Abstract
While laparoscopic surgery has become increasingly widely used, many laparoscopic procedures are time-consuming and difficult to accomplish compared to open surgery. One such procedure is the ligation of endless organs. In this paper, the development and prototyping of a laparoscopic instrument that could significantly increase the efficiency of laparoscopic ligation is outlined. The mechanism is based on a snake-like flexible structure which is actuated by control wires. A simple simulation was carried out by both experienced surgical staff as well as non-surgical persons to confirm the effectiveness of the proposed mechanism.
Background
Since the first acknowledged laparoscopic surgery in 1987 supported by the development of a video computer chip camera that allowed the surgeon to view operative maneuvers on a TV screen in 1986, laparoscopic surgery has made rapid development [Citation1]. Compared to conventional open surgery, laparoscopic surgery results in less trauma as it uses several small incisions ranging from several millimeters to several centimeters. This kind of minimally invasive surgery typically results in less blood loss during surgery and a reduced period of hospitalization. On the other hand, laparoscopic surgery typically requires about 50% more operating time, requires a high level of surgical skill and conversion to open surgery is required in the case of unexpected findings or complications the surgery cannot be satisfactorily and/or safely carried out with laparoscopic surgery alone. Despite the limitations, laparoscopic surgery has become the standard for many procedures and is used extensively [Citation2,Citation3]. () shows a comparison of laparoscopic and open resections for colon cancer in the case of elective surgery from 2006 to 2012 [Citation4].
Figure 1. Comparison of laparoscopic and open resections for colon cancer, 2006–2012 [Citation4].
![Figure 1. Comparison of laparoscopic and open resections for colon cancer, 2006–2012 [Citation4].](/cms/asset/2355fc7b-b4c8-4fce-87d2-8e6d415d0ee5/icsu_a_1378790_f0001_c.jpg)
As we enter the second decade of the 21st century, significant effort continues towards the optimization of laparoscopic instruments and procedures towards reduced invasiveness, increased safety, increased functionality and reduced operating time. The range of surgeries possible laparoscopically continues to expand. Laparoscopic Surgery at the time of writing is divided as follows: Single-Incision Laparoscopic Surgery (SILS), Single-Port Surgery (SPS) and Laparoendoscopic Single Site Surgery (LESS) [Citation5], Mini-Laparoscopic Surgery and minilaparoscopy-assisted natural orifice surgery (MANOS) [Citation6] and both Hand assisted laparoscopic surgery (HALS) and Conventional Laparoscopic Surgery (CLS).
The difference between the types of laparoscopic surgeries are in the number of ports and the port sizes, shown in .
Table 1. Types of laparoscopic surgery, number of ports and port size.
While laparoscopic surgery has become mainstream, research and development has plateaued due to the inherent difficulties in reducing the physical size of surgical instruments without compromising robustness, safety and at the same time attempting to increase functionality.
Introduction
With the development of laparoscopic surgery during the past 30 years, the devices built for laparoscopic surgery have continued to progress through both technological innovation and the recycling of former technology. CCD camera technology contributed significantly to the success of the 1st generation of laparoscopic cholecystectomy. The progress of modern laparoscopic surgical devices has been largely driven by needs.
