ABSTRACT
With the atypical rise of Mycoplasma pneumoniae infection (MPI) in 2023, prompt studies are needed to determine the current epidemic features and risk factors with emerging trends of MPI to furnish a framework for subsequent investigations. This multicentre, retrospective study was designed to analyse the epidemic patterns of MPI before and after the COVID-19 pandemic, as well as genotypes and the macrolide resistance-associated mutations in MP sampled from pediatric patients in Southern China. Clinical data was collected from 133674 patients admitted into investigational hospitals from June 1, 2017, to November 30, 2023. Metagenomic next-generation sequencing (mNGS) data were retrieved based on MP sequence positive samples from 299 pediatric patients for macrolide resistance-associated mutations analysis. Pearson’s chi-squared test was used to compare categorical variables between different time frames. The monthly average cases of pediatric common respiratory infection diseases were increased without enhanced public health measures after the pandemic, especially for influenza, respiratory syncytial virus infection, and MPI. The contribution of MPI to pneumoniae was similar to that in the outbreak in 2019. Compared mNGS data between 2019-2022 and 2023, the severity of MP did not grow stronger despite higher rates of macrolide-resistance hypervariable sites, including loci 2063 and 2064, were detected in childhood MP samples of 2023. Our findings indicated ongoing surveillance is necessary to understand the impact of post pandemic on MP transmission disruption on epidemic season and severity of clinical outcomes in different scenarios.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
From October to December 2023, outbreaks of childhood respiratory diseases were reported in China with some hospitals being overwhelmed by pediatric emergency admissions. One or more infections, such as rhinovirus, adenovirus, respiratory syncytial virus (RSV), and influenza or Mycoplasma pneumoniae (MP), are the common causes of the upsurge in respiratory illnesses among children. More specifically, MP represents a predominant form of respiratory infection in pediatric populations in this period, thereby posing a significant threat to pediatric health. MP is a common cause of community-acquired respiratory infections such as pneumonia, particularly in children and adolescents [1]. MP infection (MPI) is a global cause of pneumonia that follows cyclical epidemic patterns every four to seven years [2]. The most recent pandemic is recorded in late 2019 globally, predominantly in Europe and Asia [3]. Regional re-emergence of MPI in 2023 have been reported in Europe [4, 5] and Singapore [3]. Additionally, WHO has paid close attention to the clusters of MPI in children in northern China [6, 7]. The impact of COVID-19 pandemic on incidence of influenza and other notifiable infectious diseases has been reported in previous studies [8-11]. Previous studies have shown that the prevalence of MPI among children is reduced during the COVID- 19 pandemic up to 2021 in China [12-14]. However, the atypical rise of MPI in 2023 remained unclear in Southern China. Given the heavy burden of pediatric MPI in recent period, it is urgent to undertake a prompt study aimed at elucidating the current epidemic features and risk factors with emerging trends, thereby furnishing a framework for subsequent investigations. Therefore, we conducted a multicentre, retrospective study to analyse the epidemic patterns of MPI before and after the COVID-19 pandemic, and analyse the genotypes and the macrolide resistance-associated mutations in MP sampled from the pediatric patients in Southern China.
Methods
Study design and approval
This retrospective, observational study was approved by the institutional review board (IRB) of Guangzhou Women and Children's Medical Center with centre ethical approval (ethical approval No.2022-246A01), and conducted at Guangzhou Women and Children's Medical Center, Guangzhou Children's Hospital, and Guangzhou Maternal and Child Health Hospital. The protocol was approved by the IRB before data collection (Text S1 in the Supplemental Online Material). Informed consent was waived since anonymised data were collected from the medical database for scientific research purpures only.
Data sources and patient population
Clinical data were collected in the investigational centres from both inpatient and outpatient databases during June 1 2017 and November 30 2023. The demographic information and monthly cases of common respiratory infectious diseases (RID) in pediatric patients were included for analysis. Clinical data were included for patients who met with the following criteria: (1) diagnosed with upper respiratory tract infection, bronchitis, or pneumonia; (2) aged between 0-18 years; and (3) positive pathogen detection for MP, influenza, parainfluenza, adenovirus, bocavirus, or respiratory syncytial virus. Data were entered by two researchers for cross-check to ensure data quality. An epidemiological statistician was involved for statistics analysis. The current study is reported based on a STROBE checklist (Table S1 in the Supplemental Online Material).
