Abstract
Background
The aim of this study was to characterise long-term neurological and neurocognitive sequelae after tick-borne encephalitis (TBE) in adults.
Methods
98 prospective consecutive TBE patients, classified by disease severity, were included. Immediate outcomes were evaluated with Glasgow Outcome Scale (GOS) and Rankin Scale (RS). After 6 and 18 months, long-term disability was evaluated using Modified Rankin Scale (MRS) and neurocognitive assessment was performed with Matrics Consensus Cognitive Battery (MCCB), measuring processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning/problem solving and social cognition. The MCCB results were compared to healthy age, gender and education-matched controls.
Results
Mild, moderate, and severe TBE was diagnosed in 53.1%, 38.8%, and 8.2% of cases, respectively. At discharge, 25.5% of the patients had major or moderate impairments (GOS) and various levels of disability in 34.7% (RS). Up to 18 months from the onset of TBE, over 20% remained with slight to moderate disability (MRS). GOS, RS and MRS scores correlated with disease severity. At 6 months after the onset, TBE patients scored significantly lower than controls in processing speed, verbal, and visual learning. Two latter domains were significantly more impaired in patients with mild TBE. Patients aged 18-39 performed significantly worse in attention/vigilance and working memory, whereas aged 60+ in verbal learning. A year later, significant improvement was observed in six of seven cognitive domains.
Conclusions
Long-term neurological sequelae persist in a substantial proportion of TBE patients with significant impairment in several cognitive domains, especially in younger patients and even after mild TBE.
Introduction
Tick-borne encephalitis (TBE) is one of the most important viral vector-borne central nervous system (CNS) infections in Europe, with 3734 cases reported in the EU/EEA in 2020 (0.9/100,000). The leading countries are Lithuania (24.3/100,000), Slovenia (8.9/100,000), Czechia (7.9/100,000) and Latvia (7.8/100,000) [Citation1]. Despite tick bites being the most common route of transmission, alimentary route through unpasteurised milk products also remains relevant. Even though a high proportion of infections are suspected to be asymptomatic [Citation2,Citation3], TBE can manifest as meningitis, meningoencephalitis, meningoencephalomyelitis or myeloradiculitis and can affect spinal and cranial nerves [Citation4–6]. Meningoencephalitis is the most frequent form, observed in around 44–64% of cases [Citation5,Citation7,Citation8]. Studies on long-term sequelae have been performed since late 1990s clearly showing long-term impact of TBE [Citation4–6]. It is known that patients with TBE develop not only objective neurological signs but also subjective symptoms and neurocognitive impairment [Citation7]. Previous prospective research indicates that neurological and neuropsychological sequelae persist in 40–46% of the patients one year after the disease [Citation4,Citation5]. Although several studies aimed to evaluate neurocognitive consequences of TBE in more detail, data is based on low sensitivity cognitive screening instruments and patients’ self-report rather than comprehensive neuropsychological assessment [Citation5,Citation7,Citation9]. To date the full spectrum of neurocognitive impairment after TBE and its dynamics over time using thorough neurocognitive assessment tools remained unexamined.
The aim of the present study was to characterise the long-term neurological and neurocognitive sequelae after TBE in adults by employing established neuropsychological tests in addition to previously used outcome measurements.
Materials and methods
Tick-borne encephalitis patients
All consecutive TBE patients ≥18 years of age admitted to the Department of Infectious Diseases of Lithuanian University of Health Sciences between June 1st, 2018 and May 31st, 2019 were included. The inclusion criteria were: clinical signs of CNS infection, CSF cell count ≥5/mm3, and positive TBEV IgM and IgG in serum using ELISA (VIROTECH FSME/TBE, Virotech Diagnostics GmbH, Germany). Vaccinated TBEV IgG positive patients with CSF inflammation were excluded if intrathecal TBEV IgM antibody production was not detected. Patients with suspected Lyme neuroborreliosis coinfection were tested for Borrelia burgdorferi antibodies in serum (Vidas LYM, bioMérieux, France) and CSF (IDEIA Lyme Neuroborreliosis, Oxoid Ltd., Ireland). Exclusion criteria were contraindications for lumbar puncture or patients declining participation. Patients were evaluated by three investigators (VG, JP, AM) and classified into three severity groups using pre-defined criteria. Mild disease was classified as producing primarily meningeal symptoms (fever, headache, neck stiffness, nausea and vomiting), moderate – manifesting with monofocal encephalitic symptoms (ataxia, dysphasia, tremor, single cranial nerve paralysis and/or moderate diffuse brain dysfunction (Glasgow Coma Scale (GCS) score 14–10)), and severe – presenting with multifocal encephalitic symptoms and/or GCS score ≤9. Spinal nerve damage was defined as a separate clinical entity, independent from the presence and severity of encephalitic symptoms [Citation5]. The residual impairments at discharge were graded according to Glasgow Outcome Scale (GOS) and Rankin Scale (RS) (Supplementary Tables 1 and 2). The patients were re-examined by the same investigators after 6 and 18 months after discharge. The long-term disability was graded according to the Modified Rankin Scale (MRS) (Supplementary Table 3) and in parallel, a wide spectrum of objective neurological signs and subjective symptoms were registered (Supplementary Table 4). At follow-ups, TBE patients were grouped by age, gender and education. Seven different age groups by decades and three groups by education level were created ().
