 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Angiosarcoma is a rare malignant tumor, even rarer particularly in the head and neck. It is considered a very aggressive neoplasm with high rates of local recurrence and a poor prognosis. We report a case of nasal angiosarcoma in which the patient underwent endoscopic surgery and postoperative chemoradiotherapy and is in good health 30 months after the treatment, without any recurrence. This result suggests that sinonasal angiosarcoma might have a better prognosis than angiosarcomas arising in other sites if it is treated appropriately. According to our review of the literature on sinonasal angiosarcomas, the 3-year overall survival rate for sinonasal angiosarcomas is 63.7%, which is better than the survival rate for angiosarcomas in other sites. Lymph node and distant metastases were rarely seen. In sinonasal angiosarcoma, appropriate resection and local control of the tumor with radiation therapy or chemoradiotherapy appear to result in a favorable outcome.
1. Introduction
Angiosarcoma is a rare malignant tumor, accounting for fewer than 2% of all soft tissue sarcomas and fewer than 1% of head and neck malignancies [Citation1]. It is considered a very aggressive neoplasm with high rates of local recurrence, early metastasis, and a poor prognosis [Citation1]. It can occur in any part of the body, but it typically arises in the skin of the head and neck. Angiosarcomas arising in the nasal cavity or sinuses are even rarer, with only a few case reports. Epistaxis and nasal obstruction are often described as chief complaints [Citation2], with a duration ranged from two months up to two years [Citation3]. The appearance of the tumor is described as nodular and polypoid, soft and friable, purple to red, and often ulcerated with associated hemorrhage or clot and necrosis [Citation3]. Therefore, when a patient with a polypoid tumor in the nose presents with long-lasting epistaxis, this disease should also be taken into consideration.
Because of its rarity, no guidelines for treatment for this disease have been established. We report a case of angiosarcoma in the nasal cavity that was treated by endoscopic resection, followed by chemoradiotherapy, in which complete remission has been maintained in the 30 months since. To investigate the treatment in detail and to compare the outcomes of treatment of angiosarcomas in the sinonasal region and in other parts of the body, we also reviewed the literature regarding sinonasal angiosarcoma.
2. Case report
A 74-year-old woman presented to our hospital with epistaxis from the left nostril that had lasted 1 month. During the endoscopic examination, we found a tumor with a smooth, irregular white surface in her left nasal cavity. No abnormal findings were noted in other otolaryngological regions. The patient had undergone bilateral Caldwell–Luc surgery for chronic rhinosinusitis at the age of 20 years and endoscopic sinus surgery (ESS) for a left-sided postoperative maxillary sinus cyst at the age of 60 years. Her medical history included both ischemic heart disease and interstitial pneumonia.
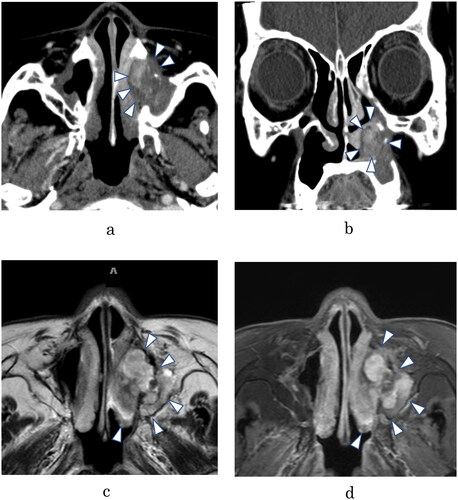
Contrast enhanced computed tomography showed that the tumor was situated mainly in the inferior meatus and was partly enhanced. Because of her past sinonasal surgery; however, determining whether bone erosion or bone destruction had occurred was difficult (). Magnetic resonance imaging also showed the mass in the same region, which was enhanced by gadolinium on T1-weighted images and represented by bright signals on T2-weighted images (). A biopsy sample from the mass revealed a proliferation of bundles of spindle cells with irregularly shaped and swollen nuclei and scattered mitotic figures, doubting a possibility of malignant tumor. Nevertheless, manifestations of inflammatory lesions such as granulation tissue were found, and so, it was unclear whether the mass was merely a benign lesion in this biopsy specimen.
Figure 1. (A) Axial contrast enhanced (CE) computed tomographic (CT) scan, showing opacification of the inferior meatus, partly enhanced. (B) Coronal CE-CT scan. It is difficult to evaluate bone erosion or bone destruction accurately, but neither is apparent. (C) T2-weighted axial magnetic resonance imaging (MRI), showing a heterogeneous high-intensity signal in the same area as on the CT scan. (D) T1-weighted MRI with gadolinium, showing heterogeneous enhancement of the lesion.
