ABSTRACT
This article reviews the literature on the circadian rhythms of body temperature and whole-organism metabolism. The two rhythms are first described separately, each description preceded by a review of research methods. Both rhythms are generated endogenously but can be affected by exogenous factors. The relationship between the two rhythms is discussed next. In endothermic animals, modulation of metabolic activity can affect body temperature, but the rhythm of body temperature is not a mere side effect of the rhythm of metabolic thermogenesis associated with general activity. The circadian system modulates metabolic heat production to generate the body temperature rhythm, which challenges homeothermy but does not abolish it. Individual cells do not regulate their own temperature, but the relationship between circadian rhythms and metabolism at the cellular level is also discussed. Metabolism is both an output of and an input to the circadian clock, meaning that circadian rhythmicity and metabolism are intertwined in the cell.
Introduction
The award of the 2017 Nobel Prize in physiology to three researchers who first identified the molecular mechanism of circadian rhythms helped bring the attention of life scientists and health professionals to the importance of circadian rhythmicity for the healthy operation of living organisms Citation[1,Citation2]. Disruption of the relationship between the internal circadian clock and the synchronizing environmental cycle (such as the disruption observed after transcontinental travel, during shift work, or even concomitantly with the extensive use of artificial light in the modern 24-hour society) has been shown to have serious negative health effects, including breast cancer, cardiovascular disease, psychiatric disorders, and the metabolic syndrome [Citation3–7].
One of the first physiological variables subjected to long-term monitoring that allowed the determination of daily rhythmicity in the mid 1800’s was body temperature [Citation8,Citation9]. Studying the rhythmicity of body temperature is important for at least two reasons: 1) the body temperature rhythm is a convenient marker of the circadian clock for studies on biological rhythms and sleep, and 2) the rhythm interacts with a concurrent rhythm of metabolism and reflects a constant conflict between homeostasis and circadian rhythmicity in the control of core temperature in mammals and birds. Two previous comprehensive literature reviews were published 28 years ago [Citation10] and 10 years ago [Citation11]. Reviews by other authors have concentrated on particular aspects of the rhythmicity of body temperature [Citation12–14].
Unlike mammals and birds, most living beings on Earth (including fungi, plants, microbes, and the majority of animal species) do not regulate the temperature of their bodies by autonomic mechanisms [Citation15,Citation16]. This means that, in the absence of effective behavioral adjustments (which are also limited to a few taxonomic groups), the body temperature of an organism will vary with the temperature of the environment. Because of the dependence of biochemical reactions on temperature, this means also that the organism’s metabolism will vary with the temperature of the environment. It is not surprising, therefore, that the body temperature and metabolism of most living beings on Earth will oscillate daily and seasonally along with the daily and seasonal oscillation in environmental temperature. However, a relatively small group of animals (primarily mammals and birds) regulates body temperature by both behavioral and autonomic mechanisms [Citation17–19]. These animals, often called endothermic homeotherms, can maintain a relatively stable body temperature while exposed to heat or cold because of their ability to modulate metabolic heat production as well as convective and evaporative heat loss. As emphasized by Claude Bernard in the 19th century, the constancy of physico-chemical properties of the internal environment of an organism is essential for a free life [Citation20]. Yet, as will be discussed here, body temperature and metabolism exhibit daily and seasonal variation in mammals and birds – not as a direct effect of variation in ambient temperature but as an endogenously controlled process. An endogenously generated physiological oscillation with a period (duration) of approximately 24 hours is called a circadian rhythm [Citation21], and this article will deal with the circadian rhythmicity of body temperature and metabolism. Seasonal oscillation in body temperature and metabolism (as reflected principally in the process of hibernation) has been reviewed by others recently [Citation22,Citation23] and will not be covered here. This review will concentrate on circadian rhythmicity.
Circadian rhythmicity of body temperature
Research methodology
A first requirement for the recording of circadian rhythms of body temperature is the possession of a thermometer. Although a standard clinical thermometer can be used if the animals are not disturbed by the frequent contact with the experimenter, more sophisticated thermometers are required for measurements taken many times a day for many consecutive days. The monitoring of body temperature in human subjects can be easily accomplished with commercially available biomonitoring systems such as those marketed by AD Instruments Inc. (Colorado Springs, Colorado), Biopac Systems Inc. (Goleta, California), Mindware Technologies Ltd. (Gahanna, Ohio), and Noldus Information Technology (Wageningen, Netherlands). Temperature-sensitive radio transmitters may be conveniently swallowed [Citation24,Citation25], although they stay in the digestive system for only a few days and are of limited use in long-term studies. Gut temperature measured with a swallowed sensor-transmitter correlates better with rectal temperature than does axillary temperature (measured under the arm) [Citation26].
Monitoring of body temperature in other animal species usually involves surgically implanted temperature-sensitive sensors for short-range telemetry in the laboratory [Citation27–32] or surgically implanted data loggers for free-ranging animals [Citation33–38] (see also in the review article by Maloney and colleagues [Citation14]). For telemetry equipment, the major manufacturers in the United States are Data Sciences Inc. (St. Paul, Minnesota), the Stellar Telemetry branch of TSE Systems (Chesterfield, Missouri), the Implantable Telemetry branch of Millar Inc. (Houston, Texas), and the E-Mitter Telemetry branch of Starr Life Sciences (Oakmont, Pennsylvania). Starr Life’s E-mitters and Millar’s Telemeters are transponder transmitters (that is, transmitters that are tele-energized by the radio receiver). This feature is especially convenient in long-term studies in which traditional transmitters will run out of battery, although transponder transmitters require maintenance after one or two years of operation, which reduces their advantage over battery-based transmitters in multi-year studies.
Table 1. Studies documenting the existence of daily rhythmicity of body temperature
An alternative to telemetry, especially for field studies, is the data logger. Data loggers are devices that can record and store data. The advantage over telemetry is that the experimental subjects can move freely over large distances without causing a loss of signal. A disadvantage is that the experimenter cannot access the data until the logger is retrieved. Manufacturers of data loggers include DataTaker Ltd. (Rowville, Australia), Onset Computer Corp. (Bourne, Massachusetts), and Pico Technology Ltd. (St. Neots, United Kingdom). A very convenient data logger is the iButton temperature logger (Maxim Integrated Products, San Jose, California). These miniature loggers (16-mm diameter) can be surgically implanted like radio transmitters. Like larger loggers, iButtons have the advantage of not requiring a separate receiver and the disadvantage of not allowing on-line access to the data. Memory limitations make them unsuitable for long studies with high temporal resolution. Available at a higher price but having the ability to record data for much longer are the miniature data loggers marketed by SubCue Dataloggers (Calgary, Canada) and Star-Oddi Ltd. (Gardabaer, Iceland).
In large animals, surgical implantation can be avoided. TekVet Technologies (Garden City, Kansas) and FeverTags (Amarillo, Texas) manufacture temperature transmitters for use in livestock. The transmitters are placed in the animal’s ear, close to the tympanic membrane, thus allowing measurement of core temperature without the need for surgical intervention. This technology has not been thoroughly evaluated in livestock, and evaluations with human subjects have not been very encouraging [Citation304,Citation305]. For smaller animals in a laboratory setting, including small rodents, temperature-sensitive PIT tags (passive integrated transponder radio-frequency identification devices) can be used [Citation306]. The tags are the size of a grain of rice and can be injected with a syringe subcutaneously or intraperitoneally without the need for a surgical procedure. Because they are transponders, PIT tags require no batteries and can be used uninterruptedly for years. Biomark Inc. (Boise, Idaho) is a major supplier of PIT tag equipment.
Endogenous determinants
Daily rhythmicity of body temperature has been extensively documented in many species of birds and mammals. More than 300 articles are listed in . Although the studies varied greatly in methodology and experimental design, they all provided evidence of a regular daily oscillation of body temperature in a variety of species.
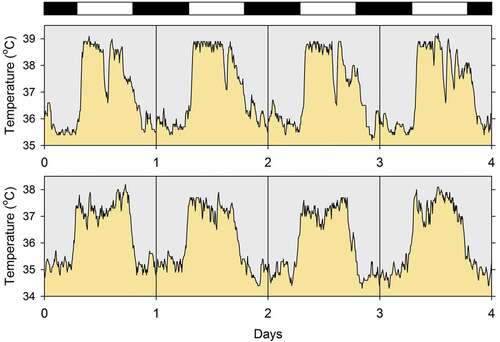
An example of the daily rhythm of body temperature is shown in . The data were obtained from two white-tailed antelope squirrels (Ammospermophilus leucurus) individually housed in the laboratory [Citation306]. The body core temperature of both animals rose daily at the time of lights-on and declined to a nighttime low shortly after lights-off. One of the squirrels (top panel) exhibited a brief temperature decline in the middle of the light phase, whereas the other squirrel (bottom panel) did not. The mean and range of oscillation of the rhythms of the two animals were similar but not identical.
Figure 1. Four-day segments of the records of body temperature of two white-tailed antelope squirrels (Ammospermophilus leucurus, 120 g average body mass) housed individually in the laboratory at 25°C. The data were collected and are plotted with 6-minute resolution. The white and black bars at the top indicate the light and dark phases of the prevailing light-dark cycle
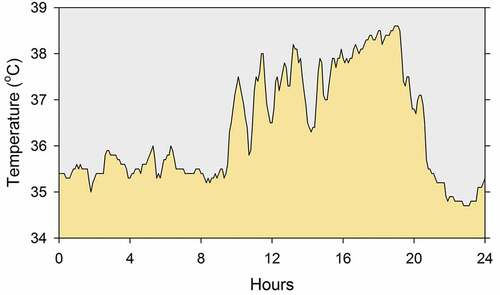
The records of body temperature of another white-tailed antelope squirrel are shown in greater temporal resolution in so that high-frequency oscillations can be seen better. Superimposed on the daily oscillation, one can see somewhat irregular oscillations, with smaller amplitude, lasting approximately 1 or 2 hours. Such ultradian oscillations in body temperature have received much less attention from researchers than circadian oscillations [Citation307], but they have been noted in reports of the rhythmicity of body temperature in rats [Citation308,Citation309], golden hamsters [Citation310,Citation311], lemmings [Citation312], squirrel monkeys [Citation313], sheep [Citation314], and dairy cows [Citation315]. Whereas the circadian rhythmicity of body temperature has been thoroughly confirmed by formal time series analysis [Citation316–319], ultradian rhythmicity has rarely been formally analyzed. Nonetheless, spectral analysis of body temperature data from rats and hamsters has been conducted and has documented statistically significant ultradian oscillation, predominantly in the range of 2 to 12 hours [Citation308–311]. Still unsettled is the question of whether the high-frequency peaks in the periodograms represent true biological rhythms or merely the harmonics needed to describe the wave form of a circadian rhythm generated by a pacemaker that does not produce an ideal sinusoidal signal. As a matter of fact, the shape (waveform) of the circadian rhythm of body temperature has not been thoroughly studied. The waveform is generally described as sinusoidal, although it is often bimodal or square, and there have been very few attempts to quantify the description of the waveform of circadian rhythms. The strength (robustness) of circadian rhythms, which is related to the stability of waveform, has received a little more attention [Citation320], but very little is known about the determinants of the waveform of circadian rhythms. As for ultradian oscillations, it has even been argued that they should be called “episodic ultradian events” (and not “ultradian rhythms”) because the oscillations are most often aperiodic [Citation321].
Figure 2. One day segment of the records of body temperature of a white-tailed antelope squirrel housed individually in the laboratory at 25°C. The data were collected and are plotted with 6-minute resolution
For investigation of the characteristics of endogenously-generated rhythms, organisms must be studied in non-rhythmic environments. Although researchers have not always made certain that environmental conditions were exactly constant, many laboratory studies have ensured the absence of cycles of ambient temperature, food availability, and predator danger. For standardization purposes, most studies maintained a light-dark cycle with 12 hours of light and 12 hour of darkness per day. Data from 218 studies with 93 species are summarized in . It can be seen that the main parameters of the body temperature rhythm – mean, range of oscillation, and acrophase (peak time expressed in hours after lights-on) – vary somewhat from study to study within the same species, possibly because of methodological differences, but intraspecies differences are most often smaller than interspecies differences. Although not inferable from the data shown in the table, it should also be pointed out that intraindividual differences within a species are usually smaller than interindividual differences [Citation322].
Table 2. Parameters of the body temperature rhythm of 93 species of mammals and birds
In terms of the mean level of body temperature, a noticeable trend in is that the body temperature of birds tends to be more than 3 °C higher than that of mammals (on average, 41°C and 37.5 °C, respectively), and the temperature of marsupial mammals tends to be about 3°C lower than that of placental mammals. The range of oscillation also varies greatly from species to species, being noticeably wider in squirrels than in other rodents of comparable body size. The acrophase (peak time) is generally consistent with the temporal niche of the species, in the sense that the acrophase usually occurs at night (i.e., more than 12 hours after lights-on) for nocturnal animals and during the day for diurnal animals, although farm animals tend to have unusually late acrophases (sometimes extending into the early dark phase).
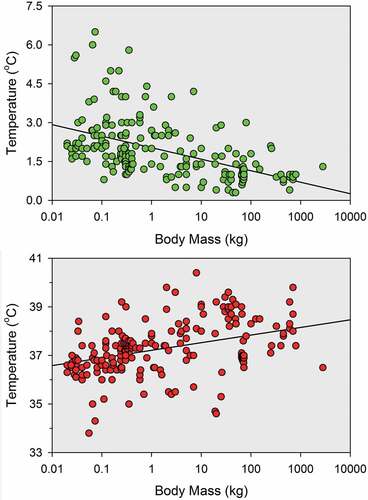
A few broad interspecies comparisons of parameters of the body temperature rhythm have been previously made by other scholars. Aschoff noticed that the amplitude of the temperature rhythm is 3 to 6 times smaller in large animals than in small animals in the body mass range from 10 g to 1 kg [Citation330]. Based on data from 206 independent studies in various laboratories, I can confirm that the amplitude is about 3 times narrower in larger mammals for the full range of body weight from 10 g to 2,000 kg, as shown in (upper panel). Presumably, large bodies can buffer the effects of the oscillations in heat production and heat loss that are responsible for the body temperature rhythm. Interestingly, body size also has an effect on the mean level of the temperature rhythm (, lower panel). Animals in the 1,000 kg range have, on average, body temperatures 1.4 °C higher than the body temperatures of animals in the 10 g range. Again, this is presumably due to the greater thermal inertia of large animals. It should be pointed out that, in both cases (amplitude and mean temperature), the correlation with body mass is statistically significant but is far from perfect (r = −0.51 and r = 0.38, respectively). This may explain why Lovegrove failed to find a correlation between body size and mean body temperature in a set of 267 studies in animals weighing under 1 kg [Citation331]. Given the very wide spread of data seen in , it is to be expected that large differences in body size would be needed for the detection of a significant correlation. Mortola and Lanthier surveyed 125 studies in mammals ranging from 10 g to 5,000 kg and did find that the amplitude of the body temperature rhythm is smaller, and the mean level is higher, in large animals than in small animals [Citation332]. Hetem and colleagues found a large reduction (rather than increase) in mean body temperature with increase in body mass above 10 kg in 17 species of large mammalian herbivores [Citation333], but the evaluated studies were conducted in the wild, where food and water shortages were likely to affect body temperature, as discussed below.
Figure 3. Parameters of the body temperature rhythm as a function of body mass as determined for 86 mammalian species in 206 published studies. Top: daily range of oscillation of the body temperature rhythm. Bottom: mean level of the body temperature rhythm
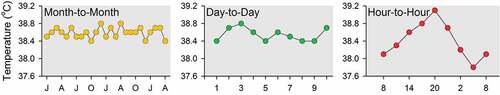
Most studies of the body temperature rhythm are conducted over a few days and cannot provide evidence regarding the long-term stability of the rhythm. A study conducted on four species of farm animals over two years provided valuable information about the variability of the parameters of the body temperature rhythm over days and months [Citation334]. As shown for a single bovine in , the normal circadian range of oscillation is wider than the range of oscillation of same-time-of-day values over days or months. This animal had full unrestricted access to food and water, but ambient temperature ranged from 8°C in the winter to 30°C in the summer, and the stability of same-time-of-day values over months emphasizes the excellence of homeothermic control of body temperature in farm animals. Of course, not all species have such good control of their core temperature, and many small rodents put homeothermy on hold during the winter and engage in hibernation [Citation22,Citation23,Citation335,Citation336]. A few studies have suggested that circadian rhythmicity of body temperature is preserved during hibernation (albeit with very small amplitude) [Citation337–340], but many other studies have found no body temperature rhythm in hibernating animals [Citation35,Citation341–348].
Figure 4. Rectal temperature of a female bovine (Bos taurus) as measured on three time scales: month-to-month (evening measurements conducted monthly for two years), day-to-day (evening measurements conducted daily for 10 days), and hour-to-hour (measurements conducted every 3 hours over a single day). The abscissas of the three plots are marked in months, days, and hours, respectively
A truly endogenous rhythm must persist (with a slightly different period) in the absence not only of environmental cycles of ambient temperature and food availability but also in the absence of a light-dark cycle. Much fewer studies have been conducted under conditions of constant darkness (or constant light), but the endogenous nature of the body temperature rhythm has been confirmed in many species of birds and animals, as shown in . Of course, the fact that the body temperature rhythm free-runs under constant environmental conditions is proof only of the existence of an endogenous circadian clock. It is not proof that the body generates a temperature rhythm as a fundamental process. The rhythmicity of body temperature could be simply a side effect of the rhythmicity of another endogenously generated process. This matter will be discussed further below.
Table 3. Studies documenting the existence of circadian (free-running) rhythmicity of body temperature
Exogenous determinants
Studies of the body temperature rhythm conducted in the outdoors have demonstrated that variations in day length, ambient temperature, food and water availability, predator danger, and other variables can affect the body temperature rhythm of free-ranging animals [Citation38,Citation370–377]. Unfortunately, studies conducted in the wild are almost always correlational and cannot differentiate the action of the various environmental factors, but studies conducted under controlled laboratory conditions have confirmed the specific effects of ambient temperature and food availability.
Regarding ambient temperature, several studies have described an increase in the amplitude of the body temperature rhythm in animals exposed chronically to ambient temperatures below thermoneutrality. This phenomenon has been described in squirrel monkeys [Citation378], tree shrews [Citation379], thirteen-lined ground squirrels [Citation380], sheep [Citation381], pigeons [Citation382], mousebirds [Citation383], and sunbirds [Citation384]. On the other hand, no effect of ambient temperature on the amplitude of the body temperature rhythm was found in rats [Citation385,Citation386], mouse lemurs [Citation387], or golden hamsters and fat-tailed gerbils [Citation379]. Genuine species differences may be responsible for the conflicting results.
Regarding food availability, it has long been known that fasted animals experience a reduction in metabolic rate and a fall in body temperature [Citation388–399]. What is especially interesting about this phenomenon is its modulation by the circadian system. The hypothermia induced by food deprivation (or chronic food restriction) does not occur indiscriminately; rather, it is restricted to the inactive phase of the circadian cycle. Some animals have a natural disposition to exhibit daily torpor even when fed regularly [Citation380,Citation400–412], but various true homeotherms exhibit circadian-modulated starvation-induced hypothermia. This has been documented in doves [Citation413], owls [Citation414], pigeons [Citation394,Citation395,Citation415–417], quail [Citation418], mousebirds [Citation383,Citation419], finches [Citation420], pygmy mice [Citation421], deer mice [Citation422], domestic mice [Citation423–425], rats [Citation396,Citation397,Citation426–430], lemurs [Citation431,Citation432], sheep [Citation433], goats [Citation434], and camels [Citation435].
