Abstract
Background
Stenotrophomonas maltophilia (S. maltophilia) is a nosocomial pathogen causing life-threatening invasive infections with a high mortality rate in some patient populations, especially those who are severely ill or immunocompromised. There is a need for data on mortality in patients with S. maltophilia bacteremia.
Objective
In this meta-analysis, we aimed to investigate risk factors for mortality in S. maltophilia bacteremia.
Methods
Studies comparing patients who died from S. maltophilia bacteremia with patients who survived were considered for inclusion. Studies were included if they reported one or more risk factors for mortality. Mortality risk factors included clinical predisposing factors, predisposing comorbidities and appropriateness of antibiotic therapy.
Results
Nineteen studies with 1248 patients were included in the meta-analysis. Five hundred and six (40.5%) patients died. The following risk factors for mortality were identified: ICU admission, septic shock, need for mechanical ventilation, indwelling central venous catheter, neutropenia, comorbid hematological malignancies, chronic kidney disease, inappropriate antimicrobial therapy and prior antibiotic use.
Conclusions
Appropriate antimicrobial therapy had a protective effect against mortality in S. maltophilia bacteremia. Indwelling central venous catheter, neutropenia, hematological malignancies and chronic kidney disease were also risk factors for mortality.
Introduction
Stenotrophomonas maltophilia (S. maltophilia) is a gram-negative bacterium closely related to Pseudomonas species but does not ferment sugars. After being identified in 1943 as Bacterium bookeri, S. maltophilia was classified as the genus Pseudomonas in 1961. In 1983, it was placed in the genus Xanthomonas, and in 1993 it was permanently assigned to the genus Stenotrophomonas [Citation1]. The bacteria easily attach to and survive on a variety of abiotic surfaces, including catheters, endoscopes, sink drains and ventilator circuits, and are widespread in the environment [Citation2]. S. maltophilia produces a biofilm which protects against damage induced by drying, impedes immune cell activities and prevents penetration of antimicrobials in vivo [Citation3–5].
S. maltophilia has been identified as a nosocomial pathogen causing life-threatening invasive infections with a high mortality rate in some patient populations, especially those who are severely ill or immunocompromised [Citation6–8]. The most frequent infections are pneumonia and bacteremia. Because S. maltophilia may colonize respiratory epithelial cells and surfaces of invasive medical devices, distinguishing between infection and colonization is difficult. Thus, appropriate antibiotic therapy can be delayed, resulting in high mortality [Citation9].
Numerous intrinsic and acquired resistance mechanisms make infections difficult to treat. S. maltophilia has significant levels of inherent resistance to a range of different antibiotics, such as tetracyclines, quinolones, aminoglycosides and beta-lactams [Citation10,Citation11]. Thus, choosing the optimal antibiotic to treat S. maltophilia bacteremia is challenging. The empirical therapy of choice for culture-proven infections and for suspected infections is trimethoprim–sulfamethoxazole (TMP–SMX) [Citation12,Citation13]. However, alternative antibiotics are required due to the rising prevalence of TMP–SMX resistance and to the common side effects [Citation14–16]. Fluoroquinolones and TMP–SMX were compared in three retrospective cohort studies and all three found no difference in efficacy between the two treatments [Citation17–19]. Fluoroquinolones also have numerous side effects, such as tendinopathy, gastrointestinal intolerance, aberrant cardiac conduction and risk of Clostridioides difficile infection [Citation20,Citation21]. However, increased resistance rates in S. maltophilia have been linked to widespread use of quinolones [Citation22]. Wei et al. found that out of 80 clinical sequentially isolated strains, 93.8% were susceptible to TMP–SMX, 95.0% to minocycline, 83.8% to tigecycline, 76.3% to levofloxacin and 20% to ceftazidime. Twenty-two TMP–SMX-resistant strains were susceptible to both minocycline and tigecycline. However, TMP–SMX-resistant bacteria were highly resistant to the other antimicrobials. The author concluded that minocycline and TMP–SMX should be first-line therapies for S. maltophilia infections, whereas tigecycline and levofloxacin should be second-line choices. The activity of ceftazidime against S. maltophilia is low [Citation23].
Mortality rates are high in S. maltophilia infections, ranging from 21% to 69%, according to a systematic review [Citation24]. Studies have shown that long-term hospitalization necessitating invasive procedures, prior use of broad-spectrum antibiotics, need for mechanical ventilation and hematologic malignancies are risk factors for infection by S. maltophilia [Citation25,Citation26] as well as septic shock, need for mechanical ventilation, admission to an intensive care unit, and hospital stay more than 30 days [Citation27–31]. However, limitations of these studies include small sample sizes and incomplete mortality risk indicators. There is no published systematic evaluation of risk factors for mortality. The purpose of this meta-analysis was to analyze risk factors for mortality in patients with S. maltophilia bacteremia.
Methods
Data search strategy
The PubMed, Web of Science and Cochrane Library databases were used to find all relevant clinical trials, meta-analyses and systematic reviews published from 1 January 2000 to 31 March 2023. The phrases searched include ‘Stenotrophomonas maltophilia bacteremia OR Stenotrophomonas maltophilia infection’ in connection with ‘mortality’, ‘attributable mortality’, ‘risk factor’, ‘outcome’ or ‘prognostic factors’. Treatment trials directly compare mortality risk variables between deceased and surviving patients. To find additional reports that might have been overlooked in the initial search, published systematic reviews and meta-analyses were examined. We also searched the reference lists of retrieved publications. Only articles in English were included.
