Abstract
Background
Only about 50% of intensive care unit (ICU) patients reach a free trough concentration above MIC (100% fT > MIC) of beta-lactam antibiotics. Although dose adjustments based on therapeutic drug monitoring (TDM) could be beneficial, TDM is not widely available. We investigated serum creatinine-based estimated GFR (eGFR) as a rapid screening tool to identify ICU patients at risk of insufficient exposure.
Method
Ninety-three adult patients admitted to four ICUs in southeast Sweden treated with piperacillin/tazobactam, meropenem, or cefotaxime were included. Beta-lactam trough concentrations were measured. The concentration target was set to 100% fT > MICECOFF (2, 4, and 16 mg/L based on calculated free levels for meropenem, cefotaxime, and piperacillin, respectively). eGFR was primarily determined via Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) and compared to three other eGFR equations. Data was analysed using logistic regression and receiver operative characteristic (ROC) curves.
Results
With intermittent standard dosing, insufficient exposure was common in patients with a relative eGFR ≥48mL/min/1.73m2 [85%, (45/53)], particularly when treated with cefotaxime [96%, (24/25)]. This eGFR cut-off had a sensitivity of 92% and specificity of 82% (AUC 0.871, p < 0.001) in identifying insufficient exposure. In contrast, patients with eGFR <48mL/min/1.73m2 had high target attainment [90%, (36/40)] with a wide variability in drug exposure. There was no difference between the four eGFR equations (AUC 0.866–0.872, cut-offs 44–51 ml/min/1.73m2).
Conclusion
Serum creatinine-based eGFR is a simple and widely available surrogate marker with potential for early identification of ICU patients at risk of insufficient exposure to piperacillin, meropenem, and cefotaxime.
Introduction
About 50% of intensive care unit (ICU) patients suffer from bacterial infections, associated with a 2–3 times increased mortality rate [Citation1,Citation2]. Beta-lactam antibiotics are a cornerstone in empiric therapy of severe bacterial infections [Citation2,Citation3]. Beta-lactam antibiotics have time-dependent efficacy, and the recommended pharmacokinetic/pharmacodynamic (PK/PD) target is to maintain free (unbound) concentrations above the minimum inhibitory concentration (MIC) of the pathogen during the entire dosing interval (100% fT > MIC) [Citation4]. Achieving this target is associated with improved clinical outcomes [Citation5,Citation6].
In many ICUs, patients are given standardised beta-lactam dosing regimens recommended by guidelines relying on data from non-critically ill patients [Citation7–9]. However, insufficient exposure has been reported in about 50% of ICU patients in Sweden and other parts of Europe [Citation10–12]. This is due to use of standardised dosing strategies and divergent pharmacokinetics in ICU patients, which results in very variable drug exposure [Citation4,Citation11–15].
To ascertain target attainment, national guidelines and expert opinions suggest dose adjustments based on therapeutic drug monitoring (TDM), preferably in combination with model-informed precision dosing including Bayesian estimation [Citation4,Citation16]. However, TDM for beta-lactam therapy is performed in approximately 10% of hospitals globally [Citation2], and even if the technical turnaround time is less than an hour, it usually takes longer from sampling to results in clinical practice. Moreover, there is a discussion on the suitable timing for the first sample after treatment initiation where a recent position paper suggests at least 24 h [Citation4]. Thus, results may not be available during the initial phase of treatment when adequate exposure is essential [Citation3,Citation17,Citation18]. Therefore, tools are needed to identify patients who require dose adjustments at an early stage. Renal function estimation may have potential in these situations.
Variations in renal function are a risk factor for insufficient exposure to beta-lactam antibiotics [Citation14,Citation19]. Renal function can be impaired or augmented by critical conditions such as systemic inflammation and/or hypoperfusion. For instance, approximately 50% of patients with severe infections or sepsis suffer acute kidney injury [Citation20–22]. However, the reported prevalence of augmented renal clearance is between 28%–56%, defined as urine creatinine clearance >130mL/min [Citation20,Citation22–24]. Detection of augmented renal clearance with serum markers alone varies between studies [Citation22–24], and is considered clinically unreliable [Citation25].
