ABSTRACT
RATIONALE: While severe asthma affects approximately 5% of all individuals with asthma, this small minority of individuals accounts for a large proportion of the asthma-related costs. Greater understanding of the pathophysiology of asthma combined with the emergence of novel biologic therapies for severe asthma supported the need for a thorough review of the diagnosis, investigation, phenotyping, and management of severe asthma.
OBJECTIVES: We aimed to propose a practical approach to distinguish uncontrolled asthma due to inadequate asthma management from severe asthma despite optimal asthma management. Moreover, based on emerging scientific evidence, we sought to provide guidance for characterizing individuals with severe asthma and considering a phenotype-specific management. We also aimed to review other novel new potential therapeutic approaches.
METHODS: We systematically reviewed the relevant literature focusing on randomized controlled trials and when available, systematic reviews of randomized controlled trials. The proposed key messages, based on scientific evidence and expert opinion, were agreed upon by unanimous consensus.
MAIN RESULTS: We defined severe asthma and outlined its significant impact from the societal and patient perspectives. We outlined a practical approach to distinguish severe from uncontrolled but not severe asthma, based on stepwise investigation and management of potential reasons for uncontrolled asthma. After reviewing the current evidence we concluded that: 1) Several biomarkers (e.g. sputum or blood eosinophil count, total IgE, or FeNO) can help identify potential responders to new therapeutic options; 2) Tiotropium may be considered as an add-on therapy for individuals 12 years of age and over with severe asthma uncontrolled despite combination ICS/LABA therapy; 3) The chronic use of macrolides may decrease asthma exacerbations in individuals 18 years of age and over with severe asthma independent of their inflammatory profile; 4) Children aged 6 years and older and adults who are sensitized to at least one relevant perennial allergen and who remain poorly controlled asthmatics despite high dose ICS and a second controller can benefit from the addition of anti-IgE therapy to reduce asthma exacerbations; due to the known risk of side effects associated with high-dose ICS in children, omalizumab should also be considered in children and adolescents who repeatedly exacerbate or have poor control when therapy is stepped down from high-dose to moderate-dose ICS and at least one other controller; 5) Anti-IL5 therapies may be considered for adults 18 years of age and over with severe eosinophilic asthma who experience recurrent asthma exacerbations in spite of high doses of ICS in addition to at least one other controller; and 6) Although bronchial thermoplasty has shown a decrease in asthma exacerbations in one study, its role in the treatment of severe asthma remains uncertain.
CONCLUSIONS: After reviewing existing and emerging therapies for severe asthma, we developed key messages for phenotyping individuals with severe asthma and suggested phenotype-specific targeted therapies. We highlighted gaps in knowledge in the pathophysiology of severe asthma, the identification of responders, and the assessment of the efficacy of novel therapies that should be targeted by future research.
RÉSUMÉ
JUSTIFICATION: Bien que l'asthme sévère touche environ 5 % des asthmatiques, une grande proportion des coûts liés à l'asthme est imputable à cette petite minorité. Une plus grande compréhension de la pathophysiologie de l'asthme, combinée à l'émergence de traitements biologiques novateurs pour l'asthme sévère, a rendu nécessaire la révision approfondie du diagnostic, de l'investigation, du phénotypage et de la prise en charge de l'asthme sévère.
OBJECTIFS: Nous avions pour but de proposer une approche pratique pour distinguer l'asthme non contrôlé dû à une prise en charge inadéquate, de l'asthme sévère dépit d'une prise en charge optimale de l'asthme. De plus, à partir des données probantes scientifiques émergentes, nous avons cherché à donner des indications pour la caractérisation des personnes souffrant d'asthme sévère et envisager une prise en charge spécifique à leur phénotype. Nous avions aussi pour but d'examiner d'autres approches thérapeutiques novatrices potentielles.
MÉTHODES: Nous avons passé en revue la littérature pertinente de façon systématique, en mettant l'accent sur les essais contrôlés randomisés et, lorsqu'elles étaient disponibles, sur les revues systématiques d'essais contrôlés randomisés.
PRINCIPAUX RÉSULTATS: Nous avons défini l'asthme sévère et décrit ses effets importants du point de vue sociétal et du point de vue du patient. Nous avons présenté une approche pratique afin de distinguer l'asthme sévère de l'asthme non contrôlé mais non sévère, fondée sur une investigation par étapes et la prise en charge des causes possibles de l'asthme non contrôlé.
Après avoir examiné les données probantes disponibles, nous avons conclu que : 1) Plusieurs biomarqueurs (ex.: numération des éosinophiles dans les expectorations ou dans le sang, IgE totales ou de FeNO) peuvent aider à répertorier les répondeurs potentiels aux nouvelles options thérapeutiques; 2) Le tiotropium peut être envisagé en tant que traitement additionnel pour les individus âgés de 12 ans et plus souffrant d'asthme sévère non contrôlé malgré une thérapie associant les CSI et les ABAP; 3) L'usage chronique des macrolides peut diminuer les exacerbations de l'asthme chez les individus âgés de 18 ans et plus souffrant d'asthme sévère indépendamment de leur profil inflammatoire; 4) Les enfants âgés de six ans et plus et les adultes sensibilisés à au moins un allergène pérenne pertinent qui demeurent des asthmatiques mal contrôlés malgré une forte dose de CSI et un deuxième contrôleur peuvent bénéficier de l'ajout d'un traitement anti-IgE afin de réduire les exacerbations de l'asthme; en raison du risque connu d'effets secondaires associés à une dose élevée de CSI chez les enfants, l'omalizumab devrait aussi être envisagé chez les enfants et les adolescents qui souffrent d'exacerbations à répétition ou dont l'asthme est mal contrôlé, lorsque le traitement passe d'une forte dose d'ICS à une dose modérée d'ICS assortie d'au moins un autre contrôleur; 5) Les traitements par anti-IL5 peuvent être envisagés pour les adultes de 18 ans et plus souffrant d'asthme éosinophilique sévère qui sont aux prises avec des exacerbations de l'asthme récurrentes malgré des doses élevées d'ICS associées à au moins un autre contrôleur; et 6) Bien que la thermoplastie bronchique ait démontré une diminution des exacerbations de l'asthme dans une étude, son rôle dans le traitement de l'asthme sévère demeure incertain.
CONCLUSION: Après avoir examiné les traitements existants et émergents pour l'asthme sévère, nous avons défini des messages-clés pour phénotyper les individus souffrant d'asthme sévère et suggérer des thérapies ciblées spécifiques au phénotype.
Introduction
Asthma is highly prevalent, affecting approximately 8.4% of CanadiansCitation1 and poses a substantial burden on individuals, and the health care system. Even though asthma mortality has declined and is now infrequent in Canada,Citation2 a recent review of asthma deaths in the United Kingdom has shown these events are often avoidable.Citation3 In addition there are accumulating data to suggest that uncontrolled asthma is highly prevalent in Canada.Citation4 While most uncontrolled asthma cases can be addressed with self-management education and pharmacologic strategies outlined in recent evidence-based guidelines,Citation5,6 a subset of individuals have severe asthma which remains poorly controlled despite these best practices. It should be noted that having severe asthma does not imply the presence of uncontrolled asthma. Severe asthma accounts for only approximately 5 to 10%Citation5 of the population with asthma, yet it is responsible for up to 50% of direct asthma costs and likely a much higher burden if one considers indirect costs.Citation7 Recognition of the significant impact of severe asthma on individuals' quality of lifeCitation6 and its associated costs occurs at a time when we have much greater understanding of the pathophysiology of asthma, particularly that of severe asthma. Consequently, many new novel therapiesCitation8 for severe asthma have emerged, some of which are currently available, with others in the late stage development. This position statement was developed to provide guidance for the management of severe asthma, to specifically address the role of new and emerging therapies, to better characterize potential responders and to provide a revised treatment algorithm accordingly.
Target population
This position statement applies to children (six years of age and over), adolescents (12 to 17 years), and adults (18 years of age and over) with asthma confirmed in accordance with published criteria,Citation5,6 and who meet the definition of ‘severe asthma’ outlined below.
Target users
The key messages provided herein are intended for use by healthcare practitioners who encounter and/or manage severe asthma including specialist physicians in respiratory medicine, pediatrics, allergy and immunology, emergency, and primary care practitioners, nurse practitioners, asthma/respiratory educators.
Methodology
Position statement development
The Canadian Thoracic Society (CTS) Asthma Clinical Assembly undertook this review of the assessment and management of severe asthma. The Assembly has a representative membership of adult and pediatric respirologists, pediatricians, specialists in Allergy and Clinical Immunology and Emergency Medicine, as well as a Primary Care physician. The document was prepared in accordance with the CTS requirements for a position statement (www.cts-sct.ca/guidelines). To answer each of the clinical questions literature reviews were conducted by the assembly members. Literature reviews included comprehensive searches of electronic databases from database inception to March 2017, focusing on randomized controlled trials (RCTs) and meta-analyses. Search strategies included keywords, with limits by study design and in some sections by specific inclusion criteria. Data from relevant studies were abstracted and summarized into evidence tables (Appendix 1) and are posted along with the guideline as supplementary material online and at www.cts-sct.ca/guidelines. The final report and its key messages were derived by consensus through a series of telephone conference calls and two face-to-face meetings. There was full consensus from Assembly members on the key messages. The completed document was reviewed by two asthma experts external to the CTS and one member of another CTS Clinical Assembly as well as the CTS Canadian Respiratory Guideline Executive Committee. One member completed the AGREE II score sheet. Original reviews and responses to reviews are posted along with the guideline and all author conflicts of interest at www.cts-sct.ca/guidelines. Based on the feedback from this process, a final document was developed and approved by the Assembly before being forwarded to the CTS Executive for final approval. Given the availability of many new treatment options for severe asthma and the evolving role for improved phenotyping of asthma, the CTS Asthma Clinical Assembly has developed this position statement to provide guidance. This position paper will be updated in accordance with the CTS Living Guideline Model www.cts-sct.ca/guidelines.
Formulation of key clinical questions
The CTS Asthma Clinical Assembly developed key clinical questions using the Problem/population Intervention/prognostic factor/exposure, Comparison, Outcome (PICO) format, which were then reviewed, revised and agreed upon by the Assembly. The Assembly agreed on prioritizing the different outcomes of the studies reviewed; the Assembly also agreed that asthma exacerbations would serve as the most relevant primary outcome in the field of severe asthma for all PICO questions. Other key secondary outcomes examined included: decreasing (or stopping) chronic oral corticosteroid use, symptoms, asthma control, quality of life and lung function parameters.
Summary of evidence and key messages
Section 1: Identification of severe asthma
What is the difference between uncontrolled asthma and severe asthma?
Definitions
Various definitions for severe asthma have been proposed, with most clinicians adopting the consensus definition developed by the European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force on Severe Asthma.Citation9 In the present document, we have adapted this definition to provide greater clarity around the inhaled corticosteroid (ICS) dose, in the context of current CTS Asthma guidelines.
Severe asthma
Asthma which requires treatment with high-dose ICS as outlined in (adults and children) and a second controller for the previous year, or systemic corticosteroids for 50% of the previous year to prevent it from becoming “uncontrolled”, or which remains “uncontrolled” despite this therapy is defined as severe asthma.
Table 1. Comparative inhaled corticosteroids (ICS) dosing categories in children, adolescents and adults.
