Abstract
One of the less common clinical manifestations of antiphospholipid syndrome (APS) is hearing loss, so early diagnosis and the use of a cochlear implant may be a viable alternative. Patient with hearing loss and anacusis, associated with positive aPL and APS, who underwent a cochlear implant (Rondo 2, Med-El) to correct hearing that is evaluated with impedance telemetry, with adequate responses were found in the 12 pairs of electrodes, a neuronal response telemetry with electrically evoked compound action potential (eCAP) and normal right hearing was achieved by audiogram (maximum phonemic discrimination threshold of 20% at 110 dB initial and 90% at 70 dB final). APS is a rare pathology which requires a high sense of alert on the health team to avoid delays in the diagnosis and treatment of hearing loss, and to establish the cochlear implant as a viable and useful alternative in the hearing recovery by APS.
Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by the presence of antiphospholipid antibodies (aPL) that induce thrombotic events [Citation1]. It is very rare in children, representing 2.8% of patients with APS. It can be primary APS in patients in whom there is no clinical or laboratory evidence suggestive of another entity or it can be associated with other diseases, mainly systemic lupus erythematosus (SLE), but also occasionally with other autoimmune diseases secondary APS [Citation2]. aPL are heterogeneous antibodies that act on phospholipids and phospholipid-binding proteins, causing hypercoagulability, venous thrombosis, and clinical manifestations such as: anticardiolipin (aCL) antibodies, anti-β 2-glycoprotein I (anti-β 2GP I) antibodies, and lupus anticoagulants (LAC) [Citation3]. Initially, the characteristics of the disease were associated with the presence of specific aPL antibodies and the development of venous or arterial thrombosis. Currently, its diagnosis is based on the Miyakiʼs criteria [Citation4], which indicates that APS can present a series of non-thrombotic manifestations such as thrombocytopenia, valvular disease, neuropathy and kidney associated aPL manifestations [Citation5], and it is associated with microthrombosis, causing skin manifestations such as livedo racemosa and skin ulcerations.
APS presents various clinical manifestations associated with the presence of aPL such as: cerebral ischemia, neuropsychiatric, chronic headache, dementia, cognitive dysfunction, psychosis, depression, transverse myelitis, disease like multiple sclerosis, chorea, and seizures [Citation4, Citation6]. Few reports have reported sensorineural hearing loss (SNHL) in association with APS and systemic lupus erythematosus [Citation3, Citation4]. SNHL is thought to be mediated by the presence of aPL, as a recent review found that most patients were male and 75% had bilateral disease, however it was difficult to treat and only 25% of patients had complete resolution or some improvement in their symptoms with anticoagulation [Citation6]. The specific pathological effect of aPL on SNHL is not yet fully understood.
At present, there are many reports of APS in adult patients, but few reports in children. This work reports a school-age patient with hearing loss, who was initially diagnosed associated with post-infection virus, and was diagnosed with APS after 12 months of symptom evolution. In addition, the cochlear implant was performed to correct hearing and to establish its usefulness in hearing loss caused by APS.
Case presentation
We report a case of a female child of 9 years and 4 months, who is admitted to the audiology service of the Poblano’s Child Hospital. She attended due to suspicion of right hearing loss of 8 months of evolution, detected by failures to phonemic discrimination when using a musical device and slowly progressive evolution. It is accompanied by episodes of bilateralear fullness that does not subside with the Valsalva maneuver. Presence of anacusis in the left ear and the time of hearing loss is unknown. Hereditary family history of no interest for the current condition and in the personal history: she presented chickenpox at 4 years of age. Previous evaluations: evaluated by a private doctor where they detected deep left hearing loss, probably post-viral, beginning follow-up with audiometry; continuous follow-up in another public hospital (IMSS) beginning the use of a left hearing aid without presenting adequate gain. Physical examination: auditory behavior of mild hearing loss; unaltered voice, speech and language. Normally implanted auricles, permeable external auditory canals at otoscopy, intact tympanic membranes with adequate anatomical projections bilaterally.
