Abstract
Toxicity from sedatives (e.g. benzodiazepines) affects cognition, behavior, and functional status. Although a direct antidote is available, it is rarely used due to fears of withdrawal and seizures. At one toxicology center, flumazenil is routinely employed in the emergency department and acute hospital setting. A dose of 0.5 mg is given IV over 30 s, with repeat doses q1-2h as needed to sedated patients with relaxed autonomic indices and peripheral neurologic status. The following reports a six-year retrospective review of the practice and a one-year close observational study of bedside use of the antidote. Flumazenil was given to 731 patients. The overall positive response rate was over 80%. There were no instances of arrhythmias or seizures. In the prospective year, there were 12 instances of side effects out of 212 patients treated. Three patients experienced drooling, 7 experienced transient anxiety, and there were 2 separate episodes of odd behavior upon awakening from coma in a patient with personality disorder. Comorbid anxiety disorders were associated with anxiety upon arousal, but no patient required medical intervention. No clinically significant adverse events occurred with flumazenil administration in patients who chronically used benzodiazepines or had a history of seizures. We conclude that flumazenil is a safe diagnostic and therapeutic antidote for cases of suspected sedative-hypnotic toxicity.
Introduction
Benzodiazepines are some of the most commonly prescribed sedative/hypnotic and anticonvulsant medications worldwide [Citation1]. The sheer availability of these medications and other sedatives makes them frequently used agents in both intentional and unintentional poisoning [Citation2]. Oversedation, confusion, and prolonged intensive care stays are common complications. Toxicity from benzodiazepines is rarely directly fatal. However, in cases of significant overdose resulting in drug-induced coma, an antidote can be used to reverse the effects of sedatives. This approach provides caregivers with means of obtaining a more accurate history of ingestion directly from the patient, as well as preventing some of the more severe complications of a comatose state, such as aspiration pneumonitis, airway obstruction, and respiratory depression. Iatrogenic and nosocomial complications may be minimized when wakefulness precludes the need for intubation, bladder catheterization, and other interventions, thus potentially reducing hospital lengths of stay.
Flumazenil is an imidazole-benzodiazepine analog, which acts as a competitive antagonist of central benzodiazepine receptors on the GABA-A complex [Citation3]. Flumazenil has been shown to work quickly and safely [Citation4], serving as both a diagnostic and therapeutic tool. Although the drug is often associated with minor side effects such as salivation, vomiting and anxiety [Citation4], only a few studies have been able to demonstrate significant adverse events such as seizures and cardiac arrhythmias [Citation5,Citation6]. In those studies, patients who suffered such adverse reactions demonstrated clinical manifestations of multiple drug ingestions (most commonly involving tricyclic antidepressants), had previous histories of excessive benzodiazepine use, and/or were given flumazenil doses of at least 1 mg at a time [Citation7,Citation8].
Although flumazenil is of obvious benefit in a pure benzodiazepine poisoning, many toxic patients presenting to the emergency department suffer sedation potentiated by combinations of prescription medications, illicit drugs and/or alcohol [Citation9,Citation10]. Therefore, the decision to use flumazenil in an unidentified poisoning may appear complex, where the benefits of rapid diagnosis and treatment seem offset by the possibility of significant adverse reactions. This perspective has resulted in underutilization of the antidote. In 2013, over 19,000 cases of benzodiazepine toxicity were reported through the National Poison Data System (NPDS) to have been treated in health care facilities, but during that same year the registry catalogued fewer than 1700 patients treated with flumazenil [Citation11]. The NPDS data almost certainly underestimate the total cases of sedative toxicity by a wide margin. Additional GABA-ergic toxicity cases involving non-benzodiazepine likely number in the thousands-per-year, too, but are not subcategorized by the American Association of Poison Control Centers’ Annual Report. Furthermore, innumerable cases of iatrogenic toxic oversedation in hospitals go unreported to the NPDS every year. In practice, there are likely to be many more patients in acute care settings whose clinical condition may be improved by the antidote, but do not receive it.
Such is not the standard of care in our toxicology practice. The setting is the highest volume inpatient toxicology practice in North America, whose senior medical toxicologists (including JWD) have nearly three decades of experience with the antidote. During the study, PinnacleHealth Toxicology served over 1000 patients per year drawn from an urban, suburban, and rural catchment population of over 4 million. With this study we present 7 years of bedside flumazenil experience in patients with diverse characteristics and ingestion circumstances.
Methods
Clinical use of flumazenil
A general outline for the use of flumazenil by this service in this setting over the course of the study period is presented in . There is usually a suspicion of access to sedative agents based on history from first responders, other providers, family and/or the patient, though the ubiquity of such compounds trumps any demand for confirmatory history. For inpatients who have been treated with benzodiazepines to treat agitation and/or maintain intubation and sedation, the correlation of clinical presentation with the effects of sedative medications is more clear. Rapid assessment by at least one member of the toxicology service including physical examination with attention to autonomic and neurologic status is performed. Typically, patients who represent good candidates for a diagnostic and therapeutic trial of flumazenil have normal to diminished deep tendon reflexes and normal or somewhat decreased heart rate and blood pressure. The antidote is not given to patients who are notably hyperreflexic, myoclonic, or tachycardic—physical findings inconsistent with sedative toxicity.
Figure 1. Clinical use of Flumazenil.
The algorithm summarizes the clinical circumstances and procedure for the use of flumazenil in the emergency department and acute inpatient areas of the study hospital setting.