Table 2. Asthma control criteria.Citation5
Uncontrolled asthma is defined as at least one of the following:
| 1) | Poor symptom control: as per Canadian Thoracic Society asthma control criteria* or other standardized questionnaires: Asthma Control Questionnaire (ACQ) consistently > 1.5, Asthma Controlled Test (ACT) <20, or child Asthma Controlled Test (cACT) < 20. | ||||
| 2) | Frequent severe exacerbations: two or more courses of systemic corticosteroids (≥3 days each) in the previous year. | ||||
| 3) | Serious exacerbations: at least one hospitalization, intensive care unit (ICU) stay or mechanical ventilation in the previous year. | ||||
| 4) | Airflow limitation: after appropriate bronchodilator withhold forced expiratory volume in one second (FEV1) <80% of personal best (or < the lower limit of normal (LLN), in the face of reduced FEV1/forced vital capacity (FVC) defined as less than the LLN). *Not meeting the criteria described in .Citation5 | ||||
An important consideration before labeling an individual as having severe asthma is a careful review of each individual's presentation. A diagnosis of asthma using objective measures, the assessment of domestic and work environment along with the verification of adherence to medication and co-morbidities is key.Citation5
While the importance of basing an asthma diagnosis on objective measures must be advised, this can often be challenging in individuals with truly severe asthma for several reasons. Adults with long-standing asthma may have limited or no reversibility due to airway remodeling, and/or may be unable to withhold bronchodilator medications to perform methacholine or exercise challenge tests. Over time, variation in airflow rates during exacerbations may be noted that meet diagnostic criteria. In the absence of such evidence, a thorough search for previous pulmonary function tests should always be undertaken, as well as a comprehensive evaluation for alternative diagnoses before making a clinical diagnosis of severe asthma.
There are reports that up to one third of individuals presumed to have a diagnosis of asthma are eventually recognized as having alternative diagnoses.Citation10 An alternate diagnosis should be considered particularly in the presence of well-preserved lung function and a lack of response to asthma therapy.
Non-adherence to prescribed medication is a major challenge to achieving asthma control and improved adherence has been shown to decrease severe exacerbations.Citation11 Careful review of an individual's pharmacy records to identify a low rate of prescription refills is particularly informative as patients overestimate their adherence.Citation12 A further major concern is the incorrect use of inhalers, which was recently shown in a systematic review to be as problematic now as it was twenty-five years ago.Citation13 Co-morbidities, that can both mimic asthma symptoms and worsen actual asthma, are highly prevalent and these include untreated upper airway disease such as rhino-sinusitis, vocal cord dysfunction (VCD), gastro esophageal reflux disease (GERD), and psychiatric disease including anxiety and depression. Careful consideration of these issues at a specialized clinic for individuals with uncontrolled asthma has shown improved outcomes.Citation14
When evaluating individuals with suspected uncontrolled severe asthma, one should review frequent causes of uncontrolled asthma outlined in , to distinguish uncontrolled asthma due to sub-optimal management from persistent uncontrolled asthma despite optimal management (i.e. even with the identification and correction of common causes for poor asthma control). This is of particular importance to those practitioners managing asthma exacerbations (e.g. emergency and primary care practitioners). The suspicion of severe asthma and the recognition of an individual with severe asthma, should prompt referrals for specialized evaluation and asthma education.Citation15 An operational definition of ‘asthma specialist’ would include specialists in asthma, general respirology, pediatrics, and/or allergy/immunology who have access to lung function, certified asthma/respiratory educators/nurse practitioners and FeNO +/− induced sputum analysis.
Figure 1. Approach to suspected uncontrolled severe asthma. *Not meeting the following 8 criteria: daytime symptoms <4 days/week; nighttime symptoms <1 night/week; normal physical activity, mild, infrequent exacerbations; no absence from work or school due to asthma; need for a fast-acting beta2-agonists <4 doses/week, forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥90% of personal best; and PEF diurnal variation <10–15%.Citation1 † Asthma Control Questionnaire on a scale of 0 (totally controlled) to 6 (severely uncontrolled) for individuals aged ≥6 years (in children ≤10 years, it must be administered by a trained interviewer).Citation2,3‡ Asthma Control Test on a scale of 0 (poor control of asthma) to 27 (complete control of asthma) for individuals aged 12 years and older.Citation4,5¶ Child Asthma Control Test on a scale of 0 to 27 for children aged 4 to 11 years old.Citation5,6** Forced expiratory volume in 1 second.†† Forced vital capacity.1Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J 2012; 19(2): 127-64.2Juniper, EF, O'byrne, PM , Guyatt, GH, Ferrie, PJ and King, DR. (1999), Development and validation of a questionnaire to measure asthma control. Eur Respir J, 14: 902–907. doi:10.1034/j.1399-3003.1999.14d29.x3Juniper EF, Svensson K, Mörk AC, Ståhl E. Modification of the Asthma Quality of Life Questionnaire (standardised) in patients 12 years and older. Health and Quality of Life Outcomes 2005, 3:58 ( 16Sep2005 )4Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65.5Koolen BB, Pijnenburg MW, Brackel HJ, Landstra AM, Van den Berg NJ, Merkus PJ, Hop WC, Vaessen-Verberne AA. Validation of a web-based version of the asthma control test and childhood asthma control test. Ped Pulmonol 2011;46:941-8.6Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007;119:817-25.
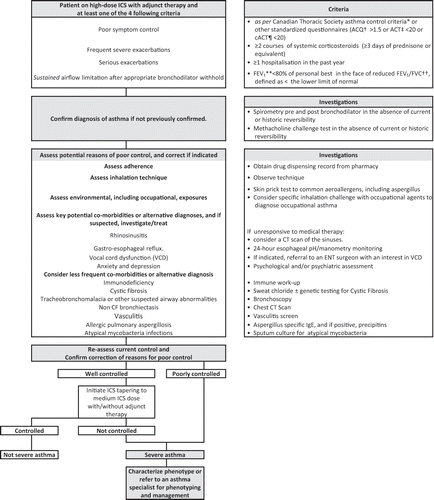
Figure 2. Management hinges upon confirming the diagnosis. All individuals with confirmed asthma should receive self-management education, including a written action plan. Very mild intermittent asthma may be treated with a short-acting beta2 agonist (SABA) taken as needed. SABAs are recommended for relief of symptoms; individuals 12 years of age and over with moderate to severe asthma (particularly those who are exacerbation prone and have poor control) who are taking an ICS/LABA formulation approved also for use as a reliever may do so. Inhaled corticosteroids (ICS) should be introduced early as the initial maintenance treatment for asthma even in individuals who report asthma symptoms less than three times a week. LTRA are second-line monotherapy for mild asthma. If asthma is not adequately controlled by low doses of inhaled corticosteroids, additional therapy should be considered. In children 6 years of age and over, the ICS should be increased to a medium dose before adding an adjunct agent such as a long-acting beta2–agonist (LABA) or LTRA. In individuals 12 years of age and over, a LABA should be considered first as adjunct therapy. A LABA should only be used in combination with an ICS. Increasing to a medium dose of ICS or the addition of a LTRA or tiotropium are third-line therapeutic options. Theophylline may be considered as a fourth-line agent in adults. Severe asthma may require additional treatment as outlined in . Exposure to asthma triggers in the environment, and the presence of co-mordibities should be reassessed at each visit and before altering the maintenance therapy. Consider also assessment of sputum eosinophils in adults with uncontrolled moderate to severe asthma managed in specialialized centres. After achieving acceptable asthma control for at least a few weeks to months, the medication should be reduced to the minimum necessary dose to achieve adequate asthma control and prevent future risk of exacerbations. HFA: Hydrofluoroalkane; mcg: Micrograms; PEF: Peak expiratory flow; yrs: Years.
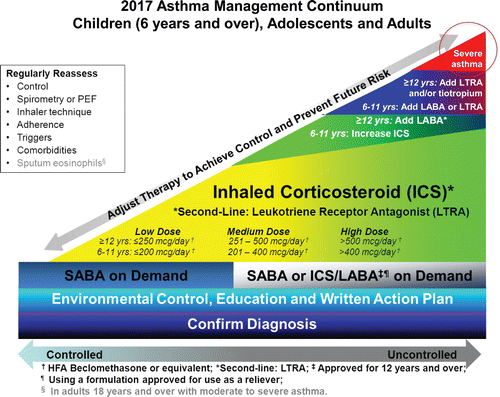
Figure 3. Management of severe asthma: The diagnosis of asthma should be objectively confirmed in all individuals with suspected uncontrolled severe asthma. Domestic and work environment as well as co-morbidities should be thoroughly assessed. Adherence to treatment should also be verified carefully. Individuals with severe asthma should be assessed by an asthma educator in order to ensure adequate inhalation technique and receive a written action plan. Individuals who experience recurrent asthma exacerbations requiring oral corticosteroids in spite of the combination of high dose ICS and long-acting beta 2 agonists +/- leukotriene inhibitor antagonists, theophylline, and/or tiotropium should be phenotyped by measuring blood eosinophil counts and serum IgE +/-sputum eosinophils and/or FeNO. Theophylline has a limited evidence base for use in severe asthma but maybe considered as part of an N-of-One therapeutic trial. Its potential benefits should be balanced against its associated adverse events. Omalizumab may be considered in individuals who experience recurrent asthma exacerbations requiring oral corticosteroids and in prednisone dependent individuals with allergic asthma with positive skin prick test to at least one perennial allergen and total serum IgE between 30 and 1300 IU/mL for children 6-11 years of age and 30 to 700 IU/mL in individuals 12 years of age and over. Anti-IL-5 therapy may be considered in individuals 18 years of age and over who experience recurrent asthma exacerbations requiring oral corticosteroids and prednisone dependent individuals and who show a pre-determined blood eosinophil count cut-off. Macrolides have been shown to decrease asthma exacerbations in only one large RCT and may be considered independently to a specific phenotype. Once asthma control has been achieved consider weaning oral corticosteroids in steroid-dependent individuals and lowering the ICS dose in the other cases.

More specialized investigations outlined in should be considered selectively when a specific co-morbidity is suspected. In particular, co-morbidities should be considered during the preliminary assessment if there is a lack of response to ICS combined with at least one other controller, despite the assessment and usual management of most frequent reasons for poor control i.e. inhaler technique and adherence. Completion of specialized investigations will depend upon local resources and the suspected co-morbidity, and in the absence of such expertise, it may justify consideration for referral to a specialized center. Sinus disease is common among individuals with severe asthma. In the absence of a response to saline irrigation with or without topical corticosteroids and/or anti–histamines, a computerized tomography (CT) scan of the sinuses and a referral to a dedicated Ear, Nose and Throat physician should be considered. GERD occurs in nearly 80% of all individuals with asthmaCitation16 but despite this high prevalence, empiric treatment of all asthma patients with a proton pump inhibitor has not proven to be effective in decreasing asthma exacerbations.Citation16 If nocturnal cough remains a predominant asthma symptom despite the absence of overt reflux, a 24 hour esophageal pH monitoring may identify previously unrecognized GERD. Upper gastrointestinal endoscopy should be considered in individuals with eosinophilia and suspected of having eosinophilic esophagitis. One may consider high resolution CT scan of the chest to exclude alternative diagnosis mimicking or complicating severe asthma, such as bronchiectasis.
Eosinophilic granulomatosis with polyangiitis and allergic bronchopulmonary aspergillosis occur in a minority of individuals with severe asthma, but recognition of these syndromes is important as they may dictate additional treatment requirements, such as oral corticosteroids (OCS) and anti-fungal treatment.