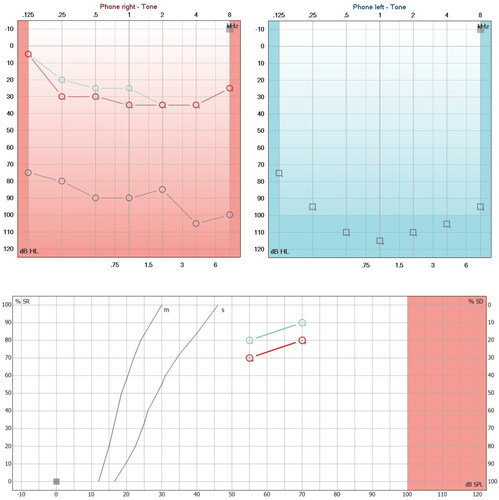
Studies carried out in audiology: tone audiometry was performed, obtaining an irregular profile curve of medium sensory hearing loss in the right ear and audiometric anacusia in the left ear (Figure ; Red and blue lines). Bilateral Jerger A curve impedancemetry. Ipsilateral stapedial reflex present in the right ear at 95 dB at 2000 and 4000 Hz, absent in the rest of the frequencies evaluated, as well as in the left ear. Transient otoacoustic emissions: inadequate overall reproducibility bilaterally. Tomography (TC) and magnetic resonance imaging (MRI) of the ears and mastoids were requested, which were reported without alterations (Figure ).
Figure 1. Hearing loss due to APS and with steroid treatment. A. First audiometry (Red and blue lines) performed at the first audiological appointment, which shows moderate right sensorineural hearing loss and left anacusis. The verbal audiometry of the right side shows a maximum phonemic discrimination of 100% at 80 dB and left anacusis. B. Second audiometry (aquamarine lines) performed 20 days after starting steroid treatment, in which profound hearing loss of the right sensorineural type and left anacusis are evident in the audiogram. The phonemic discrimination on the right side dropped dramatically until it discriminated 20% at 110 dB.
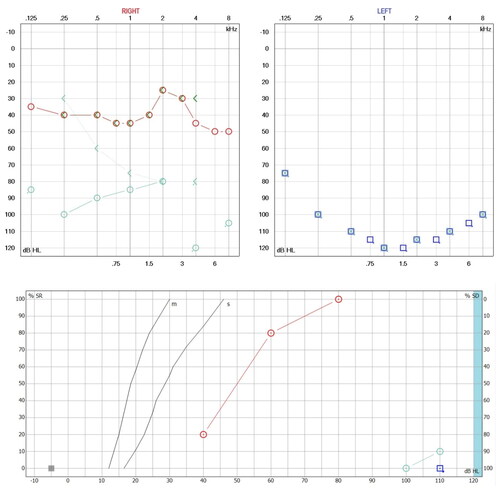
Figure 2. Tomography (TC) and magnetic resonance imaging (MRI) without alterations. (A) CT Axial reconstruction of the cochlea on the right side and lateral semicircular canal, with adequate diameter of the internal auditory canal (normal ear). (B) CT Axial reconstruction of the left side cochlea and lateral and superior semicircular canals (normal ear). (C) CT Axial reconstruction of the left side cochlea and lateral and superior semicircular canals (normal ear). (D) MRI reconstruction of the cochlea and semicircular canals in right ear. (E) MRI reconstruction of the cochlea and semicircular canals in left ear.
