?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A review and discussion of some of the literature on the subject of serious nuclear reactor accidents. This review addresses some biological issues such as the influence of dose rate on the ability of radiation to cause harm, the chemistry of a selection of serious accidents and the behaviour of a series of important fission products and the actinides. This review is intended for scientists with a professional interest in nuclear reactors, chemists in general and I expect that it will be of use to historians with an interest in the nuclear industry and its relationship with wider society.
Public Interest Statement
Radiation and nuclear accidents are of great interest to the general public, the great concern relates in part to the fact that radiation cannot be observed with a human bodies senses. It is likely that the inability to see/hear/feel/smell or taste the threat coupled with the comparative novelty of radiological accidents does heighten the degree of concern. As I wish to see nuclear/radioactive issues discussed honestly with arguments based on facts rather than feelings, half-truths and even complete fabrications I have chosen to write a large review of the phenomena (mainly chemical) associated with serious accidents. I wanted to write a review which would be of use to scientists, both supporters and opponents of “nuclear power”, future historians of the nuclear age and interested members of the general public. By providing others with better access to the truth and explaining some matters I want to improve the way in which nuclear technology is discussed in society.
1. Introduction
The use of nuclear energy for the production of electricity is a divisive matter, it is clear that “nuclear power” is a polarizing topic. One of the great problems of our age is the supply of energy for industrial and household purposes, in her book Clare Smallman pointed out that modern humans use far more energy than that is required for their bodies to function (Smallman, Citation1981). While a return to a more primitive way of living might offer a means of reducing our energy use, it is a lifestyle change which few people want to undertake. It is important to consider both the energy which is delivered to our homes and vehicles as well as the energy required by industry to produce the products which many of us use and consume. For example, aluminium metal requires a very large amount of energy to produce. Nuclear power offers a low carbon alternative to coal as an energy source; however, the waste is the subject of considerable concern by the public, pressure groups and governments. Additionally the consequences of accidents at nuclear sites is of great concern, the problem of a “nuclear accident” is one of the barriers to widespread use of nuclear technology in some parts of the world. After a radiological accident two pathways for exposure exist, firstly radioactivity can be scattered over surfaces. The radiation from contaminated surfaces and sometimes from a passing cloud of radioactivity can expose people to radiation, even without need for a person to inhale, swallow or otherwise incorporate radioactivity into their body. For many radioisotopes it is relatively easy to calculate the exposure for a person standing on an infinite area of flat land which is uniformly contaminated using conversion factors such as those of Beck (Citation1980). One great problem is making a good estimate or measurement of the contamination level (Bq m−2) and also very few people spend their time on a perfectly flat uniformly contaminated surface of land. The other type of exposure scenario is one in which a person absorbs radioactivity and then undergoes internal exposure. The internal exposure cases are often more complex than the external exposures.
Sadly I am unable to review all radiological misadventures, in order to create a review paper which can be read a need exists to exclude some events. I have chosen to exclude most of the sealed source accidents which have occurred all too frequently in industrial radiography and radiotherapy. In many ways these smaller accidents tend to be more deadly than nuclear power reactor accidents, while these accidents have caused more deaths and injuries due to acute effects among the general public than events such as the Windscale fire, Three Mile Island, Chernobyl and Fukushima I suspect that these accidents are less able to capture the attention of the public. A good review (Coy et al., Citation1998) of such events already exists which reduces the need for me to write about them. I would like to warn my reader to be careful of what you look at and what you search for, some things cannot be unseen. Some radiographic accidents such as the lost 30 Ci 192Ir source accident in Morocco (1984) which killed eight members of a family are to me the radiological equivalent to a horror film. I will not be showing shocking photographs of radiation injuries or including graphic descriptions of horrible injuries. I have also chosen to exclude the majority of events where a deliberate or reckless exposure of people to radiation has occurred. Thus I will not be discussing “radiological assault”, nuclear warfare or nuclear bomb tests in length. One major reason is that these events are not accidents, but occasionally these events will be discussed when an accident occurred during a planned nuclear or radiological event. For example during one hydrogen bomb test due to an incorrect assumption the yield of the bomb was far greater than expected.
By providing this review and my earlier review (Foreman, Citation2015) I hope that knowledge and reasoned thought on this subject will be of some use to both specialists within the radioactivity and nuclear sectors, other scientists and members of the public. Rather than make a simple list of papers on the subject (Bujdosó, Citation1987) I have chosen where possible to discuss the sources which I have found in the literature. In the ideal world men and women would reason clearly and discuss all manner of topics in a civil and honest manner, but sadly this utopia has not come to be. Instead we live in an age where falsehoods are sometimes deliberately propagated, where through recklessness or carelessness incorrect information is disseminated and where all the horrible tricks of bad argument documented by Thouless (Citation1953) are being used. I hope that this review enlightens the reader and makes life harder for those who for various reasons attempt to influence others through misinformation on nuclear matters. I have wanted to write a document which is useful both to the supports and the opponents of the nuclear industry. At times I have chosen to criticize some organizations and persons for either recklessly or deliberately spreading falsehoods or for other reasons, I want the reader of this review to understand such criticism is not ideologically motivated. I disapprove equally of pro- and anti-nuclear campaigners who misbehave as described above. I am sure that both those who read this review shortly after publication and those who read it years and decades afterwards will be aware of the problems of “alternative facts”, “fake news” and “the rejection of expert opinion in favour of that of the layperson”. I do not want to diverge too far into the world of politics, but it will be clear to many of my readers that some political leaders and other public figures are “economical with the truth” while others go beyond failing to tell the whole truth by knowingly or recklessly disseminating falsehoods.
Some of my readers will be aware of a sorry tale from Soviet botany from the era when Josef Stalin was the leader of the Union of Soviet Socialist Republics (USSR) of lysenkoism. The core of this pseudoscience was that it is possible to train plants in much the same way as Pavlov and Skinner trained dogs and other animals. Pavlov with his famous experiments with bells (and other stimuli) and Skinner with his “Skinner Box” (operant conditioning chamber) trained animals. Part of the problem was that Lysenko and Stalin indulged in violence and other forms of repression against geneticists. In today’s society I see a disturbing parallel. I am aware of intimidation campaigns being waged against scientists and other intellectuals who happen to be publishing or expressing views which challenge the ideas, political agenda or economic activities of another person (natural or otherwise). I will go quickly through some examples of this. Caroline Criado-Perez the journalist along with Stella Creasy campaigned for more women to appear on Bank of England banknotes, unfortunately they were subject to harassment in the form of menacing and grossly offensive electronic communications. At least two people who threatened Caroline Criado-Perez have been sent to prison (Cockerell, Citation2014). One case which is closer intellectually to the subject of this paper is the tale of what happened to Jay Cullen. He is an academic who works on the chemistry of the oceans, after the Fukushima event he measured the cesium content of sea water and came to the conclusion that the radioactive cesium level in the water was too low to be harmful. An activist named Dana Durnford issued death threats against Jay Cullen and another academic, he has now been convicted in a criminal court case (Wanklyn, Citation2016). I imagine that the vast majority of my readers are decent and moral people, but just in case you know someone who is considering embarking on a campaign of harassment or intimidation then I would like to point out that such a course of action can result in serious harmful consequences.
Before we go on, it is important to make something clear. Within this review the word “significant” is used to indicate something which is statistically significant. As the UK’s health and safety executive summed it up crisply my stating “statistical significance” should not be confused with the significance of each injury. Every casualty is a tragedy and has both a social cost and a personal cost to those “directly affected”. I have to admit that regardless of the statistical significance any fatality, injury or loss of the use of land or other property is significant to those affected.
In this review I will start by considering the question of how bad is radiation, before considering the circumstances and chemistry of a selection of reactor accidents. After considering the resuspension of radioactivity I will consider the chemistry of a series of important radioactive elements in turn. Sadly I am unable to make a totally comprehensive review, but I have attempted to write about what I think are the most important and interesting issues.
2. Review
2.1. How dangerous is radiation
One can argue that ionizing radiation should be treated like a chemical poison as gamma rays, beta particles, alpha particles and neutrons cause harm by delivering energy to water and other substances inside the body thus forming reactive species which in turn damage biomolecules including DNA. Debate rages over the question of “how harmful is radiation”.
It is important to note that the harmful effects of radiation to any population should not be exaggerated regardless of how noble the purpose of the exaggeration is in the mind of the exaggerator. One problem is that if risk is exaggerated then in an attempt to avoid one risk people may be exposed to other (and greater risks). Secondly in the long run by exaggerating risk the exaggerator and the cause that they represent may be discredited. Equally it would be deeply wrong to deliberately downplay the harmful effects of radiation or radioactivity, again regardless of how noble the purpose of the exaggeration is.
The mainstream scientific opinion on how harmful radiation is expressed by a series of different bodies such as the ICRP (International Commission on Radiological Protection). When considering other opinions is important to keep in mind the question of “who is expressing an alternative view” and “what is the alternative view based on”. It is clear to me that radiological protection and the creation of standards/dose limits is politically sensitive at times and can become exceptionally controversial at times. In the popular media a range of ideas and alternative views are circulating. Some of these include claims that conspiracies exist to influence radiation protection standards in a particular way to suit the interests of the “nuclear industry”. For example it has been claimed by some that an agreement exists between the IAEA (International Atomic Energy Authority) and the WHO (World Health Authority). Normally I restrict myself to what is accepted by mainstream science, but occasionally I may mention and discuss some of the alternative points of view on some issues when some valuable thing can be learnt by considering them.
Regarding the accusation about the IAEA and the WHO, it relates to a 1959 agreement (WHA12-40) between the two bodies. The text is rather long, it contains 13 articles. A selective reading (cherry picking) of the agreement is required to come to the conclusion that the IAEA is able to inhibit the WHO from an activity. The first article states in clause three.
“3. Whenever either organization proposes to initiate a program or activity on a subject in which the other organization has or may have a substantial interest, the first party shall consult the other with a view to adjusting the matter by mutual agreement.”
I have highlighted in bold the text which could be understood as meaning that the WHO can be controlled by the IAEA. It is interesting to point out that clause 2 (as seen below) contains text which indicates that the WHO recognizes that the IAEA has primary responsibility for nuclear matters but the WHO still has the right to work on any aspect of health work.
“2. In particular, and in accordance with the Constitution of the World Health Organization and the Statute of the International Atomic Energy Agency and its agreement with the United Nations together with the exchange of letters related thereto, and taking into account the respective co‐ordinating responsibilities of both organizations, it is recognized by the World Health Organization that the International Atomic Energy Agency has the primary responsibility for encouraging, assisting and co‐ ordinating research and development and practical application of atomic energy for peaceful uses throughout the world without prejudice to the right of the World Health Organization to concern itself with promoting, developing, assisting and co‐ordinating international health work, including research, in all its aspects.”
I hold the view that the agreement was a sensible choice in the 1950s for the two bodies to agree to cooperate to prevent unnecessary duplication of work. The important message is when reading a document such as statute it is important to read the whole of the document and judge it as a whole rather than concentrating on a small part of the text. Similar misunderstandings can occur as a result of cherry picking text from religious texts (such as the Bible), legal statutes or almost any document. But now back to the chemistry, as we are mainly water we need to consider the effect of radiation on water.
When water is subject to irradiation then a series of reactive species are formed. When pure water is irradiated the solvated electron and the H2O+ cation are formed. These then can recombine to form an excited state of water (H2O*). The H2O* can then fragment forming neutral radicals such as HO∙ (hydroxyl radicals) and H∙ (hydrogen atoms). The hydroxyl radicals and hydrogen atoms can combine to form hydrogen peroxide, hydrogen gas and water.
A large number of rate constants have been measured for the reaction of the reactive species formed by the irradiation of water (for example e−, HO∙ and H∙) (Buxton, Greenstock, Helman, & Ross, Citation1988). Often when water containing a solute is irradiated then the situation is more complex, for example when oxygenated water is irradiated then the reducing radicals such as solvated electrons and hydrogen atoms tend to react with the oxygen to form oxidizing radicals (Baxendale & Smithies, Citation1956).
The oxidizing radical can then react with biomolecules. The oxygen centred radicals can remove (abstract) hydrogen atoms from organic species. The resulting carbon centred radicals can then undergo a range of reactions.
This oxygen effect may be responsible for the fact that deoxygenated cancer cells are more resistant to gamma rays and X-rays than oxygenated cancer cells. Often a tumour has an outer layer which is well supplied with oxygen and nutrients, deeper within the tumour is an area which is poorly supplied with oxygen and nutrients. Deeper still within the tumour is sometimes an area of dead cells, cells which have died from a lack of oxygen and nutrients. If the oxygenated outer layer of a tumour is killed by means of radiation then the less oxygenated cells (which may be in suspended animation) can start to grow again. One solution to this oxygen effect is to use a high LET (Linear Energy Transfer) radiation such as alpha particles (Tinganelli et al., Citation2015). With alpha particles oxygen level has less effect on the ability of radiation to kill cells. The idea is to attach an alpha emitter to a molecule which seeks a specific biological target. Another approach is to administer a boron containing drug which preferentially absorbs into cancer cells, then to subject the patient to neutron irradiation. The idea is that the neutrons will cause the boron to generate alpha particles according to the following reaction.
Attempts are being made to create drugs containing boron (Bonjoch et al., Citation2008), one approach is to attach polyhedral boron hydride groups to antibodies (Alam, Soloway, & Barth, Citation1987) or nucleosides (Wojtczak, Andrysiak, Gruener, & Lesnikowski, Citation2008). Several reviews of boron neutron capture therapy for cancer exist (Barth, Soloway, Fairchild, & Brugger, Citation1992; Nedunchezhian, Aswath, Thiruppathy, & Thirugnanamurthy, Citation2016). The radicals such as HO∙ can attack biomolecules (including DNA). This can lead to two things, if a large amount of damage is done in a short time to the biomolecules of a cell then the cell may die. It is the death of cells in vital organs which is responsible for the radiation injuries and deaths which have resulted from some accidental radiation exposures. These acute effects of radiation are deterministic effects, the severity of the clinical effects often are dependent on the radiation dose. This is similar to the fact that the effects of alcoholic drinks on human behaviour often become more dramatic as the volume of alcohol ingested increases.
When less damage is done to cells or when the damage occurs at a sufficiently low rate that the self-repair mechanisms in the cells prevent the radiation killing the cells then the effect which concerns us most is the modification of the DNA in the cells. It is impossible to argue that ionizing radiation is incapable of inducing cancer, for example internal exposure to 90Sr induces bone cancer in dogs (Gillett, Pool, Taylor, Muggenburg, & Boecker, Citation1992) and it is interesting to note that a Scottish industrial radiographer who had a lifetime whole body dose in the range of 10–15 Gy died of a form of leukaemia (acute myeloid) (Lloyd et al., Citation1994) which is a common second cancer in cancer patients treated with radiotherapy. When the DNA is damaged sometimes the cell will correctly repair it and sometimes during the attempt at repair the cell will fail to repair it correctly. If we ignore cases where the DNA is so hopelessly damaged that the cell dies quickly, we can have cells which have altered DNA which are able to continue to divide. A change of DNA is known as a mutation. While feature films often contain “mutants” with a wide range of bizarre appearances and abilities, a typical mutation will only make a less dramatic change. For example if sufficient changes to the DNA of a cell occur then the normal cell will change in to a cancer cell. A malignant cancer cell differs from a normal cell in three ways.
The cell divides when it should not do so.
The cell become immortal, instead of having a limited number of times it can divide it can divide forever.
The cell no longer respects the rules which govern where it should be in the body, a great problem with cancer is that the primary tumour releases cells into the blood and lymph systems which migrate to other parts of the body where they start dividing thus creating secondary tumours. Often these secondary tumours are more dangerous than the primary tumour.
In contrast the acute effects of radiation the cancers induced by radiation do not become worse in terms of symptoms or prognosis as the radiation dose which induced them becomes larger. The only thing which becomes larger is the likelihood of developing a cancer. As the clinical course of a radiation induced cancer is the same as that of a case of the same cancer induced by something else it can be very difficult to prove that a given cancer case was induced by radiation. It is often difficult to prove that an increase in the incidence of cancer in a population of humans is due to the effect of radiation. When the incidence of an unusual cancer increases, such as either Kaposi sarcoma or Clear-cell adenocarcinoma of the vagina in young people then the change is very noticeable. These two cancers are noteworthy as the first helped to establish the existence of AIDS while the second lead to the withdrawal of an artificial oestrogen drug (diethylstilbestrol) from the market. While there are some cancers for which an association with radiation can be observed, for many cancers it is harder to find a link.
We also have to ask the question of “should society outlaw a thing when it can be shown that it might be linked to cancer?”. I hold the view that the answer should not automatically be yes, for example some treatments for cancer are likely to have induced other cancers. The creation of one cancer by a treatment which cures another might be a tolerable side effect for some people (Pedersen-Bjergaard et al., Citation1981). By curing one cancer the cancer sufferer had their life extended. Another example is a substance which many people choose to consume as a food (grapefruit). I am aware that grapefruits are a key part of a weight (mass) loss diet which is known as the “Grapefruit diet” (Hollywood diet). It is interesting that grapefruit consumption has been linked in a study of 50,000 postmenopausal women to an increase in the incidence of breast cancer (Monroe, Murphy, Kolonel, & Pike, Citation2007). The study indicated that consumption of a quarter of a grapefruit per day increases the relative risk of breast cancer by a factor of 1.3 (95% confidence interval of 1.06–1.58). While this clearly indicates that eating grapefruits can have a harmful effect and despite the fact that I loathe the taste of grapefruit I could not justify a ban on the importation, sale or consumption of grapefruits. It is noteworthy that a European study of 114,504 women found no link between grapefruit eating and breast cancer (Spencer et al., Citation2009). The reason why the grapefruit has been of such interest is that it is known that this fruit (and St-John’s wort) can alter the behaviour of a range of drugs (Mouly, Lloret-Linares, Sellier, Sene, & Bergmann, Citation2017), and it has been shown that grapefruit juice containing naringenin, quercetin and kaempherol (flavonoids) inhibits the metabolism of 17-beta-estradiol in women (Schubert, Cullberg, Edgar, & Hedner, Citation1994) ().
While naringenin and quercetin are present in large amounts in grapefruit juice as these compounds do not have an effect on the oral bioavailability of nifedipine, nisoldpine or felodipine (dimethyl-2,6-dimethyl-4-(2,3-dichlorophenyl)-1,4-dihydropyridine-3,5-dicarboxylate). Tassaneeyakul, Guo, Fukuda, Ohta, and Yamazoe (Citation2000) disregarded them in their search for the substance which inhibits the different forms of the P450 enzyme. Also it has been shown that the naringin and naringenin in grapefruit juice is not responsible for its ability to inhibit the metabolism of testosterone by the CYP3A version of P450 (Edwards & Bernier, Citation1996) (Figure ).
Figure 2. Nifedipine (dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate) and nisoldpine (3-isobutyl 5-methyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate).
It has been shown in chemistry experiments by Tassaneeyakul et al. that furanocoumarins present in grapefruit juice do have a great ability to inhibit the CYP1A2, CYP2C9, CYP2D6 and CYP3A4 forms of the P450 enzyme. While these compounds might be present at lower concentrations than naringenin in grapefruit juice they are far stronger inhibitors of the P450 enzymes. In the paper by Tassaneeyakul et al. (Citation2000), a series of compounds isolated from grapefruit juice such as bergamottin, 6ʹ,7ʹ-dihydroxy bergamottin and two bis-furanocoumarins were identified as far stronger inhibitors of these enzymes. These four compounds are likely to be the main inhibitors of the P450 enzymes in grapefruit juice. It is reasoned that by inhibiting these enzymes in women that their oestrogen level will rise, the increase in oestrogen will then favour the development of breast cancer (Figure ).
One might wonder why I have chosen to consider grapefruit in so much detail, the reason is that it illustrates several things. Firstly there is the follies of inductive reasoning, inductive reasoning works in the following way. We start with two of more statements which we believe are true, from these we create a new statement. For example:
Mark ate a stale prawn sandwich,
The next day Mark was very ill.
The new statement or idea is “Eating stale prawn sandwiches can make you ill”. The problem with this system of reasoning is that if one of the original statements is wrong then errors can propagate forwards and corrupt our thinking. Also even if all the original statements are correct it is possible to make an incorrect statement which might not be easily noticed as wrong. Consider the following:
A Geiger-Muller detector can detect fast moving electrons,
Beta emitters are radionuclides which emit fast moving electrons.
Thus we can reason “Geiger-Muller detectors can be used to search for contamination with beta emitters”. The only problem is that while GM tubes can detect beta emitters such as 14C, 32P and 90Sr/90Y they are unable to detect the lower energy beta particles from 3H (tritium) and 63Ni.
We can continue:
Dr Foreman is an Englishman,
Dr Foreman’s first name is Mark.
Thus “All Englishmen have the same first name (Mark)”
Grapefruit inhibits P450 enzymes.
Grapefruit contains naringenin.
Thus “Naringenin inhibits the P450 enzymes”.
Using inductive reasoning we have come up with three incorrect statements (one of which is rather comical). A better system of doing science would be create a hypothesis (maybe using inductive reasoning) and then to put the hypothesis to the test (an experiment designed to show if it is not true). This is the falsificationist method in which one attempts to disprove a theory, the more times one fails to disprove a theory the better and the more useful the theory becomes. The theory that the GM detector is a universal detector for beta emitters can be tested by attempting to measure a range of different beta emitters, the hypothesis regarding the universal first name of Englishmen could be tested by asking 100 randomly chosen Englishmen their names while the hypothesis that naringenin is the major P450 inhibitor in grapefruit was tested by Edwards and others. I am sure that it will be possible with inductive reasoning to make some dire mistakes in human radiation biology. One problem is that the study of an exposed group means little unless it is compared with an unexposed group. The problem is that unless the right control group is chosen then differences other than radiation exposure may cause both false positive and false negative results. For example it would be of little use to compare men who worked at Sellafield in the 1970s with women working in the 1980s in China as dentists. In this example, thankfully hypothetical, I can see four differences between the populations. A better comparison was male radiologists and male psychiatrists who worked in the USA. The most heavily exposed radiologists (graduated before 1940) show an excess of deaths due to some forms of cancer, in particular melanoma (a skin cancer), cancer of oesophagus and leukaemias other than chronic lymphocytic leukaemia (CLL) (de González et al., Citation2016). When a population of male radiologists and psychiatrists who graduated between 1960 and 1979 are compared their rates of melanoma, cancer of oesophagus and leukaemias other than CLL. It can be reasoned that the main difference between the working lives of the two types of medical doctor is that the radiologists tend to be radiation workers while the psychiatrists do not work with radiation. The reduction of the difference between the two populations as the year of graduation increases can be ascribed to the decrease in occupational radiation exposure of the radiologists as work practices changed.
When people who took part in the British nuclear bomb tests in the 1950s were compared with people who had experienced similar work but had not been present near the test sites during the tests some differences can be observed. The population exposed to the tests included members of the British military [army, navy and air force (RAF)] and workers from the atomic weapons research establishment (AWRE). The AWRE was replaced by the atomic weapons establishment (AWE) and the United Kingdom Atomic Energy Authority. The controls were members of the military who were elsewhere in the world at the time of the tests but had served in the tropics (Kendall, Muirhead, Darby, Doll, Arnold and O’Hagan, Citation2004). The population which had been exposed to the nuclear weapon tests did have an excess of some forms of cancer (leukaemia and cancer of the liver, bladder, prostrate and melanoma) (Muirhead et al., Citation2004).
The population in Japan who survived the two atomic bombings have been the subject of a large number of scientific studies regarding the question of how harmful is radiation. In contrast to the US doctors and the study on British atomic bomb veterans, in the work on the Hiroshima and Nagasaki populations rather than being lumped into exposed and unexposed populations these people have been divided up according to their dose estimates. Based on their locations at the moment of detonation attempts have been made to reconstruct their doses. The incidence of solid cancers in the survivors between 1958 and 2009 has been recently considered. In women the excess relative risk (ERR) increased in a linear manner (0.64 per Gy), while for men the increase in ERR is better modelled with a linear-quadratic (LQ) model (Grant et al., Citation2017). The different forms of leukaemia appear to obey different mathematical models (Richardson et al., Citation2009).
For chronic myeloid leukaemia (CML) and acute lymphatic leukaemia (ALL) the ERR increases in a linear manner as the dose in grays (D) increases according to the equation (β = 6.39 CML and β = 3.70 ALL):
For acute myeloid leukaemia (AML) the relationship between dose and ERR is different. As well as the dose (D), the age of exposure and time since exposure all matter for this disease. The expression for k is rather complex:
The younger a person is at the age of exposure the greater the risk of AML. This alone is a good argument for setting a minimum age limit for radiation work. In the UK a person must be at least 16 to be allowed to do radiation work, until they reach the age of 18 they are restricted to a lower annual dose limit to persons over 18. The BEIR VII committee were reported to have favoured a ERR model for leukaemia which is given by the following equation:
The quadratic relationship between the cancer rate and the dose is interesting, it does conflict with the linear no threshold (LNT) model which is commonly used. The LNT model is a key plank of the argument that “collective dose” can be used to predict the number of cancer cases among the general public or a group of workers after an exposure to radiation. If the LNT model is correct then it would be reasonable to assume that 100 people exposed to 100 mSv each, 1,000 people exposed to 10 mSv each or 10,000 people exposed to 1 mSv each then the same number of cancer cases would be induced. In all three cases I have used the collective dose would be 10 manSv. If a 1 Sv dose increases the absolute lifetime risk of developing cancer by 5% then the 10 manSv dose would induce 0.5 cancer cases. These cancer cases will be randomly distributed among the exposed population. If the additional risk of cancer was proportional to the square of the dose or some other non-linear mathematical function then the collective dose will be of little or no use.
In a very good book (Coggle, Citation1971) on radiation biology John Coggle mentioned that different bacteria have different graphs of log (surviving fraction; SF) vs dose (D). For a bacteria in which all cells are identical and a single event (single hit) is able to disable the cell then the graph is a straight line which obeys the following equation:
In a Monte Carlo simulation of 1,001 cells I was able to generate such a line in a system where it was assumed that in each exposure to radiation a cell had a given chance of being inactivated by the radiation. We can take a similar view to the induction of cancer, if we assume a single mutation is able to cause carcinogenesis in an organism then we can have a similar graph of the fraction of animals (or people) who are free of cancer as a function of the radiation dose.
However if two events are required to either kill the cell or induce cancer then the graph will be different. A common model used for understanding radiotherapy is the LQ model which has two constants (α and β), these constants have nothing to do with alpha and beta particles. It is yet another unfortunate case of symbol reuse. To me symbol or term reuse is where two (or more) areas of science/engineering use the same symbol or term for two (or more different) things. For example activity in solvent extraction can mean either a radioactivity level (Bq kg−1 or Bq l−1) or a chemical activity. In this LQ model the SF is given by the following equation:
It is possible for to have cells which can as a result of a single mutation or two mutations become cancer cells. Such a population of cells could be dealt with using this equation with the α and β constants. If α is zero while β is above zero then we would have a graph which would be described by the following equation:
This would describe a system where more than one hit on a cell is required to create a cancer cell. We need to consider the question of how long lasting is the sublethal or subcarcinogenesis damage is. If we were to divide a dose (D) into n equal fractions each containing dose (d) then assuming that we allow sufficient time for complete repair of the sublethal damage then according to Zachrisson et al. the following equation would hold true (Mu, Löfroth, Karlsson, & Zackrisson, Citation2003):
But if we do not leave sufficient time for the sublethal damage to be repaired then according to Zachrisson et al., we need to insert a new term into the equation to correct for this.
One great problem is what value should G have, from experimental work in the 1960s Elkind et al. exposed mammalian cells to radiation with and without a rest between parts of the dose (Elkind, Suttongi, Moses, Alescio, & Swain, Citation1965), it can be argued that the value of G can decrease with increasing time, then decrease again and then increase again. Coggle explains this by stating that the first part of the dose will kill off the cells which are in the most radiation sensitive stages of the cell cycle which will partly synchronize the cells. Also he stated that the radiation will inhibit the progression of the cell through the cell cycle for a few hours. The initial decrease of G with time is due to the repair of the cells. Next as the cells restart their cell cycle and move into a more radiosensitive part of the cycle the value of G rises. As cells progress into a less radiosensitive part of their cycle G decreases again and then it then stays low as the cells desynchronize and start to divide again.
If we ignore the Elkind recovery effect and assume that the sublethal damage is repaired at a rate proportional the amount of sublethal damage then we can use the ideas proposed by Lea and Catheside (Citation1942) which they used to explain the change in ease with which they could induce mutations when the dose rate changed. In the following equation Tδ is the time required to deliver a dose, while t and x are given by further equations (Mu et al., Citation2003):
Now we use the following equation to work out the SF:
While these equations about self-repair and the LQ model might seem rather dry and far from real life, I hold the view that they are very important as they explain some things. For example in the accident in Goiânia (1987 Brazil) a man and his wife were both exposed to radiation from a large 137Cs source which was stolen and brought into their home. The man (Devair Ferreira) had a dose of 7.0 Gy while his wife (Gabriela Maria Ferreira) had a dose of 5.7 Gy. His wife died while he lived for about 6 years after his exposure, before dying of cirrhosis of the liver (He was a heavy drinker).
If we assume for the sake of argument that α = 0.16 Gy−1, β = 0.016 Gy−2, t½ = 0.4 h then we can consider the effects of dose fractionation. If we choose a dose of 15 Gy delivered under different conditions then we should be able to see a difference in the effect. For a single dose delivered at a rate of 1,000 Gy h−1 we can see that it eliminates almost all the cells, while at 0.01 Gy h−1 a much larger fraction of the cells survive.
From our knowledge of cancer radiotherapy we know that cells divide and the cell division can offset the cell killing effect of the radiation. To deal with this we can use the equation where γ is a growth constant for the division of the cells.
If we set the doubling time (Tp) at 3 days, then γ will be equal to 0.009627 h−1. We can see at low dose rates that cell division offers another means of self-repair. Howard Thames worked out an equation for G for a system which is subject to short intense pulses of radiation (no self-repair during the irradiation) where the radiation is broken up into nʹ equal fractions delivered δT apart then the following holds true (Mu et al., Citation2003):
If we now consider a 7 Gy dose delivered in four equal fractions of 1.75 Gy we can see what the effect of altering the delay between the dose fractions will be. What we can see is that as the time between the doses increases the ability of the radiation to kill the cells decreases. We can next consider the effectiveness of radioactivity in the form of a sealed source implanted into a person. Dale (Citation1985) in an exceptional paper provided a series of equations which provide the relative effectiveness of radiation to kill cancer cells.
For a single dose:
While we normally write:
The following expression for the SF is also true:
While for an implanted radioactive seed which is allowed to decay away totally while inside a tumour the following equation is suggested by Dale in the list of useful equations towards the end of the paper:
Thus we can now write:
As d0 = Dλ we can now write the following equations:
The equations are not perfect when the radioactive decay constant is similar or larger than the self-repair constant the equations for the implanted radioactive “seed” predict a survival fraction which is unreasonable. This is likely to be due to the (λ–λ) term. Using a dose of 5 Gy, α = 0.16 Gy−1, β = 0.016 Gy−2 and t = 0.577 h−1 I was able to obtain this rather nonsensical graph which suggests that the use of a very short lived radioisotope (very high dose rates) will greatly increase the number of cells present, even without the need for cell division (Figure ).
A better equation is the full equation for the decaying source given by Dale:
With these equations we do not get a nasty kink in the line of the graph. Alternative methods of calculation exist we could use a Monte-Carlo method based on the idea that we have cells in three groups, unharmed, damaged and dead. I suspect that such a method would be more able to make good predictions when the values of τ and λ are similar. Another method would be to use a similar method to address the problem where the flow of cells between the three compartments as to the method used to model americium in humans. This problem in some ways is similar to the problem of calculating the fission product mixture very shortly after a nuclear detonation, in his masters thesis Captain Logan Harr of the USAF compared the different methods of calculating complex radioactive decay chains (Harr, Citation2007). He pointed out that the Bateman equations are an easy way to calculate the decay of radioisotopes, but it is sometimes impossible to use the Bateman equations. I see here a problem that the equations for cell survival fractions with a decaying source may not be perfect solutions, they may well be simplifications of more complex equations (which are general solutions) which work well under the conditions which can be found in radiotherapy. While I hold the view that the following method is not elegant and requires a lot of computer processing power it does offer a good solution. I can summarize it with a BASIC program which should run on an Amstrad 6128 (Locomotive BASIC).Footnote1
10 DIM celllive(4000); DIM cellhurt(4000); DIM celldead(4000); SF(4000)20 Let celllive(0) =1e6; Let alpha=0.158; Let bcon=0.296; Let dosestep=0.00530 For x=1 to 400040 cellhurt(x)=cellhurt(x-1)+(dosestep*bcon*celllive(x-1)-(dosestep*bcon*cellhurt(x-1)50 celldead(x)=celldead(x-1)+(dosestep*bcon*cellhurt(x))+(dosestep*alpha*celllive(x-1))60 celllive(x)=celllive(0)-(cellhurt(x)+celldead(x))70 SF(x)=1-(celldead(x)/celllive(0))80 Next
Using the same computer the program required for the SF at a given dose using a more mathematically elegant solution would be.
10 Let alpha=0.1620 Let Beta=0.01630 DIM SF(2000)30 For d=0–200040 dose=d/10040 SF(d)=exp(−1*((alpha*dose)+(beta*dose*dose))50 Next
One problem is that it is impossible to know if the ability of radiation to cause sublethal damage to a cell is the same as the ability of radiation to kill a sublethally damaged cell. If we assume that the two are equal we can use the BASIC program shown above. The values for the two constants in the first of the two programs have been chosen to give the same final result as would be obtained with α = 0.16 Gy−1 and β = 0.016 Gy−2.
Returning to the problem of the implanted seed (or radioactivity dispersed in a person) decaying away, we can see that the answers obtained by the simple Dale equation, the full Dale equation and the iterative method are different. With very short half-lives the full Dale and the iterative method give answers which are closer than those predicted by the simple Dale equation. It is clear that when a dose of 5 Gy is given to the cells that the irradiation caused by the shorter lived radionuclides is more able to kill the cells than the more prolonged irradiation at a lower dose rate.
In the case of a radioactive source implanted in a person for a time and then removed the equation for the survival fraction given by Dale is more complex. Dale offers the following expression where T is the time that the source is present inside the person:
Which then allows us to write:
As D = d0/λ, we can now write:
So we can now write:
The maths becomes much more complex if we attempt to include repopulation (cell division as a means of self-repair), this has been done by King, DiPetrillo, and Wazer (Citation2000). but I will not be showing these equations here. While humans are larger than cells it is important to keep in mind that we are a collection of different types of cells which perform different tasks. Many different types of cells are required to support life, it is possible to consider the acute effects of whole body irradiation by considering the most radiosensitive cells which perform vital actions. The reader might be excused for asking the question of “why is the survival of cancer cells being considered rather than normal cells”, there are reasons why I have chosen to consider the results generated by radiotherapy workers.
They have done a vast number of experiments on the survival rates of cells using radiation under different conditions.
They have managed to obtain a good mathematical model.
Evidence exists that healthy tissues obey the LQ model (Stewart et al., Citation2012).
What we can conclude from both the science of radiotherapy and experience from non-medical radiation exposures (such as accidents) is that the higher the dose rate the greater the ability of radiation is to cause acute effects such as radiation burns, damage to the digestive system and damage to the blood cell forming tissues. While it can be argued that the loss of life, limb or function due to radiation exposure is simply the loss of life, limb or function an alternative argument is that the loss which occurs shortly after exposure is more serious than one which occurs later in life as a result of the induction of cancer by the radiation. The reason for this second argument is that the person loses more years of either life or years of having a good quality of life. It is of the greatest importance that a situation in which members of the public suffer acute effects is never allowed to exist. Neither during the Fukushima or Chernobyl accidents were any members of the public exposed to a sufficient dose to cause acute effects.
One problem is that newspapers and other popular media are driven by a desire to be the first to publish news, while the academic and scientific world is driven more by the desire to publish results (news) which has been passed through a quality control process. As a result some rather lurid and implausible results have appeared in the media. For example in Koriyama (Koryama) in Japan on 16 March 2011, the dose rate was 240 microGy per day (10 μGy h−1), the Guardian newspaper printed an article claiming that this caused a 12-year-old girl to have nosebleeds (McCurry, Citation2017). I have to admit that this is a higher than normal dose rate which would provoke some investigation from me if I was to encounter it in a place where the normal dose rate was 0.5 μGy h−1. But even a whole year of exposure to this level of radiation (88 mGy) of gamma is too small a dose to cause the changes to the blood which would decrease the clotting of blood. Even if the dose was delivered in seconds or minutes. However in the Guardian newspaper of the UK it was claimed that this radiation caused a child to suffer nosebleeds. While it would be unreasonable for me to claim that newspapers are never right, I would like to take this chance to warn the reader to be careful of newspapers and other non-peer reviewed sources. But I do need to warn that sometimes the peer review system does not work. While Winston Churchill has been reported to have said:
“that democracy is the worst form of Government except for all those other forms that have been tried from time to time”
I would say that peer review is a bad system for quality control but it is the least bad system so far. It has failed from time to time in different ways. For example Mangano and Sherman (Citation2012) published a paper which claimed that Fukushima fallout caused infant deaths in the western part of the USA. A key part of the paper was the claim that the rate of death had increased after the Fukushima event. I have to admit that there was a small increase of the infant death rate, but an examination of the data does not indicate that the increase was due to the Fukushima event (Moyer, Citation2011). The raw data can still be obtained from the Center for Disease Control (CDC) in the USA (Anon, Citation2011). The paper of Mangano and Sherman contained a series of problems. For example it was pointed out by Gale that the deaths claimed by Mangano and Sherman were occurring after trivial exposures, while larger exposures (medical diagnostic X-ray examinations and diagnostic nuclear medicine) do not cause deaths due to acute radiation effects (Gale, Citation2012). Gale also pointed out that in Japan no reactor site workers had died despite being exposed to far greater doses than the American general public. It has been pointed out that the changes in death rate reported by Mangano and Sherman were not statistically significant (Körblein, Citation2012). While it was pointed out by another person that no mechanism is known that explains how such small doses might cause death and no sensible dose/response relationship can be seen (unexposed populations in the USA had the highest death rate increases after Fukushima) (Wolf, Citation2012). I also note that the paper by Mangano and Sherman rather than considering the degree of exposure often simply considered the fraction of air, precipitation (rain. etc.), milk and drinking water in which radionuclides could be detected. I would like the reader to consider the fact that in all fruit, vegetables, fish and meat that it will be possible to detect 14C and 40K. This does not indicate that all fruit, vegetables, fish and meat contains dangerous amounts of radioactivity. I hold the view that the peer review and editorial processes failed to prevent the publication of a paper which has multiple deep flaws which are easily apparent.
Doses of radiation too low to induce the acute effects, or exposures which are too protracted to cause acute effects can cause harm by inducing cancer. It is important to note that an acute exposure can induce cancer, but here we will consider the only late effects such as cancer. In Japan when the atomic bombs were dropped the population on the ground nearby received various doses, this dose was delivered over a very short time. In some of the data the relationship between the cancer incidence and the dose appears to obey a LQ or quadratic model. If we assume for a moment that the LNT model applies than for both atomic bomb survivors and radiation workers who receive large doses then we need to correct the LNT model. Instead of being able to write:
We need to correct the LNT model to take account of the cell killing effect of the radiation. If the radiation is delivered in a short pulse as an atomic bombing, a single pulse criticality accident or being present in the shielded cell of an irradiator or radiographic enclosure for minutes or seconds while the source is exposed then we can use the following equation where μ is a constant for the induction of cancer by radiation.
If the dose is fractionated into smaller doses or delivered over a long time then we need to consider the replacement (repopulation) and self-repair of the cells. This dose fractionation represents an exposure more similar to the commonly used fractionated radiation therapy for cancer or an occupational exposure in the early days of radiation work. I sincerely hope that radiation workers are not being currently exposed to doses which kill a significant fraction of their cells. If we assume that the likelihood of developing cancer is proportional to the number of mutated cancerous and precancerous cells which are generated by radiation. Then for a fractionated dose of radiation where the cells are allowed to repair all their sublethal damage between doses we have to consider the ability of cells in the tissue to repair themselves and/or the ability of the tissue to replace dead cells by cell division. This ability in the equations is taken care of by a constant x, I strongly suspect that if the conditions of the irradiation were to be changed than x will also change. To my mind while x is a useful constant it is a fudge factor.
The loss of the original cells is given by the equation:
αʹ is given by the following equation where df is the dose per fraction:
The appearance of new cells (repaired or replacement cells) is given by the following equation:
While the number of mutated cells which have the potential to develop into a carcinoma is given by:
While the number of mutated cells which have the potential to develop into a sarcoma is given by:
When the dose is small the equation for carcinoma induction will give an answer which is similar to that given by the LNT model. Another version of the equation for the induction of carcinomas exists. For a tissue which is unable to repair itself or for cells to repopulate R = 0 while for a tissue which can fully repair (or repopulate) itself R = 1 (Schneider, Stipper, & Besserer, Citation2010).
This model can be fitted to the statistics for the induction of breast (Schneider et al., Citation2011) and lung (Schneider et al., Citation2010) cancers during radiotherapy. The value of R in this equation can be found using the following expression:
These equations which consider the effects of cell death and repopulation which are used to model carcinomas have at their core the assumption that the likelihood of carcinogenesis is proportional to the dose at small doses. In the case of radiation delivered over a very short time the carcinogenesis (Japanese atomic bombings) seemed to obey the LQ model. There is a problem that the population which was exposed to high doses and survived the heat, blast and acute radiation effects was relatively small. As a result the statistical error on the number of cancers will be large. If Poisson statistics are assumed for the appearance of cancer cases then simply by virtue of the small number of people who received large doses and then lived sufficiently long to have the opportunity to develop the uncertainty on the cancer risks of the larger exposures are large.
