Abstract
Nowadays, diabetes and diabetes-mediated dysfunctions are overwhelming at every nook and corner of the world which has been a sober concern to the current health care professionals. However, chronic hyperglycemic subjects often suffer from hypertension, atherosclerosis, insulin resistance, brain injury, and other dysfunctions due to high glucose level which lead to kidney failure. Altogether, diabetic nephritis, fibrosis, stenosis, iron overload, and hypertrophy may often escort towards diabetic nephropathy. Furthermore, hyperglycemia-generated free radical-mediated oxidative stress plays the central role to aggravate the condition. Oxidative stress also inhibits production of several natural antioxidant genes like Nrf2, Sirt1, PGC-1α, superoxide desmutase, and catalase. Similarly, production of pro-inflammatory cytokines such as IL, TNF-α, MIP, α-SMA, and NF-κβ has been observed high in the diabetic subjects. High glucose inside the body also activates mitogen-activated protein kinase family and facilitates diabetic nephropathy. On the other hand, green tea (GT) is a widely used drink which has several protective functions. This plant possesses multiple cathecin, theoflavins, flavonoids, flavinol, caffeine, and other biological active components. Thus, this review will try to explain how GT-derived molecules prevent diabetic nephropathy, both by reducing free radical generation and improving insulin secretion. The molecular interactions between antioxidant genes and free radical-mediated oxidative stress will be explained through activation of AMPK and mTOR. Finally, this study will try to correlate the possible therapy strategy and molecular mechanism of GT to reduce the pathogenesis of diabetic nephropathy.
Public Interest Statement
Diabetes is one of the most growing concerns of the world, and everyday more and more people are getting affected by diabetes. Thus, the risks of diabetes associated diseases are also on the rise. diabetic nephropathy is one of the most common diabetes-associated diseases, and just by developing a small habit people who are suffering from diabetes can avoid diabetic nephropathy. In this study, we tried to emphasize on how green tea is conducive to prevent diabetic nephropathy.
Competing Interests
The authors declare no competing interest.
1. Introduction
In this current era, it is often heeded that consumption of high fat diet and fructose-containing beverages, cigarette smoking, alcoholism, less or no physical exercise are rising in urban areas at an alarming rate which lead to several disorders like insulin resistance, obesity, hyperlipidemia, metabolic syndrome, and diabetes (Guh, Zhang, Bansback, Amarsi, Birmingham, & Anis, Citation2009). Diabetes, a heterogeneous disorder which is primarily characterized by impaired hormone secretion, in addition it is also caused by several impairments like protein, fat, and carbohydrate metabolism by either insufficient amount of insulin production or reduced sensitivity of tissue to insulin (Pistrosch et al., Citation2015). According to WHO report 2011, 9% of the total population above 18 years are suffering from Diabetes Mellitus (DM) (Alwan, Citation2011). If this scenario continues, the projected number of diabetic patients would be approximately 552 million in 2030 (Reno et al., Citation2015; Whiting, Guariguata, Weil, & Shaw, Citation2011). Evidences also documented that around one-third of diabetic subjects suffer from diabetic nephropathy (DN) resulting in the overall cost of the treatment beyond reach (Atkins & Zimmet, Citation2010).
DN is being considered as one of the major microvascular complications of DM and it has been claimed as a primary cause of end-stage renal diseases (Jin et al., Citation2012). Hyperglycemia-induced DN creates long-term complications which lead to high mortality and morbidity rate (Kim, Davis, Zhang, He, & Mathews, Citation2009). Several studies suggested that diabetes is also affiliated with other complications like retinopathy, cardio-myopathy, neuropathy, atherosclerosis, systemic hypertension, stroke, coronary ischemia, and most importantly diabetic kidney failure (Kupelian, Araujo, Wittert, & McKinlay, Citation2015; Rutter & Nesto, Citation2011). However, study also reported that renin–angiotensin system (RAS) plays a pivotal role in the pathogenesis of DN (Peti-Peterdi, Kang, & Toma, Citation2008). In the diabetic subjects, hyperglycemia often stimulates pro-inflammatory cytokines, neutrophil infiltration, and other pathogenic factors (Chow, Ozols, Nikolic-Paterson, Atkins, & Tesch, Citation2004) which generate reactive oxygen species that further exacerbates the situation (Ha, Yu, Choi, Kitamura, & Lee, Citation2002). On top of that, recent studies proved that diabetic subjects often lack antioxidant activities which may begin defenseless oxidative stress and progress diabetic complexity (Nourooz-Zadeh et al., Citation1997; Santini et al., Citation1997). Sometimes hypertension may develop DN by influencing inflammatory cytokines as well as generating free radicals (Lopes de Faria, Silva, & Lopes de Faria, Citation2011). In fact, DN kidney mostly lacks of AMPK/Sirt1 expression on experimented animal model (Chuang et al., Citation2011). Besides, diabetic kidneys also suffer from low level of TIMP3 and FoxO1; conversely, STAT1 level was noticed high (Fiorentino et al., Citation2013).
In recent years, green tea (GT) has become a very popular drink in several regions like South-East Asia (Wolfram, Citation2007). GT extracts possess several antioxidant group of molecules like flavonoids, flavonols, polyphenols, theaflavins, tannins and other important components (Lin, Juan, Chen, Liang, & Lin, Citation1996) which control several biological mechanisms (Polychronopoulos et al., Citation2008) like increased expression of antioxidant genes (Nomura et al., Citation2015), protect glomerulas (Peng et al., Citation2011), promote insulin sensitivity (Nomura et al., Citation2015), suppress pro-inflammatory cytokines (Kim, Murakami, Miyamoto, Tanaka, & Ohigashi, Citation2010), prevent RAS (Kurita, Maeda-Yamamoto, Tachibana, & Kamei, Citation2010), augment insulin production (Ortsäter, Grankvist, Wolfram, Kuehn, & Sjöholm, Citation2012), decrease α-amylase level (Gao, Xu, Wang, Wang, & Hochstetter, Citation2013), lower lipids levels (Ramadan, El-Beih, & Abd El-Ghffar, Citation2009), prevent free radical generation (Yokozawa, Noh, & Park, Citation2012), cyto-protective (Shin, Chung, Lee, & Kim, Citation2009), improve and protect podocyte production (Peixoto et al., Citation2015), enhance mitochondrial biogenesis (Rehman et al., Citation2013), stabilize cellular signaling (Kim, Quon, & Kim, Citation2014), protect genetic materials (Glei & Pool-Zobel, Citation2006), and inhibit cancer (Darvesh & Bishayee, Citation2013). In addition, experiment revealed that GT extract was able to reduce proteinurea on tacrolimus-induced nephrotoxic mice (Back et al., Citation2015). Reduced p-ERK1/2, MAPKp38, p-JNK, and p-AKT have been showed when EGCG 50 mg/kg/day was given to rats-induced crescentic glomerulonephritis (Ye et al., Citation2015). Similarly, another study described that long-term dietary antioxidant treatment lowers kidney inflammatory cytokines and oxidative stress markers on diabetic mice (Park, Park, & Lim, Citation2011). Restoration of antioxidant genes can be targeted as pharmacological approach for DN which can help in cell survival against diabetes-mediated dysfunctions (He et al., Citation2010). Therefore, this review will try to make a correlation among hyperglycemia, antioxidant genes, free radicals, and GT.
2. Pathology of diabetic nephropathy
Diabetes is often known as metabolic disorder which explains the inability of endocrine glands or hormonal secretion. Several approaches have been explained to develop diabetes inside a subject. Study described that diabetes is the outcome of either improper hormone secretion or insufficient and defective hormone production. However, it is also explained that improper Ca++ signaling or defective insulin mRNA are responsible for the development of diabetes (Kabir et al., Citation2015). Not only the clinical features of DN are 3P (Polyurea, Poly phasia, and Polydypsia) but also showed higher albumin elimination, abnormal glomerular filtration rate, and rapid decreasing renal functions which finally lead to end-stage renal failure. Besides, hyperglycemia may also induce oxidative stress by generating free radicals, advanced glycation end-products and activating protein kinase C to further aggravate diabetic kidney (Giacco & Brownlee, Citation2010). With the help of free radical, advanced glycation end-products (Lacmata et al., Citation2012) are formed that later interacts with its receptor RAGE and develop DN. It is suggested that blocking of RAGE or deletion of RAGE can be an effective approach in preventing diabetes-mediated complications at initial stage (Tan et al., Citation2010; Wendt et al., Citation2003). It has been evaluated that higher glucose in the body often stimulates diacylglycerol to increase the vascular permeability for inviting immune cell infiltration like neutrophil, monocyte, leukocytes, macrophage, and others. Taken together, protein kinase C also participates to activate local myofibroblastic cells which further secret collagen and extra cellular matrix that leads to kidney fibrosis. Furthermore, these pathways also regulate cell growth, cytokine and chemokine release, vasoconstrictions, apoptosis, and finally cell death (Noh & King, Citation2007). It was also noticed that RAS is highly responsible for DN by changing hemodynamic alteration and activating iNOS as well as endothelin (ET-1) in diabetic mellitus subjects (Har et al., Citation2013; Mohib et al., Citation2016; Ruggenenti, Cravedi, & Remuzzi, Citation2010). Likewise, hyperglycemia also found responsible for nephropathy by controlling blood flow and blocking small vessels (Elmarakby & Sullivan, Citation2012). Not only glucose or insulin is involved in the patho-physiology of DN, but family history and environmental factors are also responsible (Martini, Eichinger, Nair, & Kretzler, Citation2008). Patients who are suffering from DN found with excess accumulation of p62/SQSTM1 (Sequestosome 1) protein in proximal tubular cells from their kidney biopsy (Yamahara et al., Citation2013). Drug-induced kidney dysfunctions are on rise (Sagor, Mohib, Tabassum, Ahmed, & Reza, Citation2016) and these complications are making difficulties for diabetic patients to achieve an effective therapy.