Table 1. Distribution of demographics and clinical features between patients and healthy controls.
Table 2. Neurological signs and subjective symptoms during the acute phase, discharge and both follow-ups.
Outcome measurements using Matrics Consensus Cognitive Battery
During both follow-up visits, neurocognitive functions were evaluated by a licenced psychologist using Matrics Consensus Cognitive Battery (MCCB) [Citation10,Citation11], measuring seven different cognitive domains: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning/problem solving and social cognition. MCCB is based on ten neuropsychological tests: Trail Making Test, Brief Assessment of Cognition in Schizophrenia, The Hopkins Verbal Learning Test-Revised, Wechsler Memory Scale-III: Spatial Span, Letter-Number Span, Neuropsychological Assessment Battery®: Mazes, Brief Visuospatial Memory Test-Revised, Category Fluency: Animal Naming, Mayers-Salovey-Caruso Emotional Intelligence Test: Managing Emotions and Continuous Performance Test – Identical Pairs. An Overall Composite Score, which included all cognitive domains was also analysed. The evaluation of each patient took one to one-and-a-half hours.
Control subjects
For each TBE group at follow-ups, sets of matched healthy volunteers without history of neuroinfection or other relevant past/present health problems, as determined by detailed verbal interview, were tested using MCCB on a one-to-one ratio (). The majority of the controls were hospital and university personnel, mainly consisting of technical and supporting staff without specific medical education. 77 subjects were tested at a single time point.
Ethics approval
The study was carried out according to the code of ethics of the World Medical Association (declaration of Helsinki) for experiments including humans [Citation12] and was approved by Kaunas Regional Bioethics Committee (protocol number BE-2-45).
Consent to participate
All participants gave written consent to use their data for scientific purposes.
Statistical methods
IBM SPSS Statistics 22 and RStudio were used for statistical calculations. P < 0.05 was considered statistically significant. The normality of variables was evaluated using Shapiro–Wilk criterion for continuous variables. Comparison between groups was performed using Student’s t-test, ANOVA for normally distributed variables and nonparametric Mann–Whitney U and Kruskal–Wallis tests for non-normally distributed variables. To measure the effect size for dependent variables Cohen’s d was used. Correlations were determined using Spearman’s criteria for non-normally distributed variables. Post-hoc analysis was performed using Tukey’s test in ANOVA and Bonferroni method to adjust for multiple testing for nonparametric tests.
Results
Demographics and epidemiology
222 patients underwent a lumbar puncture for suspected neuroinfection, 177 displayed inflammatory changes in the CSF and were tested for TBE. In five vaccinated patients, breakthrough TBE was excluded by a negative intrathecal antibody test. 106 patients were confirmed as TBE cases, eight of them were excluded due to declination to participate (n = 5); insufficient amount of CSF for analysis (n = 2); unmet inclusion criteria (n = 1). The gender and age distributions of the 98 included patients are displayed in . All patients were fully autonomous before the infection. 45.9% of the patients were active workers, 15.3% were unemployed but of active working age, 3.1% were students, and 31.6% retired. 45.9% of the patients noticed a tick bite prior to the disease. Out of those, 7 (7.1%) also reported ingesting unpasteurised milk, the remainder had tick exposure risk in nature. For 12.2% of the patients, ingestion of raw milk was the only identified risk factor.