To avoid misunderstandings, it should be stressed that any event in the environment, whether rhythmic or not, can disturb (“mask”) circadian rhythms. True synchronization (“entrainment”) of circadian rhythms has long been known to be produced by the light-dark cycle [Citation436–438], but it can also be produced by cycles of ambient temperature [Citation439–455] and food availability [Citation456–481]. A study in mice provided the suggestion that cycles of ambient temperature may be as effective as light-dark cycles in producing entrainment but may not be as effective in the production of masking [Citation452], and further studies are needed to confirm this observation. The control of circadian rhythms in the wild is likely determined just as much by the influence of entrainment as by the influence of masking on the endogenously-generated rhythms [Citation482,Citation483].
Circadian rhythmicity of metabolism
Research methodology
For the monitoring of whole-organism metabolism, three techniques are well established: direct calorimetry, indirect calorimetry, and the isotopic tracer technique [Citation484]. The isotopic tracer technique is convenient for field studies, but it does not provide the temporal resolution needed for the study of circadian rhythms. In laboratory studies, direct calorimetry is the “gold standard” for accurate measurement of whole-organism metabolism, but indirect calorimetry is by far the most commonly used technique [Citation485].
Indirect calorimetry is based on the measurement of oxygen consumed (and carbon dioxide produced) by the organism and on the chemical properties of oxidation. Knowledge of the stoichiometric properties of oxidative processes makes it possible to calculate the amount of nutrient being combusted, and the amount of heat being released, by measuring only the amount of oxygen being consumed. To measure the concentration of oxygen in the air used by the organism (as well as the concentration of carbon dioxide, if greater accuracy is needed in the computation of metabolic rate), gas analyzers are employed. Suppliers of gas analyzers for biomedical research include Servomex (Crowborough, England), Columbus Instruments (Columbus, Ohio), Sable Systems International (North Las Vegas, Nevada), and Qubit Systems (Kingston, Canada). For data collection the animal of interest is placed inside a sealed chamber, and a measured volume of air is passed through the chamber. By determining the difference in the concentration of oxygen in the air that enters the chamber and in the air that leaves the chamber, one can determine the percentage of oxygen consumed by the organism. The percentage can then be converted into amount of oxygen (and corresponding amount of heat produced) if the exact flow of air through the chamber is known [Citation486,Citation487]. A computerized system that activates the air-switch valves and collects the data is needed for the monitoring of metabolism with adequate temporal resolution for long-term studies of circadian rhythmicity.
Endogenous determinants
Daily and/or circadian rhythmicity in whole-organism metabolism has been documented in mammals and birds, as shown in . Most studies have been conducted on rodents, but other animals have been studied as well.
Table 4. Studies documenting the existence of daily rhythmicity of whole-organism metabolism
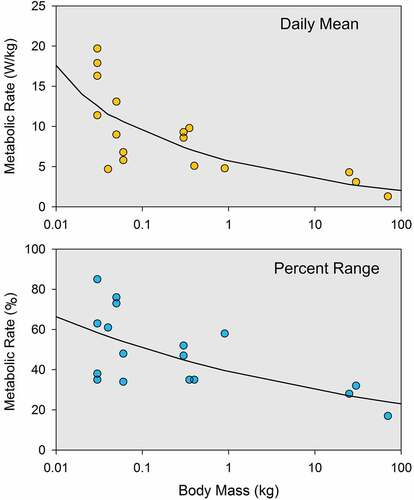
It has long been known that, when expressed per unit of body mass, metabolic rate is higher in small animals than in large animals [Citation516–519]. It is not surprising, therefore, to find out that the mean level of the daily rhythm of metabolism is higher in small animals than in large animals, as shown in (upper panel). This figure was prepared with data from 17 of the 61 studies listed in . Only studies that involved mammals and provided sufficient information for conversion of the results to the common unit of W/kg were used. Of special interest are the data on percent range shown in the lower panel of the figure. Percent range is the range of daily oscillation expressed as a percentage of the daily mean, and it is used instead of the absolute value because the interspecies differences in mean values are quite large, so that the range for each species is meaningful only in reference to the mean for that species. Thus, for example, humans (70 kg) had a mean metabolic rate of 1.3 W/kg with a range of oscillation of 0.2 W/kg, so that the percent range was 15% [Citation520]. In contrast, domestic mice (30 g) had a mean metabolic rate of 17.9 W/kg with a range of oscillation of 15.2 W/kg, so that the percent range was 85% [Citation521], which is much greater than the percent range in humans. As was the case for the body temperature rhythm, different studies on the same species were somewhat variable but relatively consistent. For example, a different study on domestic mice reported a mean metabolic rate of 19.7 W/kg with a range of oscillation of 12.4 W/kg, so that the percent range was 63%[Citation424], thus smaller than in the other mouse study but still larger than in humans.
Figure 5. Mean level (upper plot) and range of oscillation (lower plot) of the daily rhythm of metabolism as a function of body mass in various mammalian species. The range of oscillation is expressed as a percentage of the daily mean (“Percent range”). The data were obtained from 17 published studies and converted to the common unit of W/kg
Although not in the scope of this article, it should be mentioned that the circadian modulation of metabolism has been studied at the cellular level, and several reviews of the literature have been written [Citation522–524].
Exogenous determinants
While scant attention has been given to environmental factors that specifically affect the circadian rhythm of metabolism, there exists an extensive literature on the general effect of environmental factors on steady-state metabolism. As was the case concerning body temperature, the effects of changes in ambient temperature and food availability have been particularly well studied.
Birds and mammals can increase metabolic heat production when exposed to a cold environment, and this is called cold-induced thermogenesis. Shivering is one form of cold-induced thermogenesis. It is a widespread mechanism of thermogenesis used to prevent the fall of body temperature in a cold environment. It consists of small-amplitude, high-frequency contractions of skeletal muscles. It is employed both by birds [Citation382,Citation392,Citation525–531] and by mammals [Citation532–547], including humans [Citation548–550]. Forms of biological thermogenesis other than shivering are collectively called non-shivering thermogenesis. Birds seem to rely primarily on shivering and, if they exhibit thermoregulatory non-shivering thermogenesis, the muscles themselves are the probable source [Citation392,Citation528–530,Citation551-554]. Mammals, on the other hand, use non-shivering thermogenesis extensively in response to cold stress, and the capacity to use it is strongly affected by acclimation or acclimatization [Citation534,Citation540–543,Citation546,Citation555–578]. Mammalian thermoregulatory non-shivering thermogenesis often relies on the activation of a specialized tissue, brown adipose tissue [Citation579,Citation580].
The effects of food availability, and food intake more specifically, are usually discussed as part of the phenomenon of diet-induced thermogenesis. Diet-induced thermogenesis is the fraction of energy expenditure induced by the ingestion of food, and some authors include basal metabolic rate in the definition of diet-induced thermogenesis [Citation581]. There are two types of diet-induced thermogenesis. The first type is called obligatory because it cannot be avoided. After a meal is ingested, metabolic rate is temporarily elevated [Citation520,Citation582–591], and this elevation is believed to be due partially to the energetic cost of digestion and partially to a cephalic component involving mastication as well as arousal [Citation592–598]. The other type of diet-induced thermogenesis is called adaptive because its magnitude can be adapted to conditions of shortage or excess of food supply. That is, diet-induced thermogenesis can be increased after overeating and be reduced during starvation or food restriction [Citation583,Citation599–608]. As was the case for cold-induced thermogenesis, diet-induced thermogenesis in mammals seems to depend strongly on the activation of brown adipose tissue [Citation609].
Relationship of body temperature and metabolism at the organismal level
Autonomy of the body temperature rhythm
The previous sections described the rhythms of body temperature and metabolism separately, but it is well known that body temperature and metabolism can affect each other in both directions. Changes in body temperature can cause changes in metabolism by affecting the rate of chemical reactions in the body (and by inducing a thermogenic response), and changes in metabolism are accompanied by changes in metabolic heat production, which, in the absence of compensatory changes in heat loss, will cause a change in body temperature. Studies in which body temperature and metabolic rate have been recorded simultaneously have shown that the two variables oscillate together through the day [Citation313,Citation520,Citation610–612], and the obvious question to ask is whether there is a causal link – and, if so, in which direction. The question must be asked because changes in body temperature are the result of the balance between heat production and heat loss, and a rise in heat production will not elevate temperature if it is compensated by an equal rise in heat loss.
Studies conducted on reptiles have shown that endogenously-controlled rhythmicity of body temperature is present in extant ectotherms, the control being achieved by behavioral selection of suitable thermal environments [Citation613–616]. For this reason, it is sensible to assume that the evolution of circadian rhythmicity of body temperature preceded the evolution of endothermy in mammals and birds. In fact, it is believed that endothermy evolved about 70 million years ago [Citation617–619], much after the evolution of circadian rhythmicity 2.5 billion years ago [Citation620]. Thus, one can suggest that the ability to adjust metabolic rate evolved to either directly or indirectly facilitate the circadian oscillation of body temperature, even if basal metabolic rate and body temperature seem to have evolved separately from each other [Citation621]. In other words, the rhythm of body temperature must not be a side effect of the rhythm of metabolic thermogenesis; rather, the rhythm of body temperature must require the rhythmic modulation of metabolic thermogenesis. That the rhythm of body temperature is not a simple side effect of the rhythm of metabolic thermogenesis associated with changes in locomotor activity has been demonstrated experimentally both in humans and in other animals, as described in the next two paragraphs.
In order to investigate the potential causal link between the locomotor activity rhythm (which is a major thermogenic process) and the temperature rhythm, several researchers recorded the body temperature rhythm of human subjects maintained in continuous bed rest [Citation622–624] or undergoing a constant routine protocol, which involves bed rest as well as sleep deprivation and the ingestion of frequent, equal-size meals [Citation520,Citation625–627]. Although the amplitude of the rhythm was reduced under this condition of constant physical inactivity, robust rhythmicity of body temperature persisted. Thus, while the activity rhythm may alter the amplitude and shape of the body temperature rhythm, it does not cause it.
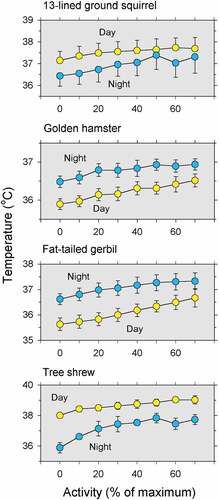
Bed rest cannot be used with animals – because they do not comply with requests for voluntary rest – but one can look at the day-night difference in the correlation between the rhythms of activity and temperature. It has been found that, although nocturnal animals are generally more active at night than during the day, their body temperature is higher at night regardless of the actual activity level [Citation628–632]. Conversely, the body temperature of diurnal animals is higher during the day regardless of the actual activity level [Citation306,Citation633]. These relationships are illustrated in for four different species of small mammals. Notice that, for the nocturnal animals (golden hamster and fat-tailed gerbil), body temperature is higher at night for all levels of activity, even though there is a small effect of activity level on body temperature. For the diurnal animals (13-lined ground squirrel and tree shrew), body temperature is higher during the day for all levels of activity [Citation633]. Thus, it can be inferred that the body temperature rhythm in animals, as in humans, is not caused by the activity rhythm. That is, the body temperature rhythm is not a side effect of the metabolism rhythm associated with changes in activity. This conclusion brings us back to the question of whether the body generates (or attempts to generate) a temperature rhythm as a fundamental process of life.
Figure 6. Mean body temperatures associated with different levels of locomotor activity during the dark phase (blue) and the light phase (yellow) of the light-dark cycle for four species. Error bars indicate SEM
In ectotherms, a rhythm of body temperature can only be produced by behavioral selection of cooler or warmer environments, but, in endotherms, endogenous heat production (through shivering or non-shivering thermogenesis) is possible. Because endogenous heat production is also at the service of homeothermy, it is not immediately evident whether the circadian system or the thermoregulatory system is in control of thermogenesis at any given time. This uncertainty is reflected in a controversy about a hypothetical circadian modulation of the thermoregulatory set point.
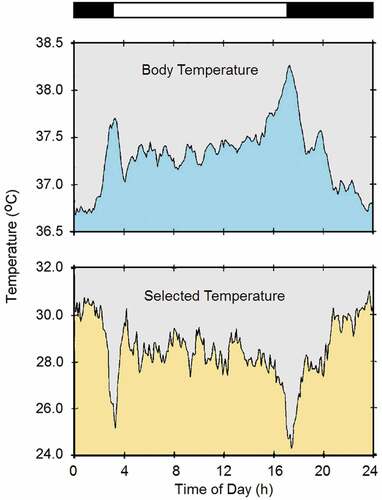
Half a century ago, most thermal physiologists endorsed the engineering model of homeostatic body temperature regulation by an adjustable set point [Citation634,Citation635]. In the 1980’s, however, a number of thermal physiologists abandoned the set point theory and adopted the viewpoint that there is no master reference-signal generator and that individual effector organs are activated directly by their sensory input [Citation636–639]. Nonetheless, many life scientists assumed that the circadian oscillation in body temperature is primarily under homeostatic control and is secondarily modulated by the circadian system through an oscillation in the thermoregulatory set point [Citation17,Citation313,Citation640,Citation641]. According to this view, the circadian rise in temperature would be a response to an elevation in the thermoregulatory set point, whereas the circadian fall in temperature would be a response to a lowering of the set point. Because circadian rhythmicity is evolutionarily older than homeothermy, however, the assumption of a set point change is contentious. To judge whether there is circadian modulation of the thermoregulatory set point, one must actually measure the set point. One way to do this is to measure the motivation of an organism to counteract an imposed deviation of its internal temperature. Research in many laboratories over the years has documented that higher ambient temperatures are preferred during the phase of low body temperature, and lower ambient temperatures are preferred during the phase of high body temperature, in rats [Citation30,Citation397,Citation642–646], mice [Citation647], golden hamsters [Citation644,Citation648,Citation649], Siberian hamsters [Citation650], fat-tailed gerbils [Citation28], degus [Citation651], stripe-faced dunnarts[Citation409], tree shrews [Citation652], flying squirrels [Citation652], mouse lemurs [Citation653], and humans [Citation654–658]. An example is given in . A degu was housed in a temperature-gradient chamber with ambient temperatures ranging from 14°C to 33°C, and its body temperature and preferred ambient temperature were recorded at 6-minute intervals for 10 or more days [Citation651]. Notice that, as expected for a diurnal animal, body temperature is high during the day and low during the night (upper panel). Also, in accordance with the animal’s crepuscular behavior, body temperature shows clear peaks at lights-on and lights-off. Importantly, the rhythm of behavioral temperature selection (lower panel) is 180° out of phase with the rhythm of body temperature, with higher ambient temperatures being selected during the night and lower temperatures during the day. The opposite movement of the two variables is particularly evident at the times of lights-on and lights-off. Clearly, higher environmental temperatures are selected when body temperature is low, and vice versa, indicating that the animal is attempting to counteract the circadian rhythm of body temperature. Thus, the oscillation of the set point cannot possibly be responsible for the temperature rhythm. As a matter of fact, there is no reason to assume that the set point oscillates at all. As body temperature oscillates, the animals behaviorally counteract the oscillation to defend the unaltered set point. The thermoregulatory system actually opposes the oscillation of body temperature imposed by the circadian system [Citation659,Citation660].
Figure 7. Daily rhythms of body temperature and selected ambient temperature of a degu (Octodon degus) housed in a temperature-gradient chamber. The white and black bars at the top indicate the light and dark phases of the prevailing light-dark cycle (14L:10D)
The existence of the body temperature rhythm is in itself proof that the thermoregulatory system’s opposition to the circadian oscillation of body temperature is not entirely successful. However, the amplitude of the temperature rhythm is effectively reduced by the action of the thermoregulatory system. There are at least two types of supportive evidence for this. One type comes from the comparison of the amplitude of the rhythm in animals maintained in a constant-temperature environment with the amplitude in animals allowed to continually select their environmental temperature in a gradient. The amplitude of the body temperature rhythm was reduced in tree shrews and flying squirrels allowed to select their environmental temperature [Citation652]. The other type of supportive evidence comes from studies in which the thermoregulatory system was impaired by surgical ablation of the main thermoregulatory center in the preoptic area of the brain. The amplitude of the body temperature rhythm was greatly enhanced in rats and golden hamsters with preoptic lesions [Citation661–663]. Thus, ablation of the preoptic area releases the circadian oscillation of body temperature from inhibitory control. This means that the thermoregulatory center in the preoptic area of unlesioned animals restricts the oscillation of body temperature to an acceptable range. That is, in normal animals, the circadian system generates an oscillatory signal that is communicated to the organs responsible for heat production and heat loss, and at the same time the thermoregulatory system generates a set point that, like most control systems, has a margin of hysteresis error; the integrated output is an oscillation whose amplitude is restricted to the boundaries of hysteresis error. For physiologists who dislike the set point model, the previous sentence can be reworded as follows: in normal animals, the circadian system generates an oscillatory signal that is communicated to the organs responsible for heat production and heat loss, and at the same time independent thermoeffectors are activated to counteract the incipient alteration in body temperature; the integrated output is an oscillation whose amplitude is restricted to the range of activation of the thermoeffectors.
Why there is a body temperature rhythm
One may wonder why should there be a circadian rhythm of body temperature at all, especially if it is opposed by the thermoregulatory system. The answer is that circadian rhythmicity of body temperature must be evolutionarily adaptive. Circadian rhythmicity is an evolutionarily old trait that most likely existed before the appearance of the first animals [Citation620]. Seeking warm temperatures in anticipation of the active phase of the circadian cycle would have provided an advantage to the original (and extant) ectothermic animals whose ability to perform bodily functions was extremely dependent on ambient temperature [Citation10]. Because homeothermy is found today only in mammals and birds (with a few exceptions), it must have appeared when circadian rhythmicity was already a property of every multicellular organism. As a matter of fact, endothermic homeothermy likely evolved gradually from an ancestral form of heterothermy [Citation619,Citation664]. Thus, in homeotherms, the more recent goal of maintaining homeostasis conflicts with the older goal of causing body temperature to oscillate, and this conflict explains the opposition between the thermoregulatory system and the circadian system in the control of body temperature. But why should the older goal of causing body temperature to oscillate have been retained during evolution? A possible reason to retain rhythmicity of body temperature in homeotherms is the ability to use body temperature as an internal non-photic zeitgeber for the entrainment of multiple slave pacemakers distributed throughout the body [Citation665–671]. Presumably, environmental light affects the master circadian pacemaker in the brain, which modulates the body temperature rhythm (through behavioral mechanisms in ectotherms and through behavioral and autonomic mechanisms in endotherms), which then non-photically modulates the peripheral clocks. Alternatively, the presence of a circadian rhythm of body temperature in extant homeothermic species may simply reflect the preservation of the body temperature rhythm as a vestigial function in animal evolution.
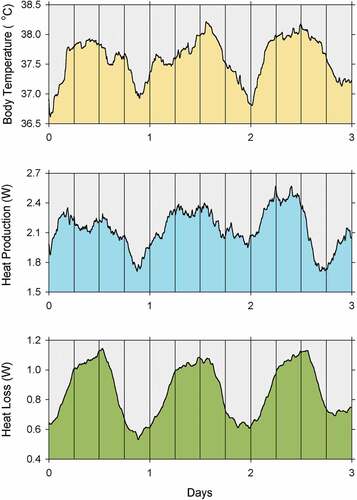
Regardless of whether the generation of the body temperature rhythm is a fundamental process or not, one can still wonder how the rhythm is produced. How does the circadian system generate the circadian rhythm of body temperature? The laws of thermodynamics require that changes in the temperature of a body be the result of changes in heat flow. Changes in body temperature must result from the balance of heat gained and heat lost. For mammals and birds housed in a thermally stable environment without direct solar radiation, heat is gained by metabolic heat production, and heat is lost by radiation, conduction, and convection [Citation17–19]. Few studies have included the simultaneous recording of body temperature, heat production, and heat loss, but one example is presented in . Shown are 3-day segments of the records of body temperature, metabolic heat production, and dry heat loss of a laboratory rat kept in constant darkness at an ambient temperature of 24°C [Citation612]. Notice that the oscillation of body temperature parallels the oscillation of heat production. Thus, the oscillation of heat production could potentially explain the oscillation of body temperature. However, notice also that heat loss parallels heat production. That is, although heat production is high when body temperature is high, heat loss is also high. This apparent paradox results from an incorrect assumption that a great amount of heat is needed to generate the body temperature rhythm. In reality, most of the energy expenditure of, say, a rat is associated with essential life processes, the maintenance of homeothermy, and energy required for locomotion. The body temperature rhythm accounts for only about 6% of the energy expended at thermoneutrality and only 3% of the energy expended in the cold [Citation612,Citation672]. Thus, most of the daily oscillation in heat production and heat loss has nothing to do with the body temperature rhythm. The mechanism responsible for the circadian rhythm of body temperature is a minor temporal mismatch between heat production and heat loss, which causes the small change in heat balance needed to generate the body temperature rhythm [Citation612].