3. Green tea history, traditional uses and its functional actives
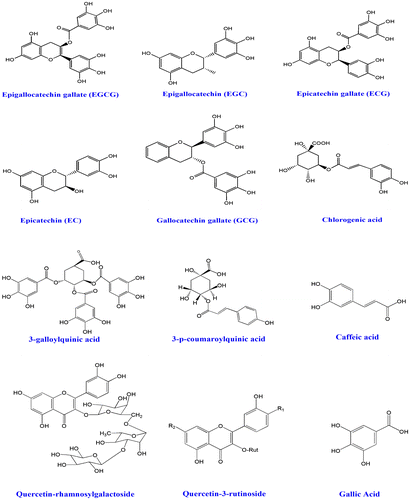
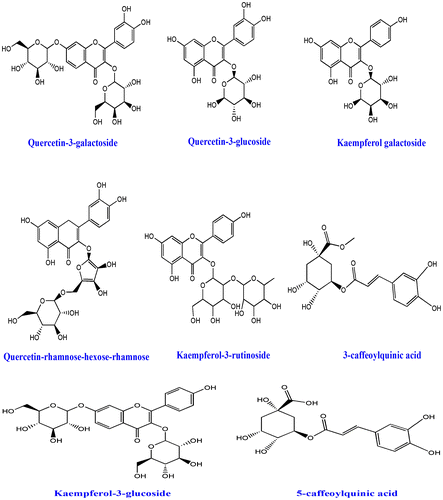
The tea plant was first noticed in China and this plant was then cultivated by the Chinese traditional inhabitant from the ancient times (International Tea Committee, Citation2009). It has been reported that GT was first exported to Japan from India during seventieth century. Around 2.5 million tons of tea is produced currently and 20% of that leaves are being processed for GT which are generally consumed by USA, Europe, Asia, and some places of North Africa (Chacko, Thambi, Kuttan, & Nishigaki, Citation2010). Tea is the second most popular drink after water (Haidari, Shahi, Zarei, Rafiei, & Omidian, Citation2012). In last few decades, several beneficial properties on human health have been noticed from the consumption of GT (Cabrera, Artacho, & Giménez, Citation2006). Traditionally, tea is classified into GT and black tea. However, tea (Camellia sinensis) belongs to Theaceae family; oolong where GT is prepared from the young green leaves. On the other hand, black tea is made by steaming. The tree normally looks green and can grow around 30 feet high in wild environment, but for commercial cultivation it is pruned to 2–5 feet. In adult age, the leaves become dark green, seen oval shape, and appear singly or cluster. After collecting the tea leaves, black tea undergoes fermentation, in contrast GT is not (Hamilton-Miller, Citation1995). Collected young leaves undergo steaming process at higher temperatures which inactivates the oxidizing enzymes thereby polyphenols remain intact (Alschuler, Citation1998). Traditionally, GT has been used on several purposes to treat viral diseases (Weber, Ruzindana-Umunyana, Imbeault, & Sircar, Citation2003), antibacterial infections (Sudano Roccaro, Blanco, Giuliano, Rusciano, & Enea, Citation2004), inflammation (Dona et al., Citation2003), cardiovascular diseases (Sueoka et al., Citation2001), obesity, and lipid lowering (Raederstorff, Schlachter, Elste, & Weber, Citation2003), angiogenesis (Sartippour et al., Citation2002), cancer (Kavanagh et al., Citation2001), neuro-protective (Weinreb, Mandel, Amit, & Youdim, Citation2004), anti-arthritic (Haqqi et al., Citation1999), antioxidant (Osada et al., Citation2001), and other health-related disorders. GT possesses diverse types of bioactive molecules which have several protective mechanisms. So far, various types of flavonoids, polyphenols, and tannins have been isolated from GT leaves (Graham, Citation1992). Classically, four types of catechins have been studied from GT extract and those potent catechins are epigallocatechin-3-gallate (EECG), (+)-catechin (CE), epicatechin-3-gallate (ECG), and epigallocatechin (EGC) (Zaveri, Citation2006). All the catechins and other isolated molecules (Figure ) from GT were found to be effective against diabetic-mediated kidney dysfunctions (Al-Attar & Zari, Citation2010).
Figure 1. Histology represents kidney dysfunctions induced by Alloxan. (A) Which was stained by Hematoxylene and Eosin shows various inflammatory components infiltration, most of the glomerulas have been destroyed by diabetes, (B) which was stained using sirius red and picric acid shows collagen deposition (red parts), and (C) which was stained Prusian blue to determine the iron overload. Inside figures ic—inflammatory cells, fb—fibrosis, and ip—iron pigments. The histology was prepared in our lab.
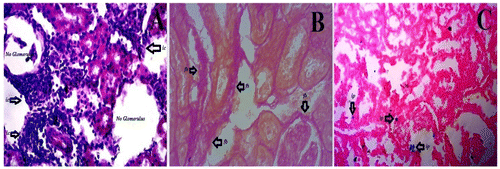
Figure 2. Molecules, which have been isolated from green tea and have biological activity.
4. Diabetes and free radical biology
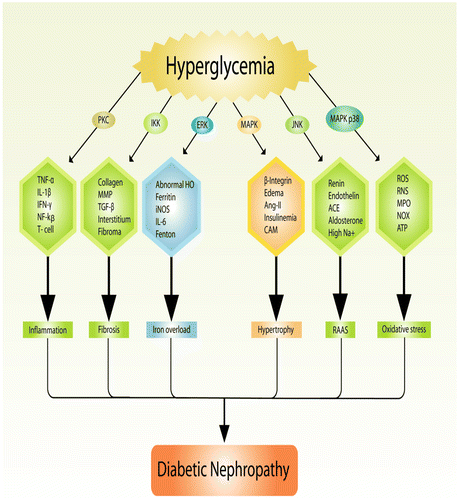
Free radicals are parts of the body, sometimes they are called byproducts. They are normally generated either during ATP production or cellular degeneration or by any harmful stimuli (Sagor, Tabassum, Potol, & Alam, Citation2015). They often produce oxidative stress which further leads to organ dysfunctions by inducing pro-inflammatory cytokines, transcription factors for fibrosis (Figure (B)), hemeoxygenase (HO) for iron overload (Figure (C)), mast cell accumulation, and other detrimental factors (Reza, Sagor, & Alam, Citation2015; Reza et al., Citation2016). Interestingly, they are responsible for cell membrane damage by oxidizing membrane lipids through malondehyde (MDA) activity. Later, free radicals react with nucleus, DNA, alter genetic codes, and oxidized regulatory protein which results in the production of advanced oxidative protein products (AOPP) that further damage various components of cell (Abu Taher et al., Citation2016; Sagor, Chowdhury, et al., Citation2015). However, nitric oxide is potent vasodilator produced from nitric oxide synthetase (NOS) can be very harmful to a system once it is produced from induced nitric oxide synthetase (iNOS) (Gupta et al., Citation2005). iNOS, a potent oxidant, is generally processed by free radical which damages cytoplasm and has a powerful role to activate NF-κβ, which further stimulate several other pro-inflammatory cytokine (Alam, Chowdhury, Jain, Sagor, & Reza, Citation2015). Unfortunately, chronic hyperglycemia is often blamed for generation of free radical-mediated oxidative stress too (Tse, Anderson, Ganini, & Mason, Citation2015). Moreover, study also explored that oxidative stress has a significant role on production of advanced glycation end products (AGE) (Pazdro & Burgess, Citation2012). It is highly suggested that NAD(P)H-mediated oxidative stress helps in disease progression of DN (Jha et al., Citation2014). NAD(P)H oxidase has been also claimed for damaging cell membrane, cytoplasm, and mitochondria through oxidative-mediated stress as the expression of NOX-4 is always found high in DN (Eid et al., Citation2010; Sedeek et al., Citation2010). Another study recommended that suppression of NOX-4 reduced glomerulas damage and protected overall kidney functions on db/db BLKS mice by decreasing albuminuria production (Figure ) (Sedeek et al., Citation2013).
Table 1. Recent protective findings of Green tea on diabetic animal studies
Table 2. Recent protective findings of green tea on diabetic patients through clinical trails
5. Role of inflammation on diabetic nephropathy
Immunity is the ultimate hero of a biological system which fights against any foreign invaders. Most importantly, inflammation is considered as primary defensive mechanism and is generally induced by any noxious or harmful stimuli. Unfortunately, it is sometimes activated against its own host (Aldhahi & Hamdy, Citation2003). The relation between DN and inflammation is common and often inflammation plays a pivotal role to develop DN, although it is very difficult to show a single molecular mechanism to understand the patho-physiology of DN through inflammatory molecules and pro-inflammatory cytokines (Figure (A)) (Ruggenenti et al., Citation2010). It is extensively studied that diabetic status of a subject often attracts several inflammatory and pro-inflammatory cytokines like nuclear factor for (NF)-κβ, tumor necrosis factor (TNF)-α, interleukin (IL)-6, interleukin (IL)-1β, α-smooth muscle actin (SMA), macrophage inflammatory protein (MIP), T-lymphocyte, matrix metalloproteinase (MMP), cyclooxygenase-2 (Cox, Abu-Ghannam, & Gupta, Citation2010), mast cell, monocytes, macrophages, myloperoxidase (Rojas, Ochoa, Ocampo, & Muñoz, Citation2006), PGE2, interferon (INF)-ϒ, and others, some of them are associated with TLR4-MyDD88 interactions (Donate-Correa, Martín-Núñez, Muros-de-Fuentes, Mora-Fernández, & Navarro-González, Citation2015). Taken together, a study found out that hyperglycemia induces PKC which further activates pro-inflammatory cytokines. Likewise, high glucose also induces oxidative stress which plays a critical role inside kidney generating various harmful free radicals (Mora & Navarro, Citation2004). Most probably, inflammatory markers like IL-1 and TNF-α were first claimed for nephropathy in diabetic condition (Ienaga & Kondo, Citation1991). Another study explained that, mRNA expression of IL-6 found high in human renal sample on a diabetic study (Suzuki et al., Citation1995). A clinical study showed that, expression of IL-18 was found remarkably high in diabetic patients (Fantuzzi, Reed, & Dinarello, Citation1999) which further linked to mitogen-activated protein kinase (MAPK) (Miyauchi, Takiyama, Honjyo, Tateno, & Haneda, Citation2009). In addition, TNF-α was also claimed to generate free radicals in rat glomeruli via MAPK and protein kinase C (PKC) pathway (Koike, Takamura, & Kaneko, Citation2007). Reduction of antioxidant gene like Nrf-2 leads to chronic kidney diseases (CKD) through oxidative stress-mediated inflammation (Ruiz, Pergola, Zager, & Vaziri, Citation2013). It was also noticed that disruption in Nrf-2 gene signaling may also create lupus-like autoimmune nephritis and induce diabetic inflammation along with nephropathy (Yoh et al., Citation2001, Citation2008).
