?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study is to analyze the risk factors associated with the development of adenomatous and malignant polyps in the gallbladder. Adenomatous polyps of the gallbladder are considered precancerous and have a high likelihood of progressing into malignancy. Preoperatively, distinguishing between benign gallbladder polyps, adenomatous polyps, and malignant polyps is challenging. Therefore, the objective is to develop a neural network model that utilizes these risk factors to accurately predict the nature of polyps. This predictive model can be employed to differentiate the nature of polyps before surgery, enhancing diagnostic accuracy. A retrospective study was done on patients who had cholecystectomy surgeries at the Department of Hepatobiliary Surgery of the Second People’s Hospital of Shenzhen between January 2017 and December 2022. The patients’ clinical characteristics, lab results, and ultrasonographic indices were examined. Using risk variables for the growth of adenomatous and malignant polyps in the gallbladder, a neural network model for predicting the kind of polyps will be created. A normalized confusion matrix, PR, and ROC curve were used to evaluate the performance of the model. In this comprehensive study, we meticulously analyzed a total of 287 cases of benign gallbladder polyps, 15 cases of adenomatous polyps, and 27 cases of malignant polyps. The data analysis revealed several significant findings. Specifically, hepatitis B core antibody (95% CI −0.237 to 0.061, p < 0.001), number of polyps (95% CI −0.214 to −0.052, p = 0.001), polyp size (95% CI 0.038 to 0.051, p < 0.001), wall thickness (95% CI 0.042 to 0.081, p < 0.001), and gallbladder size (95% CI 0.185 to 0.367, p < 0.001) emerged as independent predictors for gallbladder adenomatous polyps and malignant polyps. Based on these significant findings, we developed a predictive classification model for gallbladder polyps, represented as follows, Predictive classification model for GBPs = −0.149 * core antibody − 0.033 * number of polyps + 0.045 * polyp size + 0.061 * wall thickness + 0.276 * gallbladder size − 4.313. To assess the predictive efficiency of the model, we employed precision-recall (PR) and receiver operating characteristic (ROC) curves. The area under the curve (AUC) for the prediction model was 0.945 and 0.930, respectively, indicating excellent predictive capability. We determined that a polyp size of 10 mm served as the optimal cutoff value for diagnosing gallbladder adenoma, with a sensitivity of 81.5% and specificity of 60.0%. For the diagnosis of gallbladder cancer, the sensitivity and specificity were 81.5% and 92.5%, respectively. These findings highlight the potential of our predictive model and provide valuable insights into accurate diagnosis and risk assessment for gallbladder polyps. We identified several risk factors associated with the development of adenomatous and malignant polyps in the gallbladder, including hepatitis B core antibodies, polyp number, polyp size, wall thickness, and gallbladder size. To address the need for accurate prediction, we introduced a novel neural network learning algorithm. This algorithm utilizes the aforementioned risk factors to predict the nature of gallbladder polyps. By accurately identifying the nature of these polyps, our model can assist patients in making informed decisions regarding their treatment and management strategies. This innovative approach aims to improve patient outcomes and enhance the overall effectiveness of care.
Introduction
Gallbladder polyps (GBPs) are characterized as polypoid elevations of the inner wall mucosa into the lumen, making their characterization challenging in medical imaging. The advent of advanced medical imaging techniques has significantly increased the detection rate of GBPs. Foreign studies report an incidence rate of GBPs in adults ranging from 0.3% to 12.3% [Citation1]. In China, during the physical examination of adults, the ultrasound detection rate of GBPs ranges from 4.2% to 6.9% and is increasing annually [Citation2]. GBPs can be categorized into two groups: non-neoplastic polyps (pseudopolyps) and neoplastic polyps (true polyps). Non-neoplastic polyps include cholesterol polyps, adenomyosis, inflammatory polyps, and hyperplastic polyps, while neoplastic polyps comprise benign tumors like gallbladder adenomas and gallbladder cancer (GC). Cholesterol polyps account for more than 80% of all GBPs, benign non-cholesterol polypoid lesions represent 10% to 15%, and tumor polyps account for approximately 5% [Citation3]. Though GBPs are primarily benign lesions, there is still a potential risk of malignancy.
Gallbladder cancer (GC) is associated with a poor prognosis and low survival rate, as its symptoms are often subtle, leading to late-stage diagnoses. The 5-year survival rate for GC patients is less than 5% [Citation4]. Considering the significant likelihood of gallbladder polyps (GBPs) progressing to GC, guidelines in several European and American countries recommend surgical resection and treatment for GBPs measuring 1 cm or larger in diameter. However, postoperative pathological diagnoses have indicated that not all polyps of this size are malignant, leading to unnecessary cholecystectomies for many patients [Citation3,Citation5]. Laparoscopic cholecystectomy is a common procedure performed to remove the gallbladder and surrounding tissues, aiming to halt the progression of malignant polyps. It is important to recognize that laparoscopic cholecystectomy carries certain risks and complications, such as bile leakage, residual common bile duct stones, biliary tract damage, and post-cholecystectomy diarrhea [Citation6]. Therefore, the accurate detection of gallbladder cancer is crucial to avoid unnecessary surgeries and associated risks.