Because of the possibility of malignancy, we conducted ESS to excise the tumor both to confirm the diagnosis and to ensure complete resection. The tumor extended from the inferior meatus to the middle meatus and was attached to both the inferior and middle turbinates. No remarkable adhesion was observed. Intraoperative rapid pathological examination of the tumor could not confirm malignancy. We resected the tumor and the turbinates, with a margin of a few millimeters, and additional rapid pathological examination of the surrounding tissues revealed no tumor cells, which indicated that the tumor was excised completely ().
Figure 2. (A) The tumor was in the inferior meatus and was in contact with the inferior turbinate and middle turbinate. (B) Appearance after resection of the tumor. The middle turbinate (*) and inferior turbinate (**) were also resected. No characteristics of malignancy were found. (C) The resected tumor. (D) Specimen stained with hematoxylin and eosin (H&E; 40), showing sheets of atypical spindle cells. Scale bar: 50 μm. (E) Stained specimen (H&E,
200), showing several mitotic figures. Scale bar: 100 μm. (F) Immunohistochemical staining for erythroblast transformation-specific-related gene (ERG;
200). Tumor nuclei are positive for ERG, which is highly expressed in endothelial cells. Scale bar: 100 μm.
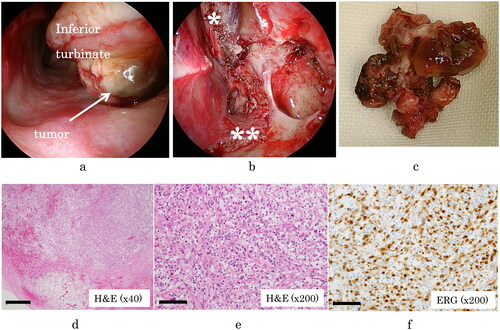
On pathological examination, the resected tumor showed atypical spindle cells growing with a solid pattern, and mitotic figures were easily identified. Few anastomotic vascular channels were observed, which was unusual for angiosarcoma. However, immunohistochemical staining yielded positive results for erythroblast transformation-specific-related gene (ERG), a sensitive marker of endothelial differentiation and for no other markers. Thus, the final conclusive diagnosis was angiosarcoma ().
Several clinical examinations were added after the surgery, and we determined that the tumor was an angiosarcoma of the nasal cavity (cT1N0M0). Hence, we planned postoperative chemoradiotherapy, starting 52 days after surgery, despite the surgically clean margin. We administered docetaxel, 30 mg/m2, every 3 weeks, and 60 Gy of radiation was delivered concurrently. After the first dose of docetaxel, a taste disorder (Common Terminology Criteria for Adverse Events [CTCAE] grade 1) and neutropenia (CTCAE grade 3) developed. No severe adverse events necessitated the discontinuation of chemotherapy, but before the second dose of docetaxel could be administered, the patient discontinued this treatment because of malaise. We continued her treatment with radiotherapy alone, which she completed. In the 30 months since her first treatment, she has maintained complete remission, without any recurrence or metastasis.
3. Discussion
Angiosarcoma is quite rare, accounting for fewer than 2% of all soft tissue sarcomas and fewer than 1% of head and neck malignancies. The risk factors for the development of angiosarcoma remain unknown, but several, such as a history of trauma, telangiectatic skin lesions, and chronic edema, are suspected to be the causes [Citation4]. Angiosarcomas can occur in any part of the body; however, 60% arise in skin or superficial soft tissue, and 15%–47% have been reported to arise in the viscera, such as those of the breast, heart, and liver [Citation5]. Half of the cutaneous angiosarcomas occur in the head and neck skin or soft tissue and frequently occur on the scalp in elderly men. The prognosis for cutaneous angiosarcomas is poor: the reported 5-year survival rate ranges from 10% to 34% [Citation6].
Few cases of sinonasal angiosarcoma have been reported, and their clinical characteristics remain unclear. As mentioned above, previous case reports have shown that patients tend to present with a chief complaint of long-lasting epistaxis and nasal obstruction, however, it is difficult to assume angiosarcoma from the clinical features or imaging studies, since similar symptoms would be observed in various kinds of tumors including pyogenic granuloma, inverted papilloma and malignant melanoma [Citation7]. Therefore, pathological findings are critical for a definitive diagnosis, and immunohistochemistry is particularly essential to distinguish this disease from similar neoplasms. As for angiosarcoma, although Factor VIII antigen and CD34 were considered to be reliable marker to indicate endothelial cells by immunohistochemistry, ERG is now considered the most sensitive and specific marker for endothelial differentiation [Citation8].