Study selection and data extraction
In order to determine the eligibility of retrieved trials, two authors independently checked and evaluated each article for eligibility. After eliminating duplicates, two researchers analyzed the titles and abstracts to identify eligible reports. After eliminating irrelevant studies, full texts of all relevant reports were read. Information on authors, year of publication, country, study design, study years, population categories, number of deceased and surviving patients, clinical predisposing factors, predisposing comorbidities and appropriateness of antibiotic therapy were extracted from the full-text articles. When disagreements arose, a third author resolved them.
Inclusion and exclusion criteria
In the current meta-analysis, we considered randomized controlled trials (RCTs), and prospective and retrospective observational studies. Only studies that explicitly compared the mortality risk variables between patients who died and those who survived were included. The four populations of patients in the literature were the general population, patients with hematological malignancy, cystic fibrosis patients and pediatric patients. Patients with malignancies of the hematopoietic and lymphoid tissues have worse clinical outcomes than non-hematological patients, and a fulminant illness known as fatal hemorrhagic pneumonia may ensue [Citation32]. Patients with cystic fibrosis are frequently colonized with S. maltophilia. Infection rates with S. maltophilia are closely linked to disease progression and loss of lung function [Citation33]. Hematological malignancy patients and cystic fibrosis patients with S. maltophilia bacteremia have clinical characteristics and outcomes that differ from those in the general population. Therefore, the current meta-analysis excluded studies focused solely on S. maltophilia bacteremia in patients with hematological malignancy and cystic fibrosis, and pediatric patients.
Definitions
S. maltophilia bacteremia was defined by culture of S. maltophilia from one or more blood samples, together with clinical signs of the systemic inflammatory response syndrome. A regimen with one or more antimicrobial agents to which the isolate was susceptible in vitro was regarded as appropriate antimicrobial therapy, whereas a regimen to which the isolate was not susceptible was regarded as inappropriate. Neutropenia was defined as an absolute neutrophil count less than 500/mm3. Polymicrobial bacteremia was defined as isolation of at least two different microorganisms from the same blood sample.
Variables assessed in analysis of risk factors
Studies were included if they reported one or more risk factors for mortality. Mortality risk factors included clinical predisposing factors, predisposing comorbidities, and appropriateness of antibiotic therapy. Clinical predisposing factors included sex, age ≥65 years, ICU admission, need of mechanical ventilation, septic shock, indwelling central venous catheter, prior chemotherapy, prior steroid therapy, neutropenia and polymicrobial bacteremia. Predisposing comorbidities included hematological malignancies, non-hematological malignancies, liver disease, chronic kidney disease (including end-stage renal disease), cardiovascular disease (excluding hypertension), pulmonary disease and diabetes mellitus. Therapeutic indicators included inappropriate antimicrobial therapy, receiving TMP–SMX, fluoroquinolones or minocycline, and prior antibiotic therapy.
Quality assessment
The Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool was used to evaluate observational studies [Citation34]. We conducted a sensitivity analysis by systematically removing each study and assessed the impact of the study quality on the effect estimates. The quality of the evidence was ranked based on the risk of bias according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach at the outcome level [Citation35,Citation36].
Statistical analysis
RevMan 5 and Cochrane Review Manager software were used for statistical analyses. Fixed-effects and random-effects were utilized for data analysis. Statistical heterogeneity was evaluated using the Q-test and I2 statistical techniques. Significant heterogeneity between studies was defined as an I2 above 50% and a p value for the Q-test less than 0.10 for each study. We tabulated the study intervention features and compared them to the scheduled groups for each synthesis using forest plots. The funnel plot was examined to determine the degree of publication bias.
Results
Characteristics of included trials
The details of the study selection process are shown in . There were 371, 410 and 53 studies in the initial search results from PubMed, Web of Science and the Cochrane Library, respectively. There were 252 duplicate articles. A total of 468 irrelevant studies were identified by reading the title and/or abstract. Thus, 114 potentially relevant articles remained. After full-text review, 95 articles were excluded due to lack of comparison of mortality risk factors between survivors and non-survivors. Finally, 19 studies were included in the meta-analysis [Citation17,Citation28–31,Citation37–50]. One study was prospective and 18 were retrospective. The main characteristics of the 19 included studies are shown in . All studies had a high risk of bias (). There were 1248 patients with S. maltophilia bacteremia, of which 506 (40.5%) died.
Figure 1. Flow diagram of the study selection process. *Exclude due to lack of comparison of survivors and non-survivors.
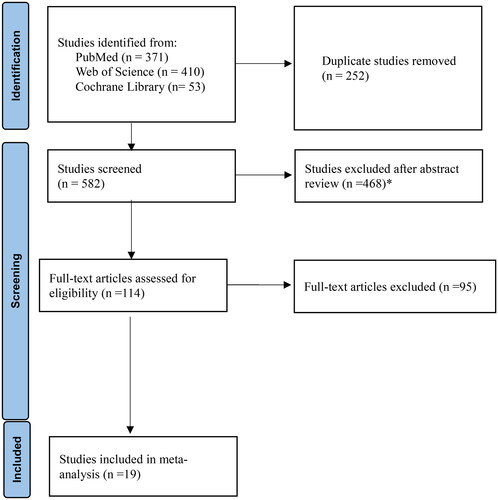
Table 1. Characteristics of the included 19 studies.