Figure 8. Records of body core temperature, metabolic heat production, and dry heat loss of a laboratory rat kept in constant darkness at an ambient temperature of 24°C for three consecutive days. The data were collected and are plotted with 6-minute resolution after smoothing by a 4 hour moving averages filter to eliminate high-frequency oscillations
Relationship of circadian rhythmicity and metabolism at the cellular level
After having discussed the relationship of body temperature and metabolism at the organismal level, it would seem reasonable to discuss the same relationship at the cellular level. However, individual cells do not regulate their own temperature. Body temperature regulation is an organismal process. In fact, the circadian clock is temperature compensated and need not make adjustments for changes in temperature [Citation673–675], even if other cellular processes are subject to temperature-induced variations in the rate of chemical reactions dictated by the Arrhenius equation. On the other hand, individual cells do possess circadian clocks, and the relationship of circadian rhythmicity and metabolism can be discussed at the cellular level.
It has been known for over 20 years that the molecular mechanism of the circadian clock in animals involves an auto-regulatory transcriptional feedback loop in which the proteins Clock and Bmal1 activate the transcription of the period and cryptochrome genes. The Period and Cryptochrome proteins then feed back and repress their own transcription by interaction with Clock and Bmal1 [Citation676,Citation677]. This is the backbone of the clock itself, but much has yet to be learned about how enzyme transcripts controlled by the clock generate circadian enzyme activity [Citation678]. One research group has found that the circadian clock generates oscillations in mitochondrial oxidative capacity via rhythmic regulation of NAD+ biosynthesis [Citation679], as diagrammed in .
Figure 9. Diagram of the cellular mechanism of circadian regulation of metabolism in the mouse. From Peek and colleagues [Citation679]. Reprinted with permission from AAAS
![Figure 9. Diagram of the cellular mechanism of circadian regulation of metabolism in the mouse. From Peek and colleagues [Citation679]. Reprinted with permission from AAAS](/cms/asset/58d16558-a967-453d-996f-2cfc677c57f9/ktmp_a_1743605_f0009_oc.jpg)
It was suggested early on that metabolism might be more than just an output of the clock and might actually be part of the clock [Citation680]. Research conducted during the past 20 years indicates that, indeed, metabolism is both an output from and an input to the circadian clock [Citation522,Citation681,Citation682], meaning that the two processes are interlinked. One example is the protein Conidial Separation Protein 1 (CSP-1) in the bread-mold fungus Neurospora crassa [Citation683]. In Neurospora, the transcriptional feedback loop of the circadian clock organizes the molecular output of the cell so that catabolic processes occur in the morning and anabolic processes occur in the evening. The csp-1 gene is directly targeted by the clock (which in Neurospora is composed primarily of the wc1-wc2 and frq genes). Interestingly, CSP-1 acts to compensate the clock for changes in metabolic conditions. Specifically, CSP-1 is regulated by glucose levels and represses the expression of wc-1 mRNA when glucose levels are high [Citation683].
A major unanswered question is, of course, how circadian rhythmicity of metabolism at the cellular level relates to circadian rhythmicity at the organismal level, particularly in complex organisms such as birds and mammals.
Conclusion
Published studies clearly document circadian rhythmicity of body temperature and metabolism. The rhythms of both variables are generated endogenously in birds and mammals, are synchronized with the Earth’s rotation by environmental cycles, and are further modulated by occasional and recurrent events in the environment, particularly variations in ambient temperature and food availability. Although modulation of metabolic heat production is a constitutive process of the body temperature rhythm, the rhythm of body temperature is not produced by the rhythm of heat production associated with physical activity and sleep-wakefulness. The body temperature rhythm is achieved by a small variation in heat balance that results from a minor temporal mismatch between heat production and heat loss. At the cellular level, the circadian clock and metabolism are deeply intertwined, although it is still not clear how this intertwining is reflected at the organismal level.
Disclosure statement
The author reports no conflicts of interest.
Additional information
Notes on contributors

Roberto Refinetti
Roberto Refinetti is Professor and Chair of the Department of Psychology at the University of New Orleans. Earlier in his career, he received his doctoral degree from the University of California at Santa Barbara, conducted postdoctoral research at the University of Illinois and the University of Virginia, and was on the faculty of the College of William & Mary, the University of South Carolina, and Boise State University. He is Editor-in-Chief of the Journal of Circadian Rhythms and of the journal Sexuality & Culture and author of the book Circadian Physiology (currently in its third edition). His laboratory research focuses on biological rhythms.
References
- Ledford H, Callaway E, Callaway E. Circadian clocks scoop nobel prize. Nature. 2017;550(7678):18.
- Stokstad E, Vogel G. Revelations about rhythm of life rewarded. Science. 2017;358(6359):18.
- Jia Y, Lu Y, Wu K, et al. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37(3):197–206.
- Lin X, Chen W, Wei F, et al. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16(11):1381–1387.
- Morris CJ, Purvis TE, Hu K, et al. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–E1411.
- Jagannath A, Peirson SN, Foster RG. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr Opin Neurobiol. 2013;23:888–894.
- Zimmet P, Albertini KGM, Stern N, et al. The circadian syndrome is the metabolic syndrome and much more! J Intern Med. 2019;286:181–191.
- Chossat C. Recherches expérimentales sur l’inanition. Annales des Sciences Naturelles, Série 2. 1843;20:293–326.
- Davy J. On the temperature of man. Philos Trans R Soc Lond. 1845;135:319–333.
- Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav. 1992;51(3):613–637.
- Refinetti R. The circadian rhythm of body temperature. Front Biosci. 2010;15(1):564–594.
- Piccione G, Refinetti R. Thermal chronobiology of domestic animals. Front Biosci. 2003;8(6):258–264.
- Waterhouse J, Drust B, Weinert D, et al. The circadian rhythm of core temperature: origin and some implications for exercise performance. Chronobiol Int. 2005;22(2):207–225.
- Maloney SK, Goh G, Fuller A, et al. Amplitude of the circadian rhythm of temperature in homeotherms. CAB Rev. 2019;14(art):19.
- Cossins AR, Bowler K. Temperature biology of animals. London: Chapman and Hall; 1987.
- Clarke A. Principles of thermal ecology. Oxford: Oxford University Press; 2017.
- Hensel H. Thermoreception and Temperature Regulation. New York: Academic Press; 1981.
- Jessen C. Temperature regulation in humans and other mammals. Berlin: Springer; 2001.
- Romanovsky AA. Thermoregulation: from basic neuroscience to clinical neurology (Handbook of Clinical Neurology Volume 156). New York: Elsevier; 2018.
- Bernard C. De la Physiologie Générale. Paris: Librairie Hachette; 1872.
- Halberg F. Physiologic 24-hour periodicity: general and procedural considerations with reference to the adrenal cycle. Z Vitam Horm Fermentforsch. 1959;10:225–296.
- Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev. 2015;90:891–926.
- Van Breukelen F, Martin SL. The hibernation continuum: physiological and molecular aspects of metabolic plasticity in mammals. Physiology. 2015;30(4):273–281.
- Kolka MA, Levine L, Stephenson LA. Use of an ingestible telemetry sensor to measure core temperature under chemical protective clothing. J Therm Biol. 1997;22(4–5):343–349.
- Byrne C, Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med. 2007;41(3):126–133.
- Edwards B, Waterhouse J, Reilly T, et al. A comparison of the suitabilities of rectal, gut, and insulated axilla temperatures for measurement of the circadian rhythm of core temperature in field studies. Chronobiol Int. 2002;19(3):579–597.
- Refinetti R, Menaker M. Body temperature rhythm of the tree shrew, Tupaia belangeri. J Exp Zool. 1992;263(4):453–457.
- Refinetti R. Homeostatic and circadian control of body temperature in the fat-tailed gerbil. CompBiochem Physiol A. 1998;119(1):295–300.
- Ishii K, Uchino M, Kuwahara M, et al. Diurnal fluctuations of heart rate, body temperature and locomotor activity in the house musk shrew (Suncus murinus). Exp Anim. 2002;51(1):57–62.
- Ray B, Mallick HN, Kumar VM. Changes in thermal preference, sleep-wakefulness, body temperature and locomotor activity of rats during continuous recording for 24 hours. Behav Brain Res. 2004;154(2):519–526.
- Sanchez-Alavez M, Alboni S, Conti B. Sex- and age-specific differences in core body temperature of C57BL/6 mice. Age (Omaha). 2011;33(1):89–99.
- Williams CT, Barnes BM, Yan L, et al. Entraining to the polar day: circadian rhythms in Arctic ground squirrels. J Exp Biol. 2017;220(17):3095–3102.
- Hahn GL, Eigenberg RA, Nienaber JA, et al. Measuring physiological responses of animals to environmental stressors using a microcomputer-based portable datalogger. J Anim Sci. 1990;68(9):2658–2665.
- Nocek JE, Allman JG, Kautz WP. Evaluation of an indewelling ruminal probe methodology and effect of grain level on diurnal pH variation in dairy cattle. J Dairy Sci. 2002;85(2):422–428.
- Gür MK, Refinetti R, Gür H. Daily rhythmicity and hibernation in the Anatolian ground squirrel under natural and laboratory conditions. J Comp Physiol B. 2009;179(2):155–164.
- Kemp R, Noakes MJ, McKechnie AE. Thermoregulation in free-ranging ground woodpeckers Geocolaptes olivaceus: no evidence of torpor. J Avian Biol. 2017;48(10):1287–1294.
- Abdo H, Calvo-Enrique L, Lopez JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365(6454):695–699.
- Thiel A, Evans AL, Fuchs B, et al. Effects of reproduction and environmental factors on body temperature and activity patterns of wolverines. Front Zool. 2019;16(1):21.
- Refinetti R. Comparison of the body temperature rhythms of diurnal and nocturnal rodents. J Exp Zool. 1996;275(1):67–70.
- Kluger MJ, Conn CA, Franklin B, et al. Effect of gastrointestinal flora on body temperature of rats and mice. Am J Physiol. 1990;258(2 Pt 2):R552–R557.
- Mistlberger RE, Lukman H, Nadeau BG. Circadian rhythms in the Zucker obese rat: assessment and intervention. Appetite. 1998;30(3):255–267.
- Shido O, Sakurada S, Nagasaka T. Effect of heat acclimation on diurnal changes in body temperature and locomotor activity in rats. J Physiol. 1991;433(1):59–71.
- Shido O, Sakurada S, Kohda W, et al. Day-night changes of body temperature and feeding in heat-acclimated rats. Physiol Behav. 1994;55(5):935–939.
- Georgiev J. Influence of environmental conditions and handling on the temperature rhythm of the rat. Biotelem Patient Monit. 1978;5(4):229–234.
- Berkey DL, Meeuwsen KW, Barney CC. Measurements of core temperature in spontaneously hypertensive rats by radiotelemetry. Am J Physiol. 1990;258(3 Pt 2):R743–R749.
- Morley RM, Conn CA, Kluger MJ, et al. Temperature regulation in biotelemetered spontaneously hypertensive rats. Am J Physiol. 1990;258(4 Pt 2):R1064–R1069.
- Peloso E, Wachulec M, Satinoff E. Stress-induced hyperthermia depends on both time of day and light condition. J Biol Rhythms. 2002;17(2):164–170.
- Bruguerolle B, Roucoules X. Time-dependent changes in body temperature rhythm induced in rats by brewer’s yeast injection. Chronobiol Int. 1994;11(3):180–186.
- Yoshida Y, Fujiki N, Nakajima T, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activitties. Eur J Neurosci. 2001;14(7):1075–1081.
- Meinrath M, D’Amato MR. Interrelationships among heart rate, activity, and body temperature in the rat. Physiol Behav. 1979;22(3):491–498.
- Kittrell EMW, Satinoff E. Diurnal rhythms of body temperature, drinking and activity over reproductive cycles. Physiol Behav. 1988;42(5):477–484.
- Refinetti R. Experimentally induced disruption of the diurnal rhythm of body temperature of the rat. Biotemas. 1990;3(2):47–58.
- Fioretti MC, Riccardi C, Menconi E, et al. Control of the circadian rhythm of body temperature in the rat. Life Sci. 1974;14(11):2111–2119.
- Halberg F, Zander HA, Houglum MW, et al. Daily variations in tissue mitoses, blood eosinophils and rectal temperatures of rats. Am J Physiol. 1954;177(3):361–366.
- Thornhill JA, Hirst M, Gowdey CW. Measurement of diurnal core temperatures of rats in operant cages by AM telemetry. Can J Physiol Pharmacol. 1978;56(6):1047–1050.
- Abrams R, Hammel HT. Cyclic variations in hypothalamic temperature in unanesthetized rats. Am J Physiol. 1965;208(4):698–702.
- Miles GH. Telemetering techniques for periodicity studies. Ann N Y Acad Sci. 1962;98(4):858–865.
- Tanaka H, Yanase M, Kanosue K, et al. Circadian variation of thermoregulatory responses during exercise in rats. Am J Physiol. 1990;258(4 Pt 2):R836–R841.
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53(5):983–993.
- Ikeda M, Inoué S. Simultaneous recording of circadian rhythms of brain and intraperitoneal temperatures and locomotor and drinking activities in the rat. Bio Rhythm Res. 1998;29(2):142–150.
- Isobe Y, Takaba S, Ohara K. Diurnal variation of thermal resistance in rats. Can J Physiol Pharmacol. 1980;58(10):1174–1179.
- Stephenson R, Liao KS, Hamrahi H, et al. Circadian rhythms and sleep have additive effects on respiration in the rat. J Physiol. 2001;536(1):225–235.
- Seifert EL, Mortola JP. The circadian pattern of breathing in conscious adult rats. Respiration Physiol. 2002;129(3):297–305.
- De Castro JM. Diurnal rhythms of behavioral effects on core temperature. Physiol Behav. 1978;21(6):883–886.
- Honma K, Hiroshige T. Internal synchronization among several circadian rhythms in rats under constant light. Am J Physiol. 1978;235(5):R243–R249.
- Ikeda M, Sagara M, Inoué S. Continuous exposure to dim illumination uncouples temporal patterns of sleep, body temperature, locomotion and drinking behavior in the rat. Neurosci Lett. 2000;279(3):185–189.
- Deprés-Brummer P, Lévi F, Metzger G, et al. Light-induced suppression of the rat circadian system. Am J Physiol. 1995;268(5 Pt 2):R1111–R1116.
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95(5):1195–1201.
- Murphy HM, Wideman CH, Nadzam GR. A laboratory animal model of human shift work. Integr Psychol Behav Sci. 2003;38(4):316–328.
- Fischette CT, Edinger HM, Siegel A. Temporary desynchronization among circadian rhythms with lateral fornix ablation. Brain Res. 1981;229(1):85–101.
- Scales WE, Kluger MJ. Effect of antipyretic drugs on circadian rhythm in body temperature of rats. Am J Physiol. 1987;253(2 Pt 2):R306–R313.
- Warren WS, Cassone VM. The pineal gland: photoreception and coupling of behavioral, metabolic, and cardiovascular circadian outputs. J Biol Rhythms. 1995;10(1):64–79.
- Refinetti R, Ma H, Satinoff E. Body temperature rhythms, cold tolerance, and fever in young and old rats of both genders. Exp Gerontol. 1990;25(6):533–543.
- Li H, Satinoff E. Changes in circadian rhythms of body temperature and sleep in old rats. Am J Physiol. 1995;269(1 Pt 2):R208–R214.
- Gordon CJ, Rezvani AH. Genetic selection of rats with high and low body temperatures. J Therm Biol. 2001;26(3):223–229.
- Spencer F, Shirer HW, Yochim JM. Core temperature in the female rat: effect of pinealectomy or altered lighting. Am J Physiol. 1976;231(2):355–360.
- Severinsen T, Øritsland NA. Endotoxin induced prolonged fever in rats. J Therm Biol. 1991;16(3):167–171.
- De Vries J, Strubbe JH, Wildering WC, et al. Patterns of body temperature during feeding in rats under varying ambient temperatures. Physiol Behav. 1993;53(2):229–235.
- Cuesta M, Clesse D, Pévet P, et al. From daily behavior to hormonal and neurotransmitter rhythms: comparison between diurnal and nocturnal rat species. Horm Behav. 2009;55(2):338–347.
- Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol. 1995;269(5 Pt 2):R1038–R1043.
- Maskrey M, Wiggins PR, Frappell PB. Behavioral thermoregulation in obese and lean Zucker rats in a thermal gradient. Am J Physiol. 2001;281(5):R1675–R1680.
- Tsai L, Tsai Y, Huang K, et al. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am J Physiol. 2005;289(2):E212–E217.
- Mortola JP. Correlations between the circadian patterns of body temperature, metabolism and breathing in rats. Respir Physiol Neurobiol. 2007;155(2):137–146.
- Mendez N, Halabi D, Spichiger C, et al. Gestational chronodisruption impairs circadian physiology in rat male offspring, increasing the risk of chronic disease. Endocrinology. 2016;157(12):4654–4668.
- Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, et al. The suprachiasmatic nucleus participates in food entrainment: a lesion study. Neuroscience. 2010;165(4):1115–1126.
- Machado FSM, Zhang Z, Su Y, et al. Time-of-day effects on metabolic and clock-related adjustment to cold. Front Endocrinol (Lausanne). 2018;9(art):199.
- Calonne J, Arsenijevic D, Scerri I, et al. Low 24-hour core body temperature as a thrifty metabolic trait driving catch-up fat during weight regain after caloric restriction. Am J Physiol. 2019;317(4):E699–E709.
- Leon LR, Walker LD, DuBose DA, et al. Biotelemetry transmitter implantation in rodents: impact on growth and circadian rhythms. Am J Physiol. 2004;286(5):R967–R974.
- Keeney AJ, Hogg S, Marsden CA. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav. 2001;74(1–2):177–184.
- Shiromani PJ, Xu M, Winston EM, et al. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am J Physiol. 2004;287(1):R47–R57.
- Berezkin MV, Kudinova VF, Batygov AN, et al. Effect of lighting conditions on circadian rhythm of rectal temperature in mice. Bull Exp Biol Med. 1989;106(3):1337–1340.
- Conn CA, Franklin B, Freter R, et al. Role of gram-negative and gram-positive gastrointestinal flora in temperature regulation of mice. Am J Physiol. 1991;261(6 Pt 2):R1358–R1363.
- Weinert D, Waterhouse J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol Behav. 1998;63(5):837–843.
- Kramer K, Voss HP, Grimbergen J, et al. Circadian rhythms of heart rate, body temperature, and locomotor activity in freely moving mice measured with radio telemetry. Lab Anim (NY). 1998;27(8):23–26.
- Connolly MS, Lynch CB. Classical genetic analysis of circadian body temperature rhythms in mice. Behav Genet. 1983;13(5):491–500.
- Sei H, Oishi K, Morita Y, et al. Mouse model for morningness/eveningness. NeuroReport. 2001;12(7):1461–1464.
- Irizarry RA, Tankersley C, Frank R, et al. Assessing homeostasis through circadian patterns. Biometrics. 2001;57(4):1228–1237.
- Weinert D, Waterhouse J. Daily activity and body temperature rhythms do not change simultaneously with age in laboratory mice. Physiol Behav. 1999;66(4):605–612.
