ABSTRACT
Background and aims: Autoimmune liver diseases are rare diseases, and population-based studies on the epidemiology of autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) are sparse. We aimed to assess the incidence of AIH, PBC, and PSC in the Faroe Islands.
Methods: All cases of AIH, PBC, and PSC diagnosed in the Faroe Islands between January 1st, 2004, and December 31st, 2021, were included in this nationwide registry-based cohort study. In addition, we searched all medical records to assess diagnostic criteria and cause of death.
Results: The incidences of AIH, PBC, and PSC in the Faroe Islands were 5.2, 2.5 and 0.7 per 100,000 population per year, respectively. Point prevalence per 100,000 population on December 31st 2021, was 71.8 for AIH, 38.5 for PBC, and 11.0 for PSC. Nine AIH patients died after a median of 3 years, three died of hepatocellular carcinoma (HCC), and two died of liver failure. Five PBC patients died after a median of 7 years, one of HCC and one of liver failure. One PSC patient died of cholangiocarcinoma.
Conclusion: The incidence and prevalence of AIH, PBC and PSC in the Faroe Islands are among the highest reported in population-based settings.
Introduction
Autoimmune liver diseases are rare diseases, and population-based studies on the epidemiology of autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) are sparse [Citation1,Citation2]. However, recent studies demonstrated an increase in the incidence of AIH, PBC, and PSC [Citation1,Citation3–5].
AIH is an autoimmune, chronic, inflammatory liver disease affecting hepatocytes in the liver. A recent literature review demonstrated an AIH incidence of approximately 1–2 per 100,000 population per year [Citation6], and a prevalence of 5 to 25 per 100,000 population in European countries and the Asia-Pacific region [Citation7]. The highest numbers came from Scandinavian countries, e.g. 24 per 100,000 population in Finland [Citation1]. The 10-year mortality ranges from 23% to 32% [Citation3,Citation8].
PBC is an autoimmune, chronic, inflammatory liver disease affecting the intrahepatic small bile ducts. The incidence of PBC is approximately 1–2 per 100,000 population per year with a variation from 0.23 to 4.3 per 100,000 population per year [Citation6], and the prevalence ranges from 1.9 to 40.2 per 100,000 population [Citation2]. The 10-year mortality ranges from 41% to 62% [Citation9].
Some patients display the characteristics of both PBC and AIH based on clinical, biochemical, immunological, or histological features at different stages during their disease. Additional diagnostic criteria report the proportion of PBC-AIH overlap syndrome (OS) among AIH patients and PBC patients between 2% and 20% [Citation10,Citation11].
PSC is an autoimmune liver disease with the affection of larger intra- and/or extrahepatic bile ducts with inflammation and liver fibrosis. PSC may occur in the presence or absence of inflammatory bowel disease (IBD) [Citation12]. The incidence of PSC ranges from 0.0 to 2.0 per 100,000 population per year. There was no incident PSC patient among natives in Alaska [Citation6], while the incidence and prevalence in Europe and North America were 0.6 and 0.5 per 100,000 population per year in Europe and North America, respectively. The prevalence of PSC shows inter-regional differences from 0.0 to 31.7 per 100,000 population per year [Citation13]. The prevalence of PSC in Europe ranges from 0.2 to 1.6 per 100,000 population [Citation2]. The 10-year mortality ranges from 8% to 15% [Citation14,Citation15].
As demonstrated above, the incidence and prevalence of autoimmune liver diseases vary, but in general, there seems to be a higher incidence and prevalence of autoimmune liver diseases in the Nordic Countries [Citation6]. However, no data on autoimmune liver diseases from the Faroe Islands exist. Epidemiological studies have shown a high incidence and prevalence of inflammatory bowel disease (IBD) in the Faroe Islands [Citation16]. Genetic studies of Familial recurrent intrahepatic cholestasis (FRIC) [Citation17], open-angle glaucoma [Citation18], and carnitine transporter defect [Citation19] have all demonstrated a high incidence of single gene disorders. Therefore, we aimed to investigate the incidence, prevalence, and cause of death in patients with autoimmune liver diseases in the Faroe Islands.
