?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ethanol is a common psychoactive substance that has been widely consumed in several parts of the world. Gas chromatography (GC) coupled with flame ionisation detector (FID) has often been used for the determination of blood alcohol concentration. The aim of this study was to develop and validate a very simple and reliable HS-GC-FID method for quantitative determination of ethanol in blood sample. Validation of the method was performed by means of Bias, Linearity, LOD, Selectivity, Specificity, Precision, Robustness and Intermediate precision. The method showed an excellent linearity with correlation coefficient (r2 = 0.993) was observed in the range from 0.1 to 3.5 mg/mL of ethanol. The percent recovery value were between 91.0 and 109.1 which was with an acceptable percent recovery, The precision (repeatability) was reported as 27 % and intermediate precision of the method resulted in 11% and 1 % for two analysts. The limit of detection (LOD) of ethanol was calculated as 0.099 mg/mL and the selectivity of the method for interferents (methanol and acetaldehyde) was totally selective. Generally, the results obtained confirmed that the method is relatively fast, precise, simple, robust and can be used in routine forensic analyses for the determination of blood alcohol concentration (BAC) at a concentration level greater than 0.13 mg/mL.
PUBLIC INTEREST STATEMENT
Alcoholic intoxication frequently results some impairments that can lead to violent crimes during driving, sexual abuse and fatal accidents. Ethanol is commonly used in forensic toxicology analysis mainly in post-mortem analysis, which causes alcohol intoxications. Several analytical methods have been used to determine the amount of ethanol in various samples. Among these gas chromatography (GC), which is most precise and reliable analytical method, has been used for the quantitative determination of alcohol in human blood, urine and oral fluids for forensic toxicological analysis. Thus, the researchers were interested and worked on the development of cheaper, environment-friendly and efficient novel methods for the fast and accurate determination of ethanol in human-based samples, which is expected to have greater importance for alleviating the risks associated to alcoholic intoxications.
Competing interests
The authors declares no competing interests.
1. Introduction
Ethanol is a psychoactive substance used worldwide more frequently in an enormous amount. It is commonly found in forensic toxicology analysis, mainly in post-mortem analysis due to alcohol intoxication which is associated with impairments that frequently lead to violent crimes such as driving under alcohol influence, sexual abuse and fatal accidents(Chun et al., Citation2016; Kovatsi et al., Citation2011; Tiscione. et al., Citation2011). Blood is the ideal human-based sample used for the analysis of alcohol and other drugs of abuse (Chun et al., Citation2016). The concentration of ethanol in blood indicates the level of intoxication of an individual and its impact on various human activities. The BAC is significantly used as a standard marker for alcohol concentrations in urine and oral fluid alcohol analysis (Chun et al., Citation2016; Görkem et al., Citation2010). Hence, a fast and accurate procedure for the quantitation of BAC is crucial (Westland & Dorman, Citation2013).
Several analytical methods have been used to determine the amount of ethanol in various samples. Among these gas chromatography (GC) (Chun et al., Citation2016; Görkem et al., Citation2010; O’Neal et al., Citation1996; Snow & Slack, Citation2002), high-performance liquid chromatography (HPLC) (Nikelly & Betz, Citation1987; Yarita et al., Citation2002) and infrared spectroscopy (IR) (Lachenmeier, Citation2007) were commonly used. However, GC is the most precise and reliable analytical method preferred for the quantitation of alcohol in human blood, urine and oral fluids in several forensic toxicological analyses (Hong-tao et al., Citation2014). For the reliability of the analysis of such complex sample matrices, sample preparation is the most important step. There are several sample preparation techniques available for the determination of ethanol in human specimens, including direct injection, static, dynamic headspace(O’Neal et al., Citation1996; Snow & Slack, Citation2002) or headspace injection using solid-phase microextraction (HS-SPME)(De Martinis & Martin, Citation2002; Zuba et al., Citation2002) and GC-HS (Diana et al., Citation2009).
The need for developing novel methods and assessing and validating the developed methodologies for faster and accurate determination of ethanol in the human-based sample is of great importance (De Martinis et al., Citation2004). The head space-gas chromatography (HS-GC-FID) is currently the most commonly used analytical technique for the analysis of volatile analytes in human specimens because the technique has tremendous capability to detect trace levels of analytes with volatility nature in the absence of complex and lengthy sample preparation techniques (Görkem et al., Citation2010). Moreover, the technique is highly preferred in the laboratories dealing with heavily routine laboratory works (Zuba et al., Citation2002).