Intercurrent evaluations: Antinuclear antibodies test (ANA) with positive levels of 1:80 and antimyeloperoxidase (MPO) and proteinase 3 (PR3) negative antibodies were requested in the otolaryngology department. Treatment with prednisone was indicated for 18 days with a reduction scheme of 50 mg to 10 mg, however, treatment was only continued for 10 days. Subsequently, she was evaluated in the rheumatology service, where a battery of studies for autoimmune disease was complemented, reporting the presence of lupus anticoagulant, with negative anti-cardiolipin IgG and IgM antibodies, negative anti-Beta 2 glycoprotein 1, negative anti-DNA, negative anti-Ro, negative anti-La, and negative anti-Sm. Lupus anticoagulant testing was made with base on 2009 International Society on Thrombosis and Haemostasis recommendations committee. The test uses a pooled normal plasma for ratio normalization, and a ratio for the mixing test interpretation. The positive ratio is considering up a 1.2 result. The dRVVT (Dilute Rusell Viper Venom Time) helps for screen and confirm the LA presence. The first determination was realized without anticoagulation therapy. The first ratio was 1.6. The posterior results were always positive with a value of 2.1. We ruled out the thrombophilia disorders. The diagnosis of non thrombotic antiphospholipid syndrome was made, starting treatment with prednisone (2 mg/kg) by 3 months, azathioprine (1.5 mg/kg/day by 6 months), hydroxychloroquine (4 mg/kg/day), aspirin antiaggregant dose (3 mg/kg/day), and rivaroxaban (15 mg for three months). Rivaroxaban was considered as an initial therapeutic option, based on its anticoagulant effect by inhibiting factor Anticoagulation based on the inhibition of vitamin K with the use of warfarin was chosen. The warfarin dose was adjusted based on the INR result, making its management dynamic. Regarding audiology, prior informed consent signed by the parents, a retroauricular curve-type digital hearing aid was adapted for moderate to profound loss, which provided adequate gain for 4 months until the hearing loss progressed to the level of profound hearing loss and the placement of a cochlear implant (strip of hearing loss) was decided (Figure ; aquamarine lines). Synchrony electrodes, flex 28 with Med-El brand Rondo 2 processor) in the right ear. The surgery was carried out without complications (Figure ), impedance telemetry was performed, finding adequate responses in the 12 pairs of electrodes, in addition to obtaining a neural response telemetry with adequate electrically evoked compound action potentials (Electrically evoked compound action potentials eCAP) (Figure ). After two audiological adjustment sessions in a period of two months, normal right hearing evidenced in the audiogram was achieved (Figure ; aquamarine lines), and the evaluation of the phonetically balanced word list according to Dr. Tato’s report [Citation7], and which as a whole shows a good gain for a person with profound hearing loss.
Figure 3. Cochlear implant electrode placement surgery. Surgical image of the right ear, posterior tympanotomy, stapedial tendon and pyramidal process with adequate visualization of the round window. (A) Previous insertion of the cochlear implant electrode. (B) Initiating cochlear implant electrode insertion. (C) Complete cochlear implant electrode insertion.
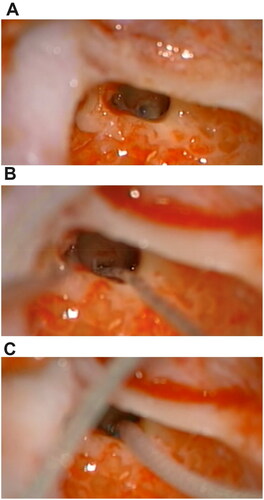
Figure 4. Adequate auditory responses after implant programming. (A) Impedance telemetry with adequate electrical responses in the 12 pairs of electrodes in the last programming session. (B) Postoperative automatic response telemetry of the auditory nerve (AutoART) which shows adequate responses of electrically evoked compound action potentials (eCAP) of the auditory nerve. The patient was found without any discomfort during the programming. (C) Levels (MCL-most comfortable loudness) in their last programming, which are balanced, sufficient and did not cause any discomfort for the patient. With these MCL levels, an audiogram is made which is within normal hearing parameters.
Figure 5. Hearing improvement by cochlear implant. (A) Audiometric study (Red Lines) performed 45 days after switching on the cochlear implant processor. Note the improvement in hearing thresholds in the sound field and the improvement in phonemic discrimination in speech audiometry (logoaudiometry). (B) Audiometric study (Aquamarine lines) carried out 30 days after the second programming of the cochlear implant processor, showing an average of 24 dB in pure audible tones (PTA), and phonemic discrimination on free field audiometry is 90% at 70 dB in the right ear.