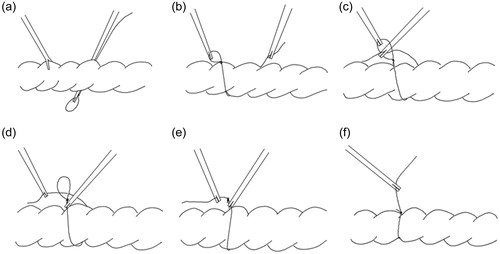
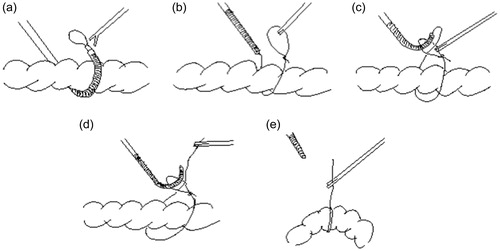
This study focuses on rectal ligation, as the surgeons at the Nagasaki University hospital have sited a need for a more efficient mechanism to carry out this procedure laparoscopically. During laparoscopic surgery ligation of terminal organs for example the appendix is typically carried out by forming a slip knot extracorporeal and then inserting the knot with forceps through a trocar into the abdomen and placing it over the organ – appendix. But for endless organs such as the sigmoid colon, initially a thread pre-prepared with a slip knot needs to be inserted under and around the organ, (in the case of such intestinal organs a small incision is required through the mesentery – sigmoid mesocolon in this case) the tip of the forceps holding the slip knot needs to be carefully manipulated to bring it through this incision into visual range of the laparoscopic camera refer (). Once the tip is sited the knot is grasped by a second set of forceps as shown in (), and then the tail of the thread is brought through the eye of the slip knot (), released and re-grasped from the opposite side of the slip knot () and the knot is tightened (). Finally, the organ may be suspended as required, in the case of the sigmoid colon typically a puncture is usually made in the abdominal wall through which the sigmoid colon will be suspended. Based on a survey of local surgeons this laparoscopic procedure typically takes about 5 minutes in the case of experienced surgeons. In the case of conventional open surgery, the time required is about 1 minute.
Laparoscopic ligation requires manipulation outside of camera range while the thread is brought behind the organ, and as such there is some risk in regard to unforeseen obstructions and/or difficulty in bringing the forceps back into view around the far side of the organ. Thus, this research focuses on developing an instrument that can both reduce the time required for the procedure as well as reducing this risk.
Development
Model 1
At the present point in time flexible snake like structured devices providing multiple degrees of freedom (DOF) are used extensively in minimally invasive surgery (MIS). Such mechanisms are typically belt [Citation7] or wire [Citation8,Citation9] controlled. Such mechanisms are essential to provide distal dexterity on such as forceps in the case of single port MIS.
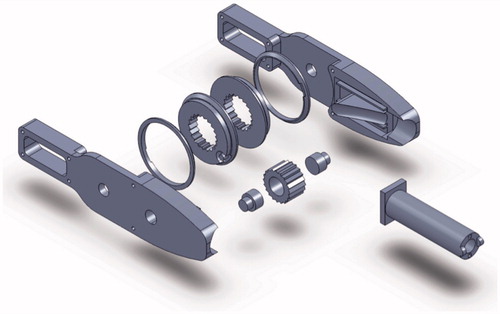
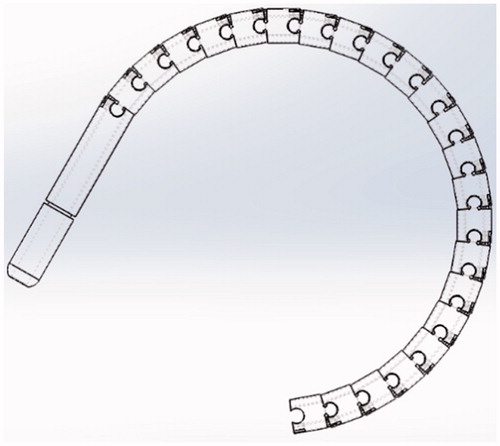
For optimizing the ligation of endless organs, the authors considered various types of flexible structures, and selected a multi-segmented snake-like structure which provides the flexion and stability necessary to guide the tip of a thread around the far side of continuous organs in this case specifically the sigmoid colon. The first prototype referred to as model 1 provided a minimum flexion of 10 centimeters – shown in ( and ). With reference to (), a pre-looped thread unit is loaded into a (larger) tube as can be seen in (), the thread itself consists of a smaller tube containing the pre-looped thread. This mechanism provides a single degree of freedom (DOF). Latitudinal movement is provided by rotating the entire unit. To actuate this single DOF mechanism, a control wheel was coupled to provide push – pull operation of the two actuating cables which feed through the shaft to the tip, refer to (). Details of this design are outlined in the following section.