In comparison with others sample preparation procedures, head space (HS) for alcohol analysis presents several advantages such as ease of sample preparation, free of contamination, reduces interferences and saves time (De Martinis et al., Citation2004). Therefore, the main aim of this study was to validate the HS-GC-FID analytical method for the determination of ethanol in blood samples.
2. Materials and methods
2.1. Chemicals and reagents
All reagents used throughout the assay were analytical reagent grade and were purchased from Fisher Scientific (Loughborough, UK). However, ASC acetaldehyde was purchased from Sigma Aldrich (Switzerland), Ethanol and Defibrinated Horse blood sample were obtained from Fisher Scientific (Loughborough, UK). Deionized water obtained from a Millipore® Milli-Q gradient system with 18 M Ω cm output was used throughout the analysis.
2.2. Instrumentation
All experiments were carried out using Perkin Elmer Clarus 500 GC coupled with FID, and DB-5 MS Elite-1701 column (30 m x 0.53 mm ID x 1 μm film thickness) all obtained from Perkin Elmer Inc., Shelton, USA. The carrier gas was Helium (He) at 10 psi and splitless injection mode was used. The GC oven (column temperature) was held constant at 40 °C for 2 min. The injector temperature was held at 150 °C while the detector temperature was set at 250 °C: range 20 attenuation:0 and the FID H2 and air at a flow rate of 30 mL/min and 300 mL/min were used respectively. The sample injection volume was 0.2 mL. The samples were prepared in headspace vials using a water bath at a temperature of 50 °C for 10 min.
2.3. Methods
2.3.1. Preparation of samples, internal standards, calibrators, controls and validation parameters
Initially, the blood sample was prepared by diluting 1: 5 with deionised water and then blank blood sample (1 mL) were placed into a clean glass headspace vial containing 1 mL of 80 mg/mL propan-1-ol as an internal standard (IS). The positive control was prepared by transferring 10.1 µL of ethanol into the 10 mL volumetric flask and dilute with blood solution to the mark. Then 80 mg/mL of acetaldehyde, methanol and butanol were prepared separately by transferring 0.01, 0.01 and 0.1 mL of stock solution acetaldehyde, methanol and butanol respectively into 10 mL volumetric flask and dilute to the mark with a blood sample.
Two different working solutions containing 0.8 and 3.5 mg/mL of ethanol were prepared by spiking 101.1 and 443.4 µL pure ethanol 99.99% (v/v) into 100 mL volumetric flask and diluting the content to the final volume with a blood sample.
Six calibration solutions with three replicates (n = 3) were prepared from independent dilution to minimize/avoid consecutive dilutions that might be a cause for the accumulation of errors due to sampling preparation. Accordingly, calibration solutions 0.2, 0.5 mg/mL were accurately prepared from 0.8 mg/mL by transferring 2.5 and 6.25 mL of 0.8 mg/mL ethanol solution respectively into 10 mL volumetric flasks and dilute to the mark with blood solution. Besides, calibration solutions of 1.07 and 2.0 mg/mL were also prepared by transferring 3.0 mL and 5.7 mL of 350 mg/mL ethanol solution respectively into 10 mL volumetric flasks and diluted to the mark with blood solution. Each vial was sealed with a rubber cap and aluminum crimp seal immediately after the addition of the analyte ethanol and propan-1-ol internal standard (IS). Finally, all samples were analysed as per the standard operating procedure for quantitation of blood using HS analysis GC-FID from Lindum toxicology services.
3. Result and discussion
The method for determination of ethanol in blood using HS-GC-FID was validated according to the guidelines established by International Conference on Harmonization (ICH), such as Accuracy, precision, specificity, limit of detection Linearity and Range and Food and Drug Administration (FDA).
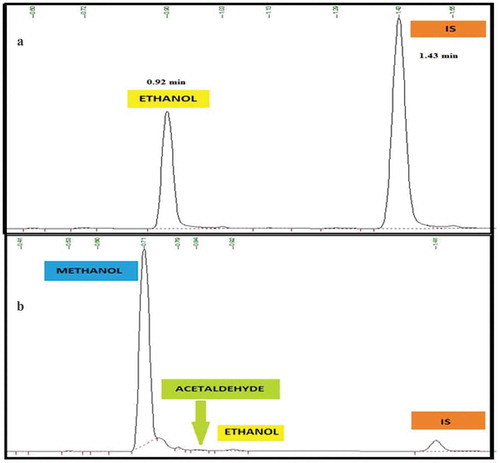
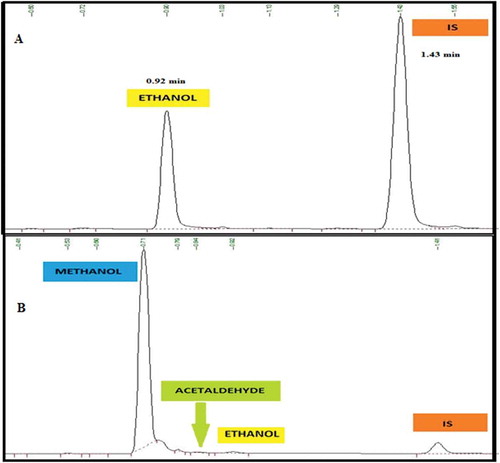
Background check: In order to establish the elution order and the retention time (tR) of the analyte, interferents and IS, five replicate bank sample was injected and the standard deviation of the blank was calculated and reported as 0.043.