The macrolide resistance-associated mutation analysis of MP was conducted with metagenomic next-generation sequencing (mNGS) technology based on bronchoalveolar lavage fluid, sputum, and throat swab samples. All the samples were collected from pediatric patients with respiratory infections during July 14 2019 and 6 December 2023 in Southern China.
Data analysis plan and definition
Clinical and mNGS data were retrieved in database of pediatric patients in Southern China. Data handling and analysis process are shown in Figure 1. The disease spectrum was analysed based on annual and monthly cases of common pediatric infectious diseases, including MPI, influenza, parainfluenza, adenovirus infection (AI), bocavirus infection (BI), and respiratory syncytial virus infection (RSVI). The impact of COVID-19 pandemic was observed by the change proportion of pediatric common RID cases during 2020 and 2022 compared with baseline before (2017-2019) and after (2023) the pandemic. During the pandemic, regional knockdown with enhanced public health interventions were identified based on the cases distribution of COVID-19. Enhanced public health measures (PHM) included non-pharmaceutical interventions, timely disinfection, travel restrictions, school closures, reginal lockdown and local quarantine strategies [15]. Additionally, epidemic patterns of MPI before and after the COVID-19 pandemic were analysed based on monthly average cases. According to the guideline of a national academic society, the Chinese Medical Association Pediatrics Branch Respiratory Group, pathogen detections were prescribed for inpatients with suspicious symptom of MPI, including respiratory infection symptom, like dry paroxysmal cough, and other immune inflammatory response [16]. The pathogen testing for MP were performed in accordance with the guidelines of a national academic society, the National Health Commission China Expert Committee on Rational Drug Use Pediatric Drug Use Professional Group [17]. Briefly, nasopharyngeal swabs were collected and detected for MP using real-time fluorescence quantitative Polymerase Chain Reaction (RT-PCR). RT-PCR detection was sensitive for current infection of MP, and recommended as first-line detection method in the guidelines [16, 17]. Therefore, serum antigenic tests were not conducted. The patients with positive MP PCR tests were included for further analysis. Specifically, a comparison was performed on the outbreak patterns of MPI between 2019 and 2023. Furthermore, MP sequences were detected with biological samples by mNGS per standard data handling process, mainly including data quality control, removal of host (human) sequences, and database matching of pathogenic bacteria genera. Among the samples positive with MP sequences, MP sequences in fastq format was aligned with a the M129 reference genome (816550 bp) using Burrows-Wheeler Aligner (version 0.7.17-r1188, Massachusetts, USA). Then the 23sRNA fragment (OU342337.1: 120031- 122935) was extracted from the alignment of each sample for mutations detection using Samtools (version 1.13, Cambriadge, UK). Further analysis was conducted for samples with a mutation rate of 10% at least. The difference of mutation frequency for common macrolide- resistance mutated loci was analysed between samples collected in 2023 and before to explore the pathology features of MP in the current outbreak.
Figure 1. Data handling process and analysis diagram. There were 133674 patients were included for data analysis. Additionally, macrolide- resistance-mutation characteristics of Mycoplasma pneumoniae were analysed based on samples from 299 patients.