The performance of a bronchoscopy maybe helpful when an upper airway disease or an atypical infection is suspected. For example, a recent Canadian study assessing the over diagnosis of asthma in CanadaCitation10 identified two individuals, among 900 subjects, with previously unrecognized sub-glottic stenosis. Similarly, assessment of the vocal cords may suggest a diagnosis of VCD. If such assessments have been completed, possible therapeutic modifications made, and the asthma remains uncontrolled, it is reasonable to consider the individual to have severe asthma.
Corticosteroid-dependent asthma
Approximately 30% of adults with severe asthma are considered corticosteroid-dependent, meaning that chronic OCS are required in addition to ICS and other controllers to achieve and maintain control.Citation17,18 Regular use of OCS is associated with significant adverse events.Citation19 There appears to be a dose-response relationship between the maintenance OCS dose and its associated side effect profile.Citation20 The use of OCS therapy chronically is not only associated with a significant increase in adverse events but is also associated with an increased economic burden.Citation21 In addition, there are individuals who elect not to use maintenance controller therapy but would rather use frequent courses of OCS to treat exacerbations. This group requires education with regard to the efficacy of maintenance controller therapy in reducing the risk of asthma exacerbations and improving asthma control. Individuals who are truly corticosteroid-dependent based on current best available evidence, may be candidates for mepolizumab and benralizumab (when approved) because of their documented steroid sparing effect. Of note, ICS may cause some of the same systemic side effects as oral corticosteroids, particularly adrenal suppression in susceptible individuals with asthma.Citation22 Limited studies suggest that the risk of systemic side effects increases dramatically when high-dose ICS are used and that children and adolescents are particularly vulnerable to effects on linear growth, bone mineral density and adrenal function.Citation23 For this reason, the prescribing of long-term high-dose ICS for children and adults should be limited to specialists.Citation5
Conclusions
Severe asthma is uncommon occurring in approximately 5% of all asthma patients. The diagnosis of asthma needs to be ascertained and confirmed by objective measures. Severe asthma has to be distinguished from uncontrolled asthma. Uncontrolled asthma is most commonly associated with non-adherence and poor inhaler technique. While frequent comorbidities, such as upper airways disease, gastro esophageal reflux, and psychological illness need to be treated, other uncommon comorbidities have to be explored when asthma remains uncontrolled. When asthma control cannot be achieved despite an optimal asthma management a comprehensive phenotyping for consideration of novel therapies for severe asthma should be undertaken.
Box 1. Identification of severe asthma
What is the difference between uncontrolled asthma and severe asthma?
Key messages:
| 1. | Confirm the diagnosis of asthma with history and objective measures of lung function in individuals old enough to reliably perform pulmonary function tests (i.e. usually 6 years of age and over). | ||||
| 2. | Individuals with either suspected or confirmed severe asthma should receive comprehensive self-management asthma education and be evaluated by an asthma specialist. | ||||
| 3. | Domestic and occupational environment, co-morbidities, adherence to treatment and inhaler technique should be carefully assessed and treated before labelling an individual as having severe asthma. | ||||
| 4. | Adherence to treatment and inhaler technique should be carefully reviewed and addressed again before labelling an individual as having severe asthma and before considering additional therapies for this condition. | ||||
Section 2: Biomarkers to predict response to biologic therapies and macrolides
PICO 1: In children and adults with severe asthma, does the measurement of:
| a) | sputum differential cell count | ||||
| b) | fraction of exhaled nitric oxide (FeNO) | ||||
| c) | blood eosinophils | ||||
| d) | blood total Immunoglobulin E (IgE) | ||||
Phenotyping asthma
Severe asthma is increasingly recognized as a heterogeneous disease with multiple phenotypes. A phenotype is defined as a set of observable characteristics that result from interactions between genes and the environment.Citation9 Recognition of phenotypes such as atopic asthma has allowed us to develop a greater understanding of the underlying mechanisms of disease. Different pathophysiologic pathways involved in these phenotypes (labelled asthma endotypes), which are characterized by specific biomarkers and a differential response to more targeted therapies are emerging. These new therapies are costly, involve a more invasive method of drug delivery than oral administration (subcutaneous or intravenous injection) and have an unknown long-term safety profile. Therefore, it is important to identify objective biomarkers that will help identify individuals who are likely to respond to these therapies. Recent or chronic use of medications such as OCS that may affect biomarkers should be taken in to consideration when phenotyping patients. For example, if peripheral eosinophils are normal in an individual on chronic OCS, consideration should be given to tapering the OCS and repeating the biomarker.
One well-studied mechanistic pathway in severe asthma is the inflammation mediated by Type 2 cytokines. The literature typically refers to the Th2 pathway or Th2 high and low groups in recognition of the Type 2 T helper cell which was thought to be main producer of these cytokines. However it is now recognized that other cell types, such as Type 2 innate lymphoid cells also produce these Type 2 cytokines. Characterization of individuals into Th2-high and Th2-low groups by the differential gene expression of molecules induced by Th2 cytokines (periostin, chloride channel regulator 1, serpin peptidase inhibitor clade B, member 2) have shown that these individuals differ in their clinical characteristics, reticular basement thickness, and response to inhaled corticosteroids.Citation24 Molecules including IgE and various cytokines (Interleukin (IL) IL-4, IL-5, IL-13) involved in this pathway have become the targets for newly developed asthma medications. Biomarkers that have been used to identify individuals with Th2-mediated airway inflammation include blood and sputum eosinophil counts, FeNO, total serum IgE, and blood periostin. However, periostin is not available in clinical practice. In contrast, less is known about individuals with a Th2-low profile and whether this is associated with Th1 mediated inflammation or a pauci-inflammatory phenotype. Currently, clinically available biomarkers for Th1 inflammation are limited to sputum neutrophils.
The pivotal clinical trials for biomarkers are summarized in Appendix 1.
Biomarkers associated with Th2 inflammation
| a) | Sputum differential cell count | ||||
Inflammatory cellular profiles have been assessed in individuals with severe asthma using sputum differential cell counts. Although there are no published national or international guidelines on sputum induction, collection, and processing procedures, multiple protocols have been published.Citation25–27 Adequate sputum samples have been obtained in up to 74% of adults and 85% of children aged 7 years of age and over with severe stable asthma in centers experienced with this test.Citation27,28
Four sputum inflammatory profiles have been identified in adults: eosinophilic (eosinophils > 1.01%), neutrophilic (neutrophils > 61%), mixed granulocytic (eosinophils > 1.01% and neutrophils >61%) and pauci-granulocytic (eosinophils < 1.01% and neutrophils <61%)Citation29 although a cut-off of >3% is often used to define sputum eosinophilia.Citation30 In children, the same profiles have been identified, using slightly different cut-offs than in adults: eosinophilic (eosinophils > 2.5%); and neutrophilic (neutrophils > 54%).Citation28 Although there is no consensus for defining sputum neutrophilic inflammation, the identification of a sputum neutrophil count greater than 65% or greater than 500 × 104/ml on two occasions has been proposed.Citation31 In adults, sputum inflammatory profiles are relatively stable over the short and long term,Citation29,32 whereas more variation is observed in children. Indeed, in a longitudinal study of children with severe asthma, 63% of children displayed a different sputum inflammatory profile over four visits, an observation that was not explained by changes in medication.Citation28
Sputum eosinophil counts have been explored as a potential predictor of response to anti-IL5 therapies and macrolides. Sputum eosinophil counts have also been used to select individuals for trials of mepolizumabCitation33,34 and reslizumab.Citation35 These trials reported clear benefits on exacerbations and quality of life (QoL) in subjects with eosinophils >3%. However, the optimal cut-off of sputum eosinophil counts predicting a response to therapy has not been identified and as discussed below, blood eosinophils are a more consistent predictor of response to anti-IL5 medications.
In contrast, a recent trialCitation36 of azithromycin showed a significant decrease in the risk of severe asthma exacerbations in individuals, independent of their level of sputum eosinophil counts.
| b) | Fractional exhaled nitric oxide (FeNO) | ||||
Nitric oxide is generated by the airway epithelium due to upregulation of inducible nitric oxide synthase which is induced by IL-13. FeNO can be measured using portable devices. This non-invasive test can typically be performed in children aged 6 years of age and over, generally the same ones who can reproducibly perform spirometry. There are published ATS/ERS standards for the performance and interpretation of this test.Citation37
FeNO has been explored as a potential predictor of response to anti-IgE and anti-IL5. In a pre-specified post hoc analysis of an omalizumab trial, individuals with a high baseline FeNO (≥19.5 parts per billion (ppb) experienced a higher reduction (53%) in exacerbation rate than individuals with a low baseline FeNO (9%).Citation38 A RCT of omalizumab in adolescents included total FeNO as one of eleven pre-specified subgroup analyses and did not find that outcomes differed according to the level of baseline FeNO.Citation39 Only one trial of anti-IL5 therapy had a pre-specified analysis by FeNO (≥ 50 ppb); high levels of FeNO did not identify responders to mepolizumab.Citation40
| c) | Blood eosinophil counts | ||||
Complete cell count (or cell differential) from peripheral blood is a routinely available test in any center. Absolute eosinophil counts are reported as cells/µL, cells/mm3 or by SI units as cells × 109/L where 100/µL = 100/mm3 = 0.1 × 109/L with normal values differing in children of various ages and adults.Citation41 It should be noted that thresholds used to define elevated eosinophils in asthma clinical trials (e.g. 300/µL) are within the normal range of laboratories and would not be flagged as elevated. Blood eosinophil counts have been explored as a potential predictor of response to macrolides, anti-IgE, and anti-IL5.
Low serum eosinophils do not consistently identify responders to macrolides. One trial concluded that subjects with blood eosinophil counts ≤200/µL receiving azithromycin experienced fewer severe exacerbations than subjects with blood eosinophil count >200 /µL,Citation36 while another trial observed a similar number of exacerbations in azithromycin-treated subjects independently of their levels of blood eosinophil counts.Citation42
Two post hoc analyses performed in two adult clinical trials found that high blood eosinophils (≥260 to 300/µL) identified subjects with a greater response to omalizumab compared with subjects with low blood eosinophil counts.Citation38,43
Finally, blood eosinophil counts have been intensively studied as predictors of response to anti-IL5 therapies (mepolizumab, benralizumab, reslizumab). High blood eosinophils counts (≥150, 300 or 400/µL, depending of the cut-offs chosen in different studies) predicted a significant reduction of asthma exacerbations in anti-IL5 treated subjects. A post hoc analysis performed in subjects treated with mepolizumab reported a greater reduction of asthma exacerbations in subjects with blood eosinophils ≥500 / µL compared to those with lower blood eosinophils levels.Citation40,44,45 Similarly, reslizumab has also been shown to lead to substantial improvements in those with eosinophils ≥400–500 cells/ µL;Citation46,47 however, trials that have included only those with blood eosinophils ≥400/ µL have not shown that further stratification of blood eosinophils beyond this cut off predicted greater improvements in FEV1,Citation48 or in the rate of exacerbations.Citation35,48 One trial assessed the effect of a single dose of benralizumab after an emergency department (ED) visit for an exacerbation and found a decreased exacerbation rate in all individuals, regardless of their baseline serum eosinophil count.Citation49 Subsequently, three trials that stratified randomization based on the levels of blood eosinophil countsCitation50–52 found that the most significant improvements in exacerbation rate, FEV1, ACQ, and ability to wean the OCS dose were in individuals with serum eosinophils ≥300 cells/ µL. Finally, a post hoc sub-analysis of the benralizumab trials SIROCCOCitation53 and CALIMACitation52 trials re-analyzed the results stratifying individuals by serum eosinophils and reported similar results for those with eosinophils ≥ 150 cells/ µL. In summary, most anti-IL5 trials demonstrate greater benefits in those with serum eosinophilia, although the best cut off has not been firmly established.
| d) | Serum total IgE | ||||
IgE has been shown to play a central role in the inflammatory cascade of allergic asthma. Cross linking of IgE bound to high affinity IgE receptors on basophils and mast cells causes the release of inflammatory mediators such as histamine and eicosanoids which cause contraction of airway smooth muscle and mucus secretion.Citation54 Recently, cross-linking of IgE has been shown to decrease interferon response to rhinovirus which provides a novel pathophysiologic mechanism for more severe viral-induced asthma exacerbations in atopic children.Citation39 IgE is reported in IU/ml, ng/ml or mcg/L (1 IU/ml = 2.4 ng/ml = 2.4 mcg/L).