Continuous cardiac monitoring is routinely performed during antidote delivery, but sometimes, patients have been seen in consultation on medical floors without telemetry available when flumazenil has been administered. The antidote is then given by intravenous (IV) infusion of 0.5 mg over 30 s into a running IV fluid line; the dose is typically not adjusted based on the weight or age of the patient, as it functions as a direct competitive antagonist to the body burden of drug. Occasionally, a patient whose physical examination is equivocal (e.g. mild hypertension or slight hyperreflexia in the context of sedation still suspicious for sedative toxicity) will be given an initial dose of 0.2 mg to test for a positive effect, and avoid agitated hyperstimulation that could result from a larger dose. A “piggyback” infusion procedure prevents the effects of pain at the IV delivery site from being confused with genuine pharmacologic arousal at the level of the CNS. The effects of this direct-acting agent are rapid once the drug reaches the brain, so assessment for response is performed within 2 min of infusion. A positive response produces improved wakefulness and/or cleared cognition. If benzodiazepine-induced delirium is suspected, a positive effect may also take the form of decreased behavioral dyscontrol. The Riker Sedation-Agitation Scale is typically used to simply and effectively describe the psychobehavioral status of patients [Citation12], though scores are not routinely recorded in our medical record, and were thus not presented in our data. In terms of this scale, patients with a positive response to antidote would be described as having a Riker score that moves from their pretreatment state nearer to 4 (the score associated with calm, cooperative wakefulness), the majority from a score below 4, improving upward. In the event of such a response, repeat doses of flumazenil are given every 1–2 h as needed to treat sedation, confusion, and/or respiratory depression and allow patients to participate in their own care so they do not require other interventions such as urinary catheterization and endotracheal intubation. The antidote is also employed with this regimen to facilitate extubation, with subsequent doses helping to maintain wakefulness and airway protection after the procedure. In general, then, our practice is to prescribe flumazenil 0.5 mg IV q1-2 h PRN confusion, failure to participate in care, sedation with Riker score < 3, and/or passage of a concerning threshold in either oxygen saturation or end-tidal carbon dioxide (ETCO2) measurement. When no improvement in cognition or ventilation status is observed within 2 min of any infused dose, the antidote is discontinued. The routine use of flumazenil in this general fashion underlies the systematic study of its safety and efficacy as outlined below.
Retrospective study
For the retrospective portion of this work, the authors reviewed the electronic medical record in the PinnacleHealth System, first identifying all patients under the care of the toxicology service from June 2003 to June 2009. The patients included in the study were seen as emergency department consultations, inpatient consultations and/or cared for primarily by the toxicology service. Within this group, patients whose treatment included flumazenil were identified through a keyword search of the electronic medical record and medication administration record. Patient demographic information, comorbidities and toxicologic diagnoses were obtained by a review of the record. In addition, for each identified patient, a separate, written database record, kept by toxicology service attending physicians during patient care, was used for supporting information about the patient’s presentation, treatment course, treatment outcome and adverse events. In accordance with clinical practice as outlined above, treatment outcome was recorded as positive on the basis of one or more of the following: 1) Riker Score moving toward 4, 2) responsiveness to questions or verbal commands if previously unable to do so, 3) cleared cognition evidenced by restored orientation and immediate recall, 4) improved ventilatory status as measured by ETCO2. A negative response to flumazenil implies no change in a patient’s level of consciousness or neurobehavioral symptomology. Adverse events that were routinely recorded in the electronic medical record and in the written service record for all toxicology patients included seizures, arrhythmias, and respiratory distress. The primary author compared accuracy of the data sources and reconciled discrepancies with assistance and clarification by the senior author when necessary.
Observational study
For the close observational leg of the study, beginning in July 2009, an intentional gathering of data on all patients treated with flumazenil was undertaken, concluding in July 2010. This period corresponded to the first year experience of two fellows in training (JJR and KKS) who, between them, saw every patient cared for by Pinnacle Toxicology in that year. As described above, patients were treated directly by the toxicology service in the two acute care hospitals of the health system. In addition to the greater detail and accuracy of data gathering, one particular advantage of this observational year of study over the previous retrospective years was to identify cases of antidote usage that occurred in the emergency department, as our electronic medical record does not capture medication orders until after the time of admission. Patient demographic information, comorbidities, toxicologic diagnoses, laboratory findings, treatment outcomes and adverse events were thoroughly gathered and carefully recorded. Particular attention was paid to information regarding psychiatric conditions and neurological illnesses, including seizure disorders and their treatment. Toxicologic diagnoses were made, as in previous years, on the basis of clinical presentation and test results, including chemistry values and drug of abuse screens by immunoassay. Occasionally, the diagnosis was supported by thin-layer chromatography or gas chromatography with mass spectrometry. Toxicologic testing in itself was never the basis for diagnosis, but sometimes helped to establish which specific compounds correlated with the clinical manifestations, course, and responses to treatment (including antidotes). Criteria for response were consistent with the retrospective study methodology (v.s.), but assessed directly by one of the toxicology fellows on the service (JJR and KKS) in addition to corroborating input from the toxicology attending in each case.
In addition to seizures, arrhythmias, and respiratory distress—the adverse events related to use of antidotes that were routinely recorded in the electronic medical record and in the written service record for all patients—other effects were ascertained, including signs of agitation or behavioral unrest. No specific laboratory or diagnostic criteria were used for identifying patients to receive flumazenil. The decision to use the drug was based upon the judgment of the treating physician evaluating the clinical data as outlined ()—the same procedure used to guide therapy during the retrospective years of the study.
Logistics and statistics
Both phases of the study were approved by the Institutional Review Board at PinnacleHealth. Results were tabulated using Microsoft Office Excel. All statistical analyses were completed using the chi-square test for results involving dichotomous categorical variables and logistical regression for results involving the independent variable of age.
Results
Retrospective review - Demographic information and overall response rates
Across the health system, 5063 patients were treated by the PinnacleHealth Toxicology Service between June 2003 and July 2009 (). Of these cases, 519 patients were identified who received flumazenil. Patients ranged from 3 to 85 years of age. 424 (81.7%) of these patients demonstrated a positive response to flumazenil therapy. There were no documented adverse events attributable to the antidote. demonstrates the annual use of flumazenil by the toxicology service. There was relatively stable employment of the antidote over time during the study period.
Figure 2. Retrospective Study Overview.
a) Summarizes the medical toxicology practice cases for the retrospective study, the number treated with flumazenil, the overall response rate, and reported side effects.
b) Reports the number of cases year-by-year over the retrospective study period, and highlights the consistent us of flumazenil each year.