Study population and material
The Faroe Islands are an archipelago between Norway and Iceland in the North Atlantic Ocean. Healthcare in the Faroe Islands is a high-quality system financed through statutory health insurance and taxes. The Faroese population is isolated and consists of descenders from Scandinavia and the British Isles, who founded the islands in the 9th century. The population is stable, and migration has been low [Citation20,Citation21].
We conducted a nationwide registry-based cohort study of Faroese inhabitants diagnosed with AIH, PBC, and PSC between January 1st, 2004, and December 31st, 2021. The diagnosis of AIH, PBC and PSC were based on their medical records and from the National Patient Registry in the Faroe Islands.
General practitioners refer patients with elevated liver enzymes to the Gastro Unit at the National Hospital of the Faroe Islands for further examination. The Gastro Unit is the only centre to diagnose, treat and follow up on patients with chronic liver diseases in the Faroe Islands.
The National Hospital of the Faroe Islands laboratory analysed blood samples for immunoglobulins; the Department of Clinical Immunology, Vejle Sygehus, Denmark, analysed blood samples for Antinuclear antibodies (ANA); and the Department of Clinical Immunology, Odense University Hospital, Denmark, analysed blood samples for smooth muscle antibodies (SMA), and antimitochondrial antibodies (AMA).
Liver biopsies were performed at the Departments of Internal Medicine and Radiology, National Hospital of the Faroe Islands. Histopathology examinations of the liver were performed at the Department of Pathology, Rigshospitalet, Copenhagen, Denmark.
Data sources
The Faroese patient registry uses The International Classification of Diseases, 10th edition (ICD-10). Therefore, we extracted and searched medical records with the diagnostic codes for AIH: K73.2 and K75.4, PBC: K74.3, and PSC: K83.0. In addition, we included all medical records of patients with the diagnosis of “Chronic hepatitis, not elsewhere classified” (K73.0 to K73.9), “Fibrosis and cirrhosis of the liver” (K74.0 to K74.6), “Other inflammatory liver diseases” (K75.0 to K75.9), and “Other diseases of biliary tract” (K83.0 to K83.9). For further classification, positive cases of AIH had to complete the Simplified Diagnostic Scoring System of the International Autoimmune Hepatitis Group (SC-IAIHG) [Citation22], positive cases of PBC had to fulfil at least two out of three criteria: Presence of AMA (>40 U/L); ALP > 2 ULN or GGT > 5 ULN; or liver biopsy specimen showing florid bile duct lesions [Citation23], and positive cases of PSC had to fulfil elevated cholestatic enzymes and cholangiogram [Citation23–25]. We evaluated all PBC and AIH cases according to the “Paris criteria” for diagnosing PBC/AIH overlap syndrome (OS), which requires at least two of three criteria for diagnosing PBC and AIH [Citation10]. We calculate positive cases of OS in the AIH cohort.
Statistical analysis
We used Rstudio version 4.1.2 for statistical analyses [Citation26].
Results
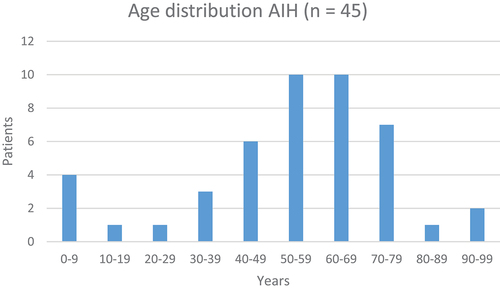
Forty-five patients fulfilled the IAIHG Score Criteria. Twenty-four scored above seven, indicating a definite diagnosis, and 21 scored above six, indicating a possible diagnosis. The female/male ratio was 3 to 1. The median age was 54 (range 3 to 93); age data are demonstrated in .
We diagnosed between 0 and 5 AIH patients yearly from 2004 to 2021. Conversion to incidence gives a mean AIH incidence of 5.2 per 100,000 inhabitants (range 0.0 to 12.5). The prevalence of AIH was 71.8 per 100,000 population on December 31st, 2021. Seven patients fulfilled the Paris PBC/AIH overlap syndrome (OS) criteria [Citation27,Citation28]. The PBC/AIH-OS patients accounted for 15.6% of the AIH cohort. Seven AIH patients died ().