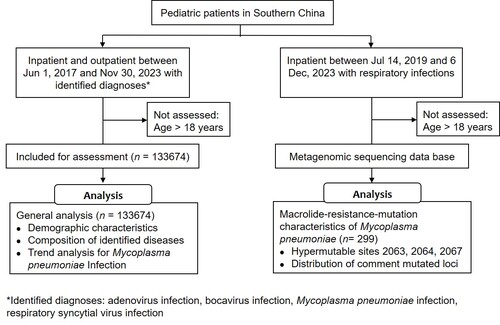
Figure 2. The changes in case distribution for common respiratory infectious diseases before and after the COVID-19 pandemic in pediatric patients. Outbreaks of MPI were noted in 2019 and 2023, while the decrease of MPI was associated with enhanced public health measures during COVID-19 pandemic (timeframes marked with shadow). Abbreviations: AI, adenovirus infection; BI, bocavirus infection; MPI, Mycoplasma pneumoniae infection; RSVI, respiratory syncytial virus infection
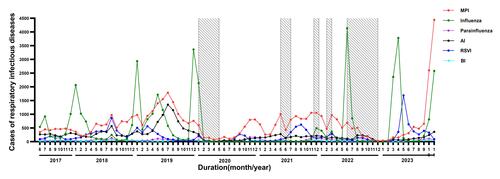
Figure 3. The case distribution of MPI in observation duration. The peak cases of MPI in 2023 was twice more than that in the outbreak in 2019 (A). The cases of MPI were accumulated specifically in Oct and Nov 2023 (B), which contributed around 60.0% of cases among RID (C). Abbreviations: MPI, Mycoplasma pneumoniae infection; RID, respiratory infectious diseases
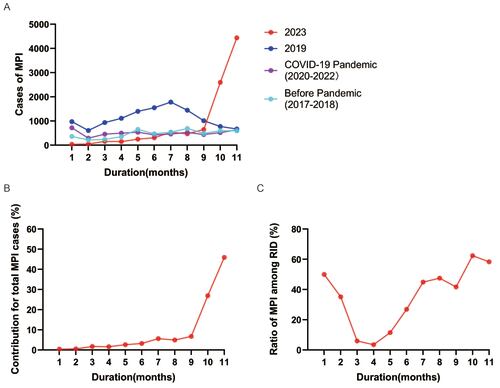
Figure 4. Disease spectrum of pediatric common respiratory infectious disease (RID) and the clinical manifestation of MPI before and after the COVID-19 pandemic. The annual proportion of MPI was stable between 34.0% and 46.0% among RID from 2017 to 2023 (A). The cases of MP associated pneumoniae were similar between the outbreaks in 2023 and 2019, while a remarkable decrease was observed with enhanced public health measures during the COVID-19 pandemic from 2020 to 2022 (B). Abbreviations: AI, adenovirus infection; BI, bocavirus infection; MPI, Mycoplasma pneumoniae infection; RSVI, respiratory syncytial virus infection; URTI, upper respiratory tract infection
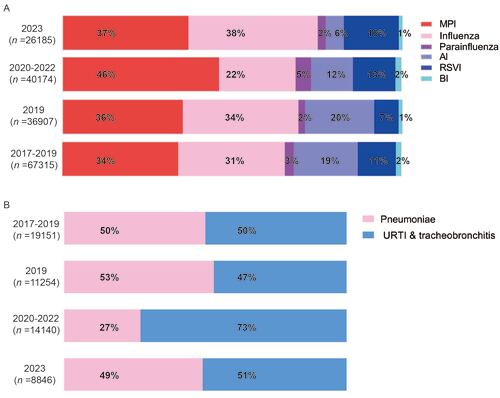
Figure 5. Macrolide-resistance-mutation characterizations of MP in the recent prevalence in Southern China. Positive MP sequences were identified in 299 samples from five provinces in Southern China (A), and mutations were detected in macrolide-resistance hypervariable sites including loci 2063 and 2064 (B).
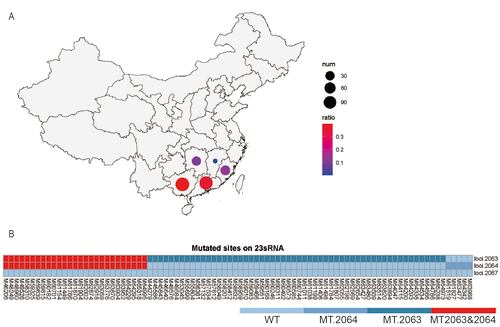
Table 1. Demographic characteristics of pediatric patients with common respiratory infectious diseases.
Table 2. Monthly average cases and change proportion of common respiratory infectious diseases in pediatric patients during observation.
Table 3. Monthly average cases and change proportion of pediatric patients with Mycoplasma pneumoniae infection.
Statistical analysis
Statistical analysis was conducted with SPSS software (version 20.0, International Business Machines Corporation, Armonk, NY, USA). Descriptive statistics were used to show the distribution features of variables. Categorical variables were presented as number and frequency. Continuous variables are presented as mean ± stand error or the median (interquartile range). No imputation was used for missing data. Pearson’s chi-squared test was used to compare categorical variables between different time frames. Statistical significance was considered if p < 0.05.
Results
Demographic characteristics of patients
From June 1, 2017, to November 30, 2023, total 133,674 patients were included for disease spectrum analysis with a median age of 3.4 (2.2 – 5.7) years, including 76614 (57.3%) males and 57060 (42.7%) females (Table1). mNGS data were retrieved based on MP sequence positive samples from 299 pediatric patients with a median age of 6.0 (2.7 – 8.3) years, including 160 (53.5%) males and 139 (46.5%) females (Table S2 in the Supplemental Online Material). All these patients were from five provinces in Southern China, including Guangxi (119 cases, 39.8%), Guangdong (110 cases, 36.8%), Fujian (38 cases, 12.7%), Hunan (31 cases, 10.4%), and Jiangxi (1 case, 0.3%).