In clinical research, the threshold for intervention in gallbladder polyps has been set at a diameter exceeding 10 mm. International recommendations advocate laparoscopic cholecystectomy for patients with polyps surpassing this threshold [Citation7]. However, accurate diagnosis of the nature of gallbladder polyps is crucial for patients to make informed treatment decisions as relying solely on polyp size for preoperative predictions of polyp nature is unsatisfactory. Various predictive models have been developed to diagnose different categories of gallbladder polyps, but these models have certain limitations. For instance, Fujiwara et al. [Citation8] collected clinical data from 227 patients with gallbladder polyps to study the predictive value of polyp size in relation to gallbladder cancer. Sung et al. [Citation9] analyzed 253 patients with gallbladder polyps and determined that a polyp diameter of 14.5 mm was the best predictor of gallbladder cancer, while a diameter of 27 mm was the best predictor for gallbladder cancer at stage T2 and above, using ROC curves. Liu et al. [Citation10] analyzed the clinical data of 2,704 patients who underwent cholecystitis surgery to determine risk factors for gallbladder tumors and identified a bulk diameter >15 mm as an independent risk factor. These studies highlight the limitations of single-factor analysis in accurately predicting the occurrence of cancer. Liu et al. [Citation11] conducted a study using univariate and multivariate analyses to determine the nature of gallbladder polyps, demonstrating the effectiveness of multivariate analyses. However, their study only considered variables extracted from ultrasound examinations, neglecting other potential risk factors.
In order to comprehensively analyze the risk factors associated with the occurrence of neoplastic and malignant polyps, we collected data from patients with gallbladder polyps, including admission records, preoperative laboratory investigations, imaging characteristics of the polyps, and postoperative pathological results. To unravel the intricate interactions among these risk factors and achieve accurate prediction of gallbladder polyp nature, we developed an advanced deep learning network called GBNet. Our approach incorporates an attention strategy to dynamically assign weights to the features, thereby enhancing the accuracy of gallbladder polyp nature prediction. Additionally, we addressed the issue of imbalanced data resulting from the prevalent occurrence of malignant lesions by developing a data balancing algorithm. To ensure the reliability of our recommended course of action, we ranked the significance of features using SHAP-values and compared them to the risk variables identified through statistical methods. This rigorous analysis helps validate the effectiveness and relevance of our predictive model.
Interactive visualization plays a vital role in effectively communicating disease prediction models to healthcare professionals, researchers, and patients alike. By presenting visual representations of performance metrics, such as sensitivity, specificity, and accuracy, users can assess the reliability of the model and make informed decisions. Moreover, interactive visualization empowers users to explore diverse input parameters and observe the resulting outputs, fostering a deeper comprehension of how different risk factors contribute to the prediction of gallbladder diseases. As shown in , interactive visualization is used in disease prediction models and diagnosis of gallbladder disease.
Methods
Study population and design
We conducted a retrospective analysis of all patients with gallbladder polyps (GBPs) who underwent cholecystectomy at the Department of Hepatobiliary Surgery of Shenzhen Second People’s Hospital between January 2017 and December 2022. The inclusion criteria consisted of patients who were pathologically diagnosed with cholesterol polyps, adenomas, or gallbladder cancer, with or without gallstones, following cholecystectomy. In cases where the specimen had multiple pathological diagnoses, the most dysplastic subtype was used for histological classification. Patients with a preoperative diagnosis of gallbladder cancer or those who underwent cholecystectomy for non-gallbladder-related surgeries were excluded from the study. The patients were then categorized into three groups based on their pathology: benign gallbladder polyp group, gallbladder adenoma group, and gallbladder cancer group. As this study was retrospective in nature, informed consent was waived. Ethical approval for the study was obtained from the Clinical Research Ethics Committee of Shenzhen Second People’s Hospital.
Laboratory tests
A nurse collected blood samples from the participants prior to cholecystectomy for laboratory testing. The following parameters were measured: fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and uric acid levels. Hepatitis B virus (HBV) infection was detected using a second-generation enzyme-linked immunosorbent assay (Abbott Laboratories, Inc., Chicago, IL, USA). Metabolic syndrome (MS) was defined as the presence of three or more of the following criteria: 1) abdominal obesity, defined as a waist circumference greater than 90 cm in men and greater than 85 cm in women; 2) hypertriglyceridemia, defined as triglyceride levels equal to or higher than 1.7 mmol/L (150 mg/dL); 3) low HDL cholesterol, defined as HDL cholesterol levels below 1.03 mmol/L (40 mg/dL) in men and below 1.29 mmol/L (50 mg/dL) in women; 4) high blood pressure, defined as a blood pressure reading of 130/85 mmHg or higher; and 5) high fasting blood glucose levels, defined as fasting blood glucose greater than mmol/L (110 mg/dL).