Discussion
Inner ear involvement has been reported in APS and may be related to antibodies directed to the small vessels of the labyrinthine circulation. Endothelial cells can be damaged by free radicals within the cochlear circulation by direct activation of antiphospholipid antibodies. These hyperstimulated endothelial cells would initiate the formation of local microthrombi and subsequent ischemia in the inner ear [Citation8].
Mouadeb & Ruckenstein conducted a prospective study with 168 patients who suffered from progressive sensorineural hearing loss. They underwent a panel to detect autoimmune diseases in their blood, including anticardiolipin (aCL) antibodies, anti-B2 glycoprotein, and lupus anticoagulant (LAC). In this population, forty-two patients (25%) had at least one elevated antiphospholipid antibody marker; twenty patients had two or more positive results, suggesting that antiphospholipid antibodies might be involved in the pathogenesis of some forms of hearing loss and inner ear involvement, related to microthrombus formation in the labyrinthine circulation [Citation9]. The treatment recommended for autoimmune hearing loss are corticosteroids in a short time, and the use of anticoagulation based on pathophysiology.
In the case of our patient, there was no clinical or serological evidence to demonstrate another connective tissue disease; the only clinical manifestation was progressive hearing loss. Cochlear implants are the mainstay of treatment for most hearing-impaired children with severe to profound hearing loss who want to acquire or recover spoken language. Implants are indicated for children (> 12 months of age) who do not make the expected progress with adequate amplification from hearing aids. Cochlear implants can be placed unilaterally or bilaterally, be simultaneous or sequential (delayed in another surgery) and can be used in combination with bimodal amplification (use a hearing aid in the contralateral ear) [Citation10].
The cochlear implant has been used in both primary and secondary autoimmune diseases, generating a marked improvement in hearing and phonemic discrimination. Early cochlear implant placement provides long-lasting hearing in addition to reducing the side effects of systemic immunosuppressants. It should also be mentioned that the hearing gained can be affected if there is ossification or cochlear fibrosis. There are no additional risks to those universally accepted for cochlear implant surgery [Citation11].
In our patient, good performance was achieved from the power on of the processor and normal hearing was achieved in the right ear in the audiogram with a phonemic discrimination of 80% at 55 dB and a normal Ling Test, which shows good audiological gain for a person with profound hearing loss. A second cochlear implant was not placed due to lack of economic resources, and the implant placed was donated by the state health system, and currently, the patient receives speech therapy and continues with her treatments and rehabilitation at the Poblano’s Child Hospital.
The incidence of idiopathic sudden sensorineural hearing loss (SNHL) is approximately 20 out of 100 000 people per year and is classified as idiopathic after careful exclusion of the many causes of SNHL. The site of the lesion resulting in sensorineural deafness can be predominantly localized to the cochlear but may also be caused by retrocochlear lesions. Much of the evidence linking the immune system with cochleovestibular dysfunction is indirect, largely as an interpretation of case reports and response to immunosuppressive treatment.
Thrombosis in APS can be a cause of hearing loss, small infarcts of the cochlear tissue have been found in subjects with multifocal microangiopathic encephalopathies and deafness associated with autoimmune diseases. In addition, in vivo animal studies have shown that vascular obstruction in the inner ear causes reduced intracochlear oxygenation and, ultimately, a significant loss of auditory response, which could be a cause of hearing loss in adults. children with primary APS [Citation12].
Vyse et al. presented a case of a 23-year-old primigravida who presented as an emergency at 27 weeks gestation with fulminant pre-eclampsia and bone marrow necrosis. As her mental state improved it became apparent that she was severely deaf, and she complained of a loss of balance. There was profound bilateral hearing loss, and the caloric response was absent bilaterally. Short term steroid therapy did not result in improvement of the auditory thresholds. IgG anticardiolipin antibody level was grossly elevated and there was a weakly positive lupus anticoagulant. Long term anticoagulation with warfarin was commenced. As expected, there was no improvement in hearing thresholds after a 5 year follow up, but she did not have recurrent thrombotic events [Citation13]. Making treatment decisions is challenging, however, there is no treatment superior to corticosteroids. Considering that hearing loss is a severe feature of APS, mixed treatment with anticoagulants and immunosuppressants might be a reasonable approach, but still with lack of evidence. Furthermore, although the effect or usefulness of azathioprine has not been demonstrated in patients with hearing loss, its use was decided based on its theoretical effect on autoimmunity processes and immunosuppressive effect.
