ABSTRACT
Response to treatment usually depends on the time of drug administration. Interleukin-22 (IL-22) is known for its protective effect against liver injury. Therefore, the aim was to study the effectiveness of the IL-22 treatment at different circadian timing on S. mansoni-infected mice. Mice grouping included; control group, mice infected with cercariae, and IL-22-treated groups. Treatment with IL-22 (0.36 µg/kg) was performed on infected mice either at 7 am, or 7 pm. Hepatic granuloma index (GI), levels of tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17), IL-22, and immunoglobulin E (IgE) were measured. In addition, hepatic expressions of signal transducer and activator of transcription 3 (STAT3) and β-catenin genes were estimated. Infection with S. mansoni increased pro-inflammatory parameters, STAT3, and β-catenin mRNA significantly (P < 0.05) compared to the control group. IL-22 groups showed a significant reduction (P < 0.05) in liver GI, TNF-α, and β-catenin mRNA compared to infected mice. Moreover, it enhanced STAT3 gene expression (P < 0.05). IL-22 administration at 7 am reduced GI, IL-17, and IgE levels significantly (P < 0.05) compared to 7 pm values. The conclusion, IL-22 might have an immunotherapeutic effect. Its administration at 7 am was more effective in reducing hepatic granuloma and IgE, but further studies are needed to support these findings.
Introduction
Human schistosomiasis is a parasitic disease caused by trematodes of the genus Schistosoma. Nearly 240 million individuals worldwide are infected with schistosomiasis, and more than 700 million people living in endemic regions are at risk of infection [Citation1]. Acute and chronic stages of schistosomiasis can be distinguished from one another [Citation2]; the acute stage might be linked with a Th1 response [Citation3]. When many Schistosoma eggs laid, a quick change from a Th1 response to a Th2-dominated response will then occur [Citation4].
Parasite treatment depends on the use of a single drug which poses serious concerns regarding the onset of resistance [Citation5–7]. Praziquantel (PZQ), a common drug in treating Schistosoma infection since 1970, due to its broader spectrum [Citation8,Citation9]. Although, it was reported to induce hemorrhage in the lungs, abdominal pain, headache, nausea, and diarrhea, and it is secreted in breast milk [Citation10]. The potential resistance problems of Schistosoma to PZQ necessitate searching for alternative drugs to be helpful in the treatment of schistosomiasis. Additionally, the search for bioactive products against Schistosoma has great importance for establishing future strategies to control schistosomiasis [Citation5–7].
IL-22 preserve hepatocytes against liver damage in various mouse models including adenovirus-induced or T cell-mediated hepatitis [Citation11,Citation12]. Additionally, IL-22 protects the host from drug-induced hepatocellular injury [Citation13] and plays a hepatoprotective role during liver inflammation/fibrosis of chronic hepatitis B [Citation14].
STAT3 is a member of the STAT protein family. In response to cytokines and growth factors, it is phosphorylated by receptor-associated Janus kinases it acts as a transcription activator in the cell nucleus. STAT3 becomes activated in response to ligands such as, interferons, epidermal growth factor (EGF), interleukin-5, IL-6, and IL-22 [Citation15,Citation16]. STAT3 mediates the expression of a variety of genes and plays a key role in many cellular processes such as, cell growth and apoptosis [Citation17]. Studies using animal models of hepatic damage suggests that STAT3 prevents fibrosis, mainly because of its hepatoprotective and proliferative properties [Citation18–20].
The β-catenin is a protein acting as an adhesion molecule and a transcription factor [Citation21]. Its function is mainly regulated by Wnt proteins [Citation22]. The β-catenin is responsible for interactions and adhesions between stellate cells [Citation23,Citation24]. The stimulation of stellate cells is related to fibrosis of liver fibrosis in several diseases, including alcoholic steatohepatitis, nonalcoholic steatohepatitis, and viral hepatitis [Citation25].
Various features, components, and functions of the immune system exhibit daily variations. Immunocompetent cell counts and cytokine levels show variations according to the time of the day and the sleep-wake cycle. Moreover, different immune cell types, such as macrophages, natural killer cells, and lymphocytes, contain circadian molecular clockwork. These clocks regulate the function of the immune system, including their response to signals and their effector functions [Citation26]. In addition, the parasites causing diseases have circadian rhythmicity. They slightly alter their composition and function according to the different hours of the day. One of the consequences of such alterations is that parasites become more sensitive to a certain drug at a specific time of the day [Citation27].