While the atomic bomb exposures occurred over a similar time to some short duration exposures which some radiation workers might experience during some types of accidents (such as those which can occur in the shielded cell of an irradiator or radiographic enclosure) in many accidents it is likely that both workers and members of the general public will be exposed to protracted exposures lasting hours, days, weeks or even longer. A meta-study of the induction of cancer by radiation in animals suggests that for protracted exposures the dose response is not LQ (Haley, Paunesku, Grdina, & Woloschak, Citation2015). I cannot hope to be able to give a detailed review of the relationship between radiation exposure and carcinogenesis in detail but I hope that I have made it clear that it is difficult to decide quite how carcinogenic radiation is. The question of does radiation cause serious harm to the circulatory system has been the subject of some papers as I have mentioned in the first review.
It is important to consider before we explore serious accident chemistry the fact that the fear and terror associated with radiation can cause harm. Even scientists and other people who regard themselves as rational can be affected, I can admit that 17 years ago when I did radioactive chemistry for the first time I experienced fear and anxiety which caused some physical symptoms of stress to appear in me. While in my case the transient terror had no lasting effect it is quite credible that in some cases the fear of radiation has been more deadly than the radiation. In Japan an increase in the death rate of elderly people who were evacuated rapidly from care homes was observed after Fukushima (Nomura et al., Citation2016). As the increase in death rate in the population who were evacuated later in a calmer more ordered manner was much smaller, we can conclude that those who were evacuated later were less likely to die.
The radiation exposure, due to the Fukushima accident, of the nursing home residents was lower (0.01 mSv) while those evacuated later received a higher average dose (0.4 mSv). If we assume that a dose of less than 1 Gy is only able to cause death by randomly inducing cancer and that the risk of developing cancer in a given year is increased throughout a person’s life. Then we would expect that the younger a person is that the greater their loss of life expectancy as a result of the radiation exposure. In the paper about the nursing home the older a person the greater the life shorting effect of the incident was. If the deaths were caused by radiation exposure then we would expect that the more exposed persons would be either more likely to suffer an adverse outcome. But in this case the persons exposed to less radiation lost more life expectancy than those exposed to the higher (but trivial dose) (Table ).
Table 1. The number of days lost from the life expectancy of a person requiring low to medium care evacuated from a care home in the Fukushima area.
The same authors considered the life shorting effects of 25 and 100 mSv doses of radiation, they calculated that these doses would cause a 80-year-old man to lose 0.2 and 1.1 days of his life. It is interesting that Greenpeace in their document entitled “Unequal Impact” (Ulrich, Citation2017) and the World Information service on Energy (Anonymous, Citation2011) (Olson, Citation2011) have stated that radiation is more harmful to women than it is to men. The paper by Murakami et al. does consider female residents, for a women aged 80 using the standard methods they predict that a dose of 25 mSv will cause the lady to lose 0.26 days of her life and the 100 mSv dose will cause her to lose 1.4 days of her life. While these values are higher than the loss of life expectancy for the 80-year-old man, the difference is small.
As the uncertainties on estimates of how carcinogenic radiation is to humans are large, I want to make a few things clear.
Ionizing radiation is carcinogenic, it is an inescapable fact that exposure to radiation doses above 1 Gy are clearly carcinogenic.
At low doses the statistical errors on the data are very large. It is not totally clear if the response for some cancers is linear or if there is some threshold or threshold like behaviour.
The younger a person is at the time of exposure the more harmful (carcinogenic) a large dose of radiation is. This is biologically plausible, children have longer life expectancies than adults (more time for radiation induced cancers to develop) and they have higher rates of growth (and cell division) than adults.
Ignoring the special case of children, anyone who claims that one group of humans are more/less sensitive than another to radiation should be viewed with a healthy degree of scepticism. My advice is ask them what evidence they base their judgement on and ask how statistically significant the difference is.
Anyone who disagrees with the ICRP on the question of how dangerous radiation is should be treated with a healthy degree of scepticism. While sadly the ICRP are not incapable of making an error, their position was arrived at after considering a lot of data. While this data may not be perfect it is likely to be some of the best data in the world. My advice if you encounter someone who states that the ICRP is wrong is to politely ask for the evidence (including statistical evidence).
Finally before we go on to consider some of the chemical (and occasionally physical) processes which govern nuclear reactor accidents and some of the other accidents in the nuclear fuel cycle. We should reflect on the question of what is an acceptable exposure. If we look at UK law we will notice that over the years the dose limits for workers has decreased they have decreased. In the time between 1961 and 1984 the legal classified worker whole body limit for gamma rays and other penetrating radiations was 120 mSv per year. From 1985 to 1999 the yearly whole body limit classified worker limit was 50 mSv while since 1999 it has been 20 mSv per year. This to me suggests that while the medical management of radiation induced diseases might be improving that both science and society in general has become stricter with regard to the question of “what is an acceptable occupational radiation exposure.”
2.2. Types of nuclear reactor accidents
Before we start to discuss the different types of accident we need to consider the scale by which reactor accidents are measured, this is the International Nuclear Event Scale (INES) (Abe et al., Citation2013). This was devised by the IAEA and the OCED after Chernobyl as a means of classifying, rating and communicating the severity of nuclear accidents. The scale has eight levels (0–7). At the bottom is a level 0 event which has no impact on health, safety or the environment. The top level is level 7. The order of events are anomaly (1), incident (2), serious incident (3), accident with local consequences (4), accident with wider consequences (5), serious accident (6) and major accident (7). Non-radioactive events such as workers falling off the roof of a nuclear plant, being shocked with electricity in the switch yard and even stubbing their toes in either the control room or the containment are not considered on this scale. Unless one of these “non-nuclear” and “non-radioactive” accidents has an effect on the safety of a nuclear or radioactive site. For example a careless HGV driver who rams a pylon causing it to crash to earth into the switch yard thus disabling the electrical power systems of a site could be accessed on the INES scale, but if this dire driver was to ram the restaurant killing the catering staff it would not be considered. The scale considers the impact of an event on three different things:
People and the environment,
Radiological barriers and controls, and
Defence in depth.
The impact on people and the environment considers the effect on the general public, a release of radioactivity into the atmosphere can be used to rank an event. The scale is based on 131I, a series of other radioisotopes are given conversion factors to allow them to be compared with 131I. For example 241Am has a value which is 8,000 times that of 131I, 32P has a value of 0.2 times that of 131I while noble gases have a value of zero. For example a release of 1 TBq of 241Am is ranked as being the same as a release of 80 PBq (8,000 TBq) of 131I. While a release of 1 TBq of 32P is regarded as being the same as a release of 200 GBq of 131I.
Based on radiation doses to humans the event can also be assessed for example if an event causes a non-lethal radiation injury then it must be rated at least three, a fatal radiation injury will result in a rating of at least four. Even if no human is harmed (or even mildly irradiated) by an accident and no radioactivity is dispersed into the environment an event can still receive a score. For example the Three Mile Island accident was thought by the IAEA to have only exposed the public to a dose not greater than 0.8 mSv. If the accident was judged on dose to the public alone it would have a very low score (maybe 0). But as part of the core was molten (more than a few percent) it was rated at level 5. Some examples of real life events which have been rated in the INES scale are included in Table .
Table 2. Examples of different accidents rated on the INES scale.
I judged the Casablanca lost 192Ir source which occurred in 1984 to be a level 5 event as it killed eight members of the public. For between 1 and 3 deaths the minimum rating is 4, but when more than a few deaths occurs then the rating is increased by one level. It is not considered likely that a radiation source accident would kill more than 30 people, but if it did so then the event would be rated at least at 6. For comparison I have added four fatal railway accidents, while they should not be rated on the INES scale based on the loss of lives I have ranked them as if they were radiation source accidents with no dispersion of radioactivity. While the IAEA do not consider a radiological accident involving a radioactive source that kills more than 30 people to be credible, I hold the view that a fatal accident which kills over 500 people then the spirt of the logarithmic INES scale would indicate that it would be rated at 4 + 3 = 7. I will not be discussing in detail the fatal and non-fatal food and medical product irradiators, these accidents have been associated with a number of fatal injuries to workers. All the irradiator accidents which I am aware of occur in the following way.
Something goes wrong inside the irradiation chamber, thus attracting the interest of an operator.
The operator then enters the room, sometimes they ignore alarms and defeat safety devices.
The operator then receives a dose of radiation.
The operator is then either dies in hospital or has some other serious life altering injury.
If the reader wishes to it is possible to have some further understanding and experience of accessing events then on-line teaching tool provided by the IAEA exists (IAEA, Citation2014).
I have chosen to write this review about fission reactors, this is because with the exception of stars it has been impossible to obtain a self-supporting sustained fission reaction in a system which emits more energy than that it consumes. The lack of fusion power plants has not totally prevented the idea of an accident in a fusion plant being considered. If an accident was to occur in a fusion plant such as ITER it could reach level 6 on the INES scale (Nie, Ni, & Wei, Citation2017). It is important to note that the radionuclides which would be important in such an accident are different to those which are important in a fission plant. For a start we will have little or no radioiodine. But in terms of safety the fusion reactors have a weak point, this is the vast tritium inventory. While the fuel in the vast majority of fission power reactors is a high melting point solid the fuel in a fusion reactor is either in the form of gas or plasma. As a result the tritium in a fusion reactor has the potential to become very mobile. It is likely that in a fusion plant much of the tritium will be stored in the form of a stable metal hydride such as uranium hydride.
In a fusion reactor accident activated metal may be liberated in the accident. Neutron activation of the water cooling loops will be able to generate some 51Cr, 54Mn, 56Mn, 55Fe, 57Co, 58Co, 60Co and 57Ni. While the plasma facing tungsten will contain 60Co, 179Ta, 182Ta, 182mTa, 183Ta, 184Ta, 186Ta, 179W, 179mW, 181W, 185W, 185mW, 187W, 184Re, 186Re, 188Re and 188mRe (Taylor & Raskob, Citation2007). During the normal life of a plant and during an accident dust can be formed by the erosion of metal surfaces, if this dust is mobilized during an accident this could contribute to either workers or members of the public being exposed to radiation. If the plant uses a lithium/beryllium fluoride blanket as a tritium breeding system then within this some 18F could be generated by the n.2n reaction. This 18F could add to the dose due to an accident. Due to the difficulty of causing a fusion reaction to occur, I do not consider an accident due to a surge in fusion rate to be reasonable. I think that the main types of accident will be a loss of cooling accident and a loss of vacuum accident (LOVA). These two types of accident have been considered (Malizia, Poggi, Ciparisse, Rossi, Bellecci and Gaudio, Citation2016). It is interesting to note that in some facilities that air can become radioactive as a result of exposure to very high energy radiations, for example 11C as CO2, 13N as N2 and NO and 15O as O2 and NO has been found in the air at a 100 MeV LINAC (LINear ACcelerator) (Endo, Kikuchi, Izawa, & Ikezawa, Citation1995). These proton rich nuclides are likely to be formed by photonuclear reactions due to very high energy gamma rays. While high energy neutrons are able to create 38Cl and 39Cl from 40Ar and 83Br and 84Br from 84Kr. These radiohalogens can become attached to existing aerosol particles (Endo et al., Citation2003). I imagine that nothing would prevent other radionuclides absorbing onto existing aerosols.
While in the high energy proton facility in Japan 7Be was observed, this radionuclide is unlikely to be formed in a nuclear reactor system. According to some Japanese workers in the proton accelerator sites primary and secondary particles with 30 MeV or more energy are able to form this beryllium radionuclide by spallation of nucleons from the nitrogen and oxygen in the air (Kondo, Muramatsu, Kanda, & Takahara, Citation1984). However some Russian workers claim that protons with little more than 10 MeV can form 7Be from 14N (Larin, Malyshevskii, & Fomin, Citation2014). Even 10 MeV protons are particles which are very high in energy compared with the typical fission neutron from 235U (2 MeV). Before we leave 7Be there are two interesting things about it.
Firstly the 7Be is thought by Kondo et al. to form either beryllium oxide or the hydroxide which then attaches to non-radioactive aerosol particles to form the radioactive aerosols. Secondly it is a radionuclide with rather interesting decay kinetics. The rate of decay is a function of the chemical environment of the nuclide. Some of the young earth creationists (Walker, Citation2000) have pointed out that the decay of 7Be is very dependent on the chemical environment of the beryllium (Huh, Citation1999). This might be a shock, but it is true (Mazzocchi, Janas, Bączyk, Fynbo, & Köster, Citation2012). The half-life of 7Be is influenced by the chemical environment of the radionuclide. It is reasonable to state that 7Be is a special case. Firstly 7Be decays by electron capture with a very low decay energy (862 keV). As the decay energy is shared between an emitted positron and the neutrino it is unlikely that the nuclide will be able to form an antielectron (positron) and eject it from the nucleus.
One consequence of this is that as the decay energy is low compared with the energy required to form a positron the 7Be is unable to decay by β+ and must undergo an electron capture reaction. Secondly as a neutral atom of beryllium has four valance electrons and can reach an oxidation state of +2, it is possible to significantly alter the electron density near the atomic nucleus. As a result different chemical forms of 7Be decay at different rates.
While 7Be in precipitation from the atmosphere (natural fallout) is important as a measure of solar activity, the decay of 40K into 40Ar is important as a radioactive reaction using for dating rocks. The reader may be interested to know that experiments have been performed in which the rate of the decay of 40K has been measured for different chemical forms, no difference was observed (Norman et al., Citation2001). As in the case of potassium, any change in the chemical environment of the potassium atom will have a far smaller effect on the electron density close to the nucleus. This is because the uncharged potassium has 21 electrons while beryllium only has four electrons per atom.
But back to reactor accidents. The first major reactor accident in the west was the Windscale reactor fire (1957), this was a fire in an air cooled graphite moderated isotope and plutonium production reactor in the north of England. The reactors were rated for 180 MW of thermal output, the fuel load was 180 tons of uranium (72,000 cartridges) and the moderator was 1,900 tons of graphite. The reactors are 15 m diameter graphite cylinders which are 8-m long. The reactor was designed for a maximum uranium temperature of 395°C (Sheil, Botzem, & Johnston, Citation1999).
The fire dispersed radioactive iodine, cesium and some other radionuclides including 210Po over much of Europe (Stewart, Crooks, & Fisher, Citation1961). The polonium was released from bismuth containing capsules which were being neutron irradiated to form polonium. The polonium was wanted as an alpha emitter for use in nuclear weapons. As much of the non-noble gas release from the reactor accident was radioactive iodine, in response to the accident a considerable amount of work was done on iodine.
Energy will accumulate in the graphite of a nuclear reactor which operates at a low temperature, if this energy was allowed to accumulate without a release then it could lead to a sudden energy release (Bell et al., Citation1962). Often when neutron irradiated graphite is heated a release of energy is observed at 200°C, a smaller amount of energy is released at higher temperatures. However when graphite is either irradiated to very high neutron doses or is irradiated at higher temperatures then the release of energy at 200°C becomes smaller when compared with the releases at higher temperatures (Mitchell & Taylor, Citation1965). It is normally considered that the annealing reactions are first order reactions whose rate can be described by the Arrhenius equation:
On heating the number of the defects will decrease. In one study the effect of the rate of heating (1–100°C min−1) was considered at length (Iwata, Citation1985), what Iwata observed was evidence that several different interstitial clusters of different size exist in neutron irradiated graphite. In one recent study of graphite subject to fast neutron bombardment (5.67 × 1020 to 1.13 × 1022 n m−2 at 7.88 × 1016 n m−2 s−1 and below 100°C) using differential scanning calorimetry energy releases at 150, 200, 230 and 280°C were observed, these peaks correspond to activation energies of 1.31, 1.47, 1.57 and 1.72 eV respectively (Lexa & Dauke, Citation2009).
With higher neutron doses it was found that the temperature at which the energy is released is shifted to a higher temperature even when the graphite was irradiated below 100°C (Lexa & Kropf, Citation2006). Other workers have also observed energy releases over the range 200–600°C (Lasithiotakis, Marsden, & Marrow, Citation2013) and it is known that the graphite interlayer spacing changes after neutron irradiation. Lexa and Kropf along with other workers have observed that the c axis of the graphite unit cell increases in length as the Wigner energy level increases. But it is important to keep in mind that the higher the irradiation temperature the less energy will be retained as Wigner energy by the graphite.
Using the graphite plunger from a control rod taken from unit 2 of the Kursk nuclear power plant (RBMK reactor) indicated that after 6.5 years of service some of the properties of this graphite had changed. For example the thermal conductivity of the graphite had decreased as a result of neutron bombardment and instead of the thermal conductivity of graphite decreasing as the temperature rises the thermal conductivity of the neutron bombarded graphite increased as the temperature rises (Platonov et al., Citation2003).
The Wigner energy is deposited in graphite by bombardment by fast neutrons. It is important to note that the action of the neutrons on the graphite create a series of different types of defects. Some of these defects are very easy to anneal out of the graphite. While some of them are so stable that they require heating to a very high temperature (1,400°C) before the graphite will relax back to normal (Telling & Heggie, Citation2007). Telling and Heggie in their extensive review considered the Windscale piles, rather than returning the graphite to the virgin state they expressed the view that during each anneal that residual defects with higher activation energies (release barriers) were formed. This contributed to an accumulation of Wigner energy in the graphite. They suggested that an aggregation of the defects was occurring.
It has been stated in interviews that the fuel at the Windscale piles had been modified in an attempt to increase the neutron flux in the reactor and thus the production of plutonium and other products. I hold the view that this could contributed to the accident. It is impossible to know exactly what caused the Windscale fire, but it is reasonable to state that during the ninth annealing of the graphite pile that the reactor overheated. It is likely that a tritium generation capsule containing Mg/Li alloy burst open before igniting. My own view is that the likely root causes of the fire were a poor reactor design combined with a misjudgement made by a senior person. This was the choice to increase the neutron flux in the reactor (and thus the power level). In an attempt to create a hydrogen bomb the UK government needed a large amount of tritium. Together with plutonium and polonium the tritium was needed for nuclear bombs.
The resulting fire caused a large release of radioactivity. While the filters on top of the chimneys would have reduced the size of the radioactive release they were not able to prevent all solid particles from escaping. It was reported that 137Cs, 103/106Ru and 95Zr were found on nearby farms after the accident (Dunster, Howells, & Templeton, Citation2007). While it is conceivable that the ruthenium could have become mobile as the tetroxide the zirconium would have had to leave the pile in the form of a solid. The krypton would have long since decayed into one of the solid forming nuclides in the following decay chain before it would have been able to diffuse out of the fuel.
The MAGNOX and AGR reactors operate with the graphite at a higher temperature than the Windscale piles, as a result the accumulation of Wigner energy in the graphite is a smaller problem in these reactors. While the existence of the Wigner energy problem might make the idea of a graphite moderated reactor appear unattractive to some readers, it is important to note that the absence of water from the core of a gas cooled reactor eliminates the possibility of a Boiling Liquid Expanding Vapour Explosion (BLEVE) in the event of a temperature increase in the reactor or as a result of a sudden pipe break. During the Chernobyl accident which is discussed later, the surge in the thermal power output of the nuclear reaction caused a steam explosion. A steam explosion is a BLEVE where the liquid is water, the steam explosion broke open the core and may have been followed by a hydrogen/air explosion at Chernobyl.
At a similar time to the Windscale fire, in the Soviet Union an accident with a rather more disagreeable effect occurred (INES level 6). This event was first brought to public attention in the west by Zhores Medvedev (An exiled Soviet geneticist) in a 1976 article (Medvedev, Citation1976) in New Scientist (Norman, Citation1983). It is not clear what was known in the west by “the establishment”, a 1982 document (Soran & Stillman, Citation1982) from Los Alamos argued that the accident at Kyshtym never occurred and that “The Soviets successfully, albeit rather unsensationally, created a contaminated area near Kyshtym through carelessness and blatant disregard for their people or their surroundings”. Ignoring the form of radiological hooliganism known as “dirty bombing” I am unsure how the creation of a contaminated area can be a success. To my mind contamination is the presence of something which is unwanted. On the other hand it has been claimed by some that the CIA have known about this accident for many years but for political reasons they choose to keep their knowledge secret.
The reason I regard the Kyshtym accident as being worse than the Windscale Fire is that the effects of the Kyshtym accident were worse due to the release of more activity which included the long lasting 90Sr. A recent Russian estimate is that 740 PBq of activity was released during the Kyshtym accident (Akleyev, Krestinina, Degteva, & Tolstykh, Citation2017). This compares with Garland and Wakefield’s estimate that 35 PBq of radioactivity was released by the Windscale fire. According to Garland and Wakefield’s estimate the Windscale fire released 8.5 PBq of non-noble gas radioactivity, which is a little more than 1% of the Kyshtym release. Much of the radioactivity released by the Windscale fire was radionuclides with short half-lives (133Xe t½ = 5.2 days, 131I t½ = 8 days and 132Te t½ = 3.2 days), while much of the Kyshtym accident release was longer lived radionuclides such as 95Zr (t½ = 64 days) and 144Ce (t½ = 285 days). All the radionuclides which were listed as major components of the release have such shorter lived daughters except for 95Zr. As a result for those other than 95Nb I have chosen to consider their radioactivity as an extension of the parent’s radioactivity.
The release was caused by a violent event inside a waste store, at Mayak PA a high level radioactive waste (a mixture of acetate and other salts) (Jones, Citation2008) was stored in metal tanks which were inside concrete vaults. Each full tank contained between 70 and 80 tons of waste. The tank was a cylinder with a plain bottom (diameter 8 m and height 6 m) which was made of stainless steel. This was within a reinforced concrete canyon (diameter 9 m and height 7.4 m). The canyon had a circular lid (circa 0.8 m) made of concrete and was then covered with between 1 and 1.5 m of soil (Avramenko et al., Citation2000). The mixture of radioactivity, water, sodium nitrate, sodium acetate and some potassium dichromate was heated by the decay heat until it boiled dry. The solid residue contained a fuel sodium acetate (22.2%, w/w) and two oxidants (sodium nitrate and potassium dichromate sum 75.8%, w/w). If we assume all of the oxidant was sodium nitrate then in 1 kilo of the solid residue 2.71 mol of sodium acetate were present and 8.92 mol of sodium nitrate were present. Total combustion of sodium acetate can occur by the following reaction:
If sodium nitrate was heated it can decompose to sodium nitrite according to the following reaction:
If sodium nitrate was subject to greater heating then it might be possible to convert it into oxygen, nitrogen and sodium oxide as according to the following reaction:
The stoichiometric mixture for the reaction in which sodium nitrate is converted into nitrogen gas will be a 4:1 (by moles) mixture of sodium nitrate and sodium acetate. Chillingly the mixture in the tank was rather close to this ratio at 3.3:1.0:
Modern nuclear fuel reprocessing uses the extraction of uranium and plutonium from nitric acid by solutions of tributyl phosphate in what is known as the PUREX process. It is normal to extract both the uranium and plutonium from the nitric acid solution of the fuel (pregnant leach liquor) and then back extract them, before reextracting them again. The process is designed to extract uranium(VI) and plutonium(IV) from the fuel. The plutonium oxidation state can be adjusted with things like sodium nitrite or nitrogen oxides before the extraction. By reducing the plutonium to the +3 oxidation state with a reducing agent such as ferrous ions, hydroxylamine or hydrazine the plutonium is converted into a form which does not extract from nitric acid with tributyl phosphate. Thus the uranium and plutonium can be separated. Chromate and acetate are not normally used in the PUREX process so the contents of the waste tank suggest to me that the Soviets at the time were not using PUREX chemistry to obtain plutonium. The method of plutonium separation is based on the formation of acetate complexes of the actinides. The uranium (Paramonova & Kalychev, Citation1966) and plutonium (Moskvin, Citation1969) form complexes with acetate (Table ).
Table 3. Thermodynamic stability constants for acetate complexes of uranium and plutonium.
It is known that uranium and plutonium can be extracted from acetate media using tri-isooctylamine mixed with xylene (Moore, Citation1960), it could have been reasoned that in the Soviet Union that such a process was used in the early days at Mayak. But the waste at Mayak associated with the accident was not from a solvent extraction process. The chromate in the waste is also of note, Moore reported that very little extraction of trivalent and tetravalent plutonium from the acetate media into the amine containing organic layer occurred. But hexavalent plutonium (PuO22+) was well extracted. It is well known that plutonium redox chemistry is very complex, it is possible for four different oxidation states of plutonium to coexist in aqueous media. Complicating the matter further plutonium tends to disproportionate according to the equation:
Things are further complicated by the fact that in a concentrated plutonium solution the water is subject to a lot of alpha radiation. The radiation creates hydrogen peroxide and other species which can alter the redox state of the plutonium. We need to consider several redox couples:
We need to consider also a more complex reaction:
The redox potential for this reaction will change as the proton concentration changes as the reaction involves protons. The Nernst equation can be used to predict the emf of a cell which does not have everything at 1 mol per litre. If we assume for a moment that all activity coefficients are 1 then we can write:
In this case Q is given by the following equation:
It can be reasoned that as the hydrogen ion concentration decreases that it will be easier and easier to oxidize plutonium(IV) to plutonium(V).The complex thing about chromium in acidic media is that a vast number of redox reactions are possible. The book by John Emsley lists a rather large number of redox reactions for chromium.
This data suggests that it is possible for chromate to oxidize plutonium into the hexavalent state.
The Russian radiochemical literature it is pointed out that an acetate precipitation method was preferred in the early Soviet nuclear industry (Zilberman & Romanovskii, Citation2003). The process formed solid sodium uranyl triacetate Na[UO2(OAc)3] which was mixed with the plutonyl compound. It has been reported that the sodium uranium(VI) triacetate has a cubic cell (a = 10.670 Å), while the neptunium(VI) and plutonium(VI) compounds are isostructural and have cubic cells which are slightly smaller (10.659 and 10.643 Å respectively) (Zachariasen & Plettinger, Citation1959). What happened in the process was that the irradiated fuel was dissolved in nitric acid yielding a solution in 1–2 M nitric acid. This was then treated with sodium dichromate to oxidize the plutonium to plutonium(VI). Then the solution was adjusted to a near neutral solution of 5 M sodium nitrate, 1 M acetic acid and 0.5 M sodium acetate. The uranium and plutonium then were allowed to precipitate. The supernatant, containing most of the fission product radioactivity, was then removed and the solid washed with an acetate solution. The mixed plutonium/uranium solid was then redissolved and the process repeated to reduce the radioactivity of the solid. When the fission product radioactivity was sufficiently low the plutonium was reduced to plutonium(IV) or plutonium(III). A wide range of reducing agents could have been used for this, the most obvious one to my mind is iron(II).
The plutonium would have remained in solution while the uranium was then precipitated with acetate to form a plutonium only solution. The plutonium was then reoxidized to plutonium(VI) which was then converted into sodium plutonium triacetate. All this precipitation and redissolving would have generated vast volumes of liquid waste, and under some conditions it could have caused the metals to deposit onto surfaces in the plant and/or formed small (and mobile particles). During the second world war in the United States of America plutonium was isolated using another precipitation process based on bismuth phosphate. As the bismuth phosphate process is better known than the acetate process I will not dwell on it.
One can imagine that in the same way as perchlorate is a large anion which makes many cations such as the iron-tris(2,2ʹ-bipyridyl) dication form water insoluble crystalline solids the uranyl triacetate anion is a large anion which has the potential to form insoluble salts with cations. But cesium and rubidium do not form solids with uranyl zinc acetate with the same ease (Barber & Kolthoff, Citation1929), thus there must be something special about sodium. A search of the crystallographic literature indicates that a vast array of uranyl triacetate complexes exist.
For cesium two cesium uranyl triacetates appear in the Cambridge database, there is a cubic (Serezhkina, Vologzhanina, Klepov, & Serezhkin, Citation2011) and an orthorhombic (Serezhkina, Vologzhanina, Klepov, & Serezhkin, Citation2010) solid. The cubic compound has 16 uranium atoms in the unit cell, the unit cell contains 12 locations which are either cesium or barium. This ratio suggests to me that some of the “cesium” locations in the solid may in fact be barium atoms. The solid is a disordered solid with sites being randomly occupied by either cesium or barium. A given site has a 0.333 probability of being occupied by a barium and a 0.666 probability of being occupied by a cesium. The orthorhombic cesium compound has uranyl triacetate groups being bridged by the cesium cations (Figure ).
Figure 5. The coordination environment of the cesium in cesium uranyl acetate. The cesium is blue, the uranium atoms are yellow, the oxygens are red, the carbons are dark grey and the hydrogens are light grey.
The cesium atoms in the orthorhombic solid have a coordination number of 7, six of the oxygens in the inner coordination sphere of the cesium are carboxylate oxygens while one is an oxide ligand from an uranyl (UO22+) group. The coordination of uranyl oxygens is not unique, 96 different structures are known which include actinyl oxygens binding to metal centres other than the actinide which they are doubly bonded to. The rubidium uranyl triacetate has been characterized, it has a tetragonal cell (a = 13.840 Å, b = 13.840 Å and c = 27.570 Å), but sadly no coordinates are present in the Cambridge database. Potassium uranyl triacetate has a six coordinate potassium, the potassium is coordinating to carboxylate oxygens only (Serezhkina et al., Citation2010) (Figure ).
Figure 6. The coordination sphere of a potassium in potassium uranyl acetate. The potassium is purple, the uraniums are yellow, the oxygens are red, the carbons are dark grey and the hydrogens light grey.
The sodium uranyl acetate has six coordinate sodiums, these sodiums are being coordinated to by carboxylate oxygens.
So it is unlikely that the cesium would have been removed from the fission products by this sodium uranyl acetate formation. In September 1957 the cooling water supply to tank 14 failed, the contents of the tank overheated to 330–350°C (Jones, Citation2008). On the 29th of September at 16:20 (local time) an explosion occurred. The majority (90%) of the radioactivity was deposited within 5 km while the rest of the radioactivity was dispersed by the north-northeast wind (Akleyev et al., Citation2017). To my mind this accident is a cogent argument for not storing highly active waste in a liquid or other easily dispersible form, I reason it is better to condition the waste into a glass or other solid which is not easily dispersed. Another key lesson is that it is best to avoid an accumulation of a mixture of fuel and oxidant which can overheat. I imagine some readers will have heard of Dr Alex Comfort or might have read some of his work. He was famous for writing a popular sex manual (The Joy of Sex), but it is noteworthy that as a youngster he seriously injured himself while experimenting with explosives. From what I have learnt of this event he had made the mistake of grinding a mixture of a fuel and an oxidant. Equally it is inadvisable to heat violently a large amount of a fuel mixed with an oxidant. But it is clear that under some conditions a fuel and an oxidant can be combined and reacted without explosion. For example a candle flame is a reaction of oxygen with the air with a fuel. While the majority of the fuel might be in contact with atmospheric oxygen it is far too cold to react, the warmth of the candle flame melts the wax. The wax is then drawn up the wick and it is heated to generate the combustible vapours which then burn in the flame thus forming light, heat and combustion products. It is clear that often a candle flame is a reaction which is in a steady state.
Equally it is possible to react cyclohexanones or formic acid with nitric acid in a denitrification process without the mixture exploding. By treating PUREX first cycle raffinate [a Highly Active Liquor (HAL] with formic acid it is possible to destroy much of the nitric acid in the HAL. It is important to note that in the nuclear reprocessing industry it is common to take the highly active raffinate (HAR) from the first cycle of the PUREX plant and concentrate this down to form a smaller volume of highly active concentrate (HAC). For example recently some Chinese workers have described how they have been able to denitrify simulated HAR (Li et al., Citation2017), this step is needed if they are to treat HAR with their TRPO process. The idea of the TRPO process is to remove the trivalent transplutonium elements (such as americium and curium), the neptunium (Li, Chen, & Wan, Citation2012) (Np) and the residual plutonium (Pu) in the HAR to form a waste which needs to be isolated from humans and their environment for a shorter time (circa 300 years). The actinides (Pu, Am, Cm, Bk, Cf) can then be disposed of in a separate smaller volume waste store built to a higher standard than that required for the high level beta/gamma waste or they could be placed in a fast reactor as fuel. The latter option will require the removal of the lanthanides from the trivalent actinide fraction. In the work by Sun et al. it was found that zirconium molybdate formed during the reaction, when the removal of nitric acid was greater they observed that tin, tellurium, ruthenium, rhodium and palladium disappeared from the solution into the precipitate. Equally at the ITU during the PARTNEW project a HAR formed by small scale treatment of a genuine fuel dissolution liquor (MOX in PWR, 30 GW day/ton metal) was concentrated by boiling and the addition of formic acid without anything untoward happening (Serrano-Purroy, Christiansen, Glatz, Malmbeck, & Modolo, Citation2005). Normally sugar is combined with high level waste (Highly Active Liquor) before it is converted by the vitrification process into the waste glass (Harrison, Citation2014). While sugar is well known to be a fuel and nitric acid is an oxidant this process is safe, the reason is that large amounts of sugar/nitric acid mixtures are not allowed to accumulate. The reason the sugar is added is to aid the denitrification of the HAL and to reduce the formation of volatile RuO4 during vitrification.
As the explosion was caused by the reaction of a rather special mixture of sodium acetate and sodium nitrate, it can be concluded that a Kyshtym like accident is not going to occur in a high level waste (HLW) tank farm associated with a PUREX plant. A while ago in Ireland a television drama was made about a serious accident at Sellafield which released vast amounts of radioactivity from the liquid HLW storage facility. In the drama, named Fallout by Raidió Teilifís Éireann, this release of radioactivity resulted in the deposition of sufficient radioactivity in Ireland to require evacuation, unrest in Dublin and widespread adverse health effects. The drama was criticized by Dick Roche (Environment Minister) of Ireland. He expressed the view that implausibility of the scenario in the drama played into the hands of the pro-nuclear lobby (Bradley, Citation2006) and that the drama depicted Irish people as being barbaric (Anonymous, Citation2006). The Radiological Protection Institute of Ireland’s chief executive (Ann McGarry) stated “the scenario envisaged in the programme is not realistic and grossly exaggerates the amount of radioactivity that could reach Ireland” (RPPI, Citation2006). I think that the lack of chemical fuel in the high active liquid wastes at Sellafield is the main reason a Kyshtym like accident is impossible. Now back to the Kyshtym accident.
The main radionuclides released by the Kyshtym accident are listed in Table .
Table 4. Main radionucldies released in the Kyshtym accident.
Recent examinations of soil samples taken from 4 km from the epicentre of the accident confirm that the accident released far more 90Sr than 137Cs (Molchanova, Mikhailovskaya, Antonov, Pozolotina, & Antonova, Citation2014). It is also noteworthy that the 90Sr is more mobile in the soil than the 137Cs in some places while in others the two radionuclides seem to have similar mobility in soil. Two explanations exist for this difference, either the nature of the soils differs greatly between the locations or the chemical form of the fallout differs between the two locations. As the conditions inside the tank represent an extreme version of a denitrification process and as the chemical reaction responsible for the explosion is likely to have generated alkali it is quite possible that the release was in the form of an aerosol of solid particles. This may account for the difference in the mobility of the radioactivity in the soil.
It appears that much of the 137Cs had been removed from the waste before it was placed in the ill-fated tank, so far I have been unable to find a flow sheet for the processing of the liquor from fuel dissolution through to depositing into the tank. In the early days the Soviets were reported to recovery radioactivity from the waste left behind from plutonium/uranium recovery. According to some Russian radiochemists (Milyutin, Gelis, Dzekun, & Malykh, Citation1995) several methods have been used to recover cesium from reprocessing waste. These are coprecipitation with transition metal ferrocyanides, coprecipitation with salts of heteropoly acids, solvent extraction with cobalt dicarbolide and adsorption onto transition metal ferrocyanides (Milyutin et al., Citation1995). The solvent extraction process using a cobalt dicarbolide is known as the UNEX (Luther et al., Citation2006) is intended for the removal of cesium, strontium and the actinides from high level liquid wastes. The cobalt dicarbolide anion is a very large and poorly coordinating anion which has also found use in non-radioactive and non-nuclear chemistry (Schweiger, Seidel, Arif, & Stang, Citation2002) (Figure ).
Figure 7. A cobalt dicarbolide anion. The cobalt atom is blue, the boron atoms are gold colour, the carbon atoms are dark grey and the hydrogen atoms are light grey.
Of the ferrocyanides tested (potassium nickel, potassium copper and potassium zinc) on silica gel the potassium copper ferrocyanide performed best. The industrial process for the removal of cesium is based on three reactions:
Strong “nitric acid” is able to oxidize the solid, this oxidation might be due to the action of nitrous acid and nitrogen oxides. Using hydrazine it is possible to reverse the oxidation. It is interesting that rather than the Russian workers reporting a process in the literature during development or even when it is at the pilot plant scale they were in 1995 reporting a process which had treated 8 million cubic meters or waste and had recovered 24 MCi between 1988 and 1993. In my efforts to understand the process I considered the solid state structures of copper ferrocyanides. A western group (France, Spain and Mexico) have reported the crystal structure of K2CuFe(CN)6 which is a layered solid formed from cupric sulphate (CuSO4) and potassium ferrocyanide (Loos-Neskovic et al., Citation2004). This clearly has anionic layers of a mixed copper/iron cyanide and layers of potassium cations. An examination of the unit cell confirms that the empirical formula is K2CuFe(CN)6. This suggests that the copper and the iron are both in the plus two oxidation state. This is a different formula to that discussed in the Russian paper. This group stated that after being in contact with dilute nitric acid that Cu2Fe(CN)6 and other materials were formed (Figure ).
Figure 8. The structure of dipotassium copper ferrocyanide. Potassium atoms are pale blue, iron atoms are green, copper atoms are orange, carbon atoms are dark grey and nitrogen atoms are dark blue.
In another paper (Ayrault et al., Citation1998) it has been reported that Cu2Fe(CN)6 does absorb cesium from 0.1 M nitric acid while Cu3Fe(CN)6 fails to absorb cesium from this medium. However this paper is spoiled by the fact that the equilibrium time for the latter solid was 5 h while that for the first was 6 months. I would be more happy with the paper if the authors had measured the adsorption of the cesium for both solids at the same time after starting the experiments. In this paper it was found that copper can be released into dilute nitric acid as the cesium is absorbed. The structures of Cu2Fe(CN)6 and Cu3Fe(CN)6 are similar to that of KNiFe(CN)6 which is shown below (Malecki & Ratuszna, Citation1999). It is important to note that the potassium sites are not fully occupied, thus giving the impression that the unit cell contains more potassium than it truly does (Figure ).
It is noteworthy that resins formed from KNiFe(CN)6 and ammonium molydophosphate supported on polyacrylonitrile can be used for the separation of cesium from waters including sea water (Kamenik, Dulaiova, Sebesta, & St’antna, Citation2013). This allows the cesium in environmental water samples to be preconcentrated thus allowing the measurement of very small amounts of 134Cs and 137Cs. Such resins are being marketed currently by Eichrom. In common with the cyanometallates ammonium molydophosphate binds the cesium by simple electrostatic attraction. The cesium is absorbed by simple ion exchange. The anion in the solid is shown here (Boeyens, McDougal and Van R. Smit, Citation1976). The anion has a phosphate at the centre of a polymobdate cage (Figure ).
While the Kyshtym accident did not involve a nuclear reactor it is an important accident as it involved waste formed from nuclear fuel cycle operations. The Kyshtym accident is a difficult accident to study in several ways, firstly it was hidden by the Soviet Union for many years and secondly unlike Chernobyl and Fukushima the radioactive release occurred close to where several other major releases had occurred. As a result the background of some radioisotopes in soil and other media nearby is elevated. For example in 1967 during the summer the wind dispersed 20 TBq of 90Sr and 137Cs from lake Karachay. While in the 1950s a vast amount of radioactivity (100 PBq) was discharged into the Techa river in a combination of accidents and planned releases.
A series of accidents of the same general class as the Kyshtym accident have occurred in nuclear fuel cycle sites, rather being induced by a surge by nuclear reactivity in or a loss of cooling of a nuclear reactor, these are accidents in which an exothermic chemical reaction occurs which scatters radioactivity in or around the plant. One recurring issue has been “red oil”, this is a poorly defined substance which forms in PUREX plants. Red oil is formed when a mixture of nitric acid, uranium nitrate, the organic diluent and tributyl phosphate is heated (Gordon, O’Dell, & Watkin, Citation1994). Red oil is thought to contain a range of nitro compounds which are able to decompose exothermically on heating to generate gas. A series of red oil accidents in uranium active areas have occurred in the USA, the most serious red oil accident so far occurred at Tomsk-7 on 6 April 1993. The accident involved a tank containing 4,000 l of nitric acid containing thorium (142 g), uranium (8,773 kg), neptunium (248 g), plutonium (310 g), some fission products and some organic matter. To this tank was added more nitric acid bringing the total to 21,000 l (Porfiriev, Citation1996). The tank has been reported by Porfiriev to contain 537 Ci of alpha activity and 22 Ci of beta activity, but I suspect that a typographical error exists in that paper which interchanged alpha and beta in that sentence. The paper later states that the beta activity in the fallout was no more than 50 Ci. The IAEA report on the event indicates that a total of 1.5 TBq (40.5 Ci) of beta/gamma activity was released, according to the this report the total beta activity of tank contents was 20 TBq (541 Ci) (Andreev et al., Citation1998). The IAEA report (Andreev et al.) stated that before the accident 8,757 ± 286 kg of uranium and 449 ± 120 g of plutonium were in the tank and that afterwards 8,707 ± 350 kg of uranium and 577 ± 117 g of plutonium were recovered from the wreckage of the damaged equipment and cell. Thus it can be concluded that the majority of the radioactive mixture remained inside the plant. In this accident a mixture of 95Nb, 106Ru, 95Zr and 103Ru with small amounts of 137Cs and 90Sr were released. A small amount of radioactivity was detected in Alaska (Larsen, Sanderson, Lee, Decker, & Beck, Citation1994), but the majority of the radioactivity was deposited in the plant or close to the plant. It is noteworthy that the accident did not expose the general public to large doses.