6. Role of green tea on diabetic nephropathy
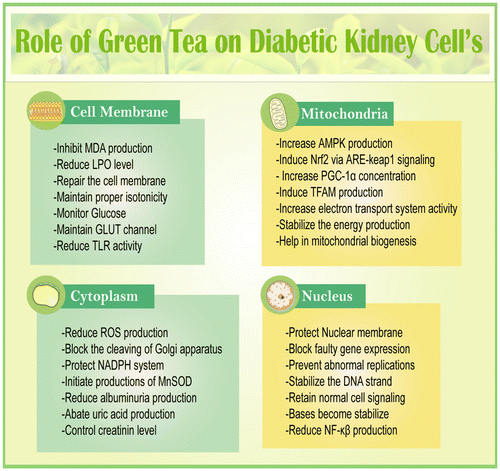
In DN, cell membrane gets damaged due to diabetic inflammation which is a result of excess glucose concentration inside the organ (Giacco et al., Citation2014). High glucose concentration always hampers multiple cellular signaling like GLUT, MAPK, and PKC (Dronavalli, Duka, & Bakris, Citation2008). On top of that, it also increases AGEs, inflammatory cytokines, tumor necrosis factors, nuclear factor-κβ, interleukin-1β, and pro-inflammatory cytokines are often induced by methylglyoxal signaling (Wang, Meng, Gordon, Khandwala, & Wu, Citation2007). (+)–Catechins have been highly effective against DN and related other complications. One of the studies found that administration of (+)–catechin for 16 weeks was quite effective against DN by diminishing renal damage and methylglyoxal signaling on db/db mice. Similarly, catechin was found to be very effective against DN on human endothelium-derived cells (Zhu et al., Citation2014). Altogether, diabetic subjects possess free radicals generation, AGEs production, HbA1C level and free glucose concentration which always hamper kidney functions by preventing antioxidant genes like Nrf2, Sirt1, PGC-α, SOD, FOXO (Ding & Choi, Citation2015), and others which are mostly mediated through mTOR (Zoncu, Efeyan, & Sabatini, Citation2011) and AMPK pathway (Lee, Park, Takahashi, & Wang, Citation2010; Price et al., Citation2012). It is significantly observed that GT protects DN by either increasing antioxidant genes (Wang et al., Citation2015), or reducing free radical production (Khan et al., Citation2009) or suppressing pro-inflammatory mediators (Figure ) (Sachdeva, Kuhad, Tiwari, Arora, & Chopra, Citation2010).
GT possesses several types of cathechins and flavonoids which generally exert multiple mechanisms to protect from DN-related complexities (Funamoto et al., Citation2016). Nrf2, the most protective gene, known as the master regulator of a cell that fights against inflammation; fibrosis and free radicals mediated oxidative stress in chronic kidney diseases (Aminzadeh et al., Citation2014). Nuclear factor-erythroid-2-related factor 2 (Nrf2) plays the central role to regulate and coordinate in the induction of more than 250 gene which protect cell through several signaling. The genes which are expressed by the help of Nrf2 are generally known as antioxidant or protective gene and some of the potent components are SOD, heme oxygenase-1, glutamate cysteine ligase, catalase, NAD(P)H: quinone oxidoreductase-1 (NQO1), thioredoxin, glutathione S-transferase, and glutathione peroxidase which mainly work by either restoring antioxidant property or help in harmful substances metabolism like phase-II drug enzymes (Li et al., Citation2008; Wakabayashi, Slocum, Skoko, Shin, & Kensler, Citation2010). Activation and production of antioxidant genes like Nrf2, ARE, and SOD can protect a cell from any kind of stress which make them a target for drug molecules (Sriram, Kalayarasan, & Sudhandiran, Citation2009). It is often observed that nephropathy subjects always lack Nrf2 production in kidney (Aminzadeh, Nicholas, Norris, & Vaziri, Citation2013). Experiment showed that expression of Nrf2 also ameliorates oxidative stress, reduces inflammation, and kidney fibrosis in animal model via Nrf2-keap1 signaling (Soetikno et al., Citation2013). It is observed that Nrf2-mediated pathway reduces NF-κβ-inflammatory signaling and thus initiates apoptosis (Li et al., Citation2008). GT flovonoids have been proposed for production of Nrf2 mRNA on DN subjects (Na & Surh, Citation2008; Yoon et al., Citation2014). One of the most potent cathecins, epigallocatechin-3-gallate protects from Cisplatin-induced nephrotoxic rats through Nrf2/HO-1signaling pathway (Sahin et al., Citation2010). In addition, another study revealed that epigallocatechin-3-gallate stops lupus nephritis development by enhancing Nrf2 and inhibiting NLRP3 (Tsai et al., Citation2011). Several protective effects of GT molecules have been summarized in Tables and .
Another important cell saving component is superoxide dismutase which protects cytoplasm, mitochondria, and nucleus of a cell (Wassmann, Wassmann, & Nickenig, Citation2004). Hyperglycemia generates several free radicals like superoxide anion which contributes in the development of DN progression (Ha & Kim, Citation1999) and damage kidney podocyte as well as glomerulas (United States Renal Data System, Citation2011). It has been also noticed that interaction of AT1R with Ang-II may generate superoxide anion which is a positive signal for pathogenesis of chronic kidney diseases through NAD(P)H oxidase (Kim, Sato, Rodriguez-Iturbe, & Vaziri, Citation2011; Vaziri, Dicus, Ho, Boroujerdi-Rad, & Sindhu, Citation2003). Diabetic kidney often suffers from various disturbances due to over production of superoxide anion and hydrohen per-oxide (Koya et al., Citation2003). SOD family exerts its protective function by residing inside membrane (EC-SO, mitochondria (Mn-SOD), and cytoplasm (Cu-Zn-SOD) (Bartz & Piantadosi, Citation2010; Rosenthal & Nocera, Citation2007). Production of mitochondrial SOD can also decrease hyperglycemia and related complications (Nishikawa et al., Citation2000).
Recently Sirt1 has achieved several protective functions like DNA repairing; neutralizing oxidants, maintaining glucose homeostasis, hormone secretion and others. Hence, it is now called silent information regulator (Weber et al., Citation2003) gene. It also regulates in the transcription of non-histone protein including FOXO1/3, p53, PPAR, and PGC-1α (Morris, Citation2013). Study also showed that expression of Sirt1 in the proximal tubules enhances glomerular function and defends against diabetes-induced renal damage (Hasegawa et al., Citation2013). A recent experiment explored that GT polyphenol (−)-epigallocatechin-3-gallate (EGCG, 50 mg/kg BW/day × 3 weeks) restored Sirt1 expression, resulting in the reduction of serum creatinine, proteinurea which ultimately protects kidney cell. The study also linked that supplementation of GT polyphenol reduced p-ERK1/2, p-Akt, p-JNK, and p-P38 signaling along with activating PPARγ activity (Ye et al., Citation2015). GT isoflavones have been investigated to induce Sirt1 and other protein content like TFAM for mitochondrial biogenesis when rat’s kidney were treated with cyclosporine A (Rehman et al., Citation2013).
AMPK, another master regulator, known as a nutrient sensor which is generally activated under energy-depleted conditions by phosphorylation of a preserved threonine residue (T172) (Alers, Wesselborg, & Stork, Citation2012). In type-1 and type-2 diabetic patients, AMPK phosphorylation and other activities were noticed lower inside both glomeruli and tubules (Kitada, Kume, Imaizumi, & Koya, Citation2011). GT treatment found to be protective against cyclosporine A-induced renal injury. Increased AMPK and PGC-1α help in the mitochondrial biogenesis and thus prevent drug-induced kidney dysfunctions (Rehman et al., Citation2013).
Beside these, epigallocatechin-3-Gallate treatment also protected the kidney function by reducing MCP-1, MCP-3, and TGF-β content on fibrotic rat model (Wang et al., Citation2015). Another study showed that GT normalizes renal kidney injury by down regulating NOX-4 production on diabetic rats (Ribaldo et al., Citation2009). It was also found out that water extracts of GT lowered DN by controlling glomerular hypertrophy and interstitial inflammation on alloxan induced diabetic rats (Shokouhi et al., Citation2015). Recent study also revealed that GT polyphenol epigallocatechin-3-gallate up-regulated heme oxygenase-1through phosphatidyl inositol 3-kinase/Akt and ERK and thereby prevent kidney damage (Wu et al., Citation2006). A proposed molecular mechanism has been explained in the (Figure ) to show the possible interaction between GT and DN.