Table 1. Death causes of patients with autoimmune liver diseases in the Faroe Islands.
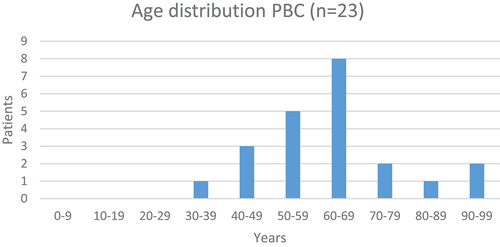
Twenty-three patients fulfilled the PBC criteria [Citation29,Citation30]. The female/male ratio was 10 to 1. The median age was 61 (range 32 to 92); age data are demonstrated in .
We diagnosed between 0 and 3 PBC patients yearly from 2004 to 2021. Conversion to incidence gives a mean PBC incidence of 2.5 per 100,000 population (range 0 to 7.6). The prevalence of PBC was 43 per 100,000 population on December 31st, 2021. Five PBC patients died ().
We identified seven PSC patients, and six of these patients had PSC-IBD. All had ulcerative colitis (UC). Two patients had UC as their first diagnosis (range 11 and 20 months from UC to PSC), and five patients had PSC as their first diagnosis (range 5 to 56 months from PSC to UC). The mean PSC incidence from 2004 to 2021 was 0.7 per 100,000 population (range 0 to 4.2). The median age was 45 years (range 8 to 59), and 86% were men. One patient died (). The prevalence of PSC was 11 per 100,000 population on December 31st, 2021.
Our extensive search of autoimmune liver diseases among liver diseases did not reveal any patient misclassified without a diagnosis of autoimmune liver diseases.
Discussion
This study is the first to report the epidemiology of autoimmune liver diseases in the Faroe Islands. The main findings demonstrated the highest nationwide incidence and prevalence of AIH. Similarly, we observed an increased incidence and prevalence of PBC, while there were only a few cases with PSC and thus an uncertain PSC incidence.
We observed a very high AIH incidence of 5.2 per 100,000 population per year, which is higher than reported in nationwide registry-based studies in Denmark (1.7 per 100,000 per year) [Citation3], Finland (1.1 per 100,000 per year) [Citation1], Iceland (2.2 per 100,000 per year) [Citation31], Sweden (0.9 per 100,000 per year) [Citation32], the United Kingdom (2.1 per 100,000 per year) [Citation4], and a centre study from Norway (1.9 per 100,000 per year) [Citation5].
Like other studies, the prevalence of OS in the AIH cohort was between 2% and 20% (15,6%). However, this study is too small and uncertain about comparing death rates between AIH and PBC/AIH overlap syndrome.
The age- and gender composition of the AIH cohort displayed a typical presentation, with a significant part being women and a minor part of men and children [Citation3].
Recent studies from Denmark, Finland and the United Kingdom showed an increased incidence of AIH [Citation1,Citation3,Citation4]. Our study, however, is too small to detect an increase.
There is a high awareness of autoimmune diseases in the Faroese population and among Faroese health professionals. The high incidence and prevalence of other autoimmune diseases in the Faroe Islands, such as inflammatory bowel disease, alopecia areata, and psoriasis, can explain the co-existing high incidence of AIH [Citation16,Citation33].
The high incidence may indicate either a genetic predisposition or an environmental factor of autoimmune diseases, as indicated in inflammatory bowel disease [Citation34,Citation35]. However, predisposition is multifactorial and complex, suggesting a combination of genetic and environmental factors [Citation36]. Of interest, an Icelandic study indicates that the increasing use of biologics may be associated with rising AIH incidence [Citation31]. In our study, only a 56-year-old woman with ulcerative colitis (UC) developed AIH.
The results of this study also demonstrate a high incidence and prevalence of PBC. The incidence and prevalence were equal to studies in North America and Europe [Citation37,Citation38]. The PBC cohort consisted of 91% women in the fifth and sixth decade of life, which is a typical PBC distribution [Citation39,Citation40].
The high incidence and prevalence of PBC and AIH in the Faroe Islands is an excellent opportunity to investigate familial aggregations and genetics using the Faroese national nationwide genealogy registry for data collection and whole-genome sequencing. The Faroese national nationwide genealogy registry is located at the Genetic Biobank and covers the Faroese population from approximately 1650 to date [Citation41].