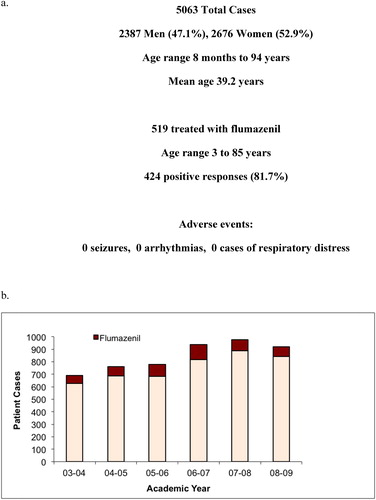
Retrospective review – flumazenil use, response rates, and adverse events
Underlying seizure disorders, chronic use of sedatives, and suspected co-ingestions are often cited as contraindication parameters for flumazenil administration. demonstrates the number of patients with multiple toxicologic diagnoses, sedative abuse and dependence, alcohol abuse, and comorbidities such as heart and lung disease and seizure disorders between 2003 and 2009. The category of seizure disorder indicates a diagnosis of this underlying condition in the medical record, but only in a small fraction of cases could the specific type be ascertained, the level of treatment adherence be deduced, or the serum concentrations of all relevant antiepileptic medications (AEDs) be gathered. Thus, these additional data are not reported for the retrospective portion of the study. Of note, though, 7 of the patients in the seizure disorder group were on benzodiazepine therapy, and 3 were on benzodiazepines alone for their epilepsy at the time of flumazenil administration. No seizure activity was seen in these patients during the course of their flumazenil treatment.
Table 1. Retrospective review of response rates and adverse events with Flumazenil.
Sedative abuse was a frequently listed diagnosis in the medical record, often rendered by a wide variety of physician specialists, including the toxicology service. Sedative/hypnotic dependence was less commonly encountered, as this particular diagnosis was rendered primarily by psychiatrists in consultation when the problem was deemed clinically relevant to the patient’s acute hospital presentation. It was highly correlated with a presenting toxicologic diagnosis of sedative withdrawal as opposed to sedative toxicity, thus these patients received flumazenil at a significantly lower rate—25.7% of patients with dependence received flumazenil compared to 79.8% with abuse.
Not listed separately in the table are patients who were treated for alcohol withdrawal and then, during the latter portion of hospitalization, were given flumazenil to reverse sedating and deliriogenic effects of benzodiazepines. Such patients were often treated with sedatives by a primary hospital service beyond the point of resolution of alcohol withdrawal. Then, persistence of abnormal behavior and mentation would prompt a toxicology consultation. Noting the lack of neurologic and autonomic findings consistent with ongoing withdrawal, our service would pursue a working diagnosis of delirium secondary to benzodiazepines, and give a test dose of flumazenil for both diagnostic and therapeutic purposes. Improved cognition without emergent side effects would both support the working diagnosis and prompt continued use of flumazenil PRN. There were 77 such cases in the retrospective study period, with no documented adverse events, and a positive antidotal response rate of 74% (n = 57). All are included in the category of alcohol abuse or dependence. Some of these cases overlap with a more focused study of the use of flumazenil in patients with alcohol withdrawal at PinnacleHealth [Citation13].
Individuals with multiple toxicologic diagnoses were statistically more likely to receive the antidote—a finding consistent with the described clinical practice () of using flumazenil diagnostically and therapeutically in cases of undifferentiated poisoning. Specific diagnoses having clinical features similar to sedative toxicity also predicted a higher rate of antidote use. These include anticonvulsant toxicity, opioid toxicity, and alcohol intoxication. Patients with stimulant toxicity, serotonin syndrome, and lithium toxicity were among those less likely to have been given flumazenil during the retrospective study period.
A positive response rate to flumazenil ranging from 76-89% was observed across the various demographic and comorbidity subgroups. Female patients were somewhat more likely to have been given flumazenil, but there was no gender difference in documented positive responses to the antidote. Patients with pulmonary disease, sedative abuse, and alcohol use disorders were more likely to receive the antidote ().
Positive response rates to flumazenil on the basis of toxicologic diagnosis varied from 58-100%, with patients having multiple diagnoses responding at rate of 80%, similar to the overall response rate of 81.7%. Patients with diagnosed sedative/hypnotic toxicity were statistically more likely to respond favorably to antidotal therapy. Response rates were lower in patients with opioid toxicity, anticonvulsant toxicity, neuroleptic toxicity, and alcohol intoxication, but there were no documented adverse events. Specifically, there were no documented cardiac arrhythmias, a particular concern in the heart and lung disease population, no instances of respiratory distress, and no seizures or acute withdrawal reported in any case ().
Observational study – flumazenil use, response rates, and adverse events
A total of 1026 patients between the ages of 2 and 89 years were treated by the PinnacleHealth Toxicology Service between July 2009 and July 2010, and each case was included in the close observation arm of this study. Of these cases, 212 received flumazenil. Patients given the antidote ranged in age from 2 to 75 years, with 84 males and 128 females. 169 (79.7%) of these cases demonstrated a clinically meaningful positive response, with no statistically significant difference in response rates between male and female patients. reports the number of patients treated by the toxicology service with multiple toxicologic diagnoses, tricyclic antidepressant (TCA) toxicity, sedative abuse, alcohol abuse, and comorbidities such as heart and lung disease and seizure disorders between July 2009 and July 2010. More details were captured with prospective study, and some differences in patient characteristics are reported, as a result.
Table 2. Response rates and adverse events with prospective use of Flumazenil.
The close observational study identified a significantly greater proportion of patients with seizure disorders, alcohol abuse, and abuse of sedative medications and illicit substances than the retrospective design. Chronic sedative use was suspected in patients who had active prescriptions for benzodiazepines, and then formally determined by clinical history reported by the patient. Over 22% of the patients treated during the observational year confirmed chronic use of sedative medications, either prescribed or illicitly obtained. Transient anxiety or behavioral unrest occurred in a fraction of these patients given flumazenil, but not at a statistically higher rate than patients without chronic sedative use. None of these patients experienced a seizure or problematic withdrawal symptoms. The same was true of patients with alcohol use disorder comorbidity. Patients with underlying seizure disorders experienced no adverse effects from flumazenil. They were, however, less likely to have a positive response to the antidote, an effect that disappears when taking into account the large number of patients in this subgroup who had a diagnosis of anticonvulsant toxicity.