Medical data and ultrasound imaging parameter collection
At the time of admission, we recorded the age, sex, and symptoms of all patients. Prior to undergoing cholecystectomy, patients were positioned in the left lateral position for ultrasound scanning, performed by a highly experienced sonographer with over five years of expertise. Doppler ultrasound was employed in both grayscale and color modes to assess the lesions. Various parameters were documented, including the width of gallbladder polyps (GBPs), size of the gallbladder, thickness of the gallbladder wall, and the number of GBPs present. The diameter of the polyp was determined based on the largest measurement provided in the pathology report. In cases where multiple polyps were present, the diameter of the largest polyp was selected.
Statistical analysis
Data analysis was performed using the SPSS software package, version 26.0. Continuous variables were presented as mean ± standard deviation (SD). To evaluate the physiological characteristics of gallbladder cancer, statistical methods such as correlation analysis, binary logistic regression analysis, and the neural network prediction model proposed in this study were employed. These analyses were used to generate precision-recall curves (PRC) and receiver operating characteristic curves (ROC).
Gallbladder classification network
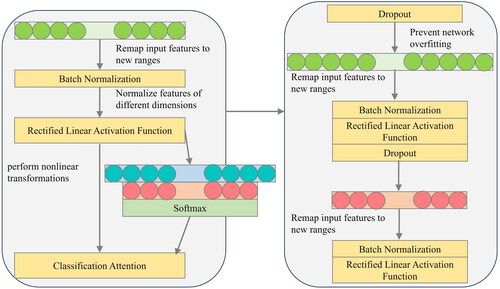
To predict and classify gallbladder lesions, this study developed a model by incorporating a fully connected layer. The model was designed to successfully classify different categories of gallbladder changes. In order to prevent overfitting during the training of the classification model, batch normalization and dropout operations were introduced into the network. These techniques help optimize the performance of the model and enhance its generalization ability.
To enhance the network’s ability to predict and classify the three categories of gallbladder variables during training, a customized classification weight attention function is incorporated into the network. This function assigns different weights to the categories, allowing the network to focus on specific aspects during training. illustrates the triple classification model for gallbladder data. This approach aims to optimize the network’s performance and improve its accuracy in predicting and classifying gallbladder variables.
The utilization of Classification Attention in learning three distinct categories of data enables the mapping of discretely distributed gallbladder features to the corresponding label space. This approach helps minimize the impact of varying feature spaces on the classification outcomes of the samples. Furthermore, incorporating activation functions enhances the nonlinear representation of the gallbladder prediction classification model, thereby improving the model’s robustness for effectively addressing classification problems.
To address the issue of excessive computation associated with full connectivity, the network incorporates dropout, which helps mitigate overfitting resulting from the abundance of data. EquationEquation (1)(1)
(1) describes the relationship between the input and output of each layer within the fully connected structure. This approach not only optimizes computational efficiency but also ensures a balanced and effective utilization of the network’s resources.
(1)
(1)
where
represents the output passing through the layer network,
represents the output data format,
represents the sample classification,
represents the sample features,
represents the layer network,
represents the input to the network,
represents the weight coefficient of the layer network,
represents the bias coefficient of the layer network.
After the network output of the first layer, the output results are subjected to batch normalization and activation function as shown in EquationEquation (2)(2)
(2) .
(2)
(2)
where
represents the input data,
represents the result after activation and normalization,
represents the activation function,
represents the batch normalization operation.
In the classification weight attention module, the calculation process of assigning classification weights is represented by EquationEquations (3)(3)
(3) and Equation(4)
(4)
(4) .
(3)
(3)
(4)
(4)
where
represents the categorical weight attention coefficient,
represents the
operation,
represents the fully connected network,
represents the bias of the categorical weight attention network.
After the network output of the second layer, the output results are subjected to batch normalization and activation function as shown in EquationEquation (5)(5)
(5) .
(5)
(5)
where represents input data,
represents output data.
The output of the network in the third layer is shown in EquationEquation (5)(5)
(5) .
(6)
(6)
where represents input data,
represents output data.
Data balance function
The gallbladder dataset used in this research comprises three categories: benign polyp, gallbladder adenoma, and gallbladder cancer. However, there is an issue of data imbalance among these categories with a distribution of 287:15:27 for benign polyp, gallbladder adenoma, and gallbladder cancer respectively. To prevent training errors caused by the insufficient representation of certain categories in the dataset, a category balancing function is customized in this study. This function dynamically balances the number of gallbladder lesions across the three categories to cater to the training and testing requirements of the network. The definition of the dynamic category balancing function is presented in EquationEquations (7)(7)
(7) and Equation(8)
(8)
(8) . This approach helps address the data imbalance problem and ensures that the network receives adequate training examples from each category for improved classification performance.
(7)
(7)
(8)
(8)
where
represents the overall gallbladder data set,
represents the set of benign polyp categories,
represents the set of gallbladder adenoma categories,
represents the set of gallbladder cancer,
、
and
represents the dynamic adjustment coefficients of the three category numbers.