Table 2. Risk bias of the included 19 studies.
Clinical predisposing factors
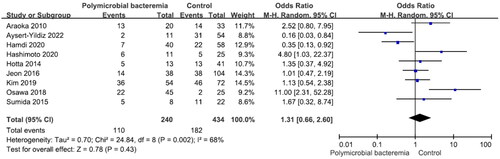
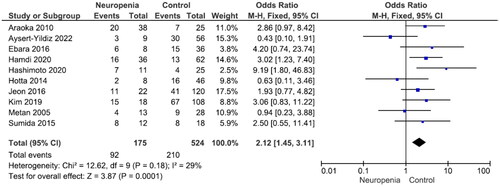
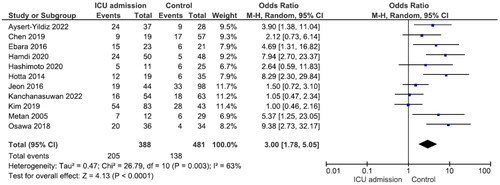
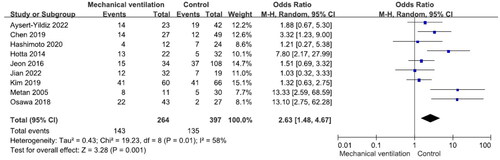
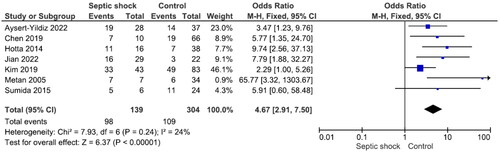
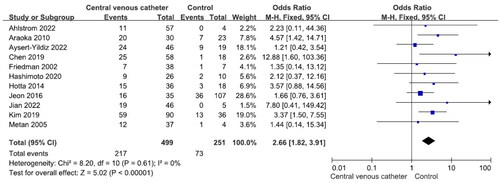
There were no significant differences in mortality between males and females and between patients aged ≥65 years and those aged <65 years (Figures S1 and S2). Nine studies reported 240 patients with polymicrobial bacteremia (mortality rate 45.8%) and 434 without (mortality rate 41.9%). There was no significant difference in mortality between the two groups (OR = 1.31, p = .43, I2 = 68%) (). Ten studies reported 175 patients with neutropenia (mortality rate 52.6%) and 524 without (mortality rate 40.1%). The difference was significant (OR = 2.12, p < .001, I2 = 29%) (). Eleven studies reported 388 patients with ICU admission (mortality rate 52.8%) and 481 without (mortality rate 28.7%). This difference was significant (OR = 3.00, p < .001, I2 = 63%) (). Nine studies reported 264 patients on mechanical ventilation (mortality rate 54.2%) and 397 without (mortality rate 34.0%). This difference was significant (OR = 2.63, p = .001, I2 = 58%) (). Seven studies reported 139 patients with septic shock (mortality rate 70.5%) and 304 without (mortality rate 35.90%). The difference was significant (OR = 4.67, p < .001, I2 = 24%) (). Eleven studies reported 499 patients with indwelling central venous catheter (mortality rate 43.52%) and 251 without (mortality rate 29.1%), which was a significant difference (OR = 2.66, p < .001, I2 = 0%) (). There was no significant difference in mortality between patients receiving and not receiving steroid therapy or between patients receiving and not receiving chemotherapy (Figures S3 and S4).
Predisposing comorbidities
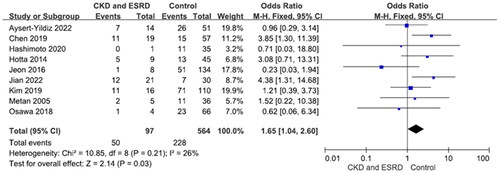
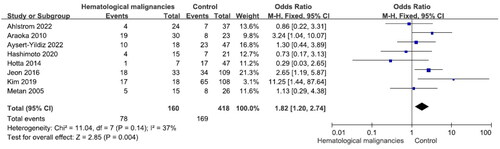
Nine studies reported 97 patients with chronic kidney disease (mortality rate 51.5%) and 564 without (mortality rate 40.4%). The difference was significant (OR = 1.65, p = .03, I2 = 26%) (). Eight studies reported 160 patients with hematological malignancy (mortality rate 48.8%) and 418 patients without (mortality rate 40.4%). The difference was significant (OR = 1.82, p = .004, I2 = 37%) (). There were no significant differences in S. maltophilia bacteremia-related mortality between patients with and without cardiovascular, pulmonary or liver diseases, diabetes mellitus and non-hematological malignancies (Figures S5–S9).
Appropriateness of antibiotic therapy
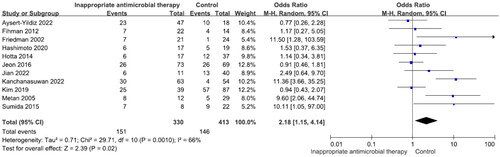
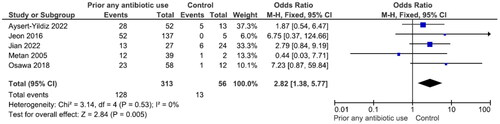
Eleven studies reported 330 patients with inappropriate antibiotic therapy (mortality rate 45.8%) and 413 with appropriate therapy (mortality rate 35.4%). The difference was significant (OR = 2.18, p = .02, I2 = 66%) (). There were no significant differences in mortality between patients receiving TMP–SMX, fluoroquinolones or minocycline (Figures S10–S12). Five studies reported 313 patients with prior antibiotic use (mortality rate 40.9%) and 56 without (mortality rate 23.2%). The difference was significant (OR = 2.82, p = .005, I2 = 0%) ().