Design
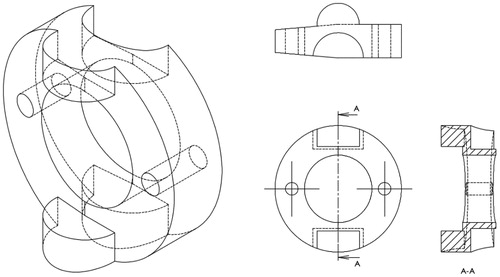
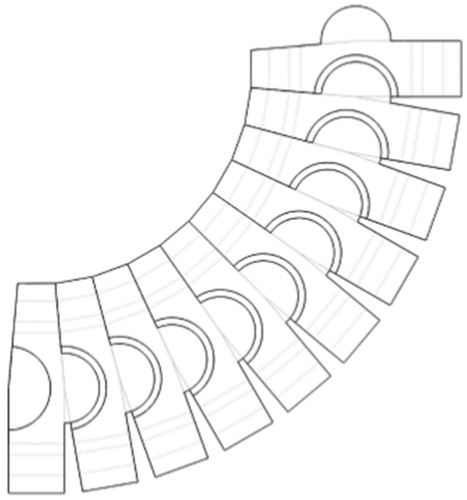
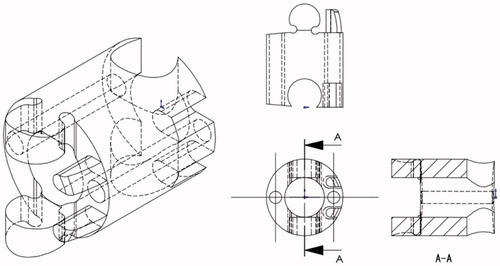
The snake-like flexible structure consists of a series of ring like units of which the design of a single ring is shown in (). Each ring has two semicircular male and female joints and provides two eyelets through which the actuating cables feed and the central cavity providing passage for the preloaded thread units. By serially connecting the rings in a configuration as shown in (). (side view of rings) the required flexion can be achieved.
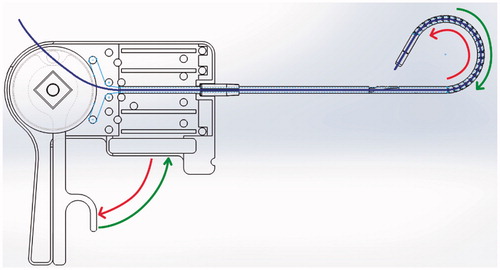
The tip is controlled by control wires configured as shown (). The respective upper and lower cables are diametrically coupled with the control wheel and thus provide smooth control of the flexion of the mechanism.
With reference to (), the flexion angle facilitated by each ring is given by α from 0 to π/18, where n is the number of rings and the total flexion angle at the tip βTip can be expressed as:
(1)
The maximum flexion angle at the tip is:
The wire’s length Lwire under flexion can be expressed as follows:
(3)
l is the distance from the ring’s center to the center of the hole provided for the actuation wire and H is the incremental thickness of each ring.
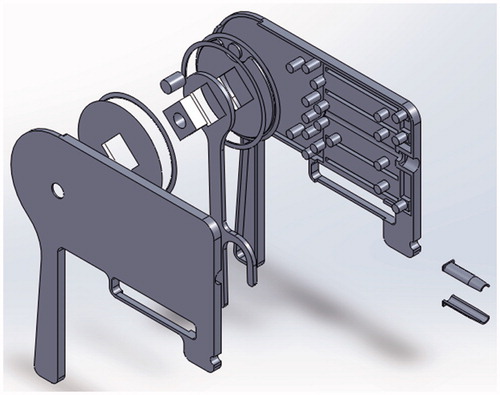
An assembly of the control unit is shown in ().