Chronotherapy is a new therapeutic dosing strategy that aims to synchronize the timing of drug administration with circadian rhythms [Citation28]. In chronotherapy, the drug availability in vivo is timed to match illness cycles to enhance therapeutic outcomes and minimize negative effects. Many medications have an interdependent relationship between their peak-to-trough rhythmic activity in illness symptoms and risk factors, as well as their pharmacologic sensitivity and pharmacokinetics [Citation29].
Our previous study, showed a protective role of IL-22 against S. mansoni soluble egg antigen-induced granuloma in vitro [Citation30]. In this work, we aimed to investigate the potential chronotherapeutics effect of IL-22.
Materials and methods
Experimental animals
Thirty-two male albino mice (8 weeks old, weighing 21.5 ± 2.5 g) were purchased from TBRI, Giza, Egypt. Animals were kept under 12 h light/12 h dark cycles with light intensity 600 lux, and at a temperature of 25 ± 3°C. The study was approved by the Animal Ethics Committee of the Zoology Department, Faculty of Science, Helwan University (approval no. HUIACUC/Z/AS2903–28).
Mice were divided into four groups. In the control group, mice were injected intraperitoneally with PBS. In the infected group, mice were injected subcutaneously with approximately 40 cercariae of S. mansoni for 45 days. IL-22 groups; infected mice were treated with IL-22 either at 7 am or 7 pm for 14 days. Recombinant mouse interleukin-22 protein was purchased from R&D systems (Catalog no. 582-ML-010/CF, USA) as a lyophilized endotoxin-free product. It was dissolved in a phosphate buffer solution, and the dosage for each mouse was 0.36 µg/kg [Citation30]. shows the animal grouping and treatment protocol.
Table 1. The animal grouping and treatment protocol.
The experiment lasted for 14 days then the animals were sacrificed by decapitation, after six h from the last treatment. Mice from the control group, infected group, and 7 am treated group were sacrificed, during artificial light (600lux). IL-22 treated group at 7 pm was sacrificed at low light intensity (3 lux). Blood samples were collected and left for half an hour and then centrifuged at 500 g for 15 min at four °C to separate serum. The liver was removed and weighed. Relative weights of the liver in the infected and treated groups were calculated relating to the control group. The liver in each mouse was divided into two parts: one part was used for the histopathological examination and the second part was kept frozen in −80 for the molecular techniques. Sera were frozen at −80 for measuring cytokines and IgE.
Histopathological examination
Samples from liver tissues (n = 5/group) were fixed in neutral formalin (10%), dehydrated, cleared, embedded in paraffin, sectioned (5 µm thick), hydrated and stained with hematoxylin and eosin (H& E) for histopathological examination [Citation31].
Measurement of granuloma area
The distance between liver sections should be at least 250 µm to avoid measuring the same granuloma. In each liver section, 10 granulomas with visible central eggs were randomly selected; their diameters were measured at 10× magnification using the Image J program (version 1. x; Image J Software, U.S). Two perpendicular maximal diameters were measured, getting the mean diameter for each granuloma and then calculating the mean granuloma area for each mouse in a group.
Measurement of cytokines and immunoglobulin E (IgE) by ELISA
TNF-α, IL-17, IL-22 and IgE were measured using Sandwich-ELISA method according to the manufacturer’s instructions. (Mouse ELISA kit, Sun Long Biotech, China, Catalog no. SL0547Mo, SL0314Mo, SL0865Mo, and SL0597Mo, respectively).
Real-time polymerase chain reaction (RT-PCR)
The extraction of mRNA was performed by the TRIzol reagent method (ThermoFisher Scientific, USA). The quality and integrity of mRNAs were determined using the Agilent RNA 6000 Nano Kit on the Agilent 2100 Bioanalyzer (Agilent Technologies). mRNA was quantified by measuring A260 nm on the ND-1000 Spectrophotometer (NanoDrop Technologies). Extracted mRNA was reversed transcribed to cDNA using the Thermo scientific reverted first stander cDNA synthesis kit (ThermoFisher Scientific, Lithuania). All PCR runs were performed on the Applied Bio-systems 7500 instrument.
Real-time PCR reactions of mRNA were performed with Applied Bio-systems 7500 system using the QuantiTeh SYBR Green PCR Master Mix Kit (QIAGEN). The relative gene expression of STAT3 and β-catenin was normalized to the level of GAPDH. The sequences of primers were showed in . Fold changes of STAT3 and β-catenin studied in the present samples were calculated using the comparative CT method, and the 2−ΔΔCt was calculated according to the formula of Pfaffl [Citation32]. All reactions were performed in triplicates for each sample. At least three independent experiments were carried out for each experimental condition. GAPDH was an internal reference for the STAT3 and β-catenin primers.
