Abstract
Orexin A and B and their receptors OX1R and OX2R modulate human sleep/wakefulness cycles, energy homeostasis, and behavior. Modafinil and suvorexant either directly or indirectly manipulate the orexin neuro-excitatory system. As such they are prototypes for modulation of this pathway and can be used to anticipate the clinical effects of exposure to the emerging drug class. We queried the National Poison Data System for exposures to both between 2005 and 2017. Single-substance exposures followed to a known outcome were included. Severity of outcome, clinical effects, healthcare facility utilization, and reason for exposure were assessed. 1616 modafinil and 83 suvorexant exposures were included. No deaths occurred. No major effects were noted in suvorexant exposures while 0.9% of exposures to modafinil resulted in major effects. Exposure to modafinil commonly resulted in stimulant-type effects: agitation (2.8%), tachycardia (1%), and hypertension (1%). Suvorexant resulted in sedation in 26.1% of exposures. Vomiting (15.9%) was also noted in suvorexant exposures. Both modafinil and suvorexant appear well-tolerated following exposure.
Introduction
In 1998, neuropeptides orexin A and B and their receptors OX1R and OX2R were isolated by a pair of research teams [Citation1,Citation2]. Subsequent research into the nature and function of these neuropeptides has revealed their role in human sleep/wakefulness cycle, energy homeostasis, and behavior [Citation3]. Since that time two therapeutic agents, modafinil and suvorexant, have received FDA approval [Citation4,Citation5]. Both either directly or indirectly manipulate the orexin neuro-excitatory pathway.
Suvorexant, marketed under the trade name Belsomra (Merck), is a dual receptor antagonist FDA approved in 2014 for the management of insomnia. Both OX1R and OX2R, receptors located primarily in the lateral hypothalamus, are antagonized by suvorexant [Citation6]. Because stimulation of OX1R and OX2R results in wakefulness, antagonism of this pathway improves both onset and maintenance of sleep [Citation6]. Suvorexant was generally well tolerated in clinical trials with a minority of individuals experiencing daytime somnolence, sedation, muscle weakness, abnormal dreams, and headache [Citation7,Citation8].
Modafinil, marketed under the trade name Provigil (Teva), received FDA approval in 1998 for the treatment of narcolepsy [Citation5]. It has been used for a wide variety of indications including excessive daytime sleepiness due to sleep apnea, shift work sleep disorder, cocaine addiction, depression, schizophrenia, attention deficit/hyperactivity disorder, and multiple sclerosis [Citation9]. Modafinil increases dopamine and norepinephrine transporter activity resulting in increased neurotransmitter release, though this mechanism is only responsible for part of modafinil’s clinical effects [Citation10]. Increased hypothalamic histamine release via the orexin pathway appears to contribute to its wakefulness promoting ability as well [Citation10].
Several clinical trials are underway investigating other orexin-modulating agents. Given the potential clinical applications of both agonism and antagonism of this neurochemical pathway, it is likely more drugs of this class will soon become available. As is the case with any drug, both accidental exposure and intentional abuse/misuse can occur. Small retrospective studies of the effects of exposure to modafinil suggested clinical outcomes were generally mild with minimal evidence of severe or life-threatening toxicity [Citation11,Citation12]. Data regarding clinical outcomes associated with suvorexant exposure has been minimal since its introduction in 2014. We utilized the National Poison Data System (NPDS) to evaluate suvorexant and modafinil exposures with regard to clinical outcomes associated with this novel pharmacologic treatment pathway.
Methods
Data source
The National Poison Data System (NPDS) was queried for reported exposures to suvorexant and modafinil between 2005 and 2017. NPDS Product codes for modafinil included: 5077436, 7808574, 7567518, 7817781, 7989861, 7527091, 7967031, 7396272, 7767952, 7767952, 7392220, 7725356, 7808293, 7567253, 7817947, 7989408, 7527083, 7967305, 7397535, 7767910, 7767910, 7392106, 7725182, and 5077436. Product codes for suvorexant included 7883930 and 78949768. Medical outcome was categorized using NPDS outcome definitions: no effect, minor effect (“minimally bothersome to the patient, symptoms resolve rapidly and usually involve skin or mucous membrane manifestations”), moderate effect (“more pronounced, more prolonged or more of a systemic nature than minor symptoms and usually some form of treatment is or would have been indicated”), and major effect (“symptoms were life-threatening or resulted in significant residual disability or disfigurement”) and death [Citation13]. We recorded the reason for exposure, proportion treated in a health care facility, and frequency of each outcome.