The number of patients treated under observational study with flumazenil in each toxicologic diagnostic group is demonstrated in . Though not statistically significant, individuals with multiple toxicologic diagnoses were somewhat more likely to receive the antidote—a finding consistent with the described clinical practice of using flumazenil diagnostically and therapeutically in cases of undifferentiated poisoning. Two hundred twenty-seven cases of sedative/hypnotic toxicity were diagnosed during the observational study year, most of these involving benzodiazepines and nonbenzodiazepine hypnotics, but also melatonin, ramelteon, gamma-hydroxybutyrate, and 27 cases involving trazodone. The majority of these patients were given flumazenil, and of course, only those involving the effects of benzodiazepines and nonbenzodiazepine hypnotics responded. Twelve cases of mild intoxication were not treated with flumazenil.
As in the retrospective study, specific diagnoses having clinical features similar to benzodiazepine toxicity predicted a higher rate of antidote use. These include opioid toxicity, anticonvulsant toxicity, and alcohol intoxication. Response rates in patients with the latter two conditions were correspondingly lower than average. Patients with stimulant toxicity, serotonin syndrome, and lithium toxicity were among those less likely to have been given flumazenil during the prospective study period, as sedative medications are part of the treatment when patients’ clinical presentations are dominated by these toxidromes. Patients with mixed ingestions, however, and those oversedated with benzodiazepines were treated with flumazenil based on the clinical pathway as described (). A very small minority of patients with TCA poisoning was treated with flumazenil, but each had a positive response that contributed to safe management while avoiding intubation.
While not given flumazenil early in their courses of therapy, patients suffering withdrawal states are regularly given benzodiazepines, and some experience oversedation and/or delirium. These patients are treated with flumazenil when seen by our toxicology service. It was not possible to verify this time course for such cases in the retrospective study, so they are not reported separately in . We suspect there were a large number of patients who underwent this course of treatment over that six-year period, and those cases are contained within those data that reflect no major side effects, including seizures. We did, however, identify 53 alcohol withdrawal cases, 9 opioid withdrawal cases, and 6 sedative withdrawal cases in the prospective year of study, a fraction of whom received flumazenil to reverse excessive effects of benzodiazepine treatment (). Response rates were high, and none of these patients suffered adverse effects. No specific toxicologic diagnoses predicted higher rates of adverse events.
In the 212 patients given flumazenil during the close observation year, 12 total adverse events were observed, the majority of which were minor psychobehavioral manifestations (). Three patients exhibited notable drooling, but in no case did it interfere with extubation procedures or airway integrity. Seven patients awoke with notable anxiety and/or emotional upset. All of the observed anxiety episodes occurred in patients from the chronic benzodiazepine use and sedative abuse groups. None of these reactions was associated with clinically relevant alterations in vital signs or physical status, and all such patients responded to verbal reassurance. Repeat doses of the antidote were given to 6 of these 7 patients; anxiety did not recur with similar intensity. The other two adverse behavioral events occurred in the same patient who presented similarly on two separate occasions after purposeful overdose of multiple medications. A 40-year-old male with spina bifida and a multi-cluster personality disorder had evidence of baclofen, pregabalin, and clonazepam toxicity. He was near coma, so flumazenil was given to promote arousal. It led to more wakefulness and increase in oxygen saturation on pulse oximetry monitoring, but the patient awakened in an altered state of mind and engaged in self-stimulatory activity for nearly a minute, and did not respond to verbal redirection from staff. Upon repeat of the same initial presentation several weeks later, despite the history and concern about a similar behavioral event, flumazenil was given again in order to avoid endotracheal intubation. The intervention was successful in that regard, but the patient engaged in similar sexual behavior in his more aroused, altered state. None of these adverse effects, however, led to clinical complications or lasting sequelae that could be attributed to the dosing of antidote.
Observational study – flumazenil use, response rates, and adverse events by comorbidity viewed as high risk
reports the number of patients treated with flumazenil during the prospective study period with conditions that could be construed as high-risk with respect to potential side effects from the antidote. The conditions include traumatic brain injury, CNS disease, psychiatric illnesses (attention-deficit hyperactivity disorder, mood disorders, anxiety disorders, psychotic disorders, and personality disorders), and seizure disorders. Data for the latter group were gathered on the use of AEDs, and patients were subcategorized based on the relation between their AED serum concentrations and therapeutic ranges.
Table 3. Patients with neurologic and psychiatric comorbidity treated with Flumazenil.
Patients with seizure disorders were statistically less likely to respond to treatment with flumazenil. Higher serum concentrations of AEDs in these patients were negatively associated with antidotal response. One seizure disorder patient with comorbid anxiety experienced emotional upset upon arousal with flumazenil. No other adverse effects were seen, including seizures. Two patients were identified with epilepsy who were taking only benzodiazepines to prevent seizures. They both had positive responses to flumazenil treatment (one received 5 doses and the other 7 doses) without adverse events.
Patients with a variety of CNS pathologies including traumatic brain injury, multiple sclerosis, dementia, Parkinson’s disease, and brain tumor had safe experiences with the antidote. As noted above, one patient with spina bifida exhibited behavioral disturbance upon wakening in the context of a polysubstance toxic ingestion on two separate occasions. Again, no seizures were observed. A variety of Axis I conditions were also associated with no significant adverse events, and response rates were not significantly different across major diagnostic categories. Anxiety was most likely to emerge in patients with an underlying anxiety disorder, but other psychiatric comorbidities were common in patients who awakened with emotional upset, as well.
Individuals with personality disorder diagnoses were less likely to have a positive response to flumazenil. However, these patients were also more likely to ingest toxic amounts of multiple compounds. Therefore, controlling for the impact of having multiple toxicologic diagnoses rendered personality disorder patients statistically no less likely to respond to flumazenil than others in the prospective study sample. In general, the underlying conditions that make some physicians reluctant to use flumazenil to promote arousal, allow for meaningful dialog with patients, and potentially avoid intubation and other instrumentation were not correlated with untoward effects that adversely impacted the management of patients in this detailed study of bedside practice.