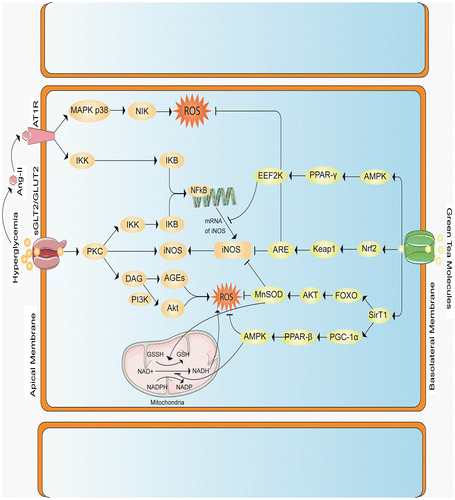
Figure 5. Proposed mechanism explained that, Hyperglycemia induces ROS in numerous ways, such as by activating Mitochondria, or by increasing the accumulation of Transforming Growth Factor Beta (TGF-β), or by triggering protein kinase C (PKC), it also enhances the accumulation of tumor necrosis factor alpha (TNF-α) and up regulates angiotensin-2 production. (1) Inside the mitochondria, hyperglycemia causes electron imbalance which converts oxygen molecule to a reactive one. (2) TGF-β generates ROS via PI3K-Smad2/3 pathway. (3) Activation of PKC by high level of glucose causes formation of ROS via different ways, PKC also possess interleukin 6, which regulates iNOS that is directly involved in the generation of ROS, PKC itself can help in the formation of iNOS directly; PKC causes activation of nuclear factor κβ via IKK-IKB pathway. (4) In addition, hyperglycemia can cause accumulation of TNF-α adjacent the cell membrane, which binds TNF-α receptor that further activates NF-κβ inside the DNA to produce iNOS gene that ultimately generates ROS. (5) Higher glucose level may facilitate the production of Angiotensin-II by Renin-Angiotensin-Aldosterone system, Ang-II binds with Ang-II Type 1 receptor(AT1R), which causes accumulation of ROS via MAPK p38- NIK (NF-κβ inducing kinase) pathway which further activates NF-κβ via IKK-IKB pathway. Green tea flavonoids and polyphenols block the activation/accumulation/generation of ROS by enhancing 5’ AMP-activated protein kinase (AMPK), nuclear factor-erythroid-2-related factor 2 (Nrf2) and Sirtuin-1 (Sirt1). (1) AMPK generally activates both Nrf2 and Sirt1, it further inhibits the expression of iNOS through Peroxisome proliferator-activated receptor gamma (PPAR-γ) in Eukaryotic elongation factor-2 kinase (EEF-2K) pathway, AMPK may also nullify the effect of TNF-α and PKC. (2) Green tea polyphenols and flavonoids induce Nrf2 which blocks both iNOS production and ROS accumulation by activating Keap1-ARE (antioxidant-responsive element) signaling. (3) Sirt1 up regulates Mn superoxide dismutase (MnSOD) production via Forkhead box O3 FOXO3-AKT (Protein kinase) pathway which prevents ROS generation, furthermore Sirt1 inhibits TNF-α and TGF-β by producing PGC-1α (Peroxisome proliferator-activated receptor gamma co-activator 1-alpha)—PPARβ (peroxisome proliferator-activated receptors-β).
7. Problem with green tea treatment
A potent molecule must exert its action on biological system being stable, at the same time it should be safe and not interfere with other signaling pathways. Sometimes it is showed that high dose of a component often produces unwanted side effects, life-threatening ADR which may lead to necrosis-mediated cell death (Elsherbiny et al., Citation2012). Similarly, a study has also found that 1% GT polyphenols with food produced nephrotoxicity by down-regulating antioxidant protein expression and heat shock protein expression (Inoue et al., Citation2011). Likewise, another study explored that high dose of GT (EECG) induced hepatic dysfunction by producing liver4-hydroxynonenal (4-HNE) and malonyldialdehyde (MDA) (Lambert et al., Citation2010). In addition, similar kind of hepatic dysfunctions have been reported when people take daily GT supplements (Mazzanti et al., Citation2009). Furthermore, a cell culture study also investigated that EECG induced oxidative stress and also enhanced the production of pro-matrix metalloproteinase-7 (Kim, Murakami, & Ohigashi, Citation2007). Another report noticed that 1% GT polyphenol treatment induced carcinoma by increasing pro-inflammatory cytokines along with reducing antioxidants like SOD and catalase (Kim et al., Citation2010). A recent meta-analysis showed that intake of GT also increased the risk of endometrial cancer (Zhou et al., Citation2016). It has been also reported that GT component EGCG, has poor absorption via oral route due to metabolization by gut microbiata (Chan, Zhang, & Zuo, Citation2007) and also found very shorter half-life (maximum 30 min in cell culture and 30–60 min on animals) in both animal and human study (Lee et al., Citation2002). It is normally conjugated with glucuronidation and easily metabolized. Study showed that one-third of the ECGE gets eliminated from the body within first 24 h of its administration (Manach, Williamson, Morand, Scalbert, & Rémésy, Citation2005). Oxidation of ECGE may produce several free radicals like superoxide anion which later hampers multiple cellular signaling by binding on epidermal growth factor receptor (Hou et al., Citation2005; Smith, Citation2011; Wein, Schrader, Rimbach, & Wolffram, Citation2013). EGCE also blocks insulin secretion from β-cell by inhibiting glutamate dehydrogenase (GDH)-mediated signaling (Li et al., Citation2006). However, a cross talk has been shown from a clinical trial where 35 obese subjects were given GT (4 cups/a day), and after 8 weeks no significant alteration in serum biomarkers of inflammation including adiponectin, C-reactive protein (CRP), interleukin-(IL-6), interleukin-(IL-1β), soluble vascular cell adhesion molecule-(sVCAM-1), leptin, or leptin: adiponectin ratio were observed, although the authors noticed reduced plasma serum amyloid alpha (Basu et al., Citation2011; Eid et al., Citation2010). Most of the studies were conducted with small number of subjects. Therefore, it is very difficult to conclude anything concrete against the obtained results. Clinical trial on a large population must be carried out to get a noble drug molecule from GT.
8. Conclusion and future directions
This study describes possible patho-physiology for kidney dysfunctions, especially in diabetic condition. As the complications of diabetes are increasing tremendously, a safe and alternative treatment must be approached. Beneficial roles of GT cathecins on diabetic-induced kidney dysfunctions have been well studied. Regular administration of GT supplements can be an effective treatment approach for the diabetic subjects. This study also provides strong evidences in favor of GT treatment in DN. The authors highly recommend for large clinical trial to establish the exact molecular mechanism of action of GT against DN.
Additional information
Funding
Notes on contributors
Iqbal Ahmed
Our group endeavors to explore the role of oxidative stress in different diseases and the potential role of antioxidant derived from natural sources. In our current study, we tried to identify the role of green tea as a potential source of antioxidant against diabetic nephropathy. We focused on possible mechanism by means of which the molecules from green tea can suppress the oxidative damage as well as how it can restore the normal antioxidant level in the system. Our study was led by Sarif Mohiuddin, lecturer at Pioneer Dental College and Hospital. His area of specialization is Diabetology, Natural Antioxidant, and Angiotensin-II. He has completed his MBBS from Dhaka University and has been awarded FRSPH from The Royal Society for Public Health (RSPH) UK. He has also completed certified course on Diabetology, and extension of diabetic Care Course from BIRDEM Hospital, Dhaka, Bangladesh.
References
- Abdel-Raheem, I. T., El-Sherbiny, G. A., & Taye, A. (2010). Green tea ameliorates renal oxidative damage induced by gentamicin in rats. Pakistan Journal of Pharmaceutical Sciences, 23, 21–28.
- Abu Taher, S., Hasan Mahmud, R., Nabila, T., Biswajit, S., Anayt, U., Nusrat, S., … Ashraful, A. (2016). Supplementation of rosemary leaves (Rosmarinus officinalis) powder attenuates oxidative stress, inflammation and fibrosis in carbon tetrachloride (CCl4) treated rats. Current Nutrition & Food Science, 12, 1–8. doi:10.2174/1573401312666160816154610
- Alam, M. A., Chowdhury, M. R. H., Jain, P., Sagor, M. A. T., Reza, H. M. (2015). DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetology & Metabolic Syndrome, 7(1), 1–10. doi:10.1186/s13098-015-0095-3
- Al-Attar, A. M., & Zari, T. A. (2010). Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi Journal of Biological Sciences, 17, 295–301.10.1016/j.sjbs.2010.05.007
- Aldhahi, W., & Hamdy, O. (2003). Adipokines, inflammation, and the endothelium in diabetes. Current Diabetes Reports, 3, 293–298.10.1007/s11892-003-0020-2
- Alers, S., Wesselborg, S., & Stork, B. (2012). Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Molecular and Cellular Biology, 32, 2–11.10.1128/MCB.06159-11
- Alschuler, L. (1998). Green tea: Healing tonic. American Journal of Natural Medicine, 5, 28–31.
- Alwan, A. (2011). Global status report on noncommunicable diseases 2010. Geneva: World Health Organization.
- Aminzadeh, M. A., Nicholas, S. B., Norris, K. C., & Vaziri, N. D. (2013). Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrology Dialysis Transplantation, 28, 2038–2045.10.1093/ndt/gft022
- Aminzadeh, M. A., Reisman, S. A., Vaziri, N. D., Khazaeli, M., Yuan, J., & Meyer, C. J. (2014). The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica, 44, 570–578.10.3109/00498254.2013.852705
- Atkins, R. C., & Zimmet, P. (2010). Diabetic kidney disease: act now or pay later—World Kidney Day, 11 March 2010. Therapeutic Apheresis and Dialysis, 14(1), 1–4.
- Back, J., Ryu, H. H., Hong, R., Han, S. A., Yoon, Y. M., Kim, D. H., … Kwon, Y. E. (2015). Antiproteinuric effects of green tea extract on tacrolimus-induced nephrotoxicity in mice. In Transplantation Proceedings (Vol. 47, pp. 2032–2034). Elsevier. 10.1016/j.transproceed.2015.06.008
- Bartz, R. R., & Piantadosi, C. A. (2010). Clinical review: Oxygen as a signaling molecule. Critical Care, 14, 234.10.1186/cc9185
- Basu, A., Du, M., Sanchez, K., Leyva, M. J., Betts, N. M., Blevins, S., … Lyons, T. J. (2011). Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition, 27, 206–213.10.1016/j.nut.2010.01.015
- Bogdanski, P., Suliburska, J., Szulinska, M., Stepien, M., Pupek-Musialik, D., & Jablecka, A. (2012). Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutrition Research, 32, 421–427.10.1016/j.nutres.2012.05.007
- Borges, C. M., et al. (2016). The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Scientific Reports, 6.