Table 2. Primers sequences of genes analyzed by real time PCR.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism (version 3.0; Graph Pad Software). One-way analysis of variance (ANOVA) was used for comparison between different treatments. T-test analysis was used for comparison between IL-22 at 7 am and 7 pm groups. P-values <0.05 were considered significant.
Results
Relative weight of liver
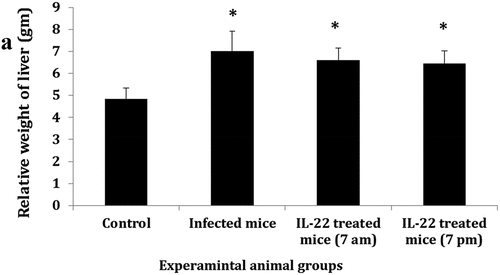
The relative weight of the liver showed a significant increase (P < 0.05) in the infected mice. IL-22 treatment either at 7 am or 7 pm also increased the liver relative weight significantly (P < 0.05) compared to the control group. In contrast, the relative weight of the liver was decreased (non-significant change) in both treated groups compared to the infected one ()
Histopathological changes and granuloma index (GI)
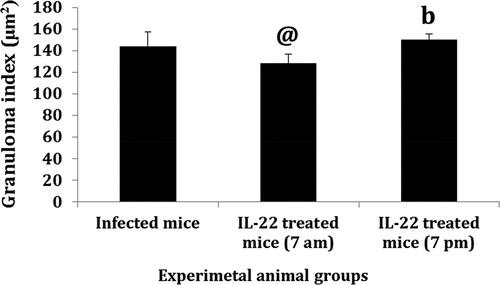
The control group showed a normal structure of the liver with hepatic lobules, and hepatic strands surrounding a central vein. In the infected and treated mice, several granulomas appear in the histological sections, but their diameters were diminished in the treated groups () Hepatic granuloma of infected mice appeared with central eggs and was surrounded by fibro-collagen bundles entangling fibroblasts and inflammatory cells (). A significant decrease (P < 0.05) in the hepatic GI was detected in S. mansoni-infected mice treated with IL-22 at 7 am, while a non-significant decrease was observed in the 7 pm treated group compared to S. mansoni- infected mice ()
Figure 2. Photomicrographs of sections of liver showing a normal liver structure with hepatic strands (H) surround central veins (v) in the control mice. Sections of S. mansoni-infected mice showing a number of granuloma (G) with eggs (E). Sizes of the granuloma in IL-22 (0.36 µg/kg) treated groups appear smaller than the infected group (H&E, scale bar = 100 μm).
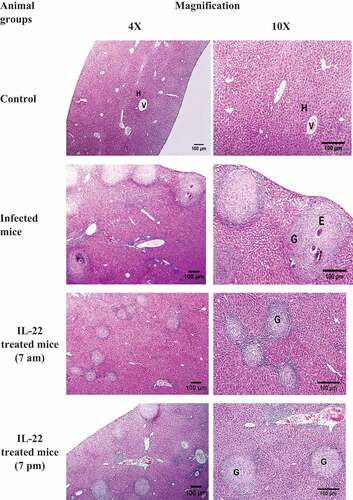
Figure 3. A photomicrograph of sections of infected liver showing a high magnification (400X) of granuloma (G) with central eggs (E), surrounded by fibro-collagen bundles entangling fibroblasts (arrows) and inflammatory cells (IC) (H&E, scale bar = 100 µm).
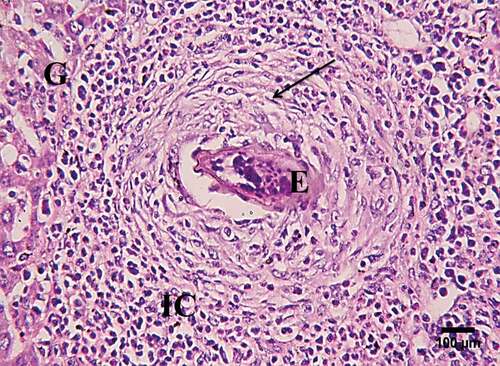
Figure 4. Granuloma index (GI) in the liver of S. mansoni-infected mice treated with IL-22 at 7 am or 7pm.