Results
In our study, we compared our method, GBNet, with various machine learning (ML) classification models, including multilayer perceptron (MLP), logistic regression, decision tree, multinomial NB, K-nearest neighbors, gradient boosting, extreme gradient boosting (XGBs), random forest, Adaboost, and Gaussian NB. To evaluate the performance of these models, we utilized accuracy, precision, recall, F1 score, and AUC as evaluation metrics. These metrics collectively assess the correctness, precision, comprehensiveness, balance, and predictive ability of the models. Based on the results presented in , GBNet achieved the following evaluation values for its three classifications: accuracy = 0.907, precision = 0.852, recall = 0.891, F1 score = 0.868, and AUC = 0.930. The analysis of the final results indicates that the model outperforms several integrated learning models in all five evaluation metrics for classification. GBNet demonstrates superior performance compared to the other models, showcasing its effectiveness and robustness in accurately classifying gallbladder lesions.
Table 1. Comparative experiments of different classification models oriented to the gallbladder categories change dataset.
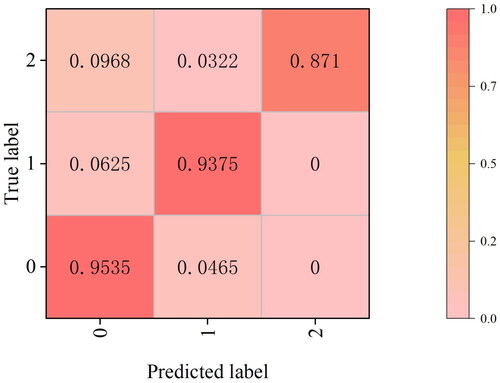
Following the training of the GBNet classification model on gallbladder data, the three categories of benign polyps, gallbladder adenomas, and gallbladder cancers were assigned as category 0, category 1, and category 2, respectively, during the experiment. To showcase the effectiveness of our model in handling imbalanced datasets, we present the classified results using a normalized confusion matrix, as illustrated in . In the normalized confusion matrix, we observe the following probabilities: for category 0, the true value is category 0, and the probability of being correctly predicted as category 0 is 95.35%; for category 1, the probability of being correctly predicted as category 1 is 93.75%; and for category 2, the probability of being correctly predicted as category 2 is 87.10%. The confusion matrix effectively demonstrates the high performance of our model in accurately identifying the categories of benign polyps, malignant polyps, and adenomas. This highlights the model’s ability to handle imbalanced datasets and achieve accurate classification results.
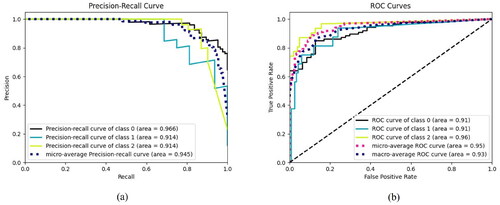
To provide a more intuitive representation of the triple classification results achieved by the GBNet model, the dataset used in this research incorporates a classification dynamic balance function. The model’s classification performance is evaluated based on the results, and further analyzed using precision-recall (PR) curves and receiver operating characteristic (ROC) curves. As depicted in Figures (a) and (b) within , the PR curve (Figure (a)) and the ROC curve (Figure (b)) clearly demonstrate the excellent classification performance of the GBNet model for the gallbladder triple classification task. The distribution direction of the PR curve and the shape of the ROC curve signify the model’s high accuracy and effectiveness in categorizing gallbladder lesions. These findings validate the outstanding performance of the GBNet model in the context of triple classification for gallbladder data.
To enhance the transparency of our model’s role in medical diagnosis and treatment, thereby increasing reliability for both doctors and patients, we utilize visualization techniques to highlight the significant features that greatly influence the model’s training process. Additionally, we perform a comparative analysis between these important features and the risk factors derived from a widely-used statistical model in clinical practices.
The purpose of this analysis is to evaluate the alignment between our model’s prediction process and the clinical recognition process. By comparing the two, we aim to gain insights into the effectiveness of our model and its agreement with established clinical practices. To determine the importance of each individual feature in contributing to the final outcome, we employ the Shapley Additive Explanations (SHAP)-value as the feature importance explainer. This approach utilizes a game-theoretic approach to measure the contribution of each feature. Through this visualization and comparative analysis, we aim to provide a clear understanding of how our model arrives at its predictions, allowing healthcare professionals and patients to have confidence in the reliability and interpretability of our model’s outcomes.
Using the proposed gallbladder lesion classification model in this study, we aimed to identify the physiological characteristics of the human body that directly influence changes occurring in the gallbladder. To visualize the results of our experiment, we depicted the sample characteristics that exhibit a higher degree of influence. illustrates the extent to which these characteristics impact the occurrence of lesion changes in different categories. In , category 0 represents benign polyps, category 1 represents gallbladder adenomas, and category 2 represents gallbladder cancer. The visualization reveals that five sample characteristics, namely polyp size, number of gallbladder polyps, polyp wall thickness, core antibodies, and gallbladder size, hold the top five positions in terms of their influence on the occurrence of gallbladder lesions.