In July 1959 at an experimental sodium cooled reactor in the USA suffered an overheating accident due to a leakage of tetralin (1,2,3,4-tetrahydronapthalene) into the sodium coolant. The tetralin formed a solid which caused some blockages inside the core. A report by Jerry D. Christian (expert witness in a 2005 court case) explains some of what happened. The uranium metal fuel overheated while in contact with the stainless steel cladding. Christian pointed out that diffusion of uranium into the stainless steel cladding and the 5% volume change caused by the alpha–beta phase transition (663°C) of uranium caused the failure of the cladding on 13 out of 43 fuel elements.
Alpha uranium is an orthorhombic solid (Eeles & Sutton, Citation1963), while beta uranium is a tetragonal solid. As the two solids have different densities it is clear that when the phase transition occurs that the volume of the uranium will change. Below are four different views of a unit cell of alpha uranium, now while some of my readers will know instantly how to obtain the density from a unit cell. I hold the view that very few things are “common sense” as to be common it would have to be universal. For example a 14-year-old child who has grown up in Sweden will have (or should have) a good understanding of how to cross the road safely but is unlikely to know how to deal correctly with a poisonous spider, scorpion or snake. On the other hand a child who lives in some remote part of Africa or Brazil with no motor traffic but plenty of poisonous creepy crawlies hopefully will have a good working knowledge of how to recognize and deal with these creepy crawlies but might not know how to cross a road (Figure ).
To do this we first need to calculate the volume of the unit cell, in the case of alpha uranium it is 83.4473 Å3 or 8.34473 × 10−29 m3. The next step is to calculate the mass of the atoms in the cell. We need to apply some simple rules when we see a unit cell with atoms in it. The unit cell is a polyhedron with six faces (Table ).
Table 5. Summary of rules for counting atoms in unit cells.
We have two uranium atoms in the cell which are completely within the cell, so we have at least two uranium atoms in the cell. The cell also has four uranium atoms which are touching only one face. Thus we have four halves of a uranium atom from these atoms which appear in the picture of the cell. As a result we have four uranium atoms in total. If the uranium is pure 238U then we have 952 atomic mass units in the cell. As there are 6.02214 × 1023 atoms in a moles of atoms we can work out that we will have 1.580833391 × 10−21 g of uranium in the unit cell. This is 1.580833391 × 10−24 kilos. From the mass and the volume of the unit cell we can work out the density will be 18.944 × 103 kilos m−3.
Christian argued that the diffusion of uranium into the stainless steel was able to form an iron-uranium eutectic with a melting point of 725°C. An examination of the phase diagram of the iron-uranium system reveals that two eutectic points exist (Chapman & Holcombe, Citation1984; Leibowitz & Blomquist, Citation1991). Uranium and iron form several crystalline alloys which appear in the crystallographic literature. These are Fe2U and FeU6 (Baenziger, Rundle, Snow, & Wilson, Citation1950), the unit cell of FeU6 is rather complex it is an example of what I would describe as “atomic fog” where the number of atoms makes it difficult to visualize the overall structure. The cubic Fe2U is somewhat easier to understand it is a layered solid (Figure ).
I reason that the formation of an iron/uranium alloy will assist in the melting of the cladding and some of the fuel. It is interesting to note that almost all metals will form alloys with mercury. The only common metal which does not do so is iron. I have always held the view that the best container to put a bottle of mercury inside as an overpack is a carbon steel jar. My reasoning is that if the inner container breaks that the carbon steel will be better able to resist the action of the mercury than stainless steel or aluminium. In some ways plastic is a reasonable overpack for mercury containers, but neither plastic or carbon steel is perfect. Given time plastics can become brittle while carbon steel will rust when exposed to water and air.
The chemistry of some of the other elements in a typical stainless steel with uranium is more complex. Wilson et al. report that nickel forms U6Ni, UNi, UNi2 and UNi5. They were unable to work out the structure of UNi. The crystal structure of U6Ni is the same as that of U6Fe while UNi2 has a different structure to UFe2. The UNi2 structure is the same as that of MgZn2. A unit cell of this solid is shown in Figure .
Figure 13. A unit cell of UNi2, the nickel atoms are in green while the uranium atoms are in yellow.
The UNi5 has the same structure as AuBe5 and PdBe5 (Figure ).
Figure 14. A unit cell of UNi5, the nickel atoms are in green while the uranium atoms are in yellow.
Noël and some other French workers have suggested that UNi does not exist but some solids with empirical formulas close to UNi exist. These include U5Ni7 and U11Ni16 (Perricone, Potel, & Noël, Citation2002). The crystal structure of U11Ni16 is another example of “atomic fog”. Here it is in all its glory (Figure ).
Figure 15. A unit cell of U11N16, the nickel atoms are in green while the uranium atoms are in yellow.
Nickel forms another crystalline solid UNi4, which has the same structure as CaCu5. In this solid uranium atoms partly replace nickel atoms at two crystallographic sites (Perricone & Noël, Citation2002). In the following diagram the sites which are uranium atoms (and nothing but uranium) are at the corners of the unit cell (thus we have 1 uranium). The green atoms on the top and bottom face of the cell are sites which are occupied 97.5% of the time with nickel atoms and 2.5% of the time with uraniums. Thus we have 0.05 uraniums and 1.95 nickels from these “green” sites. The blue sites are occupied with nickel atoms 95% of the time and uranium 5% of the time. Thus from these nickel sites we have 2.85 nickel atoms and 0.15 uraniums. Overall we have 1.2 uranium atoms and 4.8 nickel atoms. Overall we have a 1:4 ratio of uranium to nickel (Figure ).
What we should understand is that when stainless steel clad uranium metal fuel is overheated that the fuel will be able to interact with the cladding. It might have been better if the reactor had contained a fuel with cladding which is less able to interact with it. One option would have been to use a cermet fuel with a metal which is unable to reduce uranium dioxide to uranium metal.
Rather than releasing iodine in the form of elemental iodine, Christian argues that it would have been present in the fuel in the form of uranium(III) iodide, cesium iodide (CsI) or another metal iodide. He pointed out that the yield of cesium atoms as a result of fission would be greater than that of iodine. He argues as a result that the iodine will be immobilized in the fuel as cesium iodide once the fuel has reached a thermodynamic equilibrium. I have considered this idea. If we assume that the only nuclear processes in the core are nuclear fission (of 235U) and the radioactive decay of short lived fission products then from the fission yield of 235U with 1 MeV neutrons of the long lived nuclides we can estimate the I:Te:Cs ratio to be 1.0:2.4:17.6. Thus if all the tellurium is in the form of cesium telluride and all the iodine is as cesium iodide then the fuel will contain Cs2Te, CsI and Cs metal in a mole ratio of 2.4:1.0:6.2. Thus 28% of the cesium is present as Cs2Te, 6% as CsI and 67% as cesium metal (Table ).
Table 6. Half-lives and fission yields of cesium, tellurium and iodine.
Christian argues that if the fuel was oxidized to that barium, strontium, lanthanum, cerium and neodymium in that order would sequester the iodine as metal iodides. If these fission products were to be consumed by oxidation then the iodine would be absorbed as uranium(III) iodide (UI3). The first metal which the fission product iodine encounters will be uranium, thus it will form a uranium iodide which can then later react with more electropositive fission products to form the thermodynamically favoured iodide according to a reaction like this.
Christian pointed out that if elemental iodine (I2) had formed then it would have been very likely to react with the cladding of the fuel to form metal iodides such as CrI2, MnI2, FeI2, NiI2 or MoI2. In a French study it was found that even without oxygen being present elemental iodine is corrosive to stainless steels (SS316 and others) (Aubert, Calais, & Beuze, Citation1975). In this study isothermal and temperature gradient experiments were conducted. In the isothermal study a series of zones were found on the surface of a sample of a low chromium 2574 stainless steel which had been heated at 700°C with iodine (Table , Figure ).
Table 7. Details of the layers on a stainless steel exposed to iodine.
Likewise when SS316 is heated with iodine a manganese and chromium depleted zone is formed on the surface. Christian also argues that any iodine which escaped from the fuel would have been converted into the very involatile sodium iodide. He cited a report by Castleman et al. which indicates that when a small amount of cesium iodide is combined with excess sodium that it is converted into cesium metal and sodium iodide. The sodium iodide is soluble in molten sodium, as the temperature of the sodium increases the solubility of sodium fluoride, chloride, bromide and iodide increases (Bredig, Johnson, & Smith, Citation1955). A further study using 22Na, 132Te, 131I and 134Cs has confirmed that iodine and tellurium are retained in hot sodium, when hot sodium containing was kept in a stream of helium gas the sodium (22Na) was lost at a higher rate than 132Te or 131I from the sample (Clough & Fraser, Citation1973). The paper by Clough and Fraser also concluded that if iodine was to be released then it would be in the form of an aerosol with sodium metal. Tellurium (as 132Te) was more difficult to study, it was found to absorb onto the stainless steel surfaces of the equipment. When wall effects were removed the results suggested that the tellurium was better retained by the sodium than iodine. In this paper the relative volatility (αE) of the elements (E) was estimated by gamma counting. The radioactivity (A) of the elements in the liquid and gas phase are written as AE liq and AE gas respectively.
Their values for iodine, tellurium and cesium obtained by measurement of the rate of loss of radioactivity from the furnace were 0.5, 0.35 and between 4 and 75. The cesium values are over a large range, they stated that the cesium attaches to the stainless steel walls of the equipment which can reduce the release of the cesium. The aerosol particles formed when the vapours from the furnace were allowed to cool in a steel box (0.1 m3 volume), when the 22Na to 131I ratio of the aerosol particles was measured a value of between 0.55 and 0.7 was obtained for αI. However using the measurements of the radioactivity of the aerosol the αTz value was found to be between 0.03 and 0.05. They reasoned that the tellurium is not uniformly incorporated into the aerosol but it is condensed onto cool surfaces first. They argue that this could either provide a useful trapping process for tellurium or a means of producing finer aerosols. Christian further argues in favour of the sodium cooled reactor, by pointing out the work of Hart et al. which indicates that the molten sodium retained strontium, iodine and cesium.
The case for the retention of iodine released as elemental iodine into the sodium coolant is strengthened further by the work of Kunkel et al. who released iodine vapour at the bottom of a container of molten sodium. Very little of the iodine was able to escape from the surface of the sodium thus demonstrating that the iodine was retained by the sodium metal. It is most likely that the iodine reacted according to the following equation with the sodium.
In January 1961 a reactivity accident occurred in the SL-1 reactor in Idaho killed three men, this accident was a steam explosion caused by a surge in reactivity. The surge in reactivity was caused by some unknown event during servicing of the reactor. This accident can be regarded as a miniature version of Chernobyl, but in this case the reactor building was able to contain the majority of the radioactive inventory in the reactor. As this accident was so similar to the more serious Chernobyl accident I will not discuss it in detail.
At Chapelcross in 1967 an overheating accident occurred in a Scottish Magnox reactor. It was predicted (Macdonald, Ballard, Thompson, Goldfinch, & Orchard, Citation1977) in the 1970s that in the event of a single channel meltdown in a Magnox reactor that all of the noble gases would be released from the damaged fuel, 10% of the non-halogen volatiles, 1% of the halogens and the mid-volatiles and finally 0.1% of the non-volatiles would be released into the coolant. However if air entered the damaged reactor and caused a fire in a single channel then it was expected then that 50% of the halogens, cesium, ruthenium and volatiles would be released, 5% of the other mid-volatiles and 0.5% of the non-volatiles would be released (Table ).
Table 8. Groups of fission products in the prediction of the behaviour of magnox fuel during an accident.
In 1977 in Czechoslovakia an accident occurred in a plant named A1 two separate accidents occurred during the working life of the plant. The A1 plant was a carbon dioxide cooled and heavy water moderated reactor (150 MWe). The fuel was uranium metal clad with a magnesium/beryllium alloy. The purpose of the beryllium was to reduce the tendency of the magnesium cladding to oxidize. When a clean magnesium surface is first exposed (12 l H2O) in ultrahigh vacuum conditions to water at 77 K according to PES it becomes covered with an oxygen species which is suspected to be Mg(OH)2 (binding energy 533.5 eV) and a small amount of MgO (531.0 eV). When more water (500 l H2O) is deposited on the surface then the main oxygen species on the surface is water (535.1 eV). On warming the surface up to 100 K this water is converted into a mixture of mainly Mg(OH)2 and a small amount of MgO. As the sample is heated further to 210, 250 and 390 K more and more of the Mg(OH)2 is converted into MgO (Fuggle, Watson, Fabian, & Affrossman, Citation1975). It has been argued that when a clean magnesium surface is exposed to oxygen that first chemisorption of oxygen occurs, which is followed by the formation of an oxide layer which then becomes thicker (Shih, Liu, & Wei, Citation2007).
While it might be possible to predict the thermodynamic outcome of a reaction, it is impossible with thermodynamics alone to predict the rate at which a reaction occurs. For the oxidation of magnesium or another metal the PB ratio is important. The Pilling-Bedworth ratio is the ratio of the volume of the metal and the volume of the oxide. In the case of magnesium as the relative density of the metal is 1.74 (Busk, Citation1952), the density of the oxide (MgO, periclase) is 3.59 (Jay & Andrews, Citation1946) and that of the hydroxide (Mg(OH)2 brucite) is 2.37 (Isetti, Citation1965). It can be calculated that one mole of magnesium has a volume of 13.97 ml, one mole of the oxide has a volume of 11.23 ml and one mole of the hydroxide has a volume of 24.60 ml. Thus if the magnesium was to be oxidized to magnesium oxide the volume of the oxide will be smaller than that of the metal. As a result of having a PB ratio of 0.80 the oxide layer will have cracks in it. As a result oxygen gas will be able to gain access to the surface and the metal will also be able to leave via the gaps in the oxide layer.
In the case of the aluminium containing magnesium alloy Shih et al. observed the existence of a aluminium/magnesium oxide close to the corroding surface, if this is formed by the migration of aluminium to the surface and then the formation of spinel (MgAl2O4) then we need to reconsider the PB ratio for this alloy. The density of spinel has been reported to be 3.57 (Fischer, Citation1967). The volume of one mole of the spinel will be 39.86. It can be reasoned that if a small amount of spinel was to form in the surface layer and aluminium is able to migrate towards the surface then it could help to seal the cracks. The addition of a small amount of beryllium to magnesium greatly reduces the ease with which it oxidizes and ignites. For example two AZ91D alloys containing 5 and 10 ppm of beryllium were compared in one paper (Czerwinski, Citation2004). Alloy B had a far greater resistance to oxidation than alloy A, it was argued that the difference was due to the change in beryllium content (Table ).
Table 9. Two magnesium alloys, the amounts of elements are in % (w/w).
In the first accident (1976) a large amount of carbon dioxide leaked out and killed several workers. The second accident (22 February 1977) was an overheating accident. Many of the details were supplied to me by the Nuclear Regulatory Authority of the Slovak Republic. Due to an operator error the silica gel used to protect the fuel during transport and storage was not removed correctly from the fuel. The bag of silica gel burst open and the silica gel spilled onto the fuel, the operators did not clean it completely from the fuel element. The fuel element with the silica gel was then loaded into the reactor. The silica gel then prevented the carbon dioxide gas from circulating properly through the reactor. This then resulted in overheating. If this had been the end of the matter then it is likely that the reactor could have been saved. However the overheating caused the heavy water moderator (21 tonnes) to leak into the gas circuit, this caused rapid corrosion of the cladding on all of the fuel in the reactor. Steam can react with magnesium in the following exothermic reactions:
As the system has a high concentration of carbon dioxide we need to consider the following reaction. Thankfully during a thermodynamic assessment of the following reaction has been done during an assessment of a fluidized bed incinerator (Denloye, Gasner, & Adamchak, Citation1984). This reaction at 850°C (1,123 K) was reported to have a DG of 90.091 kJ mol−1. This suggests that during an overheating event the magnesium oxide formed will not react with carbon dioxide to form magnesium carbonate. Magnesium carbonate is less thermally stable than calcium carbonate.
I have been told that circa 150 kilos of loosened cladding and 100 kilos of used fuel were distributed into the primary circuit. This caused a release of 3.7 × 1015 Bq (100 kCi) from the used fuel and 5.5 × 1014 Bq (14.9 kCi) from the cladding into the primary cooling loop. Over the next days, circa 360 Ci of noble gases and aerosols escaped from the plant and about 100 Ci of liquid discharges occurred.
It is interesting that the security and fire protection company Chubb have published a film which shows the effect of spraying water onto a pile of 100 g burning magnesium turnings, putting it mildly the results are rather dramatic (Chubb, 2018). The A-1 accident resulted in dire contamination of the primary coolant circuit and due to a steam generator fault the secondary circuit was contaminated. The A-1 accident might offer an insight into what would have happened in a MAGNOX plant if a large water leak had occurred from a steam generator into the carbon dioxide cooling circuit.
On 28 March 1979, an overheating accident occurred in the Three Mile Island plant, this was largely due to poor design of the control room and a horrible error in the wiring of the plant. These both contributed to the operators of the plant misunderstanding the situation which resulted in incorrect actions being taken. As a result of an event in a non-nuclear part of the plant (water feed to the steam generators) provoked an emergency shutdown (SCRAM) of the reactor in unit 2. As planned the control rods inserted and the nuclear reaction was stopped. What should have happened was that a pressure release valve on top of the pressurizer should have opened for a moment after the SCRAM to release some steam. Instead the valve opened and then jammed open. The problem was that the indicator light for this valve in the control room showed the intended state of the valve rather than its real condition. A second indictor lamp which could have alerted the operators to the fact that the valve was stuck open was hidden from their view by a poor design of the control room.
Some other issues in the design of the plant contributed to the accident, for example the plant lacked a sensor to measure the water level in the core. Such a sensor might have provided a warning to the operators which would have changed the course of the event. But with hindsight everything is always clear.
In 1986 the Chernobyl accident occurred, as a result of a poorly planned safety experiment a large reactivity surge occurred in a graphite moderated light water reactor. This reactor design had a dangerous positive void coefficient when operating at low power. One of the design flaws was that the moderator was not in good thermal contact with the fuel. If the fuel and the moderator had been in intimate contact such as that found in a pebble bed reactor during the power surge as the fuel heated up the temperature of the moderator would have increased. This would have increased the temperature of the neutrons. By increasing the speed of the thermal neutrons the fission cross section of the fuel would have become smaller thus limiting the size of the power excursion.
If we assume that a nuclear reactor is infinite in size and moderated then the neutron multiplication factor k∞ which is defined as:
Can be calculated with a four factor equation:
Where η is the number of neutrons generated per neutron capture in the fuel, for example if a single thermal neutron when captured by the fuel always causes the release of three neutrons in a fission event then the value of η would be three. In real systems we need to know the ratio of nγ reactions and neutron induced fission events. If we know the capture (σg) and the fission (σf) cross sections for the nuclide then we can calculate η from these cross sections and the number of neutrons released per fission event (n).
When thermal neutrons cause fission in the fuel, sometimes the fast neutrons induce fission reactions in the fuel without first being slowed down (moderated). These extra fissions are taken care of by a constant z.
For various nuclides large resonance peaks exist in the graph of σg against neutron energy in the epithermal energy range (1–105 eV), the probability that a neutron is able to avoid simple capture in a ng reaction as it zooms around the core bouncing between moderator atoms is given the symbol p.
The symbol f is for the fraction of thermal neutrons which avoid being captured in nuclei other than the fissile fuel.
The boiling of the water in the fuel channels increased the reactivity of the reactor by increasing the fraction of the fast fission neutrons which after escaping from the fuel which return to the fuel after scattering off the graphite. It is during this scattering process that their excess energy is lost in the moderation process.
At Chernobyl as the core started to heat up during the power excursion as the water boiled this removed a neutron absorber from the core, thus increasing the value of f. Also the burning up (destruction of xenon) in the core will have suddenly caused f to increase. These increases in f would have caused k∞ to increase. As a result of the power surge a steam explosion occurred, this ejected about 3.5% of the fuel from the plant. It also ejected other materials such as graphite blocks from the core. This ejection of materials from the core and the rearrangement of the core caused by the explosion altered the geometry of the core. All the fission reactors I have heard of are non-infinite in nature. From these reactors the loss of neutrons is important. We need to consider the “effective multiplication factor” (keff) where Pt and Pf are the probability that thermal and fast neutrons do not leak out of the core.
I do not wish to discuss in great detail the equations for calculating the values for Pt and Pf, but by increasing the surface area to volume ratio of a reactor the values of Pt and Pf will decrease. Conversely by placing a neutron reflector around fissile material the values of Pt and/or Pf will increase. In several criticality accidents neutron reflectors have caused otherwise subcritical masses of fissile materials to become critical. For example the demon core which killed two people (Harry Daghlian and Louis Slotin) in two separate and horrible accidents became critical due to neutron reflectors being placed around it. The demon core was a sphere of the gallium-plutonium alloy which is used in nuclear (fission) bombs. It would have been a solid sphere with a hole to allow a neutron source to be placed at its centre.
The report (Harding et al., Citation1948) on the doses of radiation delivered to the people in the room used neutron activation of human tissue as a method of determining the neutron dose. Both 32P and 24Na in urine and blood plasma were considered as methods for determining the neutron dose. The 32P can be formed by at least three reactions:
Due to the complexities of the biochemistry of phosphorus the report concluded that 24Na was a better radionuclide for estimating neutron exposure after an accident. One method of improving the estimate of the neutron dose is for a person to wear a crisis dosimeter. One option is to wear or carry an object which will undergo neutron activation for example the late John Pecket told me that at Harwell he wore an accident dosimeter which included a vial of sulphur which would have helped reconstruct fast neutron exposure in the event of a criticality accident. Another thing which could be used would be hair (Lebaron-Facobs et al., Citation2007), fingernail and toenail clippings. These parts of the body contain a sulphur rich protein which could be examined for radioactivity (32P). Using longer lived radionuclides it is possible to reconstruct neutron doses from the atom bombs dropped on Hiroshima (Marchetti & Straume, Citation1996). Due to the great differences in design the neutron to gamma ratio at Hiroshima was higher than at Nagasaki. The bomb dropped on Hiroshima had a large amount of steel surrounding the highly enriched uranium, this would have adsorbed gamma rays but allowed the fast neutrons to escape with ease. The Nagasaki bomb had a plutonium core which was surrounded with plenty of organic matter (explosives) which would have adsorbed neutrons but would have been transparent to gamma rays. A review (Ainsbury et al., Citation2011) of the methods which can be used for retrospective dose estimation was published recently so I will not dwell further on this topic.
A survey of early film badge dosimeters was made by the journal Nucleonics back in 1955 (Anonymous, Citation1955), if you want to look at an on line museum of dosimeters then Oak Ridge Associated Universities has a very good one (Oak Ridge, Citation1999). But now back to the Chernobyl accident.
Due to a defect in the design of the control rods of the RBMK reactor at Chernobyl an attempt to SCRAM the reactor during the accident has been suggested by some to have been the event which started the power surge. It is not clear if the control room workers attempted to SCRAM the reactor before the power surge or if the workers pressed the SCRAM button after the power surge occurred. At Chernobyl the control rods had graphite tips, thus as they were inserted at first they would have replaced water with graphite rather than replacing water with a neutron absorbing material. According to Landeyro and Buccafurni the RBMK reactor had four types of control rod, these were manual, emergency, automatic and local automatic (Landeyro & Buccafurni, Citation1991). The manual and emergency control rods had graphite containing displacers attached. These would have displaced water from the channels where the control rods went. The graphite containing displacers were separated from the adsorbing section of the control rod by 1 m. Landeyro and Buccafurni used as a starting model a reactor which is not homogenous, they argued that the “positive scram” effect of the insertion of the control rods was small compared with the effect of the formation of voids in the reactor.
I reason that if an attempt was made at a SCRAM that for a moment that graphite displacer section would have increased the reactivity in the lower part of the reactor. In the following diagram the graphite displacer sections are shown in grey while the boron carbide containing neutron absorber sections are shown in black. At the far left the arrangement of the control rod when it was fully withdrawn is shown and on the right it is fully inside the reactor. When I saw withdrawn and inside I am making a reference to the neutron absorbing section of the rod. As the rod moves from the fully withdrawn position to the fully inserted position the graphite section will for a time increase the moderation in the lowest part of the core (Figure ).
Figure 18. A diagram of the Chernobyl core and the control rods. H are the areas of the core with the highest K∞ due to the lowest burnup, M are areas with medium K∞ and L are the areas with the lowest K∞. The boron containing adsorbing sections are shown in black and the graphite displacers in grey.
It is important to keep in mind that the neutron flux in a reactor is not constant throughout the whole of the reactor, a simple case is a reactor which is an infinite slab of thickness H. For this reactor the flux distribution in reactor will be given by the following equation where distance z from the centre of the slab. Here Hex is a thickness which is slightly larger than the thickness of the slab, this is due to the factor that some neutrons will leak out of the reactor.
If we assume that the reactor has a cylindrical shape (height H and radius R) then the axial and the radial neutron flux distributions are given by the following equation if we assume that the neutron flux is zero at the surface of the reactor. The J is for the zero order Bessel function of the first kind.
For a reactor which is box shaped (parallelepiped) which has the dimensions 2X, 2Y and 2Z the flux distribution at a point x,y,z from the centre of the reactor will be given by:
According to Buki and Bede (Citation1965) the flux distribution in a spherical reactor of radius R at a point distance r from the centre will be given by:
We can continue with our geometric fun, by considering a sphere (R) with a central cavity (ro). The equation for this system for the flux density is not quite so easy. For a spherical reactor with no cavity the geometric buckling B is given as.
The total volume of the sphere (active part and cavity) is given by the equation:
The relationship between Bg0 for the simple sphere and Bg for the sphere with the hollow (which has volume V) can be worked out by rearrangement of the following equation:
Which we rearrange into:
As V for this reactor is given by:
We can write:
Then
As expected the sphere reactor has a greater and greater geometric buckling value as the size of the cavity inside increases. Within the central cavity the neutron flux is constant at the same value (f0) as at the edge of the cavity (r0).
The ratio of C1 to C2 can be calculated from the ratio of ro to R using the following equation:
Buki and Bede also consider cylinder shaped reactors with axial holes and longitudinal holes. For the reactor which is a cylinder of active matter with a coaxially arranged cylindrical cavity (longitudinal cavity) and the reactor with a cylindrical cavity at 90° to the axis of the main cylinder which passes through the centre of the cylinder the equations are rather complex. For the coaxial reactor the equation for the flux is.
The ratio of C1 to C2 changes as a function of the value of r0/R and the constant a changes as a function of the overall radius (R) of the reactor. I will not discuss them further but I want my reader to take away the message that in all practical homogenous reactors it is not possible to have a neutron flux density which is uniform.
The fuel at the top and the bottom of the Chernobyl reactor had a lower burnup than the fuel in the middle. As a result the fuel was more reactive. From the point of view of a reactivity accident the worst part of the core in which to add voids or increase moderation was the bottom or the very top of the reactor. While defect in the control rods did increase the reactivity in this part of the reactor but Landeyro and Buccafurni reason that the void formation made a greater contribution the power surge than the control rod defect. They also considered a second reactivity surge which was caused by the fragmentation of the fuel.
In the 2015 review I commented on the origins of the term SCRAM. I would like to thank Jerry Christian who brought to my attention a paper on the history of the term SCRAM. This paper (Murray, Citation1988) identifies Norman Hilberry as the axe wielding man who was ready to cut a rope which would have caused a rapid insertion of a control rod. In the designs of the experiment the term SCRAM (Safety Control Rod Ax Man) was present. I would conclude that the Chernobyl accident was caused by a combination of the poor design of the reactor, errors made by the operators and also a failure of the regulatory body to ensure that the reactor was constructed and operated in a safe manner.
In 2011 the Fukushima accident occurred, this was an event caused not by an event inside the nuclear site but by an event (earthquake) outside the site. What happened was that an almighty earthquake shook Japan making buildings shake, this earthquake also caused a very large tsunami. The earthquake and the tsunami caused almost 30,000 people to die or disappear (Takewaki, Citation2011). The earthquake also triggered units 1, 2 and 3 at the Fukushima Daiichi plant to SCRAM. The other units (4–6) there were not in operation when the earthquake occurred. The great problem with the Fukushima accident is that the accident was initiated by an event outside the reactor site. Almost all serious radiological accidents before it have had an initiating event which occurs within the nuclear site.
2.3. Forrest fires and other resuspension
One issue is the resuspension of long lived radioactive materials, here we are considering the return of radioactivity into the air. The a wide range of potential events exist which can resuspend radioactivity, one unusual event which was planned but never carried out was the “Divine Strike” non-nuclear test planned at the former Nevada Test site. The “Divine Strike” was to have been a large non-nuclear explosion, to test the effects of a “bunker buster” which would have occurred on land which had been previously contaminated by nuclear bomb tests.
Less dramatic events include forest fires in the area which was strongly contaminated by the Chernobyl accident and the collapse of buildings. While the collapse of any radioactively contaminated building could in principle cause a release of radioactivity, a collapse of the Chernobyl object shelter (also known as the sarcophagus) was of great concern. If the object shelter had collapsed it could have released a large amount of radioactive dust, this possible event was one of the motivations for the construction of the arch which further protects the radioactive wreckage at the site. An even less dramatic event is the driving of a vehicle along a road or agricultural management work. Such activities can increase the amount of dust which is present in the air. It has been shown that grass cutting in the Chernobyl exclusion zone (Zapolie 14 km from the reactor) causes a small increase in the 137Cs level in the air compared to that observed while equipment was being assembled (Garger, Paretzke, & Tschiersch, Citation1998). However harrowing and driving trucks causes a far greater increase in the 137Cs level in the air. It is important that people be able to work in the exclusion zone, trucks need to be driven to deliver supplies, fire prevention work needs to be done and also decontamination work will sometimes need to be done.
Measurements made in the 30 km exclusion zone around Chernobyl between September 1986 and June 1993. The measurements were made at Pripyat (close to the reactor site) and at Zapolie. It was found that even without obvious human dust raising activities that the air still contained radioactivity which had been released by the reactor accident. During 1992 and 1993 soil samples were taken at Zapolie and it was found that the specific activity (Bq g−1) of 137Cs in soil increased greatly as the particle size was reduced while for 144Ce little difference in the specific activity of different size fractions was observed (Garger, Kashpur, Paretzke, & Tschiersch, Citation1998). As a result it can be concluded that the cesium is able to spread in the finer dust from soil. It was also found by Tschiersch et al. that in 1986 at Zapolie that the majority of the 137Cs and 144Ce were in the form of coarse particles (>10 μm) while in later years the contribution due to finer particles becomes more important. The fraction of the radioactive ruthenium (106Ru) present in 1986 as finer particles is far greater suggesting that the ruthenium behaved differently in the accident.
The reduction in the size of the cesium bearing particles can be explained by several hypotheses, firstly the fixing of the larger particles without the generation of any new smaller particles will cause the average size of the cesium containing particles to become smaller. Secondly if the particles of cesium from the reactor accident are subject to a leaching reaction which releases cesium which then is deposited onto small clay particles. These small clay particles (colloidal particles) are mobile both in soil and can be resuspended with ease.
This reminds me of the fractionation of radionuclides which occurs in the moments after a nuclear bomb detonation in the atmosphere. Those radionuclides which form solids in the seconds after detonation tend to be present in finer solids than those radionuclides which were present at the start of the particle formation time as volatile substances.
It has been found that the behaviour of a radioisotope in fallout depends on the chemical and physical state of it about 20 s after detonation as the fireball cools and the fission products condense, For example if we consider the isobars 95, 131 and 137 then the physical forms of the fission products 20 s after detonation will be very different. All of the 95Zr will be inside the solid particles formed soon after detonation, while part of the 131I will be found in these quickly formed particles while almost none of the 137Cs will be in these particles.
To do this we need to start with the yields for each fission product and their half-lives (Table ).
Table 10. Fission yields for nuclides with a mass of 131.
As the yield of 131In is so small and its half-life is so short we can ignore it. We start by calculating the decay of the 131Sn, tin will form an involatile oxide when heated to a high temperature in air. So any of the atoms in isobar 131 present as tin at 20 s post-detonation will be in an involatile form.
Fractionation has been observed during studies of fine particles of fallout collected by a Swedish jet aircraft far from Soviet thermonuclear tests. In the Swedish study particles were collected on a filter, using autoradiography the larger particles were identified and separated. The larger particles and the smaller particles were then gamma counted. It was found that with increasing time the larger particles were no longer found, and that the fraction of 103Ru present in the larger particles was smaller than the fractions of 95Zr and 141Ce which were in the larger particles (Edvarson, Löw, & Sisefsky, Citation1959). This can be explained by the fact that during the important condensation time the majority of the fission products with an atomic mass of 103 will be in the form of 103Mo. Molybdeum forms a volatile oxide (MoO3), on the other hand 20 s after the detonation 95Y and 141Ba will be the main nuclides for the fission product decay chains in the isobars at 95 and 141 amu respectively. Both barium and yttrium oxides are involatile refractory solids (Tables and ).
Table 11. Fission products with a mass of 103.
Table 12. Fission products with a mass of 99.
There is a problem, while the Bateman equations might appear to be an attractive way of dealing with these problems there can be a problem. The problem relates to the dynamic range of a digital computer. This problem is mentioned in the master’s thesis of Captain Logan J. Harr of the USAF (Harr, Citation2007). He used the delightful words:
“catastrophic cancellation occurs resulting in a shrinking denominator which causes the loss of digits of precision”
To describe what happens when two decay constants are similar in size
If we consider an eight bit system in which we want to subtract one decay constant from another. If we have λ1 of 200 (11,001,000) and λ2 of 198 (11,000,110) then in our system we can start with 200 and 198, by subtracting one from the other then we will end up with 2. If we were to change to a 16 bit system which records 200 as 0100111000100000 (200 × 102) and 198 as 0100110101011000 (198 × 102) then any rounding errors which will occur during the subtraction of one number from the other will be smaller. As a result the computer system will be better able to accurately generate small numbers by subtracting one large number from another large number. The problem with the method based on the Bateman equations is that these errors can cause the vast errors in the calculated activity. To explain this problem we can use excel or another spreadsheet system. Place the top left cell in cell A1 (Table ).
Table 13. Details of excel calculations used to test the dynamic range of the calculation process.
If the number in box b1 is increased then the error value in B8 should increase.
As a demonstration of this problem we can consider the decay chain 131In →131Sn → 131Sb → 131Te. If we consider the effect of reducing the dynamic range of excel by adding an additional term Q to the Bateman equations we can quickly see the effect. For the third radioisotope in a chain we normally write:
But we can write the following in place of the more conventional exponential parts of the equation:
When time (t) is zero we should calculate AC to be zero, we will use this as the test of the calculations. As the value of Q increases the size of the error increased. I have defined the error as the value using the following equation:
It is clear that as the value of Q increases the absolute value of the error increases (Figure ).
After having used the Bateman equations to model a range of things, my advice is to try to limit the number of steps along a radioactive decay chain that you model using these equations. For example you could if you knew the neutron flux in a reactor consider the evolution of the transuranium elements in the following series using the Bateman equations. This series can be explained by neutron capture, beta decays and one alpha decay at the end.
While with the perfect computer it could be done, due to the limits of the computer very quickly along the line we will run into trouble. A better way to deal with the problem is to leave out the short lived nuclides to try to reduce the length of the chain. For example one can regard the radioactive decay of 239Np as an extension of that of 239U as long as you are willing to make a sacrifice of the accuracy of the predictions in the first days after the reactor is started with fresh fuel. I would suggest that modelling the following would be better for many purposes. Now the 238Pu is nuclide 6 in the chain rather than nuclide 10.
Using real data from nuclear bomb tests done in the USA, Hicks was able in the early 1980s to write an exceptional paper in which this fractionation effect is explained (Hicks, Citation1982). Using the following equation the enrichment of a radionuclide relative to a case where no fractionation occurs (wi) can be calculation from the refractory to volatile deposition ratio (z) and a constant for each fission product (ri):
If a perfect fractionation of fallout occurred into volatile and refractory (non-volatile) fractions was to occur then after 4 h the majority of the radionuclides which are responsible for the external gamma dose would be in the involatile fraction. Such a total fractionation would require the formation of two fallouts with z values of zero and infinity. While such an extreme fractionation is not possible it has been shown by Hicks that the smaller a fallout particle, the further a location is from a bomb test and the longer it takes for fallout to arrive then the higher the refractory to volatile deposition ratio (z) is. One consequence of this is that the people who were close to the bomb tests would have been disproportionately exposed to the refractory class of radionuclides. Using the logic of Hicks it can be reasoned that if radioactive ruthenium was to remain in the gas phase longer after release from the damaged fuel at Chernobyl than the cesium then it would be able to form smaller particles.
Lurid accounts have appeared that suggest that radioactivity from forest fires near to the Chernobyl site poses a grave threat to people living far from the accident site. In particular, Yablokov, Nesterenko, and Nesterenko (Citation2009) stated that “On 6 September 1992, radioactive aerosols lifted by a strong wind from the 30-km Chernobyl zone reached the vicinity of Vilnius, Lithuania (about 300 km away) in 5–7 h, where the 137Cs concentration increased 100-fold”. The great problem is what consequence does a short duration 100-fold increase in the 137Cs content of the air have on public health. This question has already been considered by Jargin (Citation2011), he argues that the amount of exposure of the public to radioactivity which escapes from the Chernobyl area as a result of wind and fire is insignificant. An increase of the 137Cs level in the air in Vilnius did occur in September 1992, and as a result of a fire in 2002 the radioactive cesium level in Vilnius did increase again. It is interesting that the chemical form of the cesium differed between the 2002 and that which arrived in Lithuania in 1986 and 1992 (Lujaniene et al., Citation2006). The cesium in 2002 was in the form of an anionic substance while much of the cesium in the other 2 years was in a cationic form. One reason might be that the 2002 cesium was absorbed onto particles which when immersed in water become anionic.
We need to be careful as the mere detection of a substance does not mean that it is present at a dangerous level. For example I have been able to detect radioactivity (40K) in a packet of low sodium salt purchased from a supermarket. Additionally an excessive intake of copper is harmful to both humans and cats, but some copper is needed to keep cats healthy. I have ashed dry cat food, extracted the ash with nitric acid and then detected copper. My detection of copper in the cat food does not automatically indicate that consumption of cat biscuits will result in copper poisoning in either cats or humans. Equally it is possible to harm yourself by drinking monumental amounts of Earl Grey tea (Finsterer, Citation2002), but moderate consumption of Earl Grey tea is a relatively harmless activity. Paracelsus stated back in the 16th century “Was ist das nit gifft ist? alle ding sind gift/und nichts ohn gifft/Allein die dosis macht das ein ding kein gift ist” which is German for “What is not poison? All things are poison and nothing [is] without poison”. His grand statement has also been summarized as “Solely the dose determines that a thing is not a poison” (Borzelleca, Citation2000).
The great problem which we face is that some things only exert an effect when the dose (or concentration) is above a given threshold while any exposure to some other things has a finite chance of inducing an effect. In the latter cases the clinical course of the effect is the same regardless of the dose required to induce it but the probability of inducing the effect increases as the dose increases.
One of the key problems is dose rate, it has been reported that in North Carolina that either 80 or 160 mmol of potassium chloride is used as part of the lethal injection protocol (Zimmers et al., Citation2007). While I do not wish to discuss the morality of and other issues associated with capital punishment here it is interesting to note that a 500 g packet of low sodium salt contains 330 g of potassium chloride which contains about 4.4 mol of potassium ions. While if one was to consume an entire packet of either normal or low sodium table salt in 1 h either by mouth or by intravenous injection I am sure that it would induce a series of dire effects. But it is clear that if you were to ingest the low sodium salt over a year, each day adding a little to your food then you would be more able to tolerate the potassium chloride.
Experiments have been done in which wood and straw which the fate of cesium, chlorine and iodine were investigated (Amiro, Sheppard, Johnston, Evenden, & Harris, Citation1996). Here it was found that the hotter the conditions the greater the fraction of these elements which was liberated into the air and the smaller the fraction of the elements which were found in the ash. The experiments at Chalk river in Canada indicate that when contaminated vegetation is burnt that very little strontium (90Sr) is released into the smoke, most of it remains in the ash. But the release of the cesium depends greatly on the temperature of the fire and the nature of the vegetation (Zhou, Rao, Corcoran, & Kelly, Citation2016). Forest and grassland fires have been lit in contaminated areas, it was shown that resuspension of cesium, strontium and plutonium did occur. But only a tiny fraction of the radioactivity on the land became airborne during the fire (Yoschenko et al., Citation2006).
Measurements have been made after the wildfires in 2015 which were close to Chernobyl (Evangeliou et al., Citation2016). It is reported that the effective dose due to the resuspension of radioactivity caused by the fire was small (up to 4% of 137Cs/90Sr and up to 1% of the Pu in a forest fire (Yoschenko et al., Citation2006)) but under some conditions a fire which involves radioactivity could have more serious consequences. Many years a series of safety experiments on nuclear weapons were performed by the British government in Australia. These were known as the minor trials, in one particular experimental series (Vixen A) plutonium was exposed to motor fuel fires. In these trials plutonium metal was exposed to petrol (gasoline) fires, during these trials relatively little of the plutonium became airborne.
The source term of a plutonium fire accident can be determined with the following equation which appears in a review on plutonium dispersion during fires (Kogan & Schumacher, Citation2008).
where Puinventory is the amount of plutonium at the site, Pufraction is the fraction of the plutonium which is involved in the fire, ARF is airborne release fraction, RF is the respirable fraction of the release and LPE is the leak path fraction.
