization treatment in advanced solid tumours.
Author contributions
Yingcan Xu: conceptualization, methodology, investigation, formal analysis, writing-original draft. Kehui Zhu: conceptualization, investigation, data curation. Jiakang Wu and Shifan Zheng: resources, investigation. Rui Zhong: conceptualization, writing—review and editing. Wentao Zhou: supervision, data curation. Ye Cao: conceptualization, writing—review and editing. Jiaxin Liu: conceptualization, supervision, and funding acquisition. Hong Wang: conceptualization and supervision. All authors have agreed to be accountable for all aspects of the work and the submitted version of the manuscript.
Supplemental Material
Download MS Word (465.9 KB)Acknowledgements
The HBOC production used in this study was provided by Redpharm (Beijing) Biotechnology Co. Ltd. We would like to thank Chengmin Yang for contributing towards the administrative aspects of this manuscript, and for his support in general throughout this process.
Data availability statement
The data supporting the findings of this study are available within the article and its Supplementary Materials.
Additional information
Funding
References
- Lang JY. Retrospect, thinking and prospect of radiotherapy in China in the past 30 years. Cancer Prev Treat. 2017;30(1):1–4 + 6.
- Cruz FD, Matushansky I. Solid tumor differentiation therapy – is it possible? Oncotarget. 2012;3(5):559–567. doi:10.18632/oncotarget.512.
- Wang H, Jiang H, Van De Gucht M, et al. Hypoxic radioresistance: can ROS be the key to overcome it? Cancers. 2019;11(1):112. doi:10.3390/cancers11010112.
- Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341(1):63–72. doi:10.1016/j.canlet.2012.11.019.
- Barker HE, Paget JT, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi:10.1038/nrc3958.
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. doi:10.1038/nrc1367.
- Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25(26):4066–4074. doi:10.1200/JCO.2007.12.7878.
- Yuan M, Liang S, Zhou Y, et al. A robust oxygen-carrying hemoglobin-based natural sonosensitizer for sonodynamic cancer therapy. Nano Lett. 2021;21(14):6042–6050. doi:10.1021/acs.nanolett.1c01220.
- Feng J, Yu W, Xu Z, et al. An intelligent ZIF-8-gated polydopamine nanoplatform for in vivo cooperatively enhanced combination phototherapy. Chem Sci. 2020;11(6):1649–1656. doi:10.1039/c9sc06337d.
- Teicher BA, Herman TS, Hopkins RE, et al. Effect of a bovine hemoglobin preparation on the response of the FSaIIC fibrosarcoma to chemotherapeutic alkylating agents. J Cancer Res Clin Oncol. 1992;118(2):123–128. doi:10.1007/BF01187500.
- Jahr JS, Guinn NR, Lowery DR, et al. Blood substitutes and oxygen therapeutics: a review. Anesth Analg. 2021;132(1):119–129. doi:10.1213/ANE.0000000000003957.
- Standl T. Haemoglobin-based erythrocyte transfusion substitutes. Expert Opin Biol Ther. 2001;1(5):831–843. doi:10.1517/14712598.1.5.831.
- Raabe A, Gottschalk A, Hommel M, et al. No effect of the hemoglobin solution HBOC-201 on the response of the rat R1H tumor to fractionated irradiation. Strahlenther Onkol. 2005;181(11):730–737. doi:10.1007/s00066-005-1418-3.
- Lee NP, Chan KT, Choi MY, et al. Oxygen carrier YQ23 can enhance the chemotherapeutic drug responses of chemoresistant esophageal tumor xenografts. Cancer Chemother Pharmacol. 2015;76(6):1199–1207. doi:10.1007/s00280-015-2897-2.
- Belcher DA, Lucas A, Cabrales P, et al. Tumor vascular status controls oxygen delivery facilitated by infused polymerized hemoglobins with varying oxygen affinity. PLOS Comput Biol. 2020;16(8):e1008157. doi:10.1371/journal.pcbi.1008157.
- Qi X, Wong BL, Lau SH, et al. A hemoglobin-based oxygen carrier sensitized cisplatin based chemotherapy in hepatocellular carcinoma. Oncotarget. 2017;8(49):85311–85325. doi:10.18632/oncotarget.19672.
- Gao YQ, Zhu KH, Xv YC, et al. Preliminary study on enhancing chemosensitivity of subcutaneous xenograft of breast cancer in nude mice with polymeric human umbilical cord hemoglobin oxygen carrier. Chinese J Blood Transf. 2022;35(05):475–479.