3-D building and testing
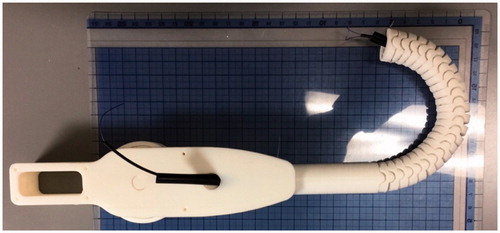
Based on the above design the first model laparoscopic ligation mechanism proof of concept model was fabricated using an MF-2200 D (Mutoh) 3 D printer using PLA plastic. While the target diameter of the mechanism was 5 to 10 mm, the resolution of this particular printer (50um max.) prevented actual size printing, after some trial and error a 20 mm diameter was selected. The fully assembled model is shown in () with no flexion and in () at maximum flexion.
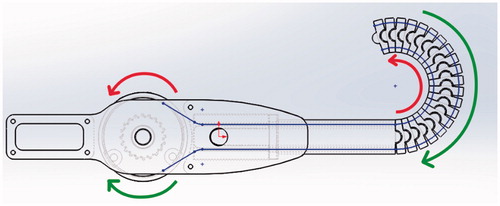
The mechanical functionality of the Model 1 laparoscopic ligation concept prototype mechanism as shown in and , and associated trials confirmed the feasibility of the mechanism. Smooth control of the flexion was achieved from 0 to π by rotating the wheel on the handle of the control unit. The pre-looped thread unit mentioned previously could be easily fed into position ready for use. Control mechanics of the tip is shown by red and green arrows, actuation is achieved by the control wires connecting the tip to the control wheel – shown by a thin blue line in ().
However, a number of difficulties with this design were encountered as follows: Some mechanical instability between the series of ring units was encountered under certain conditions, the cause was the hemispherical nature of the male female connectors. Specifically operating on its side with respect to gravity the tip drooped in the straight configuration as well as resulting in some rotational error. The mount through tube was located on the right-hand side of the control unit, making operation easy for right handed persons but somewhat awkward for left handed users. Finally, the snake-like structure assembled with only 24 rings provided a maximum angle of π, which provided an insufficient range margin for the experiments.
Model 2
Model 2 was designed and built to deal with the limitations of Model 1.
Design
While the fundamental snake-like flexible structure was unchanged, redesign of the ring units and the use of a higher resolution 3-D printer made a 5 mm (3 mm inner) ring unit possible, furthermore an improved ring design has resulted in a significant increase in operational stability in all three dimensions. The total flexion angle for Model 2, can be calculated in the same was as for Model 1, giving Model 2 a maximum tip angle βTip of 4π/3 based on n, No. of ring units =24 and the flexion angle per unit is as shown in () π/18.
Improvements include fundamental redesign to be symmetrical and thus catering for both left and right-handed users by feeding the thread through the center of the mechanism as shown in () by the blue line. Further by using a single trigger to control flexion this brings the mechanism closer to the final goal of being able to complete the entire ligation procedure with this single device. The exploded view of the Model 2 control unit is shown in (). Control dynamics of the tip is shown by red and green arrows, actuation is achieved by the control wires connecting the tip to the control wheel – shown by a thin blue line in (). Redesign of the unit rings as shown in () from a ½ circle overlap to a ¾ circle overlap inherently provides increased inter-ring stability.