3.1. Bias
The bias of the method was examined from the recovery experiments using spiked samples in the absence of appropriate reference material according to EURACHEM (Magnusson, Citation2014) and SWGTOX guidelines (Scientific Working Group for Forensic Toxicology, S, Citation2013). Accordingly, the blood samples fortified at a concentration of 0.5, 1.07 and 3.5 mg/mL were analysed (n = 3) to assess the trueness of the method. For the above-fortified blood samples, a bias of 8.7 %, 9.4 %, and 0.8 % were obtained respectively which was consistent with the SWGTOX guidelines that establish the maximum acceptable bias should be ±20% at each concentration level. Moreover, the SWGTOX guidelines explicitly recommended less bias value (±10%) for ethanol analysis, which agreed with the result obtained for the three concentration levels. As can be seen from the result the bias for 1.07 mg/mL is larger than the other two concentration levels. Which probably could be due to the random and systematic errors compared to the other calibration solutions. The results are tabulated in Table .
Table 1. Bias data of blood spiked with ethanol and IS at three concentration levels
3.2. Linearity
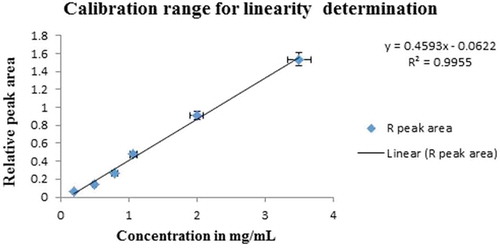
The calibration curve was plotted by running a series of standard solutions containing ethanol at six concentration levels and IS (Figure ). The highest and lowest concentration ranges tested were 0.2–3.5 mg/mL by a series of three injections. After an outlier has been removed from the data, the correlation coefficient (r2) of 0.993 was obtained indicating the proposed analytical method is appropriate for the determination of BAC. (The detailed linear regression data are given in Appendix I.) To assess the acceptance criteria for linearity, visual evaluation, and residual plots are useful in clear-cut situations. For this method, the correlation coefficient (r2) values are not less than 0.99 this indicates the linearity was fit. However, in complex analysis, complementary to the visual evaluation of linearity, it is recommended to use statistical (F-test) such as Mandel’s fit test, goodness-of-fit test or lack of fit test, which compares the variances.
3.3. Limit of detection (LOD)
The ICH guideline (International Conference on Harmonization [ICH], Citation2005) suggested that the LOD could be determined by using the residual standard deviation (SD) of a regression line or the SD of Y-intercept of regression lines may be used as a standard deviation. Accordingly, the LOD was calculated as 0.13 mg/mL. Therefore, the developed method can be used for the determination of BAC in England and Wales as the legal limit is 0.8 mg/mL. However, the LOD value calculated in this study was much higher than those obtained in other studies 0.0148 mg/mL (Görkem et al., Citation2010), 0.001 mg/mL (De Martinis & Martin, Citation2002).
3.4. Selectivity/specificity
Selectivity study of the method was carried out by injecting a sample matrix spiked with possible interferents. Accordingly, interferents that could possibly be obtained in the matrix components such as acetaldehyde and methanol, that can co-elute with ethanol were evaluated. The method confirmed excellent chromatographic selectivity with no interferents from the spiked matrix component at the retention times of ethanol, acetaldehyde, methanol and IS (0.92, 0.83, 0.71 and 1.41 min, respectively) Figure ). As the acceptance criteria are the existent compounds that must not interfere with the analysis of the targeted analyte, none of the interferents affects the ethanol determination. Therefore, the absence of the interfering signal with the analyte of interest agreed with the recommendations of both ICH (Citation2005) and (Scientific Working Group for Forensic Toxicology, Citation2013) guidelines. Therefore, matrix components were not expected to interfere in the determination of ethanol in blood samples.