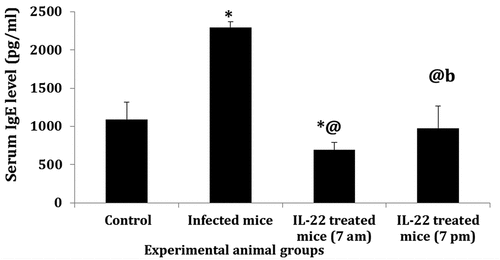
Sera cytokines’ level
A significant increase (P < 0.05) in the serum level of TNF-α was recorded in the infected mice and IL-22 at 7 pm treated group compared to the control mice. In contrast, a significant reduction in its level was observed after IL-22 treatment (P < 0.05) compared to the infected mice. Non-significant changes were detected between the two treated groups (). On the other hand, the serum level of IL-17 showed a significant increase (P < 0.05) in S. mansoni-infected mice and mice treated with IL-22 compared to the control group. In comparison with the infected group, IL-17 levels significantly (P < 0.05) increased after the treatment with IL-22 either at 7 am or 7 pm. In addition, IL-17 was significantly elevated in the 7 pm treated group compared with the 7 am one (). Moreover, the serum IL-22 levels increased significantly (P < 0.05) in the infected mice compared to the control. On the contrary, a significant decrease (P < 0.05) in its level was recorded, in both treated groups, compared to the control and infected groups. Levels of IL-22 were more reduced (P < 0.05) in the 7 am treated group than the 7 pm treated one ().
Figure 5. Serum cytokine level in S. mansoni-infected mice treated with IL-22 at 7 am or at 7pm. a: TNF-α (Tumor necrosis factor alpha), b: IL-17(Interleukin 17), c: IL-22 (Interleukin 22).
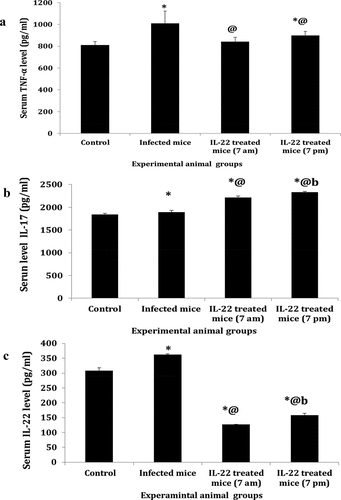
Serum level of immunoglobulin E (IgE)
A significant increase (P < 0.05) in the serum IgE level was recorded in S. mansoni–infected mice compared to the control group. On the contrary, its levels were decreased in mice treated with IL-22 at 7 am or 7 pm compared to the infected mice. On the other hand, serum IgE levels showed a significant increase (P < 0.05) in mice treated with IL-22 at 7 pm as compared to mice treated at 7 am ()
Figure 6. Serum IgE level in S. mansoni-infected mice treated with IL-22 at 7 am or at 7pm.
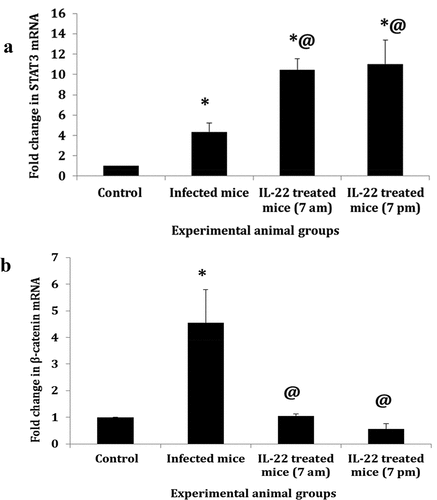
Hepatic STAT3 and β-catenin gene expression
The present data showed a significant upregulation (p < 0.05) of hepatic STAT3 mRNA in the infected mice (fold, 4.3) and those treated with IL-22 either at 7 am (fold, 10.4) or at 7 pm (fold, 11.0) compared to control mice. Significant upregulation of STAT3 mRNA was also recorded in both treated groups compared to the infected mice (). Additionally, the expression level of β-catenin mRNA was upregulated significantly in the infected mice, compared to the control, by fold change 4.3. Significant downregulation in β-catenin mRNA was observed after IL-22 treatment either at 7 am or 7 pm compared to the infected group ().
Figure 7. The expression level of mRNA STAT3 (a) and β-catenin (b) in S. mansoni-infected mice treated with IL-22 at 7 am or at 7 pm.