Pediatric common respiratory infectious disease spectrum analysis
The monthly average cases from 2017 to 2019 were set as a baseline before the COVID-19 pandemic (Table 2). Among the pediatric common RID, the top six diseases in occurrence included MPI (742 cases), influenza (683 cases), AI (409 cases), RSVI (244 cases), parainfluenza (58 cases), and BI (35 cases) before the pandemic. Five periods of local outbreak with enhanced PHM were identified in Guangzhou according to the confirmed cases of COVID-19 (Figure S1 in the Supplemental Online Material). During the pandemic with enhanced PHM, the monthly average cases of these diseases decreased remarkably, with a proportion as 30.6% for MPI, 63.1% for influenza, 12.1% parainfluenza, 66.3% for AI, 42.6% for RSVI, and 42.9% for BI. However, after the pandemic with relaxed PHM in 2023, the monthly average cases increased evidently compared with that during the pandemic except for AI. The top three diseases with remarkable increase in occurrence were influenza (259.5%), RSVI (174.3%), and MPI (70.9%). It was indicated that the PHM played an important role in disease control for pediatric common RID based on the occurrence analysis with or without enhanced PHM.
The epidemic features of MPI
The epidemic patterns of MPI before and after the COVID-19 pandemic were analysed. Before the pandemic, an outbreak of MPI was observed in 2019 with an average of 1087 cases (Table S3 in the Supplemental Online Material) and a peak near 2000 cases monthly (Figure 2). The cases of MPI decreased during the pandemic of COVID-19 (2020-2022) owing to the intervention of PHM, while a regional resurgence of MPI in southern China was noticed in 2023 with relaxed PHM (Table 3). Worth noting is that the epidemic pattern of MPI in 2023 is different from that in previous years. Commonly, MPI is more prevalent in autumn and winter, although it is not seasonal and can be contracted throughout the year. However, the MPI cases began to increase significantly since May 2023 in China (Table 3), and reached a peak of 4443 cases in Nov up to the data cut-off date (Figure 2), which was even more than twice the peak cases in its outbreak in 2019 (Figure 3 panel A). In addition, 80.0% of MPI cases were reported specifically in Oct and Nov (Figure 3 panel B). Along with the increasing cases, MPI contributed around 60.0% of cases among RID in Oct and Nov 2023 (Figure 3 panel C, Table S4 in the Supplemental Online Material), although the proportion of MPI was stable annually between 34.0% and 46.0% among RID (Figure 4 panel A). Regarding the contribution of MPI to pneumoniae, little difference was noticed between 2023 (49.0%) and 2019 (53.0%) although the peak cases in 2023 were more than twice compared to those of 2019. Of note, the positive rate of MP in patients with pneumoniae decreased remarkably during the COVID-19 pandemic with PHM in place (Figure 4 panel B). It was indicated that the severity of MP did not grow stronger despite regional epidemics in 2023.
Macrolide-resistance-mutation analysis of MP
After a standard process of mNGS and screening, 299 samples from southern China (Figure 5 panel A) were identified as positive with MP sequences. Among these samples, macrolide- resistance hypervariable sites were detected in 124 samples, including loci 2063, 2064, and 2067 (Figure 5 panel B). Furthermore, mutations were identified in 88 (71.0%) samples (Table S5 in the Supplemental Online Material), which were primarily composed of base substitution A to G mutation in loci 2063 (33.9%). The mutation rate of common macrolide- resistance loci of MP (loci 2063 and 2064) was significantly higher in 2023 than that during 2019 to 2022 (χ2 = 8.783, P = 0.003, Table S6 in the Supplemental Online Material). Further analysis was performed to find out any impact of the increase macrolide-resistance mutations on clinical outcomes based on the data in 2023 and 2019 (Table S7 in the Supplemental Online Material). Compared with MPI cases in 2019, no significant difference was detected for cases in 2023 regarding main clinical outcomes, including average length of hospital stays (6.5 ± 0.3 days in 2023 vs 7.6 ± 1.1 days in 2019), time to defervescence (1.5+0.1 days in 2023 vs 1.9+0.3 days in 2019), and time to resolution of rales (5.2+0.3 days in 2023 vs 6.4+0.9 days in 2019).
Discussion
Previous studies have reported the impact of COVID-19 pandemic on incidence of common respiratory infectious diseases [9-11]. Additionally, we have analysed the impact of PHM on the common infectious disease portfolio in children and adolescents [18]. In the current study, we conducted a further analysis on the pathogenology of pediatric common respiratory infectious diseases in southern China before and post pandemic. Specifically, we focus on the epidemic patterns of childhood MPI and macrolide-resistance-mutation characterizations of MP in the recent prevalence in Southern China, in order to provide valuable strategy for infectious disease control.