Figure 5. Visualization of the influence of physiological characteristics on the occurrence of lesions in the gallbladder.
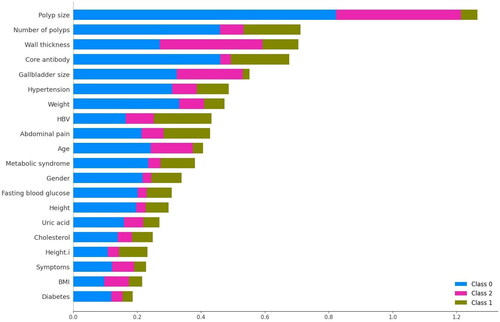
This visual representation provides valuable insights into the significant factors that contribute to gallbladder lesion formation. By identifying these influential sample characteristics, healthcare professionals can gain a better understanding of the underlying factors that drive such changes in the gallbladder.
For the analysis of the data involved, the software tool used is SPSS, which is used to analyze various physiological indicators. presents the results of a correlation analysis conducted to examine the factors influencing the development of gallbladder carcinoma. The analysis reveals a significant correlation between several physiological factors and the occurrence of gallbladder cancer. Specifically, the characteristics of core antibody, number of polyps, polyp size, wall thickness, gallbladder size, and symptoms (p < 0.001) exhibit a highly significant correlation with the development of gallbladder cancer.
Table 2. Correlation analysis of physiologic features affecting carcinogenesis of the gallbladder.
This study employed binary logistic regression analysis to examine the impact of physiological factors on gallbladder carcinogenesis. The results are presented in , revealing significant correlations between core antibody, number of polyps, polyp size, wall thickness, and gallbladder size with the development of gallbladder cancer (p < 0.001).
Table 3. Binary logistic regression analysis of physiological characteristics influencing the development of carcinoma in the gallbladder.
The machine learning classification program used in this study for predicting gallbladder disease is based on Python 3.9. It utilizes an NVIDIA GeForce RTX 3060 graphics card. The program has a total runtime of 26.21 s and a total parameter count of 1590. The memory usage of the program, excluding the Python interpreter, is 1136 bytes. These results suggest that the program optimizes resource consumption in terms of both time and memory during the prediction and classification process.
The proposed network model in this study, designed for the three classification predictions of gallbladder lesions, has been experimentally validated to align with the outcomes of mathematical and statistical analyses. The clinical physiological features that exhibit a substantial impact on the occurrence of gallbladder lesions, as identified by the proposed classification model, demonstrate high consistency with the clinical physiological features that significantly differ from those in the statistical model. This finding serves as evidence for the robust interpretability and reliability of the prediction classification model proposed in this study. The strong alignment between the network model’s predictions and the established clinical understanding of physiological features further strengthens the trustworthiness and applicability of our proposed model in gallbladder lesion classification and diagnosis.
Discussion
While ultrasonography, CT, and MRI are commonly used in clinical practice for diagnosing gallbladder polyps (GBPs) before surgery, distinguishing between benign and malignant GBPs remains challenging. When gallbladder cancer (GC) is detected in patients with GBPs, simple cholecystectomy is often not a viable treatment option, and the surgical approach is closely linked to the cancer staging. For patients with GC stages of Tis or T1a, laparoscopic simple cholecystectomy is recommended to avoid the need for extensive surgical resection. However, when the stage of gallbladder cancer is T1b or higher, open radical cholecystectomy is recommended, which involves a wider resection area, potentially including partial hepatectomy (segments 4b and 5). The poor overall prognosis of gallbladder cancer, with a high likelihood of recurrence, often necessitates further surgeries [Citation12]. In 1982, Kozuka et al. [Citation13] first proposed the idea that polyps can progress from adenomas to carcinomas, which sparked increased research attention on the management of GBPs. Boulton and Adams published a study on managing GBPs using GC risk factors in 2007 [Citation14], which further contributed to the existing literature on GBPs management. Subsequently, numerous researchers have published articles focusing on GBPs management, further enhancing our understanding of the subject.