Clinical presentation in the acute phase
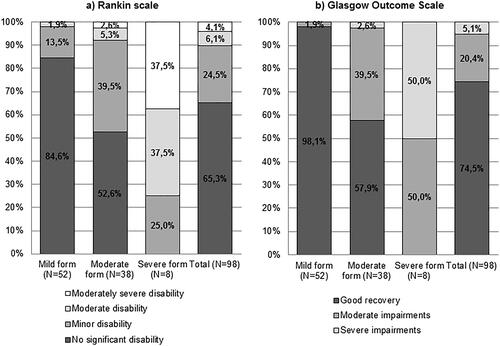
The disease manifested with biphasic course in 72.5% of the cases, monophasic – in 25.5%, and 2% could not specify. 52 patients (53.1%) were classified as experiencing a mild form (meningitis), 38 (38.8%) as having a moderate form (meningoencephalitis of moderate severity), and eight (8.2%) had severe encephalitis (). Twelve patients (12.2%) showed signs of spinal nerve damage, and twelve (12.2%) patients suffered from cranial nerve impairment; of those, three patients (3.1%) had both. Out of eight tested patients due to clinical suspicion, no coinfections with Lyme borreliosis were diagnosed. All symptoms with frequencies are listed in .
Median CSF cell count was 67x106/l (range 5–782 × 106/l), with mononuclear leukocytes predominating in 81.6% of cases. Median CSF protein was 0.77 g/l (range 0.31–2.03 g/l), glucose − 2.86 mmol/l (range 2.19–4.39 mmol/l), and lactate − 1.6 mmol/l (range 1.2–3.8 mmol/l, n = 50). Median WBC count in serum was 10.4 × 109/l (range 4.8–22.1 × 109/l) and CRP − 5.65 mg/l (range 0.1–44.1 mg/l). Disease severity correlated with CSF protein (Spearman’s Rho 0.296, p = 0.003) and lactate (Spearman’s Rho 0.387, p = 0.006).
Eight patients (8.2%) were treated in the ICU and three of them (3.1%) required mechanical ventilation. Average duration of hospitalization was 10 days (median − 9, range 3-69), average ICU stay − 11 days (median – 3, range 1–50). None of the patients died during the acute phase of the disease. 56.1% of the patients were discharged home, 40.8% required either outpatient (n = 11) or inpatient (n = 29) rehabilitation. At discharge, 5.1% were classified as having severe, and 20.4% – as moderate impairments on GOS. 4.1% remained with moderately severe disability, 6.1% with moderate disability and 24.5% had minor disability on RS. The RS and GOS evaluations correlated with disease severity (Spearman’s Rho −0.651 and 0.522 respectively, p < 0.001, ). At discharge, 45.9% of the patients had objective neurological signs, most commonly ataxia and tremor (), 77.8% of them also had subjective complaints. 25.5% of the patients complained of having only subjective symptoms at discharge, most commonly headache and decreased concentration ().
Long term outcome of tick-borne encephalitis
77 patients (78.6%) came for the first follow-up visit, median time since discharge was 154 days (range 87–299 days). 61 patients (62.2%) returned for the second follow-up, median time since discharge was 541 days (range 453–765 days). There was no difference between TBE patients who returned for follow-ups and those who did not in terms of TBE severity (p = 0.256), neurological symptoms at discharge (p = 0.417), subjective symptoms (p = 0.348), GOS (p = 0.909), RS (p = 0.989) or age (p = 0.782). However, patients with higher levels of education were more likely to return for the follow-up visits (p = 0.024).
First follow-up
During the first follow-up, 36.4% (n = 28) of the patients had objective neurological signs, most frequently tremor and ataxia (), and all of them reported subjective symptoms. Two patients displayed signs of central paresis, which was not noted during the acute phase. Imaging studies revealed signs of intracranial bleeding/hemosiderin deposits; however, the role of TBE for these findings is unclear. 36.4% (n = 28) of the patients reported only subjective symptoms, most common being decreased memory, increased irritability and headaches (). 6.5% (n = 5) of the TBE cases who came for the first follow-up remained with moderate disability, and 15.6% (n = 12) with a slight disability on MRS. MRS correlated with the disease severity during the acute stage (Spearman’s Rho 0.362, p < 0.001).
Second follow-up
On the second follow-up, 67.2% (n = 41) of the patients still reported subjective symptoms, most commonly memory impairment, sleep disturbances and headaches (). 39.3% (n = 24) still had objective neurological signs, most frequent being tremor and spinal nerve damage (). 4.9% (n = 3) remained with moderate disability and 18% (n = 11) with slight disability on MRS. MRS still correlated with the disease severity during the acute stage (Spearman’s Rho 0.415, p < 0.001).