- Cabrera, C., Artacho, R., & Giménez, R. (2006). Beneficial effects of green tea—A review. Journal of the American College of Nutrition, 25, 79–99.10.1080/07315724.2006.10719518
- Chacko, S. M., Thambi, P. T., Kuttan, R., & Nishigaki, I. (2010). Beneficial effects of green tea: A literature review. Chinese Medicine, 5(1), 1–9. doi:10.1186/1749-8546-5-13
- Chan, K., Zhang, L., & Zuo, Z. (2007). Intestinal efflux transport kinetics of green tea catechins in Caco-2 monolayer model. Journal of Pharmacy and Pharmacology, 59, 395–400.10.1211/jpp.59.3.0009
- Chow, F., Ozols, E., Nikolic-Paterson, D. J., Atkins, R. C., & Tesch, G. H. (2004). Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney International, 65, 116–128.10.1111/j.1523-1755.2004.00367.x
- Chuang, P. Y., Dai, Y., Liu, R., He, H., Kretzler, M., Jim, B., Cohen, C. D., … Dryer, S. E. (2011). Alteration of forkhead box o (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS ONE, 6, e23566.10.1371/journal.pone.0023566
- Cox, S., Abu-Ghannam, N., & Gupta, S. (2010). An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. International Food Research Journal, 17, 205–220.
- Darvesh, A. S., & Bishayee, A. (2013). Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutrition and Cancer, 65, 329–344.10.1080/01635581.2013.767367
- Ding, Y., & Choi, M. E. (2015). Autophagy in diabetic nephropathy. Journal of Endocrinology, 224, R15–R30.
- Dona, M., Dell'Aica, I., Calabrese, F., Benelli, R., Morini, M., Albini, A., & Garbisa, S. (2003). Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. The Journal of Immunology, 170, 4335–4341.10.4049/jimmunol.170.8.4335
- Donate-Correa, J., Martín-Núñez, E., Muros-de-Fuentes, M., Mora-Fernández, C., & Navarro-González, J. F. (2015). Inflammatory cytokines in diabetic nephropathy. Journal of Diabetes Research, 2015, Article ID: 948417. doi:10.1155/2015/948417
- Dostal, A. M., Samavat, H., Espejo, L., Arikawa, A. Y., Stendell-Hollis, N. R., & Kurzer, M. S. (2016). Green tea extract and catechol-O-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. Journal of Nutrition, 146, 38–45.10.3945/jn.115.222414
- Dronavalli, S., Duka, I., & Bakris, G. L. (2008). The pathogenesis of diabetic nephropathy. Nature Clinical Practice Endocrinology & Metabolism, 4, 444–452.10.1038/ncpendmet0894
- Eid, A. A., Ford, B. M., Block, K., Kasinath, B. S., Gorin, Y., Ghosh-Choudhury, G., & Abboud, H. E. (2010). AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. Journal of Biological Chemistry, 285, 37503–37512.10.1074/jbc.M110.136796
- Elmarakby, A. A., & Sullivan, J. C. (2012). Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovascular Therapeutics, 30, 49–59.10.1111/cdr.2012.30.issue-1
- Elsherbiny, N. M., El Galil, K. H. A., Gabr, M. M., Al-Gayyar, M. M.,Eissa, L. A., & El-Shishtawy, M. M. (2012). Reno-protective effect of NECA in diabetic nephropathy: Implication of IL-18 and ICAM-1. European Cytokine Network, 23, 78–86. doi:10.1684/ecn.2012.0309
- Fantuzzi, G., Reed, D. A., & Dinarello, C. A. (1999). IL-12–induced IFN-γ is dependent on caspase-1 processing of the IL-18 precursor. Journal of Clinical Investigation, 104, 761–767.10.1172/JCI7501
- Faria, A. M., Papadimitriou, A., Silva, K. C., Lopes de Faria, J. M., & Lopes de Faria, J. B. (2012). Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes, 61, 1838–1847.10.2337/db11-1241
- Fiorentino, L., Cavalera, M., Menini, S., Marchetti, V., Mavilio, M., Fabrizi, M., … Federici, M. (2013). Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Molecular Medicine, 5, 441–455.10.1002/emmm.201201475
- Funamoto, M., Masumoto, H., Takaori, K., Taki, T., Setozaki, S., Yamazaki, K., … Sakata, R. (2016). Green tea polyphenol prevents diabetic rats from acute kidney injury after cardiopulmonary bypass. The Annals of Thoracic Surgery, 101, 1507–1513. doi:10.1016/j.athoracsur.2015.09.080
- Gao, J., Xu, P., Wang, Y., Wang, Y., & Hochstetter, D. (2013). Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against α-amylase and α-glucosidase in vitro. Molecules, 18, 11614–11623.10.3390/molecules180911614
- Giacco, F., & Brownlee, M. (2010). Oxidative stress and diabetic complications. Circulation Research, 107, 1058–1070.10.1161/CIRCRESAHA.110.223545
- Giacco, F., Du, X., D'Agati, V. D., Milne, R., Sui, G., Geoffrion, M., & Brownlee, M. (2014). Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes, 63, 291–299.10.2337/db13-0316
- Glei, M., & Pool-Zobel, B. (2006). The main catechin of green tea,(−)-epigallocatechin-3-gallate (EGCG), reduces bleomycin-induced DNA damage in human leucocytes. Toxicology in Vitro, 20, 295–300.10.1016/j.tiv.2005.08.002
- Graham, H. N. (1992). Green tea composition, consumption, and polyphenol chemistry. Preventive Medicine, 21, 334–350.10.1016/0091-7435(92)90041-F
- Guh, D. P., Zhang, W., Bansback, N., Amarsi, Z., Birmingham, C. L., & Anis, A. H. (2009). The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health, 9(1), 1–20. doi:10.1186/1471-2458-9-88
- Gupta, M., Solís, P. N., Calderón, A. I., Guinneau-Sinclair, F., Correa, M., Galdames, C., … Ocampo, R. (2005). Medical ethnobotany of the teribes of bocas del toro, panama. Journal of Ethnopharmacology, 96, 389–401.10.1016/j.jep.2004.08.032
- Ha, H., & Kim, K. H. (1999). Pathogenesis of diabetic nephropathy: The role of oxidative stress and protein kinase C. Diabetes Research and Clinical Practice, 45, 147–151.10.1016/S0168-8227(99)00044-3
- Ha, H., Yu, M. R., Choi, Y. J.,, Kitamura, M., & Lee, H. B. (2002). Role of high glucose-induced nuclear factor-κB activation in monocyte chemoattractant protein-1 expression by mesangial cells. Journal of the American Society of Nephrology, 13, 894–902.
- Haidari, F., Shahi, M. M., Zarei, M., Rafiei, H., & Omidian, K. (2012). Effect of green tea extract on body weight, serum glucose and lipid profile in streptozotocin-induced diabetic rats. A dose response study. Saudi Medical Journal, 33, 128–133.
- Hamilton-Miller, J. (1995). Antimicrobial properties of tea (Camellia sinensis L.). Antimicrobial Agents and Chemotherapy, 39, 2375–2377.10.1128/AAC.39.11.2375
- Haqqi, T. M., Anthony, D. D., Gupta, S., Ahmad, N., Lee, M.-S., Kumar, G. K., & Mukhtar, H. (1999). Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proceedings of the National Academy of Sciences, 96, 4524–4529.10.1073/pnas.96.8.4524
- Har, R., Scholey, J. W., Daneman, D., Mahmud, F. H., Dekker, R., Lai, V., … Cherney, D. Z. I. (2013). The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia, 56, 1166–1173.10.1007/s00125-013-2857-5
- Hasegawa, K., Wakino, S., Simic, P., Sakamaki, Y., Minakuchi, H., Fujimura, K., … Itoh, H. (2013). Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nature Medicine, 19, 1496–1504.10.1038/nm.3363
- He, W., Wang, Y., Zhang, M.-Z., You, L., Davis, L. S., Fan, H., … Hao, C.-M. (2010). Sirt1 activation protects the mouse renal medulla from oxidative injury. Journal of Clinical Investigation, 120, 1056–1068.10.1172/JCI41563
- Hou, Z., Sang, S., You, H., Lee, M. J., Hong, J., Chin, K. V., & Yang, C. S. (2005). Mechanism of action of (−)-epigallocatechin-3-gallate: Auto-oxidation–dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Research, 65, 8049–8056. doi:10.1158/0008-5472.CAN-05-0480
- Hsu, C.-H., Tsai, T.-H., Kao, Y.-H., Hwang, K.-C., Tseng, T.-Y., & Chou, P. (2008). Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clinical Nutrition, 27, 363–370.10.1016/j.clnu.2008.03.007
- Hua, C. H., Liao, Y. L., Lin, S. C., Tsai, T. H., Huang, C. J., & Chou, P. (2011). Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebocontrolled clinical trial. Alternative Medicine Review, 16, 157–163.
- Ienaga, K., & Kondo, M. (1991). Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney international, 40, 1007–1012.
- Inoue, H., Akiyama, S., Maeda-Yamamoto, M., Nesumi, A., Tanaka, T., & Murakami, A. (2011). High-dose green tea polyphenols induce nephrotoxicity in dextran sulfate sodium-induced colitis mice by down-regulation of antioxidant enzymes and heat-shock protein expressions. Cell Stress and Chaperones, 16, 653–662.10.1007/s12192-011-0280-8
- International Tea Committee. (2009). International tea committee: Annual bulletin of statistics. London: Author.