Comprehensive guidelines for head and neck angiosarcoma have not been established; previous cases of sinonasal origin had been treated with strategies similar to those used for dermatological angiosarcomas. The reported treatment comprised surgery, chemotherapy, and radiotherapy, among which complete surgical resection was considered especially important [Citation6]. Treatment was most effective when surgical margins by wide excision had no cancer cells, but because of the anatomical difficulty of accessing the sinonasal region and the extensive vascular spread of these tumors, achieving complete resection was often difficult [Citation9]. The most efficient treatment strategy for angiosarcoma may be resection that is as complete as possible, followed by radiotherapy or chemoradiotherapy to cover the possibility of remaining cancerous microlesions. In our patient, complete surgical resection was achieved without pathological remnant, however adequate surgical margin was not obtained due to sinonasal anatomical traits together with her previous surgical history. Therefore, we considered postoperative some additional therapies were necessary to get complete remission. Chemoradiotherapy which had a highest treatment intensity was chosen at first. Unfortunately, she quit the chemotherapy at one time due to unbearable side effects. We continued postoperative additional therapy to carry out radiotherapy alone.
Between 1979 and 2020, only 22 references reported angiosarcomas primary in the nasal cavity and paranasal sinuses (). In almost all cases, the tumor was localized at the first examination; distant metastasis was present in only two cases, and no lymph node metastasis was observed. In cases reported until the 1980s, treatment involved mainly radiotherapy, but more recently, especially after 2000, most cases were treated first with surgery and postoperative radiotherapy or chemoradiotherapy. This strategy was based on the assumption that wide excision was the most efficient treatment of angiosarcoma and that chemotherapy and radiotherapy would destroy any remaining tumor cells. Surgery followed by radiotherapy (or chemoradiotherapy) was more effective than earlier strategies: in the 1970s and 1980s, of the five patients who underwent first radiotherapy and then surgery, two died, whereas after the 1990s, of the 15 patients who underwent first surgery and then radiotherapy, only two died.
Table 1. Literature review of 23 cases of sinonasal angiosarcoma, including that of our patient.
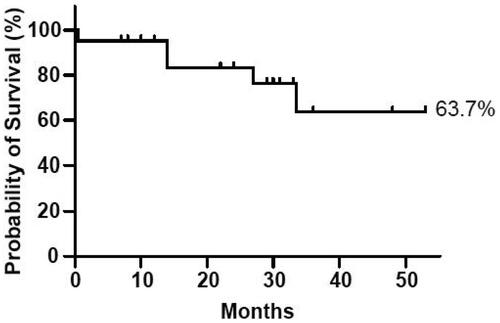
With appropriate treatment, the prognosis may be better for patients with sinonasal angiosarcoma than for those with angiosarcoma in another region. In 2019, Lee et al. reported that among 1250 patients with head and neck angiosarcomas (93% of which were cutaneous), the 2-year overall survival rate was 47.3% [Citation10]. Sinnamon et al. reporting about patients with clinically localized cutaneous and soft tissue angiosarcoma who underwent resection, calculated the 5-year overall survival rate as 39.7% [Citation11]. Investigations of localized nasal or paranasal angiosarcoma are sparse in the English literature because only a few cases have been reported [Citation1,Citation2,Citation4,Citation9-10,Citation12-18], but according to one 2009 report, the rate of 5-year overall survival with sinonasal angiosarcomas was 22% [Citation2]. Despite the paucity of data regarding sinonasal angiosarcoma, treatment seems to have improved during the past several decades. Summarizing the data from the collected literature, the 3-year survival rate was estimated at 63.7%. (). Although the rate of 5-year overall survival is more typically used to estimate prognosis, this 3-year rate indicates that the prognosis is better than that of other angiosarcomas.
Figure 3. Kaplan–Meier survival curve representing the patients in our review, generated with GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). We calculated the probability of 3 year survival as 63.7%.
In our patient, since the biopsy results before treatment were inconclusive and malignancy could not be ruled out, we performed ESS as diagnostic treatment while preparing for maxillectomy if necessary. Although intraoperative rapid pathological diagnosis could not determine whether the tumor was malignant or not, no malignant cells were found in the surrounding tissues, suggesting that sufficient resection had been achieved. As the tumor was found to be angiosarcoma after the surgery, considering its high grade of malignancy, we planned postoperative chemoradiotherapy with docetaxel, which was reported to be an efficient agent for head and neck tumors. This strategy would have enabled more precise and adequate treatment. The prognosis of sinonasal angiosarcoma could be good with tight control of local cancer and multidisciplinary treatment.
4. Conclusion
Treatment of sinonasal angiosarcoma requires a multidisciplinary strategy, and adequate and precise resection is especially important. Local control with both surgery and chemoradiotherapy can help manage the disease. Because of the rarity of this tumor, more detailed reports are needed to establish an accurate treatment strategy.