It is important to consider that the decision to perform cholecystectomy or follow-up for gallbladder polyps (GBPs) is often based on the size of the polyp. However, this approach raises concerns that some gallbladder adenomas with a diameter of less than 10 mm may go undetected and progress to become malignant GBPs before developing into gallbladder cancer (GC). The 2022 guidelines from the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) recommend cholecystectomy for GBPs larger than 10 mm in diameter when combined with other potentially malignant factors [Citation7]. However, this guideline recommendation lacks high-quality evidence, as some GBPs with a diameter below 10 mm may be overlooked due to the absence of clinical symptoms. These smaller polyps are more likely to be malignant, progress to GC, and have a poor prognosis if delayed treatment allows the disease to worsen. For GBPs with a diameter of 6–9 mm and no risk factors, cholecystectomy is not necessary. Instead, ultrasound follow-up of the gallbladder is advised at 6 months, 1 year, and 2 years, with a cessation of follow-up after 2 years if there is no growth. If a patient has no risk factors for malignancy and a gallbladder polypoid lesion of 5 mm or less, follow-up is not required [Citation7]. A retrospective study reported that adenomas accounted for 19.5% of all GBPs, and the incidence of malignant transformation in adenomas was 7.0% [Citation15]. The consensus reached by the Society of Radiologists in Ultrasound (SRU) identified polyp size, growth rate, follow-up time, and morphologic features as risk factors for malignant GBPs or polyps that may transform into cancer [Citation16]. Malignant GBPs are also associated with other clinical factors such as primary sclerosing cholangitis, polyp number, shape, and patient age [Citation17–19]. The chances of early diagnosis for GC are exceedingly slim, with only 15% to 47% of cases being medically treatable and a 5-year survival rate of less than 12% following surgery [Citation20]. Once GC has advanced to later stages, the prognosis becomes extremely poor, with a 5-year survival rate of less than 25% [Citation21]. Therefore, more efficient treatment methods are needed. The most effective method to prevent neoplastic polyps from progressing into malignant tumors is cholecystectomy, which improves long-term survival and prognosis. Thus, regular follow-up is recommended for benign polyps, while adenomatous gallbladder polyps and GCs typically require surgical excision due to the limitations of preoperative imaging in accurately diagnosing these conditions and the potential risks associated with surgical injury. Cost-effectiveness analysis has shown that following European consensus guidelines for surveying 1000 GBPs can lead to annual savings of £209,163. Additionally, adherence to these recommendations would result in a 12.5% increase in the number of people requiring cholecystectomy under observation [Citation22].
In our study, we observed differences in the ultrasound characteristics of gallbladder adenomas, malignant polyps, and benign polyps. We found that 18.5% (53/287) of benign polyps had a diameter greater than 10 mm, 40% (6/15) of adenomatous polyps had a diameter below 10 mm, and 8% (2/25) of malignant polyps had a diameter below 10 mm. These findings suggest that relying solely on the size of the diameter is challenging when differentiating the nature of the polyps. Wennmacker et al. [Citation23] reported a predictive model for identifying the nature of gallbladder polyps with a diameter greater than 1 cm. This model demonstrated a sensitivity of 68.1% and a specificity of 70.2% in predicting neoplastic polyps. However, many unnecessary cholecystectomies may result from treatment decisions based solely on polyp diameter. Postoperative pathological diagnosis often reveals benign, non-neoplastic polyps with no malignant potential, warranting regular ultrasound follow-up [Citation24]. Yang et al. [Citation25] collected clinical data from 1976 patients with gallbladder polyps and established risk factors for tumor polyps (age, number, presence or absence of tips, and polyp size) through multifactorial analysis. They developed a correlation probability model using these risk factors, which predicted tumor polyps with a sensitivity of 78.5% and a specificity of 77.5%. Similarly, Liu et al. [Citation26] collected data from 423 patients with gallbladder polyps and established risk factors for neoplastic polyps (polyp number, size, and morphology) through univariate and multivariate analyses. They developed a logistic regression equation prediction model with these risk factors, estimating the sensitivity of adenomatous polyps to be 79.5%, specificity to be 70.6%, and accuracy to be 73.3%. In our study, we developed a prediction model combining preoperative patient laboratory tests and imaging performance. This model demonstrated a significantly higher accuracy compared to previous models. It achieved a diagnostic accuracy of up to 90.7% for benign polyps, adenomatous polyps, and malignant polyps, surpassing the accuracy based solely on a 10 mm cutoff value. Our prediction model outperformed diameter alone in distinguishing between benign, adenomatous, and malignant polyps. By using this predictive algorithm, doctors can make informed decisions on the best course of treatment for patients with gallbladder polyps, avoiding unnecessary surgical procedures.
We also identified several risk factors for the development of gallbladder adenomatous and malignant polyps, including core antibodies, the number and size of polyps, the thickness of the gallbladder wall, and the size of the gallbladder itself. Based on these results, we developed a predictive classification model for gallbladder polyps: GBPs = −0.149 * Core antibody + 0.033 * Number of polyps + 0.045 * Polyp size + 0.061 * Wall thickness + 0.276 * Gallbladder size − 4.313. We evaluated the predictive efficiency of the model using PR curves and ROC analysis, and the area under the curve (AUC) was 0.945 and 0.930, indicating a high predictive accuracy.
