Abstract
Epithelial carcinoma of the nasopharynx originates from the mucosal lining of this region. The etiology of the disease involves a complex interplay of environmental factors, genetic predisposition, and infection with the Epstein Barr virus. In this article, we present a case of nasopharyngeal carcinoma (NPC) that presented with right orbital proptosis and ocular symptoms, without any of the typical signs of nasopharyngeal carcinoma. However, imaging studies revealed subtle findings of NPC. Isolated neuro-ophthalmologic symptoms are not a common manifestation in NPC patients. Proptosis is a rarer finding among ophthalmologic symptoms, usually associated with tumor invasion into the orbit. However, in the presented case, unilateral proptosis was observed without orbital tumor invasion.
Background
Epithelial carcinoma of the nasopharynx originates from the mucosal lining of this region. Within the nasopharynx, the tumor is frequently detected in the pharyngeal recess, which is also known as the fossa of Rosenmuller [Citation1,Citation2].
NPC is a relatively uncommon type of epithelial tumor that occurs at a rate of approximately 1 per 100,000 individuals, accounting for 0.7% of all annual cancer diagnoses [Citation3]. This particular cancer is prevalent in regions of Southern China and North Africa, where dietary habits have been identified as a possible contributing factor, but it is infrequent in other parts of the world [Citation4].
The etiology of the disease involves a complex interplay of environmental factors, genetic predisposition, and infection with the Epstein Barr virus [Citation5,Citation6]. Regardless of race or ethnicity, the incidence of NPC is higher in men than in women [Citation7]. There is no discernible difference in the risk of NPC between urban and rural populations in Southern China, including those living in Southeast Asia [Citation8,Citation9].
The etiology of nasopharyngeal cancer is multifactorial and involves various environmental factors, such as tobacco and alcohol use, exposure to occupational hazards like formaldehyde, consumption of certain Chinese herbs, inadequate consumption of fresh fruits and vegetables, early consumption of preserved foods, and lower socioeconomic status [Citation7].
Ophthalmologic and neurologic manifestations may be the initial and sole symptoms of NPC. This can be misleading unless a high level of suspicion exists [Citation10]. In this article, we present a case of NPC that presented with right orbital proptosis and ocular symptoms, without any of the typical signs of NPC. However, imaging studies revealed subtle findings of NPC.
Case report
A 61-year-old Asian woman with no previous medical history presented to an ophthalmologist with a primary complaint of right orbital proptosis, as well as ptosis and diplopia. During the physical examination, it was observed that the patient experienced impairment in right eye adduction and upward and downward gaze. This finding is typically indicative of third cranial nerve palsy. She underwent an ophthalmologic examination and was subsequently referred to the neurology service due to the absence of a clear cause for her symptoms and the presence of headaches. Neuro-ophthalmologic causes such as cerebral venous thrombosis, Tolosa-Hunt syndrome, etc., were considered and treated with corticosteroids, but there was no significant improvement. Further work-up was performed, and the aforementioned diagnoses were ruled out. No clear pathological findings were identified on the contrast-enhanced CT scan that could explain the patient’s neurologic symptoms (Figure ).
Figure 1. Brain CT Scan (A) No pathological inter- and extra-axial findings that could explain the patient’s neuro-ophthalmological symptoms were observed in the axial CT scan images of the brain. (B and C) Evidence of proptosis was found in the right orbit. Proptosis is diagnosed in cross-sectional images by measuring the distance between the anterior and posterior edges of the globe to the intrazygomatic line (green line) at the level of the lens and optic nerve head. The upper limit of the normal distance is 21 mm, and any distance greater than 21 mm indicates proptosis [Citation11]. The lower limit of the normal distance between the intrazygomatic line and the posterior edge of the globe (yellow line) is 5.9 mm, and any distance less than 5.9 mm also indicates proptosis [Citation12]. In the axial CT scan images of the patient taken at the level of the lens and the optic nerve head on the right side, the distance between the anterior edges of the globe to the intrazygomatic line (red line) was 21 mm and the distance measured between the intrazygomatic line and the posterior edge of the globe (yellow line) was 4.9 mm (B). Due to the patient’s rotation for comparing the two eyes in a same-plane cut with a bilateral lens, evidence of a more anterior position of the right globe compared to the left was observed (C).