It is important to keep in mind that depending on the conditions in the fire, either a small or a large fraction of the plutonium present will be released. Also the respirable fraction of plutonium can vary according to the conditions. When plutonium is burnt in air under static conditions, such as a pool of molten plutonium in crucible then only a small amount of the plutonium is released. But when droplets of molten plutonium burn as they fall through the air a large fraction of the plutonium is released. The accidental fires which involve plutonium metal are most likely to be nuclear weapon accidents but it is possible in principle within the civilian nuclear industry for plutonium to be dispersed during an accidental fire. The two scenarios within nuclear sites which I think are most important are fires which involve solids such as plastics and cellulose or liquids such as the aqueous liquids or process solvent in a PUREX plant. In their review Kogan and Schumacher offer Table for plutonium in liquids.
Table 14. ARF and RF values for plutonium compounds in fires.
For boiling aqueous liquids the following equation is suggested by Kogan and Schumacher. Where B is the boil off rate ranges between 0.0004 and 0.0011 l cm−2 min−1. The maximum value of ARF from this equation is 0.02.
2.4. Charcoal
Charcoals are used in the nuclear sector as absorbant materials in order to perform a range of tasks, they are used in protective clothing, sampling and for treating gases before release from a nuclear power plant.
Charcoal has been used in protective clothing for a long time, a first world war UK military respirator (the small box respirator) used charcoal. The latest UK military respirator (General Service Respirator) also uses a charcoal. While some interesting protective filters such as unlit cigarettes (Kumana & Lemlich, Citation1974), zeolites (Ning, Qiu, Wang, Liu, & Chen, Citation2013) and mesoporous silica (Hudson et al., Citation2004) have been suggested for respirators charcoal together with fine filters is very common. Charcoal is used in many individual and collective respiratory protection systems, individual protection refers to personal protective equipment such as an air purifying filter respirator while collective protection refers systems such as the air purification system used to supply purified air to a bunker or other shelter. The use of a purification system to prevent the release of radioactivity or some other harmful substance to the environment is strictly speaking a collective protection system. For example at Chalmers in the nuclear chemistry unit all radiochemical fumehoods are fitted with a HEPA filter to prevent radioactive dust leaving the fumehood via the extraction system. An additional set of HEPA filters are present in a plant room on the floor above the laboratory which would provide protection to the general public and the environment if the HEPA filter on a fumehood was to fail.
A combination of a particle arresting mechanical filter and a pad of charcoal offers protection against some airborne threats. These include some forms of radioactivity which can be present in air. It is important to keep in mind that charcoal is not a panacea for all harmful substances in air. It has been shown that DABCO is needed in a charcoal to remove methyl iodide from air, while for the removal of elemental iodine from air no DABCO is needed (Wood, Citation1981). In many mines (Gillmore, Phillips, Denman, Sperrin, & Pearce, Citation2001), some caves (Sánchez, de la Torre Pérez, Sánchez, & Correa, Citation2013) and now in the Chernobyl object shelter (Ogorodnikov et al., Citation2009) (known commonly as the sarcophagus) high concentrations of radon and its radioactive daughters can be found. Gavin Gillmore at Kingston-upon Thames has suggested that industrial archaeologists should take care to avoid excessive radon exposure while working underground in places such as disused mines (Gillmore, Gharib, Denman, Phillips, & Bridge, Citation2011). While it should be clear that 222Rn is formed by the alpha decay of 226Ra present in the minerals in caves and mines, the 220Rn (Thoron) at the Chernobyl site is due to the decay of the 224Ra formed from the radioactive decay of 232U. A range of radioactive decays and neutron induced reactions in uranium and MOX fuel form 232U (Yamamoto & Okumura, Citation2014). While some of these reactions will be predominately caused by fast neutrons (such as the n.3n reaction on 234U), some of these reactions can be caused by slower neutrons (such as the n.g. reactions of 230Th and 231Pa). While it might be harder to remove radon from air than dust particles, it is possible to greatly reduce the lung dose due to radon by removing smoke and dust particles which have absorbed radon daughters onto their surfaces. Dust masks have been shown to intercept a large fraction of the alpha emitting radon daughters from air (Wake, Brown, Trottier, & Liu, Citation1992), also it has been shown in uranium miners that a synergy between cigarette smoking and radon exists (Saccomanno, Huth, Auerbach, & Kuschner, Citation1988). It can be reasoned that the radon daughters are able to bind to smoke particles and these particles are more likely to lodge in the lungs than radon daughters present in clean air.
During a reactor accident or other radiological accident the air can become contaminated with radioactive aerosols and volatile radioactive substances. It is important to bear in mind that the nature of the airborne threat will depend on the physical and chemical properties of the radioactivity present. It is also important to consider the non-radioactive threats which might be present in a radioactive (or potentially radioactive) environment. For example pathogens, asbestos, lead, electrical hazards and confined spaces may exist in a building with suspected radioactive contamination (SRP, Citation2011), it should be clear that it would be pointless to don a cumbersome respirator, a heavy lead apron and a plastic suit to protect against radioactivity if the person then comes to some other unfortunate end. For example one could fall from a great height, get heat stroke or be attacked by a dangerous animal while performing the work.
In the event of an easily dispersed source being broken open during an accident it is possible for respirable radioactive particles to be liberated or formed. For example 226Ra, as radium sulphate, has been inhaled after some accidents (Marinelli, Norris, Gustafson, & Speckman, Citation1953) as has 227Ac (Newton, Citation1966). In the event of a radioactive source being melted in a steel scrap metal furnace (Neuschütz et al., Citation2005) then 137Cs tends to be found in the dust which comes out of the furnace with the off gases and sometimes as well in the slag, while the actinides (such as plutonium) tend to concentrate in the slag (Sappok, Citation1991). If a dust is raised during the handling of either a contaminated off gas dust or slag then it is possible for workers to inhale radioactive dust. Here again a dust mask can greatly reduce the internal dose which a worker is subjected to. It is noteworthy that 60Co tends to become incorporated into the steel, unless the product is cut up with flame cutting or some other method which generates dust this radionuclide will be less able to become airborne. It is interesting that authors of the paper about the inhalation of radium sulphate includes Marinelli, he was the inventor of a very special beaker which is still used in a modified way. The original beaker was a glass beaker with a central hollow glass tube projecting up from the bottom. The idea was that a GM tube could be placed in the central tube where it is surrounded by the sample. The modern version of this beaker is a plastic cylinder with a cavity in the bottom which fits over the head of a sodium iodide or germanium detector. Again this is done to increase the amount of sample which can be placed around the detector (Figure ).
Figure 20. On the left the original Marinelli beaker with a GM tube in the central cavity while on the right is the modern version with a solid state detector in the cavity at the bottom.
But back to reactor accidents, in the early weeks after a reactor accident the radioactive iodine can pose an airborne threat to workers. It is important to understand that iodine is often present both as particulate matter and in the form of volatile substances such as elemental iodine and methyl iodide. Luckily iodine binds very strongly to charcoal (Reyerson & Cameron, Citation1936) while for good protection against methyl iodide and the other volatile organic forms of radioactive iodine the charcoal needs to contain some additives.
A classic method of taking samples of volatile organic compounds is to use a charcoal or other porous material (Bianchi & Varney, Citation1993; Franco, Correa, Marques, & Perez, Citation2014; Ramirez, Cuadras, Rovira, Borrull, & Marce, Citation2010) to collect organic compounds from the air before they are introduced into a gas chromatography machine. These methods have been used for the detection of halogenated compounds including vinyl chloride (Miller, Kane, Robinson, & Whittingham, Citation1978) and the organochlorine compounds from a charcoal grilled chicken restaurant along with a vast array of other organic compounds, some differences in the emissions from the wood oven roasted piglet restaurant were noted (Alves et al., Citation2015). Returning to compounds which are more relevant to serious nuclear reactor accidents it is noteworthy that natural methyl iodide, ethyl iodide, chloromethyl iodide and other halogen compounds have been observed in the marine environments (Yokouchi et al., Citation1997). In the study by Yokouchi et al. the volatile organics were trapped on Tenax TA® which is a porous resin made from poly(2,6-diphenylene oxide). The idea of natural methyl iodide might be a surprise to many, one might think that organohalogen compounds are exclusively or mainly artificial, but this is clearly wrong. Gribble (Citation1994, Citation2003) pointed out that a large number of natural organohalogens exist. Methyl iodide has a role in the natural iodine cycle, it is formed in the sea. The methyl iodide is then converted in the air into inorganic iodine compounds which then fall in rain onto the land. Lovelock, Maggs, and Wade (Citation1973) noticed back in the 1970s that methyl iodide was being formed in the sea. In recent years methyl iodide was used as a pesticide in the Californian strawberry industry. The use of methyl iodide in strawberry farming is an interesting tale.
For many years methyl bromide had been used as a fumigation agent to kill unwanted organisms in soil and to kill a range of pests. In some ways methyl bromide was a horrible choice of reagent, it has no strong warning odour and it is also harmful to the ozone layer. The great problem is that it is too stable in the lower atmosphere. It can reach the stratosphere where ultra-violet light breaks it down releasing a bromine atom. The bromine atom then acts as a catalyst for the degradation of ozone in a cycle similar to the ClOx cycle.
The idea of using methyl iodide was that it would have similar chemistry in soil, woodworm infested wood and other materials which were to be fumigated. Methyl iodide has been shown to have an antifungal activity which is better than that of methyl bromide (Hutchinson, McGiffen, Oh, Sims, & Becker, Citation2000). The stability of it in the troposphere is low thus protecting the ozone layer. For methyl iodide released at mid-latitudes the atmospheric lifetime is 13.6 days and the ozone depletion potential is 0.017, interestingly the iodine replacement for Halon-1301 (CF3Br) when released from mid-latitudes has a atmospheric lifetime of 5.0 days and an ODP (Ozone Depletion Potential) of 0.008 (Youn, Patten, Wuebbles, Lee, & So, Citation2010). In contrast methyl bromide has an atmospheric lifetime of 256 days and an ODP of 0.39 (Ko et al., Citation1998). Halon-1301 has an atmospheric lifetime of 75 years and an ODP of 18.6 (Bernard, McGillen, Fleming, Jackman and Burkholder Citation2015). While from the ozone layer damage point of view these two iodine compounds are ideal replacements for the bromine compounds, things have not always worked out well for the iodine compounds.
I will not be discussing the iodine firecontrol chemicals, it is interesting to discuss the use of methyl iodide in farming. It is interesting to look at some of the arguments regarding methyl iodide in fruit farming. For example, the pesticide action network issued the following comment http://www.panna.org/pan-news/strawberry-fields-poisoned-forever:
“The state that supplies 90 percent of U.S. strawberries has approved a fumigant pesticide that chemists are hesitant to handle in the lab. One more reason to buy organic. While it is unlikely that methyl iodide injected into strawberry fields pre-planting will wind up on or in the nonorganic strawberries you buy at the store, rest assured those conventionally grown berries could be teeming with other toxic compounds. That’s because many chemical farmers rely on a cocktail of pesticides to help compensate for chemically destroyed soil quality. For example, according to Pesticide Action Network’s What’s on my food? tool, which uses U.S. Department of Agriculture (USDA) Pesticide Data Program numbers, 55 percent of conventional strawberries contained residues of the fungicide Captan, a probable human carcinogen, according to the EPA.”
I will examine some of the statements in this comment.
The statement “chemists are hesitant to handle in the lab” is to my mind a scary sounding non-statement, the problem is does hesitant mean. Some sloth like chemists might hesitate to do anything in the lab or elsewhere. Also the chemist who stops and considers what they are going to do in the labFootnote2 before they do it might be regarded as a hesitant person compared with a person who just does whatever comes into their mind. I have a preference for students who stop and think through what they want to do rather than just act on impulse and then have to live (or die) with the consequences.
The statements “those conventionally grown berries could be teeming with other toxic compounds” and “55 percent of conventional strawberries contained residues of the fungicide Captan, a probable human carcinogen, according to the EPA” suggest that substances which are harmful are present in strawberries, but they do not indicate that these substances are present in sufficient amounts to be of concern. With the improvements which have been made over the years it is possible to measure many harmful substances at concentrations far below those at which they are harmful.
Susan Kegley gave her expert opinion https://www.youtube.com/watch?v=I4uK9Blj5ms (also see http://sagri.senate.ca.gov/sites/sagri.senate.ca.gov/files/TranscriptFinal_Methyl_Iodide.pdf at a hearing considering if methyl iodide should be licensed as a pesticide in California. She makes a series of claims I will consider some of them. At 2 min she states that methyl iodide vapour can drift in the air, I have to say she is right. Methyl iodide has a lifetime in the air which is measured in days thus allowing it to migrate from place to place. At 4 min and 15 s she states that methyl iodide is a carcinogen, and a proposition 65 carcinogen. The problem is that many things are carcinogenic, but not all carcinogens are equally able to induce cancer. In California a law exists (prop 65) which requires a warning to be issued even for carcinogens which have very weak carcinogenic effects. These include oestrogen in oral contraceptive pills and an attempt was made recently to force Starbucks and other coffee houses to label their coffee with this warning. As a result I hold the view that the Californians are bombarded with warning which have little meaning. If we consider a sign for a dangerous animal, it might seem like a good idea to mark the entrance to a crocodile pit with a warning. But if the same sign is used to warn us about the presence of a scary looking (but harmless spider) and a domestic cat (it might scratch) then the warning will lose its ability to protect us from crocodiles and other very dangerous animals. To me the use of proposition 65 dilutes the message that methyl iodide is either a carcinogen or likely to be one.
At 4 min and 30 s she states that methyl iodide can cause spontaneous abortion in animals (in humans this is known as miscarriage). But when reading the literature the link between methyl iodide and foetal death is not clear. In rabbits it is possible to cause foetal death by exposing pregnant rabbits to methyl iodide vapour (Sloter et al., Citation2009), but an injection of sodium iodide induced the same effects in the foetus. The paper concluded that the methyl iodide caused foetal death by overloading the foetus with iodide. To me this is rather different to methyl iodide killing the foetus, to me it is inorganic iodide killing the foetus. The methyl iodide just happened to be the delivery mechanism for the iodide. As stable iodine (potassium iodide) prophylaxis has been recommended for children and pregnant women (Agopiantz et al., Citation2016) this suggests to me that a threshold dose of iodide is needed to cause foetal death. The study using rabbits used 4 days of exposure at 25 ppm. I think that 25 ppm of methyl iodide is a very high level which humans should not be exposed to.
Kathleen Collins https://www.youtube.com/watch?v=uhR-Y1MrHaI talked about how alkylation agents can induce cancer, she also commented that she is not allowed to dispose of the compound to the environment in any way (5 min and 40 s) and “way higher than all the radioactivity we use”. As I have no knowledge of what radionuclides she uses or the amounts I cannot make much of a judgement on this matter, also without her being willing to give out details of what radionuclides she has at her lab and the amounts (Such a disclosure is inadvisable for nuclear/radioactivity security reasons) it is impossible for anyone to make a judgement as to how bad the methyl iodide is compared with her radioactivity. If she has 100 kBq of tritium then this is close to harmless, if she has 2 GBq of 131I then this is something to worry about more. Let alone the risks posed by 100 MBq of 238Pu or 226Ra.
At 6 min and 15 s she states that to use methyl iodide she would have to do a risk assessment which would consider why she needs to use methyl iodide. I have to point this out but in both the UK and Sweden the regulations on the use of chemicals in the workplace require me as a chemist where possible to use less hazardous chemicals where possible. Thus methyl iodide should not be regarded as being special in this way. Her comments about being not allowed to release the compound are in some ways a bit of a red herring, while methyl iodide should not be released in a wanton manner into the environment. It is possible by using tarpaulins, thiourea (Zheng, Papiernik, Guo, Dungan, & Yates, Citation2005) and thiosulphate (Xuan, Yates, Ashworth, & Luo, Citation2012) to reduce the escape of volatile alkylating agents from soil, what I found interesting about the campaign against methyl iodide use in California is that it seemed to concentrate on the idea that “methyl iodide is dangerous” rather than considering the questions of “can people work safely with methyl iodide?” and “can we trust farm workers to work safely with methyl iodide?”
While for the measurement of an organic chemical such as benzene or vinyl chloride the substance must be liberated from the porous material to allow it to be directed into a device such as a gas chromatography machine to enable it to be separated and then measured, in the case of a radionuclide which emits gamma rays it can be measured while it is still absorbed in the charcoal (or other solid). The gas chromatography can be compared with the radiochemistry used to purify radioactivity in an environmental sample into a form which can be measured. For example one method for the measurement of plutonium in rainwater the plutonium is separated using chromatography on ion exchange resin from iron before depositing the plutonium onto the metal disks used in an alpha spectrometer (Montero & Sánchez, Citation2001).
When radioactive samples consisting of radioactivity absorbed onto charcoal are counted with gamma spectroscopy, the charcoal is used to improve the efficiency of the counting by concentrating the radioactivity into a small volume. For example if a gamma emitter was dispersed evenly throughout a cylinder which is 2 m in radius (Rs) and 4 m tall (h) then ignoring absorption effects then the counting efficiency will be very low as the vast majority of the gamma photons will never enter a modest sized detector. Using the equation (Aguiar, Galiano, & Fernandez, Citation2006) the difference in geometric efficiency between the counting efficiency of the cylinder with a point detector arranged coaxially at distance d from the circular surface of the cylinder is given by:
where zt is the efficiency for the cylinder source, zp is the efficiency for a point source at d+(h/2) from the detector, fgeo is given by:
Using this relationship, and assuming d to be 0.03 m, it can be shown that the geometric efficiency is circa 60% of that of a point source which is placed 2.03 m from the point detector. While 60% might not appear to be a bad efficiency, it is important to note that a point source 203 cm from the detector will give a counting efficiency 841 times lower than a point source only 7 cm from the point detector. Thus the cylinder of air (4 m high with a radius of 2 m) will give a 1,401 times lower counting efficiency than the point source at 7 cm from the point detector. If the point source at 7 cm from the detector centre is replaced with a cylinder with a radius of 3 cm and 8 cm high which has the detector on its axis (3 cm from the centre of the circular face of the cylinder), the large cylinder will cause the detector to register 1,251 times fewer counts per second than the large cylinder. Thus it should be clear that by concentrating the radioactivity from a large cylinder of air into a small cylinder of charcoal then the measurement will be far easier.
In some cases separation of different elements using charcoal is possible, one problem in nuclear reactors are the leaks in cladding of fuel. Despite the best efforts of the fuel makers some fuel pins develop leaks during use. Charcoal can be used to improve the measurements of the radioactivity which is leaking from the fuel. In the 1980s an interesting gamma spectrometry experiment was done in Japan at a research reactor (JRR-3), here the helium cover gas over the reactor was examined using a coal based charcoal (Sakai, Citation1984). One of the great problems in gamma ray spectroscopy is that it can be hard to measure a low energy gamma emitter at the same time as a high energy gamma emitter is present. One of the reasons is that the high energy gamma emitter causes the background in the detector for low energies to become high as a result of the Compton effect. Now here is a spectrum which I recorded recently using a large bottle of K2CO3. What you can see is a smile shaped curve to the left of the photopeak from the electron capture decay of the 40K (Figure ).
This curve in the spectrum is caused by the Compton scattering process, what happens is that gamma rays interact with electrons, they change direction when they strike electrons. As momentum is conserved the electron accepts some momentum and energy from the gamma photon. The fast moving electron tends to give up its energy in the detector while sometimes the product gamma photon escapes from the detector. As a result only part of the energy of the photon is delivered to the detector, as a result a wide range of different fractions of the energy of the original photon can be delivered to the detector. This increases the background in the detector for low energy events. It should be clear to the reader that the presence of a large amount of 41Ar in charcoal pad will make it hard to measure the low energy lines of nuclides such as 85mKr. Below I have shown some of the key properties of some noble gas radionuclides which are formed in a nuclear reactor (Table ).
Table 15. Details of some noble gas radionuclides formed in a fission reactor.
The 41Ar is generated by the action of neutrons on 40Ar (neutron activation) while the krypton and xenon are formed by fission. As a result in reactors in which air cannot be excluded totally from the system it is possible to generate some of this high energy gamma emitter. The measurement of free fission gas levels in nuclear reactors can be used to estimate how much fuel has damaged cladding, it is important to note that water cooled reactors tend to have a problem with “tramp uranium”. Tramp uranium is uranium which was in the original water used to fill up the reactor or uranium which has escaped from damaged fuel in the past, this tramp uranium can undergo fission in the core and generate the radioactive fission products which include noble gases.
A small hole in a fuel rod can increase the radioactivity level of coolant, for example Lin and Chao reported that before the leaks occurred in some fuel about 100 pCi g−1 of 91Sr and 92Sr was present in the coolant of a reactor, while only 1 pCi g−1 of 131I was present in the water (Lin & Chao, Citation2006). Around week 16 of cycle 16 a leak occurred which increased the 131I level to almost 100 pCi g−1 (the level of some of the other iodines 132I, 133I, 134I and 135I also increased slightly at this time). But the 91Sr and 92Sr levels only increased months later. It can be argued that the strontium can be used as a means of sensing a different fuel failure to pin holes which are assumed to release the iodine.
To explain these changes we can use the empirical relationship which Lin and Skarpelos (Citation1997) reported that General Electric used:
In their 1990s paper Lin and Skarpelos divided releases of fission products into the water of a BWR into three classes, recoil (from tramp uranium b = 0), equilibrium (from pin holes b = 1) and diffusion (split cladding b = 0.5). Here they came up with an equation for the release rate which is:
where Kr, Ke and Kd are constants which relate to the three different sources of the fission product. Now if we subject uranium to a neutron flux (σ) and the fission yield (Y) for a given nuclide is known then it will be possible to estimate the ratio of the radionuclides which will be present when the reactor has reached equilibrium, for this calculation we will ignore neutron capture by fission products. For iodine we get the following results (expressed as an activity ratio) when b = 0.
For this ratio I have greatly simplified the calculations by assuming that the only formation of the iodines is fission directly to the iodines, in reality the iodines are largely formed by the beta decay of precursors such as 131Te (t½ = 25 min) and 131Sb (t½ = 23 min). While I have presented data for iodine, I could have presented data for any element formed in the fuel. The key thing here is that we are looking at the ratio between short and long lived radionuclides (Figure ).
The fission products released by damaged fuel, will give a different activity ratio. This is because time is required for the fission product to be transferred out of the fuel and into the coolant. In the event of an accident which did not cause damage to fuel the radioactivity release would be higher as the coolant (heat transfer fluid) would be have a higher radioactivity level. An example of such an accident would be a spillage of coolant. The degree of core damage can be estimated by taking a sample of the water from a light water reactor and measuring the radioactivity level.
It should be clear to the reader that even if leaking fuel (damaged fuel) is unlikely to worsen a serious accident it is not desirable to operate a reactor with leaking fuel inside it, now we have the question of how does one find the damaged fuel in a reactor. While in theory one could unload all the fuel into a hotcell (taking care to avoid a criticality accident) and examine or test it all for leaks, it should be clear that this would be a slow, expensive and tiresome process. The reader will be glad to know that there are better methods of checking fuel. One method is known as flux tilting, the idea is that the reactivity of the reactor as a whole is kept constant, but by moving the control rods it is possible to increase or lower the flux in part of the reactor.
As the tramp uranium which is in the water is still subject to the same neutron flux (on average) the contribution to the coolant radioactivity from the tramp uranium will not change. But if the release of radioactivity from a fuel pin changes as a function of the neutron flux it is subject to (and thus its thermal power output) then the coolant radioactivity will then change. By performing a series of flux tilting operations it is possible to eliminate many of the fuel assemblies from the hunt for the defective one.
3. Other fission products and activation products
In the early days of the nuclear industry an atomic utopia was offered to the general public, in some books from the 1950s it was promised that nuclear technology would offer us a far better life. This was supposed to be a world of electricity too cheap to metre. However all was not to stay well forever, events such as the contamination of the Lucky Dragon fishing vessel during the castle bravo hydrogen bomb test in 1954 changed public opinion. While the castle bravo bomb was an intentional nuclear detonation in some ways it was a nuclear accident. The reason I would consider it to be an accident is as a result of an unexpected nuclear reaction. The neutron bombardment of 7Li instead of forming 8Li (t½ = 838 ms) which would decay via 8Be to a pair of alpha particles formed a tritium nuclei, an alpha particle and a neutron.
The more common reaction for the production of tritium is:
This addition of extra neutrons and tritium into the secondary of the bomb would have increased both the amount of fusion which occurred and the neutron flux on the uranium tamper which is thought to surround the fusion fuel. As the castle bravo event was a military accident (unexpectedly high yield) we will leave it alone now. Together with other events in society the castle bravo event may well have made the public less trusting of their governments and their representatives. It may be as a result in this lack of trust in governments that the public have turned to other sources of advice and information. Sometimes members of the public make some choices regarding information sources which are surprising.
Some of the people who the public trust make statements which cannot be reconciled with the results of experiments, my advice on fission product chemistry is to be careful who you trust and to where possible check the facts for yourself. Also be careful of those who might choose to deliberately not tell the whole truth but only the small fraction of the truth which suits their political purposes. For example Caldicott, (Citation2006) wrote in chapter 3 of her book entitled “Nuclear Power is not the answer” the following about cesium.
“Cesium 137 is an isotope with a half-life of 30 years, radioactive for 600 years. As a potassium analogue, it is present in every cell of the body. Cesium 137 tends to concentrate in animal muscle and fish, and it deposits in human muscles where it irradiates muscle cells and other nearby organs. It is a dangerous beta and high-energy gamma emitter and is very carcinogenic”
I am now going to consider the following statements which I can extract from this short bit of text.
137Cs has a half-life of 30 years;
It is radioactive for 600 years;
It is present in every cell of the body;
It tends to concentrate in animal muscle and fish;
It is very carcinogenic.
I would agree that the half-life of 137Cs is 30 years, but the idea that it is radioactive for 600 years is a rather odd idea to me. After 600 years the activity of 137Cs remaining will be about 9.5 × 10−7 times that which is present now. But in year 601 the activity will not suddenly drop to zero, thus it is unreasonable to say it will be radioactive for 600 years. A better way of expressing the lifetime of a nuclide is the “average lifetime” of the radioactive atoms which in the case of 137Cs will be 43.2 years.
We have to ask ourselves the question of how much must be present for it to be in a cell, one of the great problems is that with more and more sensitive measurement equipment it is possible to detect things at lower and lower concentrations. The great problem is that since the nuclear bomb tests everybody now contains a trace of 137Cs along with wine. Gamma counting in an ultralow background system has been used to detect some cases of wine fraud. One method of wine fraud is to mislabel a new wine as an older (and more expensive one), Philippe Hubert has been using a very low background high purity germanium detector to measure the tiny trace of 137Cs in wines (Hubert, Perrot, Gaye, Medina, & Pravikoff, Citation2009). Rather than trying to date wine using beta emitters such as tritium (3H) and 14C he has used a beta-gamma radionuclide as the measurement does not require the bottle to be opened. In a paper he described how he uses an inner shield of very old lead (no 210Pb), a layer of boron loaded polyethene, a layer of normal lead, another layer of boron loaded polyethene. On the outside of this system some plastic scintillation detectors are used to veto the germanium detector when cosmic rays strike the shielding. With this system it is possible to detect 137Cs down to a few mBq per litre. The problem is that if it is possible to detect 137Cs in many wines it is reasonable to assume that in most people it would be possible to detect this nuclide if you were to try sufficiently hard. However it is not reasonable to assume that the mere detection of 137Cs will render the wine dangerous to drink. Using the data in Prof Hubert’s paper and the assumption that a wine glass holds 100 ml, I have calculated that a person drinking a Bordeaux from the height of the cold war (Citation1962) that get a dose of about 1.3 nSv from the cesium in the wine. At nuclear chemistry in Chalmers this would correspond to about 4 s of background radiation. To reach the occupational limit (1 mSv year−1) for radiation exposure for a non-radiation worker from this wine cesium, one would have to drink a staggering 2,106 glasses of this rather expensive wine per day. I am sure the alcohol will kill you long before the cesium! While this example might be somewhat bizarre it does prove the point that mere detection should not be equated with danger. As a result I think that statement three while being true it is of little note.
The idea that cesium tends to concentrate in animal muscle and fish can be considered. Some evidence exists that in some food chains that cesium can become concentrated. By spiking a lake with stable cesium (133Cs) and then following the cesium concentration in a series of different animals the biomagnification of cesium was observed in two food chains (Pinder, Hinton, Taylor, & Whicker, Citation2011). But inn a recent study it was shown in India that the concentration of 137Cs in grass is lower than that of the soil (Karunakara et al., Citation2013) (Table ).
Table 16. 137Cs activities in soil and grass in India.
Also the grass to milk transfer coefficient was low, the following equation was used to calculate the transfer coefficient (FM d l−1), AM is the radioactivity of the milk (Bq l−1), Ag is the specific radioactivity of the grass (Bq kg−1) and QM is the daily intake of grass (kg d−1).
Using two cows it was estimated that a cow eats 2.2% of its body mass per day, it was found that cows which graze on common grazing areas have a FM value between 0.030 and 0.64, while cows from a dairy farm have a FM value of 0.050–0.15. The geometric means of these values are 0.12 and 0.092 respectively. These results suggest that cesium does not biomagnify through all food chains. The dairy farm cows (Holstein Fried, commonly known as Jersey) produce 12–15 litres per day. As the 137Cs content of the milk from the farm was 0.04 Bq kg−1, we can estimate that the cows release 0.48–0.60 Bq of radioactive cesium per day in their milk. As the cows ate 13 kilos per day of grass (dry matter) they would have ingested 16.9 Bq of radioactive cesium per day. I suspect that much of the cesium which each cow eats is excreted in the urine.
While much of the cesium in a human will be in the muscle tissue cesium has a relatively short biological half-life in humans, thus I think it is unreasonable to state that “that it concentrates in animal muscle” as this statement strongly suggests that it accumulates in this tissue. Observations made on workers from the Chernobyl accident site indicated that a fraction of the cesium in a human is retained (effective half-life 360 days) (Kutkov, Citation2000). This long retention time is very likely to be due to the fact that the 137Cs is within aerosol particles formed from spent nuclear fuel. Kutkov also gives the ratio between six radionuclides which were found in workers at Chernobyl (Table ).
Table 17. Radionuclide ratio in workers at the Chernobyl accident.
After the accident in Brazil (Goiânia) in humans the fraction of cesium which is retained can be approximated by the equation. Where a1 = 0.15, a2 = 0.85 and a3 = 0.001. The constants k1, k2 and k3 are calculated from the half-lives of the three different pools of radioactivity 3, 90 and 500 days respectively (Melo et al., Citation1997).
It is important to note that diet and other things can alter the biological half-life of cesium (and I assume other elements). In sheep it has been shown that by increasing the amount of potassium in the diet of sheep increases the rate at which 42K and 134Cs is excreted. But a change in the amount of sodium in the diet of the sheep has no effect on the rate of loss of 42K and 134Cs (Mraz, Citation1959). It is also interesting that on average the biological half-life of cesium in 27 ± 8 year old women is 84 days (ESD [Estimated Standard Deviation] = 27 days) while in pregnant women (25 ± 5 years of age) is only 49 days (ESD = 16 days) (Zundel et al., Citation1969). These papers on sheep and women are good reminders of several things, firstly the habits and diet of an animal can alter the metabolism (and thus the biological half-lives of elements). Secondly between different individuals there are some variations.
I hold the view that a good case exists for limiting the amount of radiation and radioactivity a pregnant woman or child is exposed to. But if the biological half-life of cesium in pregnant women is shorter then this may mitigate the consequences of an intake of radioactive cesium by women to their children. It is also noteworthy that the in a study of 12 German women that it was found that cesium is not reconcentrated in breast milk. Only circa 15% of a woman’s daily intake of 137Cs in food and drink is transferred into one litre of breast milk. While only circa 0.25% of a woman’s total body 137Cs activity is transferred into the litre of breast milk (Gall, Mahler, & Wirth, Citation1991). Depending on what part of the diet the radioactive cesium is delivered in under some conditions, such as radiocesium in drinking water, it may be possible for the breastfeeding woman to act as a filter which reduces the intake of radioactivity by her child. This is compared to the scenario in which an infant is fed baby formula which has been reconstituted with contaminated drinking water.
When lead is compared with cesium it is far clearer that lead accumulates in some organs (such as bones). Using a compartment model for lead in human beings I have made some predictions about the way in which lead would behave in a human being (Leggett, Citation1993). I think that her statement about where the cesium goes in an animal is not untrue but it is misleading.
The question of how carcinogenic 137Cs is not an easy matter to consider, but it is interesting that she also states that 131I is very carcinogenic. The problem is that Holm showed that moderate (MBq) doses of 131I does not induce thyroid cancer when it is used for thyroid examinations. If Helen Caldicott considers 137Cs and 131I to be comparable in terms of carcinogenic strength then on this scale 137Cs is a very weak carcinogen. I then choose to look at the references in her book, reference [45a] in chapter 3 is to a Suffolk County resolution which relates to an aggressive malignant muscle cancer (In the references written as Rhabdo-myoma, I assume she means rhabdomyoma). In the body of her book she wrote of rhabdomyosarcoma. A newspaper report on subject of one of these resolutions stated it was the sarcoma (Tuma, Citation2000). Now I have to confess that I dislike the excessive use of hyphens, but I hold the view that nothing would be gained by nit picking on punctuation. But lets consider something more important.
She claims that exposure to radioactive cesium could cause this form of cancer. I hold the view that this is a poor statement, one could argue that in theory that all forms of cancer could be induced by anything which damages DNA. This is because radiation alters DNA, cancer cells contain DNA which is different to that of the healthy cells from which the cancer evolved. But we need to ask the question of “is radiation a common cause of rhabdomyoma or rhabdomyosarcoma?”. I made a web of science search and found no link between “rhabdomyoma and radiation”. One of the two papers which has both words simply mentions radiation as a treatment for cardiac tumours and also states in the abstract that rhabdomyoma is a benign disease (Gowdamarajan & Michler, Citation2000). On the other hand rhabdomyosarcoma is a different disease to rhabdomyoma which can be induced in mice by irradiation with beta particles (Gupta, Andrews, McDaniel Nagle and Bowden, Citation1999), and it has been observed in radiotherapy patients years after treatment for an earlier cancer. But these two diseases are different conditions, I would be much happier if Helen was to use a reference to the study of a cancer in the peer reviewed literature or an easily available report from a national radiological protection organization rather than a local law. I have looked at the resolutions and they are not scientific documents or statements of what does (or what is thought to) cause cancer. Both resolutions are merely statements that an enquiry (Gaffney, Citation2000a) will be made into the incidence of a form of cancer in the county and some details of how this will be done (Gaffney, Citation2000b). To my mind her book misrepresents the contents of the two resolutions. A charitable view can be taken that she made an error in writing the book, but I hold the view that it is easily avoided mistake.
She also makes the bold statement that inhalation of a microgram of plutonium will result in lung cancer. I will not deal with this statement here, I have commented on the risk of inhaling a microgram of plutonium later in this review. Overall I think that many of the statements on matters of science within chapter 3 of her book are either wrong or misleading. Also her choice and use of references are not good. In general I would suggest that you the reader should be careful with all information sources, even what I write. I would suggest that when you encounter some idea or claim in the secondary literature that you should trace it back to the primary literature. In this way form a judgement about the source in the secondary literature based on facts rather than emotions. Now suitably warned we will start to look at some fission products.
4. Cesium
Cesium was released at Fukushima from the fuel, it then escaped from the plant. While much of the cesium was either in the form of a water soluble compound or associated with organic matter (Xu et al., Citation2015) some cesium in the form of water insoluble spheres was released (Adachi, Kajino, Zaizen, & Igarashi, Citation2013). There spheres contain silicon and are likely to be some silicate spheres. Radiometric measurements on seven of the most heavily internally contaminated TEPCO workers indicate that inside their chests is an insoluble cesium compound (Nakano et al., Citation2016). This observation can be explained by the inhalation of the cesium containing silicate spheres. It is not clear yet how the presence of the insoluble cesium will alter the effective dose which the workers receive as a result of inhalation of the radioactivity. This long-term retention of some insoluble cesium in the lungs is not unique, both at Chernobyl and in an accident in Germany the unusually long retention of cesium in humans was observed. In common with the long retention in TEPCO workers this can be explained by the inhalation of insoluble cesium compounds.
Several independent reports exist of microscopic radioactive particles being emitted by Fukushima, in some cases attempts have been made to chemically characterize these particles. One very good study was conducted by Abe et al. (Citation2014) in this study XRF and XANES were used to detect the elements in the particles and to make an attempt at identifying the chemical environment of the elements. This study indicates that sodium, potassium, iron, zinc, rubidium, zirconium, molybdenum, tin, antimony, tellurium, cesium and barium were found in the particles. In some particles it was possible to detect magnesium, aluminium, lead, chlorine, calcium, chromium, manganese and uranium. The XANES indicated that that iron, zinc, molybdenum and tin were present in a soda-lime glass. This observation of a glass is consistent with the abnormally long retention of the cesium in humans. In another paper (Kaltofen & Gundersen, Citation2017) it is claimed that the microparticles contain tellurium (up to 48%), cesium (up to 15.6%), rubidium (up to 1.22%), polonium (up to 1.19%), dysprosium (up to 0.18%) together with radium, tin, lead, nickel, iron and chromium all detected by scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDX). I have several criticisms of this work, firstly it is exceptionally difficult to be able to measure with SEM/EDX a concentration of an element to three significant figures. Secondly I do not believe the observation of the tellurium, polonium and radium by SEM/EDX.
The published X-ray spectra which the authors interpret as indicating the presence of a large amount of tellurium, contains X-ray lines which are at the correct energy for the L lines of tellurium. However the K lines of calcium are at very similar energies to the Te-L lines, I suspect that the authors may have mistaken the calcium X-rays for tellurium X-rays. I do not believe that sufficient radium would be present in the microparticles in an environmental sample for it to be detectable by SEM/EDX. The authors stated that they had used gamma spectroscopy to examine the samples. I reason that if the samples had contained a large amount of radium together with radon then the radium (and its daughters) should be very noticeable by gamma spectroscopy. This brings us to the problem of a person getting the “right” answer by the “wrong method”. I reason that if a person using a totally inappropriate process in an attempt to obtain an answer or make a choice, and by some miracle they arrive at the correct answer then while it was good that they obtained the correct answer they may well lack the ability to consistently get it right. Thus their work or method is not trustworthy.
Experiments on the leaves of trees indicate that the Fukushima cesium is mobile within a tree. One problem with cesium is that multiple releases have occurred. For example nuclear bomb detonations have released 137Cs while Chernobyl released both 134Cs and 137Cs. The shorter half-life of 134Cs (2 years) means that the Chernobyl 134Cs has almost totally decayed away by now. Over 15 half-lives have passed since the Chernobyl accident, thus this cesium radionuclide has decayed by a factor of more than 32,768. As 134Cs is formed by neutron activation of stable 133Cs it can never observed in the fallout from a nuclear bomb, unless for some reason a bomb was to be fitted with a jacket of a stable cesium compound. As a result the 134Cs to 137Cs ratio in the leaves of the trees is an important clue to the origin of the cesium. The 134Cs:137Cs ratio in the leaves which were exposed to fallout was the same as the leaves which grew on the tree after the accident (Xu et al., Citation2016).
Radioactive fallout can be either deposited under dry conditions or during rain, or other precipitation, under wet conditions. The transfer of radioactivity to fruit is often higher when the radioactivity is deposited under dry conditions. It can be reasoned that rain will wash the radioactivity off the surfaces of a tree and into the soil. In the early years after an accident the main source of cesium which goes into the fruit is the cesium which was deposited directly onto the trees and was then stored inside the trees. In the early years the adsorption of cesium via the roots is not very important (Antonopoulos-Domis, Clouvas, & Gagianas, Citation1991).
The aggregated transfer factor (Tag) is the activity of the fruit (Bq kg−1) for a given amount of radioactivity deposited onto the land (Bq kg−1 m−2). This equation is normally used for fruit growing on the tree in the first year. The aggregated transfer factor is given by the following equation (Renaud & Gonze, Citation2014). Where IF is the interception factor (area of foliage for a given area of land), t is the current time, ta is the time of the accident, Tfr(t − ta) is the term containing the true translocation factor while λ is the physical decay constant (days−1) for the nuclide.
This equation suggests that the transfer of radioactivity from the leaves (and other surfaces) of the tree to the fruit is proportional to the time between the deposition and harvesting of the fruit. I suspect that under extreme conditions that the following equation might be more correct. As a plant with very slow growing fruit which is contaminated with a radionuclide which relocates very quickly will come to an equilibrium in which the fruit will have a constant radioactivity (Afruit) at a time after the deposition of radioactivity (Aland).
It is important to understand that the transfer of radioactivity to the fruit in the first years after an accident will not be the same as the degree of transfer to fruit from the same tree many years later, nor will it be the same as that for a new fruit tree which is planted after the accident. Antonopoulos-Domis, Clouvas, and Gagianas (Citation1996) found that the radioactivity of fruit grown in the 1990s on trees planted after the Chernobyl accident was both lower than that of fruit from trees planted before the accident, and the 137Cs level of the fruit on the trees planted after Chernobyl declined more slowly. These authors found that the radioactivity of the fruit was well described by the double exponential equation when t was greater than 1 year.
In this equation A is a constant for the cesium deposited directly onto the tree while γ1 is a constant (years−1), B is the constant for the cesium adsorbed through the roots of the tree and γ2 is another constant. Finally t is the time in years. It is clear that in the years after an accident the entry of the cesium (or most other radionuclides) will occur via the roots from the soil. For example lemon trees growing in Brazil close to where a cesium accident occurred in the 1980s were found to produce lemons with much lower radioactivity (83 Bq per kilo of dry mass) than the corresponding activity of the soil (3,659 Bq kg−1) (Velasco et al., Citation2012). The soil to fruit transfer factor did decrease as the fruit developed on the tree.