Glomerular filtration rate (GFR) is commonly estimated with serum creatinine in routine practice. Serum creatinine varies over time for critically ill patients [Citation15], and estimation of GFR based on serum creatinine has been found less accurate in critically ill patients compared to more accurate markers such as iohexol clearance and 24-h creatinine clearance in urine [Citation26]. However, more accurate markers are not rapid, and therefore clinically less applicable in the early, critical stages of ICU care [Citation27,Citation28]. The potential of serum creatinine-based estimated GFR (eGFR) to predict patients with insufficient beta-lactam exposure at admission to the ICU has not been well studied. Early identification of such patients could aid in selection of early optimal dosing regimens and selection of patients for TDM.
Therefore, the aim of this study was to investigate serum creatinine-based eGFR as a rapid and simple screening tool for identifying ICU patients at risk of insufficient exposure, defined as not reaching a target of 100% fT > MIC, when treated with piperacillin/tazobactam (piperacillin), meropenem, or cefotaxime.
Materials and methods
Study design
The patient population has previously been described by Woksepp et al. [Citation10]. In summary, adult patients treated with intravenous (IV) beta-lactam antibiotics were included after admission to four ICUs (Kalmar, Växjö, Linköping, and Jönköping) in southeast Sweden from September 2014 to July 2015. Patients receiving renal replacement therapy during the first 24 h and those not treated with piperacillin/tazobactam (piperacillin), meropenem, or cefotaxime were excluded.
Data collection
Sample collection, drug concentration analyses and data collection have been described in detail previously [Citation10]. Serum creatinine was analysed at the inclusion day according to clinical routine at accredited laboratories in each hospital. Drug concentrations were analysed in blood collected just prior to the next dose of antibiotics after inclusion. The samples were centrifuged 2000 x g for 10 min within one hour of sampling, before being stored at −80 0C and analysed using liquid chromatography-mass spectrometry. The measurement range was 0.2–100 mg/L for piperacillin (PIP) and 0.2–50 mg/L for meropenem (MER) and cefotaxime (CTX). Total beta-lactam therapy duration was recorded. Missing body weight data was imputed if individual weight was recorded the following day, by adjusting the weight using a gaussian-distributed mean weight difference in the population between day 1 and 2.
Dosing regimens were recorded and compared to maximum daily dose information found in the product information and in the European Committee on Antimicrobial Susceptibility testing (EUCAST) rationale documents [Citation8,Citation9]. Dosing adjustments for renally impaired patients were compared to national recommendations from the Swedish strategic program against antibiotic resistance (STRAMA) [Citation7].
eGFR estimation
Individual eGFR was calculated using Chronic Kidney Disease Epidemiology Collaboration (2021) [CKD-EPI] [Citation29], Lund-Malmo revised (2011) [Citation30], 4-item Modification of Diet in Renal Disease [MDRD-4] [Citation31], and Cockcroft-Gault formulae [Citation32]. Comparisons of eGFR equations were performed with regard to relative and absolute eGFR. All further eGFR analyses were performed using CKD-EPI. Body surface area was estimated using Du Bois and Du Bois formula [Citation33].
Target selection
The exposure target was set to 100% fT > MIC, a target recommended for ICU patients in a collaboration between multiple international expert groups specialised in intensive care, infectious disease, and therapeutic drug monitoring [Citation4]. Targets were based on epidemiological cut-offs (ECOFF), as determined by EUCAST, and were chosen as previously described from a ‘worst-case’ scenario (MICECOFF); Pseudomonas aeruginosa piperacillin MIC 16 mg/L and meropenem MIC 2 mg/L, and Staphylococcus aureus cefotaxime MIC 4 mg/L [Citation34]. The free antibiotic concentration was calculated from measured total concentrations using published values for the free fraction (0.7 for piperacillin, 0.98 for meropenem, 0.6 for cefotaxime) [Citation35–37]. Insufficient exposure was defined as not reaching the target of 100% fT > MICECOFF. The percentages of individuals with calculated free trough concentration below and above 100% fT > MICECOFF were recorded.