3-D building and testing
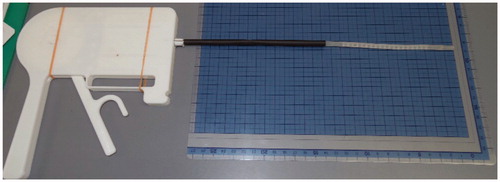
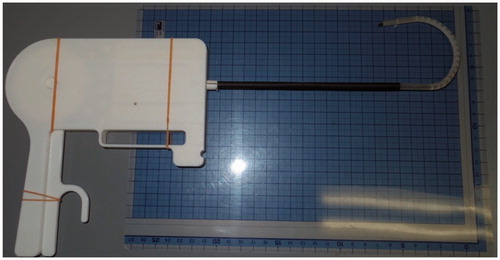
For model 2, in order to create an actual size proof of concept model an Objet260 Connex (Stratasys) 3-D printer using VeroClear resin was used to provide the necessary precision (15 um). Using this 3-D printer a 5 mm diameter mechanism was prototyped as shown in () – straight and () at full flexion.
Simulations
Method
In this section, the new procedure for laparoscopic ligation is described based on the newly developed mechanisms. The current procedure was outlined in the introduction section.
Firstly, a thread with a slipknot is pre-loaded into a tube, the tube is then fed into the mechanism as can be seen in and illustrated in (), so that at least half of the slip knot is exposed. The ligating mechanism is then set to the straight position for insertion through a trocar, into the patient’s body and through an incision in the sigmoid mesocolon in the case of ligating the sigmoid colon. The ligating mechanism is then flexed as it is brought around the far side of the colon back into view as is shown in (). A set of forceps are then used to take hold of the loop extending from the ligating mechanism and the ligating mechanism is retracted and straightened as shown in () whereby the tip of the ligating mechanism is brought to the near side of the organ and then once again is flexed to bring the tail of the thread through the loop as is shown in (). Finally, the forceps release the loop and take hold of the tail of the thread through the loop, after which the ligating mechanism can be straightened and returned through the trocar and the ligated organ can be suspended as required ().
The following methods were used verify the validity of this ligation mechanism for laparoscopic surgery. A laparoscopic surgical simulator as shown in () was used.
a) A comparison was made between the time taken for surgeons to ligate using the conventional ligating method and ligation using the newly developed prototype mechanism.
b) Further a comparison was also made between the time taken by experienced surgeons and non-surgical persons (engineering students) to use the newly developed prototype mechanism.
Comparison
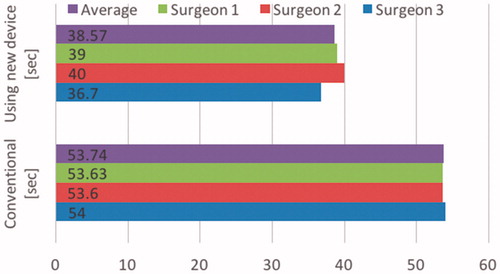
Three surgeons were asked to ligate a simulated endless organ using a laparoscopic simulator. The surgeons requested to ligate the simulated organ using both the conventional approach and to ligate using the newly developed prototype mechanism. The results are shown in (). The time taken to ligate using the conventional method averaged 53.74 seconds (sd = 0.22 sec.), the time taken to ligate using the newly developed prototype mechanism was 38.57 seconds (sd = 1.69 sec.), thus representing an average reduction of 15.17 seconds or 28% when expressed a percentage of time saved for the process of ligation. This reduced time taken is comparing a first attempt at using the newly developed mechanism compared to the regular procedure for which the surgeons are experienced with. All things being equal experience in using this new device would result in further time reductions. This also indicates the newly developed mechanism is “user friendly” that is the usage is intuitive, for many laparoscopic instruments this is an issue.
Figure 17. Comparison of time taken to ligate organs using conventional ligation and ligation using the newly developed mechanism in the case of experienced surgeons.