Discussion
Chrono-therapeutic drug delivery offers a novel strategy in the planning of pharmacologic interventions for the efficient treatment of numerous diseases. Drug efficiency increased when delivered to the target organ at the optimum timing [Citation33,Citation34]. Several studies indicated the protective function of IL-22 through the inflammation of the liver [Citation11,Citation35,Citation36]. Therefore, the present work studied the efficiency of IL-22 administration at different times of the day for treating schistosomiasis. First, the study surveyed the inflammatory status in the infected mice after IL-22 treatment. A reduction in the pro-inflammatory cytokine levels, TNF-α, was found which is in agreement with Prado et al. [Citation37] who found an elevation in TNF-α and IL-1 β levels in IL-22−/− mice which was associated with an increase in lung inflammation. Further, the treatment with IL-22 in this study induced an elevation in the IL-17 while reducing IL-22 levels in the serum compared to infected mice. IL-17 and IL-22 act in concert as they provide a barrier against extracellular pathogens. IL-17A and IL-17F are common pro-inflammatory mediators. While, IL-22 seems to be a new type of immune mediator that enhances the immune defenses of tissue cells, inhibits tissue damage, and promotes their regeneration [Citation38]. The reduction in IL-22 level after its external administration may be due to a negative feedback mechanism. Additionally, the half-life of recombinant human IL-22is less than 2 h in animals [Citation39].
Further, IL-17 and IL-22 were more elevated after the administration of IL-22 at night onset than during the day in this study. Similarly, Wang et al. [Citation40] found a circadian rhythm in IL-17 and IL-22 secretion in type 3 innate lymphoid cells (ILC3) with a peak at the onset of the dark phase. In addition, Yu et al. [Citation41] reported that Th17 frequency which is characterized by expressing many regulatory cytokines, including IL-17 was increased in mice with colitis in the active cycle (at night). Circadian clock modulates Th 17 function. The retinoic acid receptor-related orphan nuclear receptor (RORγt) regulates the differentiation of the pro-inflammatory Th17 cells and induces the expression of IL-17 and IL17F, whereas Rev-erbα antagonizes the function of RORγt by binding the same DNA motif [Citation42,Citation43] Accordingly, Th17 lineage specification varies diurnally and is altered in Rev-erbα−/− mice [Citation41]. Moreover, IL-22 production is impaired in Rev-erbα−/− mice [Citation40].
IgE antibody is mainly involved in allergic reactions to environmental allergens, though it is an important component of host-protective immune responses against helminthic parasites [Citation44]. In the present study, infection with S. mansoni increased IgE level. Lynch et al. [Citation44] suggested that parasitic infections enhance the generation of anti-parasite IgE antibodies and the nonspecific polyclonal IgE that causes an elevation in total serum IgE levels. Herein, IL-22 treatment diminished serum IgE levels to reach normal levels, which may be valuable for defense against parasites. Consistent with this result, Amiri et al. [Citation45] found that the anti-IgE treatment of S. mansoni-infected mice decreased worm burden and egg production.
IL-22 administration at the light onset reduced IgE levels more than when it administered at night. This result agreed with Zheng et al. [Citation46] who found that IgE-mediated acute-type allergy response in the skin was elevated in the active phase (at night) with immune response increase. Moreover, IgE/mast cell-mediated allergic reactions in vivo are temporally controlled by the circadian clock [Citation47].
In the present study, STAT3 gene expression was higher in IL-22-treated groups than in infected ones. Several studies revealed a protective role of STAT3 in the intestine during experimental colitis [Citation48–50]. The mechanism involves the secretion of IL-22 by innate immune cells which evokes STAT3 signaling. Herein, the elevation of STAT3 gene expression was linked with the reduction of TNF-α and the elevation of IL-17. Previous studies revealed the anti-inflammatory role of STAT3 [Citation51,Citation52]. Its deficiency caused excessive production of pro-inflammatory cytokines, including TNF-α, IL-6, IL-12, and IFN-γ in macrophages, neutrophils, and DCs [Citation53]. At the molecular level, STAT3 stimulates the expression of the Th17 lineage-specifying factors RORᵧ and RORα, which are required for Th17 development and IL-17 expression [Citation54–59].
β-catenin is a protein with dual functions, acting as both an adhesion molecule and a transcription factor [Citation21]. In this study, a significant downregulation in β-catenin expression was observed after IL-22 treatment. Similarly, Li et al. [Citation60] revealed a reduction of β-catenin protein levels in the cytosol by IL-22 administration. Matsu-Ura et al. [Citation61] stated that the WNT/β-catenin pathway regulates β-catenin expression under circadian control. On the other hand, the present result detected an inverse correlation between the expression levels of β-catenin and STAT3 upon IL-22 treatment. Similarly, Hu et al. [Citation62] recorded an increase in p-STAT3 and a decline in β-catenin gene expressions in hepatic stellate cells after IL-22 treatment. Thus the decline in β-catenin gene expression during the dark phase may be via the corresponding increase in IL-22 directly after its administration.