Ethics
The original study was approved by the Regional Ethical Review Board, Linköping University, Sweden (DNR 2014/236-31) [Citation10]. Analyses in the current study were performed on data collected and pseudonymized in the original study. The objectives of the current study are part of the original ethical approval.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation if normally distributed and as median and interquartile range (IQR) if not normally distributed. Categorical variables are expressed as numbers and percentages.
Differences between groups were analysed with ANOVA or Kruskal–Wallis test for continuous and χ2 test for categorical variables. A p value less than 0.05 was considered to indicate statistical significance. A post hoc analysis was performed for χ2-test using adjusted standardised residuals and corrected for type I error with Bonferroni [Citation38]. Logistic univariate regression (logit model) and receiver operation characteristic (ROC) analysis were performed to investigate eGFR as a tool to detect insufficient exposure. Area under the curve (AUC) of ROC was analysed with DeLong testing and verified with Hanley & McNeil testing. Positive and negative predictive values were calculated, assuming that the study population correctly reflected the prevalence in the population. The χ2, ANOVA, Kruskal–Wallis and logistic regression analyses were performed with Statistica (TIBCO Software, Version 13.5.0.17) and receiver operation characteristic (ROC) curve analysis was performed using MedCalc (MedCalc Software, Version 20.110).
Results
Patient characteristics
A total of 93 individuals were included in the analyses (piperacillin = 40, cefotaxime = 37, meropenem = 16). Individual characteristics are listed in . The median time of treatment prior to inclusion in the ICU was approximately 24 h (range 4–720). The proportion of patients with insufficient exposure differed between the antibiotics (p = 0.0357, piperacillin 48%, meropenem 31%, cefotaxime 68%, post hoc analysis: not significant). Sixty-six percent of individuals had renal impairment, defined as eGFR <60mL/min/1.73m2.
Table 1. Demographic and laboratory data.
Dosages prescribed were 4 g piperacillin q6–12h, 0.5–2 g meropenem q6–12h and 1–2 g cefotaxime q6–24h (Table S1). Ninety-two individuals (99%) received doses equal to or less than the maximum dosing according to the product information or to the EUCAST rationale documents () [Citation8,Citation9].
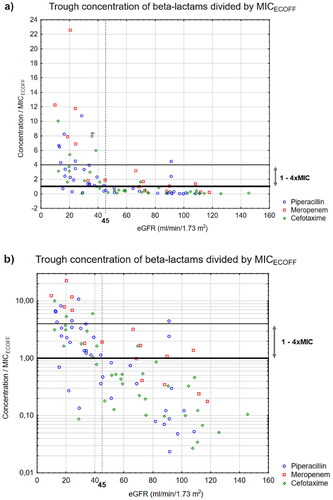
Figure 1. Calculated free beta-lactam trough concentration divided by MICECOFF for piperacillin (○), meropenem (□) and cefotaxime (◊) versus relative eGFR* (mL/min/1.73 m2) presented with (a) a normal and (b) a logarithmic y-axis.MIC: Minimum inhibitory concentration, eGFR: estimated glomerular filtration rate, ECOFF: Epidemiological cut-offs. Lower line equals 1xMIC, higher line equals 4 × MIC.*Calculated with Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).
eGFR as a predictor of insufficient exposure
Logistic regression showed a significant relationship between insufficient exposure and increasing eGFR (p < 0.001). ROC analysis showed that eGFR predicted insufficient exposure with a sensitivity of 92%, a specificity of 82% and an AUC of 0.87 (CI95 0.79–0.93, p < 0.0001) at the cut-off ≥48 mL/min/1.73m2 (). The positive predictive value was 85% and the negative predictive value was 90% (). ROC analysis for target attainment gave inverted results for the cut-off <48mL/min/1.73m2 (sensitivity 82%, specificity 92%, AUC: 0.87 (CI95 = 0.79–0.93), positive predictive value 90%, negative predictive value 85%).