![Figure 1. Brain CT Scan (A) No pathological inter- and extra-axial findings that could explain the patient’s neuro-ophthalmological symptoms were observed in the axial CT scan images of the brain. (B and C) Evidence of proptosis was found in the right orbit. Proptosis is diagnosed in cross-sectional images by measuring the distance between the anterior and posterior edges of the globe to the intrazygomatic line (green line) at the level of the lens and optic nerve head. The upper limit of the normal distance is 21 mm, and any distance greater than 21 mm indicates proptosis [Citation11]. The lower limit of the normal distance between the intrazygomatic line and the posterior edge of the globe (yellow line) is 5.9 mm, and any distance less than 5.9 mm also indicates proptosis [Citation12]. In the axial CT scan images of the patient taken at the level of the lens and the optic nerve head on the right side, the distance between the anterior edges of the globe to the intrazygomatic line (red line) was 21 mm and the distance measured between the intrazygomatic line and the posterior edge of the globe (yellow line) was 4.9 mm (B). Due to the patient’s rotation for comparing the two eyes in a same-plane cut with a bilateral lens, evidence of a more anterior position of the right globe compared to the left was observed (C).](/cms/asset/a9d95615-87cf-4221-bd05-54f735b91a73/icro_a_2332591_f0001_c.jpg)
In the evaluation of the patient’s paranasal sinuses CT scan, subtle asymmetry in the nasopharynx was observed. Additionally, evidence of right orbital proptosis and effusion in mastoid air cells was observed. However, no evidence of effusion was observed in either middle ear or bone destruction (Figure ). In consultation with an otorhinolaryngologist, the possibility of primary nasopharynx pathology was deemed unlikely given the isolated neuro-ophthalmologic symptoms, as well as the lack of evidence of involvement of the ear, nose, or neck masses. Therefore, a biopsy of the nasopharynx was not performed. Due to the ongoing nature of the patient’s symptoms, an MRI with contrast was performed, which revealed a subtle mucosal enhancement in the right nasopharynx that extended towards the cavernous sinus (Figure ). While other typical findings of nasopharyngeal cancer were not observed in the MRI images, a biopsy of the right nasopharynx mucosa was performed for further evaluation.
Figure 2. Paranasal sinuses CT scan. (A and B) In the coronal and axial Paranasal sinuses CT scan images of the bone and soft tissue window at the level of the nasopharynx, evidence of asymmetry of the right superolateral wall compared to the left (indicated by arrows) was observed. (C) In the axial CT scan images obtained at the level of the mastoid and middle ear cavity, evidence of effusion was observed in the right mastoid air cells (indicated by arrow number 1) without any evidence of effusion in the middle ear (indicated by arrow number 2).
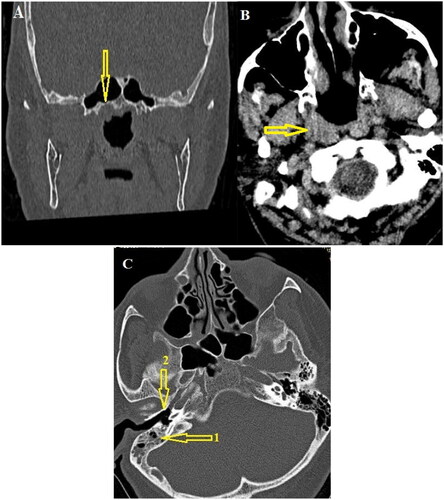
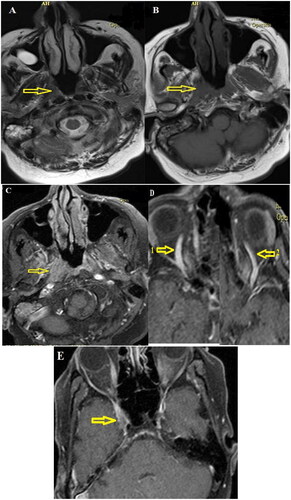
Figure 3. Brain MRI with IV gadolinium injection. (A and B) In the T1- and T2-weighted axial images without contrast at the level of the nasopharynx, minor isosignal asymmetry was present in the nasopharynx (indicated by arrows). (C) In the axial fat-saturated T1-weighted post-gadolinium injection images, evidence of asymmetry of the right lateral wall of the nasopharynx compared to the left wall (indicated by an arrow) with enhancement was observed. (D) In the evaluation of other areas, evidence of prominent right-sided superior ophthalmic vein (indicated by arrow number 1 compared to arrow number 2) with (E) involvement of a small part of the right side of the cavernous sinus (indicated by arrow) was observed. No evidence of invasion into the nasal cavity, paranasal sinuses, or orbit was observed.