Assessment of neurocognitive functioning using Matrics Consensus Cognitive Battery
The majority of MCCB test results did not show evidence of non-normality (p > 0.05). Only scores of verbal learning during the second follow-up departed from normality (W = 0.961, p < 0.01), however skewness and kurtosis did not exceed 1, thus parametric tests were chosen for analysis of cognitive scores.
During the first follow-up, TBE patients scored significantly lower than healthy controls in three cognitive domains: speed of processing, verbal learning, and visual learning (Supplementary Table 5). A similar trend was observed for attention/vigilance, but the difference was not statistically significant (Supplementary Table 5). Analysis within different age groups was performed using Mann–Whitney U test and it revealed that the youngest TBE patients performed most poorly compared to healthy controls in six out of seven domains ( and ). The difference was significant for attention/vigilance (p = 0.004, ) and working memory (p = 0.03, ). The eldest age group scored significantly lower in the verbal learning domain (p = 0.03, ). After p-value adjustment using the Bonferroni method, significance remained for attention/vigilance in the youngest group (p = 0.014). The effect of illness severity on cognitive functioning, controlling for age effect, was evaluated; two age groups were formed based on age median (≤53 and >53 years). As the number of patients with severe encephalitis was small, groups of moderate and severe disease were merged and compared to mild TBE and healthy controls. The impairment of verbal learning (p = 0.001) and visual learning (p = 0.019), were significantly related to illness severity in the acute phase (). However, Tukey’s post-hoc tests showed no significant differences between mild and moderate/severe disease groups in verbal learning (p = 0.274) and visual learning (p = 0.188), significant difference was observed only between healthy controls and mild TBE (verbal learning p = 0.002; visual learning p = 0.022). Illness severity also showed a trend to affect both speed of processing (p = 0.06) and working memory (p = 0.079) (). Post-hoc analysis using Tukey’s test revealed that patients with moderate/severe disease differed significantly in speed of processing from healthy controls (p = 0.027) but not from patients with mild TBE (p = 0.272). Tukey’s post-hoc tests showed no significant differences between mild and moderate/severe disease groups in working memory domain (p = 0.362).
Figure 2. Comparison of different cognitive domains between tick-borne encephalitis (TBE) patients and healthy controls depending on age, 6 months after TBE (*p < 0.05, **p < 0.01).
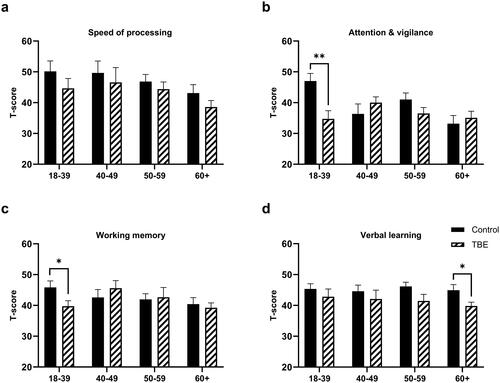
Figure 3. Comparison of different cognitive domains and Overall composite score between tick-borne encephalitis (TBE) patients and healthy controls depending on age, 6 months after TBE (*p < 0.05, **p < 0.01).
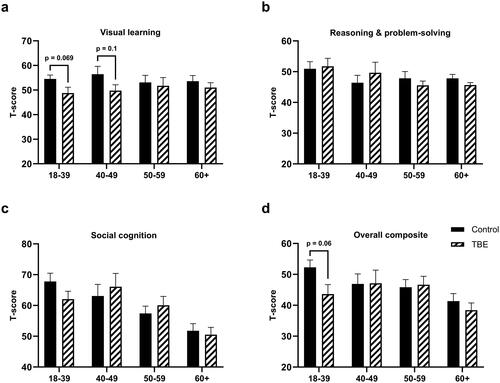
Table 3. Comparison of neurocognitive domains according to the severity of TBE at the time of first (6 months after the acute phase) follow-up controlling for age effect.
Neurocognitive functioning significantly improved from the first to the second follow-up (). Significant positive change was observed in six out of seven domains, with an exception of the social cognition (). At the second follow-up TBE patients did not differ in any cognitive domain from healthy controls (Supplementary Table 5). Similar results were observed when different age groups were compared. Disease severity no longer had an effect on cognitive functioning during the second follow-up.
Table 4. Changes in TBE patients’ neurocognitive functioning from 1st to 2nd follow-up. Paired sample t-test.