Ethical statement
The authors have obtained informed consent from the patient.
Informed consent
Authors confirm that consent was obtained from the patients for the study.
Acknowledgements
The authors would like to thank Xtra (https://www.x-tra.jp/vision) for the English language review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kurien M, Nair S, Thomas S. Angiosarcoma of the nasal cavity and maxillary antrum. J Laryngol Otol. 1989;103(9):874–876. doi: 10.1017/s0022215100110369,
- Treviño-González JL, Santos-Lartigue R, González-Andrade B, et al. Angiosarcoma of the nasal cavity: a case report. Cases J. 2009;2(1):104. doi: 10.1186/1757-1626-2-104,
- Nelson BL, Thompson LD. Sinonasal tract angiosarcoma: a clinicopathologic and immunophenotypic study of 10 cases with a review of the literature. Head Neck Pathol. 2007;1(1):1–12. doi: 10.1007/s12105-007-0017-2, PMID: 20614274.
- Fukushima K, Dejima K, Koike S, et al. A case of angiosarcoma of the nasal cavity successfully treated with recombinant interleukin-2. Otolaryngol Head Neck Surg. 2006;134(5):886–887. doi: 10.1016/j.otohns.2005.03.053.
- Juan CJ, Yu CY, Hsu HH, et al. Visceral and non-visceral angiosarcoma: imaging features and clinical correlation. Chin J Radiol. 2000;25:183–189.
- Olcott CM, Karakla DW. Innovative application of intraoperative laser-assisted fluorescence angiography in resection of an angiosarcoma of the scalp. Am J Otolaryngol. 2017;38(6):710–712. doi: 10.1016/j.amjoto.2017.08.006,
- Sireci F, Dispenza F, Lorusso F, et al. Tumours of nasal septum: a retrospective study of 32 patients. Int J Environ Res Public Health. 2022;19(3):1713. doi: 10.3390/ijerph19031713. PMID: 35162736.
- Ronchi A, Cozzolino I, Zito Marino F, et al. Primary and secondary cutaneous angiosarcoma: distinctive clinical, pathological and molecular features. Ann Diagn Pathol. 2020;48:151597. doi: 10.1016/j.anndiagpath.2020.151597. PMID: 32829071.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19(3):191–195. doi: 10.1055/s-0035-1547520,
- Lee KC, Chuang SK, Philipone EM, et al. Characteristics and prognosis of primary head and neck angiosarcomas: a surveillance, epidemiology, and end results program (SEER) analysis of 1250 cases. Head Neck Pathol. 2019;13(3):378–385. doi: 10.1007/s12105-018-0978-3,
- Sinnamon AJ, Neuwirth MG, McMillan MT, et al. A prognostic model for resectable soft tissue and cutaneous angiosarcoma. J Surg Oncol. 2016;114(5):557–563. doi: 10.1002/jso.24352,
- Tomovic S, Kalyoussef E, Mirani NM, et al. Angiosarcoma arising from the frontal sinus. Am J Otolaryngol. 2014;35(6):806–809. doi: 10.1016/j.amjoto.2014.08.003,
- Es-Sbissi F, Nitassi S, Boulaadas M, et al. Sinonasal angiosarcoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(3):161–163. doi: 10.1016/j.anorl.2015.01.006,
- Deshmukh SD, Gaurish SKS, Pathak N, et al. High grade angiosarcoma of nasal cavity and paranasal sinuses: a rare case with immuno-histopathological study. Indian J Pathol Microbiol. 2015;58(4):570–572. doi: 10.4103/0377-4929.168878,
- Sun D, Yu R, Chen J, et al. A rare case of primary paranasal sinus angiosarcoma with pulmonary metastasis detected by 18F-FDG PET/CT. Clin Nucl Med. 2015;40(3):286–288. doi: 10.1097/RLU.0000000000000694,
- Williamson IG, Ramsden RT. Angiosarcoma of maxillary antrum—association with vinyl chloride exposure. J Laryngol Otol. 1988;102(5):464–467. doi: 10.1017/s0022215100105365,
- Gravvanis A, Lagogiannis G, Kyriakopoulos A, et al. Angiosarcoma of the nasal septum mimicking reticulohistiocytoma. J Craniofac Surg. 2013;24(3):e276–e279. doi: 10.1097/SCS.0b013e31828f2a19,
- Chung HJ, Koh MJ, Kim CH, et al. A patient with submerged sinonasal angiosarcoma after resection of underlying organizing hematoma. J Craniofac Surg. 2018;29(3):645–647. doi: 10.1097/SCS.0000000000004144,