Discussion
The current meta-analysis of 19 studies provides evidence that the risk factors for mortality in S maltophilia bacteremia are ICU admission, septic shock, need for mechanical ventilation, indwelling central venous catheter, neutropenia, hematological malignancies, chronic kidney disease (including ESRD), inappropriate antimicrobial therapy and prior antibiotic use.
Clinical factors predisposing to mortality
There was no significant difference in mortality between males and females. Thirteen studies showed that sex was not a risk factor for mortality in S. maltophilia bacteremia [Citation28–31,Citation37–40,Citation44–48].
In the study by Aysert-Yildiz et al., the median age of deceased patients was 65 years (56–77), and of survivors 57 years (52–70) (p = .001). The author concluded that advanced age was an independent risk factor for mortality [Citation39]. In the current meta-analysis, four studies showed that old age was not a risk factor for mortality [Citation28,Citation30,Citation45,Citation46].
Polymicrobial infections were common in patients with S. maltophilia bacteremia. Other nonfermenting gram-negative bacteria (Pseudomonas aeruginosa, Acinetobacter spp. and Burkholderia cepacia complex) or Staphylococcus aureus are frequent co-pathogens [Citation51,Citation52]. The current meta-analysis shows that Acinetobacter baumannii, Candida spp., Pseudomonas aeruginosa and Klebsiella pneumoniae are common pathogens in order of common occurrence. Mortality was not higher in patients with polymicrobial bacteremia than in those with solely S. maltophilia [Citation29,Citation30,Citation38,Citation45,Citation49] which can be attributed to the following three factors. First, S. maltophilia bacteremia was largely polymicrobial, the concomitant, more virulent pathogens may incur a higher mortality risk. Second, polymicrobial bacteremia may have developed as a result of the first empirical broad-spectrum antibiotic therapy that was successful in treating concomitant microorganisms [Citation53,Citation54]. Third, patients with S. maltophilia bacteremia were more likely to die from their illness than those with polymicrobial bloodstream infections, as demonstrated in the study by Hamdi et al. Nonetheless, it is possible that other host features are responsible for the disparity in death rates in patients with monomicrobial infections [Citation43].
Neutropenia and mortality were not significantly correlated in the study by Paez et al. on patients with S. maltophilia infection [Citation55]. However, patients with S. maltophilia bacteremia and neutropenia at the time of bacteremia had a high risk of mortality in two studies [Citation54,Citation56]. In multivariate analysis, neutropenia was an independent predictor of unfavorable prognosis [Citation38]. Six other studies showed that patients with neutropenia had a poor prognosis in the current meta-analysis [Citation30,Citation40,Citation43–45,Citation49]. Thus, we concluded that neutropenia is a mortality risk factor in S. maltophilia bacteremia.
Septic shock is a potentially fatal medical condition characterized by organ injury as a result of infection with severe circulatory, cellular and metabolic abnormalities and is associated with a high risk of mortality. In numerous studies on S. maltophilia bacteremia, septic shock was an independent risk factor for mortality [Citation51,Citation55,Citation56]. Seven studies in the current meta-analysis showed that septic shock is a risk factor for mortality in S. maltophilia bacteremia [Citation28–30,Citation39,Citation46,Citation48,Citation49].
Few studies have investigated the use of steroids. Steroid treatment did not increase the risk of mortality in patients with S. maltophilia infection in the study by Paez et al. [Citation55]. The risk of mortality may be affected by the use of corticosteroids, which inhibit cell-mediated immunity. In our meta-analysis, steroid usage was not a risk factor for mortality. However, only 62 patients received steroid treatment.
In the current meta-analysis, chemotherapy was not a risk factor for mortality in patients with S. maltophilia bacteremia. Only 112 patients received chemotherapy; therefore, the result should be interpreted with care.
Garazi et al. found that patients with S. maltophilia bacteremia admitted to an ICU had a high risk of mortality [Citation56]. According to a systematic analysis by Paez et al., admission to an ICU was an independent risk factor for mortality in infections caused by S. maltophilia [Citation24]. Del-Toro et al. found that, in univariate analysis, need for mechanical ventilation and ICU stay were associated with mortality in S. maltophilia infection [Citation57]. In our meta-analysis, ICU admission and need for mechanical ventilation were associated with mortality. This emphasizes how crucial it is to account for serious underlying risk factors when evaluating prognosis in these patients.
When a central venous catheter is in place, S. maltophilia may adhere to the catheter and form a biofilm, which may increase the risk of bacteremia. Central venous catheters are associated with increased risk of bacteremia and mortality [Citation52,Citation58,Citation59]. Eleven studies in the current meta-analysis showed that an indwelling central venous catheter increases the mortality risk [Citation28–30,Citation37–39,Citation42,Citation44–46,Citation48]. It may be beneficial to remove the central venous catheter in patients with S. maltophilia bacteremia [Citation30,Citation38,Citation42,Citation45,Citation60,Citation61].