Presence of remaining subjective symptoms at the second follow-up predicted slower processing speed independently from patients’ age (M = 42.93, SD = 11.61 and M = 51.75, SD = 12.88 in patients with and without subjective symptoms, respectively F(1)=5.91, p < 0.05, partial η2=0.09), and lower social cognition (M = 53.95, SD = 11.49 and M = 64.00, SD = 15.53 in patients with and without subjective symptoms, respectively F(1)=5.41, p < 0.05, partial η2=0.09). Specifically, reported irritability was related to lower social cognition scores and memory complaints – to slower processing speed (Partial correlation controlling for age r = 0.318, df = 42, p = 0.036, and r = 0.331, df = 42, p = 0.028, respectively).
Discussion
In this study, we have shown that several cognitive domains remained impaired after TBE for a prolonged period of time, even though there was a tendency for significant improvement over time. This raises high concern as decreased cognitive functioning may lead to life quality impairment, and, as already known from previous studies, causes increased health care use, sick leave and even early retirement, leading to significant societal costs [Citation13,Citation14].
Our data confirms that TBE mainly affects middle-aged working people, is usually transmitted by an unnoticed tick bite, and the course of the disease is usually biphasic. The distribution of clinical forms matched previous studies and there is a lack of correlation between routine CSF analytes and outcomes [Citation5,Citation6,Citation8]. During the acute phase, a large number of patients are affected by severe neurological impairment – limb paresis, cranial nerve involvement, alteration of consciousness, etc. The severity of the acute disease puts a significant burden on hospitals due to prolonged in-hospital stays, with almost 10% of patients requiring expensive and protracted ICU treatment.
For this research, we used GOS and RS for the first time to classify the outcome and disability after TBE. Our results show that GOS and RS may be useful for characterising TBE's short-term outcomes because their measurements are consistent with the immediate outcomes of other TBE studies that employed different methodologies [Citation5]. The continued application of these scales in future TBE studies would provide researchers with the ability to compare outcomes of different neuroinfections or non-infectious neurological diseases and to evaluate the importance of TBE in the context of all CNS diseases.
We classified long-term sequelae and disability at two time-points using two distinct methods: first, registering objective neurological signs and subjective symptoms, second – using MRS. We discovered that even after 1.5 years, 7 out of 12 patients presenting with pareses of the limbs during the acute phase remain with significant loss of function of the affected limb. Additionally, in a substantial part of the cohort, tremor and ataxia persisted. Subjective symptoms mainly consisted of headaches, sleep disturbances and problems with concentration, memory and emotional state. However, despite long-lasting neurological signs and subjective complaints, only a small percentage (4.9%) of the patients fall into the moderate disability category using MRS. None of the follow-up patients showed severe impairment on MRS, which was reported to occur in up to 10% of patients in a previous Lithuanian study [Citation5]. This discrepancy may be due to a smaller proportion of severe cases in the present cohort, or an increase in the accessibility and effectiveness of physical rehabilitation. Also, a quite substantial part of the subjects was lost to follow-up, even though all severe TBE cases returned for the visits. Additionally, sequelae classification previously used in our centre included both moderate and moderately severe disability (as specified on MRS) as severe sequelae, whereas slight disability on MRS was an equivalent of moderate sequelae and those results remain proportionate [Citation5]. Moreover, we observed a phenomenon when the amount and types of subjective complaints changed over time but the shift did not affect the patient’s MRS score. Taking all of these findings into account, we do not believe that MRS is an ideal tool to use solely in TBE patients’ long-term sequelae evaluation, as it does not reflect subtle persisting symptoms, including impaired cognition, which affects life quality in other ways than physical disability. However, its use paired with neurocognitive evaluation is feasible.
Consequently, our main focus was to evaluate and specify the neurocognitive impairment after TBE, and to elucidate what exactly comprises the term ‘post-encephalitic syndrome’, which has not been achieved despite multiple and extensive studies [Citation4–6,Citation8,Citation15]. A recent study of over 500 TBE cases in Germany revealed that half of the adult patients report persisting sequelae after 18 months, including fatigue (17.0%), weakness (13.4%) and concentration deficit (13.0%) [Citation13], whereas Veje et al. discovered that even several years after the acute disease (median time to follow-up − 5.5 years, N = 92), TBE survivors still reported more memory, executive functions, vigilance and physical complaints compared to healthy controls [Citation7]. Both of these studies were, however, based on self-reports. In a study by Gustaw-Rothenberg [Citation9], 40 patients with severe TBE were evaluated using a variety of neuropsychological tests to determine the type and degree of cognitive impairment. 55% of the patients met the criteria for mild cognitive impairment; however the researchers used relatively low-volume cognitive evaluation tools. An extended evaluation by Quist-Paulsen et al. provided data that cognition is impaired in encephalitis patients more than in aseptic meningitis, but the research did not focus on different aetiologies, including TBE [Citation16]. Studies using high-quality tools in the paediatric population by Schmolck et al. showed that children with TBE were more likely to have attention and psychomotor speed issues [Citation17], Engman et al. found difficulties with perception, memory, emotion, and executive function [Citation18], whereas Ullman et al. also discovered working memory deficits [Citation19].