However in a greenhouse experiment on orange and olive trees it was found that the transfer factors were higher. For an orange tree which had been growing on a 134Cs contaminated calcareous soil (pH of a 1:1 slurry 7.5, CEC 182 mmol kg−1, 6.8 mmol exchangeable potassium kg−1) the transfer factor (for the edible part of an orange) increased from 0.7 in the first year, to 1.6 in the second year and in the third year it was 2.8. This increase was surprising as in the vast majority of studies I have seen the transfer of cesium to plants from soil decreases with time. When the orange tree had been placed in an acidic light soil (pH of a 1:1 slurry 5.6, CEC 56 mmol kg−1, 2.2 mmol exchangeable potassium kg−1) the transfer factor was 90.6 in the third year (Skarlou, Nobeli, Anoussis, Haidouti, & Papanicolaou, Citation1999). Again with the olive trees the transfer factor for cesium from the soil to the fruit increased with time, and the transfer factors observed with the acidic soil were higher. This clearly illustrates that transfer factor will change when the soil or the species of plant is changed. The increase in transfer factor was surprising as in the vast majority of studies I have seen the transfer of cesium to plants from soil decreases with time. This is likely to be due to the gradual increase in KD as the cesium becomes more strongly bonded to the soil minerals (Sanzharova et al., Citation1994).
It is possible to change the soil by adding potassium to the soil. Currently on the Bikini Atoll 137Cs in food would be responsible for the majority of the dose which would be delivered if people were to live on the island. The 137Cs level of the trees on the island is decreasing faster than it would if the only loss of the cesium was radioactive (physical) decay (Robison, Conrado, Bogen, & Stoker, Citation2003). But the 137Cs level in the plants growing on the island is still high. One of the problems is that the islands are made of a soil which was formed from coral, this is low in potassium.
It has been estimated that in 1999 an average person living on the island would get 0.42 mSv year−1 from gamma photons from the 137mBa formed by the decay of 137Cs outside their body. If the island was cleaned up by removing soil from inside and around the houses the external dose could be decreased. The soil would have to be replaced with crushed coral or some other nonradioactive material. This would reduce the external dose from the cesium to 0.170 mSv year−1. For a person living on the island who is supplied with food imported onto the island the internal dose would be 4 mSv year−1. This dose is mainly due to ingestion and very little is due to inhalation of radioactivity. The 4 mSv year−1 dose assumes no clean-up of the island. A person living only on locally growth food would get four times as much exposure to internal cesium (Robison et al., Citation2009). The 90Sr, 239+240Pu and 241Am only make a small contribution to the effective dose which people living on the island for 30 years would get regardless of whether they ate imported food or locally grown food. If the soil removal work was done and the soil of the island was treated with potassium then the sum of the external and internal dose received by a person living on the island for 30 years would be reduced in either food scenario by a factor of about 10.
Sadly potassium treatment of soil is not a panacea for cesium contaminated land. The application of potassium has been shown to reduce the incorporation of 137Cs into coconuts (Robison et al., Citation2009), and the plants which sheep eat (Jones, Paul, & Mitchell, Citation1999). But the application of potassium to land with low potassium soil can alter the plants. In a study on the reestablishment of plants after a severe moorland fire it was found that potassium fertilizer alters the root/shoot ratio of Calluna-Vulgaris (Legg, Maltby, & Proctor, Citation1992). I can imagine that the application of a potassium fertilizer to a low potassium soil could alter the land by allowing plants which were unable to thrive due to the lack of potassium to grow much faster.
Incineration offers a means of compacting waste and a method of recovering energy from waste, after a radiological accident radioactive waste may be burnt either by design or inadvertently. A possibility exists that cesium will migrate through incinerator or power station ash. If waste or biomass from a contaminated area is used as a fuel then radioactive cesium can appear in the ash. Sadly it is impossible to give an easy answer as to what will happen, what happens depends on the waste/fuel and the form of the cesium when it enters the plant. When tree bark (either from deciduous or evergreen trees) was burnt the mass was reduced by a factor of circa 20 (Parajuli et al., Citation2013). The fly ash formed in this incineration when exposed to water released the majority of the radioactive cesium with great ease. Likewise when contaminated municipal solid waste (MSW) was burnt the fly ash formed when treated with water released its radiocesium with great ease. In contrast when sewage sludge was burnt in a fluidized bed furnace the fly ash retained its cesium when it was treated with water. Even treatment with nitric acid (0.5 M) with a liquid to solid ratio of 100:1 for 1 h at 50°C only released 14% of the radiocesium. These results with wood ash, MSW ash and sewage sludge ash strongly suggest either the fluidized bed combustor or the sewage sludge itself creates an ash which does not release cesium with ease. Parajuli et al. pointed out that the sewage sludge contained sand and soil, this might explain why the cesium behaviour was different. If the cesium had been given time to exchange with other cations in fine particles of clays then it would be in a non-water leachable form when it entered the incinerator. It is a pity that the authors did not use the same furnace type for all the wastes which they studied. The chemical form of the cesium in the ashes from MSW and sewage sludge were examined with XANES. It was found that 80% the cesium in MSW fly ash was in the form of cesium chloride while the remaining 20% was in the form of a cesium exchanged zeolite (pollucite). Two samples of fly ash from sewage sludge were examined, in one all the cesium was in the zeolite while in the other 90% of the cesium was in the zeolite and the rest was in the form of cesium carbonate (Cs2CO3) (Shiota, Takaoka, Fujimori, Oshita, & Terada, Citation2015). These XANES results support the hypothesis of Parajuli et al. that the clay did alter the cesium leaching of the ash.
When radiocesium contaminated MSW was burnt in a stoker furnace 40% of the radioactive cesium was routed into the bottom ash while the remainder was found in the fly ash. However when decontamination waste, partly composted vegetable matter and soil, was burnt the majority of the radioactive cesium was found in the fly ash. In this paper it was also found that the ashes from MSW and the decontamination waste were different. The results of X-ray fluorescence analysis indicated that the decontamination waste contained plenty of silicon and aluminium but contained very little chlorine. The MSW contained much more chlorine and calcium (Nomura, Maeseto, & Osako, Citation2017).
It was also found that it was much easier to leach cesium from the fly and bottom ashes formed from the MSW than those from burning the decontamination waste. The authors concluded that this was due to the fact that the very low chlorine content of the decontamination waste prevents the formation of cesium chloride. The cesium chloride evaporates in the heat of the furnace before condensing in the fly ash. From this work we can draw the conclusion that it is best not to burn decontamination waste with a low chlorine content together with MSW. Even if a dedicated incinerator for the decontamination waste is not available it would be better to burn the two types of waste on different days at the incinerator.
5. Iodine
As I have commented on before during a reactor accident radioactive iodine can be released first into the containment and then out of the plant. It is important that the amount of radioactive iodine be measured. The reason is that without measurement it will be impossible to know if protective measures such as sheltering, evacuation or changes in diet should be used to protect the general public. Shortly after the Windscale fire in the UK the question of how to measure and determine radioactive iodine was considered. During the Windscale fire emergency it is possible that the iodine release was underestimated. Garland and Wakefield (Citation2007) expressed the opinion that during the underestimate may have been made of the amount of radioactive iodine which was released. This was because the methods and devices used to collect the radioactive iodine were not able to capture the organic iodines.
We need to consider how do we sample the different forms of radioactive iodine. Megaw and May described two sampling devices which are filters, the simple one was two charcoal loaded filter papers separated by an asbestos filter paper (Megaw & May, 1962). This combination of three layers is able to capture iodine vapour and particles. The second sampler they described contained a millipore filter, two charcoal impregnated filter papers and a pack (25 g) of activated charcoal. The Millipore filter was shown to be able to capture radioactive particles, the charcoal papers were shown to capture elemental iodine and finally the activated charcoal was shown to capture iodine compounds (Figure ).
Figure 23. The samplers used by MeGaw and May. On the left is the simple iodine vapour and particles filter. The bold line is the asbestos paper filter while the two normal vertical lines are the charcoal impregnated paper filters. On the right is the more complex device which can provide more details of the speciation of the iodine. The dotted vertical line is the Millipore filter (particles), the two blue vertical lines are the charcoal impregnated paper filters (elemental iodine) while the charcoal pad is used to capture iodine compounds.
The more complex filter was referred to as the “May pack” by Chamberlain, Eggleton, Megaw, and Morris (Citation1963), a year later, the may pack was modified to include copper mesh disks to remove elemental iodine from the air. These copper disks were placed in front of the Millipore filter. Now with this improved design the device is able to distinguish between elemental iodine which is captured in the copper gauzes, particles are still captured in the Millipore filter. In the 1970s it was intended that a Maypack would be used to sample iodine in air in the event of a serious accident at a nuclear power plant (Macdonald et al., Citation1977) (Figure ).
Figure 24. The improved “May Pack” from 1963. The device was improved by the addition of the copper gauzes (dotted red lines) which are in front of the Millipore filter.
The “May pack” was improved further in Japan, in 1988 the improved design of Noguchi and Murata (Citation1988) was used to study the iodine from the Chernobyl accident. The design was changed two different versions were created. In one by adding a series of silver coated copper gauzes after the Millipore filter any radioactive elemental iodine formed by the decomposition of particles on the Millipore filter were prevented from reaching the charcoal impregnated filter papers. A second charcoal pad was placed behind the first one to enable a check to be made that all of the organic iodine was being captured in the first pad (Figure ).
The second version of the filter by Noguchi et al. has a Millipore filter, five silver coated copper gauzes, the two charcoal loaded filter papers and finally the two pads of activated charcoal. The second version has the advantage that radioactive particles cannot be deposited onto the silver/copper gauzes.
Using the Japanese version of the May-pack it was possible to determine that much of the radioactive iodine (131I) which travelled from Chernobyl to Japan was in the form of organic iodine compounds. The May-pack of Noguchi used charcoal which is impregnated with an amine, it is not clear at what point these impregnated charcoals replaced simple charcoals. It is possible that even the first Maypack used an impregnated charcoal but the details of the charcoal were not in the early papers. A range of substances have been tested for their ability to enable charcoal to intercept radioactive methyl iodide (Kitani, Noro, & Kohara, Citation1972). Without methyl iodide only 7% of the radioactivity was intercepted, while when DABCO was present 98% of the methyl iodide radioactivity was retained.
While this is an interesting observation it is eclipsed by a German study on the speciation of 131I, 132Te, 132I and 133I from Chernobyl over many days (Winkelmann et al., Citation1986). It is clear that the particulate/elemental/organic ratios of 131I and 133I are similar. But the behaviour of the 132I appears to be different. With time for 131I the organic iodine become more and more important in West Germany after Chernobyl, the 133I decayed away rather quickly but the 132I which is the daughter of 132Te appeared to have behaved in a very different way. When the iodine (and tellurium) was divided into three fractions (aerosol particles), elemental iodine and compounds which absorb into a charcoal then the 132I and 132Te remained mostly in the aerosol fraction.
The most likely explanation is that 132I was formed by the beta decay of 132Te, but it was trapped in the solid particles of the tellurium compound. The 131I (t½ = 8 days) is formed by the beta decay of 131Te which has a half-life of 25 min, some 18% of the 131I will be generated via 131mTe which has a half-life of 30 h. In contrast the 132I (t½ = 8 days) is generated by the beta decay of 132Te which has a half-life of 3.2 days. So while the 131I emitted by Chernobyl was released as iodine (in whatever form) the 132I in Germany was delivered there as the precursor nuclide.
In a 1973 study on the release of 131I from neutron irradiated tellurium it was shown that tellurium oxides and telluric acid (Te(OH)6) need to be heated to over 400°C to release the radioactive iodine (Shikata & Amano, Citation1973). The telluric acid decomposes in a series of reactions.
It is clear that the tellurium compounds emitted by the Chernobyl accident when they were in Germany were far too cold to release the 132I within them. I suspect that some of the 132I being measured in during the counting of the sample will have been generated after the sample had been taken. But the division of 132Te and 132I between the three fractions in the German study was slightly different. This suggests to me that some iodine was able to diffuse out of the tellurium containing particles or was otherwise separated from the tellurium.
6. Noble gases
Of all the fission products the noble gases are the most mobile, of all the reactor accidents which I am aware of the most gentle are a subset of criticality accidents. It is a sad fact that in terms of the number of deaths per joule of energy released by fission or the amount of radioactivity involved they are unusually deadly. I will not discuss the entire litany of woes associated with the 51 criticality accidents which I am aware of. But I will consider some issues associated with the 1999 accident in Japan as it include some interesting chemistry. Criticality accidents can be divided in several ways, one classification is “Process Accidents” vs “Reactor and Critical Experiment Accidents”. In a process accident there is never an intention to have a self-sustaining nuclear fission reactor while in some of the reactor and critical experiment accidents there was an intention to have some nuclear fissions. But during the accident far more fissions occurred than were intended thus resulting in the accident occurring.
I hold the view that a fine line exists between a criticality accident and a reactivity initiated accident. For example, SL1 accident, the Chernobyl accident and the 1954 explosion in the Borax reactor were all caused by an excessive nuclear fission rate in the reactors. In all three cases the reactors quickly self-terminated the nuclear reaction, steam explosions typically changed the geometry of the reactors. The January 1958 accident at Mayak was a deeply sad event, in common with the Chernobyl accident it was an experiment intended to improve safety which went horribly wrong. But we will be considering the Japanese accident in 1999. In common with the Mayak accident in early 1958 the Japanese workers decided to alter a safe system of work to make the work more convenient. In both cases people paid the ultimate price for the shortcut. But back to the chemistry.
Now as I have stated before the more volatile a fission product the greater the fraction released from an accident. If we look at the fission products observed in the air after the 1999 accident in Japan we find some rare radionuclides. We find 91Sr, 131I, 133I, 135I, 138Cs, 140Ba and 140La in air samples (Inada, Citation2000). While the release of the iodine from a solution of uranium in nitric acid is not a great surprise the release of the strontium, cesium, barium and lanthanum might be more unexpected. I hold the view that no strontium, cesium, barium or lanthanum was released from the uranyl nitrate solution, instead the noble gases 91Kr (t½ = 8.6 s), 138Xe (t½ = 14 min), 140Xe (t½ = 14 s) were emitted from the aqueous uranyl nitrate solution. The decay products (91Rb, 91Sr, 138Cs, 140Ba, 140La) of these noble gases would have deposited onto dust particles to form radioactive aerosols. I am sure that some 135Cs, 90Sr and 89Sr would have been released by the same mechanism in the form of aerosols but the activity in terms of Bq or Bq m−3 would have been much lower for these cesium and strontium nuclides as their half-lives are longer. Also 89Sr and 90Sr are pure beta emitters, to measure low levels of these a chemical separation from the other elements in the collected sample is needed. While the event in Japan was a prolonged criticality lets consider for a moment a single pulse of fission activity. We can use the Bateman equations to deal with this problem.
If we consider the decay chain 89Kr–89Rb–89Sr–89Y then if we were to have a fission event in which 235 g of 235U undergoes fission as a result of bombardment with thermal neutrons in a tank of water in which noble gases are lost by a first order process into the headspace (half-life in tank of 60 s). Then the number of atoms in the air above the liquid (headspace) can be predicted, we assume that the aerosols do not return into the liquid (Figure ).
Figure 26. Prediction of the number of atoms of different nuclides with a mass of 89 in both the liquid and the headspace above a liquid.
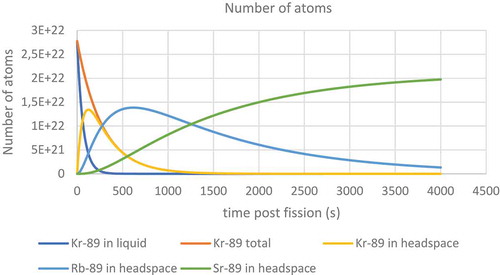
It can be seen that the majority of the 89Kr atoms become 89Sr atoms in the aerosol, but 89Sr has quite a long half-life (50 days) so the radioactivity of the 89Sr in the aerosol will not be high. While some of my readers might be thinking 3.3 PBq is rather high, I can promise you that we will consider a higher activity for a strontium soon. As can be seen in the following graph in which the radioactivity of the different nuclides are shown (Figure ).
Figure 27. Prediction of the radioactivity associated with different nuclides with a mass of 89 in the liquid and the headspace.
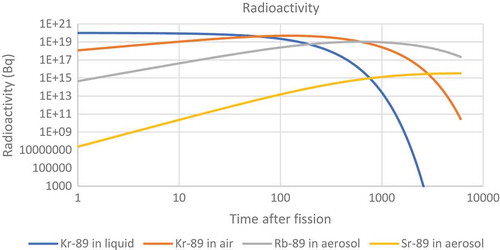
If we repeat the calculations for the decay chain 91Kr-91Rb-91Sr then at 10,000 s we have a much higher radioactivity for the strontium in the air (46.3 PBq). The graph can be seen in Figure .
Figure 28. Prediction of the radioactivity of different nuclides with a mass of 91 in both the liquid and the headspace above a liquid.
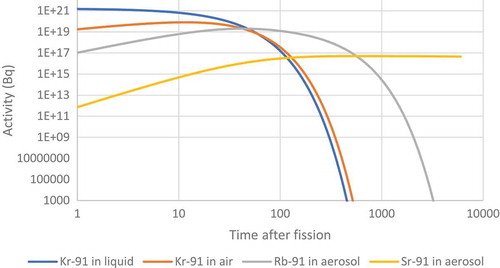
This is despite the fact that a far smaller fraction of the 91Kr atoms end up as 91Sr atoms in the aerosol. Below the numbers of the atoms at different times can be seen in a graph of Figure .
Figure 29. Prediction of the number of atoms of different nuclides with a mass of 91 in both the liquid and the headspace above a liquid.
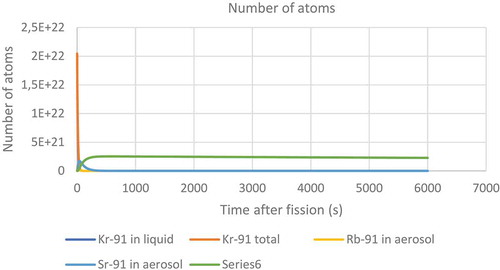
If we consider a longer lived strontium product in the 90Kr-90Rb-90Sr decay chain then we can see that more of the strontium is found in the aerosol. But the final radioactivity of the strontium in the aerosol will be much lower at “only” 9.49 TBq. If the reader wants to read more about such events then I suggest that they read the review by McLaughlin et al. (Citation2000).
7. Tellurium
During both the Chernobyl and Fukushima accidents radioactive tellurium was released. Tellurium is an element of concern as one of its isotopes (132Te) decays into a radioactive iodine (132I). When both uranium dioxide (UO2) and a mixture of uranium dioxide and graphite are heated in oxygen free helium after having been neutron irradiated then the 131I is released at about 500°C while the 132Te is released at 1,000°C. When oxygen (10−5 atm.) is present in the gas then part of the tellurium is released at 550°C while the majority is released at 1,000°C. When the oxygen level is much higher (10−3 atm or 0.1%, v/v) the tellurium is released at 550°C. In separate experiments with 132TeO2 and 132Te metal in pure helium it was shown that the 132I is released at 300°C, the 132Te from tellurium dioxide was released at 550°C and the tellurium metal released at 1,000°C (Shiba, Hanada, & Yajima, Citation1969). The results of this study suggest that under the oxidizing conditions found at Chernobyl that tellurium will be released from fuel at a lower temperature than under the more reducing conditions found during Fukushima.
The release of tellurium from used fuel depends on the oxygen concentration around the fuel pins. When zircaloy-4 is heated to 900°C in 20% oxygen in argon no segregation of tin from the zirconium is observed during the oxidation. But at higher temperatures (such as 1,300°C) tin separated from the zirconium in the oxide layer. When a sealed zircaloy tube containing tellurium, on the inner surface as Zr5Te4, was subject to prolonged oxidation (1,200°C in 20% O2 for 5 or 10 h) the remains of the tubes contained ZrO2, ZrTe3O8 and SnO2 according to powder diffraction. While a deposit of tellurium dioxide (TeO2) was found in a cold trap. When the zircaloy tube containing tellurium was subject to a shorter oxidation (3 h 20 min) under the same conditions the oxide layer was found by SEM with EDX to contain inclusions of tin with tellurium. It was reasoned that tin telluride (SnTe) was formed during the oxidation process. These tin telluride particles can be oxidized if they are heated further to form tin and tellurium dioxides according to the following equation:
When mixtures of zirconium powder (85 w/w%), tin powder (5 w/w%) and a zirconium telluride (Zr5Te4 10 w/w%) were heated at 1,000°C in mixtures of steam and hydrogen tellurium and tin telluride were found in the condensed solids while the solid remains contained zirconium dioxide (sometimes with tin dioxide). In the experiments with small amounts of hydrogen the mixture of solids emitted tellurium vapour while in those experiments in which larger amount of hydrogen were present the hot mixture of solids emitted tin telluride vapour (DeBoer & Cordfunke, Citation1997). When the conditions are oxidizing both the tin and the zirconium are oxidized and elemental tellurium is released. As tellurium is more electronegative than tin and zirconium it is reasonable that tin telluride can react with water according to the following reaction.
In another study with the same grade of zircalloy the formation of zirconium tellurides (ZrTen) was observed when the oxygen concentration was low. Here the zircalloy surface had a coating of zirconium tellurides were seen, on top of the telluride surface was observed a layer of zirconium oxide. By X-ray powder diffraction both ZrTe and ZrO2 were observed on the surface. When the oxygen concentration was higher the zirconium surface was covered only with a thin layer zirconium oxide and the tellurium was present as a vapour. In this higher oxygen level experiment alpha-zirconium and zirconium dioxide were observed by powder diffraction (Arima, Masuzumi, Furuya, Idemisu, & Inagaki, Citation2002). It is important to note that tellurium has a high affinity for the platinum group metals such as palladium which are found in used fuel. In a high burnup fuel metallic particles containing palladium and ruthenium are commonly found. These particles are known as epsilon particles, already the thermodynamics of the interaction of palladium and tellurium has been considered (Gosse & Gueneau, Citation2011). It might be possible to improve nuclear fuel by the addition of palladium, Kolarik and Renard (Citation2005) have reviewed the potential uses of the platinum group metals formed by nuclear fission. One suggestion they pointed out in the review is to use palladium (as described in a patent) (Grubb, Citation1976) to prevent fission cadmium embrittleing the zircaloy cladding. Very little cadmium is formed as a fission product from uranium, but the cadmium has a very harmful effect on the properties of the zircaloy (Shann & Olander, Citation1983). Shann and Olander also pointed out that iodine, iron iodide and aluminium iodide also increase the rate of stress corrosion cracking (SCC) of zircaloy but cesium iodide does not have a harmful effect. SCC is a corrosion process which occurs when a metal is subject to both tension and a chemical attack which causes the surface to become brittle. What happens is that the surface of the metal becomes covered with a brittle layer of a corrosion product such as metal oxide, a small crack in the surface then locally increases the stress. The corrosion product cracks at this point and when the crack reaches the ductile metal surface it stops growing. Then the chemical reaction of the corrosion converted the newly exposed metal surface into a brittle substance, then the crack can grow again thus exposing the metal surface again. This process can continue until the metal object fails. A nuclear fuel pin inside an element will be subject to tension due to the internal pressure, this can then lead to SCC.
While we can accept that cadmium metal is harmful to zircaloy cladding we need to ask ourselves the question of why is cadmium so harmful. The first reason is that cadmium is volatile, it has a boiling point of only 770°C. In a typical oxide nuclear fuel much of the uranium dioxide will be much hotter than this. As a result the fission cadmium can be driven out of the fuel pellets and into the gap between the pellets and the cladding. Secondly the cadmium is a very noble metal compared with many of the others present in spent fuel as can be seen in the Ellingham diagram (Figure ) in the paper by Cubicciotti and Sanecki (Citation1978). Thirdly exposure to cadmium together with mechanical stress can cause SCC (Cahn, Citation1977). The palladium is likely to act by forming an alloy with the cadmium and thus lowering the vapour pressure of the cadmium, this is conceptually similar to the use of zinc dust to make mercury spills less dangerous. If a wooden floor with cracks becomes contaminated with mercury, one treatment method to remediate the room is to rub zinc dust into the cracks in the floor. The zinc will then form zinc amalgam which has a lower vapour pressure than pure mercury.
Additionally palladium has a high affinity for tellurium, if a combination of tellurium and palladium powders are heated in a quartz tube it is relatively easy to form palladium tellurides (PdTe and PdTe2) (Meijer, Citation1955). In addition to altering the cadmium and tellurium behaviour the addition of palladium powder to fuel might be able to increase the thermal conductivity of the fuel. One enduring problem is that uranium dioxide has a very low thermal conductivity, one solution I once encountered was the idea of blending beryllium oxide (beryllia) into fuel (Ishimoto, Hirai, Ito, & Korei, Citation1996). I think that this is a bad idea as it would increase the chemical toxicity of fuel production and also the used fuel would emit more neutrons. A combination of an alpha emitter and beryllium generates neutrons.
Several methods exist for the separation of palladium from used nuclear fuel, recently it was suggested that a BTBP dissolved in chloroform could be used to extract palladium from nitric acid (2 M) (Zhang, Xu, & Lei, Citation2016). Zhang et al. used thiourea to strip the palladium from the organic phase, this is thermodynamically similar to the work by Emma Aneheim, myself and Christian Ekberg in which we used a different sulphur reagent (BIMET) to prevent the extraction of palladium from nitrate media by a BTBP dissolved in cyclohexanone (Aneheim, Ekberg, & Foreman, Citation2012). Having been involved with the development of BTBP as a solvent extraction reagent I am happy to see further work being done on BTBP, but I strongly believe that a solution of a BTBP in chloroform is not a suitable organic phase for the industrial scale extraction of palladium from either a fuel dissolution liquor or a PUREX first cycle raffinate. This is for several reasons.
Firstly chlorinated solvents when exposed to ionizing radiation they tend to form chloride anions, chloride contaminated nitric acid is very corrosive to SS316 which is the grade of stainless steel which much of a reprocessing plant is typically made from. Secondly chloroform is too volatile for use in a large scale industrial metal extraction process I would be more happy if they had developed chemistry using aliphatic kerosene or octanol. Lastly a cheaper extraction agent exists, both in Russia (Torgov et al., Citation1994; Torgov, Tatatchuk, Druzhinina, Korda, & Renard, Citation2000) and Japan (Baba, Eguchi, & Inoue, Citation1986) the solvent extraction of palladium using simple cheap dialkyl sulphides has been developed. The Russian process using poor quality (high sulphur) diesel fuel does appeal to me as the organic phase is particularly cheap. Other ideas for the separation of palladium separation which are based on BTBP (Zou, Liu, Ning, Wang, & Wei, Citation2017) or BTP (Liu et al., Citation2017) on silica have been suggested but again I think that it will be difficult to devise a process based on silica which is better than the Russian solvent extraction system.
An alternative method of avoiding the dire effect of cadmium on the zircalloy would be to use some other cladding, a range of alternative materials (fuel and cladding) have been considered for use in more accident tolerant fuel (Zinkle, Terrani, Gehin, Ott, & Snead, Citation2014). I will not attempt to discuss all of these suggests in turn but it is noteworthy that silicon carbide has been considered as an alternative to zircalloy (Kim, Kim, & Park, Citation2013). Silicon carbide has already been used in pebble bed reactor fuel, in the pebbles each contain a large number of small spheres of fuel which have several layers of coating including one of silicon carbide. The diffusion of both iodine and silver through silicon carbide has been studied. At 1,100°C very little diffusion occurred (diffusion coefficients below 10−21 m2 s−1) but at higher temperatures diffusion of these elements was observed (Friedland et al., Citation2011). One of the reasons for replacing zirconium cladding in nuclear reactors is to design out of the reactor two chemical reactions. The first is the zirconium cladding/fuel interaction which can generate additional heat during an accident but lower the melting point of the uranium fuel.
The second reaction is the zirconium/steam reaction which can generate both heat and hydrogen gas.
For silicon carbide it is possible to react it with hot (1,000–1,300°C) steam according to the following reaction (Cheng & Tortorelli, Citation2013).
We can calculate the relative heat yield for zirconium and silicon carbide using Hess’s law. According to Chase (Citation1998) and Cox, Wagman, and Medvedev (Citation1984) at 25°C and 1 atm the heats of formation of the important substances are listed in Table .
Table 18. Heats of formation.
It is clear that the oxidation of silicon carbide will release far less heat per mole than that of zirconium. The density of silicon carbide is 3.16 while that of zirconium is 6.52. Thus one litre of silicon carbide is 78.8 mol of silicon carbide while one litre of zirconium is 71.5 mol. Thus if the zirconium in the cladding in the reactor was replaced with the same volume of silicon carbide then during an accident far less heat would be generated by chemical reactions in the core. We can also calculate the heats of the following reactions:
It appears with the silicon carbide cladding that fuel cladding interactions like those of uranium dioxide and zircalloy would not occur during an accident. The formation of carbon monoxide is something which needs to be considered for at least two reasons. Firstly in modern water cooled reactors it is normal to include hydrogen recombiners, one common type is the catalytic type using finely divided platinum group metals to cause a flameless combustion of the hydrogen to form water. The carbon monoxide could poison the surface of the catalysts. It is well known that the platinum electrodes used in fuel cells for the oxidation of hydrogen gas are poisoned by carbon monoxide (Jambunathan, Shah, Hudson, & Hillier, Citation2001). While carbon monoxide was found to inhibit the oxidation of hydrogen by oxygen on the surface of a palladium/platinum alloy dispersed on either stainless steel (SS316) or the silicate mineral cordierite, it was found that the poisoning by carbon monoxide was not total. The catalyst using the cordierite was still able to oxidize hydrogen when the carbon monoxide concentration was 1,000 ppm (Sanap, Varma, Waghmode, & Bharadwaj, Citation2015). As a small amount of carbon monoxide is unable to strongly inhibit the removal of hydrogen from the air in a reactor building using passive recombiners I do not think that a core loaded with a mixture of zircalloy and silicon carbide clad fuel is more likely during an accident to lead to hydrogen explosions like those seen at Fukushima than a reactor loaded only with zircalloy clad fuel. I would like to point out that the Fukushima plants were not equipped with passive recombiners, if they had been present in the reactor hall then the hydrogen-air explosions are unlikely to have occurred.
Secondly if elemental iodine is released into an oxygen containing environment then the radiolysis of the mixture would generate iodine oxide aerosols. This conversion of elemental iodine into areosols could reduce the amount of iodine which escapes from a damaged plant. However if carbon monoxide is released into the containment and reactor building of a stricken plant then it could well reverse the radiolytic oxidation of iodine into iodine oxides. It is well known that iodine(V) oxide reacts with carbon monoxide to form elemental iodine (Adams & Simmons, Citation1951).
But back to tellurium chemistry, once the tellurium escapes from the fuel in the reactor and leaves into the containment. It was suggested that the tellurium could form hydrogen telluride (Beahm, Citation1987). The heats of formation of the chalcogen hydrides decrease going down the oxygen group. As hydrogen telluride is an endothermic substance with a heat of formation of 99.7 kJ mol−1 (Chattopadhyay & Juneja, Citation1993) I hold the view that it will not be thermodynamically stable in the containment. As the heats of formation of water vapour and tellurium vapour (Te2) and at 25°C are −241.818 and 163.176 kJ mol−1 it is possible to calculate the enthalpy of the following reaction to be −519.9 kJ mol−1
As the reaction below has the reaction enthalpy of −272.8 kJ mol−1 I think it is reasonable to assume that hydrogen telluride (or tellurided hydrogen) will react with the air to form tellurium dioxide which will condense into solid tellurium dioxide.
The problem with thermodynamic instability is that countless examples of materials and systems exist which are thermodynamically unstable relative to their decomposition products. For example acetylene, picric acid and trinitrotoluene (TNT) both all decompose in exothermic reactions without any oxidant present while cotton and other cellulose clothing in air is thermodynamically unstable when compared with the combustion products. Plenty of soldiers walk around while carrying explosive products (briquettes of TNT, and grenades filled with picric acid) while wearing clothing made of cotton and other forms of cellulose without spontaneously exploding or bursting into flames. While oxy-acetylene welding has been partly replaced by methods such as MIG (Metal In Gas), I know civilian (and I assume military) motor mechanics often store acetylene in cylinders normally without explosions or other misadventures. By the way acetylene cylinders normally contain a solution of acetylene under moderate pressure in acetone dispersed on a porous solid.
Sadly while I have been unable to find papers on the mechanism and kinetics of the reaction of hydrogen telluride with common oxidants such as ozone and nitrogen oxides, I have been able to find papers on the subject of the reactions of hydrogen sulphide and hydrogen selenide with such gases.
It has been reported that hydrogen sulphide reacts with the hydroxyl radical in the air to form the HS∙ radical according to the following reaction:
The fate of the resulting HS∙ radical has been considered by a computational study (Resende, Citation2007) which suggested that nitrogen dioxide was the substance most likely in the air to react with this sulphur centred radical. The reaction would be one of three reactions:
Of these Resende predicted that the first one forming HSO and NO would be the most favoured reaction. Son et al. in their work on the destruction of sulphur compounds in air by electron beam irradiation stated that the HSO radical reacts with ozone to form a series of new species (HO∙, SO, HS∙, HSO2∙ and O2). Through further reactions these form sulphur dioxide, sulphurous acid and sulphuric acid (Son, Jung, Lee, Koutrakis, & Kim, Citation2015). It has also been shown that nitrogen dioxide can react with the HSO radical to form the HSO2 radical. The HSO2 radical then reacts with oxygen to form sulphur dioxide and the HOO∙ radical according to the following equations (Lovejoy, Wang, & Howard, Citation1987):
I reason that if a mechanism exists for rapidly converting hydrogen sulphide to sulphur dioxide with ozone and nitrogen oxides, then it is likely that hydrogen telluride is also likely to be converted quickly into the oxide if it was to form in a reactor building. The reason why ozone and the nitrogen oxides are important is that during a reactor accident a large amount of radioactive noble gases are released into the containment. The action of these radionuclides on the air then generates air radiolysis products which include ozone, nitrogen oxides and hydroxyl radicals.
A wide range of studies have been done on the chemical effect of radiation on air, for example radon has been shown to generate HO∙ and HO2∙ radicals when humid air is exposed to the alpha particles (G for the formation of HOx∙ is 7.86 ± 0.13 radicals per 100 eV of energy) (Ding & Hopke, Citation1993). Also nitric acid formation has been observed in a proton accelerator facility used for particle physics experiments in Japan (Kanda, Miura, & Nakajima, Citation2005). This nitric acid formation was explained by Kanda et al. by reference to the work of Willis et al. here a series of reactions form nitrogen oxides through a vast web of reactions which start with the N2+ formed by the ionization of nitrogen molecules are considered (Willis, Boyd, & Young, Citation1970). These reactions include.
The nitric oxide thus generated can then go onto form other nitrogen oxides and nitric acid if water is present. The formation of nitric acid, nitrous acid and a series of nitrogen oxides in a high energy electron—positron (antielectron) facility has been reported and a series of equations offered which explain the changes in concentration of these air radiolysis products as a function of time (Kanda, Momose, & Taira, Citation1996). These equations are likely to be of great use for considering the formation of nitrogen oxides and nitrogen oxoacids in an air filled containment during an accident.
When oxygen is irradiated it has been shown that ozone is formed (Willis, Boyd, Young, & Armstrong, Citation1970). Back to reactor accidents it has been argued that the air radiolysis products can alter the iodine chemistry (Bosland, Funke, Girault, & Langrock, Citation2008). In Finland a large amount of work has been done at VTT on this issue, for example Karkela et al. (Citation2015) exposed methyl iodide to beta particles in an oxygen containing environment. They found that the volatile methyl iodide was converted into non-volatile iodine oxide particles. With a knowledge of the results of the Finnish experiments it is reasonable to me that hydrogen telluride will be oxidized if it is in air to which a large radiation dose is delivered.
8. Ruthenium
The releases of radioactivity from a reactor accident are largely controlled by the volatility of the element and its simple compounds. However in the case of Chernobyl a large amount of ruthenium was released. The NEA (Nuclear Energy Agency, part of the Organisation for Economic Cooperation and Development [OECD]) report (Métivier & Waight, Citation2002) on page 35 in chapter 2 provides a table of how much of each of a series of key radionuclides was released into the air. It is very interesting that more than 3.5% of the 99Mo, 103Ru and 106Ru in the core inventory was released. From the releases of lanthanides and actinides it can be estimated that 3.5% of the fuel in the reactor was ejected into the air. But the great question is why was more ruthenium than cerium/plutonium was released?
It is commonly accepted within the nuclear community that under oxidizing conditions that ruthenium is released from fuel under oxidizing conditions. It is likely that the ruthenium forms the volatile tetraoxide (RuO4) which then migrates within the nuclear power plant. Particles with very large amounts of ruthenium were observed after Chernobyl which contained few other radionuclides in large amounts (Sandalls, Segal, & Victorova, Citation1993). It is important to note that some ruthenium hot particles have been examined years later and were found to contain 60Co and 63Ni (Kleszcz & Mietelski, Citation2010), this suggests to me that these particles may have been formed from stainless steel which was in or close to the core of the nuclear reactor. In addition to the 60Co and 63Ni the gamma emitter 125Sb has been observed in these hot particles (Broda, Mietelski, & Sieniawski, Citation1992).
Recently a PhD was defended at Chalmers on the subject of ruthenium chemistry under the conditions of a serious nuclear accident. During this study it was found that ruthenium tetroxide reacts with a range of surfaces (zinc, copper, aluminium and epoxy paint), using EXAFS it was determined that the ruthenium on these surfaces was in the form of the dioxide (either tetragonal or orthorhombic) (Kajan, Lasseson, Persson, & Ekberg, Citation2016). This observation of ruthenium dioxide is not unreasonable as the tetroxide is a strong oxidant which is able to oxidize many organic substances. Osmium tetroxide is well known as an oxidant which is able to convert alkenes into 1,2-diols. The ruthenium compound is a stronger oxidant which is able cleave alkenes. The conversion of the tetroxide into the dioxide is in agreement with the hypothesis that ruthenium vapour deposits onto existing particles and surfaces which are present during an accident. However care should be taken with chemistry which is done using high concentrations of ruthenium tetroxide, computational chemistry suggests that ruthenium tetroxide reacts with alkenes to form a five membered heterocycle in a [3+2] cycloaddition (Frunzke, Loschen, & Frenking, Citation2004). This then reacts with a second alkene in another cycloaddition to form a spiro compound. This is then oxidsed by more ruthenium tetroxide to form a Ru(VII) compound which is able to decompose into organic carbonyl compounds. This idea that after the initial reaction of the ruthenium tetroxide with the surface that a reoxidation with more ruthenium tetroxide is required for some oxidation reactions to occur suggests that the chemistry of ruthenium tetroxide with a surface may be dependent on the amount of ruthenium present. It is important to note that the reaction of ruthenium tetroxide with water to form ruthenium dioxide is catalysed by the surface of ruthenium dioxide (McMurray, Citation1993). It is also important to note that not all surfaces have the same ability to react with ruthenium tetroxide, it was found that metal oxide surfaces, borosilicate glass and quartz are more reactive towards ruthenium tetroxide than clean metal surfaces (Swider-Lyons, Love, & Rolison, Citation2005).
The oxidation of ruthenium or molybdenum metal in the presence of cesium iodide has been reported to result in the formation of elemental iodine according to the following equation (DiLemma, Colle, Benes, & Konings, Citation2015).
It is interesting that if iodine has been deposited on a surface that it increases the absorption of ruthenium, delivered as ruthenium tetroxide vapour, onto the surface (Kajan, Tietze, & Ekberg, Citation2016). Nitrogen oxides were found to increase the mobility of ruthenium as a vapour through an air filled tube furnace, it was argued that nitrogen dioxide is able to oxidize species such as ruthenium trioxide to ruthenium tetroxide (Kajan et al., Citation2017).
9. Silver
The origin of the silver in the Chernobyl fallout has been the subject of a lively debate in the letters pages of Nature (Flowers, Citation1986; VanDam, Citation1986). In the fallout from both Chernobyl (Papastefanou, Manolopoulou, & Charalambous, Citation1988) and Fukushima (Yu et al., Citation2015) some radioactive silver was observed, two sources of radioactive silver exist in a nuclear reactor firstly it is a very minor fission product. It is important to keep in mind that when uranium or plutonium undergoes fission the nucleus splits into a small and a larger fragment. In terms of fission yield for these actinides a peak occurs around zirconium/ruthenium and a second peak occurs in the early lanthanides. It is interesting to note that as the energy of the nucleus which fissions is increased the fission becomes more and more symmetric. When the lower energy excited state of 236U is replaced with the higher energy excited state of 240Pu the fission yield in the gap between the two peaks (around silver and cadmium) increases.
The second source of radioactive silver is as an activation product, silver is sometimes uses in control rods and other equipment which is used inside nuclear reactors. Due to its very noble nature silver is more likely to exist in the elemental form than cesium. However the boiling point of silver is 2,162°C which is much higher than that of cesium iodide (1,280°C). The boiling point of silver iodide is 1,506°C while this is higher than that of cesium iodide it is lower than that of elemental silver.
The radioactive silver from Fukushima along with 90Sr, 110mAg, 134Cs and 137Cs (58Co and 60Co below detection limits) was measured both in seawater and squid was measured by Yu et al. while these radionuclides were detectable the level of these were too low to have a significant adverse effect on the population of marine animals. Radioactive silver was also found in spiders (Nakanishi et al., Citation2015), it was found that the spiders contained more radioactivity (Bq per kilo) than soil which was surprising as silver is not considered to be an essential element for spiders.