Discussion
The safety profile of benzodiazepines is vastly superior to that of older classes of sedative compounds, thus giving them a wide range of applications in both inpatient and outpatient medicine. Still, they are far from benign, having the potential to produce complications in the acute care setting [Citation14], as well as in chronic use with adverse effects on cognition and ambulatory function [Citation15,Citation16]. Their wide distribution makes them readily available for an increasing share of the purposeful overdoses presenting to emergency departments [Citation11, Citation17]. Yet, the approved direct acting antidote to their primary toxic effects, which has been available for over 25 years, remains underutilized [Citation11].
Fears of inducing severe side effects based on remotely gathered poison center data often prevent the proper use of antidotes at the bedside. Regarding flumazenil, a recent study warned of a 1.4% seizure rate in 10 years of poison center recommended use [Citation18]. One death was reported to have been caused by the antidote, as well. Such data and corresponding rates of complications rely on remote sharing of information about history and clinical presentation, as well as the reporting of adverse events. Not only is there a bias in favor of over-reporting of adverse outcomes with such a methodology, but an even more important limitation with serious clinical implications is the delivery of treatment recommendations via telephone consultation. This practice, by its very nature, allows more focus to be placed on fragments of history and suspected ingestion than upon direct assessment and examination of patients.
Even in studies of patients assessed and treated directly with flumazenil, seizures have been reported [Citation7,Citation8]. However, the majority of the reported flumazenil-induced seizures in these early studies occurred in patients who co-ingested toxic amounts of TCAs and/or were given single doses of flumazenil in excess of 0.5 mg at a time [Citation8]. A recent meta-analytic review suggested that not only treatment emergent anxiety but even seizures or arrhythmias could emerge with flumazenil treatment, but the maximum dose allowed in all the clinical trials in the review was 1 mg or higher, and specific details about methodology, including attention to aspects of physical examination of patient subjects was lacking. [Citation19] The administration of flumazenil to a sedate or comatose patient involves a clinical decision that should emphasize objective aspects of patient presentation. Patients with toxicity from TCAs or other epileptogenic compounds rarely appear purely sedate. Even if they are obtunded, these patients often display tachycardia, autonomic instability, myoclonic jerks, hyperreflexia, hypertonia, and other neurologic disturbances. Indeed, in 1996 Gueye and colleagues presented a study of 35 patients treated with flumazenil, of whom 5 had seizures following antidote administration. In all 5 cases, the investigators reported neurologic and EKG abnormalities suggestive of clinically meaningful stimulatory neurotransmitter activity at the time of flumazenil administration [Citation7]. Consistent with these data, our experience (communicated through the present study) indicates that a thorough vital sign review and physical examination provides sufficient information to identify patients in whom flumazenil use could unmask the epileptogenic potential of co-ingestants.
In support of this assertion, we have presented 7 years of experience with flumazenil in multiple settings and circumstances using a protocol based centrally on direct clinical assessment of the potentially toxic patient (). Bedside use of the antidote remained steady over that time period on the basis of its observed utility and safety profile. With our retrospective review and prospective observation we have reported on a total of 731 patients treated with flumazenil for suspected GABA-ergic toxicity. The overall positive response rate exceeded 80% with a total of just 12 minor adverse reactions, and no seizures or arrhythmias. It should be noted that while many textbooks of emergency medical and toxicologic practice warn against the use of flumazenil in cases of “undifferentiated poisoning” or “suspected polysubstance ingestion,” [Citation20,Citation21] over 38% of the patients in our practice had multiple toxicologic diagnoses and were treated safely with the antidote. Nearly 75% of those had a clinically meaningful positive response, as well. Our data indicate that flumazenil can be used frequently for diagnostic purposes in cases of sedation and coma of unclear etiology, and then continued as targeted treatment when GABA-ergic toxicity is identified by the favorable response. Planning to continue dosing as needed going forward is important, as its utility could be dismissed on the basis of a short duration of action if orders for repeat treatment are not instituted. Our practice is to discontinue the often-automatic use of supplemental oxygen therapy (which may mask ventilatory compromise) and prescribe flumazenil 0.5 mg IV q1-2 h PRN confusion, failure to participate in care, sedation with Riker score < 3, and/or passage of a concerning threshold in either oxygen saturation or end-tidal carbon dioxide measurement.
Based upon the treatment practice outlined above, the only patients who would receive a diagnosis of benzodiazepine toxicity and not receive at least one dose of flumazenil would be those with very mild symptoms whose ability to participate in their own care is unimpaired. In this study, we assigned a diagnosis of GABA-ergic toxicity to all patients who had a demonstrable positive response to flumazenil. One could contend that flumazenil might function as an analeptic even in cases in which compounds with GABA-ergic activity are not present and responsible for altered mentation. Some research indicates that flumazenil may have inverse agonist activity [Citation22,Citation23], perhaps enhanced in patients with underlying anxiety disorders, but this effect is typically seen at doses higher than those used in our clinical practice [Citation23,Citation24]. Nevertheless, we do appreciate that the assignment of the diagnosis of sedative toxicity in this clinical sample may be viewed as dependent upon a degree of circular logic, so the report of all the positive responses in this diagnostic subgroup may not be statistically meaningful (). There are agents commonly classified as sedative/hypnotics for which flumazenil does not provide clinical improvement, like trazodone, ramelteon, gamma-hydroxybutyrate, and some anticholinergic antihistamines. Cases of toxicity from these agents account for the 20-25% of patients with the diagnosis of sedative/hypnotic toxicity who did not have positive responses in the study. However, the safety (and, in our estimation, utility) of the antidote cannot be denied, especially noting the high rate of documented positive responses, absence of significant side effects, and range of commonly used compounds for which flumazenil is effective ().
Table 4. Sedating Compounds for which Flumazenil is Antidotal The table lists the intoxicants for which flumazenil was used as antidotal therapy for sedation, coma, and delirium by the medical toxicology service during the seven study years.
We suspect that the majority of differences in patient characteristics between the observational and retrospective arms of the study relate to our ability to gather more complete clinical datasets during the prospective year. It is unlikely, for instance, that rates of alcohol use and seizure disorders would have increased significantly in one year’s time without changes in practice or referral patterns, which were not observed. The only notable exception was a rise in the use of synthetic cannabinoids and cathinones whose clinical presentations would not be expected to prompt the use of flumazenil during initial assessment and management.