- Dong DC, Gao YQ, Wang H, et al. Preliminary experiment on enhancing the therapeutic effect of lenvatinib on subcutaneous xenograft of liver cancer in nude mice using aggregated human umbilical cord blood hemoglobin oxygen carriers. Chinese J Blood Transf. 2021;34(5):456–460.
- Belcher DA, Ju JA, Baek JH, et al. The quaternary state of polymerized human hemoglobin regulates oxygenation of breast cancer solid tumors: a theoretical and experimental study. PLoS One. 2018;13(2):e0191275. doi:10.1371/journal.pone.0191275.
- Niemoeller OM, Belka C. Radiotherapy and TRAIL for cancer therapy. Cancer Lett. 2013;332(2):184–193. doi:10.1016/j.canlet.2011.07.003.
- Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173(6):719–728. doi:10.1667/RR1984.1.
- Huo D, Liu S, Zhang C, et al. Hypoxia-targeting, tumor microenvironment responsive nanocluster bomb for radical-enhanced radiotherapy. ACS Nano. 2017;11(10):10159–10174. doi:10.1021/acsnano.7b04737.
- Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–437. doi:10.1038/nrc2397.
- Horsman MR, Mortensen LS, Petersen JB, et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674–687. doi:10.1038/nrclinonc.2012.171.
- Lucas A, Belcher DA, Munoz C, et al. Polymerized human hemoglobin increases the effectiveness of cisplatin-based chemotherapy in non-small cell lung cancer. Oncotarget. 2020;11(42):3770–3781. doi:10.18632/oncotarget.27776.
- Heldin C-H, Rubin K, Pietras K, et al. High interstitial fluid pressure-an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi:10.1038/nrc1456.
- Wang X, Wu M, Li H, et al. Enhancing penetration ability of semiconducting polymer nanoparticles for sonodynamic therapy of large solid tumor. Adv Sci. 2022;9(6):2104125. doi:10.1002/advs.202104125.
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–239. doi:10.1007/s10555-007-9055-1.
- Martin AR, Ronco C, Demange L, et al. Hypoxia inducible factor down-regulation, cancer and cancer stem cells (CSCs): ongoing success stories. Medchemcomm. 2017;8(1):21–52. doi:10.1039/c6md00432f.
- Tsai YP, Wu KJ. Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci. 2012;19(1):102. doi:10.1186/1423-0127-19-102.
- Zhu M, Yin F, Fan X, et al. Decreased TIP30 promotes snail-mediated epithelial-mesenchymal transition and tumor-initiating properties in hepatocellular carcinoma. Oncogene. 2015;34(11):1420–1431. doi:10.1038/onc.2014.73.
- Cserni G, Vörös A, Liepniece-Karele I, et al. Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast. 2014;23(3):59–63.
- Tiburcio PD, Choi H, Huang LE. Complex role of HIF in cancer: the known, the unknown, and the unexpected. Hypoxia. 2014;2:59–70.
- Nowak A, Grzegrzolka J, Paprocka M, et al. Nestin-positive microvessel density is an independent prognostic factor in breast cancer. Int J Oncol. 2017;51(2):668–676. doi:10.3892/ijo.2017.4057.
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–634. doi:10.1038/onc.2009.441.
- Yang J, Li W, Luo L, et al. Hypoxic tumor therapy by hemoglobin-mediated drug delivery and reversal of hypoxia-induced chemoresistance. Biomaterials. 2018;182:145–156. doi:10.1016/j.biomaterials.2018.08.004.
- Tao J, Yang G, Zhou W, et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J Hematol Oncol. 2021;14(1):14. doi:10.1186/s13045-020-01030-w.
- Sun Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016;380(1):205–215. doi:10.1016/j.canlet.2015.07.044.
- Yamamoto M, Izumi Y, Horinouchi H, et al. Systemic administration of hemoglobin vesicle elevates tumor tissue oxygen tension and modifies tumor response to irradiation. J Surg Res. 2009;151(1):48–54. doi:10.1016/j.jss.2007.12.770.
- Menegakis A, Klompmaker R, Vennin C, et al. Resistance of hypoxic cells to ionizing radiation is mediated in part via hypoxia-induced quiescence. Cells. 2021;10(3):610. doi:10.3390/cells10030610.
- Hawley L. Principles of radiotherapy. Br J Hosp Med. 2013;74(11):C166–C169. doi:10.12968/hmed.2013.74.sup11.c166.