While 110mAg was measured in the spiders the paper did not contain an assessment of the possible health effects of a person being bitten by a radioactive spider. I hold the view that it is unlikely that the radioactivity of a spider contaminated by Fukushima will contain sufficient radioactivity to be of concern to a human. The most radioactive spider in the study contained 2,866, 11,464 and 17,445 Bq/kg of dry mass of 110mAg, 134Cs and 137Cs. The spider in question was an Atypus karschi. So far I have been unable to find out the mass of this spider but I expect it would be smaller than the Goliath Birdeater (Theraphosa blondi) which has a mass of 175 g. If we were to be sufficiently unlucky that the spiders in Japan were to grow to the size of the alleged birdeater from South America and to become aggressive to humans then we can calculate the radiological consequences of a spider bite.
A spider with a mass of 175 g and a specific activity of 17,445 Bq per kilo of dry mass would contain 3,053 Bq of 110mAg if we assumed that the dry mass was the same as the mass of the living spider. This I imagine is clearly impossible as a totally dehydrated spider would be unable to live. Now if the spider was to bite some unlucky person and to be able to inject all its inventory of radioactive silver into that person then using the ICRP 119 dose coefficients (Eckerman, Harrison, Menzel, & Clement, Citation2012) then the dose to the unlucky person will be 0.171 mSv. This is a small dose. I worked it out by assuming that an injection (spider bite) would be a 20 times higher a dose per Bq as the f1 value for silver listed was 0.05. As the dose to the unlucky person who was to be bitten by a spider is far below 1 mSv then I think we do not need to worry about 110mAg containing spiders biting either workers or members of the public. I also considered a person who eats a “spider burger”, if we assume that a spider burger contains 250 g of spider meat which will contain 4,361 Bq of 110mAg, then each spider burger will result in a person getting a 12.2 µSv dose. Thus a member of the public could eat 82 spider burgers made using the most radioactive spiders mentioned in the paper per year before they would reach an additional dose of 1 mSv in 1 year as a result of the consumption of this rather bizarre food.
While the fission products are normally discussed in this review in the context of the threat which they pose, we should consider how some fission products have a beneficial effect. In high burnup fuel research has suggested that palladium and silver could be able to sequester iodine under accident and repository conditions (Buck, Mausolf, McNamara, Soderquist, & Schwantes, Citation2016).
10. Strontium
In the immediate aftermath of a nuclear accident such as Fukushima the gamma emitting radionuclides such as 131I, 132Te, 132I, 134Cs, 137Cs, 140Ba and 140La can be measured by means of gamma spectroscopy. While the measurement of gamma emitters is not a trivial matter it is possible to make the measurement without doing any radiochemistry or much of another form of sample preparation. On the other hand the analysis of the pure beta strontium radionuclides is rather more complicated. As a gamma photon is emitted by a nucleus it is a two body system, as the energy of the excited state is well defined, and the energy of the state the nucleus is relaxing into is also well defined the gamma photons for a given transition are monenergistic. However when a beta decay (electron emission) occurs the energy is shared between the daughter nucleus, the electron and the neutrino. As the angle between path of the electron and the neutrino is variable the relative share of the energy taken away by the electron is not well defined. Beta decay spectra tend to be broad smears instead of the sharp peaks seen in alpha and gamma spectra. Even if the radioactive decay occurs within a detector which would be able to sense the sum of the energy of the recoiling daughter atom and the electron the fact that the amount of energy taken away by the neutrino varies will make the beta spectrum a board peak rather than a sharp set of lines.
It is often important to separate the strontium from the other elements in an environmental sample before the strontium is measured. For example soil can be digested in acid before the solution of the strontium (and other elements) in nitric acid (8 M) is applied to a polymer resin bearing 40% (w/w) of a 1 M solution of 4,4ʹ(5ʹ)-di-t-butylcyclohexano 18-crown-6 (crown ether) in octanol. The resin column was first washed with nitric acid (8 M), nitric acid (3 M) with oxalic acid (50 mM) and finally the strontium was eluted with dilute (50 mM) nitric acid. The resulting strontium solution was measured by means of liquid scintillation counting (LSC) (Sahoo et al., Citation2016). The separation of the strontium using such a resin (Horwitz, Dietz, & Fisher, Citation1991), the chemistry of the separation is very similar to that of the SREX solvent extraction process for the removal of strontium from liquid radioactive waste (Wood & Law, Citation1997). While the resin is able to reject calcium, sodium and cesium it has a very high KD value for lead. Thus any 137Cs will be eluted quickly through the column while any 210Pb in the sample will be retained by the resin. When the nitric acid concentration is 1 M the KD value of polonium(IV) is about 100, when the nitric acid concentration is 10 M the KD for polonium is about 1. Thus much of the 210Po in the sample will never adsorb onto the column, that which is absorbed is likely to be washed out by the nitric acid (8 M) washing (Horwitz, Chiarizia, & Dietz, Citation1992). Thus with this resin it is possible to separate the strontium from many of the radionuclides which could interfere with the final LSC measurement.
The methods of separation of strontium and the measurement of radioactive strontium have been reviewed (Vajda & Kim, Citation2010). Much of this review is very good but I hold the view that the statement that “Couple of years after a nuclear incident 89Sr, 90Sr, 90Y and 91Y will be present together in the environment” which appears in the review is misleading.
If we consider the case of Chernobyl, reactor 4 at the moment of the accident is thought to have had an inventory of 2,300 PBq 89Sr and 200 PBq 90Sr, of which circa 5% was released. If were to have assumed that the reactor had been running at a constant power before the accident then it is quite easy to calculate the activity of 91Sr which would have been present. To a first approximation the number of atoms of a given activation or fission product (N2) are given by the following equation while the fuel is in the pile. Where N1 is the number of atoms of the target, R1 is the rate constant for the generation of the atoms of N2 in the neutron field, λ2 is the decay constant of N2 and t is the irradiation time.
If we assume that only one fissile nuclide is present then the equation can be used, in real life a reactor fuel contains both 235U and 239Pu. Much of the energy released by the fuel in a reactor is due to plutonium fission. If we assume that thermal neutrons are responsible for all fissions then the equation can be kept simple. It can be calculated that the fuel would have been in the pile for about 1,100 days and it would have contained 2,787 PBq of 91Sr and 91Y at the moment of the accident.
We can add a term to our equation to consider the decay after an object comes out of a nuclear reactor. Now we have two times, treactor is the time in the reactor while tcool is the cooling time after it comes out of the reactor.
Assuming that at tcool = 0 we have 2,300 PBq 89Sr, 200 PBq 90Sr, 2,787 PBq 91Sr and 2,787 PBq 91Y then after 2 years (730 days) we would have 112 TBq 89Sr, 190 PBq 90Sr, no 91Sr and 522 TBq 91Y. While the 89Sr and 91Y might be possible to measure, their effect on a measurement of 90Sr after 2 years would be very small after a reactor accident with relatively old fuel such as Chernobyl. On the other hand after an accident with a younger fuel (less time in the pile) such as submarine accident at Chazhma Bay near to Vladivostok in August 1985 would have a 89Sr:90Sr:91Y ratio much closer to that of an atomic bomb. Shortly after a reactivity spike in fresh fuel I would expect an activity ratio of 166:1:174 for these radionuclides.
The alternatives to the use of the crown ether bearing resin include precipitation. One method is to use nitric acid to form solid strontium nitrate while the calcium nitrate remains in solution (Willard & Goodspeed, Citation1936). To my mind this method sounds rather disagreeable, it has the potential to generate large amounts of acidic waste.
It is important to note that barium chromate and lead chromate both have much lower solubility products than that of strontium chromate. This offers a means of removing any 140Ba which might be present in a sample of 89Sr and 90Sr from a reactor accident.
11. Actinides and lanthanides
Of all the elements plutonium provokes great public excitement and horror. It is interesting to note that plutonium is poorly adsorbed when swallowed in food, water or mucus. For example when humans in 1995 ate cockles from the Irish Sea which contained plutonium, americium, cobalt, cesium and technetium (as a result of discharges from Sellafield) very little radioactivity was incorporated into the people (Hunt, Citation1998). Measurements were made of faeces and urine to determine what fraction of the radioactivity in the meal of cockles (375 g) was adsorbed by the digestive system into the body. It was found that for plutonium on average only 0.34% of the plutonium was adsorbed by the human digestive system. The highest f1 value observed was 7 × 10–4 which indicated for one person that 0.7% of the plutonium was adsorbed from the digestive system. This study suggests that even soluble plutonium is poorly adsorbed by humans. The measurements for americium were also reassuring, the average f1 value was 5.4 × 10–4 suggesting that only 0.54% of the americium swallowed was adsorbed.
However the f1 values for 60Co and 99Tc were about 0.2 (20% adsorbed) and 0.6 (60% adsorbed) suggesting that one cannot always eat radioactive cockles with total impunity. One interesting observation in the study was that the transfer factor for 137Cs was 0.21 thus indicating that the adsorption of cesium from the digestive system is not total. The number of subjects in this experiment was small and it is not clear what the chemical form of the cesium contamination of the seafood was. If the cockles had contained small particles of clay minerals on which were adsorbed cesium then this could well reduce the adsorption of the cesium into the body.
It is well known that many samples of plutonium are in the form of plutonium dioxide, I hold the view that the f1 values for plutonium dioxide will be lower than plutonium in seafood. As plutonium dioxide is exceptionally insoluble, I reason that it will not dissolve in the stomach or in lung fluid. Instead of being a threat when humans are exposed in their diet I reason that it is more able to pose a threat when it is inhaled. The insoluble particles could remain in the lungs for some time where they are then able to deliver a dose of alpha particles to the lung tissue. I reason that injections of plutonium are exceptionally rare, I am aware of some ethically questionable human experiments and some workplace accidents where different forms of plutonium have been injected into humans.Footnote3 We will concentrate on the threat posed by inhaled plutonium. Greenpeace have made the following bold statement about it (Greenpeace, Citation2006).
“Inhalation of a single microgram of plutonium, smaller than a speck of dust, can cause fatal lung cancer. There is no safe dose of exposure for humans, and once it is inside the body, it will remain there for a very long time—longer than the average human life span.”
I will subject the statements in this paragraph of text to a detailed analysis, but before we go any further it is interesting to consider the number of pages within the domain of the environmental organization (www.Greenpeace.org) which mention plutonium compared with some other elements. A random list of elementsFootnote4 including plutonium was made, when a series of internet searches were made for Greenpeace pages mentioning these elements a total of 103,684 pages were found, the search for plutonium yielded 2,510 pages (2.4%) which is higher than the average for the elements which was 2.0%. You might be interested to know that 30,600 pages (29.5%) mentioning carbon were found in this search. While this experiment is far from perfect it does indicate that some elements are mentioned more than others by Greenpeace and that the word plutonium appears on more pages than if the mention of elements was totally random.
I have to ask the question of “can a single microgram of plutonium induce lung cancer”. I have to conclude that the answer is yes but the statement is misleading. A single atom of plutonium could in principle induce cancer if it was to undergo a radioactive decay when it was in the wrong place at the wrong time. But this says nothing about the likelihood that a single microgram (or single atom) will induce cancer.
Using recent dose coefficients from the ICRP (Eckerman et al., Citation2012) I calculated that 1 µg of plutonium (239Pu) in the form of a powder of a substance (1 µm particles) which slowly transfers into the blood (class S) will deliver an effective dose of 34 mSv to a person. If the material is in the form of a substance which transfers into blood at a moderate rate (class M) then the committed dose due to this 1 µg of plutonium will be 108 mSv. While these doses are not trivial, these doses are far below the doses which would be required to have at least a 50% chance of inducing cancer. I expect that if a person was to get a 108 mSv effective dose then they would have an addition 0.5% chance of developing cancer. As a result I think that the first statement while technically being true is one which is misleading.
A great problem is that very few samples of plutonium only contain a single isotope of plutonium, for example I saw a paper on a release of weapons grade plutonium (238Pu 0.0099% w/w, 239Pu 93.7% w/w, 240Pu 5.6% w/w, 241Pu 0.47% w/w) (Lee & Clark, Citation2005). Then it can be calculated with the same ICRP coefficients it can be seen that bomb grade plutonium is slightly more toxic than pure 239Pu plutonium. The committed effective dose from the inhalation of class M bomb grade plutonium in the form of 1 µm particles will be 141 mSv. Even while it is higher than the dose of 108 mSv it is not a dose which has a high probability of causing cancer or a serious disease. I repeated the calculations with a reactor grade plutonium isotope signature (238Pu 2.947% w/w, 239Pu 42.401% w/w, 240Pu 33.562% w/w, 241Pu 10.372% w/w and 242Pu 10.715% w/w) (Serrano-Purroy et al., Citation2005). I choose a very poor quality reactor grade plutonium which would be very unsuitable for building a bomb, it is important to bear in mind that a range of plutonium isotope signatures can be found both in “weapons grade” and “reactor grade” plutonium. The committed effective dose from 1 µg of this plutonium in 1 µm particles of the M type is 1,318 mSv. While this is a much larger dose than can be expected with a weapons grade plutonium it is important to note that this dose increases a person’s lifetime risk of cancer by 6.6% which is a dose which I would strongly wish to avoid but it does not make death by cancer a certainty. I note that much of the alpha activity in the reactor plutonium is due to 238Pu which has a relatively short half-life.
Helen Caldicott made an even more bold statement, she claimed that inhalation of less than a single microgram of plutonium will induce lung cancer in her book (Caldicott, Citation2006). Sadly this statement does not have a reference associated with it, and she makes some further claims about plutonium and other elements. I find it interesting that during her public debate with George Monbiot she told him on several occasions to read one of her books. The problem I see is that if her books are poorly referenced and she has not generated any results through her own research thus making her a poor source. For various reasons I am unable to repeat all the research mentioned in this review, but I make a point of providing reliable references to support the ideas which are presented in this review. I also would like to stress the importance of checking your references, where possible I tend to read more than one paper on a subject and when I do calculations if I obtain an unusual result I check my work with great care.
Helen continues to state that white blood cells translocate plutonium from the lungs. I am aware that macrophages do engulf and attempt to digest foreign bodies and microbes, some literature does exist on plutonium and macrophages. In the 1960s, it was shown by Sanders and Adee (Citation1968) in rats that macrophages present in the lungs do engulf and accumulate fine particles of plutonium dioxide. So on the issue of white blood cells Helen is right, but I do so wish she would reference her books better. It is interesting that chronic exposure to cigarette smoke reduces the clearance of plutonium dioxide from the lungs of rats (Finch et al., Citation1998), which is consistent with the observation that smoking harms the ability of the body to remove dirt from the lungs.
Helen also states that plutonium can migrate into the lymph nodes, this is true for example recently it was shown using tissue from a former nuclear worker, who had been heavily exposed to plutonium, by laser ablation inductively coupled plasma mass spectroscopy (LA-ICPMS) that plutonium can be found in lymph nodes (Hare et al., Citation2010). Helen next states that plutonium causes lymphoma or leukaemia. In the Mayak workers plutonium exposure is associated with lung, liver and skeletal tumours but not with leukaemia (Shilnikova et al., Citation2003). In that population no statistically significant link exists between plutonium exposure and either lymphoma or leukaemia (Kuznetsova, Labutina, & Hunter, Citation2016).
She also makes the bold claim that plutonium is appearing in the testicles of men and then harming the health of their offspring. Using urethane and X-rays Nomura did observe an excess of tumours and abnormalities in mice as a result of preconception exposure of both male and female mice (Nomura, Citation1982). Likewise with neutron irradiation it has been shown that the offspring of the irradiated male mice have shorter lifespans (Russell, Citation1957). The male offspring of male mice irradiated with fission neutrons from 252Cf have a higher rate of liver cancer than the male control mice (Takahashi, Watanabe, Dohi, & Ito, Citation1992). It is interesting to note that these neutrons are more able to induce liver cancer in mice than 60Co gamma rays.
But with plutonium different results were obtained, male mice who had been injected with plutonium citrate (3 or 40 mGy testicle dose) after mating (8–10 weeks later) with female mice had offspring who had no excess of leukaemia or other diseases (Selby & Priest, Citation2005). Some attempts to repeat Nomura’s observation have failed to observe an excess of disease in the offspring, for example when male mice were treated with 600 R of X-rays, these male mice went on to father offspring which were no different to unirradiated mice (Cosgrove et al., Citation1993). When Cattanach et al. (Citation1995) investigated the incidence of lung cancer in the offspring of male mice irradiated with 0, 2.5 or 5.0 Gy of X-rays they failed to find evidence that the preconception irradiation harmed the offspring. In one early US study by what I would describe as very patient workers, it was found that neither 10 or 35 inbreed generations (brother/sister matings) in which all the males were irradiated with 200 rads of X-rays caused a shortening of the lifetimes of the females when they were compared with a control experiment using no irradiation (Spalding, Brooks, & Tietjen, Citation1969).
But in another study (Lord et al., Citation1998) it was found that male mice injected with 128 Bq g−1 of 239Pu (as the citrate) these male mice had offspring who were more sensitive to the induction of cancer when they were exposed to N-methyl-N-nitrosourea. However male mice injected with 256 Bq g−1 of plutonium citrate had offspring who did not clearly show an increased sensitivity to the chemical carcinogen.Footnote5 However with exposure to urethane Cattanach et al. (Citation1998) were able to see little or no effect of preconception irradiation of male mice on the incidence of lung tumours in their offspring (Table ).
Table 19. Details of rodent plutonium injection experiments.
However with tritium (HTO) exposed male mice it has been found that an increase of the leukaemia rate in their offspring does occur (Daher, Varin, Lamontagne, & Oth, Citation1998). Thus it does appear that contradictory results on preconception irradiation exist in the literature. It is interesting that a defect may exist in the transgenerational carcinogenesis experiments using radiation, with a high dose of radiation which appears to result in unhealthy offspring the high dose of radiation decreases fertility. Thus the litter of mice is smaller, the young mice have higher body masses and a high body mass in mice is linked to an increased rate of cancer (Selby, Earhart, & Raymer, Citation2005). As a result I would be very careful not to make a claim that plutonium is harming human health by damaging the testicles of men.
In the case of the mice the internal exposure of their fathers to plutonium may have altered their sperm in some way that their offspring were more susceptible to cancer. Sadly there are people who have defective copies of some genes which predispose them to cancer, for example the actress Angelina Jolie has a defective BRCA1 gene which causes her cells to be less able to repair defective DNA. But I find it hard to imagine that the random DNA damage by the plutonium would have a high probability of damaging a given gene. But we could imagine that if a series of n changes are needed to the DNA of a cell to create a cancer cell that if a damaged sperm cell was fused with an egg to form an embryo that the damaged sperm could pass on some damaged dominate gene which promotes the formation of cancer. This would then appear in every cell of the animal which is born, but these changes might only matter in some tissues. But overall it is not clear that this works in mice and the birth number/weight effect might create some false positives.
I am aware of a person who lived for almost 40 years after being exposed to an aerosol of acidic plutonium(IV) nitrate solution. This person at autopsy had 0.83 Bq of plutonium in his testicles, while he had 937 Bq in his liver, 1,170 Bq in his skeleton (Total body burden of 2,317 Bq from an intake of 58,000 Bq) (Nielsen et al., Citation2012). This suggests that very little of the water soluble plutonium was deposited in his testicles.
The second statement by Greenpeace that “There is no safe dose of exposure for humans” is an interesting statement. If we assume that the LNT model applies to the induction of serious diseases by radiation then it can be easily argued that while any exposure to plutonium has the potential to cause a harmful effect. Some very small exposures cause such a small harmful effect that they should not be of concern to either a person or a regulatory body. I am unsure if it is possible to die as a result of the acute effects of a large number of paper cuts but I can imagine that it might in theory be possible to induce a serious disease by suffering a papercut while handling some unpleasant substance. I have to ask if it is reasonable for the citizen, regulatory bodies and industry to attempt to take steps to prevent some horrible but exceptionally unlikely event. For example while either reading a printed copy of this article or a pamphlet from an environmentalist a person might cut their hand and infect themselves with some unusually harmful microbes which then cause some horrible outcome. I hold the view that some risks are sufficiently small that they can be ignored.
The last statement is also interesting, the short paragraph includes the text “once it is inside the body, it will remain there for a very long time—longer than the average human life span”. The first problem is the question of “what is inside the body”. From an anatomical point of view the contents of the digerstive system is outside the body as is the air which is inside the lungs. However for many people after an item is swallowed or a substance inhaled it is regarded as being inside the body. I sincerely wish that Greenpeace’s writer had been more clear on what is “inside the body”. I know from cases in which people have been injected with plutonium that the plutonium can remain inside a person for a very long time. For instance at Hanford in 1985 a man accidently cut himself while working with plutonium, he injected himself with 48 kBq of alpha activity (mainly 239/240Pu and 241Am) (Carbaugh, Lynch, Antonio, & Medina-Del Valle, Citation2010). By means of surgery the long-term activity in the wound was reduced to 5.4 kBq, and using DTPA (Diethylene Triamine Penta Acetic acid) it was possible to remove circa 7 kBq of alpha activity was removed from the man but much of the alpha activity remained inside the man. Despite this plutonium injection accident the man is healthy 24 years after the accident.
After an interesting trip down a rabbit hole of often repeated claims about plutonium toxicity we can conclude that plutonium exposure is harmful to health and is most likely to affect the lungs, liver and the skeleton. But back to reactor accidents, if a violent event occurs inside the core which is able to eject fuel in the form of fine particles (as happened at Chernobyl) then it is possible for appreciable amounts of plutonium to escape from a reactor. It has been estimated that circa 3% of the plutonium in the core at Chernobyl was ejected from the plant. I hold the view that in the event of a reactor accident it is likely that the curium (242Cm) release and the growth of americium (241Am) could be just as important as the plutonium release. Using data from Finland (Salminen, Paatero, Jaakkola, & Lehto, Citation2005) we can see the importance of the 242Cm and the ingrowth of 241Am in the following charts. In the first chart we can see the radiotoxicity of the transuranium elements decreases in the first 3 years before increasing again (Figure ).
The decrease in the first 3 years is due to the decay of the 242Cm, the increase which occurs up to about 36 years is due to the formation of the alpha emitting 241Am by the beta decay of the 241Pu. The decrease at the right hand side of the graph is due to the decay after 40 years is largely due to the decay of the 238Pu and the 241Am. The final transuranium radionuclides which will be left in the distant future will be 239Pu, 240Pu and 242Pu.
In the following graph we can see that for the much of the first 650 years the 241Am is the most important radionuclide, we can see it growth and then start to decay in Figure .
Figure 31. The relative contribution of plutonium, americium and curium to the radiotoxicity of transuranium elements released by Chernobyl.
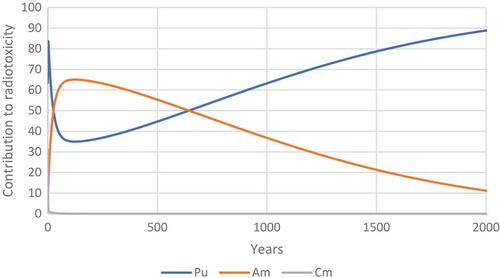
In Figure , I am showing the first decade to show how the curium decays away within the first 3 years.
Figure 32. The relative contribution of plutonium, americium and curium to the radiotoxicity of transuranium elements released by Chernobyl.
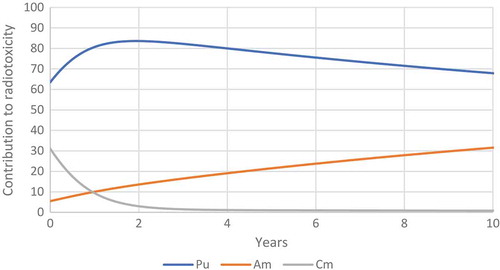
While Chernobyl released about 3.5% of its plutonium, the Fukushima accident released far less of the transuranium actinides. By examining a soil sample taken 300 km from the stricken plant it was possible to find the more mobile fission products (131I 114 kBq m−2, 132I 9.74 kBq m−2, 134Cs 7.31 kBq m−2, 136Cs 0.976 kBq m−2, 137Cs 7.49 kBq m−2, 132Te 13.2 kBq m−2, 129mTe 6.10 kBq m−2, 129Te 9.62 kBq m−2, 140Ba 0.00749 kBq m−2 and 140La 2.83 kBq m−2) but it was not possible to find either plutonium or americium in the sample by alpha spectroscopy (Zhang, Friese, & Ungar, Citation2013). The NEA report on Chernobyl estimated that circa 900 TBq of 242Cm was released while only 35, 30 and 42 TBq of 238Pu, 239Pu and 240Pu were released by the accident.
The tiny trace of transuranium radionuclides has been measured in Japan in some very careful work (Yamamoto et al., Citation2014). When I repeat the calculations using data for unit 1, I saw that 242Cm make a similar addition to the toxicity in the first years after the accident but there the americium would be slightly less important. This is likely to be due to the fact that 238Pu content in the Chernobyl fuel was lower than it was at Fukushima. In Table , some of the atomic ratios in the fuels at Chernobyl and Fukushima are shown.
Table 20. Plutonium isotope signatures for Chernobyl and the Fukushima reactors.
Currently in the basements underneath unit 4 at Chernobyl the water contains a mixture of 90Sr, 137Cs, 234U, 238U, 238Pu, 239+240Pu, 241Am and 244Cm (Odintsov, Khan, Krasnov, Pazukhin, & Shcherbin, Citation2009). According to Odintsov et al. the activity ratio of the transuranium elements is different in the fuel and the water (Table ).
Table 21. Activity ratios in the water samples from the basement of unit 4 of Chernobyl and the fuel.
It can be reasoned that the post-plutonium elements (Am and Cm) which normally form trivalent cations are more able to dissolve out of the wreckage than the plutonium which tends to be in the tetravalent state. This can be rationalized by the fact that the tetravalent plutonium will absorb more strongly onto surfaces and its water complexes are more likely to undergo hydrolysis. While the trivalent actinides are highly charged and will tend to absorb and precipitate these effects are weaker than those for the tetravalent plutonium. But much of the alpha activity in the waste water from the basement of unit 4 at the Chernobyl site is not in the form of free (or hydrated) cations. While very little of the 137Cs (beta/gamma) or the uranium is removed by filtration through a 0.01 µm (10 nm) filter, when the water is filtered through a 0.1 µm (100 nm) filter about half of the plutonium is retained by the filter. On passing the filtrate from the 0.1 µm filter through the 0.01 µm filter a little more plutonium is removed from the water. I think that the waste water from the site can be freed of plutonium by filtration through a very fine filter. However the 137Cs and 90Sr will need to be removed using an ion exchange resin. The americium in one water sample (room 001/3) behaved in a similar manner to the plutonium, while the americium in the water sample from room 012/13 was more able to pass through the filter (only 18% removed by filtration) (Rudenko & Khan, Citation2005). Odintsov et al. reported that the pH of the water from the object shelter was between 8 and 10 and that the carbonate and hydrogen carbonate anions were present. This combination of pH and carbonate explains the solubility of the uranium as uranium(VI) forms water soluble anionic carbonate complexes such as [UO2(CO3)n](n−2) −. The pH of the water is so high that americium and curium should be extensively hydrolysed to form hydroxides or other compounds.
The chemical form of the cesium, strontium, uranium, plutonium and americium was subject to a more detailed examination by Rudenko and Khan (Citation2005a), they were able to measure separately the amounts of the radionuclides in the different particle size and ionic forms. As expected much of the cesium was in the form of cations and the vast majority of the uranium was in an anionic form. While the plutonium was as expected largely in the form of particles, the finding which surprised me was that much of the americium was in an anionic form. I expect that the plutonium was in the form of some polymer, it is well known that plutonium compounds can form polymers when the acid concentration is low (Bell, Costanzo, & Biggers, Citation1973). The plutonium in soil at Rocky Flats (Lopresti, Conradson, & Clark, Citation2007) and Taranaki (Maralinga, Australia) (Ikeda-Ohno et al., Citation2016) is in the form of particles of tetravalent plutonium. It has also been found that plutonium(V/VI) can react with hematite colloids to form very insoluble nanoparticles of consisting of PuO2+x·H2O on the surface of the particles of the iron mineral (Romanchuk et al., Citation2013). Rather than the oxide particles containing plutonyl (O=Pu=O) plutonium atoms in the (V) or (VI) state it has been found by EXAFS that the increased oxidation is spread over multiple atoms (Ekberg, Larsson, Skarnemark, & Ödegaard-Jensen, Citation2013). The same paper in Dalton indicates that storage for 5 years of colloidal plutonium (94.4% 239Pu, 5.5% 240Pu and 0.1% 241Pu) causes the appearance of smaller colloids. It can be reasoned that inside the object shelter at Chernobyl that similar plutonium colloids (or nanoparticles) have formed. These can be more mobile in soil than ionic plutonium. Under some conditions the formation of colloidal particles increases the mobility of plutonium (Wolfsberg et al., Citation2017) and some other metals.
The americium may be in the form of anionic complexes of carboxylates, at Oak Ridge (USA) close to a transuranic waste store the migration of actinides (241Am and 244Cm) has been observed (McCarthy, Sanford, & Stafford, Citation1998). The saprolite minerals which are present in the site have a very high affinity for europium and americium as long as “natural organic matter” is absent. But in the field it has been shown that the americium, curium and neodymium are very mobile. This mobility is thought to be due to the organic materials.
In a paper about electrophoresis-ICPMS experiments it was shown that europium binds to humic acids. One of the problems is that humic acids are very large macromolecules which are polycarboxylic acids. Thus there is not a “humic acid” but many humic acids. It can be reasoned that if the water in the basement of the Chernobyl accident site contained some humic acids then the americium would be bound by some of the carboxylic acid groups, some of the other carboxylic acid groups will deprotonate in the alkaline water and thus make the americium-humic acid complex an anion.
The use of europium as a surrogate for americium is an interesting matter, often it is assumed that the lanthanide above a give actinide will be a good surrogate. When considering actinide chemistry it can be argued that the results of an experiment are more relevant if the actinide concerned is used rather than a surrogate. But as many of the actinides are very radiotoxic alpha emitters so sometimes it is more reasonable to do an experiment to investigate an effect using either a less radiotoxic beta emitter or even a nonradioactive metal. But sometimes the lanthanide above the actinide is a poor choice, for example above plutonium is samarium. It should be clear that while plutonium can exist in an oxidation state of +6 that the maximum oxidation state of samarium is only +3. In the case of plutonium for the purpose of studying the dioxide, thorium and cerium are better surrogates. Recently it has been argued that neodymium(III) is a better surrogate for americium(III) as the size of the cations (1.161 and 1.157 Å respectively) are more similar than those of europium(III) (1.116 Å) and americium(III) (Lundberg & Persson, Citation2016). Incidentally europium(III) may well be a better surrogate for einsteinium(III) (1.116 Å) than holmium(III). However of the early and middle lanthanides very few have a convenient radionuclide suitable for use as a radiotracer. The ideal radiotracer will be something cheap, easy to make, easy to detect (gamma emitters are easier to count than pure beta emitters) (Table ).
Table 22. Details of radiotracers of the lanthanides.
While nuclides with a mass of 152 and 154 can be formed by nuclear fission the stable 152Sm and 154Sm will minimize the formation of 152Eu and 154Sm by simple nuclear fission. This is because the main fission products (152Pr and 152Nd) with this mass are more neutron rich nuclides than the europium. Another method for the formation of 152Eu is the neutron activation of the 151Eu formed as a stable fission product in the reactor. The kinetics of the formation of this stable 151Eu will be a little complex as its precursor 151Sm has a long half-life (90 years). Also 151Sm has a large cross section for thermal neutrons (>10,000 barns) so in a reactor with a high thermal neutron flux it might not survive long enough to undergo the beta decay into 151Eu. The cross section for the n.2n reaction on 153Eu is very small unless the neutron energy is very high (>10 MeV) so it is unlikely that much 152Eu will be formed by spallation of neutrons from 153Eu.
While the fission might be a poor source of 152Eu and 154Eu, it can form for other reasons inside a nuclear reactor. Some reactors use europium in their control rods, for example some Russian naval propulsion reactors use such control rods (Klochkov, Risovanyi, Vaneev, & Dorofeev, Citation2002). Also in PWRs burnable poisons are used to reduce the change in the reactivity of the fuel during its life, a series of different materials have been considered as burnable poisons (Galahom, Citation2017). Gadolinium is commonly used as 155Gd and 157Gd have truly immense cross sections for thermal neutrons. As the burnable poison is bombarded with neutrons these two isotopes of gadolinium are converted into 156Gd and 158Gd which have much smaller cross sections for neutrons which might make this seem like the perfect element for a burnable poison. However the neutron activation of 154Gd and 156Gd will generate fresh 155Gd and 157Gd which will tend to poison the fuel towards the end of the life of the fuel when the poisoning is no longer desired. While stable europium has a smaller cross section for thermal neutrons than some of the gadolinium isotopes it can be used as a burnable poison according to Galahom. Galahom expresses the view that for a fuel which spends a long time in a reactor that europium may be superior to gadolinium as a burnable poison (Table ).
Table 23. Details of europium and gadolinium isotopes.
If we consider europium and gadolinium in a thermal neutron flux then it is possible to work out what will happen, while the gadolinium will tend to undergo neutron capture to form heavier isotopes it will form no radionuclide with a half-life of greater than 1 year. Also the stable 158Gd with a small cross section for neutrons will tend to reduce the amount of radioactivity which is formed in the gadolinium. In contrast many of the europium isotopes with high neutron cross sections form on activation radioactive isotopes of europium. If we ignore radiation damage due to the intense neutron and gamma flux in a reactor then it can be reasoned that the Gd2O3 will be chemically unchanged as it performs this useful function in the fuel. Galahom expresses the view that pyrex (borosilicate) glass is also a good burnable neutron poison, one disadvantage of boron containing poisons is that on bombardment with neutrons they form helium gas.
It can be reasoned that if europium containing parts are used as core components that 152Eu and 154Eu will form in the core, also even without a deliberate addition of europium the fission process will generate 155Eu. As a result I conclude that the europium is very relevant to the study of used fuels and reactor accidents. As a less toxic surrogate for 241Am, I reason that while not perfect that 152Eu is a useful mimic.
While most attention regarding actinides and nuclear accidents has been payed to the transuranic elements uranium can pose a threat. It is important to keep in mind that natural and depleted uranium has a small ability to pose a threat. The problem uranium isotope is 232U, this can be formed in a range of different ways. The bombardment of 232Th by neutrons can form 233Th by simple neutron capture, the 233Th then decays via 233Pa to the fissile nuclei 233U. However high energy neutrons can form 231Th by a n.2n reaction. The 231Th decays (t½ = 26 h) by beta emission into 231Pa which has a long half-life (32,760 years). By neutron capture it is converted into 232Pa which decays by beta decay (t½ = 1.3 days) into 232U. The decay of 234U forms 230Th (t½ = 75,400 years) which undergoes neutron capture to form 231Th which then by means of beta decays and neutron capture forms 232U. Fast neutrons can form 232U from 233U and 235U in n.2n and n.4n reactions. The n.4n reaction on 235U together with the pathway starting with 234U is responsible for forming the majority of 232U in UO2 fuel (Yamamoto & Okumura, Citation2014).
In MOX made from plutonium and depleted uranium the main source of 232U is a pathway starting with 238U. What happens is that fast neutrons create 237U by a n.2n reaction (Yamamoto & Okumura, Citation2014). The 237U (t½ = 6.8 days) decays by beta emission to 237Np (t½ = 2,144,000 years). Then a second n.2n reaction forms 236mNp (t½ = 22.5 h) which decays into 236Pu (t½ = 2.9 years) which in turn decays by alpha emission into 232U. If we start with MOX made using uranium recovered from a PUREX plant then the pathway starting with 238U is important but a pathway starting with neutron activation of 236U is even more important (Yamamoto & Okumura, Citation2014). If we were to make a MOX fuel and cram into it neptunium (237Np) and americium (241Am and 243Am) then even more 232U would form in the fuel. The 232U (t½ = 69 years) will decay by alpha emission to 228Th (1.9 years) which likewise decays by alpha emission to form 224Ra (3.7 days) which emits an alpha particle to form 220Rn (thoron). Already at the Chernobyl accident site inside the object shelter the daughters of 220Rn have started to pose a threat to workers (Ogorodnikov et al., Citation2009). As 232U has a longer half-life than 137Cs and 90Sr as the cesium and strontium decay away the thoron daughters will become more important. I predict that at Fukushima in the future that the thoron problem will also become one of the dominant threats at the site unless the fuel is recovered in the near future.
Competing interests
The author declares no competing interests.
Citation information
Cite this article as: Reactor accident chemistry an update, Mark R. St J. Foreman, Cogent Chemistry (2018), 4: 1450944.
Additional information
Funding
Notes on contributors
Mark R. St J. Foreman
The author is a chemist who has a professional interest in the phenomena associated with radiological accidents. He has worked with other scientists to better understand these events and to try to reduce their ability to harm the general public. In recent times he has performed research considering how trustworthy respiratory protection and iodine sampling devices based on charcoals are. He has been working recently with Aldo Jesorka on miniature devices designed to assist in the sampling and measurement of radioactivity during serious radiological accidents. Both these projects were funded by the Swedish radiation protection authority (SSM). Additionally Mark has academic interests in recycling, solvent extraction, novel solvents and other areas.
Notes
1. The reader can be forgiven for asking why I expressed myself in Locomotive BASIC, the reason is that as a youngster it was a computer language which I was heavily exposed to. Having written FORTRAN as an undergraduate I came to the conclusion that the two were similar.
2. I will not discuss in detail the ethical issues associated with the human radiation and radiaoctivity experiments performed many years ago in the USA. For the plutonium injection experiments the intention was to inject people who were terminally ill with plutonium to determine how it would behave in a human body. It is noteworthy that in this experiment (and some others) that informed consent was not obtained from the human test subjects. It is noteworthy that at least one unethical experiment was resulted in a successful lawsuit by family members against researchers, the University of Cincinnati, and the City of Cincinnati.
3. Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Flourine, Neon, Sodium, Magnesium, Aluminium, Silicon, Phosphorus, Sulphur, Chlorine, Argon, Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Ruthenium, Rhodium, Palladium, Silver, Cadmium, Osmium, Iridium, Platinum, Gold, Mercury, Thallium, Radon, Radium, Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium.
4. I have made the simple assumption that testicular dose is proportional to the activity of plutonium injected into the mouse and the time between the injection and mating with the female mouse.
5. Huet, Trompier, Clairand, Queinnec, and Bottollier-Depois (Citation2008).
References
- Abe, K., DonSantos, R., Gauvain, J., Jones, C.G., Jouve, A., Ramirez, M.L., ...,Wheatley, J. (2013). The international nuclear and radiological event scale, user’s manual 2008 edition (2013 extended edition). Austria: IAEA. Retrieved from https://www-pub.iaea.org/MTCD/Publications/PDF/INES2013web.pdf
- Abe, Y., Iizawa, Y., Terada, Y., Adachi, K., Igarashi, Y., & Nakai, I. (2014). Detection of uranium and chemical state analysis of individual radioactive microparticles emitted from the Fukushirna nuclear accident using multiple synchrotron radiation X-ray analyses. Anayltical Chemistry, 86, 8521–100. doi:10.1021/ac501998d
- Adachi, K., Kajino, M., Zaizen, Y., & Igarashi, Y. (2013). Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Scientific Reports, 3, 2554. doi:10.1038/srep02554
- Adams, E. G., & Simmons, N. T. (1951). The determination of carbon monoxide by means of iodine Pentoxide. Journal of Applied Chemistry, 1, S20–S40.
- Agopiantz, M., Elhanbali, O., Demore, B., Cuny, T., Demarquet, L., Ndiaye, C., … Klein, M. (2016). Thyroid side effects prophylaxis in front of nuclear power plant accidents. Annales d Endocrinologie, 77, 1–6. doi:10.1016/j.ando.2015.12.003
- Aguiar, J. C., Galiano, E., & Fernandez, J. (2006). Peak efficiency calibration for attenuation corrected cylindrical sources in gamma ray spectrometry by the use of a point source. Applied Radiation and Isotopes, 64, 1643–1647. doi:10.1016/j.apradiso.2006.05.014
- Ainsbury, E. A., Bakhanova, E., Barquinero, J. F., Brai, M., Chumak, V., Correcher, V., … Rothkamm, K. (2011). Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiation Protection Dosimetry, 147, 573–592. doi:10.1093/rpd/ncq499
- Akleyev, A. V., Krestinina, L. Y., Degteva, M. O., & Tolstykh, E. I. (2017). Consequences of the radiation accident at the Mayak production association in 1957 (the ‘Kyshtym Accident’). Journal of Radiological Protection, 37, R19–R42. doi:10.1088/1361-6498/aa7f8d
- Alam, F., Soloway, A. H., & Barth, R. F. (1987). Boronation of antibodies with mercaptoundecahydro-closo-dodecaborate(2-) anion for potential use in boron neutron-capture therapy. Applied Radiation and Isotopes, 38, 503–506. doi:10.1016/0883-2889(87)90195-X
- Alves, C. A., Evtyugina, M., Cerqueira, M., Nunes, T., Duarte, M., & Vicente, E. (2015). Volatile organic compounds emitted by the stacks of restaurants. Air Quality Atmosphere and Health, 8, 401–412. doi:10.1007/s11869-014-0310-7
- Amiro, B. D., Sheppard, S. C., Johnston, F. L., Evenden, W. G., & Harris, D. R. (1996). Burning radionuclide question: What happens to iodine, cesium and chlorine in biomass fires? The Science of the Total Environment, 187, 93–103. doi:10.1016/0048-9697(96)05125-X
- Andreev, G., Antipin, Y., Bennett, B., Chizhikov, V., Gonzalez, A. J., Gordeev, A., … Webb, G. A. M. (1998). The radiological accident in the reprocessing plant at Tomsk. Austria: IAEA.
- Aneheim, E., Ekberg, C., & Foreman, M. R. S. (2012). Aqueous complexation of palladium to prevent precipitation and extraction in a group actinide extraction system. Hydrometallurgy, 115, 71–76. doi:10.1016/j.hydromet.2011.12.018
- Anonymous. (2011). Retrieved October 26, 2017, from https://wonder.cdc.gov/mmwr/mmwrmort.asp
- Anonymous. (1955). Commercial film-badge services. Nucleonics, 13(2), 86–87.