- Jha, J. C., Gray, S. P., Barit, D., Okabe, J., Namikoshi, T., Thallas-Bonke, V., … Jandeleit-Dahm, K. A. (2014). Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. Journal of the American Society of Nephrology, 25, 1237–1254.10.1681/ASN.2013070810
- Jin, D. C., Ha, I. S., Kim, N. H., Lee, S. W., & Yoon, S. R.Kim, B. S. (2012). Brief report: Renal replacement therapy in Korea, 2010. Kidney Research and Clinical Practice, 31, 62–71.10.1016/j.krcp.2012.01.005
- Kabir, A. U., Samad, M. B., Ahmed, A., Akhter, F., Jahan, M. R., Akhter, F., … Hannan, J. M. A. (2015). aqueous fraction of beta vulgaris ameliorates hyperglycemia in diabetic mice due to enhanced glucose stimulated insulin secretion, mediated by acetylcholine and GLP-1, and elevated glucose uptake via increased membrane bound GLUT4 transporters. PLOS ONE, 10, e0116546.10.1371/journal.pone.0116546
- Kang, M.-Y., Park, Y. H., Kim, B. S., Seo, S. Y., Jeong, B. C., Kim, J.-I., & Kim, H. H. (2012). Preventive effects of green tea (Camellia sinensis var. Assamica) on diabetic nephropathy. Yonsei Medical Journal, 53, 138–144.10.3349/ymj.2012.53.1.138
- Kavanagh, K. T., Hafer, L. J., Kim, D. W., Mann, K. K., Sherr, D. H., Rogers, A. E., & Sonenshein, G. E. (2001). Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. Journal of Cellular Biochemistry, 82, 387–398.10.1002/(ISSN)1097-4644
- Khan, S. A., Priyamvada, S., Khan, W., Khan, S., Farooq, N., & Yusufi, A. N. K. (2009). Studies on the protective effect of green tea against cisplatin induced nephrotoxicity. Pharmacological Research, 60, 382–391.10.1016/j.phrs.2009.07.007
- Kim, H. J., Sato, T., Rodriguez-Iturbe, B., & Vaziri, N. D. (2011). Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. Journal of Pharmacology and Experimental Therapeutics, 337, 583–590.10.1124/jpet.110.175828
- Kim, H.-S., Quon, M. J., & Kim, J.-A. (2014). New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biology, 2, 187–195.10.1016/j.redox.2013.12.022
- Kim, M., Murakami, A., & Ohigashi, H. (2007). Modifying effects of dietary factors on (−)-epigallocatechin-3-gallate-induced pro-matrix metalloproteinase-7 production in HT-29 human colorectal cancer cells. Bioscience, Biotechnology, and Biochemistry, 71, 2442–2450.10.1271/bbb.70213
- Kim, M., Murakami, A., Miyamoto, S., Tanaka, T., & Ohigashi, H. (2010). The modifying effects of green tea polyphenols on acute colitis and inflammation‐associated colon carcinogenesis in male ICR mice. Biofactors, 36, 43–51. doi:10.1002/biof.69
- Kim, T., Davis, J., Zhang, A. J., He, X., & Mathews, S. T. (2009). Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochemical and Biophysical Research Communications, 388, 377–382.10.1016/j.bbrc.2009.08.018
- Kitada, M., Kume, S., Imaizumi, N., & Koya, D. (2011). Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes, 60, 634–643.10.2337/db10-0386
- Koike, N., Takamura, T., & Kaneko, S. (2007). Induction of reactive oxygen species from isolated rat glomeruli by protein kinase C activation and TNF-α stimulation, and effects of a phosphodiesterase inhibitor. Life Sciences, 80, 1721–1728.10.1016/j.lfs.2007.02.001
- Koya, D., Hayashi, K., Kitada, M., Kashiwagi, A., Kikkawa, R., & Haneda, M. (2003). Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. Journal of the American Society of Nephrology, 14(suppl 3), 250S–253.10.1097/01.ASN.0000077412.07578.44
- Kupelian, V., Araujo, A. B., Wittert, G. A., & McKinlay, J. B. (2015). Association of moderate to severe lower urinary tract symptoms with incident type 2 diabetes and heart disease. The Journal of Urology, 193, 581–586.10.1016/j.juro.2014.08.097
- Kurita, I., Maeda-Yamamoto, M., Tachibana, H., & Kamei, M. (2010). Antihypertensive effect of benifuuki tea containing O -methylated EGCG. Journal of Agricultural and Food Chemistry, 58, 1903–1908.10.1021/jf904335g
- Lacmata, S. T., Kuete, V., Dzoyem, J. P., Tankeo, S. B., Teke, G. N., Kuiate, J. R., & Pages, J. M. (2012). Antibacterial activities of selected Cameroonian plants and their synergistic effects with antibiotics against bacteria expressing MDR phenotypes. Evidence-Based Complementary and Alternative Medicine, 2012, Article ID: 623723. doi:10.1155/2012/623723
- Lambert, J. D., Kennett, M. J., Sang, S., Reuh, K. R., Ju, J., & Yang, C. S. (2010). Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food and Chemical Toxicology, 48, 409–416.10.1016/j.fct.2009.10.030
- Lee, M. J., Maliakal, P., Chen, L.,Meng, X., Bondoc, F. Y., Prabhu, S., … Yang, C. S. (2002). Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans formation of different metabolites and individual variability. Cancer Epidemiology Biomarkers & Prevention, 11, 1025–1032.
- Lee, J. W., Park, S., Takahashi, Y., & Wang, H.-G. (2010). The association of AMPK with ULK1 regulates autophagy. PLoS ONE, 5, e15394.10.1371/journal.pone.0015394
- Lee, S.-Y., Park, S.-Y., Nam, Y.-D., Yi, S.-H., & Lim, S.-I. (2013). Anti-diabetic effects of fermented green tea in KK-A y diabetic mice. Korean Journal of Food Science and Technology, 45, 488–494.10.9721/KJFST.2013.45.4.488
- Li, C., Allen, A., Kwagh, J., Doliba, N. M., Qin, W., Najafi, H., … Smith, T. J. (2006). Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. Journal of Biological Chemistry, 281, 10214–10221.10.1074/jbc.M512792200
- Li, W., Khor, T. O., Xu, C., Shen, G., Jeong, W.-S., Yu, S., & Kong, A. N. (2008). Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochemical Pharmacology, 76, 1485–1489.10.1016/j.bcp.2008.07.017
- Lin, Y.-L., Juan, I.-M., Chen, Y.-L., Liang, Y.-C., & Lin, J.-K. (1996). Composition of polyphenols in fresh tea leaves and associations of their oxygen-radical-absorbing capacity with antiproliferative actions in fibroblast cells. Journal of Agricultural and Food Chemistry, 44, 1387–1394.10.1021/jf950652k
- Lopes de Faria, J. B. L., Silva, K. C., & Lopes de Faria, J. M. L. (2011). The contribution of hypertension to diabetic nephropathy and retinopathy: The role of inflammation and oxidative stress. Hypertension Research, 34, 413–422.10.1038/hr.2010.263
- MacKenzie, T., Leary, L., & Brooks, W. B. (2007). The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: double-blind randomized study. Metabolism, 56, 1340–1344.10.1016/j.metabol.2007.05.018
- Manach, C., Williamson, G., Morand, C., Scalbert, A., & Rémésy, C. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American journal of clinical nutrition, 81(1), 230S–242S.
- Martini, S., Eichinger, F., Nair, V., & Kretzler, M. (2008). Defining human diabetic nephropathy on the molecular level: Integration of transcriptomic profiles with biological knowledge. Reviews in Endocrine and Metabolic Disorders, 9, 267–274.10.1007/s11154-008-9103-3
- Mazzanti, G., Menniti-Ippolito, F., Moro, P. A., Cassetti, F., Raschetti, R., Santuccio, C., & Mastrangelo, S. (2009). Hepatotoxicity from green tea: A review of the literature and two unpublished cases. European Journal of Clinical Pharmacology, 65, 331–341.10.1007/s00228-008-0610-7
- Miyauchi, K., Takiyama, Y., Honjyo, J., Tateno, M., & Haneda, M. (2009). Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-β1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Research and Clinical Practice, 83, 190–199.10.1016/j.diabres.2008.11.018
- Mohib, M. M., Hasan, I., Chowdhury, W. K., Chowdhury, N. U., Mohiuddin, S., Sagor, M. A. T., … Alam, M. A. (2016). Role of angiotensin ii in hepatic inflammation through MAPK pathway: A review. Journal of Hepatitis, 2, 13–20.
- Mora, C., & Navarro, J. F. (2004). Inflammation and pathogenesis of diabetic nephropathy. Metabolism, 53, 265–266.10.1016/j.metabol.2003.11.005
- Morris, B. J. (2013). Seven sirtuins for seven deadly diseases ofaging. Free Radical Biology and Medicine, 56, 133–171.10.1016/j.freeradbiomed.2012.10.525
- Mozaffari-Khosravi, H., Ahadi, Z., & Tafti, M. F. (2014). The effect of green tea versus sour tea on insulin resistance, lipids profiles and oxidative stress in patients with type 2 diabetes mellitus: A randomized clinical trial. Iranian Journal of Medical Sciences, 39, 424–432.
- Na, H.-K., & Surh, Y.-J. (2008). Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food and Chemical Toxicology, 46, 1271–1278.10.1016/j.fct.2007.10.006
- Nishikawa, T., Edelstein, D., Du, X. L., Yamagishi, S. I., Matsumura, T., Kaneda, Y., … Giardino, I. (2000). Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature, 404, 787–790. doi:10.1038/35008121
- Noh, H., & King, G. (2007). The role of protein kinase C activation in diabetic nephropathy. Kidney International, 72, S49–S53.10.1038/sj.ki.5002386
- Nomura, S., Monobe, M., Ema, K., Matsunaga, A., Maeda-Yamamoto, M., & Horie, H. (2015). Effects of flavonol-rich green tea (Camellia sinensis L. cv. Sofu) on blood glucose and insulin levels in diabetic mice. Integrative Obesity and Diabetes, 1, 109–111.