In accordance with the study design, we also suspect that overall use of flumazenil was not markedly higher in the observational year as compared to the years of the retrospective study, as it appears. Rather, the close observational study captured cases in which the antidote was used in the emergency department but then not used again after admission. This interpretation is consistent with the overall lower rate of positive response in the observational year, as a lack of improvement with a single dose of flumazenil in the emergency setting would be documented as a negative response, and repeat doses would not be given later in the hospital course after admission. The antidote would have served a diagnostic purpose to rule out toxicity from certain sedatives (), but would not be of further therapeutic value to warrant subsequent use. Such was likely the case in our practice from 2003-2009, as well, but simply not captured by the retrospective methodology. Single use cases also occurred with positive results, as well though, in exposures involving short acting toxins like temazepam and zaleplon. The use of flumazenil in these cases would have been captured in the observational year, but not the retrospective review.
With respect to side effects, in the observational study year the majority of those reported pertain to the first dose of antidote given to any particular patient. In the event of an adverse reaction in the absence of positive response, no further doses of flumazenil would be administered in accordance with clinical practice (). Patients who both responded favorably to the antidote and developed anxiety or behavioral disturbance, however, were treated with flumazenil during the remainder of hospitalization as deemed clinically necessary. Such sensitivity was more common in patients with underlying anxiety disorders, as previously reported [Citation24]. Therapeutic presence with reassurance was sufficient to help patients navigate these generally minor discomforts. Only major adverse reactions were documented in the medical record for the years of retrospective study; no such events were identified.
Apart from direct clinical assessment, the other key aspect that defines the safety of flumazenil in the protocol outlined herein is the dose (). The standard intervention is 0.5 mg IV that is not repeated or increased within an hour unless the patient’s clinical status demands it to avoid intubation. Meta analysis of the cases of emergent seizures reported during the first 5 years after flumazenil’s FDA approval reveals that doses of 1 mg at a time or higher were given in most of those cases of seizures [Citation8]. Underlying seizure disorders appeared to represent a risk, as well, but the data presented here reflecting our extensive clinical experience suggest otherwise, regardless of whether or not a given patient is adherent to therapeutic doses of AEDs or not at the time of antidote delivery (). This is the case in patients with seizure disorders who chronically take no other AEDs apart from benzodiazepines, as well.
Flumazenil, properly dosed to be an antagonist and not an inverse agonist, is not directly neurostimulatory and only reverses a portion of the inhibitory tone of a GABA-ergic overdose. Even 1.5 mg occupies only 55% of potential GABA-A binding sites in the adult brain, and the antidote also has a short CNS half-life [Citation25]. Therefore, if an ictus were to occur, protracted seizure activity would not result. Furthermore, since flumazenil works competitively at GABA receptors, treatment with benzodiazepines should halt an emergent seizure if it failed to resolve quickly on its own. It is for this same reason that proper use of the antidote is safe even when clinical assessment confuses benzodiazepine effects for other toxidromes that appear as differential diagnoses, including alcohol intoxication, opioid toxicity, and anticonvulsant toxicity. Precipitation of even mild withdrawal using our flumazenil dosing protocol is much less common than precipitated opioid withdrawal that occurs commonly with the very liberal use of naloxone as it is currently employed in acute care and pre-hospital medical practice.
Dose-governed safety also extends to patients with documented underlying withdrawal vulnerabilities. History of significant withdrawal from sedating medications, especially recent (i.e. during the current episode of acute care) could be viewed as a risk factor for complications from flumazenil administration. We report systematically studied cases of such vulnerability in the close observational year, involving alcohol, opioid, and sedative withdrawal. None of the patients experienced adverse events, and the antidote, administered based upon the patients’ presenting symptoms, vital signs, and examination as above (), was deemed clinically useful in almost every case (). It should be noted that these withdrawal cases were all moderate to severe, as a large number of milder cases did not come to the attention of the medical toxicology service. There were certainly a large number of withdrawal cases over the course of the 6 retrospective years of study, as well, captured in the cohort of patients with alcohol and substance use disorders. They are not reported separately because this common clinical course could not be definitively established through retrospective methodology (v.s. Results). However, we published a study of the antidote’s specific use in managing delirium during the latter portion of treatment for alcohol withdrawal [Citation12]. Oversedation and frank delirium were the targets for intervention that were safely addressed with flumazenil.
As delirium, regardless of cause, confers an increased risk of morbidity and mortality, its prompt identification and treatment is a central concern in acute medical practice. Although debate continues regarding the most effective management strategies for symptoms of the syndrome, it has been well established that the most important intervention for any delirious state is treatment of the underlying cause. For benzodiazepine-induced delirium, there is a direct antidote available that addresses the neurochemical etiology, but it has been vastly underutilized for decades due to concerns about adverse effects. The clinical data outlined above should largely dispel any myth that flumazenil is an unsafe antidote for suspected benzodiazepine toxicity. Some physicians choose not to treat delirial symptoms unless they appear to be a threat to life and limb as a result of agitation, or at the very least, pose challenges to nursing care. However, recent studies indicate that any untreated delirium increases the risk of long-term poor health. Post-traumatic stress disorder (PTSD) as a consequence of medical trauma is much more likely in patients who suffer delirium regardless of the cause, and the more severe and longer lasting the delirium, the greater that risk [Citation26]. Furthermore, other independent predictors of PTSD one and two years after discharge include amnesia for the early portion of hospitalization, youth, female gender, low education level, trait anxiety, and lack of social support [Citation26]—all characteristics that are more common in the acute toxicology patient than those hospitalized for other reasons.
The risk of functionally meaningful depression during the years following hospitalization also increases greatly as a function of delirium and its severity. Depression prevalence following a hospital stay with delirium is 31% [Citation27], an estimated two-fold increase in mood disorder diagnosis as compared to those whose hospitalizations proceed without similar CNS insult. As with PTSD, common characteristics of toxicology patients—female gender and lack of social support—increase this depression risk [Citation28,Citation29]. Additionally, the cumulative dose of benzodiazepines during an intensive care unit stay is positively correlated with both depression and PTSD rates in the months to years following discharge [Citation29]. This is the class of medications most commonly employed to manage agitation and maintain sedation in acute hospital settings. Benzodiazepines are often given in continuous and escalating infusions to calm delirial agitation, but their use is associated with higher rates of delirium when the cumulative dose in hospitalized patients exceeds just a few milligram lorazepam equivalents per day [Citation30].