- Tankersley CG, Irizarry R, Flanders SE, et al. Unstable heart rate and temperature regulation predict mortality in AKR/J mice. Am J Physiol. 2003;284(3):R742–R750.
- Nomoto S, Ohta M, Kanai S, et al. Absence of the cholecystokinin-A receptor deteriorates homeostasis of body temperature in response to changes in ambient temperature. Am J Physiol. 2004;287(3):R556–R561.
- Filipski E, King VM, Etienne MC, et al. Persistent twenty-four hour changes in liver and bone marrow despite suprachiasmatic nuclei ablation in mice. Am J Physiol. 2004;287(4):R844–R851.
- Gebczynski AK. Daily variation of thermoregulatory costs in laboratory mice selected for high and low basal metabolic rate. J Therm Biol. 2005;30(3):187–193.
- Nagashima K, Matsue K, Konishi M, et al. The involvement of Cry1 and Cry2 genes in the regulation of the circadian body temperature rhythm in mice. Am J Physiol. 2005;288(1):R329–R335.
- Castillo MR, Hochstetler KJ, Greene DM, et al. Circadian rhythm of core body temperature in two laboratory mouse lines. Physiol Behav. 2005;86(4):538–545.
- Wolff G, Duncan MJ, Esser KA. Chronic phase advance alters circadian physiological rhythms and peripheral molecular clocks. J Appl Physiol. 2013;115(3):373–382.
- Studholme KM, Gompf HS, Morin LP. Brief light stimulation during the mouse nocturnal activity phase simultaneously induces a decline in core temperature and locomotor activity followed by EEG-determined sleep. Am J Physiol. 2013;304(6):R459–R471.
- Gerhart-Hines Z, Feng D, Emmett MJ, et al. The nuclear receptor Rev-erb-alpha controls circadian thermogenic plasticity. Nature. 2013;503(7476):410–413.
- Helwig BG, Ward JA, Blaha MD, et al. Effect of intraperitoneal radiotelemetry instrumentation on voluntary wheel running and surgical recovery in mice. JAALAS. 2012;51(5):600–608.
- Liu C, Li S, Liu T, et al. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481.
- Mendoza J, Pévet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586(24):5901–5910.
- Filipski E, King VM, Li XM, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94(9):690–697.
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314(5800):825–828.
- Lee H, Iida T, Mizuno A, et al. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25(5):1304–1310.
- Riley E, Esterman M, Fortenbaugh FC, et al. Time-of-day variation in sustained attentional control. Chronobiol Int. 2017;34(7):993–1001.
- Smarr BL, Grant AD, Zucker I, et al. Sex differences in variability across timescales in BALB/c mice. Biol Sex Differ. 2017;8(1):7.
- Smarr BL, Grant AD, Perez L, et al. Maternal and early-life circadian disruption have long-lasting negative consequences on offspring development and adult behavior in mice. Sci Rep. 2017;7(1):3326.
- Folk GE. Twenty-four hour rhythms of mammals in a cold environment. Am Natur. 1957;91(858):153–166.
- DeCoursey PJ, Pius S, Sandlin C, et al. Relationship of circadian temperature and activity rhythms in two rodent species. Physiol Behav. 1998;65(3):457–463.
- Conn CA, Borer KT, Kluger MJ. Body temperature rhythm and response to pyrogen in exercising and sedentary hamsters. Med Sci Sports Exerc. 1990;22(5):636–642.
- Chaudhry AP, Halberg F, Keenan CE, et al. Daily rhythms in rectal temperature and in epithelial mitoses of hamster pinna and pouch. J Appl Physiol. 1958;12(2):221–224.
- Watts RH Jr., Refinetti R. Circadian modulation of cold-induced thermogenesis in the golden hamster. Bio Rhythm Res. 1996;27(1):87–94.
- Boulos Z, Macchi M, Houpt TA, et al. Photic entrainment in hamsters: effects of simulated twilights and nest box availability. J Biol Rhythms. 1996;11(3):216–233.
- Hashimoto H, Moritani N, Saito TR. Comparative study on circadian rhythms of body temperature, heart rate, and locomotor activity in three species of hamsters. Exp Anim. 2004;53(1):43–46.
- Kronfeld-Schor N, Dayan T, Elvert R, et al. On the use of the time axis for ecological separation: diel rhythms as an evolutionary constraint. Am Natur. 2001;158(4):451–457.
- Rubal A, Choshniak I, Haim A. Daily rhythms of metabolic rate and body temperature of two murids from extremely different habitats. Chronobiol Int. 1992;9(5):341–349.
- Elvert R, Kronfeld N, Dayan T, et al. Telemetric field studies of body temperature and activity rhythms of Acomys russatus and A. cahirinus in the Judean Desert of Israel. Oecologia. 1999;119(4):484–492.
- Haim A, Yedidia I, Haim D, et al. Photoperiodicity in daily rhythms of body temperature, food and energy intake of the golden spiny mouse (Acomys russatus). Isr J Zool. 1994;40:145–150.
- Haim A, Zisapel N. Oxygen consumption and body temperature rhythms in the golden spiny mouse: responses to changes in day length. Physiol Behav. 1995;58(4):775–778.
- Lovegrove BG, Heldmaier G. The amplitude of circadian body temperature rhythms in three rodents (Aethomys namaquensis, Thallomys paedulcus and Cryptomys damarensis) along the arboreal-subterranean gradient. Aust J Zool. 1994;42(1):65–78.
- Golightly RT, Ohmart RD. Heterothermy in free-ranging Abert’s squirrels (Sciurus aberti). Ecology. 1978;59(5):897–909.
- Pauls RW. Body temperature dynamics of the red squirrel (Tamiasciurus hudsonicus): adaptations for energy conservation. Can J Zool. 1979;57(7):1349–1354.
- Gebczynski AK, Taylor JRE. Daily variation of body temperature, locomotor activity and maximum nonshivering thermogenesis in two species of small rodents. J Therm Biol. 2004;29(2):123–131.
- Haim A, McDevitt RM, Speakman JR. Thermoregulatory responses to manipulations of photoperiod in wood mice Apodemus sylvaticus from high latitudes (57°N). J Therm Biol. 1995;20(6):437–443.
- McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62(1):91–96.
- Blanchong JA, McElhinny TL, Mahoney MM, et al. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14(5):364–377.
- Akita M, Ishii K, Kuwahara M, et al. The daily pattern of heart rate, body temperature, and locomotor activity in guinea pigs. Exp Anim. 2001;50(5):409–415.
- Hayes SR. Daily activity and body temperature of the southern woodchuck, Marmota monax monax, in northwestern Arkansas. J Mammal. 1976;57(2):291–299.
- Weinert D, Nevill A, Weinandy R, et al. The development of new purification methods to assess the circadian rhythm of body temperature in Mongolian gerbils. Chronobiol Int. 2003;20(2):249–270.
- Kas MJH, Edgar DM. Crepuscular rhythms of EEG sleep-wake in a hystricomorph rodent, Octodon degus. J Biol Rhythms. 1998;13(1):9–17.
- Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58(3):573–585.
- Kas MJH, Edgar DM. A nonphotic stimulus inverts the diurnal-nocturnal phase preference in Octodon degus. J Neurosci. 1999;19(1):328–333.
- Goel N, Lee TM. Social cues accelerate reentrainment of circadian rhythms in diurnal female octodon degus (Rodentia-Octodontidae). Chronobiol Int. 1995;12(5):311–323.
- Sacher GA, Duffy PH. Age changes in rhythms of energy metabolism, activity, and body temperature in Mus and Peromyscus. In: Samis HV, Capobianco S, editors. Aging and biological rhythms. New York: Plenum; 1978. p. 105–124.
- Steinlechner S, Stieglitz A, Ruf T. Djungarian hamsters: a species with a labile circadian pacemaker? Arrhythmicity under a light-dark cycle induced by short light pulses. J Biol Rhythms. 2002;17(3):248–258.
- Halberg E, Halberg F, Timm RM, et al. Socially-related and spontaneous circadian thermo-acrophase shifts in Rhabdomys pumilio: complications for chronopharmacologists. In: Takahashi R, Halberg F, Walker CA, editors. Toward Chronopharmacology. Oxford, UK: Pergamon; 1982. p. 357–368.
- Haim A, Ellison GTH, Skinner JD. Thermoregulatory circadian rhythms in the pouched mouse (Saccostomus campestris). CompBiochem Physiol A. 1988;91(1):123–127.
- Muchlinski AE, Baldwin BC, Padick DA, et al. California ground squirrel body temperature regulation patterns measured in the laboratory and in the natural environment. CompBiochem Physiol A. 1998;120(2):365–372.
- Lee TM, Holmes WG, Zucker I. Temperature dependence of circadian rhythms in golden-mantled ground squirrels. J Biol Rhythms. 1990;5(1):25–34.
- Refinetti R. Body temperature and behaviour of golden hamsters (Mesocricetus auratus) and ground squirrels (Spermophilus tridecemlineatus) in a thermal gradient. J Comp Physiol A. 1995;177(6):701–705.
- Haim A, Downs CT, Raman J. Effects of adrenergic blockade on the daily rhythms of body temperature and oxygen consumption of the black-tailed tree rat (Thallomys nigricauda) maintained under different photoperiods. J Therm Biol. 2001;26(3):171–177.
- Levy O, Dayan T, Rotics S, et al. Foraging sequence, energy intake and torpor: an individual-based field study of energy balancing in desert golden spiny mice. Ecol Lett. 2012;15(11):1240–1248.
- Cohen R, Kronfeld-Schor N. Individual variability and photic entrainment of circadian rhythms in golden spiny mice. Physiol Behav. 2006;87(3):563–574.
- Lovegrove BG. Modification and miniaturization of Thermochron iButtons for surgical implantation into small animals. J Comp Physiol B. 2009;179(4):451–458.
- Schrader JA, Walaszczyk EJ, Smale L. Changing patterns of daily rhythmicity across reproductive states in diurnal female Nile grass rats (Arvicanthis niloticus). Physiol Behav. 2009;98(5):547–556.
- Gür MK, Bulut S, Gür H, et al. Body temperature patterns and use of torpor in an alpine glirid species, the woolly dormouse. Acta Theriologica. 2014;59(2):299–309.
- Pereira ME, Aines J, Scheckter JL. Tactics of heterothermy in Eastern gray squirrels (Sciurus carolinensis). J Mammal. 2002;83(2):467–477.
- Weinert D, Weinandy R, Gattermann R. Photic and non-photic effects on the daily activity pattern of Mongolian gerbils. Physiol Behav. 2007;90(2–3):325–333.
- Long RA, Martin TJ, Barnes BM. Body temperature and activity patterns in free-living Arctic ground squirrels. J Mammal. 2005;86(2):314–322.
- Barak O, Kronfeld-Schor N. Activity rhythms and masking response in the diurnal fat sand rat under laboratory conditions. Chronobiol Int. 2013;30(9):1123–1134.
- Wilson WA, O’Riain MJ, Hetem RS, et al. Winter body temperature patterns in free-ranging Cape ground squirrel, Xerus inauris: no evidence for torpor. J Comp Physiol B. 2010;180(7):1099–1110.
- Dausmann KH, Wein J, Turner JM, et al. Absence of heterothermy in the European red squirrel (Sciurus vulgaris). Mamm Biol. 2013;78(5):332–335.
- Haupt M, Bennett NC, Oosthuizen MK. Locomotor activity and body temperature patterns over a temperature gradient in the Highveld mole-rat (Cryptomys hottentotus pretoriae). PLoS One. 2017;12(1):e0169644.
- Tachinardi P, Bicudo JEW, Oda GA, et al. Rhythmic 24 h variation of core body temperature and locomotor activity in a subterranean rodent (Ctenomys aff. knighti), the tuco-tuco. PLoS One. 2014;9(1):e85674.
- Scott GW, Fisher KC, Love JA. A telemetric study of the abdominal temperature of a hibernator, Spermophilus richardsonii, maintained under constant conditions of temperature and light during the active season. Can J Zool. 1974;52(5):653–658.
- Coleman JC, Downs CT. Daily rhythms of body temperature and activity in free-living Black-tailed tree rats (Thallomys nigricauda) along an aridity gradient. Physiol Behav. 2010;99(1):22–32.
- Boratynski JS, Iwinska K, Bogdanowicz W. Body temperature variation in free-living and food-deprived yellow-necked mice sustains an adaptive framework for endothermic thermoregulation. Mammal Res. 2018;63(4):493–500.
- Rawson RO, Stolwijk JAJ, Graichen H, et al. Continuous radio telemetry of hypothalamic temperatures from unrestrained animals. J Appl Physiol. 1965;20(2):321–325.
- Hawking F, Lobban MC, Gammage K, et al. Circadian rhythms (activity, temperature, urine and microfilariae) in dog, cat, hen, duck, Thamnomys and Gerbillus. J Interdiscip Cycle Res. 1971;2(4):455–473.
- Kuwabara N, Seki K, Aoki K. Circadian, sleep and brain temperature rhythms in cats under sustained daily light-dark cycles and constant darkness. Physiol Behav. 1986;38(2):283–289.
- Johnson RF, Randall W. Freerunning and entrained circadian rhythms in body temperature in the domestic cat. J Interdiscip Cycle Res. 1985;16(1):49–61.
- Randall W, Cunningham JT, Randall S, et al. A two-peak circadian system in body temperature and activity in the domestic cat, Felis catus L. J Therm Biol. 1987;12(1):27–37.
- Refinetti R, Piccione G. Daily rhythmicity of body temperature in the dog. J Vet Med Sci. 2003;65(8):935–937.
- Hilmer S, Algar D, Plath M, et al. Relationship between daily body temperature and activity patterns of free-ranging feral cats in Australia. J Therm Biol. 2010;35(6):270–274.
- Piccione G, Giudice E, Fazio F, et al. Association between obesity and reduced body temperature in dogs. Int J Obesity. 2011;35(8):1011–1018.
- Piccione G, Fazio F, Giudice E, et al. Body size and the daily rhythm of body temperature in dogs. J Therm Biol. 2009;34(4):171–175.
- Piccione G, Caola G, Refinetti R. Daily rhythms of blood pressure, heart rate, and body temperature in fed and fasted male dogs. J Vet Med A. 2005;52(8):377–381.
- Mohr EG, Krzywanek H. Endogenous oscillator and regulatory mechanisms of body temperature in sheep. Physiol Behav. 1995;57(2):339–347.
- Recabarren SE, Vergara M, Llanos AJ, et al. Circadian variation of rectal temperature in newborn sheep. J Dev Physiol. 1987;9(5):399–408.
- Jessen C, Dmi’el R, Choshniak I, et al. Effects of dehydration and rehydration on body temperatures in the black Bedouin goat. Pflügers Archiv. 1998;436(5):659–666.
- Bligh J, Ingram DL, Keynes RD, et al. The deep body temperature of an unrestrained Welsh mountain sheep recorded by a radiotelemetric technique during a 12-month period. J Physiol. 1965;176(1):136–144.
- Lowe TE, Cook CJ, Ingram JR, et al. Impact of climate on thermal rhythm in pastoral sheep. Physiol Behav. 2001;74(4–5):659–664.
- Ayo JO, Oladele SB, Ngam S, et al. Diurnal fluctuations in rectal temperature of the Red Sokoto goat during the harmattan season. Res Vet Sci. 1998;66(1):7–9.
- Jessen C, Kuhnen G. Seasonal variations of body temperature in goats living in an outdoor environment. J Therm Biol. 1996;21(3):197–204.
- Mphahlele NR, Fuller A, Roth J, et al. Body temperature, behavior, and plasma cortisol changes induced by chronic infusion of Staphylococcus aureus in goats. Am J Physiol. 2004;287(4):R863–R869.
- Signer C, Ruf T, Arnold W. Hypometabolism and basking: the strategies of Alpine ibex to endure harsh over-wintering conditions. Funct Ecol. 2011;25(3):537–547.
- De K, Kumar D, Saxena VK, et al. Study of circadian rhythmicity of physiological response and skin temperature of sheep during the summer and winter in a semi-arid tropical environment. Physiol Behav. 2017;169:16–21.
- Fuchs B, Sørheim KM, Chincarini M, et al. Heart rate sensor validation and seasonal and diurnal variation of body temperature and heart rate in domestic sheep. Vet Anim Sci. 2019;8:100075.
- Piccione G, Caola G, Refinetti R. The circadian rhythm of body temperature of the horse. Bio Rhythm Res. 2002;33(1):113–119.
- Piccione G, Caola G, Refinetti R. Daily and estrous rhythmicity of body temperature in domestic cattle. BMC Physiol. 2003;3:7.
- Piccione G, Caola G, Refinetti R. Feeble weekly rhythmicity in hematological, cardiovascular, and thermal parameters in the horse. Chronobiol Int. 2004;21(4–5):571–589.
- Araki CT, Nakamura RM, Kam LWG. Diurnal temperature sensitivity of dairy cattle in a naturally cycling environment. J Therm Biol. 1987;12(1):23–26.
- Hahn GL, Chen YR, Nienaber JA, et al. Characterizing animal stress through fractal analysis of thermoregulatory responses. J Therm Biol. 1992;17(2):115–120.
- Piccione G, Caola G, Refinetti R. Temporal relationships of 21 physiological variables in horse and sheep. CompBiochem Physiol A. 2005;142(4):389–396.
- Green AR, Gates RS, Lawrence LM. Measurement of horse core body temperature. J Therm Biol. 2005;30(5):370–377.
- Smith JE, Barnes AL, Maloney SK. A nonsurgical method allowing continuous core temperature monitoring in mares for extended periods, including during endurance exercise. Equine Vet J Suppl. 2006;36(S36):65–69.
- Vaidya MM, Kumar P, Singh SV. Circadian changes in heat storage and heat loss through sweating and panting in Karan Fries cattle during different seasons. Bio Rhythm Res. 2012;43(2):137–146.
- Piccione G, Giannetto C, Bertolucci C, et al. Daily rhythmicity of circulating melatonin is not endogenously generated in the horse. Bio Rhythm Res. 2013;44(1):143–149.
- Ammer S, Lambertz C, Gauly M. Comparison of different measuring methods for body temperature in lactating cows under different climatic conditions. J Dairy Res. 2016;83(2):165–172.
- Hoban TM, Levine AH, Shane RB, et al. Circadian rhythms of drinking and body temperature of the owl monkey (Aotus trivirgatus). Physiol Behav. 1985;34(4):513–518.
- Winget CM, Card DH, Hetherington NW. Circadian oscillations of deep-body temperature and heart rate in a primate (Cebus albafrons). Aerosp Med. 1968;39(4):350–353.
- Takasu N, Nigi H, Tokura H. Effects of diurnal bright/dim light intensity on circadian core temperature and activity rhythms in the Japanese macaque. Jpn J Physiol. 2002;52(6):573–578.
- Simpson S, Galbraith JJ. Observations on the normal temperature of the monkey and its diurnal variation, and on the effect of changes in the daily routine on this variation. Trans R Soc Edinburgh. 1906;45(1):65–104.
- Tapp WN, Natelson BH. Circadian rhythms and patterns of performance before and after simulated jet lag. Am J Physiol. 1989;257(4 Pt 2):R796–R803.
- Perret M, Aujard F, Séguy M, et al. Olfactory bulbectomy modifies photic entrainment and circadian rhythms of body temperature and locomotor activity in a nocturnal primate. J Biol Rhythms. 2003;18(5):392–401.
- Moore-Ede MC, Kass DA, Herd JA. Transient circadian internal desynchronization after light-dark phase shift in monkeys. Am J Physiol. 1977;232(1):R31–R37.
- Fuller CA, Sulzman FM, Moore-Ede MC. Thermoregulation is impaired in an environment without circadian time cues. Science. 1978;199(4330):794–796.
- Fuller CA, Edgar DM. Effects of light intensity on the circadian temperature and feeding rhythms in the squirrel monkey. Physiol Behav. 1986;36(4):687–691.