The NPDS is maintained by the American Association of Poison Control Centers (AAPCC) and receives near real-time data collected from calls to all regional poison control centers in the US and its territories and comprises information from more than two million human exposures per year. The Institutional Review Board of the Research Institute of Nationwide Children’s Hospital approved the use of NPDS data as IRB-exempt de-identified data.
Case selection criteria
Inclusion criteria were (1) human patient, (2) reported single-substance ingestion of either modafinil or suvorexant, and (3) patient followed to a known outcome. Exclusion criteria were (1) exposure to more than a single substance, (2) patient not followed to a known outcome, or (3) clinical outcome judged to be unrelated to the exposure.
Results
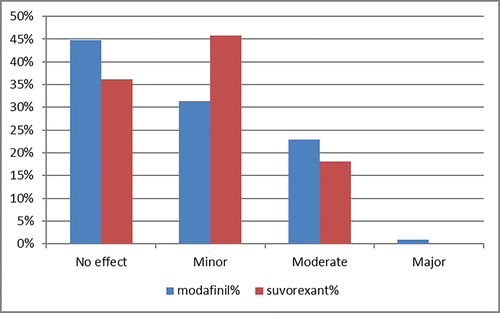
During the study period, a total of 3124 exposures to modafinil and 157 exposures to suvorexant were reported to NPDS. Of these, 86 modafinil exposures and 5 suvorexant exposures developed clinical outcomes that were felt to be unrelated to the exposure and were thus excluded. A total of 1400 modafinil and 69 suvorexant exposures were not followed to a clinical outcome (44.8% and 43.9%, respectively). Both the unrelated clinical outcomes and cases not followed to be a known outcome were excluded, leaving 1616 modafinil and 83 suvorexant exposures (). There were no significant differences between-age groups regarding the risk for moderate vs. minor clinical outcomes. No clinical effects were observed in 723 exposures to modafinil (44.7%), while 507 (31.4%) experienced minor effects, 371 (23%) patients experienced moderate effects, and 15 (0.9%) experienced major effects. Thirty (36.1%) patients exposed to suvorexant experienced no clinical effects. Minor effects were seen in 38 (45.8%), moderate effects in 15 (18.1%), and there were no major effects. There were no deaths following exposure to either substance ().
Table 1. Age, gender, and clinical outcomes of modafinil and suvorexant exposures.
The majority of exposures to modafinil and suvorexant resulted in no clinical effects or minor clinical effects. However, within the modafinil group three clinical effects occurred in 1% or more of total exposures: agitation (2.8%), tachycardia (2.6%), and hypertension (1%). All other clinical effects occurred in less than 1% of the modafinil group. These findings are consistent with those discovered previously [Citation11,Citation12]. In reported exposures to suvorexant drowsiness was common—26.1% of exposures resulted in sedation. Also prevalent were vomiting (15.9%), confusion (3.2%), agitation (2.5%), dizziness (2.5%), slurred speech (1.9%), hypotension (1.9%), hallucinations (1.9%), and headache (1.9%).
The most common reason for exposure to modafinil was unintentional-general with 564 exposures (34.9%). Also common were therapeutic error (459/28.4%) and intentional-suicide (248/15.3%). The most common reason for exposure to suvorexant was intentional-suicide (33/39.8%). Similar to modafinil, the two other most common reason for exposure were unintentional-general (17/20.5%) and therapeutic error (13/15.7%). Intentional abuse accounted for 80 (5%) and 4 (4.8%) of modafinil and suvorexant exposures, respectively.