Comorbidities predisposing for mortality
Few studies have explored predisposing comorbidities of S. maltophilia bacteremia related to mortality risk. Ebara et al. found that a high Charlson Comorbidity Index was associated with mortality [Citation40]. According to Paez and Costa, underlying hematological malignancies were independent predictors of mortality [Citation24]. In univariate analysis, Hotta et al. found that continuous renal replacement therapy was associated with 30-day mortality in patients with S. maltophilia bacteremia. Solid tumors, hematological malignancies, diabetes mellitus, cardiac disease, liver disease, autoimmune disease and lung disease were not associated with 30-day mortality [Citation29]. Multivariate analysis did not detect any significant association, while univariate analysis showed that only hematologic malignancy was significantly associated to mortality. Solid tumors, diabetes mellitus, cardiovascular disease, chronic liver disease, chronic kidney disease and chronic lung disease, were not associated with 28-day mortality [Citation45]. Kim et al. found that hematological malignancy was an independent risk factor for mortality in patients with S. maltophilia bacteremia. Solid tumors, diabetes mellitus, cardiovascular disease, chronic kidney disease, end-stage renal disease and pulmonary disease were not associated with mortality [Citation30]. In the current meta-analysis, hematological malignancies and chronic kidney disease (including ESRD) were associated with mortality in patients with S. maltophilia bacteremia.
Appropriateness of antibiotic therapy
Many infectious diseases, especially bacteremia, require early and effective antibiotic treatment. However, there is contradictory information about appropriate antibacterial therapy for S. maltophilia bacteremia. Several studies found no difference in mortality between patients who received appropriate therapy and those who did not [Citation28–30,Citation38,Citation40,Citation41,Citation44–46,Citation51,Citation52,Citation55,Citation56]. In other studies, appropriate antibacterial therapy was significantly associated with lower mortality [Citation42,Citation48,Citation49,Citation54,Citation62]. Therefore, medical experts have suggested that further research be conducted to evaluate the efficacy of appropriate antibacterial treatment. There are theoretical explanations of the contradictory results. Survival may be directly influenced by underlying medical conditions. There was heterogeneity in the definition of appropriate antimicrobial therapy and no consensus on the optimal time between obtaining blood cultures and initiation of appropriate empiric therapy. There is no consensus on the most effective antimicrobial therapy for S. maltophilia bacteremia, and different antimicrobial regimens significantly impact survival. Antimicrobial susceptibility patterns differed between patients and countries, which should impact on survival. In the current meta-analysis, 11 studies reported appropriate antimicrobial therapy. Only three found that appropriate antibacterial therapy was significantly associated with higher mortality [Citation42,Citation48,Citation49]. Eight studies found that appropriate antimicrobial therapy was associated with lower mortality [Citation29,Citation41,Citation42,Citation44,Citation46–49]. There was a trend toward a lower mortality risk in patients receiving appropriate antimicrobial therapy. In this meta-analysis, patients with inappropriate antimicrobial therapy had higher mortality (RR = 2.18, p = .02).
Few studies have assessed the relationship between prior antibiotic use and mortality. There was no difference between patients previously treated with vancomycin and carbapenem and those who were not [Citation38]. Early effective antimicrobial treatment and different antimicrobial regimens, such as TMP–SMX, fluoroquinolones and tigecycline, did not significantly affect survival [Citation39]. In the study by Garazi et al., patients treated with carbapenem during the previous 30 days were at risk of mortality [Citation56]. Patients with S. maltophilia bacteremia often received prior antibiotic therapy, indicating underlying medical conditions and the infection was mostly nosocomial. The current meta-analysis with a large number of patients provides a more reliable assessment of the relationship between mortality and prior antibiotic use.
Our meta-analysis evaluated TMP–SMX, fluoroquinolones and minocycline. There were no significant differences in mortality. The factors affecting outcome of antibiotic therapy were underlying medical conditions, appropriateness of antimicrobial therapy; and antimicrobial susceptibility patterns of S. maltophilia. We could not further analyze the impact of these factors on the risk of mortality. There is no ‘standard of care’ antibiotic regimen for S. maltophilia infections. Rigorous trials comparing the efficacy of the most commonly used antibiotics for S. maltophilia are lacking. There is still a lack of information on which antibiotics are most effective and how well-tolerated combination regimens are. Based on recommendations from the Infectious Diseases Society of America, Treatment recommendations: the following regimens are advised: mild infections can be treated with TMP–SMX, minocycline, tigecycline, levofloxacin or cefiderocol monotherapy; among these, the IDSA preferably recommends TMP–SMX and minocycline. Regardless of severity, ceftazidime is not recommended since it is likely to be ineffective due to the intrinsic production of Beta-lactamases by S. maltophilia. Any one of the following three methods is advised for moderate to severe infections: (1) the preferred combination treatment is TMP–SMX and minocycline; (2) if clinical improvement is delayed on TMP–SMX monotherapy, a second drug (minocycline (recommended), tigecycline, levofloxacin or cefiderocol) might be added; (3) ceftazidime/avibactam and aztreonam in combination when other agents are expected to be inactive or intolerable [Citation63].