- Erkert HG, Cramer B. Chronobiological background to cathemerality: circadian rhythms in Eulemur fulvus albifrons (Prosimii) and Aotus azarai boliviensis (Anthropoidea). Folia Primatologica. 2006;77(1–2):87–103.
- Hetherington CM. Circadian oscillations of body temperature in the marmoset, Callithrix jacchus. Lab Anim. 1978;12(2):107–108.
- Hoffmann K, Coolen A, Schlumbohm C, et al. Remote long-term registrations of sleep-wake rhythms, core body temperature and activity in marmoset monkeys. Behav Brain Res. 2012;235(2):113–123.
- Barger LK, Hoban-Higgins TM, Fuller CA. Assessment of circadian rhythms throughout the menstrual cycle of female rhesus monkeys. Am J Primatol. 2008;70(1):19–25.
- Weed MR, Hienz RD. Effects of morphine on circadian rhythms of motor activity and body temperature in pig-tailed macaques. Pharmacol Biochem Behav. 2006;84(3):487–496.
- Perret M, Gomez D, Barbosa A, et al. Increased late night response to light controls the circadian pacemaker in a nocturnal primate. J Biol Rhythms. 2010;25(3):186–196.
- Mitchell D, Fuller A, Maloney SK. Homeothermy and primate bipedalism: is water shortage or solar radiation the main threat to baboon (Papio hamadryas) homeothermy? J Hum Evol. 2009;56(5):439–446.
- Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381):1232.
- Serón-Ferré M, Forcelledo ML, Torres-Farfan C, et al. Impact of chronodisruption during primate pregnancy on the maternal and newborn temperature rhythms. PLoS One. 2013;8(2):e57710.
- Ishikawa A, Sakai K, Maki T, et al. Investigation of sleep-wake rhythm in non-human primates without restraint during data collection. Exp Anim. 2017;66(1):51–60.
- Aschoff J, Gerecke U, Wever R. Desynchronization of human circadian rhythms. Jpn J Physiol. 1967;17(4):450–457.
- Wever R, Zink RA. Fortlaufende Registrierung der Rectaltemperatur des Menschen unter extremen Bedingungen. Pflügers Archiv. 1971;327(2):186–190.
- Stephenson LA, Wenger CB, O’Donovan BH, et al. Circadian rhythm in sweating and cutaneous blood flow. Am J Physiol. 1984;246(3 Pt 2):R321–R324.
- Sharp GWG. Reversal of diurnal temperature rhythms in man. Nature. 1961;190(4771):146–148.
- Benedict FG. Studies in body temperature. I. Influence of the inversion of the daily routine; the temperature of night-workers. Am J Physiol. 1904;11(2):145–169.
- Ogle W. On the diurnal variations in the temperature of the human body in health. St George’s Hosp Rep. 1866;1:221–245.
- Lee KA. Circadian temperature rhythms in relation to menstrual cycle phase. J Biol Rhythms. 1988;3(3):255–263.
- Mellette HC, Hutt BK, Askovitz SI, et al. Diurnal variations in body temperature. J Appl Physiol. 1951;3(11):665–675.
- Tsujimoto T, Yamada N, Shimoda K, et al. Circadian rhythms in depression. Part I: monitoring of the circadian body temperature rhythm. J Affect Disord. 1990;18(3):193–197.
- Souetre E, Salvati E, Wehr TA, et al. Twenty-four-hour profiles of body temperature and plasma TSH in bipolar patients during depression and during remission and in normal control subjects. Am J Psychiatry. 1988;145:1133–1137.
- Scales WE, Vander AJ, Brown MB, et al. Human circadian rhythms in temperature, trace metals, and blood variables. J Appl Physiol. 1988;65(4):1840–1846.
- Elliott AL, Mills JN, Minors DS, et al. The effect of real and simulated time-zone shifts upon the circadian rhythms of body temperature, plasma 11-hydroxycorticosteroids, and renal excretion in human subjects. J Physiol. 1972;221(1):227–257.
- Aschoff J, Gerecke U, Wever R. Phasenbeziehungen zwischen den circadianen Perioden der Aktivität und der Kerntemperatur beim Menschen. Pflügers Archiv. 1967;295:173–183.
- Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (Type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333.
- Kriebel J. Changes in internal phase relationships during isolation. In: Scheving LE, Halberg F, Pauly JE, editors. Chronobiology. Tokyo: Igaku Shoin; 1974. p. 451–459.
- Weitzman ED, Moline ML, Czeisler CA, et al. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3(4):299–309.
- Nakazawa Y, Nonaka K, Nishida N, et al. Comparison of body temperature rhythms between healthy elderly and healthy young adults. Jpn J Psychiatry Neurol. 1991;45(1):37–43.
- Kleitman N, Ramsaroop A. Periodicity in body temperature and heart rate. Endocrinology. 1948;43(1):1–20.
- Cisse F, Martineaud R, Martineaud JP. Circadian cycles of central temperature in hot climate in man. Archives Internationales De Physiologie, De Biochimie Et De Biophysique. 1991;99(2):155–159.
- Lobban MC. The entrainment of circadian rhythms in man. Cold Spring Harb Symp Quant Biol. 1960;25:325–332.
- Shanahan TL, Czeisler CA. Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrinol Metab. 1991;73:227–235.
- Honma K, Honma S, Kohsaka M, et al. Seasonal variation in the human circadian rhythm: dissociation between sleep and temperature rhythm. Am J Physiol. 1992;262(5 Pt 2):R885–R891.
- Barrett J, Lack L, Morris M. The sleep-evoked decrease of body temperature. Sleep. 1993;16(2):93–99.
- Lee YH, Tokura H. Circadian rhythm of human rectal and skin temperatures under the influences of three different kinds of clothing. J Interdiscip Cycle Res. 1993;24(1):33–42.
- Pollak CP, Wagner DR. Core body temperature in narcoleptic and normal subjects living in temporal isolation. Pharmacol Biochem Behav. 1994;47(1):65–71.
- Honma K, Honma S, Nakamura K, et al. Differential effects of bright light and social cues on reentrainment of human circadian rhythms. Am J Physiol. 1995;268(2 Pt 2):R528–R535.
- Van Dongen HPA, Kerkhof GA, Souverijn JHM. Absence of seasonal variation in the phase of the endogenous circadian rhythm in humans. Chronobiol Int. 1998;15(6):623–632.
- Leproult R, Van Reeth O, Byrne MM, et al. Sleepiness, performance, and neuroendocrine function during sleep deprivation: effects of exposure to bright light or exercise. J Biol Rhythms. 1997;12(3):245–258.
- Callard D, Davenne D, Lagarde D, et al. Nycthemeral variations in core temperature and heart rate: continuous cycling exercise versus continuous rest. Int J Sports Med. 2001;22(8):553–557.
- Dijk DJ, Neri DF, Wyatt JK, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol. 2001;281(5):R1647–R1664.
- Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526(3):683–694.
- Moussay S, Dosseville F, Gauthier A, et al. Circadian rhythms during cycling exercise and finger-tapping task. Chronobiol Int. 2002;19(6):1137–1149.
- Cagnacci A, Arangino S, Tuveri F, et al. Regulation of the 24-h body temperature rhythm of women in luteal phase: role of gonadal steroids and prostaglandins. Chronobiol Int. 2002;19(4):721–730.
- Varkevisser M, Kerkhof GA. 24-Hour assessment of performance on a palmtop computer: validating a self-constructed test battery. Chronobiol Int. 2003;20(1):109–121.
- Gradisar M, Lack L. Relationships between the circadian rhythms of finger temperature, core temperature, sleep latency, and subjective sleepiness. J Biol Rhythms. 2004;19(2):157–163.
- Heikens MJ, Gorbach AM, Eden HS, et al. Core body temperature in obesity. Am J Clin Nutr. 2011;93(5):963–967.
- Grimaldi D, Provini F, Pierangeli G, et al. Evidence of a diurnal thermogenic handicap in obesity. Chronobiol Int. 2015;32(2):299–302.
- Baker FC, Waner JI, Vieira EF, et al. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530(3):565–574.
- Mackowiak PA, Wasserman SS, Levine MM. A critical appraisalof 98 upper Limit ofthe normal body temperature, and other legacies of Carl Reinhold August Wunderlich. J Am Med Assoc. 1992;268(12):1578–1580.
- Shechter A, Boudreau P, Varin F, et al. Predominance of distal skin temperature changes at sleep onset across menstrual and circadian phases. J Biol Rhythms. 2011;26(3):260–270.
- Bratzke D, Rolke B, Ulrich R, et al. Central slowing during the night. Psychol Sci. 2007;18(5):456–461.
- Gubin DG, Weinert D, Rybina SV, et al. Activity, sleep and ambient light have a different impact on circadian blood pressure, heart rate and body temperature rhythms. Chronobiol Int. 2017;34(5):632–649.
- Kolodyazhniy V, Späti J, Frey S, et al. Estimation of human circadian phase via a multi-channel ambulatory monitoring system and a multiple regression model. J Biol Rhythms. 2011;26(1):55–67.
- Acosta FM, Martinez-Tellez B, Blondin DP, et al. Relationship between the daily rhythm of distal skin temperature and brown adipose tissue 18F-FDG uptake in young sedentary adults. J Biol Rhythms. 2019;34(5):533–550.
- Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, et al. Body temperature of the camel and its relation to water economy. Am J Physiol. 1957;188:103–112.
- Körtner G, Geiser F. Body temperature rhythms and activity in reproductive Antechinus (Marsupialia). Physiol Behav. 1995;58(1):31–36.
- Rose RW, Swain R, Bryant SL. Body temperature: rhythm and regulation in the Tasmanian bettong (Bettongia gaimardi) (Marsupialia: potoroidae). CompBiochem Physiol A. 1990;97(4):573–576.
- Bakko EB, Porter WP, Wunder BA. Body temperature patterns in black-tailed prairie dogs in the field. Can J Zool. 1988;66:1783–1789.
- Harlow HJ, Phillips JA, Ralph CL. Circadian rhythms and the effects of exogenous melatonin in the nine-banded armadillo, Dasypus novemcinctus: a mammal lacking a distinct pineal gland. Physiol Behav. 1982;29:307–313.
- Gemmell RT, Turner SJ, Krause WJ. The circadian rhythm of body temperature of four marsupials. J Therm Biol. 1997;22:301–307.
- Riccio AP, Goldman BD. Circadian rhythms of body temperature and metabolic rate in naked mole-rats. Physiol Behav. 2000;71:15–22.
- Wells RT. Thermoregulation and activity rhythms in the hairy-nosed wombat, Lasiorhinus latifrons (Owen), (Vombatidae). Aust J Zool. 1978;26:639–651.
- McCarron HCK, Buffenstein R, Fanning FD, et al. Free-ranging heart rate, body temperature and energy metabolism in eastern grey kangaroos (Macropus giganteus) and red kangaroos (Macropus rufus) in the arid regions of South East Australia. J Comp Physiol B. 2001;171:401–411.
- Chevillard-Hugot MC, Müller EF, Kulzer E. Oxygen consumption, body temperature and heart rate in the coati (Nasua nasua). CompBiochem Physiol A. 1980;65:305–309.
- Varosi SM, Brigmon RL, Besch EL. A simplified telemetry system for monitoring body temperature in small animals. Lab Anim Sci. 1990;40:299–302.
- Jones ME, Grigg GC, Beard LA. Body temperatures and activity patterns of Tasmanian devils (Sarcophilus harrisii) and eastern quolls (Dasyurus viverrinus) through a subalpine winter. Physiol Zool. 1997;70:53–60.
- Macari M. Efeito do cruzamento de suínos sobre o comportamento termoregulador. Ciência E Cultura. 1983;35:1145–1150.
- Ingram DL, Mount LE. The effects of food intake and fasting on 24-hourly variations in body temperature in the young pig. Pflügers Archiv. 1973;339:299–304.
- Bligh J, Harthoorn AM. Continuous radiotelemetric records of the deep body temperature of some unrestrained African mammals under near-natural conditions. J Physiol. 1965;176:145–162.
- Hildwein G, Kayser C. Relation entre la température colonique et la consommation d’oxygène d’un insectivore, le Tenrec, au cours du nycthémère. Comptes Rendus des Séances de la Societé de Biologie de Strasbourg. 1970;164:429–432.
- Peters DG, Rose RW. The oestrous cycle and basal body temperature in the common wombat (Vombatus ursinus). J Reprod Fertil. 1979;57:453–460.
- Sargeant GA, Eberhardt LE, Peek JM. Thermoregulation by mule deer (Odocoileus hemionus) in arid rangelands of southcentral Washington. J Mammal. 1994;75:536–544.
- Hanneman SK, McKay K, Costas G, et al. Circadian temperature rhythm of laboratory swine. Comp Med. 2005;55:249–255.
- Coolen A, Hoffmann K, Barf RP, et al. Telemetric study of sleep architecture and sleep homeostasis in the day-active tree shrew Tupaia belangeri. Sleep. 2012;35:879–888.
- Warnecke L, Withers PC, Schleucher E, et al. Body temperature variation of free-ranging and captive southern brown bandicoots Isoodon obesulus (Marsupialia: paramelidae). J Therm Biol. 2007;32:72–77.
- Busse S, Lutter D, Heldmaier G, et al. Torpor at high ambient temperature in a neotropical didelphid, the grey short-tailed opossum (Monodelphis domestica). Naturwissenschaften. 2014;101:1003–1006.
- Christian N, Geiser F. To use or not to use torpor? Activity and body temperature predictors. Naturwissenschaften. 2007;94:483–487.
- Al-Haidary AA, Abdoun KA, Samara EM, et al. Daily rhythms of physiological parameters in the dromedary camel under natural and laboratory conditions. Res Vet Sci. 2016;107:273–277.
- Mole MA, D’Araujo SR, van Aarde RJ, et al. Savanna elephants maintain homeothermy under African heat. J Comp Physiol B. 2018;188:889–897.
- Oshima I, Ebihara S. The measurement and analysis of circadian locomotor activity and body temperature rhythms by a computer-based system. Physiol Behav. 1988;43:115–119.
- Oshima I, Yamada H, Goto M, et al. Pineal and retinal melatonin is involved in the control of circadian locomotor activity and body temperature rhythms in the pigeon. J Comp Physiol A. 1989;166:217–226.
- Zivkovic BD, Underwood H, Siopes T. Circadian ovulatory rhythms in Japanese quail: role of ocular and extraocular pacemakers. J Biol Rhythms. 2000;15:172–183.
- Underwood H, Edmonds K. The circadian rhythm of thermoregulation in Japanese quail. II. Multioscillator control. J Biol Rhythms. 1995;10:234–247.
- Kadono H, Besch EL, Usami E. Body temperature, oviposition, and food intake in the hen during continuous light. J Appl Physiol. 1981;51:1145–1149.
- Bobr LW, Sheldon BL. Analysis of ovulation-oviposition patterns in the domestic fowl by telemetry measurement of deep body temperature. Aust J Biol Sci. 1977;30:243–257.
- Kadono H, Besch EL. Influence of laying cycle on body temperature rhythm in the domestic hen. In: Tanabe Y, editor. Biological rhythms in birds: neural and endocrine aspects. Berlin: Springer; 1980. p. 91–99.
- Wilson HR, Mather FB, Brigmon RL, et al. Feeding time and body temperature interactions in broiler breeders. Poult Sci. 1989;68:608–616.
- Winget CM, Card DH. Daily rhythm changes associated with variations in light intensity and color. Life Sci Space Res. 1967;5:148–158.
- Berger RJ, Phillips NH. Constant light suppresses sleep and circadian rhythms in pigeons without consequent sleep rebound in darkness. Am J Physiol. 1994;267:R945–R952.
- Aschoff J, von Saint Paul U. Brain temperature as related to gross motor activity in the unanesthetized chicken. Physiol Behav. 1973;10:529–533.
- Michels H, Herremans M, Decuypere E. Light-dark variations of oxygen consumption and subcutaneous temperature in young Gallus domesticus: influence of ambient temperature and depilation. J Therm Biol. 1985;10:13–20.
- Yang J, Morgan JLM, Kirby JD, et al. Circadian rhythm of the preovulatory surge of luteinizing hormone and its relationships to rhythms of body temperature and locomotor activity in turkey hens. Biol Reprod. 2000;62:1452–1458.
- Fuller A, Kamerman PR, Maloney SK, et al. Variability in brain and arterial blood temperatures in free-ranging ostriches in their natural habitat. J Exp Biol. 2003;206:1171–1181.
- Smit B, McKechnie AE. Do owls use torpor? Winter thermoregulation in free-ranging pearl-spotted owlets and African scops-owls. Physiol Biochem Zool. 2010;83:149–156.
- Zungu MM, Brown M, Downs CT. Seasonal thermoregulation in the burrowing parrot (Cyanoliseus patagonus). J Therm Biol. 2013;38:47–54.
- Dawson A. Daily cycles in body temperature in a songbird change with photoperiod and are weakly circadian. J Biol Rhythms. 2017;32:177–183.
- Teller J, Ragazzi M, Simonetti GD, et al. Accuracy of tympanic and forehead thermometers in private paediatric practice. Acta Paediatrica. 2014;103(2):e80–e83.
- Mogensen CB, Wittenhoff L, Fruerhøj G, et al. Forehead or ear temperature measurement cannot replace rectal measurements, except for screening purposes. BMC Pediatr. 2018;18(1):15.
- Refinetti R, Kenagy GJ. Circadian rhythms of body temperature and locomotor activity in the antelope ground squirrel, Ammospermophilus leucurus. J Therm Biol. 2018;72:67–72.
- Blessing W, Ootsuka Y. Timing of activities of daily life is jaggy: how episodic ultradian changes in body and brain temperature are integrated into this process. Temperature. 2016;3(3):371–383. doi: 10.1080/23328940.2016.1177159.
- Benstaali C, Bogdan A, Touitou Y. Effect of a short photoperiod on circadian rhythms of body temperature and motor activity in old rats. Pflügers Archiv. 2002;444(1–2):73–79.
- Blessing W, Mohammed M, Ootsuka Y. Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats. Physiol Behav. 2013;121:61–69.
- Golombek DA, Ortega G, Cardinali DP. Wheel running raises body temperature and changes the daily cycle in golden hamsters. Physiol Behav. 1993;53(6):1049–1054.
- Refinetti R. Circadian modulation of ultradian oscillation in the body temperature of the golden hamster. J Therm Biol. 1994;19(4):269–275.
- Haim A, Saarela S, Hohtola E, et al. Daily rhythms of oxygen consumption, body temperature, activity and melatonin in the Norwegian lemming Lemmus lemmus under northern summer photoperiod. J Therm Biol. 2004;29(7–8):629–633.
- Robinson EL, Fuller CA. Endogenous thermoregulatory rhythms of squirrel monkeys in thermoneutrality and cold. Am J Physiol. 1999;276(5 Pt 2):R1397–R1407.
- Mohr E, Krzywanek H. Variations of core-temperature rhythms in unrestrained sheep. Physiol Behav. 1990;48(3):467–473.
- Lefcourt AM, Huntington JB, Akers RM, et al. Circadian and ultradian rhythms of body temperature and peripheral concentrations of insulin and nitrogen in lactating dairy cows. Domest Anim Endocrinol. 1999;16(1):41–55.
- Refinetti R, Cornélissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Bio Rhythm Res. 2007;38(4):275–325.
- Leise TL, Harrington ME. Wavelet-based time series analysis of circadian rhythms. J Biol Rhythms. 2011;26(5):454–463.
- Dowse HB. Maximum entropy spectral analysis for circadian rhythms: theory, history and practice. J Circadian Rhythms. 2013;11:6.
- Agostinelli F, Ceglia N, Shahbaba B, et al. What time is it? Deep learning approaches for circadian rhythms. Bioinformatics. 2016;32(12):i8–i17.
- Refinetti R. Non-stationary time series and the robustness of circadian rhythms. J Theor Biol. 2004;227(4):571–581.
- Goh GH, Maloney SK, Mark PJ, et al. Episodic ultradian events: ultradian rhythms. Biology (Basel). 2019;8(1):15.