The current result revealed a decline in GI after IL-22 treatment. Consistent with this result Dkhil [Citation63] found a reduction in granuloma size after berberine treatment which reflects antifibrotic effects. Furthermore, a significant reduction in the liver GI was detected when it was administered at 7 am rather than at 7 pm. This result may be explained firstly by the worms’ active phase (night time), which is a period of stress, increased egg-laying, and physical activity. The worm’s increased egg-laying rates may coincide with the periods of higher host insulin levels [Citation64,Citation65]. This means the number of eggs increased at night during the active phase of the worm. Secondly, the circulating numbers of some immune cells (such as monocytes, neutrophils, and lymphocytes) exhibit a 24-hour daily rhythm, which is translated into variations in the body’s acute response to infection [Citation26,Citation66,Citation67]. Immune response activity peaks at the beginning of the active phase [Citation68–70].
According to Scheiermann et al. [Citation68], the central suprachiasmatic nucleus clock regulates circadian rhythms in the adhesion molecules expression like ICAM-1 and VCAM-1, chemokines, and chemokine receptors on leukocytes like CCL2 and CXCR4, which helps to regulate leukocyte recruitment depending on the time of the day. Thus, the controlled leukocyte migrations increases during the active phase of the host with increasing immune response. Taken together, IL-22 administration at night triggers more immune responses leading to an elevation in liver GI during the active phase (at night) of the host.
In conclusion
Herein it’s the first time to study the chrono-therapeutic effect of IL-22 treatment on schistosomiasis. IL-22 treatment of S.mansoni-infected mice, at the light onset, reduced GI and IgE levels, which may be due to synchrony with the host circadian rhythm. These results reflect a potential chrono-therapeutic effect of IL-22. Further studies were needed to investigate the mechanism of IL-22 action on both the host and the parasite and its relation to the host circadian rhythm.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. Guideline on control and elimination of human schistosomiasis in. 2022. who.int/publication/i/item/9789240041608
- Pisarski K. The global burden of disease of zoonotic parasitic diseases: top 5 contenders for priority consideration. Trop Med Infect Dis. 2019;4(1):44. doi: 10.3390/tropicalmed4010044
- Figliuolo da Paz VR, Figueiredo-Vanzan D, Santos Pyrrho A D. Interaction and involvement of cellular adhesion molecules in the pathogenesis of schistosomiasis mansoni. Immunol Lett. 2019;206:11–18. doi: 10.1016/j.imlet.2018.11.011
- Cortes-Selva D, Elvington AF, Ready A, et al. Schistosoma mansoni infection-induced transcriptional changes in hepatic macrophage metabolism correlate with an athero-protective phenotype. Front Immunol. 2018;9:2580. doi: 10.3389/fimmu.2018.02580
- Ali SA, El-Regal NS, Saeed SM. Antischistosomal activity of two active constituents isolated from the leaves of Egyptian medicinal plants. Infect Dis (Auckl). 2015;8:5–16. doi: 10.4137/IDRT.S24342
- Utzinger J, Raso G, Brooker S, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136(13):1859–1874. doi: 10.1017/S0031182009991600
- Alwan SN, Taylor AB, Rhodes J, et al. Oxamniquine derivatives overcome Praziquantel treatment limitations for schistosomiasis. PLOS Pathog. 2023;19(7):e1011018. doi: 10.1371/journal.ppat.1011018
- Erko B, Degarege A, Tadesse K, et al. Efficacy and side effects of praziquantel in the treatment of schistosomiasis mansoni in schoolchildren in Shesha Kekele Elementary School, Wondo Genet, Southern Ethiopia. Asian Pac J Trop Biomed. 2012;2(3):235–239. doi: 10.1016/S2221-1691(12)60049-5
- Shao B, Gui X, Lu Z, et al. Praziquantel promotes protection against Schistosoma japonicum infection in mice. Acta Trop. 2023;241:106874. doi: 10.1016/j.actatropica.2023.106874
- Kabatereine NB, Kemijumbi J, Ouma JH, et al. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at Lake Albert, Uganda. Trans R Soc Trop Med Hyg. 2003;97(5):599–603. doi: 10.1016/S0035-9203(03)80044-5
- Radaeva S, Sun R, Pan HN, et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39(5):1332–1342. doi: 10.1002/hep.20184