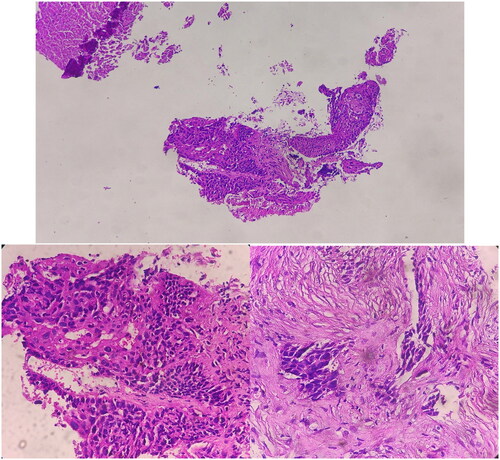
Upon macroscopic examination of the biopsy sample taken by the pathologist, multiple pieces of soft, brownish-yellow tissue measuring a total of 12x9x4 millimeters were observed. Microscopic examination revealed sections of stratified squamous mucosa and underlying fibrotic tissue involved by malignant neoplastic lesion composed of sheets and nests of atypical pleomorphic cells with high nuclear to cytoplasmic ratio and acidophilic cytoplasm with rare mitosis; foci of necrosis and hemorrhage. The findings indicated moderately differentiated squamous cell carcinoma (Figure ). Based on the histopathological analysis, the observed characteristics of the lesion strongly indicate a diagnosis of non-keratinizing squamous cell carcinoma (type 2) within the classification of nasopharyngeal cancers as defined by the World Health Organization. Consequently, considering the histological type of the tumor, there is a significant likelihood that the occurrence of this cancer is associated with an EBV virus infection.
Figure 4. Sections show stratified squamous mucosa and underlying fibrotic tissue involved by malignant neoplastic lesion composed of sheets and nests of atypical pleomorphic cells with high nuclear to cytoplasmic ratio and acidophilic cytoplasm with rare mitosis; foci of necrosis and hemorrhage.
Following the confirmation of nasopharyngeal malignancy through pathological examination, further investigations were conducted to determine the stage of the cancer according to TNM classification by UICC 8th edition. The lesion was classified as T4 due to involvement of the cranial nerve. However, there was no evidence of metastasis or lymphatic involvement based on the imaging studies. According to the TNM classification, the patient was diagnosed with Stage IVA.
Subsequently, the patient was referred to an oncologist for additional management of the nasopharyngeal cancer. Taking into account the stage of the disease, a treatment plan consisting of chemo-radiation therapy was chosen. The radiation dosage administered was between 60 to 70 Gy, targeting the nasopharynx and both sides of the neck. Concurrently, the patient received chemotherapy which included cisplatin and 5-fluorouracil.
Discussion
The most frequent symptoms of NPC are dependent on the affected anatomical regions and include cervical mass, epistaxis, nasal obstruction, and hearing loss [Citation3]. While nasal and otological symptoms are the most common (80%), intracranial extension is present in 8% of cases, and pituitary localization is rarely reported in the literature [Citation5].
Approximately half of all patients with NPC experience neurological complications, and these are the initial presenting symptoms in 34% of cases [Citation13]. Other common symptoms of NPC include trismus, pain, otitis media, nasal regurgitation (due to paresis of the soft palate), hearing loss, and cranial nerve palsies. The incidence of cranial nerve involvement ranges from 12% to 35% [Citation14].