- Nourooz-Zadeh, J., Rahimi, A., Tajaddini-Sarmadi, J., Tritschler, H., Rosen, P., Halliwell, B., & Betteridge, D. J. (1997). Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia, 40, 647–653.10.1007/s001250050729
- Ortsäter, H., Grankvist, N., Wolfram, S., Kuehn, N., & Sjöholm, Å. (2012). Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutrition & Metabolism, 9(1), 1. doi:10.1186/1743-7075-9-11
- Osada, K., et al. (2001). Tea catechins inhibit cholesterol oxidation accompanying oxidation of low density lipoprotein in vitro. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 128, 153–164.
- Park, N.-Y., Park, S.-K., & Lim, Y. (2011). Long-term dietary antioxidant cocktail supplementation effectively reduces renal inflammation in diabetic mice. British Journal of Nutrition, 106, 1514–1521.10.1017/S0007114511001929
- Pazdro, R., & Burgess, J. R. (2012). The antioxidant 3H-1, 2-dithiole-3-thione potentiates advanced glycation end-product-induced oxidative stress in SH-SY5Y cells. Experimental Diabetes Research, 2012, Article ID: 137607. doi:10.1155/2012/137607
- Peixoto, E., Papadimitriou, A., Teixeira, D. A. T., Montemurro, C., Duarte, D. A., Silva, K. C., … Lopes de Faria, J. B. (2015). Reduced LRP6 expression and increase in the interaction of GSK3β with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. The Journal of Nutritional Biochemistry, 26, 416–430.10.1016/j.jnutbio.2014.11.012
- Peng, A., Ye, T., Rakheja, D., Tu, Y., Wang, T., Du, Y., … Zhou, X. J. (2011). The green tea polyphenol (−)-epigallocatechin-3-gallate ameliorates experimental immune-mediated glomerulonephritis. Kidney International, 80, 601–611.10.1038/ki.2011.121
- Peti-Peterdi, J., Kang, J. J., & Toma, I. (2008). Activation of the renal renin–angiotensin system in diabetes—new concepts. Nephrology Dialysis Transplantation, 23, 3047–3049.10.1093/ndt/gfn377
- Pistrosch, F., Ganz, X., Bornstein, S. R., Birkenfeld, A. L., Henkel, E., & Hanefeld, M. (2015). Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease: A cohort study under real-world conditions. Acta Diabetologica, 52, 889–895. doi:10.1007/s00592-015-0727-y
- Polychronopoulos, E., Zeimbekis, A., Kastorini, C.-M., Papairakleous, N., Vlachou, I., Bountziouka, V., & Panagiotakos, D. B. (2008). Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study). European Journal of Nutrition, 47, 10–16.10.1007/s00394-007-0690-7
- Price, N. L., Gomes, A. P., Ling, A. J. Y., Duarte, F. V., Martin-Montalvo, A., Agarwal, B., … Sinclair, D. A. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabolism, 15, 675–690.10.1016/j.cmet.2012.04.003
- Raederstorff, D. G., Schlachter, M. F., Elste, V., & Weber, P. (2003). Effect of EGCG on lipid absorption and plasma lipid levels in rats. The Journal of Nutritional Biochemistry, 14, 326–332.10.1016/S0955-2863(03)00054-8
- Ramadan, G., Nadia, .M., & El-Ghffar, E. A. A. (2009). Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. British Journal of Nutrition, 102, 1611–1619.
- Ramadan, G., El-Beih, N. M., & Abd, E. A. (2009). El-Ghffar, Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. British Journal of Nutrition, 102, 1611–1619.
- Rehman, H., Krishnasamy, Y., Haque, K., Thurman, R. G., Lemasters, J. J., Schnellmann, R. G., & Zhong, Z. (2013). Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin a treatment in rats. PLoS One, 8(6), e65029. doi:10.1371/journal.pone.0065029
- Rehman, H., Krishnasamy, Y., Haque, K., Thurman, R. G., Lemasters, J. J., Schnellmann, R. G., & Zhong, Z. (2013). Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin A treatment in rats. PLoS ONE, 8, e65029.10.1371/journal.pone.0065029
- Renno, W. M., Abdeen, S., Alkhalaf, M., & Asfar, S. (2008). Effect of green tea on kidney tubules of diabetic rats. British Journal of Nutrition, 100, 652–659.10.1017/S0007114508911533
- Reno, F. E., et al. (2015). A novel nasal powder formulation of glucagon: Toxicology studies in animal models. BMC Pharmacology and Toxicology, 16(1), 937.10.1186/s40360-015-0026-9
- Reza, H. M., Sagor, M. A. T., & Alam, M. A. (2015). Iron deposition causes oxidative stress, inflammation and fibrosis in carbon tetrachloride-induced liver dysfunction in rats. Bangladesh Journal of Pharmacology, 10, 152–159.
- Reza, H. M., Tabassum, N., Sagor, M. A. T., Chowdhury, M. R. H., Rahman, M., Jain, P., & Alam, M. A. (2016). Angiotensin-converting enzyme inhibitor prevents oxidative stress, inflammation, and fibrosis in carbon tetrachloride-treated rat liver. Toxicology Mechanisms and Methods, 26, 46–53. doi:10.3109/15376516.2015.1124956
- Ribaldo, P. D., Souza, D. S., Biswas, S. K., Block, K., de Faria, J. M. L., & de Faria, J. B. L. (2009). Green tea (Camellia sinensis) attenuates nephropathy by downregulating Nox4 NADPH oxidase in diabetic spontaneously hypertensive rats. The Journal of Nutrition, 139, 96–100.
- Rojas, J. J., Ochoa, V. J., Ocampo, S. A., & Muñoz, J. F (2006). Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complementary and Alternative Medicine, 6(1), 43.10.1186/1472-6882-6-2
- Rosenthal, J., & Nocera, D. G. (2007). Role of proton-coupled electron transfer in O–O bond activation. Accounts of Chemical Research, 40, 543–553.10.1021/ar7000638
- Ruggenenti, P., Cravedi, P., & Remuzzi, G. (2010). The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nature Reviews Nephrology, 6, 319–330.10.1038/nrneph.2010.58
- Ruiz, S., Pergola, P. E., Zager, R. A., & Vaziri, N. D. (2013). Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney International, 83, 1029–1041.10.1038/ki.2012.439
- Rutter, M. K., & Nesto, R. W. (2011). Blood pressure, lipids and glucose in type 2 diabetes: How low should we go? Re-discovering personalized care European Heart Journal, 32, 2247–2255.10.1093/eurheartj/ehr154
- Ryu, H. H., Kim, H. L., Chung, J. H., Lee, B. R., Kim, T. H., & Shin, B. C. (2011). Renoprotective effects of green tea extract on renin-angiotensin-aldosterone system in chronic cyclosporine-treated rats. Nephrology Dialysis Transplantation, 26, 1188–1193.10.1093/ndt/gfq616
- Sachdeva, A. K., Kuhad, A., Tiwari, V., Arora, V., & Chopra, K. (2010). Protective effect of epigallocatechin gallate in murine water-immersion stress model of chronic fatigue syndrome. Basic & Clinical Pharmacology & Toxicology, 106, 490–496.10.1111/pto.2010.106.issue-6
- Sagor, A. T., Chowdhury, M. R. H., Tabassum, N., Hossain, H., Rahman, M. M., & Alam, M. A (2015). Supplementation of fresh ucche (Momordica charantia L. var. muricata Willd) prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. BMC Complementary and Alternative Medicine, 15, 474.10.1186/s12906-015-0636-1
- Sagor, M. A. T., Tabassum, N., Potol, M. A., & Alam, M. A. (2015). Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxidative Medicine and Cellular Longevity, 2015, 9.
- Sagor, M. A. T., Mohib, M. M., Tabassum, N., Ahmed, I., & Reza, H. M. (2016). Fresh seed supplementation of Syzygium cumini attenuated oxidative stress, inflammation, fibrosis, iron overload, hepatic dysfunction and renal injury in acetaminophen induced rats. Journal of Drug Metabolism & Toxicology, 7, 2. doi:10.4172/2157-7609.1000208
- Sahin, K., Tuzcu, M., Gencoglu, H., Dogukan, A., Timurkan, M., Sahin, N., … Kucuk, O. (2010). Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sciences, 87, 240–245.10.1016/j.lfs.2010.06.014
- Santini, S. A., Marra, G., Giardina, B., Cotroneo, P., Mordente, A., MartoranaG. E., … Ghirlanda, G. (1997). Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes, 46, 1853–1858.10.2337/diab.46.11.1853
- Sartippour, M. R., Shao, Z. M., Heber, D., Beatty, P., Zhang, L., Liu, C., … Brooks, M. N. (2002). Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. The Journal of Nutrition, 132, 2307–2311.
- Sedeek, M., Callera, G., Montezano, A., Gutsol, A., Heitz, F., Szyndralewiez, C., … Hebert, R. L. (2010). Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. AJP: Renal Physiology, 299, F1348–F1358.10.1152/ajprenal.00028.2010
- Sedeek, M., Gutsol, A., Montezano, A. C., Burger, D., Nguyen Dinh Cat, A., Kennedy, C. R. J., … Touyz, R. M. (2013). Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clinical Science, 124, 191–202.10.1042/CS20120330
- Shin, B. C., Chung, J. H., Lee, B. R., & Kim, H. L. (2009). The protective effects of green tea extract against L-arginine toxicity to cultured human mesangial cells. Journal of Korean Medical Science, 24(Suppl 1), S204–S209.10.3346/jkms.2009.24.S1.S204
- Shokouhi, B., AbediGaballu, F., Khyavy, O. M., Mamandy, H., Rasoulian, H., Hajizadeh, N., & Mardomi, A. (2015). Effects of Nigella sativa and Camellia sinensis water extracts on alloxan induced diabetic nephropathy in rats. Advances in Bioresearch, 6, 128–132.