- Anonymous. (2006). Retrieved from https://www.independent.ie/irish-news/sellafield-drama-a-bizarre-slur-26391469.html
- Anonymous. (2011, November 11). Atomic radiation is more harmful to women. Nuclear Monitor, issue #736, number 6192, 7.
- Antonopoulos-Domis, M., Clouvas, A., & Gagianas, A. (1991). Radiocesium dynamics in fruit-trees following the Chernobyl accident. Health Physics, 61, 837–842. doi:10.1097/00004032-199112000-00015
- Antonopoulos-Domis, M., Clouvas, A., & Gagianas, A. (1996). Long term radiocesium contamination of fruit trees following the Chernobyl accident. Health Physics, 71, 910–914. doi:10.1097/00004032-199612000-00007
- Arima, T., Masuzumi, T., Furuya, H., Idemisu, K., & Inagaki, Y. (2002). Reaction of Zircaloy-4 with tellurium under different oxygen potentials. Journal of Nuclear Materials, 301, 90–97. doi:10.1016/S0022-3115(02)00711-0
- Aubert, M., Calais, D., & Le Beuze, R. (1975). Role of iodine in development of reaction between fuel and its 316 stainless-steel cladding in reactors. Journal of Nuclear Materials, 58, 257–277. doi:10.1016/0022-3115(75)90118-X
- Avramenko, M. I., Averin, A. N., Drozhko, E. G., Glagolenko, Y. V., Filin, V. P., Loboiko, B. G., … Romanov, G. N. (2000). Radiation accident of 1957 and Eastern-Urals radioactive trace: Analysis of measurement data and laboratory experiments. Atmospheric Environment, 34, 1215–1223. doi:10.1016/S1352-2310(99)00303-9
- Ayrault, S., Jimenez, B., Garnier, E., Fedoroff, M., Jones, D. J., & Loos-Neskovic, C. (1998). Sorption mechanisms of cesium on (Cu2FeII)-Fe-II(CN)(6) and Cu-3(II)[Fe-III(CN)(6)](2): Hexacyanoferrates and their relation to the crystalline structure. Journal of Solid State Chemistry, 141, 475–485. doi:10.1006/jssc.1998.7997
- Baba, Y., Eguchi, T., & Inoue, K. (1986). Solvent-extraction of palladium with dihexyl sulfide. Journal of Chemical Engineering of Japan, 19, 361–366. doi:10.1252/jcej.19.361
- Baenziger, N. C., Rundle, R. E., Snow, A. I., & Wilson, A. S. (1950). Compounds of uranium with the transition metals of the 1st long period. Acta Crystallographica, 3, 34–40. doi:10.1107/S0365110X50000082
- Barber, H. H., & Kolthoff, I. M. (1929). Gravimetric determination of sodium by the uranyl zinc acetate method. II. Application in the presence of rubidium, cesium, potassium, lithium, phosphate or arsenate. Journal of the American Chemical Society, 51, 3233–3237. doi:10.1021/ja01386a008
- Barth, R. F., Soloway, A. H., Fairchild, R. G., & Brugger, R. M. (1992). Boron neutron-capture therapy for cancer—realities and prospects. Cancer, 70, 2995–3007.
- Baxendale, J. H., & Smithies, D. (1956). Die Strahlenchemie in Wasser gelöster organischer Verbindungen [The radiation chemistry in water of dissolved organic compounds]. Zeitschrift für Physikalische Chemie (Frankfurt), 7, 242–264. doi:10.1524/zpch.1956.7.3_4.242
- Beahm, E. C. (1987). Tellurium behavior in containment under light water reactor accident conditions. Nuclear Technology, 78, 295–302. doi:10.13182/NT87-A15995
- Beck, H. L. (1980). Exposure rate conversion factors for radionuclides deposited on the ground. New York, NY: Environmental Measurements Laboratory, Department of Energy.
- Bell, J. C., Bridge, H., Cottrell, A. H., Greenough, G. B., Reynolds, W. N., & Simmons, J. H. W. (1962). Stored energy in graphite of power-producing reactors. Philosophical Transactions of the Royal Society of London, 254, 361–395. doi:10.1098/rsta.1962.0002
- Bell, J. T., Costanzo, D. A., & Biggers, R. E. (1973). Plutonium polymerization. 2. Kinetics of plutonium polymerization. Journal Inorganic Nuclear Chemical, 35, 623–628. doi:10.1016/0022-1902(73)80576-7
- Bernard, F., McGillen, M. R., Fleming, E. L., Jackman, C. H., & Burkholder, J. B. (2015). CBrF3 (Halon-1301): UV absorption spectrum between 210 and 320 K, atmospheric lifetime, and ozone depletion potential. Journal of Photochemistry and Photobiology A: Chemistry, 306, 13–20. doi:10.1016/j.jphotochem.2015.03.012
- Bianchi, A. P., & Varney, M. S. (1993). Sampling and analysis of volatile organic-compounds in estuarine air by gas-chromatography and mass-spectrometry. Journal of Chromatography, 643, 11–23. doi:10.1016/0021-9673(93)80536-H
- Boeyens, J. C. A., McDougal, G. J., & Van R. Smit, J. (1976). Crystallographic study of ammonium-potassium 12-molybdophosphate ion-exchange system. Journal of Solid State Chemistry, 18, 191–199. doi:10.1016/0022-4596(76)90095-5
- Bonjoch, J., Drew, M. G. B., Gonzalez, A., Greco, F., Jawaid, S., Osborn, H. M. I., … Yaqoob, P. (2008). Synthesis and evaluation of novel boron-containing complexes of potential use for the selective treatment of malignant melanoma. Journal of Medicinal Chemistry, 51, 6604–6608. doi:10.1021/jm8007745
- Borzelleca, J. F. (2000). Paracelsus: Herald of modern toxicology. Toxicological Sciences, 53, 2–4. doi:10.1093/toxsci/53.1.2
- Bosland, L., Funke, F., Girault, N., & Langrock, G. (2008). Paris project: Radiolytic oxidation of molecular iodine in containment during a nuclear reactor severe accident. Part 1. Formation and destruction of air radiolysis products—Experimental results and modelling. Nuclear Engineering and Design, 238, 3542–3550. doi:10.1016/j.nucengdes.2008.06.023
- Bradley, L. (2006). Retrieved from https://www.independent.ie/irish-news/nuclear-docudrama-slammed-by-minister-26411084.html
- Bredig, M. A., Johnson, J. W., & Smith, W. T. (1955). Miscibility of liquid metals with salts. 1. The sodium–sodium halide systems. Journal of the American Chemical Society, 77, 307–312. doi:10.1021/ja01607a016
- Broda, R., Mietelski, J. W., & Sieniawski, J. (1992). Radioactive SB-125 and Co-60 in ruthenium hot particles from Chernobyl fallout. Journal of Radioanalytical and Nuclear Chemistry Letters, 166, 173–180. doi:10.1007/BF02164740
- Buck, E. C., Mausolf, E. J., McNamara, B. K., Soderquist, C. Z., & Schwantes, J. M. (2016). Sequestration of radioactive iodine in silver-palladium phases in commercial spent nuclear fuel. Journal of Nuclear Materials, 482, 229–235. doi:10.1016/j.jnucmat.2016.10.029
- Bujdosó, E. (1987). The Chernobyl reactor accident. Journal of Radioanalytical and Nuclear Chemistry, 116, 223–231. doi:10.1007/BF02037225
- Buki, G., & Bede, G. (1965). Critical size and flux distribution of hollow spherical and cylindrical reactors. Periodica Polytechnica-Engineering, 9, 227–242.
- Busk, R. S. (1952). Effect of temperature on the lattice parameters of magnesium alloys. Journal of Metals, 4, 207–209.
- Buxton, G. V., Greenstock, C. L., Helman, W. P., & Ross, A. B. (1988). Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (∙OH/ ∙O−) in aqueous-solution. Journal of Physical and Chemical Reference Data, 17, 513–886. doi:10.1063/1.555805
- Cahn, R. W. (1977). Metal bites metal. Nature, 265, 11–12. doi:10.1038/265011b0
- Caldicott, H. (2006). Nuclear power is not the answer. New York, NY: The New Press.
- Carbaugh, E. H., Lynch, T. P., Antonio, C. L., & Medina-Del Valle, F. (2010). Twenty-four years of follow-up for a Hanford plutonium wound case. Health Physics, 99, 483–494. doi:10.1097/HP.0b013e3181d96381
- Cattanach, B. M., Papworth, D., Patrick, G., Goodhead, D. T., Hacker, T., Cobb, L., & Whitehill, E. (1998). Investigation of lung tumour induction in C3H/HeH mice, with and without tumour promotion with urethane, following paternal X-irradiation. Mutation Research, 403, 1–12. doi:10.1016/S0027-5107(97)00322-9
- Cattanach, B. M., Patrick, G., Papworth, D., Goodhead, D. T., Hacker, T., Cobb, L., & Whitehill, E. (1995). Investigation of lung-tumor induction in balb cj mice following paternal X-irradiation. International Journal of Radiation Biology, 67, 607–615. doi:10.1080/09553009514550721
- Chamberlain, A. C., Eggleton, A. E. J., Megaw, W. J., & Morris, J. B. (1963). Physical chemistry of iodine and removal of iodine from gas streams. Reactor Science and Technology (Journal of Nuclear Energy Parts A/B), 17, 519–550. doi:10.1016/0368-3230(63)90066-1
- Chapman, L. R., & Holcombe, C. E., JR. (1984). Revision of the uranium iron phase-diagram. Journal of Nuclear Materials, 126, 323–326. doi:10.1016/0022-3115(84)90046-1
- Chase, M. W. (1998). NIST-JANAF themochemical tables, fourth edition. Journal Physical Chemical Ref Data, Monograph 9, 1–1951.
- Chattopadhyay, G., & Juneja, J. M. (1993). A thermodynamic database for tellurium-bearing systems relevant to nuclear technology. Journal of Nuclear Materials, 202, 10–28. doi:10.1016/0022-3115(93)90024-S
- Cheng, T., & Tortorelli, P. F. (2013). Silicon carbide oxidation in high-pressure steam. Journal American Ceram Social, 96, 2330–2337. doi:10.1111/jace.12328
- Chubb. Retrieved from http://www.chubb.ie/Pages/ChubbFirePyrometPowderExtinguisher.aspx (Note that COSHH paperwork for the pyromet system existed in 2003).
- Clough, W. S., & Fraser, A. (1973). Tellurium, cesium, iodine and methyl-iodide in fast-reactors. Journal of Nuclear Energy, 27, 1–14. doi:10.1016/0022-3107(73)90050-6
- Cockerell, J. (2014). Retrieved from http://www.independent.co.uk/news/uk/crime/twitter-trolls-isabella-sorley-and-john-nimmo-jailed-for-abusing-feminist-campaigner-caroline-criado-9083829.html
- Coggle, J. E. (1971). Biological effects of radiation. London: Wykeham Publications (London) Ltd (A member of the Taylor and Francis Group).
- Cosgrove, G. E., Selby, P. B., Upton, A. C., Mitchell, T. J., Steele, M. H., & Russell, W. L. (1993). Life-span and autopsy findings in the 1st-generation offspring of x-irradiated male-mice. Mutation Research, 319, 71–79. doi:10.1016/0165-1218(93)90032-9
- Cox, J. D., Wagman, D. D., & Medvedev, V. A. (1984). CODATA Key values for thermodynamics (Vol. 1). New York, NY: Hemisphere Publishing Corp.
- Coy, K., Ghosh, P., Kaituri, M., McCready-Shea, S., Oresegun, M., Piccone, J., … Zamora, F. (1998). Lessons learned from accidents in industrial radiography. Safety Reports Series No. 7. Vienna: International Atomic Energy Agency, IAEA.
- Cubicciotti, D., & Sanecki, J. E. (1978). Characterization of deposits on inside surfaces of LWR cladding. Journal of Nuclear Materials, 78, 96–111. doi:10.1016/0022-3115(78)90508-1
- Czerwinski, F. (2004). The early stage oxidation and evaporation of Mg–9%Al–1%Zn alloy. Corrosion Science, 46, 377–386. doi:10.1016/S0010-938X(03)00151-3
- Daher, A., Varin, M., Lamontagne, Y., & Oth, D. (1998). Effect of pre-conceptional external or internal irradiation of N5 male mice and the risk of leukemia in their offspring. Carcinogenesis, 19, 1553–1558. doi:10.1093/carcin/19.9.1553
- Dale, R. G. (1985). The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. The British Journal of Radiology, 58, 515–528. doi:10.1259/0007-1285-58-690-515
- de González, B., Ntowe, E., Kitahara, C. M., Gilbert, E., Miller, D. L., Kleinerman, R. A., & Linet, M. S. (2016). Long-term mortality in 43 763 US radiologists compared with 64 990 US psychiatrists. Radiology, 281, 847–857. doi:10.1148/radiol.2016152472
- DeBoer, R., & Cordfunke, E. H. P. (1997). The chemical form of fission product tellurium during reactor accident conditions. Journal of Nuclear Materials, 240, 124–130. doi:10.1016/S0022-3115(96)00600-9
- Denloye, A. O., Gasner, L. L., & Adamchak, F. R. (1984). Thermodynamic analysis of a fluidized-bed combustor. Thermochimica Acta, 75, 9–22. doi:10.1016/0040-6031(84)85002-9
- DiLemma, F. G., Colle, J. Y., Benes, O., & Konings, R. J. M. (2015). A separate effect study of the influence of metallic fission products on CsI radioactive release from nuclear fuel. Journal of Nuclear Materials, 465, 499–508. doi:10.1016/j.jnucmat.2015.05.037
- Ding, H. L., & Hopke, P. K. (1993). HO(X) production due to radon decay in air. Journal of Atmospheric Chemistry, 17, 375–390. doi:10.1007/BF00696855
- Dunster, H. J., Howells, H., & Templeton, W. L. (2007). District surveys following the windscale incident, October 1957. Journal of Radiological Protection, 27, 217–230. doi:10.1088/0952-4746/27/3/001
- Eckerman, K., Harrison, J., Menzel, H.-G., & Clement, C. H. (2012). Compendium of Dose Coefficients based on ICRP Publication 60. Annals ICRP, 41, 1–130. doi:10.1016/j.icrp.2012.06.038
- Edvarson, K., Löw, K., & Sisefsky, J. (1959). Fractionation phenomena in nuclear weapons debris. Nature, 184, 1771–1774. doi:10.1038/1841771a0
- Edwards, D. J., & Bernier, S. M. (1996). Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sciences, 59, 1025–1030. doi:10.1016/0024-3205(96)00417-1
- Eeles, W. T., & Sutton, A. L. (1963). X-ray determination of atomic positions in alpha-uranium at 22 degrees C and 600 degrees C. Acta Crystallographica, 16, 575. doi:10.1107/S0365110X63001511
- Ekberg, C., Larsson, K., Skarnemark, G., & Ödegaard-Jensen, A. (2013). The structure of plutonium(IV) oxide as hydrolysed clusters in aqueous suspensions. Dalton Transactions, 42, 2035–2040. doi:10.1039/C2DT32185H
- Elkind, M. M., Suttongi, H., Moses, W. B., Alescio, T., & Swain, R. W. (1965). Radiation response of mammalian cells grown in culture. V. Temperature dependence of repair of X-ray damage in surviving cells (aerobic and hypoxic). Radiation Research, 25, 359–376. doi:10.2307/3571978
- Endo, A., Kikuchi, M., Izawa, S., & Ikezawa, Y. (1995). Characteristics of the chemical forms of C-11, N-13, and O-15 induced in air by the operation of A 100-MEV electron linear-accelerator. Health Physics, 68, 80–88. doi:10.1097/00004032-199501000-00010
- Endo, A., Sato, K., Noguchi, H., Su, T. T., Furuichi, I. S., Kanda, Y., & Oki, Y. (2003). Study of particle size distribution and formation mechanism of radioactive aerosols generated in high-energy neutron fields. Journal of Radioanayltical and Nuclear Chemistry, 256, 231–237. doi:10.1023/A:1023925031655
- Evangeliou, N., Zibtsev, S., Myroniuk, V., Zhurba, M., Hamburger, T., Stohl, A., … Kireev, S. I. (2016). Resuspension and atmospheric transport of radionuclides due to wildfires near the Chernobyl Nuclear Power Plant in 2015: An impact assessment. Scientific Reports, 6, 26062. doi:10.1038/srep26062
- Finch, G. L., Lundgren, D. L., Barr, E. B., Chen, B. T., Griffith, W. C., Hobbs, C. H., … Mauderly, J. L. (1998). Chronic cigarette smoke exposure increases the pulmonary retention and radiation dose of Pu-239 inhaled as (PuO2)-Pu-239 by F344 rats. Health Physics, 75, 597–609. doi:10.1097/00004032-199812000-00003
- Finsterer, J. (2002). Earl Grey tea intoxication. The Lancet, 359(9316), 1484. doi:10.1016/S0140-6736(02)08436-2
- Fischer, P. (1967). Neutronenbeugungsuntersuchung der strukturen Von MGAL2O4-und ZNAL2O4-spinellen in abhangigkeit von der vorgeschichte [Neutron binding study of the structures of MGAL2O4 and ZNAL2O4 spinelles in relation to the previous story]. Zeitschrift fuer Kristallographie Kristallgeometrie Kristallphysik and Kristallchemie, 124, 275–302. doi:10.1524/zkri.1967.124.4-5.275
- Flowers, F. H. (1986). Dragons exhalations give clue to Chernobyl. Nature, 323, 208. doi:10.1038/323208a0
- Foreman, M. R. S. (2015). An introduction to serious nuclear accident chemistry. Cogent Chemistry, 1. doi:10.1080/23312009.2015.1049111
- Franco, M. G., Correa, S. M., Marques, M., & Perez, D. V. (2014). Emission of volatile organic compounds and greenhouse gases from the anaerobic bioremediation of soils contaminated with diesel. Water, Air and Soil Pollution, 225, 1879. doi:10.1007/s11270-014-1879-z
- Friedland, E., Van der Berg, N. G., Matherbe, J. B., Hancke, J. J., Barry, J., Wendler, E., & Wesch, W. (2011). Investigation of silver and iodine transport through silicon carbide layers prepared for nuclear fuel element cladding. Journal of Nuclear Materials, 410, 24–31.
- Frunzke, J., Loschen, C., & Frenking, G. (2004). Why are olefins oxidized by RuO4 under cleavage of the carboncarbon bond whereas oxidation by OsO4 yields cis-diols? Journal of the American Chemical Society, 126, 3642–3652. doi:10.1021/ja039921a
- Fuggle, J. C., Watson, L. M., Fabian, D. J., & Affrossman, S. (1975). X-ray photoelectron studies of reaction of clean metals (MG, AL,CR,MN) with oxygen and water-vapor. Surface Science, 49, 61–76. doi:10.1016/0039-6028(75)90328-3
- Gaffney, R. J. (2000a). Retrieved from https://apps2.suffolkcountyny.gov/legislature/resos/resos2000/i1767-00.htm
- Gaffney, R. J. (2000b). Retrieved from https://apps2.suffolkcountyny.gov/legislature/resos/resos2000/i1971-00.htm
- Galahom, A. A. (2017). Study of the possibility of using Europium and Pyrex alloy as burnable absorber in PWR. Annals of Nuclear Energy, 110, 1127–1133. doi:10.1016/j.anucene.2017.08.052
- Gale, R. P. (2012). Response to “an unexpected mortality increase in the United States follows arrival of the radioactive plume from Fukushima: Is there a correlation?” International Journal of Health Services, 42, 557–559. doi:10.2190/HS.42.3.l
- Gall, M., Mahler, S., & Wirth, E. (1991). Transfer of CS-137 into mothers milk. Journal of Environmental Radioactivity, 14, 331–339. doi:10.1016/0265-931X(91)90023-9
- Garger, E. K., Kashpur, V., Paretzke, H. G., & Tschiersch, J. (1998). Measurement of resuspended aerosol in the Chernobyl area—Part II. Size distribution of radioactive particles. Environment Biophysics, 36, 275–283. doi:10.1007/s004110050082
- Garger, E. K., Paretzke, H. G., & Tschiersch, J. (1998). Measurement of resuspended aerosol in the Chernobyl area Part III. Size distribution and dry deposition velocity of radioactive particles during anthropogenic enhanced resuspension. Radiational, Environmental, Biophysics, 37, 201–208. doi:10.1007/s004110050118
- Garland, J. A., & Wakefield, R. (2007). Atmospheric emissions from the Windscale accident of October 1957. Atmospheric Environment, 41, 3904–3920. doi:10.1016/j.atmosenv.2006.12.049
- Gillett, N. A., Pool, R. R., Taylor, G. N., Muggenburg, B. A., & Boecker, B. B. (1992). SR-90 induced bone-tumors in beagle dogs—effects of route of exposure and dose-rate. International Journal of Radiation Biology, 61, 821–831. doi:10.1080/09553009214551701
- Gillmore, G., Gharib, H. A., Denman, A., Phillips, P., & Bridge, D. (2011). Radon concentrations in abandoned mines, Cumbria, UK: Safety implications for industrial archaeologists. Natural Hazards and Earth System Sciences, 11, 1311–1318. doi:10.5194/nhess-11-1311-2011
- Gillmore, G. K., Phillips, P., Denman, A., Sperrin, M., & Pearce, G. (2001). Radon levels in abandoned metalliferous mines, Devon, southwest England. Ecotoxicology and Environmental Safety, 49, 281–292. doi:10.1006/eesa.2001.2062
- Gordon, P. L., O’Dell, C., & Watkin, J. G. (1994). Synthesis and energetic content of red oil. Journal of Hazardous Materials, 39, 87–105. doi:10.1016/0304-3894(94)00061-1
- Gosse, S., & Gueneau, C. (2011). Thermodynamic assessment of the palladium-tellurium (Pd-Te) system. Intermetallics, 19, 621–629. doi:10.1016/j.intermet.2010.12.014
- Gowdamarajan, A., & Michler, R. E. (2000). Therapy for primary cardiac tumors: Is there a role for heart transplantation? Current Opinion in Cardiology, 15, 121–125. doi:10.1097/00001573-200003000-00010
- Grant, E. J., Brenner, A., Sugiyama, H., Sakata, R., Sadakane, A., Utada, M., … Ozasa, K. (2017). Solid cancer incidence among the life span study of atomic bomb survivors: 1958–2009. Radiation Research, 187, 513–537. doi:10.1667/RR14492.1
- Greenpeace. (2006) Retrieved September 17, 2017, from http://www.greenpeace.org/international/en/campaigns/nuclear/proliferation/plutonium/
- Gribble, G. W. (1994). Natural organohalogens—many more than you think. Journal Chemical Editors, 71, 907–911. doi:10.1021/ed071p907
- Gribble, G. W. (2003). The diversity of naturally produced organohalogens. Chemosphere, 52, 289–297. doi:10.1016/S0045-6535(03)00207-8
- Grubb, W. T. (1976). Nuclear fuel assembly and process. US patent, 1976, US4097402 A.
- Gupta, A., Andrews, K. L., McDaniel, K. M., Nagle, R. B., & Bowden, G. T. (1999). Experimental induction of rhabdomyosarcoma in mice with fractionated doses of beta-irradiation. Journal of Cancer Research and Clinical Oncology, 125, 257–267. doi:10.1007/s004320050272
- Haley, B. M., Paunesku, T., Grdina, D. J., & Woloschak, G. W. (2015). The increase in animal mortality risk following exposure to sparsely ionizing radiation is not linear quadratic with dose. PLOS ONE, 10, e0140989. doi:10.1371/journal.pone.0140989
- Harding, M., Manley, J., Wright, C., Buckland, C., Evans, C., Barschall, H., … Whipple, H. O. (1948). Radiation doses in the Pajarito accident of May 21 1946, LA-687. Los Alamos: Los Alamos Scientific Laboratory of the University of California.
- Hare, D., Tolmachev, S., James, A., Bishop, D., Austin, C., Fryer, F., & Doble, P. (2010). Elemental bio-imaging of thorium, uranium, and plutonium in tissues from occupationally exposed former nuclear workers. Analytical Chemistry, 82, 3176–3182. doi:10.1021/ac902650w
- Harr, L. J. (2007). Precise calculation of complex radioactive decay chains. Ohio: Air Force Institute of Technology. http://dtic.mil/dtic/tr/fulltext/u2/a469273.pdf
- Harrison, M. T. (2014). Vitrification of high level waste in the UK. Procedia Materials Science, 7, 10–15. doi:10.1016/j.mspro.2014.10.003
- Hicks, H. G. (1982). Calculation of the concentration of any radionuclide deposited on the ground by offsite fallout from a nuclear detonation. Health Physics, 42, 585–600. doi:10.1097/00004032-198205000-00003
- Horwitz, E. P., Chiarizia, R., & Dietz, M. L. (1992). A novel strontium-selective extraction chromatographic resin. Solvent Extraction and Ion Exchange, 10, 313–336. doi:10.1080/07366299208918107
- Horwitz, E. P., Dietz, M. L., & Fisher, D. E. (1991). Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using a crown-ether. Analytical Chemistry, 63, 522–525. doi:10.1021/ac00005a027
- Hudson, M. J., Knowles, J. P., Harris, P. J. F., Jackson, D. B., Chinn, M. J., & Ward, J. L. (2004). The trapping and decomposition of toxic gases such as hydrogen cyanide using modified mesoporous silicates. Microporous and Mesoporous Materials, 75, 121–128. doi:10.1016/j.micromeso.2004.07.023
- Huet, C., Trompier, F., Clairand, I., Queinnec, F., & Bottollier-Depois, J. F. (2008). Physical dosimetric reconstruction of a radiological accident at Fleurus (Belgium) on 11 March 2006. Radiation Measurements, 43, 845–848. doi:10.1016/j.radmeas.2007.12.018
- Huh, C.-A. (1999). Dependence of the decay rate of Be-7 on chemical forms. Earth and Planetary Science Letters, 171, 325–328. doi:10.1016/S0012-821X(99)00164-8
- Hunt, G. J. (1998). Transfer across the human gut of environmental plutonium, americium, cobalt, caesium and technetium: Studies with cockles (Cerastoderma edule) from the Irish Sea. Journal Radiology Protection, 18, 101–109. doi:10.1088/0952-4746/18/2/005
- Hutchinson, C. M., McGiffen, M. E., Oh, H. D., Sims, J. J., & Becker, J. O. (2000). Efficacy of methyl iodide and synergy with chloropicrin for control of fungi. Pest Management Science, 56, 413–418. doi:10.1002/(ISSN)1526-4998
- IAEA. (2014). INES Rating Interactive Learning Tool. Retrieved from https://iec.iaea.org/inesrilt/
- Ikeda-Ohno, A., Shahin, L. M., Howard, D. L., Collins, R. N., Payne, T. E., & Johansen, M. P. (2016). Fate of plutonium at a former nuclear testing site in Australia. Environmental Science & Technology, 50, 9098–9104. doi:10.1021/acs.est.6b01864
- Inada, J. (2000). Radiological and environmental aspects of the criticality accident in Tokai-mura. Radiation Protection Dosimetry, 92, 239–246. doi:10.1093/oxfordjournals.rpd.a033277
- Isetti, G. (1965). Ricerche sulla struttura della Brucite. Periodico di Mineralogia, 34, 327–335.
- Ishimoto, S., Hirai, M., Ito, K., & Korei, Y. (1996). Thermal conductivity of UO2-BeO pellet. Journal of Nuclear Science and Technology, 33, 134–140. doi:10.1080/18811248.1996.9731875
- Iwata, T. (1985). Fine-structure of wigner energy-release spectrum in neutron-irradiated graphite. Journal of Nuclear Materials, 133–134, 361–364. doi:10.1016/0022-3115(85)90168-0
- Jambunathan, K., Shah, B. C., Hudson, J. L., & Hillier, A. C. (2001). Scanning electrochemical microscopy of hydrogen electro-oxidation. Rate constant measurements and carbon monoxide poisoning on platinum. Journal of Electroanalytical Chemistry, 500, 279–289. doi:10.1016/S0022-0728(00)00344-2
- Jargin, S. V. (2011). Forest fires in the former Soviet Union: No reasons for radiophobia. Journal of Environmental Radioactivity, 102, 218–219. doi:10.1016/j.jenvrad.2010.10.001
- Jay, A. H., & Andrews, K. W. (1946). Note on oxide systems pertaining to steel-making furnace slags—FEO-MNO, FEO-MGO, CAO-MNO, MGO-MNO. Journal of the Iron and Steel Institute, 152, 15–18.
- Jones, D. R., Paul, L., & Mitchell, N. G. (1999). Effects of ameliorative measures on the radiocaesium transfer to upland vegetation in the UK. Journal of Environmental Radioactivity, 44, 55–69. doi:10.1016/S0265-931X(98)00065-4
- Jones, S. (2008). Windscale and Kyshtyrn: A double anniversary. Journal of Environmental Radioactivity, 99, 1–6. doi:10.1016/j.jenvrad.2007.10.002
- Kajan, I., Kärkelä, T., Tapper, U., Johansson, L.-S., Gouello, M., Ramebäck, H., … Ekberg, C. (2017). Impact of Ag and NOx compounds on the transport of ruthenium in the primary circuit of nuclear power plant in a severe accident. Annals of Nuclear Energy, 100, 9–19. doi:10.1016/j.anucene.2016.10.008
- Kajan, I., Lasseson, H., Persson, I., & Ekberg, C. (2016). Interaction of ruthenium tetroxide with surfaces of nuclear reactor containment building. Journal of Nuclear Science and Technology, 53, 1397–1408. doi:10.1080/00223131.2015.1120245
- Kajan, I., Tietze, S., & Ekberg, C. (2016). Interaction of ruthenium tetroxide with iodine-covered surfaces of materials in nuclear reactor containment building. Journal of Nuclear Science and Technology, 53, 1889–1898. doi:10.1080/00223131.2016.1174627
- Kaltofen, M., & Gundersen, A. (2017). Radioactively-hot particles detected in dusts and soils from Northern Japan by combination of gamma spectrometry, autoradiography, and SEM/EDS analysis and implications in radiation risk assessment. Science of the Total Environment, 607–608, 1065–1072. doi:10.1016/j.scitotenv.2017.07.091
- Kamenik, J., Dulaiova, H., Sebesta, F., & St’antna, K. (2013). Fast concentration of dissolved forms of cesium radioisotopes from large seawater samples. Journal of Radioanalytical and Nuclear Chemistry, 296, 841–846. doi:10.1007/s10967-012-2007-4
- Kanda, Y., Miura, T., & Nakajima, H. (2005). Observation of gaseous nitric acid production at a high-energy proton accelerator facility. Radiation Physics and Chemistry, 73, 213–217. doi:10.1016/j.radphyschem.2004.08.009
- Kanda, Y., Momose, T., & Taira, M. (1996). Characterization of radiolytic products from air at a high-energy electronpositron storage ring. Radiation Physical Chemical, 48, 49–54. doi:10.1016/0969-806X(95)00430-6
- Karkela, T., Auvinen, A., Kekki, T., Kotiluoto, P., Lyyranen, J., & Jokiniemi, J. (2015). Radiolytical oxidation of gaseous iodine by beta radiation. Radiochimica Acta, 103, 719–728. doi:10.1515/ract-2015-2417
- Karunakara, N., Ujwal, P., Yashodhara, I., Rao, C., Sudeep Kumara, K., Dileep, B. N., & Ravi, P. M. (2013). Studies on soil to grass transfer factor (F-v) and grass to milk transfer coefficient (F-m) for cesium in Kaiga region. Journal of Environmental Radioactivity, 124, 101–112. doi:10.1016/j.jenvrad.2013.03.008
- Kendall, G. M., Muirhead, C. R., Darby, S. C., Doll, R., Arnold, L., & O’Hagan, J. A. (2004). Epidemiological studies of UK test veterans: I. General description. Journal of Radiological Protection, 24, 199–217. doi:10.1088/0952-4746/24/3/001
- Kim, W.-J., Kim, D., & Park, J. Y. (2013). Fabrication and material issues for the application of sic composites to LWR fuel cladding. Nuclear Engineering and Technology, 45, 565–572. doi:10.5516/NET.07.2012.084
- King, C. R., DiPetrillo, T. A., & Wazer, D. E. (2000). Optimal radiotherapy for prostate cancer: Predictions for conventional external beam, IMRT, and brachytherapy from radiobiologic models. International Journal Radiation Oncology Biologic Physical, (46), 165–172. doi:10.1016/S0360-3016(99)00406-X
- Kitani, S., Noro, T., & Kohara, T. (1972). Removal of methyl lodide by impregnated charcoals from flowing air under humid condition. Journal of Nuclear Science and Technology, 9, 197–202. doi:10.1080/18811248.1972.9734831
- Kleszcz, K., & Mietelski, J. W. (2010). Ni-63 in Chernobyl “ruthenium hot particles” and in forest soil samples. Radiochimica Acta, 98, 377–379. doi:10.1524/ract.2010.1727
- Klochkov, E. P., Risovanyi, V. D., Vaneev, Y. E., & Dorofeev, A. N. (2002). Radiation characteristics of europium-containing control rods in a SM-2 reactor after long-term operation. Atomic Energy, 93, 656–660. doi:10.1023/A:1021096715382
- Ko, M. K. W., Size, N. D., Scott, C., Rodriguez, J. M., Weisenstein, D. K., & Sander, S. P. (1998). Ozone depletion potential of CH3Br. Journal of Geophysical Research—Atmospheres, 103, 28187–28195. doi:10.1029/98JD02537
- Kogan, V., & Schumacher, P. M. (2008). Plutonium release fractions from accidental fires. Nuclear Technology, 161, 190–202. doi:10.13182/NT08-A3922
- Kolarik, Z., & Renard, E. V. (2005). Potential applications of fission platinoids in industry. Platinum Metals Review, 49, 79–90. doi:10.1595/147106705X35263
- Kondo, K., Muramatsu, H., Kanda, Y., & Takahara, S. (1984). Particle-size distribution of BE-7-aerosols formed in high-energy accelerator tunnels. International Journal Applications Radiation and Isotopes, 35, 939–944. doi:10.1016/0020-708X(84)90206-0
- Körblein, A. (2012). Response to “An unexpected mortality increase in the United States follows arrival of the radioactive plume from Fukushima: Is there a correlation?” International Journal of Health Services, 42, 553–555. doi:10.2190/HS.42.3.k
- Kumana, J. D., & Lemlich, R. (1974). Unlit cigarette or cigar as a possible expedient gas mask. Environmental Letters, 6, 19–23. doi:10.1080/00139307409437341
- Kutkov, V. A. (2000). Results of in vivo monitoring of the witnesses of the Chernobyl accident. Radiation Protection Dosimetry, 89, 193–197. doi:10.1093/oxfordjournals.rpd.a033063
- Kuznetsova, I. S., Labutina, E. V., & Hunter, N. (2016). Radiation risks of leukemia, lymphoma and multiple myeloma incidence in the Mayak Cohort: 1948–2004. PLOS ONE, 11, e0162710. doi:10.1371/journal.pone.0162710
- Landeyro, P. A., & Buccafurni, A. (1991). Time-independent neutronic analysis of the Chernobyl accident. Nuclear Science and Engineering, 108, 126–149. doi:10.13182/NSE91-A23813
- Larin, A. E., Malyshevskii, V. S., & Fomin, G. V. (2014). Altitude and latitude distribution of the production rate of BE-7 in the earth’s atmosphere in the parma model. Russian Physics Journal, 57, 803–808. doi:10.1007/s11182-014-0308-5
- Larsen, R. J., Sanderson, C. G., Lee, H.-N., Decker, K. M., & Beck, H. L. (1994). Fission-products detected in Alaska following the Tomsk-7 accident. Journal of Environmental Radioactivity, 23, 205–209. doi:10.1016/0265-931X(94)90061-2
- Lasithiotakis, M., Marsden, B. J., & Marrow, T. J. (2013). Annealing of ion irradiation damage in nuclear graphite. Journal of Nuclear Materials, 427, 95–109. doi:10.1016/j.jnucmat.2012.04.013
- Lea, D. E., & Catheside, D. G. (1942). The mechanism of the induction by radiation of chromosome aberrations inTradescantia. Journal of Genetics, 44, 216–254. doi:10.1007/BF02982830
- Lebaron-Facobs, L., Gaillard-Lecanu, E., Briot, F., Distinguin, S., Boisson, P., Exmelin, L., … Fottorino, R. (2007). Hair dosimetry following neutron irradiation. Health Physics, 92, S98–S104. doi:10.1097/01.HP.0000256282.71542.19
- Lee, M. H., & Clark, S. B. (2005). Activities of Pu and Am isotopes and isotopic ratios in a soil contaminated by weapons-grade plutonium. Environmental Science & Technology, 39, 5512–5516. doi:10.1021/es0486115
- Legg, C. J., Maltby, E., & Proctor, M. C. F. (1992). The ecology of severe moorland fire on the North York moors—seed distribution and seedling establishment of calluna-vulgaris. Journal of Ecology, 80, 737–752. doi:10.2307/2260863
- Leggett, R. W. (1993). An age-specific kinetic-model of lead metabolism in humans. Environmental Health Perspectives, 101, 598–616. doi:10.1289/ehp.93101598
- Leibowitz, L., & Blomquist, R. A. (1991). Thermodynamics and phase-equilibria of the iron uranium system. Journal of Nuclear Materials, 184, 47–52. doi:10.1016/0022-3115(91)90531-B
- Lexa, D., & Dauke, M. (2009). Thermal and structural properties of low-fluence irradiated graphite. Journal of Nuclear Materials, 384, 236–244. doi:10.1016/j.jnucmat.2008.11.013
- Lexa, D., & Kropf, A. J. (2006). Thermal, structural, and radiological properties of irradiated graphite from the ASTRA research reactor—implications for disposal. Journal of Nuclear Materials, 348, 122–132. doi:10.1016/j.jnucmat.2005.09.010
- Li, S. W., Chen, J., & Wan, J. C. (2012). Reduction and removal of neptunium from synthetic high level liquid waste by TRPO process. Journal of Radioanalytical and Nuclear Chemistry, 292, 697–704. doi:10.1007/s10967-011-1480-5
- Li, W., Duan, W., Sun, T., Liu, C., Wang, J., & Chen, J. (2017). Denitration of simulated high-level liquid waste by formic acid for the connection of PUREX process with TRPO process. Journal of Radioanalytical and Nuclear Chemistry, 314, 221–229. doi:10.1007/s10967-017-5357-0
- Lin, C. C., & Chao, J. H. (2006). Radiochemical analysis in fuel integrity evaluation. Nuclear Technology, 160, 244–250. doi:10.13182/NT07-A3896
- Lin, C. C., & Skarpelos, J. M. (1997). Monitoring of fission product release in a boiling water reactor. Journal of Radioanalytical and Nuclear Chemistry, 220, 173–181. doi:10.1007/BF02034852
- Liu, R. Q., Zou, Q., Zu, J. H., Wei, Y. Z., Ding, Y. Q., & Zhao, Y. P. (2017). Feasibility studies on the selective separation of fission palladium(II) by isoHex-BTP/SiO2-P adsorbent from HLLW. Journal of Nuclear Science and Technology, 54, 899–907. doi:10.1080/00223131.2017.1323687
- Lloyd, D. C., Edwards, A. A., Fitzsimons, E. J., Evans, C. D., Railton, R., Jeffrey, P., … Sumitomo, H. (1994). Death of classified worker probably caused by overexposure to gamma-radiation. Occupational and Environmental Medicine, 51, 713–718. doi:10.1136/oem.51.10.713
- Loos-Neskovic, C., Ayrault, S., Badillo, V., Jimenez, B., Garnier, E., Fedoroff, M., … Merinov, B. (2004). Structure of copper-potassium hexacyanoferrate(II) and sorption mechanisms of cesium. Journal of Solid State Chemistry, 177, 1817–1828. doi:10.1016/j.jssc.2004.01.018
- Lopresti, V., Conradson, S. D., & Clark, D. L. (2007). XANES identification of plutonium speciation in RFETS samples. Journal of Alloys and Compounds, 444–445, 540–543. doi:10.1016/j.jallcom.2006.10.101
- Lord, B. I., Woolford, L. B., Wang, L., Stones, V. A., McDonald, D., Lorimore, S. A., … Scott, D. (1998). Tumour induction by methyl nitroso-urea preconceptional paternal contamination with plutonium-239. British Journal of Cancer, 78, 301–311. doi:10.1038/bjc.1998.491
- Lovejoy, E. R., Wang, N. S., & Howard, C. J. (1987). Kinetic-studies of the reactions of HSO with NO2, NO, and O2. Journal Physical Chemical, 91, 5749–5755. doi:10.1021/j100306a046
- Lovelock, J. E., Maggs, R. J., & Wade, R. J. (1973). Halogenated hydrocarbons in and over the Atlantic. Nature, 241, 194–196. doi:10.1038/241194a0
- Lujaniene, G., Šapolaite, J., Remeikis, V., Lujanas, V., Jermolajev, A., & Aninkevičius, V. (2006). Cesium, americium and plutonium isotopes in ground level air of vilnius. Czechoslovak Journal of Physics, 56, D55–D61. doi:10.1007/s10582-006-1077-3
- Lundberg, D., & Persson, I. (2016). The size of actinoid(III) ions—structural analysis vs. common misinterpretations. Coordination Chemistry Reviews, 318, 131–134. doi:10.1016/j.ccr.2016.04.003
- Luther, T. A., Herbst, R. S., Peterman, D. R., Tillotson, R. D., Garn, T. G., Babain, V. A., … Antono, N. G. (2006). Some aspects of fundamental chemistry of the Universal Extraction (UNEX) process for the simultaneous separation of major radionuclides (cesium, strontium, actinides, and lanthanides) from radioactive wastes. Journal of Radioanaytical and Nuclear Chemistry, 267, 603–613. doi:10.1007/s10967-006-0114-9
- Macdonald, H. F., Ballard, P. J., Thompson, I. M. G., Goldfinch, E. P., & Orchard, H. C. (1977). Development and current status of emergency monitoring procedures at CEGB nuclear-power stations. Journal of the British Nuclear Energy Society, 16, 177–186.