The use of IgE levels to predict the effect of omalizumab has been assessed in a pooled post hoc analysis of seven RCTs.Citation55 Based on the INNOVATE trial,Citation56 the only consistent predictor of response was serum IgE level ; those with a baseline value in the lowest quartile (≤75 IU/mL) had consistently a smaller treatment benefit compared to those with IgE in the three higher quartiles (76–147 IU/mL, 148–273 IU/mL, ≥274 IU/mL). Although a subgroup analysis of the pooled data from seven clinical trials (including INNOVATECitation56) observed a decrease in severe exacerbation rates and improvement in physician's overall assessment across all IgE ranges, significant improvements in the Asthma Quality of Life Questionnaire (AQLQ), asthma exacerbations, and ED visits were only seen in individuals with an IgE level greater than 76 IU/ml.
As for anti-IL5, a pre-specified subgroup analysis of the DREAM studyCitation40 did not find that stratification by baseline serum IgE level predicted a decrease in the rate of severe exacerbation with mepolizumab.
Blood periostin
Periostin is a protein that acts as an extracellular and matricellular matrix protein and its production is induced by IL-13 and IL-4 which are both cytokines that figure prominently in Th2 inflammation.Citation57 Immunohistochemical studies have identified periostin deposition on the basement membrane of individuals with asthma, suggesting that it contributes to sub-epithelial fibrosis in asthma.Citation57 Periostin does not appear to be as a useful biomarker in childhood asthma because periostin levels are high normally in growing children such that differences between healthy children and those with asthma is comparatively small or not significant.Citation58 Although it might be a useful biomarker of response to drugs that might be commercialized in the future, there is currently no clinically available commercial assay for periostin.
Biomarkers associated with Th1 inflammation
Although a neutrophilic inflammatory pattern has been well described in the sputum of some individuals with severe asthma,Citation17,29 the Th1 pathway has been less studied in these individuals. Clinically accessible biomarkers other than sputum neutrophils are currently lacking to identify individuals with this type of inflammation.
Only one small RCT of clarithromycin has stratified individuals by neutrophilic airway inflammation. A pre-planned analysis of those with non-eosinophilic asthma (either neutrophilic: neutrophils >61% or pauci-granulocytic: sputum neutrophils <61% and sputum eosinophils <1.01%) observed that the treatment group had an improved QoL regardless of baseline airway neutrophilia, yet only those with non-eosinophilic asthma showed a decrease in airway IL-8, neutrophil elastase and neutrophil numbers.Citation59
Conclusion
With the emergence of a better understanding of the asthma pathophysiology, a number of biomarkers have emerged with the potential to predict response to therapies. This is especially the case with the emergence of anti-IL therapies where peripheral, as well as, sputum eosinophil assessments can be used to identify subjects who are likely to respond. Similarly in atopic asthma, serum IgE levels can be used to predict candidates for omalizumab. As newer therapies emerge other biomarkers predictive of responsiveness to therapies should become available.
Box 2. Biomarkers to predict response to biologic therapies and macrolides
PICO 1: In children and adults with severe asthma, does the measurement of:
| a) | sputum differential cell count | ||||
| b) | fraction of exhaled nitric oxide (FeNO) | ||||
| c) | blood eosinophils | ||||
| d) | blood total Immunoglobulin E (IgE) | ||||
Key messages:
| 1. | Individuals with confirmed severe asthma should undergo specific testing such as total IgE, and peripheral eosinophil count and where available sputum eosinophils and FeNO to characterize phenotype. | ||||
| 2. | Sputum eosinophils, although availability is limited, may be useful in identifying responders to anti-IL5 therapies but has not been shown to be helpful in identifying responders to macrolides. | ||||
| 3. | There is inconclusive evidence for the use of FeNO to predict response or responders to omalizumab or anti-IL5 therapies. | ||||
| 4. | Blood eosinophil counts have a reasonable ability to identify those who will experience fewer exacerbations with anti-IL-5 therapies and omalizumab. | ||||
| 5. | Serum IgE does not predict response to anti-IL5 therapies. Within the approved range for omalizumab, there is no association between higher IgE level and a greater magnitude of benefit. | ||||
Section 3: Long acting muscarinic antagonists (LAMA) in severe asthma: Tiotropium bromide inhalation therapy
PICO 2: What is the efficacy and safety of adding inhaled tiotropium bromide in children and adults with severe asthma uncontrolled despite receiving maintenance therapy with ICS ± a second controller therapy?
Introduction
Tiotropium bromide belongs to a class of medications called long-acting muscarinic antagonists (LAMA). LAMA achieve bronchodilation by a mechanism that is distinct from the direct smooth muscle relaxation that occurs with long-acting beta agonist (LABA) therapy. LAMA prevent acetylcholine-mediated bronchoconstriction by competitively antagonizing M3-receptors in the airways, and thereby permitting bronchodilation. The prolonged half-lives of LAMA mean that these agents only need to be administered once or twice daily. LAMAs have emerged initially as a therapy for chronic obstructive lung disease (COPD), with comparable efficacy to LABA.Citation60 Some children and adults with asthma will remain uncontrolled despite the use of ICS +/− LABA or other add-on therapy. Hence, in recent years many clinical trials have been conducted to determine the role of tiotropium in the management of moderate to severe persistent asthma. Potential side effects specific to LAMAs include dry mouth, mydriasis, urinary retention and metallic taste.
Various formulations of tiotropium bromide have been available for the treatment of COPD for several years. In 2015, tiotropium soft mist inhaler (Respimat®) was approved by Health Canada for the add-on long term once daily maintenance therapy of adult patients (18 years plus) with asthma who remain symptomatic on a combination of high dose ICS/LABA and who experience one or more severe exacerbations in the previous year. In 2017, the United States food and drug administration (FDA) has extended the indication of tiotropium Respimat® to adults and children with asthma aged 6 years and over who have poorly controlled asthma despite other maintenance therapy. Tiotropium Respimat® is currently approved for asthma only in 5 mcg dosing once daily (2.5 mcg per inhalation × 2), therefore, in Canada, individuals eligible for this therapy will need to take it in addition to their usual controller inhaler therapy device (ICS/LABA combination).
The pivotal clinical trials for tiotropium bromide are summarized in Appendix 1.
Efficacy in adults (18 years of age and over)
Two separate meta-analyses each including over 1,000 individuals with predominantly severe asthma have evaluated the addition of tiotropium to LABA/ICS combination therapy (i.e. triple therapy with ICS/LABA/LAMA).Citation61,62 Both meta-analyses show significant improvements in lung function. Although one of these meta-analysesCitation61 showed a reduction in exacerbations with tiotropium bromide compared to placebo (OR 0.76, 95% CI 0.57 to 1.02) as add-on therapy to ICS/LABA, these results need to be interpreted cautiously considering the width of the confidence intervals.
We were unable to find any published studies comparing the use of tiotropium bromide to biologic therapy as add-on therapy to individuals with severe asthma who remain uncontrolled on high dose ICS +/− a second controller therapy.
Efficacy in adolescent and pediatric individuals
A published meta-analysis of three clinical trials including 1,001 adolescents with asthma aged 12–17 yearsCitation63 presents results similar to those observed in adult studies;Citation62 the tiotropium treatment group displayed modest improvements in lung function (though FEV1 increased by 100 mL compared to placebo; p < .0001) and exacerbation frequency (17.6% vs 23.8% in the placebo group; numbers needed to treat (NNT) = 16; p = 0.04). The trials included only individuals with moderate to severe asthma who remained symptomatic despite at least medium dose ICS +/− LABA.
The results of two clinical trials in children aged 6–11 years have been published and documented the safety and efficacy of tiotropium in improving lung function, but with no clear reduction in exacerbations.Citation64,65 An additional study in new onset asthmaCitation66 did not meet our inclusion criteria. The limited number of published pediatric data preclude making any firm recommendations for children aged 6–11 years. Of note, the recent Food and Drugs Administration (FDA) indication for children aged 6 years and over is based on RCT data from additional trials that are currently unpublished in full text, and hence not included in this review.
Safety
A systematic review and meta-analysis designed to specifically address the safety of tiotropium in adults did not show a difference in adverse events, serious adverse events or even the side effects specific to adverse events (e.g. dry mouth) compared to placebo (further details in Appendix 1).Citation67 Multiple other systematic reviews with meta-analyses in adults and adolescents which focused on specific treatment comparisons in mild, moderate and severe asthma patients also demonstrate the same safety outcomes.Citation61–63,68–71 Published safety data in children is limited to date, but appears similar to adult and adolescent data.Citation64–66 No deaths related to treatment were reported.
Conclusions
Tiotropium bromide soft mist inhalation therapy is safe and effective in improving lung function and reducing severe asthma exacerbations in adults and adolescents with uncontrolled asthma despite ICS and LABA. In considering its position in asthma guideline add-on therapy, its efficacy must be balanced with pragmatic issues such as:
| 1) | availability only as a single drug inhaler compared to LABA therapy which is combined with ICS in the same inhaler; and | ||||
| 2) | relatively modest cost and convenience (ease of administration) compared to biologic therapy. | ||||
Box 3. Long acting muscarinic antagonists (LAMA) in severe asthma: Tiotropium bromide inhalation therapy
PICO 2: What is the efficacy and safety of adding inhaled tiotropium bromide in children and adults with severe asthma uncontrolled despite receiving maintenance therapy with ICS +/- a second controller therapy?
Key messages:
| 1. | Tiotropium bromide 5 mcg (2 inhalations of 2.5 mcg) once daily by soft mist inhaler may be considered as an add-on therapy for individuals 12 years of age and over with severe asthma, who remain uncontrolled despite combination ICS/LABA therapy. Of note, tiotropium bromide is not currently approved by Health Canada for individuals aged 6-17 years. | ||||
| 2. | Tiotropium bromide 5 mcg (2 inhalations of 2.5 mcg) once daily appears to be safe and well tolerated. | ||||
Section 4: Macrolides
PICO 3: What is the efficacy and safety of adding macrolides in children and adults with severe asthma uncontrolled or requiring high-dose ICS +/− second controller therapy?
Introduction
Macrolides have both anti-microbial as well as anti-inflammatory effects. They have been used chronically to treat neutrophilic airway diseases such as cystic fibrosis and diffuse respiratory panbronchiolitis. In individuals with asthma, macrolides have been shown to decrease neutrophil numbers and IL-8 and have been studied in individuals with both eosinophilic and neutrophilic airway inflammation.Citation42,59 Macrolides, such as azithromycin and clarithromycin, are currently only approved in Canada for their antimicrobial properties.