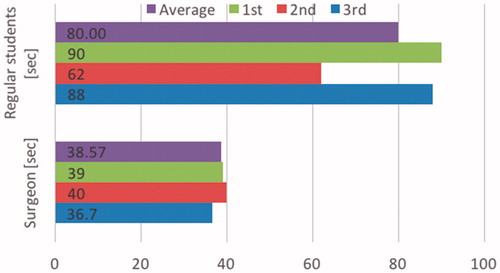
() shows a comparison of surgical staff and non-surgical persons (regular engineering students) performance in using the developed mechanism. The surgical staff on average completed the procedure in 38.57 seconds (sd = 1.69 sec.), while the non-surgical volunteers averaged 80 seconds (sd = 15.6 sec.). The results of () reflect surgical staff experience in using laparoscopic instruments in a laparoscopic environment and yet also show that despite a lack of such experience the proposed mechanism is sufficiently user friendly to be used by non-medical persons.
Discussion
This paper has described a laparoscopic surgical device for ligating endless organs based on the use of a snake like flexible structure. The purpose of this development is to provide a mechanism and procedure that allows surgeons to more efficiently and safely ligate continuous organs, and to do so without the need for a high level of skill and/or experience. The helpful advice and feedback from the surgeons who assisted with this research was essential in the development of the mechanism. The proof of concept prototypes was made possible using 3-D printers.
The concept of using pre-looped tubes attracted significant interest from the surgical staff. Furthermore, as mentioned above the aim of this development is to create a laparoscopic ligating mechanism that can facilitate safe and efficient ligation single handedly with a single laparoscopic device.
Regarding the simulation model, while this time a 3 D printed replica organ was used that was on hand, however clearly a more realistic result would be obtained by using something that more closely replicated a human colon such as a porcine colon ex-vivo.
Conclusion
The need has been cited for a safer and more efficient means of ligating continuous organs during laparoscopic surgery. Based on a snake like mechanism two proof of concept mechanisms have been designed, prototyped and tested in regard to ligation efficiency. A simple laparoscopic simulation test was set up and the results of conventional ligation and that using the newly proposed mechanism were compared. Based on the results the proposed mechanism resulted in significantly improved efficiency in regard to this procedure. Future work will focus on making the entire procedure possible single handed and with a single device.
Acknowledgements
This research was partially supported by the Medical-Engineering Hybrid Professional Development Program, Graduate School of Biomedical Sciences, Nagasaki University. We also wish to express our thanks to the surgeons who are also part of the same course for their helpful advice and feedback.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Spaner SJ, Warnock GL. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J Laparo-endosc Adv Surg Tech A. 2009;7:369–373.
- Nguyen NT, Silver M, Robinson M, et al. Result of a national audit of bariatric surgery performed at academic centers: a 2004 University Health System Consortium Benchmarking Project. Arch Surg. 2006;141:445–449.
- Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs [with discussion]. Ann Surg. 2001;234:279–291.
- Laudicella M, Walsh B, Munasinghe A, et al. Impact of laparoscopic versus open surgery on hospital costs for colon cancer: a population-based retrospective cohort study. BMJ Open. 2016;6:e012977.
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Single-incision laparoscopic surgery (SILS) vs. conventional multiport cholecystectomy: systematic review and meta-analysis. Surg Endosc. 2012;26:1205–1213.
- Alhashemi M, Almahroos M, Fiore JF, et al. Impact of miniport laparoscopic cholecystectomy versus standard port laparoscopic cholecystectomy on recovery of physical activity: a randomized trial. Surg Endosc. 2017;31:2299–2309.
- Chen SP, Li YX, Li GB, et al. Flexible laparoscopic forceps manipulator using synchronous belt mechanism, applied mechanics and materials. Trans Tech Publ. 2014;607:303–306.
- Tan Z, Ren H. Design and actuation of a snake-like robot for minimally invasive surgeries. In: Goh J, editor. The 15th International Conference on Biomedical Engineering. IFMBE Proceedings; vol 43. New York: Springer; 2014. p. 28–31.
- Jelínek F, Tom D, Sander D, et al. Method for minimising rolling joint play in the steerable laparoscopic instrument prototype DragonFlex. Minim Invasive Ther Allied Technol. 2015;24:181–188.