Orbitocranial recurrence of NPC is an infrequent occurrence, and ocular symptoms may be the initial manifestation of the disease [Citation15]. Certain studies have reported unusual manifestations of nasopharyngeal cancer, which can lead to delayed or incorrect diagnoses. In a study by Rego-Lorca et al. a case report of isolated sixth cranial nerve palsy as a manifestation of nasopharyngeal cancer was published. While the manifestation of this cancer is uncommon with the involvement of isolated cranial nerves [Citation3]. Our patient presented with ocular symptoms and headaches for several months. An interesting point about the case presented in this study was the occurrence of unilateral right-sided proptosis as the initial sign of NPC, which was accompanied by ptosis and diplopia. Orbital involvement in NPC was observed in 3.2% of cases [Citation16]. The occurrence of these symptoms led to clinical confusion and deviation from the diagnosis of NPC. Another point that delayed the diagnosis of this cancer was the absence of any common ear or nose symptoms in the patient, which are usually seen in patients with NPC.
Based on the imaging studies in this case, the presence of cavernous sinus involvement suggests that the patient’s proptosis is likely due to elevated venous pressure in the cavernous sinus and ophthalmic veins. Additionally, given that the ocular motor nerves pass through the cavernous sinus, the patient’s diplopia is likely caused by cranial nerve involvement. Based on the patient’s symptoms, the involvement of the third cranial nerve is the most probable cause.
Some additional infrequent manifestations of this cancer include sclerosis of the anterior arch of the atlas, odontoid process, and the base of the skull in the vicinity of the tumor [Citation17], hypopituitarism caused by tumor extension to the hypophysis [Citation5], Garcin syndrome [Citation18], the coexistence of unilateral abducens nerve palsy and ipsilateral Horner’s syndrome [Citation19], epiglottic metastasis [Citation20], optic neuritis [Citation21,], mass in the neck and intraoral involvement of the soft palate [Citation4], metastasis to the parotid gland [Citation22], and cutaneous metastases [Citation23].
The World Health Organization recognizes three pathological subtypes of NPC: keratinizing squamous, non-keratinizing, and basaloid squamous [Citation24]. The non-keratinizing subtype accounts for the majority of cases in endemic regions (>95%) and is primarily linked to infection with the Epstein-Barr virus (EBV) [Citation25,Citation26]. The histological examination of the present case also suggests non-keratinizing squamous cell carcinoma. Therefore, with 100% probability, the patient’s cancer is related to EBV virus [Citation27].
MRI, CT, and 18 F-fluorodeoxyglucose (18 F-FDG)-PET/CT are currently the most widely used imaging techniques for staging and radiotherapy planning in NPC. With its superior soft-tissue resolution, MRI is more effective than CT in assessing primary tumor extension and retropharyngeal lymph node metastasis, although both have similar accuracy in detecting cervical lymph node metastasis [Citation28,Citation29].
There can be variations in the CT scan findings among different individuals. In some cases, small lesions may be localized within the nasopharynx. However, larger and more aggressive tumors have the potential to extend in multiple directions, eroding the base of the skull and traversing through structures such as the Eustachian tube, foramen lacerum, foramen ovale, cavernous sinus [Citation30,Citation31]. The CT scan findings of our patient were initially overlooked by the radiologist due to their subtlety, and the patient’s confusing symptoms. However, during a second review of the patient’s CT scan, brief asymmetry in the nasopharynx was observed. It’s worth noting that evidence of effusion in the right mastoid air cells was observed, although there was no effusion in the middle ear. Additionally, no evidence of bone destruction or invasion into the orbital and nasal cavities was observed.
Magnetic resonance imaging (MRI) is a valuable tool for detecting NPC. Typically, on T1-weighted images, the tumor appears similar in signal intensity to muscle, while on T2-weighted images, the signal intensity is typically slightly higher than that of muscle. While fluid in the middle ear may be a common finding, it is not specific to NPC. On fat-saturated T1-weighted images with gadolinium contrast, prominent heterogeneous enhancement is often observed, which is a characteristic feature of NPC. In addition, it is important to evaluate for perineural extension [Citation32]. Our patient’s MRI showed slight enhancement in the soft tissue of the right nasopharynx on T1 fat-saturated sequence after gadolinium injection, as well as asymmetry and a brief extension into the right cavernous sinus, and subtle prominence of the right superior ophthalmic vein. Evidence of effusion was also noted in the right mastoid air cells without effusion in the right middle ear. Furthermore, there was no indication of bone destruction or invasion into the orbital or nasal cavities.