Several studies have investigated the impact of the number of gallbladder polyps (GBPs) on their nature. Bhatt et al. developed a risk assessment model for GBPs through meta-analysis and found that solitary polyps smaller than 10 mm in diameter had a higher risk of malignancy compared to multiple polyps. Kwon et al. [Citation27] studied the postoperative pathology of 291 GBPs and observed that malignant polyps were more likely to be solitary. In our study, we observed that a majority of gallbladder adenomas and malignant polyps presented as solitary polyps (13/15, 86.7% and 23/27, 85.2%, respectively), whereas there were fewer benign polyps presenting as solitary (124/287, 43.2%). The diameter of gallbladder adenomas and malignant polyps was larger compared to benign polyps (9.86 ± 4.745 and 21.26 ± 10.589 vs. 5.41 ± 3.699). Among patients with GBPs measuring 10 mm or more in diameter, 18.5% (53/287) were diagnosed with benign polyps, 74.8% (9/15) with gallbladder adenomas, and 92.6% (25/27) with malignant polyps. Park et al. [Citation28] studied 689 patients and concluded that there was no direct relationship between solitary polyps and malignancy. The distinction between solitary and multiple polyps regarding malignant features remains unclear, and there is insufficient evidence to recommend cholecystectomy for all solitary polyps, regardless of size. Terzioglu et al. [Citation19] analyzed 278 patients and identified thickening of the gallbladder wall as a risk factor for neoplastic polyps. Yang et al. [Citation25] conducted a study with 1,976 patients, and 71% of those with malignant polyps had thicker gallbladder walls. In our study, through binary logistic regression analysis, we also found that gallbladder wall thickening is a risk factor for the development of malignant polyps. International guidelines recommend cholecystectomy for all patients with polyps and a thickened gallbladder wall [Citation7]. Notably, we discovered that hepatitis B core antibody and gallbladder size are risk factors for the development of malignant polyps in the gallbladder. To our knowledge, no previous study has reported the relationship between hepatitis B core antibody, gallbladder size, and the development of malignant polyps in the gallbladder. Our findings indicate that cholecystectomy in patients with GBPs should consider multiple factors before making a decision.
This study has several limitations that should be acknowledged. Firstly, the number of cases involving patients with benign polyps, gallbladder adenomas, and malignant polyps who underwent cholecystectomy was relatively small, limiting the generalizability of the predictive model to the entire population. Future prospective studies with larger sample sizes are required to validate and refine the findings. Secondly, the retrospective nature of the study introduces the possibility of selection bias, as the data collection relied on existing medical records. Prospective studies would help mitigate this limitation and provide more robust evidence.
Conclusion
This study revealed significant associations between the occurrence of gallbladder adenomas and malignant polyps and various factors, including hepatitis B core antibody, number of polyps, diameter size, gallbladder wall thickness, and gallbladder size. The predictive model developed in this study outperformed models based solely on polyp diameter, number, and shape, demonstrating higher sensitivity, specificity, and accuracy. Moreover, the prediction model encompassed not only non-neoplastic and adenomatous polyps but also malignant polyps, providing a more comprehensive approach to predict the neoplastic potential of GBPs based on multiple risk factors. Implementing this predictive model could potentially reduce unnecessary cholecystectomies caused by benign polyps. However, large-scale prospective studies are needed in the future to validate and further investigate the findings of this study.
Author contributions
Conception and design: Xuesong Deng, Xiangyun Liao and Weixin Si. Administrative support: Xuesong Deng and Xiangyun Liao. Provision of study materials or patients: Kerong Yuan and Xuesong Deng. Collection and assembly of data: Kerong Yuan, Zhe Deng and Xuesong Deng. Data analysis and interpretation: Xiaofeng Zhang, Qian Yang, Xiangyun Liao and Weixin Si. Manuscript writing: All authors. Final approval of manuscript: All authors.
Ethical approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- McCain RS, Diamond A, Jones C, et al. Current practices and future prospects for the management of gallbladder polyps: a topical review. World J Gastroenterol. 2018;24(26):1–12. doi: 10.3748/wjg.v24.i26.2844.
- Xu Q, Tao LY, Wu Q, et al. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB (Oxford). 2012;14(6):373–381. doi: 10.1111/j.1477-2574.2012.00457.x.
- Wiles R, Thoeni RF, Barbu ST, et al. Management and follow-up of gallbladder polyps: joint guidelines between the European Society of gastrointestinal and abdominal radiology (ESGAR), European association for endoscopic surgery and other interventional techniques (EAES), International society of digestive surgery–European Federation (EFISDS) and European society of gastrointestinal endoscopy (ESGE). Eur Radiol. 2017;27(9):3856–3866. doi: 10.1007/s00330-017-4742-y.
- Zhou W, Li G, Ren L. Triphasic dynamic contrast-enhanced computed tomography in the differentiation of benign and malignant gallbladder polypoid lesions. J Am Coll Surg. 2017;225(2):243–248. doi: 10.1016/j.jamcollsurg.2017.04.014.
- Overby DW, Apelgren KN, Richardson W, et al. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc. 2010;24(10):2368–2386. doi: 10.1007/s00464-010-1268-7.
- Ahmad DS, Faulx A. Management of postcholecystectomy biliary complications: a narrative review. Am J Gastroenterol. 2020;115(8):1191–1198. doi: 10.14309/ajg.0000000000000704.
- Foley KG, Lahaye MJ, Thoeni RF, et al. Management and follow-up of gallbladder polyps: updated joint guidelines between the ESGAR, EAES, EFISDS and ESGE. Eur Radiol. 2022;32(5):3358–3368. doi: 10.1007/s00330-021-08384-w.