- Megaw, W.J. & May, F.G., (1962). Behaviour of iodine released in reactor containers. Journal of nuclear energy parts a and b-reactor science and technology, 16, 427, doi:10.1016/0368-3230(62)90195-7
- Malecki, G., & Ratuszna, A. (1999). Crystal structure of cyanometallates Me-3[Co(CN)(6)](2) and KMe[Fe(CN)(6)] with Me = Mn2+, Ni2+, Cu2+. Powder Diffraction, 14, 25–30. doi:10.1017/S0885715600010265
- Malizia, L. A., Poggi, J.-F., Rossi, C. R., Bellecci, C., & Gaudio, P. (2016). A review of dangerous dust in fusion reactors: From its creation to its resuspension in case of LOCA and LOVA. Energies, (9), 578. doi:10.3390/en9080578
- Mangano, J. J., & Sherman, J. D. (2012). An unexpected mortality increase in the United States follows arrival of the radioactive plume from Fukushima: Is there a correlation? International Journal of Health Services, 42, 47–64. doi:10.2190/HS.42.1.f
- Marchetti, A. A., & Straume, T. (1996). A search for neutron reactions that may be useful for Hiroshima dose reconstruction. Applied Radiation and Isotopes, 47, 97–103. doi:10.1016/0969-8043(95)00240-5
- Marinelli, L. D., Norris, W. P., Gustafson, P. F., & Speckman, T. W. (1953). Transport of radium sulfate from the lung and its elimination from the human body following single accidental exposures. Radiology, 61, 903–915. doi:10.1148/61.6.903
- Mazzocchi, C., Janas, Z., Bączyk, P., Fynbo, H. O. U., & Köster, U. (2012). Precision half-life measurement of Be-7 implanted in different materials. Acta Physica Polonica B, 43, 279–284. doi:10.5506/APhysPolB.43.279
- McCarthy, J. F., Sanford, W. E., & Stafford, P. L. (1998). Lanthanide field tracers demonstrate enhanced transport of transuranic radionuclides by natural organic matter. Environmental Science and Technology, 32, 3901–3906. doi:10.1021/es971004f
- McCurry, J. (2017, March 10). Fukushima evacuees face ‘forced’ return as subsidies withdrawn. The Guardian. Retrieved from https://www.theguardian.com/world/2017/mar/10/japan-fukushima-nuclear-disaster-evacuees-forced-return-home-radiation
- McLaughlin, T. P., Monahan, S. P., Pruvost, N. I., Frolov, V. V., Ryazanov, B. G., & Sviridov, V. I. (2000). A review of criticality accidents 2000 review. Los Alamos, NM: Los Alamos National Laboratory.
- McMurray, H. N. (1993). Uniform colloids of ruthenium dioxide hydrate evolved by the surface-catalyzed reduction of ruthenium tetroxide. Journal Physical Chemical, 97, 8039–8045. doi:10.1021/j100132a038
- Medvedev, Z. A. (1976). Two decades of dissidence. New Scientist, 72(1025), 264–267.
- Meijer, W. O. J. (1955). Synthesis, structures, and properties of platinum metal tellurides. American Mineralogist, 40, 646–657.
- Melo, D. R., Lipsztein, J. L., Oliveira, C. A. N., Lundgren, D. L., Muggenburg, B. A., & Guilmette, R. A. (1997). A biokinetic model for Cs-137. Health Physics, 73, 320–332. doi:10.1097/00004032-199708000-00004
- Métivier, H., & Waight, P. (2002). Chernobyl: Assessment of radiological and health impacts 2002 update of Chernobyl: Ten years on. Boulogne-Billancourt: Nuclear Energy Agency OCED (Organisation for Economic Co-operation and Development).
- Miller, B., Kane, P. O., Robinson, D. B., & Whittingham, P. J. (1978). Determination of a 24-hour time-weighted average value of environmental vinyl-chloride monomer concentration by charcoal adsorption followed by gas-chromatography. Analyst, 103, 1165–1172. doi:10.1039/an9780301165
- Milyutin, V. V., Gelis, V. M., Dzekun, E. G., & Malykh, Y. A. (1995). Sorption technology for removal of cesium-137 from solutions of spent nuclear-fuel reprocessing. Radiochemistry, 37, 85–87.
- Mitchell, E. W., & Taylor, M. R. (1965). Mechanism of stored-energy release at 200degrees C in electron-irradiated graphite. Nature, 208, 638–641. doi:10.1038/208638a0
- Molchanova, I., Mikhailovskaya, L., Antonov, K., Pozolotina, V., & Antonova, E. (2014). Current assessment of integrated content of long-lived radionuclides in soils of the head part of the East Ural Radioactive Trace. Journal of Environmental Radioactivity, 138, 238–248. doi:10.1016/j.jenvrad.2014.09.004
- Monroe, K. R., Murphy, S. P., Kolonel, L. N., & Pike, M. C. (2007). Prospective study of grapefruit intake and risk of breast cancer in postmenopausal women: The Multiethnic Cohort Study. British Journal of Cancer, 97, 440–445. doi:10.1038/sj.bjc.6603880
- Montero, M. P. R., & Sánchez, A. M. (2001). Activity of Pu239+240 and Pu-238 in atmospheric deposits. Applied Radiation and Isotopes, 55, 97–102. doi:10.1016/S0969-8043(00)00365-1
- Moore, F. L. (1960). Liquid-liquid extraction of uranium and plutonium from acetate solution with triiso-octylamine—separation from thorium and fission products. Anayltical Chemistry, 32, 1075–1079. doi:10.1021/ac60165a007
- Moskvin, A. I. (1969). On the complex formation of actinides with acid anions in aqueous solutions. Radiokhimiya, 11, 458.
- Mouly, S., Lloret-Linares, C., Sellier, P. O., Sene, D., & Bergmann, J. F. (2017). Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and Saint-John’s Wort? Pharmacological Research, (118), 82–92. doi:10.1016/j.phrs.2016.09.038
- Moyer, M. (2011). Retrieved October 26, 2017, from https://blogs.scientificamerican.com/observations/researchers-trumpet-another-flawed-fukushima-death-study/
- Mraz, F. R. (1959). Influence of dietary potassium and sodium on cesium-134 and potassium-42 excretion in sheep. Journal of Nutrition, 68, 655–662. doi:10.1093/jn/68.4.655
- Mu, X., Löfroth, P.-O., Karlsson, M., & Zackrisson, B. (2003). The effect of fraction time in intensity modulated radiotherapy: Theoretical and experimental evaluation of an optimisation problem. Radiotherapy and Oncology, 68, 181–187. doi:10.1016/S0167-8140(03)00165-8
- Muirhead, C. R., Kendall, G. M., Darby, S. C., Doll, R., Haylock, R. G. E., O’Hagan, J. A., … Hunter, N. (2004). Epidemiological studies of UK test veterans: II. Mortality and cancer incidence. Journal of Radiological Protection, 24, 219–241. doi:10.1088/0952-4746/24/3/002
- Murray, R. L. (1988, August). The etymology of “scram”. Nuclear News, pp. 106–107.
- Nakanishi, H., Mori, A., Takeda, K., Tanaka, H., Kobayashi, N., Tanoi, K., … Mori, S. (2015). Discovery of radioactive silver (110mAg) in spiders and other fauna in the terrestrial environment after the meltdown of Fukushima Dai-ichi nuclear power plant Proceedings of the Japan Academy Series B: Physical and Biological Sciences, 91, 160–174.
- Nakano, T., Tani, K., Kim, E., Kurihara, O., Sakai, K., & Akashi, M. (2016). Three-year retention of radioactive caesium in the body of tepco workers involved in the Fukushima Daiichi nuclear power station accident. Radiation Protection Dosimetry, 170, 315–317. doi:10.1093/rpd/ncw036
- Nedunchezhian, K., Aswath, N., Thiruppathy, M., & Thirugnanamurthy, S. (2016). Boron neutron capture therapy—a literature review. Journal of Clinical and Diagnostic Research, 10, ZE1–ZE4.
- Neuschütz, D. S., Quade, U., Meier-Kortwig, J., Holappa, L., Hämäläinen, M., Lozano, M. A. H., & Bonany, M. J. G. (2005). Inadvertent melting of radioactive sources in BOF or EAF: Distribution of nuclides, monitoring, prevention. ISIJ International, 45, 288–295. doi:10.2355/isijinternational.45.288
- Newton, D. (1966). A case of accidental inhalation of actinium-227. Health Physics, 12, 1129–1138. doi:10.1097/00004032-196608000-00015
- Nie, B., Ni, M., & Wei, S. (2017). Individual dose due to radioactivity accidental release from fusion reactor. Journal of Hazardous Materials, 327, 135–143. doi:10.1016/j.jhazmat.2016.12.018
- Nielsen, C. E., Wilson, D. A., Brooks, A. L., McCord, S. L., Dagle, G. E., James, A. C., … Morga, W. F. (2012). Microdistribution and long-term retention of Pu-239 (NO3)(4) in the respiratory tracts of an acutely exposed plutonium worker and experimental beagle dogs. Cancer Research, 72, 5529–5536. doi:10.1158/0008-5472.CAN-12-1824
- Ning, P., Qiu, J., Wang, X. Q., Liu, W., & Chen, W. (2013). Metal loaded zeolite adsorbents for hydrogen cyanide removal. Journal of Environmental Sciences, 25, 808–814. doi:10.1016/S1001-0742(12)60138-7
- Noguchi, H., & Murata, M. (1988). Physicochemical speciation of airborne I-131 in japan from Chernobyl. Journal of Environmental Radioactivity, 7, 65–74. doi:10.1016/0265-931X(88)90042-2
- Nomura, H., Maeseto, T., & Osako, M. (2017). Behavior of radioactive cesium during incineration of radioactively contaminated wastes from decontamination activities in Fukushima. Journal of Environmental Radioactivity, 178, 290–296.
- Nomura, S., Blangiardo, M., Tsubokura, M., Nishikawa, Y., Gilmour, S., Kami, M., & Hodgson, S. (2016). Post-nuclear disaster evacuation and survival amongst elderly people in Fukushima: A comparative analysis between evacuees and non-evacuees. Preventative Medicine, 82, 77–82. doi:10.1016/j.ypmed.2015.11.014
- Nomura, T. (1982). Parental exposure to X-rays and chemicals induces heritable tumors and anomalies in mice. Nature, 296, 575–577. doi:10.1038/296575a0
- Norman, C. (1983). The Kyshtym mystery (CONTD). Science, 221, 138. doi:10.1126/science.221.4606.138
- Norman, E. B., Rech, G. A., Browne, E., Larimer, R.-M., Dragowsky, M. R., & Chan, Y. D. (2001). Influence of physical and chemical environments on the decay rates of Be-7 and K-40. Physics Letters B, 519, 15–22. doi:10.1016/S0370-2693(01)01097-8
- Oak Ridge. (1999). Retrieved September 30, 2017, from http://www.orau.org/ptp/collection/dosimeters/dosimeters.htm
- Odintsov, A. A., Khan, V. E., Krasnov, V. A., Pazukhin, E. M., & Shcherbin, V. N. (2009). Transuranium elements in liquid radioactive wastes from the Shelter. Radiochemistry, 51, 383–389. doi:10.1134/S1066362209040109
- Ogorodnikov, B. I., Budyka, A. K., Khan, V. E., Pazukhin, E. M., Koval’chuk, V. P., & Krasnov, V. A. (2009). 220Rn as an additional factor of radiation hazard in the Shelter. Radiochemistry, 51, 390–399. doi:10.1134/S1066362209040110
- Olson, M. (2011). Atomic radiation is more harmful to women. Nuclear Information and Resource Service Briefing Paper. Retrieved from https://www.nirs.org/wp-content/uploads/2017/02/corrected-radiationwomenfinal.pdf
- Papastefanou, C., Manolopoulou, M., & Charalambous, S. (1988). Silver-110m and Sb-125 in Chernobyl fallout. Science of the Total Environment, 72, 81–85. doi:10.1016/0048-9697(88)90008-3
- Parajuli, D., Tanaka, H., Hakuta, Y., Minami, K., Fukuda, S., Umeoka, K., … Kawamoto, T. (2013). Dealing with the aftermath of Fukushima Daiichi nuclear accident: Decontamination of radioactive cesium enriched ash. Environmental Science and Technology, 47, 3800–3806. doi:10.1021/es303467n
- Paramonova, V. I., & Kalychev, V. B. (1966). Some additional notes to the theory of method of relative absorption using U(VI) in acetate-nitrate solutions as an example. Radiokhimiya, 8, 304.
- Pedersen-Bjergaard, J., Philip, P., Mortensen, B. T., Ersb, J., ll, Jensen, G., Panduro, J., & Thomsenen, M. (1981). Acute nonlymphocytic leukemia, preleukemia, and acute myeloproliferative syndrome secondary to treatment of other malignant diseases. Clinical and cytogenetic characteristics and results of in vitro culture of bone marrow and HLA typing. Blood, 57, 712–723.
- Perricone, A., & Noël, H. (2002). Crystal structure and magnetic properties of the binary uranium-nickel alloy UNi4. Intermetallics, 10, 519–521. doi:10.1016/S0966-9795(02)00015-8
- Perricone, A., Potel, M., & Noël, H. (2002). Crystal structure and magnetic properties of the binary uranium-nickel alloy U11Ni16. Journal of Alloys and Compounds, 340, 39–42. doi:10.1016/S0925-8388(02)00009-9
- Ph Hubert, F., Gaye, P. J., Medina, B., & Pravikoff, M. S. (2009). Radioactivity measurements applied to the dating and authentication of old wines. Comptes Rendus Physique, 10, 622–629. doi:10.1016/j.crhy.2009.08.007
- Pinder, J. E., III, Hinton, T. G., Taylor, B. E., & Whicker, F. W. (2011). Cesium accumulation by aquatic organisms at different trophic levels following an experimental release into a small reservoir. Journal of Environmental Radioactivity, 102, 283–293. doi:10.1016/j.jenvrad.2010.12.003
- Platonov, P. A., Burlakov, E. V., Chugunov, O. K., Dostov, A. I., Alekseev, V. M., & Smorodkin, E. I. (2003). Computational-experimental investigation of the Wigner energy and the physical properties of plunger graphite in the safety and control system of an RBMK reactor. Atomic Energy, 94, 225–237. doi:10.1023/A:1024737702092
- Porfiriev, B. N. (1996). Environmental aftermath of the radiation accident at Tomsk-7. Environmental Management, 20, 25–33. doi:10.1007/PL00006699
- Ramirez, N., Cuadras, A., Rovira, E., Borrull, F., & Marce, R. M. (2010). Comparative study of solvent extraction and thermal desorption methods for determining a wide range of volatile organic compounds in ambient air. Talanta, 82, 719–727. doi:10.1016/j.talanta.2010.05.038
- Renaud, P., & Gonze, M.-A. (2014). Lessons from the Fukushima and Chernobyl accidents concerning the Cs-137 contamination of orchard fresh fruits. Radioprotection, 49, 169–175. doi:10.1051/radiopro/2014004
- Resende, S. M. (2007). The atmospheric oxidation of the HS radical: Reaction with NO2. Journal Atmos Chemical, 56, 21–32. doi:10.1007/s10874-006-9040-z
- Reyerson, L. H., & Cameron, A. E. (1936). The sorption of bromine and iodine by activated charcoal. Journal Physical Chemical, 40, 233–237. doi:10.1021/j150371a009
- Richardson, D., Sugiyama, H., Nishi, N., Sakata, R., Shimizu, Y., Grant, E. J., … Kasagi, F. (2009). Ionizing radiation and leukemia mortality among Japanese Atomic Bomb Survivors, 1950–2000. Radiation Research, 172, 368–382. doi:10.1667/RR1801.1
- Robison, W. L., Brown, P. H., Stone, E. L., Hamilton, T. F., Conrado, C. L., & Kehl, S. (2009). Distribution and ratios of Cs-137 and K in control and K-treated coconut trees at Bikini Island where nuclear test fallout occurred: Effects and implications. Journal of Environmental Radioactivity, 100, 76–83. doi:10.1016/j.jenvrad.2008.10.016
- Robison, W. L., Conrado, C. L., Bogen, K. T., & Stoker, A. C. (2003). The effective and environmental half-life of Cs-137 at Coral Islands at the former US nuclear test site. Journal of Environmental Radioactivity, 69, 207–223. doi:10.1016/S0265-931X(03)00080-8
- Romanchuk, A. Y., Kalmykov, S. N., Egorov, A. V., Zubavichus, Y. V., Shiryaev, A. A., Batuk, O. N., … Presnyakov, I. A. (2013). Formation of crystalline PuO2+x center dot nH(2)O nanoparticles upon sorption of Pu(V,VI) onto hematite. Geochimica et Cosmochimica Acta, 121, 29–40. doi:10.1016/j.gca.2013.07.016
- RPPI. (2006). Retrieved from https://web.archive.org/web/20131015024743/http://www.rpii.ie/Site/Media/Press-Releases/RTE-s-docudrama-scenario-could-not-happen-accordin.aspx
- Rudenko, L. I., & Khan, V. E. (2005). Membrane methods for treating liquid radioactive wastes from the shelter to remove transuranic elements. Radiochemistry, 47, 89–92. doi:10.1007/s11137-005-0054-1
- Rudenko, L. I., & Khan, V. E. (2005a). Contribution of radionuclides associated with microparticles to their migration from the shelter and its service area to groundwater. Radiochemistry, 47, 93–95. doi:10.1007/s11137-005-0055-0
- Russell, W. L. (1957). Shortening of life in the offspring of male mice exposed to neutron radiation from an atomic bomb. Proceedings of the National Academy of Sciences of the United States of America, 43, 324–329. doi:10.1073/pnas.43.4.324
- Saccomanno, G., Huth, G. C., Auerbach, O., & Kuschner, M. (1988). Relationship of radioactive radon daughters and cigarette-smoking in the genesis of lung-cancer in uranium miners. Cancer, 62, 1402–1408. doi:10.1002/(ISSN)1097-0142
- Sahoo, S. K., Kavasi, N., Sorimchi, A., Arae, H., Tokonami, S., Mietelski, J. W., … Yoshida, S. (2016). Strontium-90 activity concentration in soil samples from the exclusion zone of the Fukushima Daiichi nuclear power plant. Scientific Reports, 6, 23925. doi:10.1038/srep23925
- Sakai, E. (1984). Performance of a nuclear-reactor cover-gas monitor using charcoal—GE gamma-ray spectrometer combination. IEEE Transactions on Nuclear Science, NS-31, 757–760. doi:10.1109/TNS.1984.4333361
- Salminen, S., Paatero, J., Jaakkola, T., & Lehto, J. (2005). Americium and curium deposition in Finland from the Chernobyl accident. Radiochimica Acta, 93, 771–779. doi:10.1524/ract.2005.93.12.771
- Sanap, K. K., Varma, S., Waghmode, S. B., & Bharadwaj, S. R. (2015). Wire gauze and cordierite supported noble metal catalysts for passive autocatalytic recombiner. Nuclear Engineering and Design, 294, 226–232. doi:10.1016/j.nucengdes.2015.09.010
- Sánchez, A. M., de la Torre Pérez, J., Sánchez, A. B. R., & Correa, F. L. N. (2013). Additional contamination when radon is in excess. Applied Radiation and Isotopes, 81, 212–215. doi:10.1016/j.apradiso.2013.03.004
- Sandalls, F. J., Segal, M. G., & Victorova, N. (1993). Hot particles from Chernobyl—a review. Journal of Environmental Radioactivity, 18, 5–22. doi:10.1016/0265-931X(93)90063-D
- Sanders, C. L., & Adee, R. R. (1968). Phagocytosis of inhaled plutonium oxide-239Pu particles by pulmonary macrophages. Science, 162(3856), 918–920. doi:10.1126/science.162.3856.918
- Sanzharova, N. I., Fesenko, S. V., Alexakhin, R. M., Anisimov, V. S., Kuznetsov, V. K., & Chernyayeva, L. G. (1994). Changes in the forms of 137Cs and its availability for plants as dependent on properties of fallout after the Chernobyl nuclear power plant accident. Science of the Total Environment, 154, 9–22. doi:10.1016/0048-9697(94)90609-2
- Sappok, M. (1991). Recycling of metallic materials from the dismantling of nuclear-plants. Kerntechnik, 56, 376–378.
- Schneider, U., Stipper, A., & Besserer, J. (2010). Dose-response relationship for lung cancer induction at radiotherapy dose. Z Medica Physical, 20, 206–214. doi:10.1016/j.zemedi.2010.03.008
- Schneider, U., Sumila, M., Robotka, J., Gruber, G., Mack, A., & Besserer, J. (2011). Dose-response relationship for breast cancer induction at radiotherapy dose. Radiation Oncology, 6(67), 1–7. doi:10.1186/1748-717X-6-67
- Schubert, W., Cullberg, G., Edgar, B., & Hedner, T. (1994). Inhibition of 17-beta-estradiol metabolism by grapefruit juice in ovariectomized women. Maturitas, 20, 155–163. doi:10.1016/0378-5122(94)90012-4
- Schweiger, M., Seidel, S. R., Arif, A. M., & Stang, P. J. (2002). Solution and solid state studies of a triangle-square equilibrium: Anion-induced selective crystallization in supramolecular self-assembly. Inorganic Chemistry, 41, 2556–2559. doi:10.1021/ic0112692
- Selby, P. B., Earhart, V. S., & Raymer, G. D. (2005). The influence of dominant lethal mutations on litter size and body weight and the consequent impact on transgenerational carcinogenesis. Mutation Research, 578, 382–394. doi:10.1016/j.mrfmmm.2005.06.025
- Selby, P. B., & Priest, N. D. (2005). First-generation offspring of male mice exposed to Pu-239-citrate show no evidence of leukaemia or life shortening. International Journal of Radiation Biology, 81, 273–290. doi:10.1080/09553000500140480
- Serezhkina, L. B., Vologzhanina, A. V., Klepov, V. V., & Serezhkin, V. N. (2011). СИНТЕЗ И РЕНТГЕНОСТРУКТУРНОЕ ИССЛЕДОВАНИЕ (CS0.5BA0.25)[UO2(CH3COO)3] И BA0.5[UO2(CH3COO)3] [Synthesis and X-ray structural study of (CS0.5BA0.25)[UO2(CH3COO)3] and BA0.5[UO2(CH3COO)3]]. Kristallografiya, 56, 290.
- Serezhkina, L. V., Vologzhanina, A. V., Klepov, V. V., & Serezhkin, V. N. (2010). КРИСТАЛЛИЧЕСКАЯ СТРУКТУРА R[UO2(CH3COO)3] (R = NH, K+ ИЛИ CS+) [Crystalline structure of R [UO2 (CH3COO) 3] (R = NH, K+ or CS+)]. Kristallografiya, 55, 822.
- Serrano-Purroy, D., Christiansen, B., Glatz, J.-P., Malmbeck, R., & Modolo, G. (2005). Towards a DIAMEX process using high active concentrate. Production of genuine solutions. Radiochimica Acta, 93, 357–361. doi:10.1524/ract.93.6.357.65645
- Shann, S. H., & Olander, D. R. (1983). Stress-corrosion cracking of zircaloy by cadmium, iodine, and metal iodides. Journal of Nuclear Materials, 113, 234–248. doi:10.1016/0022-3115(83)90148-4
- Sheil, A. E., Botzem, W., & Johnston, C. K. (1999). Decommissioning of windscale pile 1. WM’99 Conference. Tucson, Arizona. Retrieved from http://www.wmsym.org/archives/1999/02/2-1.pdf
- Shiba, K., Hanada, M., & Yajima, S. (1969). Chemical state of fission tellurium released from irradiated UO2 and UO2-graphite mixtures. Journal of Nuclear Science and Technology, 6, 333–337. doi:10.1080/18811248.1969.9732897
- Shih, T.-S., Liu, J.-B., & Wei, P.-S. (2007). Oxide films on magnesium and magnesium alloys. Materials Chemistry and Physics, 104, 497–504. doi:10.1016/j.matchemphys.2007.04.010
- Shikata, E., & Amano, H. (1973). Dry-distillation of iodine-131 from several tellurium compounds. Journal of Nuclear Science and Technology, (10), 80–88. doi:10.1080/18811248.1973.9735382
- Shilnikova, N. S., Preston, D. L., Ron, E., Gilbert, E. S., Vassilenko, E. K., Romanov, S. A., … Koshurnikova, N. A. (2003). Cancer mortality risk among workers at the Mayak nuclear complex. Radiation Research, 159, 787–798. doi:10.1667/0033-7587(2003)159[0787:CMRAWA]2.0.CO;2
- Shiota, K., Takaoka, M., Fujimori, T., Oshita, K., & Terada, Y. (2015). Cesium speciation in dust from municipal solid waste and sewage sludge incineration by synchrotron radiation micro-X-ray analysis. Analytical Chemistry, 87, 11249–11254. doi:10.1021/acs.analchem.5b03298
- Skarlou, V., Nobeli, C., Anoussis, J., Haidouti, C., & Papanicolaou, E. (1999). Transfer factors of Cs-134 for olive and orange trees grown on different soils. Journal of Environmental Radioactivity, 45, 139–147. doi:10.1016/S0265-931X(98)00098-8
- Sloter, E., Nemec, M., Stump, D., Holson, J., Kirkpatrick, D., Gargas, M., & Kinzell, J. (2009). Methyl iodide-induced fetal hypothyroidism implicated in late-stage fetal death in rabbits. Inhalation Toxicology, 21, 462–479. doi:10.1080/08958370802596942
- Smallman, C. (1981). Biology for you book one (pp. 142). London: Hutchinson.
- Son, Y.-S., Jung, I.-H., Lee, S.-J., Koutrakis, P., & Kim, J.-C. (2015). Decomposition of sulfur compounds by radiolysis: II. By-products and mechanisms. Chemical Engineering Journal, 269, 27–34. doi:10.1016/j.cej.2015.01.079
- Soran, D. M., & Stillman, D. B. (1982). An analysis of the alleged Kyshtym disaster, LA-9217-MS. Los Alamos: Los Alamos National Lab.
- Spalding, J. F., Brooks, M. R., & Tietjen, G. L. (1969). Lifetime body weights and mortality distributions of mice with 10 generations to 35 generations of ancestral X-ray exposure. Genetics, 63, 897–906.
- Spencer, E. A., Key, T. J., Appleby, P. N., van Gils, C. H., Olsen, A., Tjønneland, A., … Riboli, E. (2009). Prospective study of the association between grapefruit intake and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes & Control, 20, 803–809. doi:10.1007/s10552-009-9310-6
- SRP. (2011). A practical guide to decommissioning for the medical. Industrial or Research Small User, can be downloaded from. Retrieved from https://srp-uk.org/resources/practical-advice
- Stewart, F. A., Akleyev, A. V., Hauer-Jensen, M., Hendry, J. H., Kleiman, N. J., MacVittie, T. J., … Wallace, W. H. (2012). ICRP statement on tissue reactions/early and late effects of radiation in normal tissues and organs—threshold doses for tissue reactions in a radiation protection context. Annals of the ICRP, 41, 118. ( ICRP Publication).
- Stewart, N. G., Crooks, R. N., & Fisher, E. M. R. (1961). Measurements of the Radioactivity of the Cloud from the Accident at Windscale: Data submitted to the I.G.Y. United Kingdom Atomic Energy Authority, AERE-M, 857, 1961.
- Swider-Lyons, K. E., Love, C. T., & Rolison, D. R. (2005). Selective vapor deposition of hydrous RuO2 thin films. Journal of the Electrochemical Society, 152, C158–C162. doi:10.1149/1.1859713
- Takahashi, T., Watanabe, H., Dohi, K., & Ito, A. (1992). CF-252 relative biological effectiveness and inheritable effect of fission neutrons in mouse-liver tumorigenesis. Cancer Research, 52, 1948–1953.
- Takewaki, I. (2011). Preliminary report of the 2011 off the Pacific coast of Tohoku Earthquake. University Science A (Applied Physical & Engineering), 12, 327–334.
- Tassaneeyakul, W., Guo, L.-Q., Fukuda, K., Ohta, T., & Yamazoe, Y. (2000). Inhibition selectivity of grapefruit juice components on human cytochromes P450. Archives of Biochemistry and Biophysics, 378, 356–363. doi:10.1006/abbi.2000.1835
- Taylor, N. P., & Raskob, W. (2007). Updated accident consequence analyses for ITER at Cadarache. Fusion Science and Technology, 52, 359–366. doi:10.13182/FST07-A1514
- Telling, R. H., & Heggie, M. I. (2007). Radiation defects in graphite. Philosophical Magazine, 87, 4797–4846. doi:10.1080/14786430701210023
- Thouless, R. H. (1953). Straight and crooked thinking. London: Pan Books.
- Tinganelli, W., Durante, M., Hirayama, R., Krämer, M., Maier, A., Kraft-Weyrather, W., … Scifoni, E. (2015). Kill-painting of hypoxic tumours in charged particle therapy. Scientific Reports, 5, 17016. doi:10.1038/srep17016
- Torgov, V. G., Tatatchuk, V. V., Druzhinina, I. A., Korda, T. M., & Renard, E. V. (2000). Possibility of a high degree of removal of silver from fission palladium by petroleum sulfides. Atomic Energy, 88, 373–377. doi:10.1007/BF02680532
- Torgov, V. G., Tatatchuk, V. V., Druzhinina, I. A., Korda, T. M., Tatatchuk, A. N., & Renard, E. V. (1994). Justification of the choice of an extraction system on the basis of petroleum sulfides for extracting fragment palladium. Atomic Energy, (76), 442–448. doi:10.1007/BF02408108
- Tuma, D. (2000). suffolk study to target rare cancer cluster. Daily News. Retrieved from http://www.nydailynews.com/archives/boroughs/suffolk-study-target-rare-cancer-cluster-article-1.884577
- Ulrich, K. (2017). Unequal impact. Edited A. Kashiwagi and K. Suzuki. Tokyo: Greenpeace. http://www.greenpeace.org/japan/Global/japan/pdf/Uequal-impact-en.pdf downloaded 30 March 2018
- Vajda, N., & Kim, C.-K. (2010). Determination of radiostrontium isotopes: A review of analytical methodology. Applied Radiation and Isotopes, 68, 2306–2326. doi:10.1016/j.apradiso.2010.05.013
- VanDam, H. (1986). Silver from Chernobyl. Nature, 324, 216. doi:10.1038/324216a0
- Velasco, H., Cid, A. S., Anjos, R. M., Zamboni, C. B., Rizzotto, M., Valladares, D. L., & Ayub, J. J. (2012). Variability of Cs-137 and K-40 soil-to-fruit transfer factor in tropical lemon trees during the fruit development period. Journal of Environmental Radioactivity, 104, 64–70. doi:10.1016/j.jenvrad.2011.09.016
- Wake, D., Brown, R. C., Trottier, R. A., & Liu, Y. (1992). Measurements of the efficiency of respirator filters and filtering facepieces against radon daughter aerosols. The Annals of Occupational Hygiene, 36, 629–636.
- Walker, T. (2000). Radioactive decay rate depends on chemical environment. CEN Technical Journal, 1, 4–5. https://creation.com/images/pdfs/tj/j14_1/j14_1_04-05.pdf
- Wanklyn, A. (2016). Retrieved from https://www.japantimes.co.jp/news/2016/09/23/national/crime-legal/canada-activist-found-guilty-harassing-scientists-fukushima-fallout/#.Wem9soVOKUk
- Willard, H. H., & Goodspeed, E. W. (1936). Separation of strontium, barium, and lead from calcium and other metals—by precipitation as nitrates. Industrial and Engineering Chemistry, 8, 414–418.
- Willis, C., Boyd, A. W., & Young, M. J. (1970). Radiolysis of air and nitrogen–oxygen mixtures with intense electron pulses: Determination of a mechanism by comparison of measured and computed yields. Canada Journal Chemical, 48, 1515–1525. doi:10.1139/v70-247
- Willis, C., Boyd, A. W., Young, M. J., & Armstrong, D. A. (1970). Radiation chemistry of gaseous oxygen: Experimental and calculated yields. Canada Journal Chemical, 48, 1505–1514. doi:10.1139/v70-246
- Winkelmann, I., Endrulat, H.-J., Fouasnon, S., Gesewsky, P., Haubelt, R., Klopfer, L., … Wolff, S. (1986). Ergebnisse Von Radioaktivitätsmessungen Nach Dem Reaktorunfall in Tschernobyl. Oberschleissheim: Institut fur Strahlenhygiene, ISH-Heft 99.
- Wojtczak, B. A., Andrysiak, A., Gruener, B., & Lesnikowski, Z. J. (2008). “Chemical Ligation”: A versatile method for nucleoside modification with boron clusters. Chemistry—A European Journal, 14, 10675–10682. doi:10.1002/chem.200801053
- Wolf, A. (2012). Response to “an unexpected mortality increase in the United States follows arrival of the radioactive plume from Fukushima: Is there a correlation?” International Journal of Health Services, 42, 549–551. doi:10.2190/HS.42.3.j
- Wolfsberg, A., Dai, Z., Zhu, L., Reimus, P., Xiao, T., & Ware, D. (2017). Colloid-Facilitated Plutonium Transport in Fractured Tuffaceous Rock. Environmental Science and Technology, 51, 5582–5590. doi:10.1021/acs.est.7b00968
- Wood, D. J., & Law, J. D. (1997). Evaluation of the SREX solvent extraction process for the removal of Sr-90 and hazardous metals from acidic nuclear waste solutions containing high concentrations of interfering alkali metal ions. Separation Science and Technology, 32, 241–253. doi:10.1080/01496399708003197
- Wood, G. O. (1981). Respirator canister testing for radioiodine. American Industrial Hygiene Association Journal, 42, 570–578. doi:10.1080/15298668191420297
- Xu, S., Cook, G. T., Cresswell, A. J., Dunbar, E., Freeman, S. P. H. T., Hou, X., … Zhang, L. (2016). Carbon, cesium and iodine isotopes in Japanese cedar leaves from Iwaki, Fukushima. Journal of Radioanalytical and Nuclear Chemistry, 310, 927–934.
- Xu, S., Zhang, L. Y., Freeman, S. P. H. T., Hou, X. L., Shibata, Y., Sanderson, D., … Tanaka, A. (2015). Speciation of radiocesium and radioiodine in aerosols from Tsukuba after the Fukushima nuclear accident. Environmental Science and Technology, 49, 1017–1024. doi:10.1021/es504431w
- Xuan, R. C., Yates, S. R., Ashworth, D. J., & Luo, L. F. (2012). Mitigating 1,3-dichloropropene, chloropicrin, and methyl iodide emissions from fumigated soil with reactive film. Environmental Science & Technology, 46, 6143–6149. doi:10.1021/es300388r
- Yablokov, A. V., Nesterenko, V. B., & Nesterenko, A. V. (2009). Atmospheric, water, and soil contamination after Chernobyl. Annals New York Academic Sciences, 1181, 223–236.
- Yamamoto, K., & Okumura, K. (2014). A study of the generation of U-232 in UO2 and MOX fuels. Journal of Nuclear Science and Technology, 51, 568–573. doi:10.1080/00223131.2014.882803
- Yamamoto, M., Sakaguchi, A., Ochiai, S., Takada, T., Hamataka, K., Murakami, T., & Nagao, S. (2014). Isotopic Pu, Am and Cm signatures in environmental samples contaminated by the Fukushima Dai-,ichi Nuclear Power Plant accident. Journal of Environmental Radioactivity, 132, 31–46. doi:10.1016/j.jenvrad.2014.01.013
- Yokouchi, Y., Mukai, H., Yamamoto, H., Otsuki, A., Saitoh, C., & Nojiri, Y. (1997). Distribution of methyl iodide, ethyl iodide, bromoform, and dibromomethane over the ocean (east and southeast Asian seas and the western Pacific). Journal of Geophysical Research—Atmospheres, 102, 8805–8809. doi:10.1029/96JD03384
- Yoschenko, V. I., Kashparov, V. A., Levchuka, S. E., Glukhovskiy, S., Khomutinin, Y. V., Protsak, V. P., … Tschiersch, J. (2006). Resuspension and redistribution of radionuclides during grassland and forest fires in the Chernobyl exclusion zone: Part II. Modeling the transport process. Journal of Environmental Radioactivity, 87, 260–278. doi:10.1016/j.jenvrad.2005.12.003
- Yoschenko, V. I., Kashparov, V. A., Protsak, V. P., Lundin, S. M., Levchuk, S. E., Kadygrib, A. M., … Tschiersch, J. (2006). Resuspension and redistribution of radionuclides during grassland and forest fires in the Chernobyl exclusion zone: Part I. Fire experiments. Journal of Environmental Radioactivity, 86, 143–163. doi:10.1016/j.jenvrad.2005.08.003
- Youn, D., Patten, K. O., Wuebbles, D. J., Lee, H., & So, C. W. (2010). Potential impact of iodinated replacement compounds CF3I and CH3I on atmospheric ozone: A three-dimensional modeling study. Atmospheric Chemistry and Physics, 10, 10129–10144. doi:10.5194/acp-10-10129-2010
- Yu, W., He, J. H., Lin, W. H., Li, Y. L., Men, W., Wang, F. F., & Huang, J. (2015). Distribution and risk assessment of radionuclides released by Fukushima nuclear accident at the northwest Pacific. Journal of Environmental Radioactivity, 142, 54–61. doi:10.1016/j.jenvrad.2015.01.005
- Zachariasen, W. H., & Plettinger, H. A. (1959). Crystal chemical studies of the 5F-series of elements. 25. The crystal structure of sodium uranyl acetate. Acta Crystallographica, 12, 526–530. doi:10.1107/S0365110X5900158X
- Zhang, A. Y., Xu, L., & Lei, G. (2016). Separation and complexation of palladium(II) with a new soft N-donor ligand 6,6ʹ-bis(5,6-dinonyl-1,2,4-triazin-3-yl)-2,2ʹ-bipyridine (C9-BTBP) in nitric acid medium. New Journal of Chemistry, 40, 6374. doi:10.1039/C5NJ03082J
- Zhang, W., Friese, J., & Ungar, K. (2013). The ambient gamma dose-rate and the inventory of fission products estimations with the soil samples collected at Canadian embassy in Tokyo during Fukushima nuclear accident. Journal Radioanal Nuclear Chemical, 296, 69–73. doi:10.1007/s10967-012-2040-3
- Zheng, W., Papiernik, S. K., Guo, M., Dungan, R. S., & Yates, S. R. (2005). Construction of a reactive surface barrier to reduce fumigant 1,3-dichloropropene emissions. Environmental Toxicology and Chemistry, 24, 1867–1874. doi:10.1897/04-488R.1
- Zhou, L., Rao, R., Corcoran, E., & Kelly, D. (2016). Distribution of radionuclides between atmosphere and ash during combustion of contaminated vegetation. Journal of Environmental Radioactivity, 165, 159–167. doi:10.1016/j.jenvrad.2016.09.017
- Zilberman, B. Y., & Romanovskii, V. N. (2003). Extraction Studies at the Khlopin Radium Institute. Radiochemistry, 45(3), 211–218. (Translation of Radiokhimiya, 2003, 45, 193–199). doi:10.1023/A:1026010706135
- Zimmers, T. A., Sheldon, J., Lubarsky, D. A., Lopez-Munoz, F., Waterman, L., Weisman, R., & Koniaris, L. G. (2007). Lethal injection for execution: Chemical asphyxiation? PLOS Medicine, 4(4), e156. doi:10.1371/journal.pmed.0040156
- Zinkle, S. J., Terrani, K. A., Gehin, J. C., Ott, L. J., & Snead, L. L. (2014). Accident tolerant fuels for LWRs: A perspective. Journal of Nuclear Materials, (448), 374–379. doi:10.1016/j.jnucmat.2013.12.005
- Zou, Q., Liu, R. Q., Ning, S. Y., Wang, X. P., & Wei, Y. Z. (2017). Recovery of palladium by silica/polymer-based 2,6-bis(5,6,7,8-tetrahydro-5,8,9,9-tetramethyl-5,8-methano-1,2,4-benzotriazin-3-yl)pyridine adsorbents from high level liquid waste. Journal of Nuclear Science and Technology, 54, 569–577. doi:10.1080/00223131.2017.1298481
- Zundel, W. S., Tyler, F. H., Mays, C. W., Lloyd, R. D., Wagner, W. W., & Pendleton, R. C. (1969). Short half-times of caesium-137 in pregnant women. Nature, 221, 89–90. doi:10.1038/221089b0


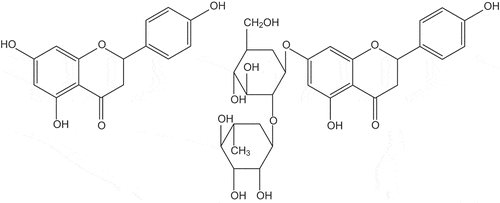
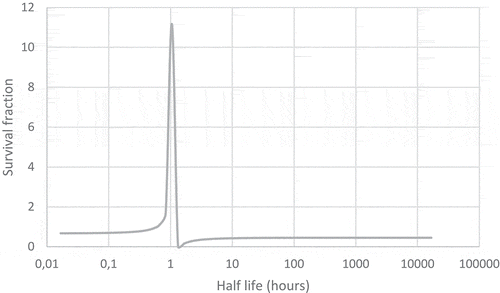
![Figure 10. The anion in ammonium molydophosphate [Mo12O36·PO4]3−.](/cms/asset/df2cc14c-f6a9-453c-9c45-495a7b9e9fec/oach_a_1450944_f0010_c.jpg)