3.5. Precision
The precision of the method was evaluated in terms of relative standard deviation (RSD). The RSD was calculated by dividing the standard deviation of six replicate analyses by the mean. The calculated RSD value for within-day precision was 0.27. According to the ICH guideline (ICH, Citation2005) and as cited in Peters et al. (Citation2007) the acceptance criteria for % RSD value should be 15%. However, the calculated % RSD value was reported as 27% which is higher than the acceptance criteria. This could probably be due to systematic cumulative errors from the analyst such as manual injection and sample preparation in the HS. Besides, it should have been done at least 9 determinations which were not the case in this experiment. Detailed data of accuracy and precision studies are tabulated in Table .
Table 2. Statistical evaluation for repeatability and intermediate precision between two analysts
3.5.1. Intermediate precision
The intermediate precision was examined by performing analyses by two different analysts with the same number of replicate analysis (n = 3), concentration (0.8 mg/mL), instrument and on the same day. The intermediate precision assessment should have been performed with at least 6 replicates. However, due to time constraints, the analysis was performed with three replicates only. As a result, comparison of the two analysts were performed by comparing their precision using simple F-test, as target value for precision was not decided, thus the F experimental was calculated as 1.5 and F critical was (α = 0.05, 2,2) is 19, therefore, F-theoretical is larger than F experimental the null hypothesis is accepted and confirmed that there is no significant difference between the two analysts.
3.6. Robustness
The robustness of the method was examined by changing the flow rate (FR) within ± 0.2 mL/min as a result no effect on the peak area of ethanol was observed.
4. Conclusions
The capability of the method adopted HS-GC-FID to determine BAC was fit for the intended purpose. The developed analytical method is reliable and robust for the determination of ethanol, it aligned with criteria stated in ICH and FDA guidelines by using bias, precision, LOD, selectivity, and Linearity. Moreover, with this method, several samples can be analysed in a very short period. Generally, the developed method is suitable for the determination of BAC below the legal limit in diffrent countries. Hence, the developed method is applicable in routine forensic toxicology laboratories. Besides, compared with the analytical methods repotrted in several literatures this method is very fast, reliable and it offered an excellent selectivity, bias and robustness.
Additional information
Funding
Notes on contributors

Libargachew Demlie Mihretu
L. D. Mihretu is Lecturer at the Department of Chemistry, Mekelle University, Ethiopia. He holds MSc degree in Analytical Chemistry & in Forensic Toxicology. He is experienced in carrying out researches aimed at the evaluation of different human specimens and environmental samples using different analytical techniques.
Asfaw Gebretsadik Gebru
A. G. Gebru is Lecturer at the Department of Chemistry, Mekelle University, Ethiopia. He holds MSc degrees in Environmental Chemistry. He has more than 3 articles published in reputable local and international journals.
Kebede Nigussie Mekonnen
K. N. Mekonnen (PhD) is working as analytical and environmental chemist at the Department of Chemistry, Mekelle University, Ethiopia. He has more than 15 articles published in reputable local and international journals.
Abraha Gebrekidan Asgedom
A. G. Asgedom (PhD) is working as analytical and environmental chemist at the Department of Chemistry, Mekelle University, Ethiopia. He has more than 30 articles published in reputable local and international journals.