- Refinetti R, Piccione G. Intra- and inter-individual variability in the circadian rhythm of body temperature of rats, squirrels, dogs, and horses. J Therm Biol. 2005;30(2):139–146.
- Cuesta M, Boudreau P, Cermakian N, et al. Skin temperature rhythms in humans respond to changes in the timing of sleep and light. J Biol Rhythms. 2017;32:257–273.
- Kattapong KR, Fogg LF, Eastman CI. Effect of sex, menstrual phase, and oral contraceptive use on circadian temperature rhythms. Chronobiol Int. 1995;12:257–266.
- Honnebier MBOM, Jenkins SL, Nathanielsz PW. Circadian timekeeping during pregnancy: endogenous phase relationships between maternal plasma hormones and the maternal body temperature rhythm in pregnant rhesus monkeys. Endocrinology. 1992;131:2051–2058.
- Fuller PM, Fuller CA. Genetic evidence for a neurovestibular influence on the mammalian circadian pacemaker. J Biol Rhythms. 2006;21:177–184.
- Satinoff E, Kent S, Hurd M. Elevated body temperature in female rats after exercise. Med Sci Sports Exerc. 1991;23:1250–1253.
- Deprés-Brummer P, Metzger G, Lévi F. Analyses des rythmes de la température corporelle du rat en libre cours. Pathol Biol. 1996;44:150–156.
- Heusner A. Variation nycthémérale de la température centrale chez le rat adapté à la neutralité thermique. Comptes Rendus de Séances de la Société de Biologie de Strasbourg. 1959;153:1258–1261.
- Aschoff J. The circadian rhythm of body temperature as a function of body size. In: Taylor CR, Johansen K, Bolis L, editors. A companion to animal physiology. Cambridge, UK: Cambridge University Press; 1982. p. 173–188.
- Lovegrove BG. The influence of climate on the basal metabolic rate of small mammals: a slow-fast metabolic continuum. J Comp Physiol B. 2003;173:87–112.
- Mortola JP, Lanthier C. Scaling the amplitudes of the circadian pattern of resting oxygen consumption, body temperature and heart rate in mammals. CompBiochem Physiol A. 2004;139:83–95.
- Hetem RS, Maloney SK, Fuller A, et al. Heterothermy in large mammals: inevitable or implemented? Biol Rev. 2016;91:187–205.
- Piccione G, Giannetto C, Giudice E, et al. Persistent homeothermy in large domestic mammals maintained under standard farming conditions. J Basic Clin Physiol Pharmacol. 2020. Doi: 10.1515/jbcpp-2018-0121
- Boyles JG, Thompson AB, McKechnie AE, et al. A global heterothermic continuum in mammals. Global Ecol Biogeogr. 2013;22:1029–1039.
- Kronfeld-Schor N, Dayan T. Thermal ecology, environments, communities, and global change: energy intake and expenditure in endotherms. Annu Rev Ecol Evol Syst. 2013;44:461–480.
- Grahn DA, Miller JD, Houng VS, et al. Persistence of circadian rhythmicity in hibernating ground squirrels. Am J Physiol. 1994;266:R1251–R1258.
- Menaker M. The free running period of the bat clock: seasonal variations at low body temperature. J Cell Comp Physiol. 1961;57:81–86.
- Ruby NF, Dark J, Burns DE, et al. The suprachiasmatic nucleus is essential for circadian body temperature rhythms in hibernating ground squirrels. J Neurosci. 2002;22:357–364.
- Larkin JE, Franken P, Heller HC. Loss of circadian organization of sleep and wakefulness during hibernation. Am J Physiol. 2002;282:R1086–R1095.
- Hut RA, Barnes BM, Daan S. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J Comp Physiol B. 2002;172:47–58.
- Fowler PA, Racey PA. Daily and seasonal cycles of body temperature and aspects of heterothermy in the hedgehog Erinaceus europaeus. J Comp Physiol B. 1990;160:299–307.
- Wollnik F, Schmidt B. Seasonal and daily rhythms of body temperature in the European hamster (Cricetus cricetus) under semi-natural conditions. J Comp Physiol B. 1995;165:171–182.
- Zervanos SM, Salsbury CM, Brown JK. Maintenance of biological rhythms during hibernation in Eastern woodchucks (Marmota monax). J Comp Physiol B. 2009;179:411–418.
- Øivind T, Blake J, Edgar DM, et al. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–909.
- Williams CT, Sheriff MJ, Schmutz JA, et al. Data logging of body temperatures provides precise information on phenology of reproductive events in a free-ling Arctic hibernator. J Comp Physiol B. 2011;181:1101–1109.
- Williams CT, Barnes BM, Buck CL. Daily body temperature rhythms persist under the midnight sun but are absent during hibernation in free-living Arctic ground squirrels. Biol Lett. 2012;8:31–34.
- Williams CT, Radonich M, Barnes BM, et al. Seasonal loss and resumption of circadian rhythms in hibernating Arctic ground squirrels. J Comp Physiol B. 2017;187:693–703.
- Refinetti R. Ultradian rhythms of body temperature and locomotor activity in wild-type and tau-mutant hamsters. Anim Biol. 1996;5:111–115.
- Pickard GE, Kahn R, Silver R. Splitting of the circadian rhythm of body temperature in the golden hamster. Physiol Behav. 1984;32:763–766.
- Refinetti R, Menaker M. The circadian rhythm of body temperature of normal and tau-mutant golden hamsters. J Therm Biol. 1992;17:129–133.
- Eastman C, Rechtschaffen A. Circadian temperature and wake rhythms of rats exposed to prolonged continuous illumination. Physiol Behav. 1983;31:417–427.
- Refinetti R, Menaker M. Independence of heart rate and circadian period in the golden hamster. Am J Physiol. 1993;264:R235–R238.
- Kas MJH, Edgar DM. Photic phase response curve in Octodon degus: assessment as a function of activity phase preference. Am J Physiol. 2000;278:R1385–R1389.
- Halberg F, Visscher MB (1954). Some physiologic effects of lighting. In: Tenobergen JE (Ed.). Proceedings of the First International Photobiological Congress. Wageningen: Veenman and Zonen, pp. 396–398.
- Kramm KR. Body temperature regulation and torpor in the antelope ground squirrel, Ammospermophilus leucurus. J Mammal. 1972;53:609–611.
- Chandrashekaran MK, Marimuthu G, Geetha L. Correlations between sleep and wake in internally synchronized and desynchronized circadian rhythms in humans under prolonged isolation. J Biol Rhythms. 1997;12:26–33.
- Reinberg A, Halberg F, Ghata J, et al. Spectre thermique (rythmes de la température rectale) d’une femme adulte avant, pendant et après son isolement souterrain de trois mois. Comptes Rendus de l’Académie des Sciences. 1966;262:782–785.
- Czeisler CA, Weitzman ED, Moore-Ede MC, et al. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267.
- Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflügers Archiv. 1981;391:314–318.
- Colin J, Timbal J, Boutelier C, et al. Rhythm of the rectal temperature during a 6-month free-running experiment. J Appl Physiol. 1968;25:170–176.
- Lund R. Personality factors and desynchronization of circadian rhythms. Psychosom Med. 1974;36:224–228.
- Campbell SS, Dawson D, Zulley J. When the human circadian system is caught napping: evidence for endogenous rhythms close to 24 hours. Sleep. 1993;16:638–640.
- Lack LC, Lushington K. The rhythms of human sleep propensity and core body temperature. J Sleep Res. 1996;5:1–11.
- Kuriyama K, Uchiyama M, Suzuki H, et al. Diurnal fluctuation of time perception under 30-h sustained wakefulness. Neurosci Res. 2005;53:123–128.
- Späti J, Münch M, Blatter K, et al. Impact of age, sleep presure and circadian phase on time-of-day estimates. Behav Brain Res. 2009;201:48–52.
- Menaker M. Endogenous rhythms of body temperature in hibernating bats. Nature. 1959;184:1251–1252.
- Jilge B, Kuhnt B, Landerer W, et al. Circadian temperature rhythms in rabbit pups and in their does. Lab Anim. 2001;35:364–373.
- Winget CM, Averkin EG, Fryer TB. Quantitative measurement by telemetry of ovulation and oviposition in the fowl. Am J Physiol. 1965;209:853–858.
- Ostrowski S, Williams JB, Ismael K. Heterothermy and water economy of free-living Arabian oryx (Oryx leucoryx). J Exp Biol. 2003;206:1471–1478.
- Fuller A, Kamerman PR, Maloney SK, et al. A year in the thermal life of a free-ranging herd of springbok Antidorcas marsupialis. J Exp Biol. 2005;208:2855–2864.
- Maloney SK, Meyer LCR, Blache D, et al. Energy intake and the circadian rhythm of core body temperature in sheep. Physiol Rep. 2013;1:e00118.
- Davimes JG, Alagaili AN, Gravett N, et al. Arabian oryx (Oryx leucoryx) respond to increased ambient temperatures with a seasonal shift in the timing of their daily inactivity patterns. J Biol Rhythms. 2016;31:365–374.
- Alagaili AN, Bennett NC, Mohammed OB, et al. Body temperature patterns of a small endotherm in an extreme desert environment. J Arid Environ. 2017;137:16–20.
- Riek A, Brinkmann L, Gauly M, et al. Seasonal changes in energy expenditure, body temperature and activity patterns in llamas (Lama glama). Sci Rep. 2017;7(art):7600.
- Riek A, Stölzl A, Bernedo RM, et al. Energy expenditure and body temperature variations in llamas living in the High Andes of Peru. Sci Rep. 2019;9(art):4037.
- Thompson DP, Barboza PS, Crouse JA, et al. Body temperature patterns vary with day, season, and body condition of moose (Alces alces). J Mammal. 2019;100:1466–1478.
- Fuller CA. Circadian brain and body temperature rhythms in the squirrel monkey. Am J Physiol. 1984;246:R242–R246.
- Refinetti R. The effects of ambient temperature on the body temperature rhythm of rats, hamsters, gerbils, and tree shrews. J Therm Biol. 1997;22:281–284.
- Refinetti R. The body temperature rhythm of the thirteen-lined ground squirrel, Spermophilus tridecemlineatus. Physiol Zool. 1996;69:270–275.
- Piccione G, Gianesella M, Morgante M, et al. Daily rhythmicity of core and surface temperatures of sheep kept under thermoneutrality or in the cold. Res Vet Sci. 2013;95:261–265.
- Graf R. Diurnal changes of thermoregulatory functions in pigeons. I. Effector mechanisms. Pflügers Archiv. 1980;386:173–179.
- McKechnie AE, Lovegrove BG. Heterothermic responses in the speckled mousebird (Colius striatus). J Comp Physiol B. 2001;171:507–518.
- Downs CT, Brown M. Nocturnal heterothermy and torpor in the Malachite sunbird (Nectarinia famosa). Auk. 2002;119:251–260.
- Roussel B, Chouvet G, Debilly G. Rythmes circadiens des températures internes et ambiance thermique chez le rat. Pflügers Archiv. 1976;365:183–189.
- Yang Y, Gordon CJ. Ambient temperature limits and stability of temperature regulation in telemetered male and female rats. J Therm Biol. 1996;21:353–363.
- Aujard F, Vasseur F. Effect of ambient temperature on the body temperature rhythm of male gray mouse lemurs (Microcebus murinus). Int J Primatol. 2001;22:43–56.
- Horst K, Mendel LB, Benedict FG. The metabolism of the albino rat during prolonged fasting at two different environmental temperatures. J Nutr. 1930;3:177–200.
- MacMillen RE. Aestivation in the cactus mouse, Peromyscus eremicus. Comp Biochem Physiol. 1965;16:227–248.
- Campbell BA, Lynch GS. Influence of hunger and thirst on the relationship between spontaneous activity and body temperature. J Comp Physiol Psychol. 1968;65:492–498.
- Bolles RC, Duncan PM. Daily course of activity and subcutaneous body temperature in hungry and thirsty rats. Physiol Behav. 1969;4:87–89.
- Duchamp C, Barré H, Delage D, et al. Nonshivering thermogenesis and adaptation to fasting in king penguin chicks. Am J Physiol. 1989;257:R744–R751.
- Klein MS, Conn CA, Kluger MJ. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol Behav. 1992;52(6):1133–1139.
- Rashotte ME, Basco PS, Henderson RP. Daily cycles in body temperature, metabolic rate, and substrate utilization in pigeons: influence of amount and timing of food consumption. Physiol Behav. 1995;57(4):731–746.
- Rashotte ME, Pastukhov IF, Poliakov EL, et al. Vigilance states and body temperature during the circadian cycle in fed and fasted pigeons (Columbia livia). Am J Physiol. 1998;275(5):R1690–R1702.
- Yoda T, Crawshaw LI, Yoshida K, et al. Effects of food deprivation on daily changes in body temperature and behavioral thermoregulation in rats. Am J Physiol. 2000;278:R133–R139.
- Sakurada S, Shido O, Sugimoto N, et al. Autonomic and behavioural thermoregulation in starved rats. J Physiol. 2000;526(2):417–424.
- Westman W, Geiser F. The effect of metabolic fuel availability on thermoregulation and torpor in a marsupial hibernator. J Comp Physiol B. 2004;174(1):49–57.
- Geiser F, Holloway JC, Körtner G. Thermal biology, torpor and behaviour in sugar gliders: a laboratory-field comparison. J Comp Physiol B. 2007;177(5):495–501.
- Vogt FD, Lynch GR. Influence of ambient temperature, nest availability, huddling, and daily torpor on energy expenditure in the white-footed mouse Peromyscus leucopus. Physiol Zool. 1982;55(1):56–63.
- Tannenbaum MG, Pivorun EB. Differential effect of food restriction on the induction of daily torpor in Peromyscus maniculatus and P. leucopus. J Therm Biol. 1987;12(2):159–162.
- Heldmaier G, Steinlechner S, Ruf T, et al. Photoperiod and thermoregulation in vertebrates: body temperature rhythms and thermogenic acclimation. J Biol Rhythms. 1989;4(2):251–265.
- Ruby NF, Ibuka N, Barnes BM, et al. Suprachiasmatic nuclei influence torpor and circadian temperature rhythms in hamsters. Am J Physiol. 1989;257(1 Pt 2):R210–R215.
- Ellison GTH, Skinner JD. The influence of ambient temperature on spontaneous daily torpor in pouched mice (Saccostomus campestris: Rodentia—Cricetidae) from Southern Africa. J Therm Biol. 1992;17(1):25–31.
- Prinzinger R, Schäfer T, Schuchmann KL. Energy metabolism, respiratory quotient and breathing parameters in two convergent small bird species: the fork-tailed sunbird and the Chilean hummingbird. J Therm Biol. 1992;17(2):71–79.
- Geiser F, Masters P. Torpor in relation to reproduction in the mulgara, Dasycercus cristicauda (Dasyuridae: marsupialia). J Therm Biol. 1994;19(1):33–40.
- Holloway JC, Geiser F. Reproductive status and torpor of the marsupial Sminthopsis crassicaudata: effect of photoperiod. J Therm Biol. 1996;21(5–6):373–380.
- Ortmann S, Heldmaier G. Spontaneous daily torpor in Malagasy mouse lemurs. Naturwissenschaften. 1997;84:28–32.
- Song X, Körtner G, Geiser F. Temperature selection and use of torpor by the marsupial Sminthopsis macroura. Physiol Behav. 1998;64(5):675–682.
- Turbill C, Law BS, Geiser F. Summer torpor in a free-ranging bat from subtropical Australia. J Therm Biol. 2003;28(3):223–226.
- Turbill C, Körtner G, Geiser F. Natural use of heterothermy by a small, tree-roosting bat during summer. Physiol Biochem Zool. 2003;76(6):868–876.
- Cooper CE, Withers PC. Patterns of body temperature variation and torpor in the numbat, Myrmecobius fasciatus (Marsupialia: myrmecobiidae). J Therm Biol. 2004;29(6):277–284.
- MacMillen RE, Trost CH. Nocturnal hypothermia in the Inca dove, Scardafella inca. Comp Biochem Physiol. 1967;23(1):243–253.
- Thouzeau C, Duchamp C, Handrich Y. Energy metabolism and body temperature of barn owls fasting in the cold. Physiol Biochem Zool. 1999;72(2):170–178.
- Rashotte ME, Henderson D. Coping with rising food costs in a closed economy: feeding behavior and nocturnal hypothermia in pigeons. J Exp Anal Behav. 1988;50(3):441–456.
- Phillips NH, Berger RJ. Regulation of body temperature, metabolic rate, and sleep in fasting pigeons diurnally infused with glucose or saline. J Comp Physiol B. 1991;161(3):311–318.
- Ostheim J. Coping with food-limited conditions: feeding behavior, temperature preference, and nocturnal hypothermia in pigeons. Physiol Behav. 1992;51(2):353–361.
- Hohtola E, Hissa R, Pyörnilä A, et al. Nocturnal hypothermia in fasting Japanese quail: the effect of ambient temperature. Physiol Behav. 1991;49(3):563–567.
- Prinzinger R, Schleucher E, Preßmar A. Langzeittelemetrie der Körpertemperatur mit synchroner Bestimmung des Energiestoffwechsels beim Blaunackenmausvogel (Urocolius macrourus) unter Normal- und Lethargiebedingungen (Torpor). Journal Für Ornithologie. 1992;133(4):446–450.
- McKechnie AE, Lovegrove BG. Facultative hypothermic responses in an Afrotropical arid-zone passerine, the red-headed finch (Amadina erythrocephala). J Comp Physiol B. 2003;173(4):339–346.
- Hudson JW. Temperature Regulation and Torpidity in the Pygmy Mouse, Baiomys taylori. Physiol Zool. 1965;38(3):243–254.
- Nestler JR. Relationships between respiratory quotient and metabolic rate during entry to and arousal from daily torpor in deer mice (Peromyscus maniculatus). Physiol Zool. 1990;63(3):504–515.
- Damiola F, Le Minh N, Preitner N, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961.
- Satoh Y, Kawai H, Kudo N, et al. Time-restricted feeding entrains daily rhythms of energy metabolism in mice. Am J Physiol. 2006;290(5):R1276–R1283.
- Zhang J, Kaasik K, Blackburn MR, et al. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439(7074):340–343.
- Liu S, Chen XM, Yoda T, et al. Involvement of the suprachiasmatic nucleus in body temperature modulation by food deprivation in rats. Brain Res. 2002;929(1):26–36.
- Pecoraro N, Gomez F, Laugero K, et al. Brief access to sucrose engages food-entrainable rhythms in food-deprived rats. Behav Neurosci. 2002;116(5):757–776.
- Nagashima K, Nakai S, Matsue K, et al. Effects of fasting on thermoregulatory processes and the daily oscillations in rats. Am J Physiol. 2003;284(6):R1486–R1493.
- Smith SE, Ramos FA, Refinetti R, et al. Protein-energy malnutrition induces an aberrant acute-phase response and modifies the circadian rhythm of core temperature. Appl Physiol Nutr Metab. 2013;38(8):844–853.
- Goh GH, Mark PJ, Maloney SK. Altered energy intake and the amplitude of the body temperature rhythm are associated with changes in phase, but not amplitude, of clock gene expression in the rat suprachiasmatic nucleus in vivo. Chronobiol Int. 2016;33(1):85–97.
- Perret M, Aujard F. Daily hypothermia and torpor in a tropical primate: synchronization by 24-h light-dark cycle. Am J Physiol. 2001;281(6):R1925–R1933.
- Génin F, Perret M. Daily hypothermia in captive grey mouse lemurs (Microcebus murinus): effects of photoperiod and food restriction. Comp Biochem Physiol B. 2003;136(1):71–81.
- Piccione G, Caola G, Refinetti R. Circadian modulation of starvation-induced hypothermia in sheep and goats. Chronobiol Int. 2002;19(3):531–541.