It was shown in previous studies that the COVID-19 pandemic lockdown policy and the associated PHM have had a significant impact on the incidence of respiratory infections [9-11]. A decrease in pediatric emergency room visits and respiratory tract infections in hospitals was observed due to the implementation of PHM [19]. However, as these measures were relaxed or cancelled, there was a resurgence in respiratory infections, including RSV and other viral infections [20]. Moreover, the emergence of COVID-19 pandemic has led to the reconsideration of surveillance strategies for respiratory viruses, indicating the crosstalk of these infectious diseases [21]. There were some other studies focusing on the effect of PHM on MPI in China during the COVID-19 pandemic; however, the representativeness of these studies was limited by single-site design [22-25], limited samples [26-29], or limited period and did not cover the post-COVID-19 period. Therefore, we conducted this multi- centre study with a large sample as 133,674 patients from 2016 to 2023, covering periods before, during and after the entire course of COVID-19 to provide more convincing evidence. In our study, the pathogenic analysis was conducted for pediatric common respiratory infectious disease, including MPI, influenza, parainfluenza, RSVI, and BI (Table 2). In accordance with previous studies, the average cases of these diseases decreased remarkably with PHM during the COVID-19 pandemic, which was rebounded without PHM after the pandemic (Figure 2). It was indicated that the rebound of pediatric common respiratory infectious diseases could be attributed to the relaxation of PHM. Therefore, a sustained and proper PHM strategy was necessary to prevent the resurgence of severe infectious diseases post COVID-19 pandemic. As PHM implementation has a double-edged effect on both infectious disease transmission and economic development, proper alternative strategies should be considered, such as hand hygiene, social distance, wearing masks, and so on.
Among these pediatric common respiratory infectious diseases, MPI have been reported to occur both endemically and epidemically worldwide with a higher prevalence in certain seasons as autumn and winter [30], and has been associated with a wide range of clinical manifestations, including pneumoniae, upper respiratory tract infection, and tracheobronchitis. Of note, rare cases of cavitary lung lesions and mucositis also shown the diverse impact of MPI on human health [31]. These recent updates highlighted the ongoing significance of MPI as a global health concern and the importance of continued surveillance and research. Particularly, the impact of COVID-19 pandemic and the PHM on the epidemiological features of MPI in children has been a subject of recent research. During the studies on infectious diseases occurrence in COVID-19 pandemic, the PHM was indicated effective to control the transmission of MP among children [12, 13]. Recently, an atypical summer rise of MPI in Northern China has drawn the attention of WHO [6, 7], which could be attributed to the relaxation of PHM. Therefore, we aim to analyse the epidemic features in Southern China as a valuable supplement to figure out the whole picture of the current prevalence in China. Based on our data, the cases of MPI decreased during the pandemic with PHM in place. In compliance with previous studies, a resurgence was noticed in 2023 without enhanced PHM (Table 3). Notably, the MPI cases began to increase since May 2023 in China (Figure 3). The earlier resurgence of MP was considered to be driven by a buildup in population susceptibility along with relaxed PHM, so-called “immunity gap”. The enhanced PHM during COVID-19 pandemic was associated with a remarkable decrease of MPI (Figure 2). Along with the persistent reduction during the COVID-19 pandemic under PHM, the suppression of MP was continued even in its prevalence season like autumn and winter. The vulnerability of individuals or populations was accumulated due to a lack of acquired immunity against MP under enhanced PHM with less chance for exposure to pathogens.
Therefore, it was likely that the relaxation of PHM following the COVID-19 pandemic could inadvertently create conditions favourable for the resurgence of infectious diseases[32] including MPI. Although MP is known to cause cyclical pandemic every four to seven years [2], the atypical outbreak in 2023 indicated that the natural disease control mechanisms could be disrupted by the enhance or relaxation of PHM under the COVID-19 pandemic. However, the shifted MP epidemic associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PHM did not result in more pneumoniae cases than that in previous outbreak in 2019 (Figure 4). Compared with upper respiratory tract infection and tracheobronchitis, the MP-related pneumoniae represented a severe outcome, the cases of which was comparable to that in 2019. Ongoing surveillance is still necessary to understand the impact of MP transmission disruption on epidemic season and severity of clinical outcomes in different scenarios.