In our study, we selected to employ the MCCB test battery, which was originally developed for cognition assessment in clinical studies of schizophrenia. In recent years MCCB battery was also used in studies focusing on other disorders, including bipolar disorder, depression or alcohol use disorder [Citation20–22]. Some tests from the battery, interchangeably with other similar types of tests, have been previously used in other studies on various types of encephalitis [Citation16,Citation23]. The battery is relatively short, comprehensive and constructed to be sensitive to change in course of the treatment, which makes it appealing for longitudinal design studies. MCCB is unique because it also includes a social cognition scale, which is still highly under-researched phenomenon although it is acknowledged that social cognition might be affected by various neurological disorders or acquired brain lesions and is significantly related to long-term functioning and quality of life [Citation10,Citation11,Citation24,Citation25].
Our findings show that some neurocognitive domains, such as processing speed, verbal learning and visual learning were affected in TBE patients when compared to healthy controls. It is worth noting that young patients were the most affected compared to other age groups, scoring lower in almost all cognitive domains and most significantly in attention/vigilance and working memory domains. It is alarming that TBE appears to disproportionally affect the most productive part of the population in terms of intellectual and physical contributions. While a decrease in the speed of processing for the most severe patients was quite predicted, a rather unexpected finding was that patients with mild disease had lower scores on memory-related domains when compared to patients with moderate/severe disease, although statistical significant difference was not confirmed by post-hoc analysis. This implies that even mild types of TBE may cause possible metabolic CNS changes that may result in long-term damage and persistent cognitive/memory issues. Similar findings were reported by Günther et al. – in their study, 28% of TBE patients with mild disease reported symptoms after one year, and decreased memory and concentration problems were the most common complaints [Citation4]. Mickienė et al. also displayed this phenomenon in ∼30% of mild TBE patients [Citation5]. According to Veje et al. disease severity was linked to short-term memory, motivation, balance, fatigue, and learning difficulties, whereas long-term memory was significantly impaired independently from disease severity [Citation7]. The exact mechanisms of cognitive impairment remains unknown, however, Quist-Paulsen et al. in their research on encephalitis of other aetiologies speculated that it might be related to the kynurenine pathway activation, which is also known to be affected during TBE [Citation16,Citation26]. This pathway generates metabolites that might impair communication between neurons and harm cognitive function, particularly memory and learning [Citation27]. While this is a promising area of research, more studies are needed to confirm a clear link between the kynurenine pathway and cognitive problems in TBE patients. The observed improving cognition over time most likely results from the overall healing process of the brain and neuroplasticity, which is expected to last up to a year after TBE as reported in previous studies [Citation5,Citation15].
Despite objectively improved cognition, measured by MCCB, a part of the cohort still complained of persisting subjective symptoms after 1.5 years. The presence of any remaining subjective symptoms at the second follow-up predicted slower processing speed and lower social cognition scores on MCCB. However, when analysing more specific complaints separately, subjective memory complaints were related to slower processing speed but not to memory scores. Weak association between subjective symptoms and objective results, obtained by neuropsychological assessment, is a known phenomenon which may reflect limited patients’ abilities to recognise and communicate their cognitive difficulties as well as the inability of objective tests to grasp subtle cognitive difficulties that become apparent only in complex daily life situations. These findings reveal that both objective assessment and subjective complaints are important when evaluating the sequelae of the disease and developing strategies for rehabilitation and later care [Citation28]. Sadly, to the best of our knowledge, only some case studies addressed neuropsychological rehabilitation challenges after TBE [Citation29]. Further research should be directed towards cognition improvement methods used in other areas such as age-related dementia and their feasibility for post-encephalitic syndrome.