An updated Cochrane review on the use of macrolides in children and adults with chronic asthma was published in 2015.Citation72 It included 18 studies, although the overall quality of evidence was deemed very low, related to concerns about publication bias, small sample sizes and variability of results. There was no subgroup analysis by asthma severity and only five studies included individuals who would be classified as having severe asthma.Citation36,42,73–75 Since the publication of that review, one additional RCT was published.Citation36 This trial included 420 individuals, which is a larger sample size than in the trials included in the above-mentioned Cochrane review.
The pivotal clinical trials for macrolides are summarized in Appendix 1.
Efficacy in adults
Effect on exacerbations
The 2015 Cochrane review found that macrolides were not associated with either a statistically significant or clinically relevant reduction in exacerbations requiring hospital admission although the imprecision of this estimate could have been related to the rarity of the event with only 4 hospitalizations (n = 143) (odds ratio (OR) 0.98, 95% confidence interval (CI) 0.13–7.23).Citation72 There were also non-conclusive results regarding exacerbations requiring ED visits or systemic corticosteroids (n = 290) (OR 0.82, 95% CI 0.43–1.57); although, the quality of evidence was recognized as low.Citation72 In contrast, the trial by Gibson and colleaguesCitation36 found a significant decrease in the number of severe exacerbations in the azithromycin vs placebo group (incidence rate ratio (IRR) 0.59, 95% CI 0.42–0.83).
Symptoms, asthma control, QoL
The 2015 Cochrane review found a modest benefit in symptom scores (standardized mean difference (SMD) −0.35, 95% CI −0.67 to −0.02) and no difference in asthma control (SMD −0.05, 95% CI −0.26 to 0.15, n = 353) or QoL (mean difference (MD) 0.06, 95% CI −0.12 to 0.24, n = 389).Citation72 Again, the quality of evidence was low.
A recently published trial found improved asthma control as measured by the ACQ6 (difference between treatment compared to placebo −0.2, 95% CI −0.34 to −0.05) and improved QoL as measured by the AQLQ (difference between treatment compared to placebo 0.36, 0.21 to 0.52, p = 0.001).Citation36
Lung function
The Cochrane review found a small improvement in FEV1 with the use of macrolides (MD 0.08, 95% CI 0.02 to 0.14, n = 600) but with a low quality of evidence.Citation72 The trial by Gibson and colleaguesCitation36 also found a small difference in FEV1 between the treatment group and placebo (adjusted mean difference between treatment compared to placebo, −0.06, 95% CI −0.12 to −0.001).Citation36
Reduction of oral corticosteroids
A small pediatric studyCitation73 reported that troleandomycin allowed for a greater reduction in oral methylprednisolone compared to placebo. In contrast, a larger adult study found that the use of troleandomycin did not allow for a decrease in oral corticosteroid dose compared to placebo, although both groups showed a decrease from baseline corticosteroid dose.Citation75 The relevance of this adult trial to current clinical practice is limited as individuals in that trial were taken off all inhaled corticosteroids.
Efficacy adolescent and pediatric individuals
Three clinical trials examined the use of macrolides recruited in pediatric and adolescent individuals,Citation73,74,76 of which two included individuals with severe asthma.Citation73,74 One trial (of individuals 6–17 years of age) included individuals requiring maintenance oral corticosteroids and found that troleandomycin allowed for a greater decrease in the oral corticosteroid dose.Citation73 The MARS trialCitation74 recruited individuals 6–17 years of age with uncontrolled asthma despite moderate to high doses of inhaled corticosteroids and LABA; they randomized them to azithromycin daily (250 mg if 25–40 kg, 500 mg if >40 kg), montelukast or placebo. This trial was terminated prior to completion because of slow randomization; however, a preliminary analysis found that there was no difference in the time to loss of asthma control in the three groups.
Safety
The Cochrane review did not find a statistically significant difference in adverse effects between those receiving macrolides or placebo (OR 0.8, 95% CI 0.24 to 2.68, n = 434).Citation72 One study reported that there was an increase in streptococci resistant to erythromycin in the azithromycin group compared to placebo (17.2% to 73.8% in azithromycin group vs. 7.9% to 17.3% in the placebo group (p < 0.001).Citation42 Another trial did not find a difference in the number of azithromycin resistant organisms detected at the end of treatment (12 vs 7 in azithromycin vs placebo, p = 0.27).Citation36 That trial noted an increased incidence of diarrhea in the azithromycin compared to placebo group (34% vs 19% in azithromycin vs placebo, p = 0.001).Citation36
Although not assessed in the systematic review, macrolides are known to prolong QT intervals which can lead to ventricular arrhythmias. Most clinical trials of macrolides in asthma exclude individuals with a prolonged QT at baseline. Macrolides should be thus used with caution or avoided in individuals with QT prolongation, hypokalemia, hypomagnesemia, bradycardia or use of other QT prolonging drugs. Post-marketing surveillance has highlighted an increased risk of cardiovascular death with the use of macrolides.Citation77 One year treatment with macrolides was associated with impaired hearing tests in 25% of COPD subjects compared to 20% in COPD subjects treated with placebo,Citation78 although this was not seen in a six month trial of macrolides in individuals with asthma (AZIZAST) and individuals with reported impaired hearing were excluded from a recent large clinical trial (AMAZES). Another concern with the use of chronic macrolides is causing macrolide resistance in individuals with nontuberculous mycobacteria infections, which is most often Mycobacterium avium intracellulare.Citation79 Thus prior to starting macrolide therapy, it may be prudent to send sputa samples for acid-fast bacillus smears and mycobacterial culture.
Predicting response to therapy
This topic has been discussed in Section 2: “Biomarkers predicting response to biologics and macrolides”.
Conclusions
Chronic use of macrolides in adults with severe asthma may decrease exacerbations and improve symptom control. Although generally well tolerated, the potential for increasing antibiotic resistant organisms, risk of QTc prolongation, and hearing loss should be considered. The data for pediatric and adolescent patients are inconclusive.
Box 4. Macrolides
PICO 3: What is the efficacy and safety of adding macrolides in children and adults with severe asthma uncontrolled or requiring high-dose ICS +/- second controller therapy?
Key messages:
| 1. | In individuals 18 years of age and over with severe asthma, there is limited evidence that the chronic use of macrolides may decrease the frequency of exacerbations. | ||||
| 2. | Inflammatory phenotype does not consistently predict response to macrolide treatment. | ||||
| 3. | Macrolides are generally well tolerated. However, they should be avoided in individuals with a prolonged QTc interval. Increased incidence of bacterial resistance and impaired hearing tests have been observed in long-term treatment with macrolides. | ||||
Section 5: Biologic therapy: Anti-IgE Omalizumab
PICO 4: What is the efficacy and safety of adding omalizumab in children and adults with severe asthma uncontrolled or requiring high-dose ICS +/− second controller therapy?
Introduction
Immunoglobulin E (IgE) plays an important role in the pathogenesis of allergic asthma. Omalizumab is a recombinant humanized monoclonal antibody that binds with high affinity to IgE.Citation80
The molecule obtained regulatory approval with a monthly or bi-monthly subcutaneous administration. Omalizumab is indicated for adult and pediatric patients (6 years of age and older) with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids.Citation81
Omalizumab is given by subcutaneous injection every 2–4 weeks depending on baseline IgE levels and body weight.
The pivotal clinical trials for omalizumab are summarized in Appendix 1.
Efficacy in adults
Effect on exacerbations
A 2014 Cochrane review pooling 25 studies, examined the efficacy of anti-IgE treatment as an adjunct to ICS therapy in children aged 6 and over and adults with moderate to severe allergic asthma.Citation82 Among individuals with moderate to severe asthma, there was a significant reduction in asthma exacerbations in the omalizumab group compared to controls with an OR of 0.55 (95% CI 0.42–0.60). There was also a significant reduction in severe exacerbations leading to hospitalization but the sample size was much smaller (OR 0.16, 95% CI 0.06–0.42).
Among the studies focusing on severe asthma (Global Initiative for Asthma (GINA) or National Heart, Lung and Blood Institute (NHLBI) stage 4 or higher), the results were also in favor of omalizumab, although the number of individuals was smaller than the studies including individuals with moderate asthma. Two studies did not show a significant reduction in exacerbations (or need for oral corticosteroids),Citation83,84 whereas most studies reported a significant reduction in exacerbations amongst individuals with severe poorly controlled atopic asthma.Citation85–88 In the INNOVATE study,Citation56 exacerbations were reduced by 26% (p = 0.042) and severe exacerbations were reduced by 50% in the omalizumab group compared to placebo, with a number needed to treat of 2.2.
Symptoms and asthma control
Omalizumab led to a significant improvement in asthma symptom scores in individuals with severe asthma in two studies, including in both the corticosteroid stable and corticosteroid reduction phases.Citation83,87 This benefit was also seen in many of the studies that included individuals with moderate to severe asthma.Citation56,86,89–92
Asthma related QoL improved significantly with omalizumab compared to placebo in the majority of studies enrolling individuals with moderate to severe asthma.Citation56,83,87,91–93
Lung function
Lung function results have been quite variable with at most small improvements in peak expiratory flow (PEF) and FEV1.Citation82 In severe asthma, several studies showed no significant improvement in FEV1 (2), whereas others showed small but significant improvements in lung function.Citation56,86 In the moderate to severe asthma group, several studies showed an improvement in FEV1 or PEF compared to either baseline or placebo in favor of omalizumabCitation90,94 whereas others did not.Citation95,96
Medication use
In the 2014 Cochrane review, individuals treated with omalizumab had a small but significant reduction in use of rescue short acting beta-agonists, both in the moderate to severe [MD −0.58 (95% CI −0.84 to −0.31)] and in the severe asthma groups on ICS, but not among those on oral corticosteroids (MD −0.30, 95% CI −0.49 to −0.10).Citation82
More individuals treated with omalizumab were able to withdraw their ICS completely, with an OR of 2.50 (95% CI 2.0–3.13) compared to placebo and there was a significant reduction in daily ICS dose in favor of omalizumab (weight mean difference −118 mcg beclomethasone dipropionate equivalents per day, 95% CI −154 to −84). However, there was no significant difference in the number of individuals that were able to come off of oral corticosteroids.Citation82,83,85,86 A pooled analysis of several RCTs showed that there was a significant median reduction in ICS dose with omalizumab compared to placebo; in addition, individuals treated with omalizumab required fewer oral corticosteroid bursts.Citation97
Efficacy in adolescent and pediatric individuals
Omalizumab is currently the only biologic approved for pediatric use in Canada. The summary below includes data from four trials of children with mild to severe asthma.Citation39,85,95,96
A total of 645 children with severe asthma (National Heart, Lung, and Blood Institute (NHLBI) step 5 or equivalent) were included in the three additional RCTsCitation39,89,95 involving children 6 to 20 years of age with predominantly moderate to severe asthma. A further publication was a subgroup analysis of individuals with severe asthma from a previous clinical trial.Citation98 Subcutaneous omalizumab decreased exacerbation rates by 50% (0.73 with omalizumab, 1.44 with placebo, (risk ratio (RR) 0.504; 95% CI 0.35–0.725, p < 0.001),Citation98 decreased the percentage of individuals with at least one exacerbation (32.6% compared to 15.1%, OR 0.37, 95% CI 0.17–0.81)Citation39 (48.8% compared to 30.3%, −18.5 difference (−28.2 to –8.8) p < 0.001),Citation95 and decreased hospitalizations (6.3 ± 1.8 versus 1.5 ± 0.9, difference −4.7 (95% CI −8.6 to −0.9, p 0.02).Citation95 There were less consistent effects on asthma controlCitation89,99 and on the ability to decrease ICS doses.Citation89,95 No study showed an improvement in lung functionCitation89,95 or QoL.Citation89
Omalizumab was associated with a significant reduction in the rate of seasonal exacerbations, occurring in the fall, in children aged 6–17 years when compared to controls (OR 0.48, 95% CI 0.25–0.92), but there was no significant difference when compared to doubling the dose of ICS.Citation39
Efficacy in special populations
Non-atopic severe asthma
In a small randomized placebo controlled study of 41 adults with severe, refractory non-atopic asthma, omalizumab led to a significant improvement in lung function (FEV1 + 250 mL, p = 0.032) and a non-significant reduction in asthma exacerbations.Citation100 A second observational registry study from Spain followed 295 individuals, of who 29 had non-allergic severe asthma (Global Initiative for Asthma (GINA) step 5 treatment) and were on omalizumab. There was an improvement in symptoms, as measured by an increase in the ACT score, and a non-significant decrease in severe exacerbations.Citation101 Thus the data is limited at this time to recommend omalizumab in this group.