Besides of the epidemiology and clinical manifestations, the genetic diversity of MP also contributed to a better understanding for the of the pathogen. The mutated loci contributing to macrolide resistance in MP have been extensively studied. The primary mechanism leading to macrolide resistance in MP is the point mutation at sites 2063 and 2064 in domain V of 23S ribosomal RNA [33]. Furthermore, the resistance-gene mutation of MP in the bronchoalveolar lavage fluid of children with MP-associated pneumonia has been investigated, highlighting the correlation between macrolide resistance and clinical characteristics [34, 35]. A2063G mutations were detected in domain V of 23S rRNA gene in a single-site study in Wuhan, China, during the COVID-19 pandemic between October 2020 and March 2022 [36]. Another single-site study had reported that A2063G mutation was overwhelmed in this winter outbreak of MPI in China [37]. However, the representativeness of these studies was restricted by single-site design, and the feature of macrolide-resistance mutation in the current resurgence remained unclear compared to previous prevalence.
Therefore, we detected MP sequences at three sites with biological samples by mNGS in the current study, and analysed the macrolide-resistance mutations based on samples collected from 2019 to 2023. Mutations were identified mainly in loci 2063 and 2064 with a higher mutation rate in samples collected in 2023 than that during 2019 and 2022 (Table S6 in the Supplemental Online Material); however, the main clinical outcomes were not affected by the higher mutation rate in 2023 (Table S7 in the Supplemental Online Material). Ongoing surveillance was necessary based on a higher mutation rate in macrolide resistance-associated loci, which indicated a rise of macrolide-resistance strains in this resurgence of MP. However, no new mutation sites associated with macrolide resistance were detected in our study. These could partially explain the reason why the severity of clinical outcomes in the current resurgence was similar with previous pandemic in 2019, even with more than twice peak cases compared to its previous outbreak.
There are some limitations in our study. First, the sample only covered the Southern region of China, and the epidemics in the North and South still have their own characteristics. So, the generalizability of our findings is limited without representative data from other regions in China, like Northern China. Further analysis combining data from the northern region is necessary in further studies. Second, the sample size for macrolide-resistance- mutation analysis was relatively limited, and subsequent research needs to further expand the sample size. Finally, due to the study’s focus on hospitalized pediatric patients, insights into community transmission of infectious diseases among children were beyond the scope of analysis. Yet community health management remains critical for pandemic mitigation from a public health standpoint.
In conclusion, the impact of COVID-19 pandemic on the epidemiological features of childhood MPI has been evident in Southern China. Although the current study did not identify any new mutation sites other than previously reported loci, a significantly higher rates of macrolide-resistance mutation were detected in MP samples collected post COVID- 19 pandemic in 2023, supporting the resurgence of childhood MPI post pandemic was at least partly attributed to macrolide-resistant MP clones. These findings underscore the critical role of ongoing public health measures in preventing and controlling infectious disease outbreaks. Nevertheless, this study didn’t examine co-infection patterns of SARS-CoV-2 and MP, as well as the impact of co-infection on disease severity in children. Further study is needed to understand the evolving epidemiology of MPI in the scenario of the SARS-CoV-2 infection.
Authorship contributions
QL, LH and YX conceived the project and manuscript. LH, CY and PPS contributed to data collection, formal analysis, investigation, and preparation of the tables and figures. FSZ, QW, CXF, CZ, YLG, XHW and XXZ accessed the clinical data and contributed to methodology. BY, JHY and PPS contributed to macrolide resistance-associated mutations analysis and mNGS technology. JLW and JPW contributed to resource and verified the data. HWZ contributed to statistics analysis as an epidemiological statistician. LH wrote the original draft manuscript. JL and JQZ contributed to data quality control and language editing for the manuscript. QL, LH, YX, JL and JQZ contributed to the review and critical editing of the manuscript. QL contributed to supervision and final validation. QL, YX and JLW contributed to funding acquisition. All authors contributed to data interpretation, article editing, and approval of the final version.
Ethics approval
The current study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institutional review board (IRB) of Guangzhou Women and Children’s Medical Center (Date 24 October 2022/ ethical approval number 2022-246A01). This was an observational study. Requirement for informed consent was waived by the IRB of Guangzhou Women and Children’s Medical Center since data were exacted from the medical database, anonymised, and collected only for scientific research.
Supplemental online material
Table S1. STROBE checklist.
Table S2. Demographic characteristics of patients included for macrolide -resistance-mutation analysis for Mycoplasma pneumoniae, presented as n (%).