NPC is more prone to distant metastases than other head and neck malignancies. Along with lymph node involvement, the high rate of proliferation may contribute to its tendency to develop distant metastases [Citation23]. Our patient showed brief involvement of the cavernous sinus without distant metastasis in other areas. An important aspect of this case was the occurrence of unilateral proptosis, as well as ptosis and diplopia in a patient with NPC without other typical signs of nasopharyngeal malignancies. Interestingly, subtle findings on CT and MRI were present without evidence of bone destruction or invasion into the orbital or nasal cavities, despite the presence of ophthalmologic symptoms.
NPC cases with reported proptosis have shown tumor invasion into the orbit from various routes, as demonstrated in Sharma et al.’s study where a NPC case with proptosis was reported, and further imaging revealed invasion into the nasal cavity, destruction of the medial wall of the left orbit, and tumor extension into the left orbit [Citation33]. However, in the presented case, evidence of right-sided proptosis was observed without tumor invasion into the orbit.
Conclusion
Isolated neuro-ophthalmologic symptoms are not a common manifestation in NPC patients. Proptosis is a rarer finding among ophthalmologic symptoms, usually associated with tumor invasion into the orbit. However, in the presented case, unilateral proptosis was observed without orbital tumor invasion.
Ethical statement
This study was approved by the institutional review board committee.
Informed consent
Informed consent was given by the patient and caregivers.
Consent
Written informed consent was obtained from the patient to publish this report in accordance with the journal’s patient consent policy.
Acknowledgments
The authors would like to express gratitude toward all who helped in the improvement of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: international agency for research on cancer https://gco.iarc.fr/today2018;2020 [cited Dec 28, 2018]. Available from: https://gco.iarc.fr/today
- Rego-Lorca D, Burgos-Blasco B, Hernández-García E, et al. Abducens palsy as first manifestation of a nasopharyngeal carcinoma. Oral Oncol. 2021; 114:105079. doi: 10.1016/j.oraloncology.2020.105079.
- Korde S, Gadbail A, Manjrekar K, et al. Undifferentiated nasopharyngeal carcinoma with oral manifestation: a case report. Acta Stomatologica Croatica: Int J Oral Scis Dent Med. 2011;45(3):209–214.
- Chatti H, Oueslati I, Azaiez A, et al. Hypopituitarism secondary to a pituitary metastasis as a first manifestation of an invasive nasopharyngeal carcinoma. Endocrine abstracts. Bioscientifica. 2021;73(558). doi: 10.1530/endoabs
- Chen Y, Chan A, Le Q, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0.
- Yu M, Yuan J. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002; 12(6):421–429. doi: 10.1016/s1044579x02000858.
- Armstrong R, Kannan Kutty M, Dharmalingam S, et al. Incidence of nasopharyngeal carcinoma in Malaysia, 1968–1977. Br J Cancer. 1979; 40(4):557–567. doi: 10.1038/bjc.1979.221.
- Yu M, Ho J, Ross R, et al. Nasopharyngeal carcinoma in Chinese—salted fish or inhaled smoke? Prev Med. 1981; /10(1):15–24. doi: 10.1016/0091-7435(81)90002-5.
- Ogunleye A, Nwaorgu O, Adaramola S. Ophthalmo-neurologic manifestation of nasopharyngeal carcinoma. West Afr J Med. 1999; 18(2):106–109.
- Haaga J, Boll D. CT and MRI of the whole body, 2-Volume set. (6th, editor). Find it at amazon: Elsevier Health Sciences; Amsterdam, The Netherlands; 2016.
- Ozgen A, Ariyurek M. Normative measurements of orbital structures using CT. AJR Am J Roentgenol. 1998;170(4):1093–1096. doi: 10.2214/ajr.170.4.9530066.
- Dunmade A, Ademola-Popoola D. Neuro-ophthalmic manifestation of nasopharyngeal carcinoma at ilorin: a five year review. Niger J Clin Pract. 2008;11(4):376–378.