Noting the risks of delirium from extended use of benzodiazepines, we recommend using flumazenil to reverse toxic effects to enhance patients’ cognitive function and facilitate and maintain extubation in patients whose sedative burden is the chief obstacle to advancing their care. Such cases represent roughly 10% of the sedative-toxic cases in the observational year of this study. In our experience, withdrawal is rare and mild, despite the fears voiced by consulting medical and surgical teams to explain why benzodiazepine infusions are continued and/or long tapers are planned. The emergence of withdrawal, however, is neither routinely observed nor largely predictable [Citation31,Citation32], so we often intervene by halting sedative drips and giving flumazenil to facilitate extubation. The purpose of our recommendation is to minimize iatrogenic consequences of assisted ventilation and prevent delirium from further lengthening and complicating hospital stays. A randomized, controlled trial would be necessary to definitively demonstrate efficacy in these arenas. Absent those data, however, we aver that it is clinically logical to reverse the effects of medications with known association to both acute and chronic adverse consequences when their immediate utility has passed. Although not routinely employed in most hospitals, flumazenil may be useful not only as a short-term diagnostic tool and treatment for sedation in toxicology patients, but also helpful in taking the long view of patients’ physical and mental resilience against the threat of delirium.
Attempts to be thorough and unbiased were pursued throughout the course of this research, but limitations of the work must be noted. The retrospective design of the first leg of this study resulted in a cohort of patients with an occasionally-incomplete medical record and reliance on a secondary written record. Some adverse events may have been missed, because they may not have been described in the medical record. As already noted the lack of an electronic medical record for the emergency department limited full evaluation of some cases and inevitably resulted in undercounting of cases of antidote use during the retrospective period. Some of those cases potentially involved adverse reactions. Such limitations and incomplete records were not an issue for the prospective year of study.
The possibility of this research, however, depended upon the invaluable resource of the toxicology practice database—a systematic documentation routine established by the medical director of the service (JWD) at its inception in June 2003 and consistently maintained for the study period. Although potentially biased by the practitioner’s perspective on the course and care of patients, the detailed notes represent extensive bedside experience in toxicology and the use of antidotes based not upon remote consultative input, but upon direct physician assessment and intervention. This system served as the model and starting point for designing the observational arm of the study. During that study year, both junior fellows (JJR and KKS) gathered data for the study, and these records were reconciled with each other and with the attending’s notes (per JWD’s established routine). Most patients were seen by both fellows during their care by the toxicology service—discrepancies were rare, and reconciled by the primary author with the documentation and guidance of the senior author. We acknowledge the potential for biased reporting in this process.
Furthermore, not every administered dose of antidote was observed directly by the authors (or even a rotating trainee physician). The use of flumazenil as outlined () is so routine in our practice, that after direct physician observation of response to an initial dose, nursing staff follow PRN orders as long as patients show improvements in mental status and behavior; a physician is contacted if adverse effects are observed, so although these reactions were recorded by study physicians, personnel may have gone to the bedside after they occurred, and would obviously not administer flumazenil again merely to reproduce the side effect for confirmation. Upon detailed review, however, it appears that one episode of sexual acting out upon awakening is the only adverse effect not directly observed by at least one of the study physicians.
The subjective nature of the clinical assessment of outcomes may have also contributed to recorder bias with respect to antidotal response. Although responses to flumazenil were recorded as simply positive or negative, the reality of bedside treatment is that some patients experienced more complete restoration of neuropsychiatric function than others. The design of this study did not account for that variability, but instead ascribed a positive response to all patients who displayed at least some clinically relevant improvement in wakefulness, cognition, or behavior. A portion of patients still required adjunctive pharmacologic and non-pharmacologic interventions for management of neurobehavioral manifestations of toxicity, even when flumazenil was deemed effective.
With respect to clinical outcomes, this study does not directly address that question. Despite its size and the report of regular clinical use based on this extensive experience, the present study does not definitively indicate whether flumazenil use is responsible for better results. Such a claim would require a placebo-controlled trial of the antidote with the clinical methodology suggested (). Nearly three decades ago, one well-designed double-blind trial in the emergency setting demonstrated high efficacy for the measures studied with a good safety profile based on having direct examination as part of the patient selection profile [Citation33]. Authors from this trial subsequently advocated for expanded use of flumazenil with careful patient selection and measured dosing [Citation34]. A meta-analysis in 2007 reported favorable outcomes across all identified studies of flumazenil involving direct clinical assessment, with minor side effects in the form of emesis or anxiety; the only serious adverse events occurred with excessive flumazenil doses in patients with physical findings inconsistent with sedative toxicity [Citation4]. Since that time, however, extension of the principles of practice into the inpatient setting has not occurred, and unfortunately, the antidote has been all but abandoned in most emergency departments. Another prospective randomized trial focused on the care of patients beyond acute overdose presentation may be needed.
Absent such results, our clinical experience documented for a wide variety of toxic patients in the retrospective portion of this study has convinced our service and numerous trainees rotating through it that flumazenil is safe and effective. Scores of patients who would have been sedate, intubated, and catheterized thereby increasing their risk of nosocomial infection and other complications from greater instrumentation and increased lengths of stay serve as testament to this perspective. Positive antidotal response has also reduced utilization of computed tomography and allowed more thorough assessment of patients through productive interviewing that revealed different acute care needs that might otherwise have been missed. It is experience with those patients that convinced the authors the prospective year would be more ethically conducted as an observational study instead of a randomized trial that would deny a large number of patients an efficacious treatment. We appreciate, however, that a placebo-controlled experiment has not been conducted to measure lengths of stay, complication rates, and long-term outcomes to support this claim; our report of positive results in this study, despite its size, lacks a comparison group.