Table S3. Monthly average cases and change proportion of common respiratory infectious diseases in pediatric patients in 2019 compared with that from 2017 to 2018, presented as n (%).
Table S4. The monthly cases and changes of common respiratory pediatric diseases in patients with pneumonia in 2023, presented as n (%).
Table S5. Distribution of mutated loci contributed for macrolide -resistance of MP in 124 samples with comment mutations (loci 2063 and 2064), presented as n (%).
Table S6. Mutation rate of common macrolide-resistance loci of MP (loci 2063 and 2064) in 124 samples, presented as n (%).
Table S7. Clinical features and outcomes of pediatric patients with MPI in 2023 compared with that in 2019 at a single site.
Figure S1. The cases distribution of COVID-19 during the pandemic.
Text S1. Study protocol.
Acknowledgments
We acknowledge the staff in information departments for help in accessing the medical database.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability
De-identified participant data supporting the conclusions of the current study are available upon publication to researchers by email to the corresponding author on reasonable request for research.
Additional information
Funding
References
- [1] Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection ofMycoplasma pneumoniaeinfections: Figure 1. FEMS Microbiology Reviews. 2008;32(6):956-73.
- [2] Vervloet LA, Marguet C, Camargos PAM. Infection by Mycoplasma pneumoniae and its importance as an etiological agent in childhood community-acquired pneumonias. Braz J Infect Dis. 2007;11(5):507-14.
- [3] Meyer Sauteur PM, Beeton ML, Pereyre S, et al. Mycoplasma pneumoniae: delayed re- emergence after COVID-19 pandemic restrictions. The Lancet Microbe. 2023.
- [4] Maeve Cullinan. Child pneumonia cases emerge in Europe as China battles wave of respiratory illness. The Telegraph. 2023. https://www.telegraph.co.uk/global- health/science-and-disease/child-pneumonia-europe-france-ireland-denmark-netherlands/.
- [5] Statens Serum Institut (Danish disease agency). Increased occurrence of Mycoplasma pneumoniae. 2023. https://en.ssi.dk/news/epi-news/2023/no-35---2023.
- [6] WHO. Upsurge of respiratory illnesses among children-Northern China. 2023. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON494.
- [7] WHO. WHO statement on reported clusters of respiratory illness in children in northern China. 2023. https://www.who.int/news/item/22-11-2023-who-statement-on-reported-clusters-of-respiratory-illness-in-children-in-northern-china.
- [8] Chen B, Wang M, Huang X, et al. Changes in incidence of notifiable infectious diseases in China under the prevention and control measures of COVID-19. Front Public Health. 2021;9:728768.
- [9] Wang L, Guo X, Zhao N, et al. Effects of the enhanced public health intervention during the COVID-19 epidemic on respiratory and gastrointestinal infectious diseases in China. J Med Virol. 2022;94(5):2201-11.
- [10] Soo RJJ, Chiew CJ, Ma S, et al. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis. 2020;26(8):1933-5.
- [11] Feng L, Zhang T, Wang Q, et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat Commun. 2021;12(1):3249.
- [12] Zhang Y, Huang Y, Ai T, et al. Effect of COVID-19 on childhood Mycoplasma pneumoniae infection in Chengdu, China. BMC Pediatrics. 2021;21(1).
- [13] Xu M, Liu P, Su L, et al. Comparison of Respiratory Pathogens in Children With Lower Respiratory Tract Infections Before and During the COVID-19 Pandemic in Shanghai, China. Frontiers in Pediatrics. 2022;10.
- [14] Chen J, Zhang J, Lu Z, et al. Mycoplasma pneumoniae among chinese outpatient children with mild respiratory tract infections during the coronavirus disease 2019 pandemic. Microbiol Spectr. 2022;10(1):e0155021.
- [15] The National Health Commission of the People's Republic of China. Prevention and control strategy of 2019-novel coronavirus (version 9). 2022. http://chrome-extension://ibllepbpahcoppkjjllbabhnigcbffpi/http://www.gov.cn/xinwen/2022-06/28/5698168/files/9585944023424f45a4b4d522b5f5c034.pdf. Accessed 23 Dec 2022.
- [16] Group CMAPBR, Board CJoPPE. Expert consensus on the diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children (2015 version). Chinese Journal of Practical Pediatrics. 2015;30(17):1304-8.
- [17] Group NHCCECoRDUPDUP. Chinese expert consensus on laboratory diagnosis standard and clinical practice for Mycoplasma pneumoniae infection in children (2019 version). Chinese Journal of Pediatrics. 2020;58(5):366-73.