Individuals with IgE above the approved range in Canada
There have been no RCTs with clinical endpoints in individuals with IgE levels above the standard dosing range. A case series of 26 individuals with IgE levels above the approved range (IgE 786–10,979 IU/ml) treated with omalizumab (dose 400–1200 mg /month) showed significant decreases in systemic corticosteroid use, ED visits and ACT score, that were similar to the decreases observed in age, sex and severity matched individuals with IgE in the approved dosing range (30–700 Iu/mL).Citation102 An open label, parallel group trial assessing the pharmacokinetics and safety of omalizumab in adults with mild to moderate asthma and IgE levels ranging from 300 IU-2,000 IU/ml and body weight up to 150 kg was conducted using omalizumab doses ranging from 450–600 mg q2 weeks.Citation103 This study showed a decrease in free IgE levels similar to what was observed in trials with clinical improvement and led to the approval of an increased dose range in Europe.
Safety
In general, omalizumab is well tolerated. The 2014 Cochrane review showed that adverse events were less frequent in the omalizumab group (OR 0.72, 95% CI 0.57–0.91) compared to placebo.Citation104 Most adverse events were minor injection site reactions. There was no difference in asthma related mortality between omalizumab and placebo in asthma. An incidence of at least 0.2% of anaphylaxis is reported in the product monograph based upon spontaneous reports. However, the Omalizumab Joint Task Force reviewed the post-marketing reports compiled by the agent's manufacturer and estimated the incidence of anaphylaxis at approximately 0.09%.Citation105 Seventy-eight percent of reactions occurred within the first two hours after the injection and within the first three doses. For this reason, omalizumab is given in a clinic setting and it is suggested that individuals are observed for two hours following the three first doses, then for 30 minutes for the following injections.Citation106
Concerns regarding the development of malignancy with omalizumab have been initially raised but one long-term cohort study of moderate to severe asthma patients aged > 12 years did not find any significant difference in the incidence of malignancy between the omalizumab patients compared to those on standard therapy.Citation107 Pooled analysis of RCTs did not show any difference in malignancy outcomes between individuals on omalizumab and placebo.Citation108,109
Predicting response to therapy
As biologic drugs are very costly, targeting individuals who are more likely to be responders is critical. Clinical patient characteristics and biomarkers have been assessed for their ability to predict response to omalizumab in post hoc subgroup analysis of clinical trials and one observational trial. The biomarkers investigated as potential predictors of response to omalizumab treatment are presented in section 2.
In a pooled analysis of 1,070 allergic asthma patients from two RCTS with symptoms despite moderate to high dose ICS, individuals with at least one of the following characteristics: budesonide dose > 800 mcg/day, frequent ED visits for asthma treatment and poor lung function (FEV1<65%) had a more than two-fold higher chance of being a responder to omalizumab compared to placebo (OR: 2.25, 95% CI 1.68 to 3.01) with the odds increasing with each additional factor present.Citation38 Response to omalizumab was defined as no exacerbations and improvement in either FEV1, beta2-agonist use, symptoms or QoL.
A post hoc analysis of a multicenter randomized double-blind placebo-controlled trial in individuals with severe asthma found that those who had more uncontrolled asthma at baseline, defined by an ACT ≤15, were more likely to have significantly larger improvements in ACT score at week 24 with omalizumab compared to placebo (change from baseline in ACT: 6.66 and 5.27, respectively; treatment difference: 1.39; 95% CI: 0.11, 2.66; p = 0.033.Citation84 In a pediatric placebo-controlled randomized double-blind trial, children were treated with omalizumab to prevent asthma exacerbations in the fall. Eleven pre-specified subgroups were identified and only children with an exacerbation during the run-in period were more likely to have a decrease in subsequent exacerbations with omalizumab compared to placebo during the 90-day trial. Other biomarkers that were explored: (eosinophil count ≥320 cells/µL, IgE ≥255 IU/L, sensitization to cockroach, FeNO ≥23.5ppb) and clinical characteristics (FEV1 ≥91%, age, race, gender, body mass index (BMI) ≥ 85 percentile) were not predictive.Citation39
Conclusions
Omalizumab is effective in reducing exacerbations, reducing ICS dose and improving patient symptoms in individuals with severe allergic and inadequately controlled asthma. Omalizumab is well tolerated with a small risk of anaphylaxis.
Box 5. Key messages: Biologic Therapy: Anti-lgE Omalizumab
PICO 4: What is the efficacy and safety of adding omalizumab in children and adults with severe asthma uncontrolled or requiring high-dose ICS +/- second controller therapy?
Key messages:
| 1. | Omalizumab may be considered in individuals 6 years of age and over with severe asthma who are inadequately controlled despite high-dose ICS* and at least one other controller and who are sensitized to at least one perennial allergen and have serum IgE level between 30-1300 IU/ml (6-11 years of age) or 30-700IU/mL (12 years of age and over). *Due to the known risk of side effects associated with high-dose ICS in children, omalizumab should be considered in children and adolescents who repeatedly exacerbate or have poor control when therapy is stepped down from high-dose to moderate-dose ICS and at least one other controller. | ||||
| 2. | There is insufficient evidence to make any recommendation regarding the use of omalizumab in individuals with severe asthma who are non-atopic or have serum IgE levels above the current dosing range. | ||||
| 3. | Predictors of response to omalizumab include a history of recurrent exacerbations or blood eosinophil counts ≥260-300 cell/µL, although the exact eosinophil cut-off remains to be clearly established. | ||||
| 4. | Omalizumab appears to be safe and well tolerated with anaphylaxis occurring rarely. Individuals should be monitored closely, usually for two hours after the first three injections (as this is the time frame when reactions most commonly occur) and for 30 minutes for subsequent injections. | ||||
Section 6: Biologic therapies in severe asthma: Anti-IL5
PICO 5: What is the efficacy and safety of anti-IL5 therapies in adults with severe eosinophilic asthma uncontrolled on high doses of ICS plus long-acting beta2-agonists and/or other add-on therapy?
Introduction
Th-2 high asthma is an endotype usually associated with a prominent airway eosinophilic inflammation. Although corticosteroids are effective in reducing eosinophilic inflammation, they fail to effectively control this inflammation in a subset of individuals with severe asthma. Interleukin (IL)-5 plays a central role for eosinophil recruitment, activation, and survival. IL-5 and its receptor (IL-5R) have been targeted for developing antagonists against this cytokine or its receptor for improving asthma control. Three drugs targeting IL-5 or its receptor have been developed to treat severe eosinophilic asthma. Two have already received a Health Canada approval (mepolizumab (Nucala™) and reslizumab (Cinqair™)) while the third one (benralizumab) is still under development but will be submitted shortly for regulatory review.
Mepolizumab
Mepolizumab is a humanized monoloclonal anti-IL5 (IgG1) antibody administered monthly. Different doses of mepolizumab have been tested for both intravenous (75 to 750 mg) and subcutaneous (100 mg) administrations. The molecule obtained regulatory approval with a monthly subcutaneous administration of 100 mg with the following indication:Citation110
Add-on maintenance treatment of adult patients with severe eosinophilic asthma who:
| • | Are inadequately controlled with high-dose inhaled corticosteroids (ICS) and an additional asthma controller(s) (e.g. LABA) AND | ||||
| • | Have a blood eosinophil count of ≥ 150 cells/μL (0.15 GI/L) at initiation of treatment OR ≥ 300 cells/μL (0.3 GI/L) in the past 12 months | ||||
It is worth emphasizing that all individuals included in the pivotal clinical trials had at least two asthma exacerbations in the year preceding the administration of mepolizumab.
Mepolizumab has been shown to be effective in reducing asthma exacerbations in individuals with severe eosinophilic asthma and two or more asthma exacerbations.Citation111,40 However, when administered to a group of individuals with moderate asthma treated with ICS who were not selected according to the presence of eosinophilic airway inflammation, it failed to show significant improvement in the clinical outcomes (change in PEF, asthma symptoms, FEV1, QoL and asthma exacerbations) even if it significantly reduced blood and sputum eosinophils.Citation112
Mepolizumab has shown good corticosteroid-sparing properties allowing for a reduction of the dose of oral corticosteroids (OCS) by 50% in corticosteroid-dependent asthmatics with persistent peripheral eosinophilia.Citation113
The administration of mepolizumab was also associated with an improvement in QoL related to asthma as measured by the AQLQCitation111 or the St. George's Respiratory Questionnaire (SGRQ).Citation44 It was also associated with an improvement in FEV1 of approximately 100 mL compared with placebo.Citation44
The optimum duration of treatment with mepolizumab remains uncertain. Cessation of mepolizumab was associated with a rise in blood eosinophils as well as with an increase in asthma exacerbations in comparison with the period during which the subjects were treated with mepolizumab.Citation114 Ongoing long-term open label safety studies may provide some indication for the best duration of therapy.
The most frequent adverse events reported in the different clinical trials were rhino-sinusitis (12–29%), and headaches (20–24%). They were not different from those reported in the placebo groups (15–24% and 17% respectively).Citation40,44 No death or anaphylaxis related to treatment was reported.
The pivotal clinical trials of mepolizumab are summarized in Appendix 1.
Two meta-analyses of RCTs comparing mepolizumab to placebo were published. The first meta-analysis included three trials in mild asthmatics and did not include the latest pivotal studies. However, this meta-analysis showed that mepolizumab reduces the risk of exacerbation and improved QoL in asthmatic subjects with eosinophilic inflammation.Citation115 A Cochrane review was published in 2015.Citation116 The literature search was performed in 2013 and updated in 2014. Eight studies on 1,707 individuals were selected. Six studies included adults, three of them included mild asthmatics. Two studies included children (over 12 years of age), but did not report separate findings for the adolescents. Two studies performed in subjects with eosinophilic asthma showed a reduction in exacerbation rates (RR 0.52, 95% CI 0.43 to 0.64; n = 690). The analysis of serious adverse events indicated a significant difference favouring mepolizumab (RR 0.49, 95% CI 0.30 to 0.80; n = 1441). The authors concluded that treatment with mepolizumab resulted in an improvement in QoL and a reduction of asthma exacerbations without significant side effects in subjects with severe eosinophilic asthma.