Management site following exposure to modafinil and suvorexant differed. Only 13 (15.7%) suvorexant exposures were managed on-site, meaning outside of a health-care facility. By contrast, 636 (39.4%) of modafinil exposures were managed outside of a health-care facility. The percentage of exposures to modafinil and suvorexant referred in to a health-care facility by Poison Control was similar between drugs (18% and 19.3%, respectively). Most suvorexant exposures (65.1%) were already in a health-care facility that the time of first contact with Poison Control as compared to less than half of modafinil exposures (41.8%).
Discussion
Orexin receptor modulation represents a promising avenue of future pharmaceutical development. Modafinil and suvorexant represent two potential therapeutic pathways with one likely agonizing the receptor complex and the other antagonizing it. Our evaluation of exposures to both drugs reveals neither seems to result in an unusual number of significant clinical effects. No deaths occurred following exposure to either drug. Major effects, defined by the American Association of Poison Control Centers (AAPCC; http://www.aapcc.org/) as generally life- or limb-threatening and requiring treatment to prevent significant disability or disfigurement, occurred in less than 1% of exposures to modafinil and were not observed after exposure to suvorexant.
Most exposures to suvorexant resulted in sedation/decreased level of consciousness to some degree, which would be expected given the purpose of the drug. Only vomiting, present in 15.9% of suvorexant exposures, was unexpected. Similarly, in modafinil exposures, most clinical effects were in the excitatory spectrum—agitation and tachycardia. No unanticipated clinical effects with a frequency of greater than 1% were noted.
As a retrospective analysis using data reported to the National Poison Data System, this study has numerous limitations. The AAPCC maintains the NPDS database of information logged by the country’s 55 poison centers (PCs). Case records in this database are from self-reported calls: they reflect only information provided when the public or health-care professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure, etc.), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s).
Modafinil exerts its clinical effects by multiple mechanisms. Both increased hypothalamic histamine release via the orexin pathway and activation of stimulatory neurotransmitters, such as dopamine and norepinephrine, contribute to its clinical effects. Therefore, the clinical effects of future orexin pathway agonists may not be accurately predicted by studying modafinil.
Though these two groups were not directly compared with each other, there was a substantial difference in included number of reported exposures to modafinil (1616) and suvorexant (83). This was expected given the relative market age of the drugs. The small number of included suvorexant exposures limits this study’s predictive ability regarding clinical effects in suvorexant exposure.
Conclusion
Manipulation of the orexin neuro-excitatory pathway is an emerging pharmaceutical trend. Modafinil and suvorexant are prototypes for orexin receptor agonism and antagonism to assess the potential clinical effects of exposure to this drug class. Both modafinil and suvorexant, while capable of generating moderate adverse effects following exposure, are well tolerated. Major adverse effects are rare (0.9% of exposures to modafinil, 0% to suvorexant) and no deaths were reported. When clinical effects occur, they are consistent with expectations given the stimulant/sedative properties of each drug. Further study of these drugs is suggested, particularly suvorexant, given the small number of exposures in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585.
- de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA.. 1998;95:322–327.
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–241.
- Suvorexant FDA Approval. 2014. US Food & Drug Administration: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=204569 [cited 2017 Dec 20]
- Modafinil FDA Approval. 1998. US Food & Drug Administration: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/020717A_Provigil.cfm [cited 2017 Dec 20]
- Bennett T, Bray D, Neville MW. Suvorexant, a dual orexin receptor antagonist for the management of insomnia. P T. 2014;39:264–266.
- Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–2274.
- Kuriyama A, Tabata H. Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2017;35:1–7.
- Golicki D, Bala MM, Niewada M, et al. Modafinil for narcolepsy: Systemic review and meta-analysis. Med Sci Monit. 2010;16:177–186.
- Ishizuka T, Murotani T, Yamatodani A. Modafinil activated the histaminergic system through the orexinergic neurons. Neurosci Lett. 2010;483:193–196.
- Spiller HA, Borys D, Griffith JR, et al. Toxicity from modafinil ingestion. Clin Toxicol (Phila). 2009;47:153–156.
- Carstairs SD, Urquhart A, Hoffman J, et al. A retrospective review of supratherapeutic modafinil exposures. J Med Toxicol. 2010;307–310.
- Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2011 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS) 29th annual report. Clin Toxicol. 2012;50:911–1164.