Figure 2. ROC analysis with CI95% of insufficient exposure to piperacillin, meropenem and cefotaxime based on (a): relative eGFR* (mL/min/1.73m2) and (b): absolute eGFR* (mL/min). ROC: Receiver Operation Characteristic, CI95: 95% Confidence Interval, eGFR: Estimated glomerular filtration rate, PIP: Piperacillin/Tazobactam, MER: Meropenem, CTX: Cefotaxime, AUC: Area Under the Curve.*Calculated with Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).
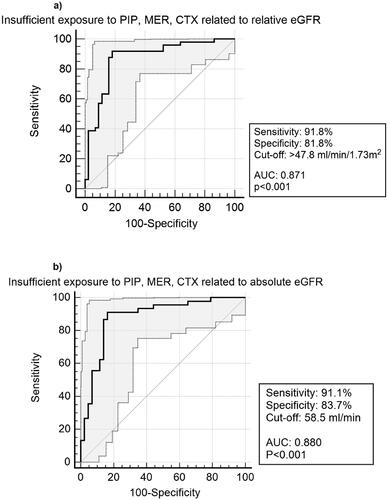
Table 2. Predictive power of insufficient exposure based on eGFR with different cut-off valuesTable Footnote*.
Calculated free concentration and target attainment
Calculated free trough concentration [median, (IQR)] for piperacillin, meropenem, and cefotaxime was 18.2 mg/L (50.9), 1.86 mg/L (6.11) and 3.48 mg/L (13.3), respectively. Forty-five individuals (85%) with an eGFR above 48 mL/min/1.73m2 did not reach target (piperacillin 16, 89%, meropenem 5, 50% and cefotaxime 24, 96%), , Table S2). Thirty-six individuals with eGFR below 48 mL/min/1.73m2 (90%) reached target (piperacillin 19, 86%, meropenem 6, 100% and cefotaxime 11, 92%), (, Table S2) and trough concentration ranged from 2–172 mg/L (piperacillin), 4–45 mg/L (meropenem), and 0.4–40 mg/L (cefotaxime).
Comparisons of absolute eGFR and eGFR equations
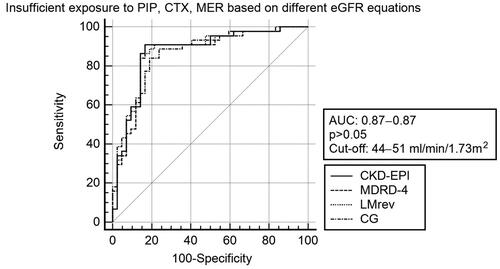
Applying absolute eGFR, the cut-off predicting insufficient exposure was 58 mL/min, with similar sensitivity (91%), specificity (84%), and AUC (0.88) as the relative eGFR (). There were no differences in sensitivity, specificity, or AUC when eGFR was calculated based on CKD-EPI, MDRD-4, Lund-Malmo (revised) and Cockcroft-Gault (p > 0.05). The number of patients predicted to be insufficiently exposed were 53 (57%), 48 (52%), 51 (55%), and 50 (57%) for CKD-EPI, Lund-Malmo (revised), MDRD-4, and Cockcroft-Gault, respectively (Table S4). With relative eGFR, AUCs ranged between 0.866–0.872 with cut-offs between 44–51 mL/min/1.73m2 ( and Figure S3). Using absolute eGFR, AUCs ranged between 0.866–0.885 with cut-offs between 54–59 mL/min. Further comparisons are found in Supplementary Appendix.
Figure 3. ROC-curves for prediction of insufficient exposure* with different relative eGFR equations**. eGFR: Estimated glomerular filtration rate, ROC: Receiver Operation Characteristics, AUC: Area Under the Curve, CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration, MDRD-4: Modification of Diet in Renal Disease – 4 item, LMrev: Lund-Malmo revised, CG: Cockcroft-Gault. *Trough concentration ≤ MICECOFF (Epidemiological cut-offs of minimum inhibitory concentrations) between dose intervals. **mL/min/1.73m2.