Reslizumab
Reslizumab is a humanized anti-IL5 (IgG4/k) antibody administered monthly. Intravenous doses of 0.3 and 3 mg/kg have been studied. The molecule was commercialized with a monthly IV administration of 3 mg/kg with the following indication:
Add-on maintenance treatment of adult patients with severe eosinophilic asthma who:
| • | Are inadequately controlled with medium-to-high-dose inhaled corticosteroids and an additional asthma controller(s) (e.g. LABA) AND | ||||
| • | Have a blood eosinophil count of ≥400 cells/μL at initiation of the treatment | ||||
All individuals included in the pivotal clinical trials had at least one asthma exacerbation in the year preceding the administration of reslizumab.
Reslizumab has shown good efficacy in reducing asthma exacerbations in subjects treated with moderate to high doses of ICS with a blood eosinophil count higher than 400 cells per µLCitation35 and at least one exacerbation in the previous year. The administration of reslizumab was also associated with significant increases in FEV1 of 115 mL [95% CI, 16–215; p = 0.0237] and 160 mL [95% CI, 60–259; P = 0.0018]) with respective doses of 0.3 and 3.0 mg/kg of reslizumab compared to placebo.Citation48 However, the administration of reslizumab did not result in a significantly greater increase in FEV1 compared with placebo after 16 weeks of treatment when subjects were not selected on the basis of blood eosinophils (p = 0.17).Citation47
Reslizumab (3 mg/kg) also improved asthma-related QoL as measured by AQLQ compared to placebo (0.359 (0.047 to 0.670) p = 0.02) although it did not reach the minimal clinically important difference of 0.5.Citation48
Upper respiratory tract infection, nasopharyngitis, sinusitis and headache were the most frequently reported adverse events and, were similar between reslizumab (3–13%, 11–19%, 4 to 9% and 8–14%), and placebo (7–13%, 14–19%, 4–9% and, 7–12 % respectively).Citation48 No death related to treatment was reported. Two anaphylactic reactions were reported in the reslizumab group.Citation35
The pivotal clinical trials of reslizumab are summarized in Appendix 1.
One meta-analysis comparing reslizumab to placebo reviewed the literature until 2016 included four publications and five RCTs with a total of 1,366 individuals. The meta-analysis showed that reslizumab decreased the risk of an exacerbation (OR = 0.46, 95% CI = 0.35 to 0.59, p < 0.001, showed a greater increase in FEV1 (SMD = 0.16, 95%CI = 0.10 to 0.23, p < 0.001, and a greater reduction in ACQ score (SMD = −0.26, 95%CI= −0.36 to −0.16, p < 0.001) compared to placebo.Citation117
Benralizumab
Benralizumab is a humanized monoclonal antibody against interleukin 5 receptor α (IgG1κ), which is expressed on eosinophils and basophils. It inhibits IL-5 mediated eosinophil activation and proliferation, and also causes antibody-dependent cell-mediated cytotoxicity of basophils and eosinophils. Single IV doses of 0.3 mg/kg or 1.0 mg/kg, and subcutaneous doses (2 mg, 20 mg, 30 mg, 100 mg and 200 mg) every 4 to 8 weeks have been studied. It is currently investigational (not yet approved in Canada for human use).
Benralizumab has been shown to reduce the rate of asthma exacerbations, time to first exacerbation and (in some of the studies) rate of exacerbations requiring an ED visit or hospitalization in subjects with severe asthma and with a history of exacerbations in the previous year despite treatment with medium- to high-dose ICS plus LABA and a blood eosinophil count greater than or equal to 300 cells/ µL.Citation52,53,118 Benralizumab has also been associated with a significant reduction in oral prednisone dose.Citation118
In phase 3 clinical trials, benralizumab also improved pre-bronchodilator FEV1 by approximately 100–160 mL compared to placebo, asthma-specific QoL symptom score as measured by the AQLQ by 0.08 – 0.23, and asthma control as measured by the ACQ-6.Citation52,53
After three induction doses at four weekly intervals the administration of benralizumab every 8 weeks seems to be more effective than every 4 weeks, which may have theoretical advantages for patient care compared to medications requiring more frequent administration.
The most common side effects were worsening asthma and nasopharyngitis, occurring in 11–14% and 12–21% of the benralizumab groups respectively, compared to 15–19% and 12–21% in the placebo groups respectively.Citation52,53,118 Serious adverse events deemed to be related to the study treatment, included: worsening asthma, allergic granulomatous angiitis, panic attack, paresthesia, pneumonia, heart failure, urticaria, and herpes zoster. Two serious adverse events deemed to be related to the study treatment in the placebo group: non-cardiac chest pain, injection-site erythema. There were no deaths deemed to be related to benralizumab in the two largest trials.Citation52,53 In the most recent trial, there were 2 deaths in individuals receiving benralizumab every 8 weeks, and the deaths were due to acute cardiac failure and pneumonia.Citation118 Overall there was no appreciable difference in side effects between active and placebo treatment groups.
The pivotal clinical trials of benralizumab are summarized in Appendix 1.
Comparison of anti-IL5 therapies
No head-to-head comparison between anti-IL5 medications has been performed to date. A recent meta-analysis reviewed the RCTs involving mepolizumab, reslizumab and benralizumab in severe asthma between 1990 and 2015. No superiority of a specific molecule over the others emerged from this analysis.Citation119 Another systematic review and meta-analysis of 20 RCTs of anti-IL5 therapies involving 7,100 individuals revealed significant improvements in FEV1 of 0.09 L (95% CI 0.06 – 0.12), FEV1% of 3.75 (95% CI 1.66 – 5.83), AQLQ score of 0.22 (95% CI 0.15–0.30), and reductions in blood and sputum eosinophils, and reductions in asthma exacerbations (RR 0.66, 95% CI 0.59–0.73) in the pooled analyses.Citation120
Adolescent and pediatric individuals
Anti-IL5 treatments have not been studied in children under 12 years of age. Although individuals 12–17 years of age were included in RCTs of mepolizumab (and not clearly specified in those of reslizumab and benralizumab), there is an insufficient number of adolescents to ascertain the safety and efficacy of these medications in individuals under 18 years of age in the published literature.
Predicting response to therapy
This topic has been discussed in Section 2: “Biomarkers predicting response to biologics and macrolides”.
Conclusions
Anti-IL5 therapies are effective in reducing asthma exacerbations in poorly controlled severe eosinophilic asthma. Since the efficacy of these molecules is dependent upon the presence of eosinophilic inflammation, ensuring that the individual has peripheral blood eosinophil levels greater than the regulatory approved levels (which are within the normative range) and with an appropriate exacerbation history is key. Prescribing these expensive medications to non-eosinophilic asthma patients will likely result in a failure of treatment.
Box 6. Biologic therapies in severe asthma: Anti-IL5
PICO 5: What is the efficacy and safety of anti-IL5 therapies in adults with severe eosinophilic asthma uncontrolled on high doses of ICS plus long-acting beta2-agonists and/or other add-on therapy?
Key messages:
| 1. | Anti-IL5 therapies may be considered for use in adults aged 18 years of age and over with severe eosinophilic asthma who experience recurrent asthma exacerbations in spite of optimal asthma treatment including high doses of ICS and at least one other controller. | ||||
| 2. | Anti-IL-5 therapies may be considered in adults 18 years of age and over with severe eosinophilic corticosteroid-dependent asthma in an attempt to decrease or withdraw oral corticosteroids. Of note, corticosteroid sparing studies have only been undertaken with mepolizumab and benralizumab. | ||||
| 3. | Blood eosinophil counts show a reasonable ability to identify responders to anti-IL5 therapy, with the greatest reduction in asthma exacerbations associated with the highest levels of blood eosinophil counts. Cut-offs of >150 cells /µL at initiation or ≥ 300 cells/μL in the past 12 months for mepolizumab ≥400 cell/µL for reslizumab and ≥/ 300 μL cells for benralizumab have been used. | ||||
| 4. | Anti-IL5 therapies have demonstrated a good safety profile in twelve month studies. | ||||
| 5. | Although infrequent, anaphylactic reactions have been reported with anti-IL5 therapies, individuals should be monitored closely for an appropriate period of time following each injection of these therapies. The time frame for observation is unclear but for at least one hour would be reasonable. | ||||
Section 7: Bronchial thermoplasty
PICO 6: What is the safety and efficacy of bronchial thermoplasty in adults with severe asthma?
Introduction
Bronchial thermoplasty (BT) is an endoscopic procedure which aims to reduce airway smooth muscle (ASM) using a radiofrequency ablation controller and a catheter (Alair, Boston Scientific).Citation121,122 The procedure targets distal 3 mm airways to proximal 10 mm bronchi. A diagnostic bronchoscope with a minimum 2 mm working channel is preferred due to better visualization of the airways. The catheter's distal tip contains an expandable basket consisting of four electrodes that deliver thermal energy at 65C for 10 seconds to the airway walls and is applied in sequence from distal to proximal airways. Three separate BT procedures are performed approximately 3 weeks apart in both lungs, sparing the right middle lobe due to concerns about the potential for stenosis to this small diameter bronchus. The procedure is performed in a standard bronchoscopy suite generally using conscious sedation. Oral corticosteroids are usually given for 5 days beginning 3 days before the procedure to reduce possible procedure related airway inflammation.
BT aims to reduce ASM to lessen bronchial constriction and airway hyper-responsiveness (AHR). The histopathological effect of BT on ASM has been confirmed in an animal model and in humans. In dogs,Citation123 BT reduced ASM mass and decreased AHR with persistent effects for 3 years. Moreover, several studies using endobronchial biopsies in humans with asthma have demonstrated consistent reductions in ASM following BT.Citation124–126 Other effects on airway remodeling include decreased type 1 collagen deposition and a reduction in reticular basement membrane thickness which persist long- term.Citation124,126 Bronchial epithelial structure, however, is not modified. Recent evidence suggests an effect of BT on airway neurological structures such as a decrease in autonomic nerve fibers in the bronchial submucosa and in ASM bundles and a reduction in neuroendocrine cells in the bronchial sub-epithelium suggesting that BT may downregulate airway neurological excitability.Citation127 Vascular and lymphatic structures appear to be unaffected by BT.Citation127 The effect on exacerbations does not appear to be related to decreased airway mucosal inflammation as mucosal eosinophils and neutrophils remain unchanged.Citation127
The pivotal clinical trials for bronchial thermoplasty are summarized in Appendix 1.
Efficacy
The clinical benefits of BT have been established in individuals with moderate and severe asthma. Three RCTs support the use of BT for individuals with uncontrolled asthma.Citation128–130 Individuals treated with BT showed fewer exacerbations, more symptom free days, improved QoL, reduction in rescue inhaler use, less days lost from work and a sustained reduction in exacerbations and overall health care useCitation131 and safety lasting up to five years.Citation132,133 Apart from the RISA trialCitation130 which was performed in individuals with more severe asthma, no improvement in lung function has been demonstrated.