In this relatively short study, five of nine AIH patients, two of five PBC patients, and two of two PSC patients died of cancer. This high cancer death rate suggests that AIH, PBC and PSC are predisposing factors for cancer [Citation42–44]. The PSC cohort demonstrated an increased link between inflammatory bowel disease and PSC [Citation2,Citation15].
The strength of this study is the nationwide population-based setting, with the referral of all cases of AIH, PBC and PSC to one department. The Faroese medical system is comparable to other Scandinavian medical systems. For future studies, MRI and other diagnostic tools will replace liver biopsies. The limitations of this study lie in the small-sized cohort, where incidence and prevalence will be significantly affected by only one additional or reduced diagnosed patient.
Conclusion
The incidence and prevalence of AIH in the Faroe Islands are the highest reported in population-based settings, and the incidence and prevalence of PBC are amongst the highest reported in population-based settings. These results call for further local studies in the Faroe Islands to focus on disease course, familial aggregation, inheritance and environmental factors.
Authorship
KRN initiated the study. KRN and JM contributed to the design and implementation of the study. KRN, JM, HLJ and HG contributed to the preparation of the manuscript. KRN did the data analyses. All authors approved the final version of the manuscript.
Abbreviations
AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease; ANA, antinuclear antibody; SMA, smooth muscle antibody; AMA, antimitochondrial antibody; ICD-10, The international classification of diseases, 10th edition; SG-IAIHG, the simplified diagnostic scoring system of the international autoimmune hepatitis group; OS, PBC/AIH overlap syndrome; UC, ulcerative colitis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Puustinen L, Barner-Rasmussen N, Pukkala E, et al. Incidence, prevalence, and causes of death of patients with autoimmune hepatitis: a nationwide register-based cohort study in Finland. Digestive Liver Dis. 2019;51(9):1294–6.
- Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188.
- Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60(3):612–617.
- Grønbæk L, Otete H, Ban L, et al. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997-2015. A population-based cohort study. Liver Int. 2020;40(7):1634–1644.
- Boberg KM, Aadland E, Jahnsen J, et al. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33(1):99–103.
- Jepsen P, Grønbæk L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(Suppl 2):2–12.
- Tanaka A. Autoimmune Hepatitis: 2019 Update. Gut Liver. 2020;14(4):430–438.
- Sharma R, Verna EC, Söderling J, et al. Increased mortality risk in autoimmune hepatitis: a nationwide population-based cohort study with histopathology. Clin Gastroenterol Hepatol. 2021;19(12):2636–2647.e13.
- Marschall HU, Henriksson I, Lindberg S, et al. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci Rep. 2019;9(1). doi:10.1038/S41598-019-47890-2
- Chazouillères O, Wendum D, Serfaty L, et al. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28(2):296–301.
- Rust C, Beuers U, Of Gastroenterology P. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14(21):3368. doi: 10.3748/wjg.14.3368. Published online 2008.
- Loftus EV, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54(1):91–96. doi: 10.1136/gut.2004.046615
- Mehta TI, Weissman S, Fung BM, et al. Global incidence, prevalence and features of primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Int. 2021;41(10):2418–2426.
- Carbone M, Kodra Y, Rocchetti A, et al. Primary sclerosing cholangitis: burden of disease and mortality using data from the national rare diseases registry in Italy. doi:10.3390/ijerph17093095
- Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58(6):2045–2055.
- Hammer T, Nielsen KR, Munkholm P, et al. The Faroese IBD study: incidence of inflammatory bowel diseases across 54 years of population-based data. J Crohns Colitis. 2016;10(8):934–942.
- Tygstrup N, Steig B, Juijn JA, et al. Recurrent familial intrahepatic cholestasis in the Faeroe Islands. Phenotypic heterogeneity but genetic homogeneity. Hepatology. 1999;29(2):506–508.
- Holm E, Holm M, Vilhelmsen K, et al. Prevalence of open-angle glaucoma in the Faroese population. J Glaucoma. 2022;31(2):72–78.