The main advantages of this study were that it was a prospective study, included all consecutive patients and was performed in a study centre with experience on TBE research. We aimed to evaluate neurocognitive sequelae not only qualitatively, but also quantitatively and compare the results to a healthy control cohort. We have achieved this goal, however the discrepancy between MCCB test results and overall recovery of the patients led us to believe that even such a thorough and detailed instrument as MCCB can hardly be used alone and, for a comprehensive and complete assessment of the patients’ recovery process, should be paired with the time-tested subjective symptoms evaluation. The limitations of our study were a relatively small percentage of severe cases and a quite substantial amount of subjects lost to follow-up, COVID-19 restrictions being the most unexpected reason. During the follow-ups, the TBE cohort was tested primarily before the widespread of COVID-19, whereas the control group was evaluated during the COVID-19 pandemic and part of them might have had clinical or asymptomatic COVID-19 infection which is now also known to impact cognitive functions in some cases [Citation30]. Unfortunately, COVID-19-related data was not collected from the control group.
In conclusion, long-term neurological sequelae persist in a substantial proportion of TBE patients with significant impairment in several cognitive domains, especially in younger patients and even in mild TBE. While a significant increase in neurocognitive performance over time provides an optimistic picture, a timely intervention might be significant to shorten that period. It is crucial to begin developing individualised neuropsychological rehabilitation programs in addition to the existing physical rehabilitation and to continue the research determining the pathogenesis of development and persistence of these symptoms for possible future creation of pharmacological measures.
Supplemental Material
Download MS Word (26.5 KB)Supplemental Material
Download MS Word (19.9 KB)Supplemental Material
Download MS Word (66.1 KB)Supplemental Material
Download MS Word (13.9 KB)Supplemental Material
Download MS Word (13.5 KB)Supplemental Material
Download MS Word (13.8 KB)Disclosure statement
M. S. and A. M. have received a Grant from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Infectious Diseases of the Brain (ESGIB). A. M. has received a Grant for the Independent Investigator Initiated Research from Pfizer R&D Investigator-Initiated Research Program, received sponsorship for participation in the international scientific conferences by MSD, Pfizer, Abbvie, Janssen, received payments for lectures at local scientific conferences and consultation fees from GSK, Sanofi, Pfizer, E-visit. M. S. has received Grants from the Swedish state under the agreement between the Swedish government and the country councils, the ALF agreement. M. V. has received speaker honoraria from Pfizer and Bavaria Nordic, support for attending medical meetings from Pfizer. V. G. has received sponsored participation in a medical conference by Sanofi-Aventis. A. P., J. P., D. G., L. L., L. S. and J. A. did not have any conflicts of interest to disclose.
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- European Centre for Disease Prevention and Control (ECDC). Tick-borne encephalitis. Annual epidemiological report for 2020 [cited 2022 Dec 13]. https://www.ecdc.europa.eu/en/publications-data/tick-borne-encephalitis-annual-epidemiological-report-2020.
- Gustafson R, Svenungsson B, Forsgren M, et al. Two-year survey of the incidence of lyme borreliosis and tick-borne encephalitis in a high-risk population in Sweden. Eur J Clin Microbiol Infect Dis. 1992;11(10):894–900. doi: 10.1007/BF01962369.
- Gustafson R, Svenungsson B, Gardulf A, et al. Prevalence of tick-borne encephalitis and lyme borreliosis in a defined Swedish population. Scand J Infect Dis. 1990;22(3):297–306. doi: 10.3109/00365549009027051.
- Günther G, Haglund M, Lindquist L, et al. Tick-borne encephalitis in Sweden in relation to aseptic meningo-encephalitis of other etiology: a prospective study of clinical course and outcome. J Neurol. 1997;244(4):230–238. doi: 10.1007/s004150050077.
- Mickiene A, Laiskonis A, Günther G, et al. Tickborne encephalitis in an area of high endemicity in Lithuania: disease severity and long-term prognosis. Clin Infect Dis. 2002;35(6):650–658. doi: 10.1086/342059.
- Kaiser R, Kaiser DR. The clinical and epidemiological profile of tick-borne encephalitis in Southern Germany 1994–98A prospective study of 656 patients. Brain. 1999;122(11):2067–2078. doi: 10.1093/BRAIN/122.11.2067.
- Veje M, Nolskog P, Petzold M, et al. Tick-borne encephalitis sequelae at long-term follow-up: a self-reported case–control study. Acta Neurol Scand. 2016;134(6):434–441. doi: 10.1111/ane.12561.
- Haglund M, Günther G. Tick-borne encephalitis – pathogenesis, clinical course and long-term follow-up. Vaccine. 2003;21(suppl 1):S11–S18. doi: 10.1016/S0264-410X(02)00811-3.