- Smith, T. J. (2011). Green tea polyphenols in drug discovery: A success or failure? Expert Opinion on Drug Discovery, 6, 589–595.10.1517/17460441.2011.570750
- Soetikno, V., Sari, F. R., Lakshmanan, A. P., Arumugam, S., Harima, M., Suzuki, K., & Watanabe, K. (2013). Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2–keap1 pathway. Molecular Nutrition & Food Research, 57, 1649–1659.10.1002/mnfr.v57.9
- Sriram, N., Kalayarasan, S., & Sudhandiran, G. (2009). Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2–Keap1 signaling. Pulmonary Pharmacology & Therapeutics, 22, 221–236.10.1016/j.pupt.2008.12.010
- Sudano Roccaro, A. S., Blanco, A. R., Giuliano, F., Rusciano, D., & Enea, V. (2004). Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrobial Agents and Chemotherapy, 48, 1968–1973.10.1128/AAC.48.6.1968-1973.2004
- Sueoka, N., Suganuma, M., Sueoka, E., Okabe, S., Matsuyama, S., Imai, K., … Fujiki, H. (2001). A new function of green tea: Prevention of lifestyle-related diseases. Annals of the New York Academy of Sciences, 928, 274–280.
- Suliburska, J., Bogdanski, P., Szulinska, M., Stepien, M., Pupek-Musialik, D., & Jablecka, A. (2012). Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biological Trace Element Research, 149, 315–322.10.1007/s12011-012-9448-z
- Suzuki, D., Miyazaki, M., Naka, R., Koji, T., Yagame, M., Jinde, K., Sakai, H., … Nomoto, Y. (1995). In Situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes, 44, 1233–1238.10.2337/diab.44.10.1233
- Tan, A. L., Sourris, K. C., Harcourt, B. E, Thallas-Bonke, V., Penfold, S., Andrikopoulos, S., … Forbes, J. M. (2010). Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. AJP: Renal Physiology, 298, F763–F770.10.1152/ajprenal.00591.2009
- Tang, W., Li, S., Liu, Y., Huang, M.-T., & Ho, C.-T. (2013). Anti-diabetic activity of chemically profiled green tea and black tea extracts in a type 2 diabetes mice model via different mechanisms. Journal of Functional Foods, 5, 1784–1793.10.1016/j.jff.2013.08.007
- Thangapandiyan, S., & Miltonprabu, S. (2014). Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: Role of Nrf2/HO-1 signaling. Toxicology Reports, 1, 12–30.10.1016/j.toxrep.2014.01.002
- Toolsee, N. A., Aruoma, O. I., Gunness, T. K., Kowlessur, S., Dambala, V., Murad, F., & Bahorun, T. (2013). Effectiveness of green tea in a randomized human cohort: Relevance to diabetes and its complications. BioMed Research International, 2013, Article ID: 412379. doi:10.1155/2013/412379
- Tsai, P.-Y., Ka, S.-M., Chang, J.-M., Chen, H.-C., Shui, H.-A., Li, C.-Y., … Chen, A. (2011). Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radical Biology and Medicine, 51, 744–754.10.1016/j.freeradbiomed.2011.05.016
- Tse, H., Anderson, B., Ganini, D., & Mason, R. (2015). Immuno-spin trapping to detect immune-derived free radicals in type 1 diabetes (TECH2P. 903). The Journal of Immunology, 194(1 Supplement), 206–213.
- United States Renal Data System. (2011). Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- Vaziri, N. D., Dicus, M., Ho, N. D., Boroujerdi-Rad, L., & Sindhu, R. K. (2003). Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney International, 63, 179–185.10.1046/j.1523-1755.2003.00702.x
- Wakabayashi, N., Slocum, S. L., Skoko, J. J., Shin, S., & Kensler, T. W. (2010). When NRF2 Talks, who's listening? Antioxidants & Redox Signaling, 13, 1649–1663.10.1089/ars.2010.3216
- Wang, H., Meng, Q. H., Gordon, J. R., Khandwala, H., & Wu, L. (2007). Proinflammatory and proapoptotic effects of methylglyoxal on neutrophils from patients with type 2 diabetes mellitus. Clinical Biochemistry, 40, 1232–1239.10.1016/j.clinbiochem.2007.07.016
- Wang, Y., Wang, B., Du, F., Su, X., Sun, G., Zhou, G., … Liu, N. (2015). Epigallocatechin-3-gallate attenuates unilateral ureteral obstruction-induced renal interstitial fibrosis in mice. Journal of Histochemistry & Cytochemistry, 63, 270–279.10.1369/0022155414568019
- Wang, Y., Wang, B., Du, F., Su, X., Sun, G., Zhou, G., … Liu, N. (2015). Epigallocatechin-3-gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic & Clinical Pharmacology & Toxicology, 117, 164–172.10.1111/bcpt.2015.117.issue-3
- Wassmann, S., Wassmann, K., & Nickenig, G. (2004). Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension, 44, 381–386.10.1161/01.HYP.0000142232.29764.a7
- Weber, J. M., Ruzindana-Umunyana, A., Imbeault, L., & Sircar, S. (2003). Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Research, 58, 167–173.10.1016/S0166-3542(02)00212-7
- Wein, S., Schrader, E., Rimbach, G., & Wolffram, S. (2013). Oral green tea catechins transiently lower plasma glucose concentrations in female db/db mice. Journal of Medicinal Food, 16, 312–317.10.1089/jmf.2012.0205
- Weinreb, O., Mandel, S., Amit, T., & Youdim, M. B. H. (2004). Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. The Journal of Nutritional Biochemistry, 15, 506–516.10.1016/j.jnutbio.2004.05.002
- Wendt, T. M., Tanji, N., Guo, J., Kislinger, T. R., Qu, W., Lu, Y., … Schmidt, A. N. (2003). RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. The American Journal of Pathology, 162, 1123–1137.10.1016/S0002-9440(10)63909-0
- Whiting, D. R., Guariguata, L., Weil, C., & Shaw, J. (2011). IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice, 94, 311–321.10.1016/j.diabres.2011.10.029
- Wolfram, S. (2007). Effects of Green Tea and EGCG on Cardiovascular and Metabolic Health. Journal of the American College of Nutrition, 26, 373S–388S.10.1080/07315724.2007.10719626
- Wolfram, S., Raederstorff, D., Preller, M., Wang, Y., Teixeira, S. R., Riegger, C., & Weber, P. (2006). Epigallocatechin gallate supplementation alleviates diabetes in rodents. The Journal of Nutrition, 136, 2512–2518.
- Wu, C., Hsu, M. C., Hsieh, C. W., Lin, J. B., Lai, P. H., & Wung, B. S. (2006). Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sciences, 78, 2889–2897.10.1016/j.lfs.2005.11.013
- Yamabe, N., Yokozawa, T., Oya, T., & Kim, M. (2006). Therapeutic potential of (-)-epigallocatechin 3-o-gallate on renal damage in diabetic nephropathy model rats. Journal of Pharmacology and Experimental Therapeutics, 319, 228–236.10.1124/jpet.106.107029
- Yamahara, K., Kume, S., Koya, D., Tanaka, Y., Morita, Y., Chin-Kanasaki, M., … Matsusaka, T. (2013). Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. Journal of the American Society of Nephrology. p. ASN. 2012111080.
- Yan, J., Zhao, Y., Suo, S., Liu, Y., & Zhao, B. (2012). Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radical Biology and Medicine, 52, 1648–1657.10.1016/j.freeradbiomed.2012.01.033
- Yapar, K., Çavuşoğlu, K., Oruç, E., & Yalçin, E. (2009). Protective effect of royal jelly and green tea extracts effect against cisplatin-induced nephrotoxicity in mice: A comparative study. Journal of Medicinal Food, 12, 1136–1142.10.1089/jmf.2009.0036
- Ye, T., Zhen, J., Du, Y., Peng, A., Vaziri, N. D., Mohan, C., & ZhouX. J. (2015). Green tea polyphenol (−)-epigallocatechin-3-gallate restores Nrf2 activity and ameliorates crescentic glomerulonephritis. PLOS ONE, 10, e0119543.10.1371/journal.pone.0119543
- Yoh, K., Itoh, K., Enomoto, A., Hirayama, A., Yamaguchi, N., Kobayashi, M., … Takahashi, S. (2001). Nrf2-deficient female mice develop lupus-like autoimmune nephritis11See Editorial by Byrd and Thomas, p. 1606. Kidney International, 60, 1343–1353.10.1046/j.1523-1755.2001.00939.x
- Yoh, K., Hirayama, A., Ishizaki, K., Yamada, A., Takeuchi, M., Yamagishi, S., … Yamamoto, M. (2008). Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes to Cells, 13, 1159–1170. doi:10.1111/j.1365-2443.2008.01234.x
- Yokozawa, T., Noh, J. S., & Park, C. H. (2012). Green tea polyphenols for the protection against renal damage caused by oxidative stress. Evidence-Based Complementary and Alternative Medicine, 2012, Article ID: 845917. doi:10.1155/2012/845917
- Yoon, S. P., Hong, R., Lee, B. R., Kim, C. G., Kim, H. L., Chung, J. H., & Shin, B. C. (2014). Protective effects of epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochemica, 116, 1210–1215.10.1016/j.acthis.2014.07.003
- Zaveri, N. T. (2006). Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sciences, 78, 2073–2080.10.1016/j.lfs.2005.12.006
- Zhou, Q., Li, H., Zhou, J. G., Ma, Y., Wu, T., & Ma, H. (2016). Green tea, black tea consumption and risk of endometrial cancer: A systematic review and meta-analysis. Archives of Gynecology and Obstetrics, 293, 143–155.10.1007/s00404-015-3811-1
- Zhu, D., Wang, L., Zhou, Q., Yan, S., Li, Z., Sheng, J., & Zhang, W. (2014). (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Molecular Nutrition & Food Research, 58, 2249–2260.10.1002/mnfr.201400533
- Zoncu, R., Efeyan, A., & Sabatini, D. M. (2011). mTOR: From growth signal integration to cancer, diabetes and ageing. Nature Reviews Molecular Cell Biology, 12, 21–35.10.1038/nrm3025