Therefore, it could be indicated that the only treatment for APS does not lead to improvements in hearing, so alternatives that allow hearing recovery should be considered, such as cochlear implants that give a good acceptance response and functional improvement. In conclusion, the APS with a single manifestation of sensory hearing loss is rare in children, so its timely detection is essential. Additionally, consider that hearing loss can result from a viral infection associated with the development of the autoimmune disease. The cochlear implant is very useful for the rehabilitation of profound hearing loss secondary to primary antiphospholipid syndrome, however, long-term evidence of the benefits of the cochlear implant in these patients is lacking.
Informed consent statement
Written informed consent was obtained from patient involved in the study.
Institution review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and ethical review and approval was waived for this study based on the design of the study and according to the national guidelines.
Acknowledgments
The authors wish to thank PhD. Julio César Tobon-Velasco (CMNBm) for supporting the writing and analysis of the work, and to audiologist Lina Rubio Gaviria (Med-El clinical support) for reviewing the language of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Madison JM, Zuo Y, Knight JS. Pediatric antiphospholipid síndrome. Eur J Rheumatol. 2019;7(Suppl1):S3–S12. doi:10.5152/eurjrheum.2019.19160.
- Madison JM, Gockman K, Hoy C, et al. Pediatric antiphospholipid syndrome: clinical features and therapeutic interventions in a single center retrospective case series. Pediatr Rheumatol. 2022;20(1):17. doi:10.1186/s12969-022-00677-8.
- Díaz-Coronado JC, Herrera-Uribe S, Hernández-Parra D, et al. Antiphospholipid syndrome (APS): clinical and immunoserological differences between primary and secondary APS in a colombian cohort. Rev. Colomb. Reumatol. 2021;28(3):191–196. doi:10.1016/j.rcreue.2021.06.003.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x.
- Sciascia S, Amigo MC, Roccatello D, et al. Diagnosing antiphospholipid syndrome: “extra- criteria” manifestations and technical advances. Nat Rev Rheumatol. 2017;13(9):548–560. doi:10.1038/nrrheum.2017.124.
- Hassan F, Naffaa ME, Saab A, et al. Cognitive impairment in anti-Phospholipid syndrome and anti-Phospholipid antibody carriers. Brain Sci. 2022;12(2):222. doi:10.3390/brainsci12020222.
- Tato JM. Características acústicas de nuestro idioma. Revista Otolaringológica. 1966;1(1):151–250.
- Ralli M, D'Aguanno V, Di Stadio A, et al. Audiovestibular symptoms in systemic autoimmune diseases. J Immunol Res. 2018;2018:5798103. doi:10.1155/2018/5798103.
- Mouadeb DA, Ruckenstein MJ. Antiphospholipid inner ear syndrome. Laryngoscope. 2005;115(5):879–883. doi:10.1097/01.MLG.0000158666.15447.37.
- Awad R, Oropeza J, Uhler KM. Meeting the joint committee on infant hearing standards in a large metropolitan children’s hospital: barriers and next steps. Am J Audiol. 2019;28(2):251–259. https://pubs.asha.org/doi/10<?sch-permit JATS-0034-007?>.1044/2019_AJA-18-0001 doi:10.1044/2019_AJA-18-0001.
- Lee J, Biggs K, Muzaffar J, et al. Hearing loss in inner ear and systemic autoimmune disease: a systematic review of post-cochlear implantation outcomes. Laryngoscope Investig Otolaryngol. 2021;6(3):469–487. doi:10.1002/lio2.563.
- Fleetwood T, Cantello R, Comi C. Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy. Front Neurol. 2018;9:1001. doi:10.3389/fneur.2018.01001.
- Man YL, Sanna G. Neuropsychiatric manifestations of antiphospholipid syndrome-a narrative review. Brain Sci. 2022;12(1):91. doi:10.3390/brainsci12010091.