Limitations
We included the findings of 19 studies. One study was prospective and 18 were retrospective. All of the included studies had a high risk of bias which was a limitation of this meta-analysis. The level of comorbidity was determined with the Charlton Comorbidity Index. A high value indicates that several concurrent conditions may influence the outcome of S. maltophilia bacteremia. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was used to determine disease severity. A score higher than 15 points is an independent risk factor for mortality in patients with S. maltophilia infection [Citation57]. The Sequential Organ Failure Assessment Score (SOFA) measures how well an organ is functioning or how quickly it is failing, and can be used to predict outcomes in critically ill patients. Paez et al. showed that 14-day mortality in patients with S. maltophilia infection correlated with SOFA score >6 as an independent risk factor [Citation55]. Information on the Charlson Comorbidity Index, APACHE II and SOFA scores was not available, which is a limitation of our study. No thorough investigation of antibiotic susceptibility, prior hospitalizations or detailed antibiotic therapy history was conducted. These variables are associated with development of antibiotic resistance and prognosis of S. maltophilia bacteremia. The information is important, thus, this is also a limitation of our study.
Conclusions
Appropriate antimicrobial therapy had a protective effect against mortality in S. maltophilia bacteremia. Patients requiring ICU admission, in septic shock, and needing mechanical ventilation had a high risk of mortality. Indwelling central venous catheter, neutropenia, hematological malignancies and chronic kidney disease were also risk factors for mortality.
Author contributions
Chienhsiu Huang: conceptualization, investigation, methodology, validation, visualization, writing-original draft, supervision, writing-review and editing. Sufang Kuo: formal analysis, project administration and data curation. Lichen Lin: data curation, resources and methodology.
Ethical approval
This is an article of meta-analysis and no need to apply for IRB.
Consent form
Not applicable.
Supplemental Material
Download MS Word (701.9 KB)Acknowledgements
The meta-analysis was registered at the Prospero international prospective register of systematic reviews (registration: CRD42023447938).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998;11(1):57–80. doi: 10.1128/CMR.11.1.57.
- Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25(1):2–41. doi: 10.1128/CMR.00019-11.
- Adegoke AA, Stenström TA, Okoh AI. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: looking beyond contemporary antibiotic therapy. Front Microbiol. 2017;8:2276. doi: 10.3389/fmicb.2017.02276.
- Carmody LA, Spilker T, LiPuma JJ. Reassessment of Stenotrophomonas maltophilia phenotype. J Clin Microbiol. 2011;49(3):1101–1103. doi: 10.1128/JCM.02204-10.
- Cervia JS, Ortolano GA, Canonica FP. Hospital tap water as a source of Stenotrophomonas maltophilia infection. Clin Infect Dis. 2008;46(9):1485–1487. doi: 10.1086/587180.
- Falagas ME, Kastoris AC, Vouloumanou EK, et al. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol. 2009;4(9):1103–1109. doi: 10.2217/fmb.09.84.
- Micozzi A, Venditti M, Monaco M, et al. Bacteremia due to Stenotrophomonas maltophilia in patients with hematologic malignancies. Clin Infect Dis. 2000;31(3):705–711. doi: 10.1086/314043.
- Labarca JA, Leber AL, Kern VL, et al. Outbreak of Stenotrophomonas maltophilia bacteremia in allogenic bone marrow transplant patients: role of severe neutropenia and mucositis. Clin Infect Dis. 2000;30(1):195–197. doi: 10.1086/313591.
- Insuwanno W, Kiratisin P, Jitmuang A. Stenotrophomonas maltophilia infections: clinical characteristics and factors associated with mortality of hospitalized patients. Infect Drug Resist. 2020;13:1559–1566. doi: 10.2147/IDR.S253949.
- Zhang L, Li XZ, Poole K. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob Agents Chemother. 2000;44(2):287–293. doi: 10.1128/AAC.44.2.287-293.2000.
- Alonso A, Martínez JL. Multiple antibiotic resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1997;44(11):3079–3086. doi: 10.1128/AAC.41.5.1140.
- Nicodemo AC, Paez JI. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur J Clin Microbiol Infect Dis. 2007;26(4):229–237. doi: 10.1007/s10096-007-0279-3.
- Chung HS, Hong SG, Kim YR, et al. Antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from Korea, and the activity of antimicrobial combinations against the isolates. J Korean Med Sci. 2013;28(1):62–66. doi: 10.3346/jkms.2013.28.1.62.
- Hu LF, Xu XH, Li HR, et al. Surveillance of antimicrobial susceptibility patterns among Stenotrophomonas maltophilia isolated in China during the 10-year period of 2005–2014. J Chemother. 2018;30(1):25–30. doi: 10.1080/1120009X.2017.1378834.
- Lawson DH, Paice BJ. Adverse reactions to trimethoprim–sulfamethoxazole. Rev Infect Dis. 1982;4(2):429–433. doi: 10.1093/clinids/4.2.429.
- Ho JM, Juurlink DN. Considerations when prescribing trimethoprim–sulfamethoxazole. CMAJ. 2011;183(16):1851–1858. doi: 10.1503/cmaj.111152.
- Cho SY, Kang CI, Kim J, et al. Can levofloxacin be a useful alternative to trimethoprim–sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother. 2014;58(1):581–583. doi: 10.1128/AAC.01682-13.
- Bonfiglio G, Cascone C, Azzarelli C, et al. Levofloxacin in vitro activity and time-kill evaluation of Stenotrophomonas maltophilia clinical isolates. J Antimicrob Chemother. 2000;45(1):115–117. doi: 10.1093/jac/45.1.115.
- Wang YL, Scipione MR, Dubrovskaya Y, et al. Monotherapy with fluoroquinolone or trimethoprim–sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother. 2014;58(1):176–182. doi: 10.1128/AAC.01324-13.