- [18] Huang L, Yang C, Pan H, et al. Effects of public health interventions and zero COVID policy on paediatric diseases: A Southern China study. Journal of Global Health. 2024;14.
- [19] Kuitunen I, Artama M, Mäkelä L, et al. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatric Infectious Disease Journal. 2020;39(12):e423-e7.
- [20] Saravanos GL, Hu N, Homaira N, et al. RSV epidemiology in Australia before and during COVID-19. Pediatrics. 2022;149(2).
- [21] Teirlinck AC, Johannesen CK, Broberg EK, et al. New perspectives on respiratory syncytial virus surveillance at the national level: lessons from the COVID-19 pandemic. European Respiratory Journal. 2023;61(4):2201569.
- [22] Cheng Y, Cheng Y, Dai S, et al. The prevalence of Mycoplasma pneumoniae among children in Beijing before and during the COVID-19 pandemic. Frontiers in Cellular and Infection Microbiology. 2022;12.
- [23] Cai F, Shou X, Ye Q. Epidemiological study on Mycoplasma pneumoniae and Chlamydia pneumoniae infection of hospitalized children in a single center during the COVID-19 pandemic. Front Cell Infect Microbiol. 2022;12:843463.
- [24] Wang H, Zheng Y, de Jonge MI, et al. Lockdown measures during the COVID-19 pandemic strongly impacted the circulation of respiratory pathogens in Southern China. Scientific Reports. 2022;12(1).
- [25] Feng Y, Zhang H, Zhang B, et al. Impact of normalized COVID-19 prevention and control measures on lower respiratory tract infection pathogenesis in hospitalized children. Frontiers in Public Health. 2024;12.
- [26] Li L, Wang H, Liu A, et al. Comparison of 11 respiratory pathogens among hospitalized children before and during the COVID-19 epidemic in Shenzhen, China. Virology Journal. 2021;18(1).
- [27] Zhang LN, Cao L, Meng LH. Pathogenic changes of community-acquired pneumonia in a children's hospital in Beijing, China before and after COVID-19 onset: a retrospective study. World journal of pediatrics : WJP. 2022;18(11):746-52.
- [28] Mai W, Ren Y, Tian X, et al. Comparison of common human respiratory pathogens among hospitalized children aged ≤ 6 years in Hainan Island, China, during spring and early summer in 2019-2021. J Med Virol. 2023;95(4):e28692.
- [29] Chen J, Zhang J, Lu Z, et al. Mycoplasma pneumoniae among Chinese outpatient children with mild respiratory tract infections during the coronavirus disease 2019 pandemic. Microbiol Spectr. 2022;10(1):e0155021.
- [30] Zhou D, del Valle-Mendoza J, Orellana-Peralta F, et al. High Prevalence of Mycoplasma pneumoniae and Chlamydia pneumoniae in Children with Acute Respiratory Infections from Lima, Peru. Plos One. 2017;12(1):e0170787.
- [31] Ambooken B, Balakrishnan PP, Asokan N, et al. Ulcerated lobular panniculitis: An unusual initial presentation of anti-Mi-2-alpha positive dermatomyositis. Indian Journal of Dermatology, Venereology and Leprology. 2022;88:381-4.
- [32] Silva SJRd, Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13:100287.
- [33] Wang A, Wu Z, Huang Y, et al. A 3D-printed microfluidic device for qPCR detection of macrolide-resistant mutations of mycoplasma pneumoniae. Biosensors. 2021;11(11):427.
- [34] Zhan X-W, Deng L-P, Wang Z-Y, et al. Correlation between Mycoplasma pneumoniae drug resistance and clinical characteristics in bronchoalveolar lavage fluid of children with refractory Mycoplasma pneumoniae pneumonia. Italian Journal of Pediatrics. 2022;48(1).
- [35] Li H, Li S, Yang H, et al. Resurgence of Mycoplasma pneumonia by macrolide-resistant epidemic clones in China. The Lancet Microbe. 2024.
- [36] Xu M, Li Y, Shi Y, et al. Molecular epidemiology of Mycoplasma pneumoniae pneumonia in children, Wuhan, 2020–2022. BMC Microbiology. 2024;24(1).
- [37] Xing F-F, Chiu KH-Y, Deng C-W, et al. Post-COVID-19 pandemic rebound of macrolide-resistant Mycoplasma pneumoniae infection: A descriptive study. Antibiotics. 2024;13(3):262. Tables