- Bhawana K, Manoj K, Vivek L. Pearls & Oy-sters: bilateral cavernous sinus syndrome as presenting manifestation of nasopharyngeal carcinoma. Neurology. 2014;82(6):e51–e54.
- Bernardini F, Croxatto J, Orcioni G, et al. Visual loss secondary to orbital apex invasion as the first manifestation of recurrent nasopharyngeal carcinoma. Ophthalmic Plast Reconstr Surg. 2009;25(3):248–250. doi: 10.1097/IOP.0b013e3181a1d48d.
- Hsu W, Wang A. Nasopharyngeal carcinoma with orbital invasion. Eye. 2004;18(8):833–838. doi: 10.1038/sj.eye.6701358.
- Potter G. Sclerosis of the base of the skull as a manifestation of nasopharyngeal carcinoma. Radiology. 1970;94(1):35–38. doi: 10.1148/10.1148/94.1.35.
- Musanip H, Anuta M, Lui A, et al. Garcin syndrome: a rare manifestation of nasopharyngeal carcinoma. J Neurol Sci. 2021;429:118458. (doi: 10.1016/j.jns.2021.118458.
- Top Karti D, Karti O, Koc A, et al. Unilateral abducens nerve palsy with ipsilateral horner’s syndrome as an initial manifestation of recurrent nasopharyngeal carcinoma. Neuroophthalmology. 2020; 44(6):379–383. doi: 10.1080/01658107.2019.1625931.
- Rajendran T, Kanapaty Y, Ambu V, et al. Epiglottic metastasis from a recurrent nasopharyngeal carcinoma: a rare manifestation. GMJ. 2019;30:205–207.
- Puri P, Puri S, Pepper I. Optic neuritis: a rare manifestation of nasopharyngeal carcinoma. Eye. 2004; 18(1):110–110. doi: 10.1038/sj.eye.6700538.
- Yeung K, Chiang P, Chang C, et al. A parotid gland mass as an initial metastatic manifestation of nasopharyngeal carcinoma. J Cancer Res Pract. 2018; 2018/09/01/5(3):123–126. doi: 10.1016/j.jcrpr.2018.03.004.
- Sawali H, Yunus M, Ai O, et al. Cutaneous metastases from nasopharyngeal carcinoma: a rare manifestation. Philipp J Otolaryngol Head Neck Surg. 2010;25(2):32–35. doi: 10.32412/pjohns.v25i2.629.
- Wang H, Chang Y, To K, et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Cancer Commun. 2016;35(1):1–16.
- Pathmanathan R, Prasad U, Chandrika G, et al. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein-Barr virus-infected neoplasia. The Am J Pathol. 1995;146(6):1355–1367.
- Young L, Dawson C. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. doi: 10.5732/cjc.014.10197.
- Su Z, Siak P, Leong C, et al. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front Microbiol. 2023;14:1116143. doi: 10.3389/fmicb.2023.1116143.
- Chen W, Li J, Hong L, et al. Comparison of MRI, CT and 18F-FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: an updated meta analysis of clinical studies. Am J Transl Res. 2016;8(11):4532–4547.
- Liao X, Mao Y, Liu L, et al. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? Int J Radiat Oncol Biol Phys. 2008;72(5):1368–1377. doi: 10.1016/j.ijrobp.2008.03.017.
- Hermans R, Baert A. Head and neck cancer imaging. Hermans R, editor.: Berlin and Heidelberg: Springer; 2006 (find it at amazon.com).
- Peter M, Som H, Curtin S. Head and neck imaging. St. Louis: Mosby-Year Book; 2003.
- King A, Wong L, Law B, et al. MR imaging criteria for the detection of nasopharyngeal carcinoma: discrimination of early-stage primary tumors from benign hyperplasia. AJNR Am J Neuroradiol. 2018;39(3):515–523. doi: 10.3174/ajnr.A5493.
- Sharma S, Ahluwalia C, Singhal S, et al. Nasopharyngeal carcinoma presenting as proptosis in a child: a diagnostic dilemma. Int J Otorhinolaryngol Head Neck Surg. 2015;1(2):81–84. doi: 10.18203/issn.2454-5929.ijohns20150905.