- Piccione G, Caola G, Refinetti R. Circadian rhythms of body temperature and liver function in fed and food-deprived goats. CompBiochem Physiol A. 2003;134(3):563–572.
- Bouâouda H, Achâaban MR, Ouassat M, et al. Daily regulation of body temperature rhythm in the camel Camelus dromedarius) exposed to experimental desert conditions. Physiol Rep. 2014;2(9):e12151.
- Daan S, Aschoff J. The entrainment of circadian rhythm. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian Clocks (Handbook of Behavioral Neurobiology Volume 12). New York: Kluwer/Plenum; 2001. p. 7–43.
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20(5):741–774.
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–1102.
- Swade RH, Pittendrigh CS. Circadian locomotor rhythms of rodents in the Arctic. American Naturalist. 1967;101(922):431–466.
- Lindberg RG, Hayden P. Thermoperiodic entrainment of arousal from torpor in the little pocket mouse, Perognathus longimembris. Chronobiologia. 1974;1(4):356–361.
- Aschoff J, Tokura H. Circadian activity rhythms in squirrel monkeys: entrainment by temperature cycles. J Biol Rhythms. 1986;1(2):91–99.
- Francis AJP, Coleman GJ. Ambient temperature cycles entrain the free-running circadian rhythms of the stripe-faced dunnart, Sminthopsis macroura. J Comp Physiol A. 1990;167(3):357–362.
- Francis AJP, Coleman GJ. The effect of ambient temperature cycles upon circadian running and drinking activity in male and female laboratory rats. Physiol Behav. 1988;43(4):471–477.
- Rajaratnam SMW, Redman JR. Entrainment of activity rhythms to temperature cycles in diurnal palm squirrels. Physiol Behav. 1998;63(2):271–277.
- Pohl H. Temperature cycles as zeitgeber for the circadian clock of two burrowing rodents, the normothermic antelope ground squirrel and heterothermic Syrian hamster. Bio Rhythm Res. 1998;29(3):311–325.
- Goldman BD, Goldman SL, Riccio AP, et al. Circadian patterns of locomotor activity and body temperature in blind mole-rats, Spalax ehrenbergi. J Biol Rhythms. 1997;12(4):348–361.
- Herzog ED, Huckfeldt RM. Circadian entrainment to temperature, but not light, in the isolated suprachiasmatic nucleus. J Neurophysiol. 2003;90(2):763–770.
- Tokura H, Aschoff J. Effects of temperature on the circadian rhythm of pig-tailed macaques Macaca nemestrina. Am J Physiol. 1983;245(6):R800–R804.
- Pálková M, Sigmund L, Erkert HG. Effect of ambient temperature on the circadian activity rhythm in common marmosets, Callithrix j. jacchus (Primates). Chronobiol Int. 1999;16(2):149–161.
- Vivanco P, Rol MA, Madrid JA. Temperature cycles trigger nocturnalism in the diurnal homeotherm Octodon degus. Chronobiol Int. 2010;27(3):517–534.
- Refinetti R. Entrainment of circadian rhythm by ambient temperature cycles in mice. J Biol Rhythms. 2010;25(4):247–256.
- Refinetti R. Comparison of light, food, and temperature as environmental synchronizers of the circadian rhythm of activity in mice. J Physiol sci. 2015;65(4):359–366.
- El Allali K, Achaâban MR, Bothorel B, et al. Entrainment of the circadian clock by daily ambient temperature cycles in the camel (Camelus dromedarius). Am J Physiol. 2013;304(11):R1044–R1052.
- Van Jaarsveld B, Bennett NC, Hart DW, et al. Locomotor activity and body temperature rhythms in the Mahali mole-rat (C. h. mahali): the effect of light and ambient temperature variations. J Therm Biol. 2019;79:24–32.
- Ota W, Nakane Y, Kashio M, et al. Involvement of TRPM2 and TRPM8 in temperature-dependent masking behavior. Sci Rep. 2019;9(1):3706.
- Jilge B. Restricted feeding: a nonphotic zeitgeber in the rabbit. Physiol Behav. 1991;51(1):157–166.
- Kennedy GA, Coleman GJ, Armstrong SM. Restricted feeding entrains circadian wheel-running activity rhythms of the kowari. American J Physiol. 1991;261(4 Pt 2):R819–R827.
- Kennedy GA, Coleman GJ, Armstrong SM. Daily restricted feeding effects on the circadian activity rhythms of the stripe-faced dunnart, Sminthopsis macroura. J Biol Rhythms. 1996;11(3):188–195.
- Challet E, Losee-Olson S, Turek FW. Reduced glucose availability attenuates circadian responses to light in mice. Am J Physiol. 1999;276(4):R1063–R1070.
- Refinetti R. Effects of prolonged exposure to darkness on circadian photic responsiveness in the mouse. Chronobiol Int. 2003;20(3):417–440.
- Edmonds SC, Adler NT. Food and light as entrainers of circadian running activity in the rat. Physiol Behav. 1977;18(5):915–919.
- Coleman GJ, Francis AJP. Food deprivation and reinstatement phase shifts rat activity rhythms in constant light but not constant dark. Physiol Behav. 1991;50(1):167–171.
- Rusak B, Mistlberger RE, Losier B, et al. Daily hoarding opportunity entrains the pacemaker for hamster activity rhythms. J Comp Physiol A. 1988;164(2):165–171.
- Challet E, Malan A, Pévet P. Daily hypocaloric feeding entrains circadian rhythms of wheel-running and body temperature in rats kept in constant darkness. Neurosci Lett. 1996;211(1):1–4.
- Holmes MM, Mistlberger RE. Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol Behav. 2000;68(5):655–666.
- Sharma VK, Chidambaram R, Subbaraj R, et al. Effects of restricted feeding cycle on the locomotor activity rhythm in the mouse Mus booduga. Physiol Behav. 2000;70(1–2):81–87.
- Sulzman FM, Fuller CA, Moore-Ede MC. Environmental synchronizers of squirrel monkey circadian rhythms. J Appl Physiol. 1977;43(5):795–800.
- Boulos Z, Frim DM, Dewey LK, et al. Effects of restricted feeding schedules on circadian organization in squirrel monkeys. Physiol Behav. 1989;45(3):507–515.
- Sulzman FM, Fuller CA, Moore-Ede MC. Feeding time synchronizes primate circadian rhythms. Physiol Behav. 1977;18(5):775–779.
- White W, Timberlake W. Two meals promote entrainment of rat food-anticipatory and rest-activity rhythms. Physiol Behav. 1995;57(6):1067–1074.
- Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005;22(11):2855–2862.
- Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6J mice. Brain Res. 1997;765(2):273–282.
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407.
- Jansen HT, Sergeeva A, Stark G, et al. Circadian discrimination of reward: evidence for simultaneous yet separable food- and drug-entrained rhythms in the rat. Chronobiol Int. 2012;29(4):454–468.
- Mendoza J, Graff C, Dardente H, et al. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light-dark cycle. J Neurosci. 2005;25(6):1514–1522.
- Abe H, Honma S, Honma K. Daily restricted feeding resets the circadian clock in the suprachiasmatic nucleus of CS mice. Am J Physiol. 2007;292(1):R607–R615.
- Feillet CA, Ripperger JA, Magnone MC, et al. Lack of food anticipation in Per2 mutant mice. Curr Biol. 2006;16(20):2016–2022.
- Mendoza J, Clesse D, Pévet P, et al. Food-reward signalling in the suprachiasmatic clock. J Neurochem. 2010;112(6):1489–1499.
- Mendoza J, Gourmelen S, Dumont S, et al. Setting the main circadian clock of a diurnal mammal by hypocaloric feeding. J Physiol. 2012;590(13):3155–3168.
- Piccione G, Bertolucci C, Caola G, et al. Effects of restricted feeding on circadian activity rhythms of sheep—A brief report. Appl Anim Behav Sci. 2007;107(3–4):233–238.
- Zhdanova IV, Masuda K, Bozhokin SV, et al. Familial circadian rhythm disorder in the diurnal primate Macaca mulatta. PLoS One. 2012;7(3):e33327.
- Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16(4):415–429.
- Helm B, Visser ME, Schwartz W, et al. Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Philos Trans Royal Soc B. 2017;372(1734):20160246.
- Lighton JRB, eds. Measuring metabolic rates. 2nd ed. Oxford: Oxford University Press; 2019.
- Kaiyala KJ, Ramsay DS. Direct animal calorimetry: the underused gold standard for quantifying the fire of life. CompBiochem Physiol A. 2011;158(3):252–264.
- Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988;47(4):608–628.
- Mansell PI, Macdonald IA. Reappraisal of the Weir equation for calculation of metabolic rate. Am J Physiol. 1990;258(6 Pt 2):R1347–R1354.
- Armitage G, Harris RBS, Hervey GR, et al. The relationship between energy expenditure and environmental temperature in congenitally obese and non-obese Zucker rats. J Physiol. 1984;350:197–207.
- Keesey RE, Swiergiel AH, Corbett SW. Contribution of spontaneous activity to daily energy expenditure of adult obese and lean Zucker rats. Physiol Behav. 1990;48:327–331.
- Stupfel M, Gourlet V, Perramon A, et al. Comparison of ultradian and circadian oscillations of carbon dioxide production by various endotherms. Am J Physiol. 1995;268:R253–R265.
- Carlisle HJ, Wilkinson CW, Laudenslager ML, et al. Diurnal variation of heat intake in ovariectomized, steroid-treated rats. Horm Behav. 1979;12:232–242.
- Williams TD, Chambers JB, May OL, et al. Concurrent reductions in blood pressure and metabolic rate during fasting in the unrestrained SHR. Am J Physiol. 2000;278:R255–R262.
- Heusner A. Mise en évidence d’une variation nycthémérale de la calorification indépendante du cycle de l’activité chez le rat. Comptes Rendus des Séances de la Société de Biologie de Strasbourg. 1956;150:1246–1249.
- Hervey GR, Hussain SH, Rayfield KM, et al. The role of thyroid hormones in the metabolic response to cold in the rat. J Physiol. 1987;386:73P.
- Yamaoka I, Hagi M, Doi M. Circadian changes in core body temperature, metabolic rate and locomotor activity in rats on a high-protein, carbohydrate-free diet. J Nutr Sci Vitaminol (Tokyo). 2009;55:511–517.
- Namvar S, Gyte A, Denn M, et al. Dietary fat and corticosterone levels are contributing factors to meal anticipation. Am J Physiol. 2016;310:R711–R723.
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913.
- Stupfel M, Gourlet V, Court L, et al. Periodic analysis of ultradian (40 min < tau < 24 h) respiratory variations in laboratory vertebrates of various circadian activities. Chronobiologia. 1987;14:365–375.
- Kenagy GJ, Vleck D. Daily temporal organization of metabolism in small mammals: adaptation and diversity. In: Aschoff J, Daan S, Groos G, editors. Vertebrate Circadian Systems. Berlin: Springer Verlag; 1982. p. 322–338.
- Bray MS, Ratcliffe WF, Grenett MH, et al. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obesity. 2013;37:843–852.
- Fabio-Braga AP, Klein W. Temperature and circadian effects on metabolic rate of South American echimyid rodents, Trinomys setosus and Clyomys bishopi (Rodentia: echimyidae). Zoologia. 2018;35:e24572.
- Welz PS, Zinna VM, Symeonidi A, et al. BMAL1-driven tissue clocks respond independently to light to maintain homeostasis. Cell. 2019;177:1436–1447.
- Adamovich Y, Ladeuix B, Golik M, et al. Rhythmic oxygen levels reset circadian clocks through HIF1-alpha. Cell Metab. 2017;25:93–101.
- Adamovich Y, Ladeuix B, Sobel J, et al. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab. 2019;29:1092–1103.
- Morris CJ, Garcia JI, Myers S, et al. The human circadian system has a dominating role in causing the morning-evening difference in diet-induced thermogenesis. Obesity. 2015;23:2053–2058.
- Zitting KM, Vujovic N, Yuan RK, et al. Human resting energy expenditure varies with circadian phase. Curr Biol. 2018;28:3685–3690.
- Randolph JC. Daily metabolic patterns of short-tailed shrews (Blarina) in three natural seasonal temperature regimes. J Mammal. 1980;61:628–638.
- Henken AM, Brandsma HA, van der Hel W, et al. Circadian rhythm in heat production of limit-fed growing pigs of several breeds kept at and below thermal neutrality. J Anim Sci. 1993;71:1434–1440.
- Verstegen MWA, van der Hel W, Duijghuisen R, et al. Diurnal variation in thermal demand of growing pigs. J Therm Biol. 1986;11:131–135.
- Kemp B, de Greef-lammers FJ, Verstegen MWA, et al. Some aspects of daily pattern in thermal demand of breeding boars. J Therm Biol. 1990;15:103–108.
- Chwalibog A, Tauson AH, Thorbek G. Diurnal rhythm in heat production and oxidation of carbohydrate and fat in pigs during feeding, starvation and re-feeding. J Anim Physiol Anim Nutr (Berl). 2004;88:266–274.
- Aschoff J, Pohl H. Rhythmic variations in energy metabolism. Federation Proc. 1970;29:1541–1552.
- Lovegrove BG, Smith GA. Is ‘nocturnal hypothermia’ a valid physiological concept in small birds? A study on Bronze Mannikins Spermestes cucullatus. Ibis. 2003;145:547–557.
- Mata A. Metabolic rate and specific dynamic action of the red-legged honeycreeper, a nectar-feeding neotropical passerine. CompBiochem Physiol A. 2010;157:291–296.
- Gavrilov VV, Veselovskaya EO, Gavrilov VM, et al. Diurnal rhythms of locomotor activity, changes in body mass and fat reserves, standard metabolic rate, and respiratory quotient in the free-living coal tit (Parus ater) in the autumn-winter period. Biol Bull. 2013;40:678–683.
- Regnault V, Reiset J. Recherches chimiques sur la respiration des animaux des diverses classes. Annales de Chimie et de Physique, Series 3. 1849;26:299–519.
- Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27(4):511–541.
- Kayser C, Heusner A. Étude comparative de métabolisme énergétique dans la série animale. J de Physiologie. 1964;56:489–524.
- Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22(3):453–460.
- Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–R829.
- Kaiyala KJ, Morton GJ, Thaler JP, et al. Acutely decreased thermoregulatory energy expenditure or decreased activity energy expenditure both acutely reduce food intake in mice. PLoS One. 2012;7(8):e41473.
- Bailey SM, Udoh US, Young ME. Circadian regulation of metabolism. J Endocrinol. 2014;222(2):R75–R96.
- Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277(5):513–527.
- Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–1015.
- West GC. Shivering and heat production in wild birds. Physiol Zool. 1965;38(2):111–120.
- Hohtola E, Stevens ED. The relationship of muscle electrical activity, tremor and heat production to shivering thermogenesis in Japanese quail. J Exp Biol. 1986;125:119–135.
- Steen J, Enger PS. Muscular heat production in pigeons during exposure to cold. Am J Physiol. 1957;191(1):157–158.
- Saarela S, Heldmaier G. Effect of photoperiod and melatonin on cold resistance, thermoregulation and shivering/nonshivering thermogenesis in Japanese quail. J Comp Physiol B. 1987;157(5):625–633.
- Barré H, Cohen-Adad F, Duchamp C, et al. Multilocular adipocytes from muscovy ducklings differentiated in response to cold acclimation. J Physiol. 1986;375(1):27–38.
- Marjoniemi K, Hohtola E. Does cold acclimation induce nonshivering thermogenesis in juvenile birds? Experiments with Pekin ducklings and Japanese quail chicks. J Comp Physiol B. 2000;170(7):537–543.
- Korhonen K. Heat loss of willow grouse (Lagopus L. Lagopus L.) in a snowy environment. J Therm Biol. 1989;14(1):27–31.
- Lim TPK. Central and peripheral control mechanisms of shivering and its effects on respiration. J Appl Physiol. 1960;15(4):567–574.
- Pohl H. Temperature regulation and cold acclimation in the golden hamster. J Appl Physiol. 1965;20(3):405–410.
- Oufara S, Barré H, Rouanet JL, et al. Adaptation to extreme ambient temperatures in cold-acclimated gerbils and mice. American J Physiol. 1987;253(1 Pt 2):R39–R45.
- Davis TRA, Mayer J. Nature of the physiological stimulus for shivering. Am J Physiol. 1955;181(3):669–674.
- Spaan G, Klussmann FW. Die Frequenz des Kältezitterns bei Tierarten verschiedener Größe. Pflügers Archiv. 1970;320(4):318–333.
- Slee J. Habituation and acclimatization of sheep to cold following exposures of varying length and severity. J Physiol. 1972;227(1):51–70.
- Fuller CA, Horwitz BA, Horowitz JM. Shivering and nonshivering thermogenic responses of cold-exposed rats to hypothalamic warming. Am J Physiol. 1975;228(5):1519–1524.
- Sellers EA, Scott JW, Thomas N. Electrical activity of skeletal muscle of normal and acclimated rats on exposure to cold. Am J Physiol. 1954;177(3):372–376.
- Griggio MA. The participation of shivering and nonshivering thermogenesis in warm and cold-acclimated rats. CompBiochem Physiol A. 1982;73(3):481–484.
- Hart JS, Heroux O, Depocas F. Cold acclimation and the electromyogram of unanesthetized rats. J Appl Physiol. 1956;9(3):404–408.
- Heroux O, Hart JS, Depocas F. Metabolism and muscle activity of anesthetized warm and cold acclimated rats on exposure to cold. J Appl Physiol. 1956;9(3):399–403.
- Moriya K, Arnold F, LeBlanc J. Shivering and nonshivering thermogenesis in exercised cold-deacclimated rats. Eur J Appl Physiol. 1988;57(4):467–473.
- Harri M, Dannenberg T, Oksanen-Rossi R, et al. Related and unrelated changes in response to exercise and cold in rats: a reevaluation. J Appl Physiol. 1984;57(5):1489–1497.
- Pohl H, Hart JS. Thermoregulation and cold acclimation in a hibernator, Citellus tridecemlineatus. J Appl Physiol. 1965;20(3):398–404.
- Heath M, Ingram DL. Thermoregulatory heat production in cold-reared and warm-reared pigs. Am J Physiol. 1983;244(2):R273–R278.
- Berthon D, Herpin P, Le Dividich J. Shivering thermogenesis in the neonatal pig. J Therm Biol. 1994;19(6):413–418.
- Israel DJ, Pozos RS. Synchronized slow-amplitude modulations in the electromyograms of shivering muscles. J Appl Physiol. 1989;66(5):2358–2363.
- Brück K, Baum E, Schwennicke HP. Cold-adaptive modifications in man induced by repeated short-term cold-exposures and during a 10-day and -night cold-exposure. Pflügers Archiv. 1976;363(2):125–133.
- Bawa P, Matthews PBC, Mekjavic IB. Electromyographic activity during shivering of muscles acting at the human elbow. J Therm Biol. 1987;12(1):1–4.
- Barré H, Bailly L, Rouanet JL. Increased oxidative capacity in skeletal muscles from cold-acclimated ducklings: a comparison with rats. Comp Biochem Physiol B. 1987;88(2):519–522.
- Duchamp C, Barré H, Rouanet JL, et al. Nonshivering thermogenesis in king penguin chicks. I. Role of skeletal muscle. Am J Physiol. 1991;261(6 Pt 2):R1438–R1445.
- Duchamp C, Cohen-Ada F, Rouanet JL, et al. Histochemical arguments for muscular non-shivering thermogenesis in muscovy ducklings. J Physiol. 1992;457(1):27–45.
- Chaffee RRJ, Mayhew WW, Drebin M, et al. Studies on thermogenesis in cold-acclimated birds. Can J Biochem Physiol. 1963;41(1):2215–2220.
- Kronfeld-Schor N, Haim A, Dayan T, et al. Seasonal thermogenic acclimation of diurnally and nocturnally active desert spiny mice. Physiol Biochem Zool. 2000;73(1):37–44.
- Haim A, Martinez JJ. Seasonal acclimatization in the migratory hamster Cricetulus migratorius —the role of photoperiod. J Therm Biol. 1992;17(6):347–351.