Ykalo Hadush Desta
Y. H. Desta Lecturer at the Department of Chemistry, Mekelle University, Ethiopia. He holds MSc degrees in Environmental Chemistry
References
- Chun, H.-J., Poklis, J. L., Poklis, A., & Wolf, C. E. (2016). Development and validation of a method for alcohol analysis in brain tissue by headspace gas chromatography with flame ionization detector. Journal of Analytical Toxicology, 40(August), 653–9. https://doi.org/10.1093/jat/bkw075
- De Martinis, B. S., & Martin, C. C. S. (2002). Automated headspace solid-phase microextraction and capillary gas chromatography analysis of ethanol in postmortem specimens. Forensic Science International, 128(3), 115–119. https://doi.org/10.1016/S0379-0738(02)00182-2
- De Martinis, B. S., Martins Ruzzene, M. A., & Santos Martin, C. C. (2004). Determination of ethanol in human blood and urine by automated headspace solid-phase microextraction and capillary gas chromatography. Analytica Chimica Acta, 522(2), 163–168. https://doi.org/10.1016/j.aca.2004.07.007
- Diana, D., Sorin, M., Cornelia, M., Elena, B., & Vasil, A.,. (2009). Development and validation of a quantitative determination method of blood ethanol by gas chromatography with headspace (GC-HS). Romanian Journal of Legal Medicine, 17(4), 303–308. doi: 10.4323/rjlm.2009.303
- Görkem, M., Zeliha, K., Emrah, D., Vugar, A., Seda, K., Serap, Y., & Tülin,. (2010). Simultaneous headspace-GC – FID analysis for methanol and ethanol in blood, saliva, and urine: Validation of method and comparison of specimens. Journal of Chromatographyonline.com, 28(7), 540–544. http://www.chromatographyonline.com/
- Hong-tao, X., Lin, H., Rong-Sheng, T., Ji-Ying, Y., Lu, C., Jing, Z., Jin-qi, L., Yuan, B., & Yuan, Z., . (2014). Rapid and Sensitive Headspace Gas Chromatography-Mass spectrometery Method for the analysis of Ethanol in the whole Blood. Journal of Clinical Laboratory Analysis, 28(5), 386–390. https://doi.org/10.1002/jcla.21698
- ICH. (2005). International conference on harmonization of technical requirements for registration of pharmaceuticals for human use topic Q2 (R1) validation of analytical procedures text and methodology. International Conference on Harmonization, 1994(November 1996), 1–17. https://www.gmp-compliance.org/guidemgr/files/Q2(R1).pdf
- Kovatsi, L., Giannakis, D., Arzoglou, V., & Samanidou, V. (2011). Development and validation of a direct headspace GC-FID method for the determination of sevoflurane, desflurane and other volatile compounds of forensic interest in biological fluids: Application on clinical and post-mortem samples. Journal of Separation Science, 34(9), 1004–1010. https://doi.org/10.1002/jssc.201000921
- Lachenmeier, D. W. (2007). Rapid quality control of spirit drinks and beer using multivariate data analysis of Fourier transform infrared spectra. Food Chemistry, 101(2), 825–832. https://doi.org/10.1016/j.foodchem.2005.12.032
- Magnusson, O. (2014). Eurachem guide: The fitness for purpose of analytical methods a laboratory guide to method validation and related topics. Eurachem Guide. Available from www.eurachem.org.
- Nikelly, J. G., & Betz, J. M. (1987). Determination of ethanol in alcoholic beverages by liquid chromatography using the UV detector. Journal of Chromatographic Science, 25(9), 391–394. https://doi.org/10.1093/chromsci/25.9.391
- O’Neal, C. L., Wolf, C. E., Levine, B., Kunsman, G., & Poklis, A. (1996). Gas chromatographic procedures for determination of ethanol in postmortem blood using t-butanol and methyl ethyl ketone as internal standards. Forensic Science International, 83(1), 31–38. https://doi.org/10.1016/0379-0738(96)02007-5
- Peters, F. T., Drummer, O. H., & Musshoff, F. (2007). Validation of new methods. Forensic Science International, 165(2–3), 216–224. https://doi.org/10.1016/j.forsciint.2006.05.021
- Scientific Working Group for Forensic Toxicology, S. (2013). Standard practices for method validation in forensic toxicology. 37(7), 452-74. doi: 10.1093/jat/bkt054
- Snow, N. H., & Slack, G. C. (2002). Head-space analysis in modern gas chromatography. TrAC - Trends in Analytical Chemistry, 21(9–10), 608–617. https://doi.org/10.1016/S0165-9936(02)00802-6
- Tiscione., N. B., IIene, A., Dustin Tate, Y., & Xiaoqin, S. (2011). Ethanol analysis by headSpace gas-chromatography with simultaneous flame ionization and mass spectrometery detection. Journal of Analytical Toxicology, 35(7), 501–511. https://doi.org/10.1093/anatox/35.7.501
- Westland, J. L., & Dorman, F. L. (2013). Comparison of SPME and static headspace analysis of blood alcohol concentration utilizing two novel chromatographic stationary phases. Forensic Science International, 231(1–3), 50–56. https://doi.org/10.1016/j.forsciint.2013.05.007
- Yarita, T., Nakajima, R., Otsuka, S., Ihara, T., Takatsu, A., & Shibukawa, M. (2002). Determination of ethanol in alcoholic beverages by high- performance liquid chromatography flame ionization detection using pure water as mobile phase. 976(1-2), 387–391. https://doi.org/10.1016/S0021-9673(02)00942-1
- Zuba, D., Parczewski, A., & Reichenbcher, M. (2002). Optimization of solid-phase microextraction conditions for gas chromatographic determination of ethanol and other volatile compounds in blood. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 773(1), 75–82. https://doi.org/10.1016/S1570-0232(02)00143-5