- Rasmussen J, Nielsen OW, Janzen N, et al. Carnitine levels in 26,462 individuals from the nationwide screening program for primary carnitine deficiency in the Faroe Islands. J Inherit Metab Dis. 2014;37(2):215–222.
- Als TD, Jorgensen TH, Børglum AD, et al. Highly discrepant proportions of female and male Scandinavian and British Isles ancestry within the isolated population of the Faroe Islands. Eur J Hum Genet. 2006;14(4):497–504.
- Jorgensen TH, Butteschön HN, Wang AG, et al. The origin of the isolated population of the Faroe Islands investigated using Y chromosomal markers. Hum Genet. 2004;115(1):19–28.
- Czaja AJ. Diagnosis and management of autoimmune hepatitis: current status and future directions. Gut Liver. 2016;10(2):177–203.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–267. doi:10.1016/J.JHEP.2009.04.009
- Björnsson ES, Kalaitzakis E. Recent advances in the treatment of primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol. 2021;15(4):413–425.
- Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020;72(2):671–722.
- R Core Team. R: a language and environment for statistical computing. www.R-project.org.
- Kuiper EMM, Zondervan PE, van Buuren HR. Paris criteria are effective in diagnosis of primary biliary cirrhosis and autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol. 2010;8(6):530–534.
- Zhang W, De D, Mohammed KA, et al. New scoring classification for primary biliary cholangitis-autoimmune hepatitis overlap syndrome. Hepatol Commun. 2018;2(3):245–253. doi:10.1002/HEP4.1148
- Hirschfield GM, Chazouillères O, Cortez-Pinto H, et al. A consensus integrated care pathway for patients with primary biliary cholangitis: a guideline-based approach to clinical care of patients. Expert Rev Gastroenterol Hepatol. 2021;15(8):929–939. doi:10.1080/17474124.2021.1945919
- Hirschfield GM, Beuers U, Corpechot C, et al. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi:10.1016/J.JHEP.2017.03.022
- Valgeirsson KB, Hreinsson JP, Björnsson ES. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int. 2019;39(12):2341–2349.
- Werner M, Prytz H, Ohlsson B, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2009;43(10):1232–1240. doi: 10.1080/00365520802130183
- Jacobsen EW, Pedersen OB, Andorsdóttir G, et al. Family recurrence risk of alopecia areata in the Faroe Islands. Clin Exp Dermatol. 2019;44(7):e224–e229.
- Hammer T, Lophaven SN, Nielsen KR, et al. Inflammatory bowel diseases in Faroese-born Danish residents and their offspring: further evidence of the dominant role of environmental factors in IBD development. Aliment Pharmacol Ther. 2017;45(8):1107–1114.
- Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63(4):588–597. doi:10.1136/gutjnl-2013-304636
- Machado M, del Pilar Fortes M, Gil G, et al. Genetic contribution of major histocompatibility complex class II region to type 1 autoimmune hepatitis susceptibility in Venezuela. Liver Int. 2007;27(10):1409–1416.
- Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565–1575.
- Gazda J, Drazilova S, Janicko M, et al. The epidemiology of primary biliary cholangitis in European countries: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2021;2021:1–11.
- Lucey MR, Neuberger JM, Williams R. Primary biliary cirrhosis in men. Gut. 1986;27(11):1373–1376.
- Neuberger J, Lombard M, Galbraith R. Primary biliary cirrhosis. Gut. 1991;Suppl(Suppl):S73–S78.
- Apol KD, Lydersen LN, Mortensen Ó, et al. FarGen – participants in the genetic research infrastructure of the Faroe Islands. Scand J Public Health. 2022;50(7):980–987. Published online 2021. doi:10.1177/14034948211046817
- Czaja AJ. Hepatocellular carcinoma and other malignancies in autoimmune hepatitis. Dig Dis Sci. 2013;58(6):1459–1476.
- Bosch DE, Zen Y, Boukhar SA, et al. Hepatocellular carcinoma in primary sclerosing cholangitis and primary biliary cholangitis: a clinical and pathological study in an uncommon but emerging setting. Virchows Arch. 2021;479(6):1131–1143.
- Trivedi PJ, Lammers WJ, Van Buuren HR, et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 2016;65(2):321–329.