Dosing according to renal function
Eighty-three of 93 individuals received dosing regimens according to national guidelines [Citation7,Citation9], and nine of the remaining ten received higher dosages than recommended (Table S1). Three of ten individuals with severe renal impairment had reduced dosages as recommended by guidelines (Table S1) [Citation7].
Discussion
Our results suggest that eGFR in clinical routine care might rapidly identify patients at risk for insufficient exposure to beta-lactam antibiotics. With a cut-off at 48 mL/min/1.73m2 (relative eGFR) or 58 mL/min (absolute eGFR), insufficient exposure and target attainment were predicted with a sensitivity >90% and a specificity >80%.
The results show a high rate of insufficient exposure, for cefotaxime and piperacillin at eGFR as low as 48 mL/min/1.73m2. Cut-offs for predicting augmented renal clearance have been estimated between 108 and 119mL/min/1.73m2 [Citation22,Citation23], suggesting that augmented renal clearance alone is not the explanation. This implies that high initial dosing should be considered in patients with mild to moderate renal impairment. At eGFR <48mL/min/1.73m2, there was high target attainment, although with wide variability in trough concentrations (). The ability of eGFR to identify insufficient exposure to beta-lactam antibiotics in ICU patients was the same, regardless of the eGFR estimation method used (). Furthermore, the predictive ability was similar when using absolute eGFR and a higher cut-off, which was expected since the mean body surface area of 1.93 m2 exceeded 1.73 m2 (, ).
In one of the few studies investigating eGFR as a single predictor of insufficient exposure to beta-lactams in ICU patients [Citation39], Imani et al. reported a lower ability of eGFR as a predictor (AUC 0.76, sensitivity 77%, specificity 65%, p < 0.001) with a higher cut-off of 72 mL/min/1.73m2 than in the present study, using similar inclusion and exclusion criteria. Possible explanations for the differing results could be that Imani et al. used measured MIC in 37% of the cases, which increases the probability of target attainment. Furthermore, target attainment is affected by dosage as well as use of prolonged infusion, neither reported by Imani et al. Prolonged infusion increases the probability of target attainment eight-fold [Citation40]. In the present study, the daily dose never exceeded 1.5 times the maximum dose of the product information or EUCAST rationale documents (Table S1). Although information about the type of infusion was not recorded in our study, intermittent injections, or short infusions up to 30 min were clinical routine at the time. This underlines the importance to compare study settings with local clinical settings, such as target selection or dosing, and to validate results before implementation.
The differences in the rates of insufficient exposure between beta-lactams found in the present study have also been reported in another study from Sweden with a similar population and target selection [Citation11], where a higher rate of insufficient exposure was found for cefotaxime (58%) compared to meropenem (30%). A difference between beta-lactam antibiotics is expected, since dosage, and risk factors for insufficient exposure vary between beta-lactam antibiotics with currently used dosing recommendations [Citation8,Citation34,Citation40]. In the present study, individuals with an eGFR above 48 mL/min/1.73m2 had the highest rate of insufficient exposure when treated with cefotaxime (24/25), with a majority receiving 1 g q8h (Table S1). Since the time of data collection, EUCAST changed the recommendations for cefotaxime to 2 g q8h as all susceptible S. aureus are now reported as ‘I’ (susceptible at increased exposure). Intermittent dosing of 2 g q8h may, however, still not be enough. A cefotaxime population PK/PD model predicted that only 60% of patients with eGFR >50mL/min treated with 2 g q8h against S. aureus infection reach 100% fT > MICECOFF [Citation41]. Using the cut-off of >48mL/min/1.73m2, in our study, none of the 4 patients with a dose of 2 g q8h reached target (Table S1). This finding must, however, be interpreted with caution due to the very small sample size.