- Fujiwara K, Abe A, Masatsugu T, et al. Effect of gallbladder polyp size on the prediction and detection of gallbladder cancer. Surg Endosc. 2021;35(9):5179–5185. doi: 10.1007/s00464-020-08010-8.
- Sung JE, Nam CW, Nah YW, et al. Analysis of gallbladder polypoid lesion size as an indication of the risk of gallbladder cancer. Korean J Hepatobiliary Pancreat Surg. 2014;18(1):9–13. doi: 10.14701/kjhbps.2014.18.1.9.
- Liu K, Lin N, You Y, et al. Risk factors to discriminate neoplastic polypoid lesions of gallbladder: a large-scale case-series study. Asian J Surg. 2021;44(12):1515–1519. doi: 10.1016/j.asjsur.2021.03.003.
- Liu XS, Chen T, Gu LH, et al. Ultrasound-based scoring system for differential diagnosis of polypoid lesions of the gallbladder. J Gastroenterol Hepatol. 2018;33(6):1295–1299. doi: 10.1111/jgh.14080.
- Feo CF, Ginesu GC, Fancellu A, et al. Current management of incidental gallbladder cancer: a review. Int J Surg. 2022;98:106234. doi: 10.1016/j.ijsu.2022.106234.
- Kozuka S, Tsubone N, Yasui A, et al. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50(10):2226–2234. doi: 10.1002/1097-0142(19821115)50:10<2226::aid-cncr2820501043>3.0.co;2-3.
- Kianmanesh R, Scaringi S, Castel B, et al. Lésions précancéreuses de la vésicule biliaire. [Precancerous lesions of the gallbladder]. J Chir. 2007;144(4):278–286.
- Sun Y, Yang Z, Lan X, et al. Neoplastic polyps in gallbladder: a retrospective study to determine risk factors and treatment strategy for gallbladder polyps. Hepatobiliary Surg Nutr. 2019;8(3):219–227. doi: 10.21037/hbsn.2018.12.15.
- Kamaya A, Fung C, Szpakowski JL, et al. Management of incidentally detected gallbladder polyps: society of radiologists in ultrasound consensus conference recommendations. Radiology. 2022;305(2):277–289. doi: 10.1148/radiol.213079.
- van Erp LW, Cunningham M, Narasimman M, et al. Risk of gallbladder cancer in patients with primary sclerosing cholangitis and radiographically detected gallbladder polyps. Liver Int. 2020;40(2):382–392. doi: 10.1111/liv.14326.
- Chou SC, Chen SC, Shyr YM, et al. Polypoid lesions of the gallbladder: analysis of 1204 patients with long-term follow-up. Surg Endosc. 2017;31(7):2776–2782. doi: 10.1007/s00464-016-5286-y.
- Terzioğlu SG, Kılıç MÖ, Sapmaz A, et al. Predictive factors of neoplastic gallbladder polyps: outcomes of 278 patients. Turk J Gastroenterol. 2017;28(3):202–206. doi: 10.5152/tjg.2017.16698.
- Yoo KS, Choi HS, Jun DW, et al. MUC expression in gallbladder epithelial tissues in cholesterol-associated gallbladder disease. Gut Liver. 2016;10(5):851–858. doi: 10.5009/gnl15600.
- Hickman L, Contreras C. Gallbladder cancer: diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am. 2019;99(2):337–355. doi: 10.1016/j.suc.2018.12.008.
- Patel K, Dajani K, Vickramarajah S, et al. Five year experience of gallbladder polyp surveillance and cost effective analysis against new European consensus guidelines. HPB (Oxford). 2019;21(5):636–642. doi: 10.1016/j.hpb.2018.10.008.
- Wennmacker SZ, van Dijk AH, Raessens JHJ, et al. Polyp size of 1 cm is insufficient to discriminate neoplastic and non-neoplastic gallbladder polyps. Surg Endosc. 2019;33(5):1564–1571. doi: 10.1007/s00464-018-6444-1.
- Jones MW, Deppen JG. Gallbladder polyp. In: statPearls. Treasure Island (FL): StatPearls Publishing; 2023 Apr 24.
- Yang JI, Lee JK, Ahn DG, et al. Predictive model for neoplastic potential of gallbladder polyp. J Clin Gastroenterol. 2018;52(3):273–276. doi: 10.1097/MCG.0000000000000900.
- Liu J, Qian Y, Yang F, et al. Value of prediction model in distinguishing gallbladder adenoma from cholesterol polyp. J Gastroenterol Hepatol. 2022;37(10):1893–1900. doi: 10.1111/jgh.15928.
- Kwon W, Jang JY, Lee SE, et al. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24(3):481–487. doi: 10.3346/jkms.2009.24.3.481.
- Park JK, Yoon YB, Kim YT, et al. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008;2(2):88–94. doi: 10.5009/gnl.2008.2.2.88.