- Gustaw-Rothenberg K. Cognitive impairment after tick-borne encephalitis. Dement Geriatr Cogn Disord. 2008;26(2):165–168. doi: 10.1159/000150443.
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/APPI.AJP.2007.07010042.
- Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–220. doi: 10.1176/APPI.AJP.2007.07010043.
- WMA. Declaration of Helsinki – ethical principles for medical research involving human subjects. Published 1964. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- Nygren TM, Pilic A, Böhmer MM, et al. Recovery and sequelae in 523 adults and children with tick-borne encephalitis in Germany. Infection. 2023;51(5):1503–1511. doi: 10.1007/s15010-023-02023-w.
- Slunge D, Boman A, Studahl M. Burden of tick-borne encephalitis, Sweden. Emerg Infect Dis. 2022;28(2):314–322. doi: 10.3201/EID2802.204324.
- Haglund M, Forsgren M, Lindh G, et al. A 10-year follow-up study of tick-borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy. Scand J Infect Dis. 1996;28(3):217–224. doi: 10.3109/00365549609027160.
- Quist-Paulsen E, Ormaasen V, Kran AMB, et al. Encephalitis and aseptic meningitis: short-term and long-term outcome, quality of life and neuropsychological functioning. Sci Rep. 2019;9(1):16158. doi: 10.1038/s41598-019-52570-2.
- Schmolck H, Maritz E, Kletzin I, et al. Neurologic, neuropsychologic, and electroencephalographic findings after European tick-borne encephalitis in children. J Child Neurol. 2005;20(6):500–508. doi: 10.1177/088307380502000606.
- Engman ML, Lindström K, Sallamba M, et al. One-year follow-up of tick-borne central nervous system infections in childhood. Pediatr Infect Dis J. 2012;31(6):570–574. doi: 10.1097/INF.0B013E31824F23C0.
- Ullman H, Fowler Å, Wickström R. Erratum to “increased working memory related fMRI signal in children following tick borne encephalitis”. Eur J Paediatr Neurol. 2016;20(4):688. doi: 10.1016/J.EJPN.2016.04.018.
- Jones BDM, Fernandes BS, Husain MI, et al. A cross-sectional study of cognitive performance in bipolar disorder across the lifespan: the cog-BD project. Psychol Med. 2023;53(13):6316–6324. doi: 10.1017/S0033291722003622.
- Shi J, Guo H, Liu S, et al. Subcortical brain volumes relate to neurocognition in first-episode schizophrenia, bipolar disorder, major depression disorder, and healthy controls. Front Psychiatry. 2021;12:747386. doi: 10.3389/FPSYT.2021.747386/BIBTEX.
- Cheng F, Cui S, Zhang C, et al. Association between cognitive function and early life experiences in patients with alcohol use disorder. Front Psychiatry. 2020;11:792. doi: 10.3389/FPSYT.2020.00792.
- Eckerström M, Nilsson S, Zetterberg H, et al. Cognitive impairment without altered levels of cerebrospinal fluid biomarkers in patients with encephalitis caused by varicella-zoster virus: a pilot study. Sci Rep. 2020;10(1):22400. doi: 10.1038/S41598-020-79800-2.
- Maggio MG, Maresca G, Stagnitti MC, et al. Social cognition in patients with acquired brain lesions: an overview on an under-reported problem. Appl Neuropsychol Adult. 2022;29(3):419–431. doi: 10.1080/23279095.2020.1753058.
- Duclos H, Desgranges B, Eustache F, et al. Impairment of social cognition in neurological diseases. Rev Neurol. 2018;174(4):190–198. doi: 10.1016/J.NEUROL.2018.03.003.
- Holtze M, Mickiené A, Atlas A, et al. Elevated cerebrospinal fluid kynurenic acid levels in patients with tick-borne encephalitis. J Intern Med. 2012;272(4):394–401. doi: 10.1111/j.1365-2796.2012.02539.x.
- Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169(6):1211–1227. doi: 10.1111/BPH.12230.
- Pranckeviciene A, Deltuva VP, Tamasauskas A, et al. Association between psychological distress, subjective cognitive complaints and objective neuropsychological functioning in brain tumor patients. Clin Neurol Neurosurg. 2017;163:18–23. doi: 10.1016/J.CLINEURO.2017.10.007.
- Ripamonti E, Gaffuri M, Molteni F. Cognitive, neuropsychiatric, and motor profile in post tick-borne flaviviral encephalomyelitis. Neurol Sci. 2020;41(12):3759–3760. doi: 10.1007/s10072-020-04531-1.
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/J.BBI.2021.12.020.