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49(4):593–596. doi: 10.1093/jac/49.4.593.
- Tandan M, Cormican M, Vellinga A. Adverse events of fluoroquinolones vs. other antimicrobials prescribed in primary care: a systematic review and meta-analysis of randomized controlled trials. Int J Antimicrob Agents. 2018;52(5):529–540. doi: 10.1016/j.ijantimicag.2018.04.014.
- Elting LS, Khardori N, Bodey GP, et al. Nosocomial infection caused by Xanthomonas maltophilia: a case-control study of predisposing factors. Infect Control Hosp Epidemiol. 1990;11(3):134–138. doi: 10.1086/646136.
- Wei C, Ni W, Cai X, et al. Evaluation of trimethoprim/sulfamethoxazole (SXT), minocycline, tigecycline, moxifloxacin, and ceftazidime alone and in combinations for SXT-susceptible and SXT-resistant Stenotrophomonas maltophilia by in vitro time-kill experiments. PLOS One. 2016;11(3):e0152132. doi: 10.1371/journal.pone.0152132.
- Paez JG, Costa S. Risk factors associated with mortality of infections caused by Stenotrophomonas maltophilia: a systematic review. J Hosp Infect. 2008;70(2):101–108. doi: 10.1016/j.jhin.2008.05.020.
- Apisarnthanarak A, Mayfield JL, Garison T, et al. Risk factors for Stenotrophomonas maltophilia bacteremia in oncology patients: a case-control study. Infect Control Hosp Epidemiol. 2003;24(4):269–274. doi: 10.1086/502197.
- Bao H, Qiao Y, Liu D, et al. The clinical impact of Stenotrophomonas maltophilia bacteremia on the 30-day mortality rate in patients with hematologic disorders: a single-institution experience. Infection. 2020;48(2):205–212. doi: 10.1007/s15010-019-01369-4.
- Tokatly Latzer I, Nahum E, Cavari Y, et al. Treatment outcomes of Stenotrophomonas maltophilia bacteremia in critically ill children: a multicenter experience. Pediatr Crit Care Med. 2019;20(5):e231–e239. doi: 10.1097/PCC.0000000000001919.
- Chen Y, Suo J, Du M, et al. Clinical features, outcomes, and risk factors of bloodstream infections due to Stenotrophomonas maltophilia in a tertiary-care hospital of China: a retrospective analysis. Biomed Res Int. 2019;2019:4931501–4931507. doi: 10.1155/2019/4931501.
- Hotta G, Matsumura Y, Kato K, et al. Risk factors and outcomes of Stenotrophomonas maltophilia bacteremia: a comparison with bacteremia caused by Pseudomonas aeruginosa and Acinetobacter species. PLOS One. 2014;9(11):e112208. doi: 10.1371/journal.pone.0112208.
- Kim EJ, Kim YC, Ahn JY, et al. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia and clinical impact of quinolone-resistant strains. BMC Infect Dis. 2019;19(1):754. doi: 10.1186/s12879-019-4394-4.
- Osawa K, Shigemura K, Kitagawa K, et al. Risk factors for death from Stenotrophomonas maltophilia bacteremia. J Infect Chemother. 2018;24(8):632–636. doi: 10.1016/j.jiac.2018.03.011.
- Kim SH, Cha MK, Kang CI, et al. Pathogenic significance of hemorrhagic pneumonia in hematologic malignancy patients with Stenotrophomonas maltophilia bacteremia: clinical and microbiological analysis. Eur J Clin Microbiol Infect Dis. 2019;38(2):285–295. doi: 10.1007/s10096-018-3425-1.
- Terlizzi V, Tomaselli M, Giacomini G, et al. Stenotrophomonas maltophilia in people with cystic fibrosis: a systematic review of prevalence, risk factors and management. Eur J Clin Microbiol Infect Dis. 2023;42(11):1285–1296. doi: 10.1007/s10096-023-04648-z.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919.
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. doi: 10.1186/1472-6963-4-38.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD.
- Ahlström MG, Knudsen JD, Hertz FB. Stenotrophomonas maltophilia bacteraemia: 61 cases in a tertiary hospital in Denmark. Infect Dis. 2022;54(1):26–35. doi: 10.1080/23744235.2021.
- Araoka H, Baba M, Yoneyama A. Risk factors for mortality among patients with Stenotrophomonas maltophilia bacteremia in Tokyo, Japan, 1996–2009. Eur J Clin Microbiol Infect Dis. 2010;29(5):605–608. doi: 10.1007/s10096-010-0882-6.
- Aysert-Yildiz P, Yildiz Y, Habibi H, et al. Stenotrophomonas maltophilia bacteremia: from diagnosis to treatment. Infect Dis Clin Microbiol. 2022;4(4):258–267. doi: 10.36519/idcm.2022.187.
- Ebara H, Hagiya H, Haruki Y, et al. Clinical characteristics of Stenotrophomonas maltophilia bacteremia: a regional report and a review of a Japanese case series. Intern Med. 2017;56(2):137–142. doi: 10.2169/internalmedicine.56.6141.
- Fihman V, Le Monnier A, Corvec S, et al. Stenotrophomonas maltophilia – the most worrisome threat among unusual non-fermentative gram-negative bacilli from hospitalized patients: a prospective multicenter study. J Infect. 2012;64(4):391–398. doi: 10.1016/j.jinf.2012.01.001.