Our finding that patients with eGFR <48mL/min/1.73m2 reached the target is in accordance with previous studies [Citation11,Citation42], and similar findings were observed with other beta-lactam antibiotics [Citation43]. We also found that even if low exposures were rare in patients with eGFR <48mL/min/1.73m2, high trough concentrations were observed in some patients, suggesting that individual beta-lactam levels cannot be precisely predicted with eGFR alone. This is of concern, since observational studies report a possible association between high trough concentrations and adverse events such as acute kidney failure and encephalopathy [Citation44–47]. To avoid overexposure, many institutional guidelines recommend dosage reductions in patients with renal impairment [Citation7,Citation9]. There is an ongoing discussion as to whether dosages should be reduced during the first 48 h of treatment [Citation48]. Camargo et al. found an association between dosage reduction and mortality in ICU patients, even after adjusting for co-factors such as disease state and antibiotic class [Citation49]. In our study, in only three of ten individuals dosage was reduced in accordance with national guidelines,(Table S1) indicating that physicians may avoid dose reductions in critically ill patients.
Our study has several limitations. Although steady-states were expected to be reached for beta-lactam antibiotics, supported by stable concentrations on three consecutive days [Citation10], some individuals may not have reached steady state. Also, the predictive ability of eGFR in a pre-ICU setting was not investigated, since blood samples for beta-lactam concentrations and creatinine were drawn at the same time at study inclusion.
Serum creatinine was the only marker available for estimating eGFR, with the risk of underestimating GFR in ICU patients [Citation26]. This could partly explain that some patients had sufficient concentrations with eGFR above 48 ml/min/1.73 m2. The predictive power may increase further if creatinine and cystatin-C combined estimation of eGFR is used, or if more accurate markers such as iohexol- or urine creatinine clearance are applied.
As with any real-world study on critically ill patients, a high heterogeneity among individuals should be expected, especially among individuals using different beta-lactams. Only piperacillin, cefotaxime, and meropenem were analysed in this study, and results cannot be directly transferred to other beta-lactam antibiotics. Additionally, this study focused on eGFR estimated from serum creatinine. Cut-offs or predictability may differ when other estimation methods are used, such as cystatin C-based eGFR, creatinine clearance using urine sampling, or iohexol clearance. The free fraction was based on calculations of measured total concentration and was used in the analysis to compare with MICECOFF. Protein binding capacity may vary between individuals, and analysing unbound concentrations could improve the accuracy in determining sufficient exposure. In a sensitivity analysis using a protein binding of 0 or 50%, there was a modest effect on the eGFR cut off for insufficient exposure (data not shown).
Although eGFR may have potential as a rapid screening tool to indicate patients at risk of inadequate beta-lactam exposures, it needs to be combined with TDM and model informed precision dosing to confirm target achievement and to individually adjust dosing regimens.
Conclusion
Serum creatinine-based eGFR has potential to simply and rapidly predict inadequate exposure to piperacillin, meropenem, and cefotaxime in ICU patients with the intermittent standard dosing as described by EUCAST. A creatinine-based eGFR of 48 mL/min/1.73m2 or 58 mL/min may be valid cut-offs to predict insufficient exposure in this patient population.
Supplemental Material
Download MS Word (246.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754.
- Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection Among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi: 10.1001/jama.2020.2717.
- Evans L, Rhodes A, Alhazzani W, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974–1982. doi: 10.1097/CCM.0000000000005357.
- Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46(6):1127–1153. doi: 10.1007/s00134-020-06050-1.
- McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–351. doi: 10.1016/j.ijantimicag.2007.12.009.
- Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. doi: 10.1093/cid/ciu027.
- Swedish Strategic Programme against antibiotic resistance (STRAMA) [Internet]. STRAMA Nationell – Behandlingsrekommendationer i app [Database application]. STRAMA Nationell; 2022; [updated 2021 Sep 21]. Available from: https://strama-nationell.infosynk.se/.
- Rationale documents. 2022; [cited 2022 Jan 01]. Available from: https://www.eucast.org/publications-and-documents/rd.
- FASS. https://www.fass.se/LIF/startpage. Läkemedelsindustriföreningen (LiF); 2023; FASS – Summary of Product Characteristics.
- Woksepp H, Hällgren A, Borgström S, et al. High target attainment for beta-lactam antibiotics in intensive care unit patients when actual minimum inhibitory concentrations are applied. Eur J Clin Microbiol Infect Dis. 2017;36(3):553–563. doi: 10.1007/s10096-016-2832-4.