- Harlow HJ. Winter body fat, food consumption and nonshivering thermogenesis of representative spontaneous and facultative hibernators: the white-tailed prairie dog and black-tailed prairie dog. J Therm Biol. 1997;22(1):21–30.
- Haim A. Thermoregulation and metabolism of Wagner’s Gerbil (Gerbillus dasyurus): A rock dwelling rodent adapted to arid and mesic environments. J Therm Biol. 1987;12(1):45–48.
- Haim A, Levi G. Role of body temperature in seasonal acclimatization: photoperiod-induced rhythms and heat production in Meriones crassus. J Exp Zool. 1990;256(3):237–241.
- Wünnenberg W. Thermosensitivity of the preoptic region and the spinal cord in the golden hamster. J Therm Biol. 1983;8(4):381–384.
- Banin D, Haim A, Arad Z. Metabolism and thermoregulation in the Levant vole Microtus guentheri: the role of photoperiodicity. J Therm Biol. 1994;19(1):55–62.
- Saarela S, Hissa R. Metabolism, thermogenesis and daily rhythm of body temperature in the wood lemming, Myopus schisticolor. J Comp Physiol B. 1993;163(7):546–555.
- Cottle WH. Calorigenic response of cold-adapted rabbits to adrenaline and to noradrenaline. Can J Biochem Physiol. 1963;41:1334–1337.
- Heldmaier G, Steinlechner S, Rafael J. Nonshivering thermogenesis and cold resistance during seasonal acclimatization in the Djungarian hamster. J Comp Physiol. 1982;149(1):1–9.
- Heldmaier G, Jablonka B. Seasonal differences in thermogenic adaptation evoked by daily injections of noradrenaline. J Therm Biol. 1985;10(2):97–99.
- Davis TRA, Johnston DR, Bell FC, et al. Regulation of shivering and non-shivering heat production during acclimation of rats. Am J Physiol. 1960;198(3):471–475.
- Hsieh ACL, Carlson LD, Gray G. Role of the sympathetic nervous system in the control of chemical regulation of heat production. Am J Physiol. 1957;190(2):247–251.
- Cottle WH, Carlson LD. Regulation of heat production in cold-adapted rats. Pro Soc Exp Biol Med. 1956;92(4):845–849.
- Davis TRA, Mayer J. Demonstration and quantitative determination of the contributions of physical and chemical thermogenesis on acute exposure to cold. Am J Physiol. 1955;181(3):675–678.
- Morimoto A, Murakami N, Nakamori T, et al. Suppression of non-shivering thermogenesis in the rat by heat-seeking behaviour during cold exposure. J Physiol. 1986;380(1):541–549.
- Arnold J, LeBlanc J, Cote J, et al. Exercise suppression of thermoregulatory thermogenesis in warm- and cold-acclimated rats. Can J Physiol Pharmacol. 1986;64(7):922–926.
- Banet M, Hensel H. The control of shivering and non-shivering thermogenesis in the rat. J Physiol. 1977;269(3):669–676.
- Vallerand AL, Pérusse F, Bukowiecki LJ. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol. 1990;259(5 Pt 2):R1043–R1049.
- McDonald RB, Horwitz BA, Hamilton JS, et al. Cold- and norepinephrine-induced thermogenesis in younger and older Fischer 344 rats. Am J Physiol. 1988;254(3 Pt 2):R457–R462.
- Nagashima T, Kuroshima A, Yoshida T. The role of beta- and alpha-adrenoceptors on blood flow and temperature of brown adipose tissue and involvement of nitric oxide in their effects. J Therm Biol. 1996;21(5–6):313–318.
- Haim A, Racey PA, Speakman JR, et al. Seasonal acclimatization and thermoregulation in the pouched mouse Saccostomus campestris. J Therm Biol. 1991;16(1):13–17.
- Haim A. Food and energy intake, non-shivering thermogenesis and daily rhythm of body temperature in the bushy-tailed gerbil Sekeetamys calurus: the role of photoperiod manipulations. J Therm Biol. 1996;21(1):37–42.
- Tomasi TE, Hamilton JS, Horwitz BA. Thermogenic capacity in shrews. J Therm Biol. 1987;12(2):143–147.
- Oelkrug R, Polymeropoulos ET, Jastroch M. Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B. 2015;185(6):587–606.
- Nedergaard J, Cannon B. Brown adipose tissue as a heat-producing thermoeffector. Handb Clin Neurol. 2018;156:137–152.
- IUPS Thermal Commission. Glossary of terms for thermal physiology. Jpn J Physiol. 2001;51:245–280.
- Wang LCH. Modulation of maximum thermogenesis by feeding in the white rat. J Appl Physiol. 1980;49(6):975–978.
- Forsum E, Hillman PE, Nesheim MC. Effect of energy restriction on total heat production, basal metabolic rate, and specific dynamic action of food in rats. J Nutr. 1981;111(10):1691–1697.
- Welle S, Lilavivat U, Campbell RG. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism. 1981;30(10):953–958.
- Rothwell NJ, Stock MJ. Acute effects of fat and carbohydrate on metabolic rate in normal, cold-acclimated and lean and obese (fa/fa) Zucker rats. Metabolism. 1983;32(4):371–376.
- Glick Z, Wickler SJ, Stern JS, et al. Regional blood flow in rats after a single low-protein, high-carbohydrate test meal. Am J Physiol. 1984;247(1 Pt 2):R160–R166.
- Maehlum S, Grandmontage M, Newsholme EA, et al. Magnitude and duration of excess postexercise oxygen consumption in healthy young subjects. Metabolism. 1986;35(5):425–429.
- Poehlman ET, Melby CL, Badylak SF. Resting metabolic rate and postprandial thermogenesis in highly trained and untrained males. Am J Clin Nutr. 1988;47(5):793–798.
- Hill JO, Anderson JC, Lin D, et al. Effects of meal frequency on energy utilization in rats. Am J Physiol. 1988;255(4 Pt 2):R616–R621.
- Romom M, Edme JJ, Boulenguez C, et al. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr. 1993;57(4):476–480.
- Maffeis C, Schutz Y, Grezzani A, et al. Meal-induced thermogenesis and obesity: is a fat meal a risk factor for fat gain in children? J Clin Endocrinol Metab. 2001;86(1):214–219.
- Diamond P, Brondel L, LeBlanc J. Palatability and postprandial thermogenesis in dogs. Am J Physiol. 1985;248(1 Pt 1):E75–E79.
- LeBlanc J, Diamond P. Effect of meal size and frequency on postprandial thermogenesis in dogs. Am J Physiol. 1986;250(2 Pt 1):E144–E147.
- Heymsfield SB, Hill JO, Evert M, et al. Energy expenditure during continuous intragastric infusion of fuel. Am J Clin Nutr. 1987;45(3):526–533.
- Diamond P, LeBlanc J. Hormonal control of postprandial thermogenesis in dogs. Am J Physiol. 1987;253(5 Pt 1):E521–E529.
- Allard M, LeBlanc J. Effects of cold acclimation, cold exposure, and palatability on postprandial thermogenesis in rats. Int J Obesity. 1988;12(2):169–178.
- Arnold J, Shipley KA, Scott NA, et al. Thermic effect of parenteral nutrition in septic and nonseptic individuals. Am J Clin Nutr. 1989;50(4):853–860.
- LeBlanc J, Mercier I, Nadeau A. Components of postprandial thermogenesis in relation to meal frequency in humans. Can J Physiol Pharmacol. 1993;71(12):879–883.
- Grande F, Anderson JT, Keys A. Changes of basal metabolic rate in man in semistarvation and refeeding. J Appl Physiol. 1958;12(2):230–238.
- Bray GA. Effect of caloric restriction on energy expenditure in obese patients. Lancet. 1969;7617(7617):397–398.
- Boyle PC, Storlien LH, Keesey RE. Increased efficiency of food utilization following weight loss. Physiol Behav. 1978;21(2):261–264.
- Dauncey MJ. Metabolic effects of altering the 24-h energy intake in man using direct and indirect calorimetry. Br J Nutr. 1980;43(2):257–269.
- Boyle PC, Storlien LH, Harper AE, et al. Oxygen consumption and locomotor activity during restricted feeding and realimentation. Am J Physiol. 1981;241(5):R392–R397.
- Gleeson M, Brown JF, Waring JJ. The effects of physical exercise on metabolic rate and dietary-induced thermogenesis. Br J Nutr. 1982;47(2):173–181.
- Stock MJ, Rothwell NJ. Evidence for diet-induced thermogenesis in hyperphagic cafeteria-fed rats. Proc Nutr Soc. 1982;41(2):133–135.
- Verboeket-van-de-Venne WPHG, Westerterp KR, Hermans-Limpens TJFMB, et al. Long-term effects of consumption of full-fat or reduced-fat products in healthy non-obese volunteers: assessment of energy expenditure and substrate oxidation. Metabolism. 1996;45(8):1004–1010.
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–214.
- Bachman ES, Dhillon H, Zhang CY, et al. Beta-AR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–845.
- Trayhurn P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2017;134:62–70.
- Fuller CA, Sulzman FM, Moore-Ede MC. Role of heat loss and heat production in generation of the circadian temperature rhythm of the squirrel monkey. Physiol Behav. 1985;34(4):543–546.
- Shido O, Sugano Y, Nagasaka T. Circadian change of heat loss in response to change in core temperature in rats. J Therm Biol. 1986;11(4):199–202.
- Refinetti R. Metabolic heat production, heat loss and the circadian rhythm of body temperature in the rat. Exp Physiol. 2003;88(3):423–429.
- Cowgell J, Underwood H. Behavioral thermoregulation in lizards: a circadian rhythm. J Exp Zool. 1979;210(1):189–194.
- Jarling C, Scarperi M, Bleichert A. Circadian rhythm in the temperature preference of the turtle, Chrysemys scripta elegans, in a thermal gradient. J Therm Biol. 1989;14(4):173–178.
- Tosini G, Menaker M. The pineal complex and melatonin affect the expression of the daily rhythm of behavioral thermoregulation in the green iguana. J Comp Physiol A. 1996;179(1):135–142.
- Refinetti R, Susalka SJ. Circadian rhythm of temperature selection in a nocturnal lizard. Physiol Behav. 1997;62(2):331–336.
- Dawson TJ. Primitive mammals and patterns in the evolution of thermoregulation. In: Bligh J, Moore RE, editors. Essays on Temperature Regulation. Amsterdam: North-Holland; 1972. p. 1–18.
- Hillenius WJ, Ruben JA. The evolution of endothermy in terrestrial vertebrates: who? When? Why? Physiol Biochem Zool. 2004;77(6):1019–1042.
- Lovegrove BG. The evolution of endothermy in Cenozoic mammals: a plesiomorphic-apomorphic continuum. Biol Rev. 2012;87:128–162.
- Edgar RS, Green EW, Zhao Y, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485(7399):459–464.
- Avaria-Llautureo J, Hernández CE, Rodríguez-Serrano E, et al. The decoupled nature of basal metabolic rate and body temperature in endotherm evolution. Nature. 2019;572(7771):651–654.
- Marotte H, Timbal J. Circadian rhythm of temperature in man: comparative study with two experiment protocols. Chronobiologia. 1981;8(2):87–100.
- Gander PH, Connell LJ, Graeber RC. Masking of the circadian rhythm of heart rate and core temperature by the rest-activity cycle in man. J Biol Rhythms. 1986;1(2):119–135.
- Golja P, Eiken O, Rodman S, et al. Core temperature circadian rhythm during 35 days of horizontal bed rest. Proc Eur Symp Life Sci Res Space. 2002;8:161–162.
- Monk TH, Buysse DJ, Reynolds CF, et al. Subjective alertness rhythms in elderly people. J Biol Rhythms. 1996;11(3):268–276.
- Carrier J, Monk TH. Estimating the endogenous circadian temperature rhythm without keeping people awake. J Biol Rhythms. 1997;12(3):266–277.
- Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiol Int. 2002;19(6):1151–1169.
- Brown CM, Refinetti R. Daily rhythms of metabolic heat production, body temperature, and locomotor activity in golden hamsters. J Therm Biol. 1996;21(4):227–230.
- Honma K, Hiroshige T. Simultaneous determination of circadian rhythms of locomotor activity and body temperature in the rat. Jpn J Physiol. 1978;28(2):159–169.
- Bolles RC, Duncan PM, Grossen NE, et al. Relationship between activity level and body temperature in the rat. Psychol Rep. 1968;23(3):991–994.
- Refinetti R. Contribution of locomotor activity to the generation of the daily rhythm of body temperature in golden hamsters. Physiol Behav. 1994;56(4):829–831.
- Gordon CJ, Yang Y. Contribution of spontaneous motor activity to the 24 hour control of body temperature in male and female rats. J Therm Biol. 1997;22(1):59–68.
- Refinetti R. Relationship between the daily rhythms of locomotor activity and body temperature in eight mammalian species. Am J Physiol. 1999;277(5):R1493–R1500.
- Hammel HT, Jackson DC, Stolwijk JAJ, et al. Temperature regulation by hypothalamic proportional control with an adjustable set point. J Appl Physiol. 1963;18(6):1146–1154.
- Hardy JD. The “set-point” concept in physiological temperature regulation. In: Yamamoto WS, Brobeck JR, editors. Physiological Controls and Regulations. Philadelphia: Saunders; 1965. p. 98–116.
- Bligh J. The central neurology of mammalian thermoregulation. Neuroscience. 1979;4(9):1213–1236.
- Werner J. The concept of regulation for human body temperature. J Therm Biol. 1980;5(2):75–82.
- Refinetti R. The concept of set point (goal value) in thermal physiology. Manuscrito. 1988;11:47–56.
- Romanovsky AA. Thermoregulation: some concepts have changed. Am J Physiol. 2007;292(1):R37–R46.
- Aschoff J. Circadian rhythm of activity and of body temperature. In: Hardy JD, Gagge AP, Stolwijk JAJ, editors. Physiological and behavioral temperature regulation. Springfield, Ill.: Charles C. Thomas; 1970. p. 905–919.
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71(1):93–128.
- Briese E. Rats prefer ambient temperatures out of phase with their body temperature circadian rhythm. Brain Res. 1985;345(2):389–393.
- Briese E. Circadian body temperature rhythm and behavior of rats in thermoclines. Physiol Behav. 1986;37(6):839–847.
- Gordon CJ. Twenty-four hour rhythms of selected ambient temperature in rat and hamster. Physiol Behav. 1993;53(2):257–263.
- Gordon CJ. 24-Hour control of body temperature in rats. I. Integration of behavioral and autonomic effectors. Am J Physiol. 1994;267(1 Pt 2):R71–R77.
- Ray B, Mallick HN, Kumar VM. Changes in sleep-wakefulness in medial preoptic area lesioned rats: role of thermal preference. Behav Brain Res. 2005;158(1):43–52.
- Gordon CJ, Becker P, Ali JS. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav. 1998;65(2):255–262.
- Refinetti R. Rhythms of temperature selection and body temperature are out of phase in the golden hamster. Behav Neurosci. 1995;109(3):523–527.
- Jefimow M, Wojciechowski M, Tegowska E. Seasonal changes in the thermoregulation of laboratory golden hamsters during acclimation to seminatural outdoor conditions. CompBiochem Physiol A. 2004;139(3):379–388.
- Mojciechowski MS, Jefimow M. Is torpor only an advantage? Effect of thermal environment on torpor use in the Siberian hamsters (Phodopus sungorus).. J Physiol Pharmacol. 2006;57 Suppl 8:83–92.
- Refinetti R. Rhythms of body temperature and temperature selection are out of phase in a diurnal rodent, Octodon degus. Physiol Behav. 1996;60(3):959–961.
- Refinetti R. Body temperature and behavior of tree shrews and flying squirrels in a thermal gradient. Physiol Behav. 1998;63(4):517–520.
- Aujard F, Séguy M, Terrien J, et al. Behavioral thermoregulation in a non human primate: effects of age and photoperiod on temperature selection. Exp Gerontol. 2006;41(8):784–792.
- Terai Y, Asayama M, Ogawa T, et al. Circadian variation of preferred environmental temperature and body temperature. J Therm Biol. 1985;10(3):151–156.
- Pöllmann L. Circadian and circannual variations in the evaluation of thermal comfort in a constant climate. Indoor Environ. 1994;3(3):145–148.
- Kim HE, Tokura H. Effects of time of day on dressing behavior under the influence of ambient temperature fall from 30 °C to 15 °C. Physiol Behav. 1994;55(4):645–650.
- Grivel F, Candas V. Ambient temperatures preferred by young European males and females at rest. Ergonomics. 1991;34(3):365–378.
- Shoemaker JA, Refinetti R. Day-night difference in the preferred ambient temperature of human subjects. Physiol Behav. 1996;59(4–5):1001–1003.
- Refinetti R. Homeostasis and circadian rhythmicity in the control of body temperature. Ann N Y Acad Sci. 1997;813:63–70.
- Briese E. Normal body temperature of rats: the setpoint controversy. Neurosci Biobehav Rev. 1998;22(3):427–436.
- Satinoff E, Liran J, Clapman R. Aberrations of circadian body temperature rhythms in rats with medial preoptic lesions. Am J Physiol. 1982;242(3):R352–R357.
- Briese E. Do medial preoptic lesions interfere with the set-point of temperature regulation? Brain Res Bull. 1989;23(1–2):137–144.
- Osborne AR, Refinetti R. Effects of hypothalamic lesions on the body temperature rhythm of the golden hamster. NeuroReport. 1995;6(16):2187–2192.
- Grigg GC, Beard LA, Augee ML. The evolution of endothermy and its diversity in mammals and birds. Physiol Biochem Zool. 2004;77(6):982–997.
- Liu Y, Merrow M, Loros JJ, et al. How temperature changes reset a circadian oscillator. Science. 1998;281(5378):825–829.
- Brown SA, Zumbrunn G, Fleury-Olela F, et al. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12(18):1574–1583.
- Ruoff P, Rensing L. Temperature effects on circadian clocks. J Therm Biol. 2004;29(7–8):445–456.
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385.
- Morf J, Ray G, Schneider K, et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science. 2012;338(6105):379–383.
- Morf J, Schibler U. Body temperature cycles: gatekeepers of circadian clocks. Cell Cycle. 2013;12(4):539–540.
- Gotic I, Schibler U. Posttranscriptional mechanisms controlling diurnal gene expression cycles by body temperature rhythms. RNA Biol. 2017;14(10):1294–1298.
- Abreu-Vieira G, Xiao C, Gavrilova O, et al. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab. 2015;4(6):461–470.
- Pittendrigh CS. On temperature independence in the clock system controlling emergence time in Drosophila. Proc Natl Acad Sci U S A. 1954;40(10):1018–1029.
- Ruby NF, Burns DE, Heller HC. Circadian rhythms in the suprachiasmatic nucleus are temperature-compensated and phase-shifted by heat pulses in vitro. J Neurosci. 1999;19(19):8630–8636.
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A. 2003;100(26):16089–16094.
- Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organisms. Adv Genet. 2011;74:175–230.
- Cox KH, Takahashi JS. Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol. 2019;63(4):R93–R102.
- Thurley K, Herbst C, Wesener F, et al. Principles for circadian orchestration of metabolic pathways. Proc Natl Acad Sci U S A. 2017;114(7):1572–1577.
- Peek CB, Affinati AH, Ramsey KM, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):591.
- Roenneberg T, Merrow M. Circadian systems and metabolism. J Biol Rhythms. 1999;14(6):449–459.
- Hurley JM, Loros JJ, Dunlap JC. The circadian system as an organizer of metabolism. Fungal Genet Biol. 2016;90:39–43.
- Putker M, Crosby P, Feeney KA, et al. Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxid Redox Signaling. 2018;28(7):507–520.
- Wu B, Wang Y, Wu X, et al. On-orbit sleep problems of astronauts and countermeasures. Mil Med Res. 2018;5(1):17.