- Friedman ND, Korman TM, Fairley CK, et al. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect. 2002;45(1):47–53. doi: 10.1053/jinf.2002.0978.
- Hamdi AM, Fida M, Abu Saleh OM, et al. Stenotrophomonas bacteremia antibiotic susceptibility and prognostic determinants: Mayo Clinic 10-year experience. Open Forum Infect Dis. 2020;7(1):ofaa008. doi: 10.1093/ofid/ofaa008.
- Hashimoto T, Komiya K, Fujita N, et al. Risk factors for 30-day mortality among patients with Stenotrophomonas maltophilia bacteraemia. Infect Dis. 2020;52(6):440–442. doi: 10.1080/23744235.2020.1734653.
- Jeon YD, Jeong WY, Kim MH, et al. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. Medicine. 2016;95(31):e4375. doi: 10.1097/MD.0000000000004375.
- Jian J, Xie Z, Chen L. Risk factors for mortality in hospitalized patients with Stenotrophomonas maltophilia bacteremia. Infect Drug Resist. 2022;15:3881–3886. doi: 10.2147/IDR.S371129.
- Kanchanasuwan S, Rongmuang J, Siripaitoon P, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. J Clin Med. 2022;11(11):3085. doi: 10.3390/jcm11113085.
- Metan G, Uzun O. Impact of initial antimicrobial therapy in patients with bloodstream infections caused by Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2005;49(9):3980–3981. doi: 10.1128/AAC.49.9.3980-3981.2005.
- Sumida K, Chong Y, Miyake N, et al. Risk factors associated with Stenotrophomonas maltophilia bacteremia: a matched case-control study. PLOS One. 2015;10(7):e0133731. doi: 10.1371/journal.pone.0133731.
- Watson L, Esterly J, Jensen AO, et al. Sulfamethoxazole/trimethoprim versus fluoroquinolones for the treatment of Stenotrophomonas maltophilia bloodstream infections. J Glob Antimicrob Resist. 2018;12:104–106. doi: 10.1016/j.jgar.2017.09.015.
- Lai CH, Chi CY, Chen HP, et al. Clinical characteristics and prognostic factors of patients with Stenotrophomonas maltophilia bacteremia. J Microbiol Immunol Infect. 2004;37:350–358.
- Muder RR, Harris AP, Muller S, et al. Bacteremia due to Stenotrophomonas (Xanthomonas) maltophilia: a prospective, multicenter study of 91 episodes. Clin Infect Dis. 1996;22(3):508–512. doi: 10.1093/clinids/22.3.508.
- Muder RR, Yu VL, Dummer JS, et al. Infections caused by Pseudomonas maltophilia. Expanding clinical spectrum. Arch Intern Med. 1987;147(9):1672–1674.
- Senol E, DesJardin J, Stark PC, et al. Attributable mortality of Stenotrophomonas maltophilia bacteraemia. Clin Infect Dis. 2002;34(12):1653–1656. doi: 10.1086/340707.
- Paez JI, Tengan FM, Barone AA, et al. Factors associated with mortality in patients with bloodstream infection and pneumonia due to Stenotrophomonas maltophilia. Eur J Clin Microbiol Infect Dis. 2008;27(10):901–906. doi: 10.1007/s10096-008-0518-2.
- Garazi M, Singer C, Tai J, et al. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect. 2012;81(2):114–118. doi: 10.1016/j.jhin.2012.02.008.
- Del-Toro MD, Rodríguez-Baño J, Martínez-Martínez L, et al. Características epidemiológicas, clínicas y pronósticas de la infecciónpor Stenotrophomonas maltophilia [Epidemiology, clinical features and prognosis of infections due to Stenotrophomonas maltophilia]. Enferm Infecc Microbiol Clin. 2006;24(1):4–9. doi: 10.1157/13083367.
- Krcmery V Jr., Pichna P, Oravcova E, et al. Stenotrophomonas maltophilia bacteraemia in cancer patients: report on 31 cases. J Hosp Infect. 1996;34(1):75–77. doi: 10.1016/s0195-6701(96)90129-2.
- Victor MA, Arpi M, Bruun B, et al. Xanthomonas maltophilia bacteremia in immunocompromised hematological patients. Scand J Infect Dis. 1994;26(2):163–170. doi: 10.3109/00365549409011780.
- Lai CH, Wong WW, Chin C, et al. Central venous catheter-related Stenotrophomonas maltophilia bacteraemia and associated relapsing bacteraemia in haematology and oncology patients. Clin Microbiol Infect. 2006;12(10):986–991. doi: 10.1111/j.1469-0691.2006.01511.x.
- Boktour M, Hanna H, Ansari S, et al. Central venous catheter and Stenotrophomonas maltophilia bacteremia in cancer patients. Cancer. 2006;106(9):1967–1973. doi: 10.1002/cncr.21846.
- Tunger O, Vural S, Cetin CB, et al. Clinical aspects and risk factors of nosocomial Stenotrophomonas maltophilia bacteremia episodes in a Turkish intensive care unit. J Chemother. 2007;19(6):658–664. doi: 10.1179/joc.2007.19.6.658.
- Tamma PD, Aitken SL, Bonomo RA, et al. Infectious diseases society of America guidance on the treatment of AmpC β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74(12):2089–2114. doi: 10.1093/cid/ciab1013.