- Smekal AK, Furebring M, Eliasson E, et al. Low attainment to PK/PD-targets for beta-lactams in a multi-center study on the first 72 h of treatment in ICU patients. Sci Rep. 2022;12(1):21891. doi: 10.1038/s41598-022-25967-9.
- Zander J, Döbbeler G, Nagel D, et al. Piperacillin concentration in relation to therapeutic range in critically ill patients–a prospective observational study. Crit Care. 2016;20(1):79. doi: 10.1186/s13054-016-1255-z.
- Sime FB, Roberts MS, Peake SL, et al. Does beta-lactam pharmacokinetic variability in critically ill patients justify therapeutic drug monitoring? A systematic review. Ann Intensive Care. 2012;2(1):35. doi: 10.1186/2110-5820-2-35.
- Boidin C, Moshiri P, Dahyot-Fizelier C, et al. Pharmacokinetic variability of beta-lactams in critically ill patients: a narrative review. Anaesth Crit Care Pain Med. 2020;39(1):87–109. doi: 10.1016/j.accpm.2019.07.016.
- De Gaetano AC,G, Gram Pedersen M, Panunzi S, et al. Modeling serum creatinine in septic ICU patients. Cardiovasc Eng. 2004;4(2):173–180. doi: 10.1023/B:CARE.0000031546.79563.bd.
- Guilhaumou R, Benaboud S, Bennis Y, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French society of pharmacology and therapeutics (societe francaise de pharmacologie et Therapeutique-SFPT) and the French society of anaesthesia and intensive care medicine (Societe Francaise d‘Anesthesie et Reanimation-SFAR). Crit Care. 2019;23(1):104. doi: 10.1186/s13054-019-2378-9.
- Kollef MH, Shorr AF, Bassetti M, et al. Timing of antibiotic therapy in the ICU. Crit Care. 2021;25(1):360. doi: 10.1186/s13054-021-03787-z.
- Nauclér P, Huttner A, van Werkhoven CH, et al. Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: implications for antimicrobial stewardship. Clin Microbiol Infect. 2021;27(2):175–181. doi: 10.1016/j.cmi.2020.02.032.
- Abdulla A, Ewoldt TMJ, Purmer IM, et al. A narrative review of predictors for beta-lactam antibiotic exposure during empirical treatment in critically ill patients. Expert Opin Drug Metab Toxicol. 2021;17(4):359–368. doi: 10.1080/17425255.2021.1879049.
- Udy AA, Roberts JA, Shorr AF, et al. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013;17(1):R35. doi: 10.1186/cc12544.
- Peerapornratana S, Manrique-Caballero CL, Gomez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099. doi: 10.1016/j.kint.2019.05.026.
- Wu CC, Tai CH, Liao WY, et al. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: a prospective observational study. Infect Drug Resist. 2019;12:2531–2541. doi: 10.2147/IDR.S213183.
- Ruiz S, Minville V, Asehnoune K, et al. Screening of patients with augmented renal clearance in ICU: taking into account the CKD-EPI equation, the age, and the cause of admission. Ann Intensive Care. 2015;5(1):49. doi: 10.1186/s13613-015-0090-8.
- Campassi ML, Gonzalez MC, Masevicius FD, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment]. Rev Bras Ter Intensiva. 2014;26(1):13–20. doi: 10.5935/0103-507x.20140003.
- Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39(3):346–354. doi: 10.1002/phar.2231.
- Sangla F, Marti PE, Verissimo T, et al. Measured and estimated glomerular filtration rate in the ICU: a prospective study. Crit Care Med. 2020;48(12):e1232–e41. doi: 10.1097/CCM.0000000000004650.
- Trevisani F, Di Marco F, Capitanio U, et al. Renal function assessment gap in clinical practice: an awkward truth. Kidney Blood Press Res. 2020;45(2):166–179. doi: 10.1159/000504649.
- Bragadottir G, Redfors B, Ricksten SE. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury–true GFR versus urinary creatinine clearance and estimating equations. Crit Care. 2013;17(3):R108. doi: 10.1186/cc12777.