- Jie Z, Liang Y, Yi P, et al. Retinoic acid regulates immune responses by promoting IL-22 and modulating S100 proteins in viral hepatitis. J Immunol. 2017;198(9):3448–3460. doi: 10.4049/jimmunol.1601891
- Lai R, Xiang X, Mo R, et al. Protective effect of Th22 cells and intrahepatic IL-22 in drug induced hepatocellular injury. J Hepatol. 2015;63(1):148–155. doi: 10.1016/j.jhep.2015.02.004
- Xiang X, Gui H, King NJ, et al. IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol Cell Biol. 2012;90(6):611–619. doi: 10.1038/icb.2011.79
- Silva CM. Role of STATs as downstream signal transducers in src family kinase-mediated tumorigenesis. Oncogene. 2004;23(48):8017–8023. doi: 10.1038/sj.onc.1208159
- Lim CP, Cao X. Structure, function, and regulation of STAT proteins. Mol Biosyst. 2006;2(11):536–550. doi: 10.1039/b606246f
- Yuan ZL, Guan YJ, Wang L, et al. Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol Cell Biol. 2004;24(21):9390–9400. doi: 10.1128/MCB.24.21.9390-9400.2004
- Horiguchi N, Lafdil F, Miller AM, et al. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology. 2010;51(5):1724–1734. doi: 10.1002/hep.23532
- Mair M, Zollner G, Schneller D, et al. Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology. 2010;138(7):2499–2508. doi: 10.1053/j.gastro.2010.02.049
- Plum W, Tschaharganeh DF, Kroy DC, et al. Lack of glycoprotein 130/signal transducer and activator of transcription 3-mediated signaling in hepatocytes enhances chronic liver injury and fibrosis progression in a model of sclerosing cholangitis. Am J Pathol. 2010;176(5):2236–2246. doi: 10.2353/ajpath.2010.090469
- Xu W, Kimelman D. Mechanistic insights from structural studies of β-catenin and its binding partners. J Cell Sci. 2007;120(19):3337–3344. doi: 10.1242/jcs.013771
- Rios-Esteves J, Resh MD. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013;4(6):1072–1081. doi: 10.1016/j.celrep.2013.08.027
- Higashi N, Kojima N, Miura M, et al. Cell-cell junctions between mammalian (human and rat) hepatic stellate cells. Cell Tissue Res. 2004;317(1):35–43. doi: 10.1007/s00441-004-0891-9
- Sodhi D, Micsenyi A, Bowen WC, et al. Morpholino oligonucleotide-triggered β-catenin knockdown compromises normal liver regeneration. Journal Of Hepatology. 2005;43(1):132–141. doi: 10.1016/j.jhep.2005.02.019
- Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45(3):401–409. doi: 10.1016/j.jhep.2006.03.016
- Cermakian N, Lange T, Golombek D, et al. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2013;30(7):870–888. doi: 10.3109/07420528.2013.782315
- Rijo-Ferreira F, Pinto-Neves D, Barbosa-Morais NL, et al. Trypanosoma brucei metabolism is under circadian control. Nat Microbiol. 2017;2(6):17032. doi: 10.1038/nmicrobiol.2017.32
- Petkovic M, Henis M, Heese O, et al. Chronotherapy in glioblastoma: state of the art and future perspectives. EBioMedicine. 2023;89:104470. doi: 10.1016/j.ebiom.2023.104470
- Traynor K, Newton DW, Hrushesky WJ, et al. A pharmacist’s primer on chronotherapeutics. Am Pharm. 1992;NS32(3):77–83. doi: 10.1016/S0160-3450(15)31188-0
- Nady S, Shata MT, Mohey MA, et al. Protective role of IL-22 against Schistosoma mansoni soluble egg antigen-induced granuloma in vitro. Parasite Immunol. 2017;39(1):39. doi: 10.1111/pim.12392
- Hirsch C, Zouain CS, Alves JB, et al. Induction of protective immunity and modulation of granulomatous hypersensitivity in mice using PIII, an anionic fraction of Schistosoma mansoni adult worm. Parasitology. 1997;115(Pt 1):21–28. doi: 10.1017/S0031182097001078
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):45e–45. doi: 10.1093/nar/29.9.e45
- Cutolo M. Circadian rhythms and rheumatoid arthritis. Joint Bone Spine. 2019;86(3):327–333. doi: 10.1016/j.jbspin.2018.09.003
- Buttgereit F, Smolen JS, Coogan AN, et al. Clocking in: chronobiology in rheumatoid arthritis. Nat Rev Rheumatol. 2015;11(6):349–356. doi: 10.1038/nrrheum.2015.31
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27(4):647–659. doi: 10.1016/j.immuni.2007.07.023
- Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology. 2011;54(1):252–261. doi: 10.1002/hep.24339
- Prado MKB, Fontanari C, Souza COS, et al. IL-22 promotes IFN-γ-mediated immunity against Histoplasma capsulatum infection. Biomolecules. 2020;10(6):10. doi: 10.3390/biom10060865
- Valeri M, Raffatellu M, Napier B. Cytokines IL-17 and IL-22 in the host response to infection. Pathog Dis. 2016;74(9):ftw111. doi: 10.1093/femspd/ftw111
- Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019;16(7):666–667. doi: 10.1038/s41423-018-0055-6
- Wang Q, Robinette ML, Billon C, et al. Circadian rhythm–dependent and circadian rhythm–independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci Immunol. 2019;4(40). doi: 10.1126/sciimmunol.aay7501
- Yu X, Rollins D, Ruhn KA, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727–730. doi: 10.1126/science.1243884
- Amir M, Chaudhari S, Wang R, et al. REV-ERBα regulates TH17 cell development and autoimmunity. Cell Rep. 2018;25(13):3733–3749 e3738. doi: 10.1016/j.celrep.2018.11.101
- Chang C, Loo CS, Zhao X, et al. The nuclear receptor REV-ERBα modulates Th17 cell-mediated autoimmune disease. Proc Natl Acad Sci U S A. 2019;116(37):18528–18536. doi: 10.1073/pnas.1907563116
- Lynch NR, Hagel I, Palenque M, et al. Relationship between helminthic infection and IgE response in atopic and nonatopic children in a tropical environment. J Allergy Clin Immunol. 1998;101(2 Pt 1):217–221. doi: 10.1016/S0091-6749(98)70386-0
- Amiri P, Haak-Frendscho M, Robbins K, et al. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. J Exp Med. 1994;180(1):43–51. doi: 10.1084/jem.180.1.43
- Zheng B, Larkin DW, Albrecht U, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740):169–173. doi: 10.1038/22118
- Nakamura Y, Harama D, Shimokawa N, et al. Circadian clock gene Period2 regulates a time-of-day–dependent variation in cutaneous anaphylactic reaction. J Allergy Clin Immunol. 2011;127(4):1038-1045 e1031–1033. doi: 10.1016/j.jaci.2011.02.006
- Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194
- Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–1472. doi: 10.1084/jem.20082683
- Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer–like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713
- Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10(1):39–49. doi: 10.1016/S1074-7613(00)80005-9
- Melillo JA, Song L, Bhagat G, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol. 2010;184(5):2638–2645. doi: 10.4049/jimmunol.0902960
- El Kasmi KC, Smith AM, Williams L, et al. Cutting edge: a transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007;179(11):7215–7219. doi: 10.4049/jimmunol.179.11.7215
- Ivanov II, McKenzie BS, Zhou L, et al. The Orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035
- Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969
- Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200
- Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586
- Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23(5):613–619. doi: 10.1016/j.coi.2011.07.006
- Li W, Li F, Lei W, et al. TRIM30 modulates Interleukin-22-regulated papillary thyroid Cancer cell migration and invasion by targeting Sox17 for K48-linked Polyubiquitination. Cell Commun Signal. 2019;17(1):162. doi: 10.1186/s12964-019-0484-6
- Matsu-Ura T, Dovzhenok A, Aihara E, et al. Intercellular coupling of the cell cycle and circadian clock in adult stem cell culture. Mol Cell. 2016;64(5):900–912. doi: 10.1016/j.molcel.2016.10.015
- Hu BL, Shi C, Lei RE, et al. Interleukin-22 ameliorates liver fibrosis through miR-200a/beta-catenin. Sci Rep. 2016;6(1):36436. doi: 10.1038/srep36436
- Dkhil MA. Role of berberine in ameliorating Schistosoma mansoni-induced hepatic injury in mice. Biol Res. 2014;47(1):8. doi: 10.1186/0717-6287-47-8
- Rawlinson KA, Reid AJ, Lu Z, et al. Daily rhythms in gene expression of the human parasite Schistosoma mansoni. BMC Biol. 2021;19(1):255. doi: 10.1186/s12915-021-01189-9
- Feillet C, Guerin S, Lonchampt M, et al. Sexual dimorphism in circadian physiology is altered in LXRα Deficient mice. PLoS One. 2016;11(3):e0150665. doi: 10.1371/journal.pone.0150665
- Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349(1):82–90. doi: 10.1016/j.mce.2011.06.039
- Shimba A, Ikuta K. Glucocorticoids regulate circadian rhythm of innate and adaptive Immunity. Front Immunol. 2020;11:2143. doi: 10.3389/fimmu.2020.02143
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. doi: 10.1038/nri3386
- Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16(5):581–622. doi: 10.3109/07420529908998730
- Haus E, Lakatua DJ, Swoyer J, et al. Chronobiology in hematology and immunology. Am J Anat. 1983;168(4):467–517. doi: 10.1002/aja.1001680406