As supported by this largest study to date, flumazenil is a safe and potentially effective medication in the undifferentiated patient with sedation, confusion, or coma and a differential diagnostic list that includes toxic etiologies for the alterations in mental status. Even in the setting of mixed drug ingestions and in patients with a variety of medical comorbidities, the antidote produces few side effects when properly dosed. The key to its safe use is clinical assessment of vital signs, cognition, and neuromuscular activity, followed, where indicated, by piggyback infusion of antidote and reassessment of neurobehavioral status. With proper use, the incidence of seizures and cardiotoxic sequelae is negligible. Other adverse effects are generally mild and self-limited; maintaining an erect posture of the torso prevents complications from drooling. With attention to the other demands of psychosomatic care, emergent anxiety and associated behavioral manifestations are manageable. Based on the ubiquity of GABA-ergic sedatives in wide medicinal, recreational, and suicidal use, the potential to diagnose toxicity, clear cognition rapidly, promote arousal, and allow more patients to participate in their own care is great. On the basis of this study, we advocate for the expanded use of flumazenil in cases of pathologic sedation and altered mental status based on proper patient selection in emergency and hospital medicine.
Author contribution
JJR and JWD conceived the study and designed the trial. JJR, KKS, and JWD supervised the conduct of the trial and collected data. JJR and VK managed and analyzed the data and assured quality control. JJR and VK drafted the manuscript, and all authors contributed substantially to its revision. JJR takes responsibility for the paper as a whole.
Acknowledgements
The authors thank Erica E. Smolcic, M.D. and Amanda Cresswell R.N., M.S.N., C.M.S.R.N. for assistance with chart review. We are also grateful to Kara Gemberling and Patti Metherell for arranging data extraction from the electronic medical and pharmacy records of PinnacleHealth.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Proudfoot AT, Park J. Changing pattern of drugs used for self-poisoning. Br Med J. 1978;1(6105):90–93.
- Henderson A, Wright M, Pond SM. Experience with 732 acute overdose patients admitted to an intensive care unit over six years. Med J Aust. 1993;158(1):28–30.
- Hunkeler W, Mohler H, Pieri L, et al. Selective antagonists of benzodiazepines. Nature. 1981;290(5806):514–516.
- Ngo AS, Anthony CR, Samuel M, et al. Should a benzodiazepine antagonist be used in unconscious patients presenting to the emergency department? Resuscitation. 2007;74(1):27–37.
- Ashton CH. Benzodiazepine overdose: are specific antagonists useful. Br Med J (Clin Res Ed). 1985;290(6471):805–806.
- Spivey WH, Roberts JR, Derlet RW. A clinical trial of escalating doses of flumazenil for reversal of suspected benzodiazepine overdose in the emergency department. Ann Emerg Med. 1993;22(12):1813–1821.
- Gueye PN, Hoffman JR, Taboulet P, et al. Empiric use of flumazenil in comatose patients: limited applicability of criteria to define low risk. Ann Emerg Med. 1996;27(6):730–735.
- Spivey WH. Flumazenil and seizures: analysis of 43 cases. Clin Ther. 1992;14(2):292–305.
- Wolf BC, Lavezzi WA, Sullivan LM, et al. Alprazolam-related deaths in Palm Beach County. Am J Forensic Med Pathol. 2005;26(1):24–27.
- Wunsch MJ, Nakamoto K, Behonick G, et al. Opioid deaths in rural Virginia: a description of the high prevalence of accidental fatalities involving prescribed medications. Am J Addict. 2009;18(1):5–14.
- Mowry JB, Spyker DA, Cantilena LR, Jr, et al. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52(10):1032–1283.
- Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol. 2014;10(2):126–132.
- Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66(Suppl 2):9–13.
- Kallin K, Jensen J, Olsson LL, et al. Why the elderly fall in residential care facilities, and suggested remedies. J Fam Pract. 2004;53(1):41–52.
- Mowry JB, Spyker DA, Cantilena LR, Jr, et al. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS):30th Annual Report. Clin Toxicol (Phila). 2013;51(10):949–1229.
- Kreshak AA, Cantrell FL, Clark RF, et al. poison center’s ten-year experience with flumazenil administration to acutely poisoned adults. J Emerg Med. 2012;43(4):677–682.
- Penninga EI, Graudal N, Ladekarl MB, et al. Adverse events associated with flumazenil treatment for the management of suspected benzodiazepine intoxication–A systematic review with meta-analyses of randomised trials. Basic Clin Pharmacol Toxicol. 2016;118(1):37–44.
- Tintinalli JE, Cline D, eds. Tintinalli’s emergency medicine manual. New York: McGraw-Hill Medical; 2012.
- Yealy DM, Callaway C, eds. Emergency department critical care. New York: Oxford University Press; 2013.
- Haefely W. The preclinical pharmacology of flumazenil. Eur J Anaesthesiol. 1988;2:25–36.
- Brogden RN, Goa KL. A preliminary review of its benzodiazepine antagonist properties, intrinsic activity and therapeutic use. Drugs. 1988;35(4):448–467.
- Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179(5):390–396.
- Savic I, Widén L, Stone-Elander S. Feasibility of reversing benzodiazepine tolerance with flumazenil. Lancet. 1991;337(8734):133–137.
- Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36:2801–2809.
- Davydow DS. Symptoms of depression and anxiety after delirium. Psychosomatics. 2009;50(4):309–316.
- Myhren H, Ekeberg O, Tøien K, et al. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14(1):R14.
- Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421–434.
- Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26.
- Buczko GB. Sedation in critically ill patients: a review. Med Health R I. 2001;84(10):321–323.
- Dominguez KD, Crowley MR, Coleman DM, et al. Withdrawal from lorazepam in critically ill children. Ann Pharmacother. 2006;40(6):1035–1039.
- Weinbroum A, Rudick V, Sorkine P, et al. Use of flumazenil in the treatment of drug overdose: a double-blind and open clinical study in 110 patients. Crit Care Med. 1996;24(2):199–206.
- Weinbroum AA, Flaishon R, Sorkine P, et al. A risk-benefit assessment of flumazenil in the management of benzodiazepine overdose. Drug Saf. 1997;17(3):181–196.