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953.
- Björk J, Grubb A, Sterner G, et al. Revised equations for estimating glomerular filtration rate based on the Lund-Malmo study cohort. Scand J Clin Lab Invest. 2011;71(3):232–239. doi: 10.3109/00365513.2011.557086.
- Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311.
- Clinical breakpoints. 2022; [cited 2022 Jan 1]. Available from: https://www.eucast.org/clinical_breakpoints.
- Wong G, Briscoe S, Adnan S, et al. Protein binding of beta-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother. 2013;57(12):6165–6170. doi: 10.1128/AAC.00951-13.
- Hayashi Y, Roberts JA, Paterson DL, et al. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin Drug Metab Toxicol. 2010;6(8):1017–1031. doi: 10.1517/17425255.2010.506187.
- Patel BM, Paratz J, See NC, et al. Therapeutic drug monitoring of beta-lactam antibiotics in burns patients – a one-year prospective study. Ther Drug Monit. 2012;34(2):160–164. doi: 10.1097/FTD.0b013e31824981a6.
- MacDonald PL, Gardner RC. Type I error rate comparisons of post hoc procedures for I x J Chi-Square tables. Educ Psychol Meas. 2000;60(5):735–754. doi: 10.1177/00131640021970871.
- Imani S, Buscher H, Day R, et al. An evaluation of risk factors to predict target concentration non-attainment in critically ill patients prior to empiric beta-lactam therapy. Eur J Clin Microbiol Infect Dis. 2018;37(11):2171–2175. doi: 10.1007/s10096-018-3357-9.
- Alobaid AS, Brinkmann A, Frey OR, et al. What is the effect of obesity on piperacillin and meropenem trough concentrations in critically ill patients? J Antimicrob Chemother. 2016;71(3):696–702. doi: 10.1093/jac/dkv412.
- Roelofsen EE, Abdulla A, Muller AE, et al. Dose optimization of cefotaxime as pre-emptive treatment in critically ill adult patients: a population pharmacokinetic study. Br J Clin Pharmacol. 2023;89(2):705–713. doi: 10.1111/bcp.15487.
- Petersson J, Giske CG, Eliasson E. Standard dosing of piperacillin and meropenem fail to achieve adequate plasma concentrations in ICU patients. Acta Anaesthesiol Scand. 2016;60(10):1425–1436. doi: 10.1111/aas.12808.
- Campbell PO, Chin PKL, Dalton SC, et al. Frequency of pharmacological target attainment with flucloxacillin and cefazolin in invasive methicillin-susceptible Staphylococcus aureus infection: a prospective cohort study in hospitalized patients. Int J Antimicrob Agents. 2023;61(1):106695. doi: 10.1016/j.ijantimicag.2022.106695.
- Beumier M, Casu GS, Hites M, et al. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81(5):497–506.
- Imani S, Buscher H, Marriott D, et al. Too much of a good thing: a retrospective study of beta-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72(10):2891–2897. doi: 10.1093/jac/dkx209.
- Vardakas KZ, Kalimeris GD, Triarides NA, et al. An update on adverse drug reactions related to beta-lactam antibiotics. Expert Opin Drug Saf. 2018;17(5):499–508. doi: 10.1080/14740338.2018.1462334.
- Venugopalan V, Casaus D, Kainz L, et al. Use of therapeutic drug monitoring to characterize cefepime-related neurotoxicity. Pharmacotherapy. 2023;43(1):6–14. doi: 10.1002/phar.2744.
- de Vroom SL, van Daalen FV, Zieck SE, et al. Does dose reduction of renally cleared antibiotics in patients with impaired renal function lead to adequate drug exposure? A systematic review. Clin Microbiol Infect. 2021;27(3):352–363. doi: 10.1016/j.cmi.2020.11.032.
- Camargo MS, Mistro S, Oliveira MG, et al. Association between increased mortality rate and antibiotic dose adjustment in intensive care unit patients with renal impairment. Eur J Clin Pharmacol. 2019;75(1):119–126. doi: 10.1007/s00228-018-2565-7.