Safety
Adverse events have been described in all of the three RCTs.Citation128,130 The most common symptoms include wheezing, cough, dyspnea and chest discomfort. Most symptoms are transient, occurring within 1 day of the procedure and usually resolving within 1 week. Severe events such as hospitalization due to asthma worsening, atelectasis and pneumonia are reported in less than 5% of procedures. Rare events such as significant hemoptysis requiring bronchial artery embolizationCitation129 and tooth aspirationCitation128 have also been reported. In a follow-up study 5 years post BT, high-resolution computed tomography data showed no structural abnormalities attributable to the procedure.Citation133
Predicting response to therapy
There remains some controversy as to which individuals can benefit the most from BT. Clinical trials have focused principally on individuals with variable airflow obstruction and an FEV1 greater than 50% predicted. Limited experience in individuals with very severe asthma have shown that some individuals do improve clinically but the procedure carries increased short-term risks such as overnight hospitalization.Citation134
Several studies have attempted to better identify the BT responder phenotype. The predictive value of imaging techniques, biomarkers and airway histology for BT response is currently under investigation. Asthmatics with significant AHR and a pauci-granulocytic endotypeCitation135 and individuals less likely to respond to pharmacological therapies including those with glucocorticoid-resistant asthma would appear to be candidates for BT but these populations also require further study.
Conclusions
International guidelines include BT as a treatment option for selected individuals with severe asthma already on optimized standard therapy.Citation9,136 The ERS and ATS recommendations fall short of endorsement and suggest limiting the use of BT to patients in institutional review board approved clinical studies or systematic registries due to concerns about increased short-term side effects, the potential for longer term side effects, the use of health care resources and the uncertainty regarding the patient population that might best benefit from BT, while encouraging further research to investigate these issues.Citation9
Currently, in Canada access to BT is limited to only a few highly-specialized centers. Since the cost is for the most part not covered by third party payers, BT remains largely an investigative tool. The role of BT vis a vis monoclonal antibody therapies in severe asthma has not been established and there have been no direct comparisons of these therapeutic modalities.
Box 7. Bronchial Thermoplasty
PICO 6: What is the safety and efficacy of Bronchial Thermoplasty in adults with severe asthma?
Key messages:
| 1. | The precise role of bronchial thermoplasty in individuals 18 years of age and over with severe asthma remains uncertain. | ||||
| 2. | Bronchial thermoplasty has a limited role and should be practiced in highly specialized centers because of the complexity of the procedure and the occurrence of severe events, such as hospitalizations due to asthma worsening, atelectasis, and pneumonia which have been reported in as many as 5% of procedures. | ||||
Summary
This article presents an approach to the diagnosis and management of severe asthma in Canada, which is summarized in and . Key messages pertaining to phenotyping individuals with severe asthma and suggested phenotype-specific targeted therapies are presented.
Current gaps and future research needs
Based on the evidence reviewed, the Clinical Assembly identifies the following research needs in severe asthma:
| • | to determine the relative efficacy and safety of anti-IgE compared to anti-IL5 therapies in individuals who are eligible for both classes of therapies | ||||
| • | to determine the relative efficacy and safety of macrolides compared to anti-IgE and anti-Il therapies | ||||
| • | to determine the relative efficacy of the subcutaneous and intravenous anti-IL5 therapies | ||||
| • | to clarify the role of bronchial thermoplasty in severe asthma, including identification of biomarkers predictive of efficacy, the relative efficacy compared to biologics, and long-term safety. | ||||
Knowledge transfer and tools for practice
| • | The present document is available for download at www.cts-sct.ca/guidelines and www.tandfonline.com | ||||||||||||||||||||||
| • | A slide deck for teaching and self-learning as well as a handout for health care professionals and students are available at www.cts-sct.ca/guidelines. | ||||||||||||||||||||||
| • | The CTS Asthma Clinical Assembly welcomes the opportunity to partner with other organizations and stakeholders in the development of educational tools and resources that support the implementation of the key messages described herein, with various targeted groups. | ||||||||||||||||||||||
| • | Successful implementation of the clinical guidance in this position statement is integral to its aims. The following parameters may be used to monitor or audit adherence with some of the key message contained in this position paper:
| ||||||||||||||||||||||
Editorial independence
The CTS Asthma Clinical Assembly is accountable to the CTS Respiratory Guidelines Committee and the CTS Board of Directors. The CTS Asthma Clinical Assembly is functionally and editorially independent from any funding sources of the CTS and does not receive any direct funding from external sources. The CTS receives unrestricted grants which are combined into a central operating account to facilitate the knowledge translation activities of the CTS Clinical Assemblies. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the key messages presented in this document.
Disclosures
Members of the CTS Asthma Clinical Assembly declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted at www.cts-sct.ca/guidelines.
Appendix_1_Severe_Asthma_Evidence_Tables.docx
Download MS Word (100.5 KB)Acknowledgments
The authors would like to thank CTS and the Executive Committee of the Canadian Respiratory Guidelines Committee for guidance throughout the process: Christopher Licskai, Samir Gupta and Louis-Philippe Boulet. We would also like to acknowledge with deep appreciation our Expert Peer Reviewers who made valuable contributions to the manuscript: Dr. Elisabeth H. Bel, Professor of Pulmonary Diseases at the Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands; Dr. Padmaja Subbarao, Assistant Professor, Department of Paediatrics, University of Toronto, Toronto, Ontario, Canada; and Dr. Donald W. Cockcroft, Professor, Division of Respirology, Critical Care and Sleep Medicine, Department of Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
References
- Government of Canada. Fast facts about asthma: Data compiled from the 2011 Survey on Living with Chronic Diseases in Canada. http://www.phac-aspc.gc.ca/cd-mc/crd-mrc/asthma_fs_asthme-eng.php. Accessed July 24, 2017.
- To T, Simatovic J, Zhu J, et al. Asthma deaths in a large provincial health system. A 10-year population-based study. Ann Am Thoracic Soc. 2014;11(8):1210–7.
- Why Asthma still Kills: The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report. London: URCoP. www.rcplondon.ac.uk/sites/default/files/why-asthma-still-kills-full-report.pdf.
- Breathe: The Lung Association. Survey: Asthma not well-controlled for most Canadians. https://www.lung.ca/news/latest-news/survey-asthma-not-well-controlled-most-canadians Accessed April 14, 2017.
- Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respiratory J. 2012;19(2):127–64. doi:10.1155/2012/635624. PMID:22536582.
- Ducharme FM, Dell SD, Radhakrishnan D, et al. Diagnosis and management of asthma in preschoolers: A Canadian thoracic society and Canadian paediatric society position paper. Paediatrics Child Health. 2015;20(7):353–71. doi:10.1093/pch/20.7.353.
- Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulmonary Med. 2009;9:24. doi:10.1186/1471-2466-9-24. PMID:19454036.
- Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunology. 2015;135(2):299–310; quiz 1. doi:10.1016/j.jaci.2014.12.1871. PMID:25662302.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respiratory J. 2014;43(2):343–73. doi:10.1183/09031936.00202013. PMID:24337046.
- Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed Asthma. Jama. 2017;317(3):269–79. doi:10.1001/jama.2016.19627. PMID:28114551.
- Morton RW, Elphick HE, Rigby AS, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax. 2017;72(4):347–54. doi:10.1136/thoraxjnl-2015-208171. PMID:27815524.
- Weinstein AG. Should patients with persistent severe asthma be monitored for medication adherence? Ann Allergy, Asthma Immunology : Official Publ Am College Allergy, Asthma, Immunology. 2005;94(2):251–7. doi:10.1016/S1081-1206(10)61304-X.
- Sanchis J, Gich I, Pedersen S. Systematic review of errors in inhaler use: Has patient technique improved over time?. Chest. 2016;150(2):394–406. doi:10.1016/j.chest.2016.03.041. PMID:27060726.
- Gibeon D, Heaney LG, Brightling CE, et al. Dedicated severe asthma services improve health-care use and quality of life. Chest. 2015;148(4):870–6. doi:10.1378/chest.14-3056. PMID:25789861.
- Schatz M, Rachelefsky G, Krishnan JA. Follow-up after acute asthma episodes: what improves future outcomes?. Proceedings of the American Thoracic Society. 2009;6(4):386–93. doi:10.1513/pats.P09ST6. PMID:19675349.
- McCallister JW, Parsons JP, Mastronarde JG. The relationship between gastroesophageal reflux and asthma: an update. Therapeutic Adv Respiratory Disease. 2011;5(2):143–50. doi:10.1177/1753465810384606. PMID:20926507.
- The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European network for understanding mechanisms of severe Asthma. Eur Respiratory J. 2003;22(3):470–7. PMID:14516137.
- Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13. doi:10.1016/j.jaci.2006.11.639. PMID:17291857.
- Zazzali JL, Broder MS, Omachi TA, Chang E, Sun GH, Raimundo K. Risk of corticosteroid-related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc. 2015;36(4):268–74. doi:10.2500/aap.2015.36.3863. PMID:26108084.
- Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J. Allergy Clin Immunol. 2015;136(6):1488–95. doi:10.1016/j.jaci.2015.07.046. PMID:26414880.
- Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respiratory Med. 2009;103(7):975–94. doi:10.1016/j.rmed.2009.01.003. PMID:19372037.
- Raissy HH, Kelly HW, Harkins M, Szefler SJ. Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med. 2013;187(8):798–803. doi:10.1164/rccm.201210-1853PP. PMID:23370915.
- Kapadia CR, Nebesio TD, Myers SE, et al. Endocrine effects of inhaled Corticosteroids in children. JAMA Pediatrics. 2016;170(2):163–70. doi:10.1001/jamapediatrics.2015.3526. PMID:26720105.
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. doi:10.1164/rccm.200903-0392OC. PMID:19483109.
- Delvaux M, Henket M, Lau L, et al. Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax. 2004;59(2):111–5. doi:10.1136/thorax.2003.011130. PMID:14760148.
- Gibson PG, Wlodarczyk JW, Hensley MJ, et al. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med. 1998;158(1):36–41. doi:10.1164/ajrccm.158.1.9705031. PMID:9655704.
- ten Brinke A, de Lange C, Zwinderman AH, Rabe KF, Sterk PJ, Bel EH. Sputum induction in severe asthma by a standardized protocol: predictors of excessive bronchoconstriction. Am J Respir Crit Care Med. 2001;164(5):749–53. doi:10.1164/ajrccm.164.5.2009035. PMID:11549527.
- Fleming L, Wilson N, Regamey N, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2012;67(3):193–8. doi:10.1136/thx.2010.156836. PMID:21825081.
- Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. doi:10.1111/j.1440-1843.2006.00784.x. PMID:16423202.
- Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet (London, England). 1999;353(9171):2213–4. doi:10.1016/S0140-6736(99)01813-9. PMID:10392993.
- Nair P, Aziz-Ur-Rehman A, Radford K. Therapeutic implications of ‘neutrophilic asthma’. Current Opinion in Pulmonary Medicine. 2015;21(1):33–38.
- van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunology. 2009;124(3):615–7, 7.e1–2. doi:10.1016/j.jaci.2009.06.029.
- Ortega H, Chupp G, Bardin P, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respiratory J. 2014;44(1):239–41. doi:10.1183/09031936.00220413. PMID:24659543.
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. New England J Med. 2014;371(13):1189–97. doi:10.1056/NEJMoa1403291. PMID:25199060.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respiratory Med. 2015;3(5):355–66. doi:10.1016/S2213-2600(15)00042-9. PMID:25736990.
- Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2017;390:659–68. doi:10.1016/S0140-6736(17)31281-3.
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi:10.1164/rccm.9120-11ST. PMID:21885636.
- Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respiratory Critical Care Med. 2013;187(8):804–11. doi:.10.1164/rccm.201208-1414OC. PMID:23471469.
- Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–85. doi:10.1016/j.jaci.2015.09.008. PMID:26518090.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet (London, England). 2012;380(9842):651–9. doi:10.1016/S0140-6736(12)60988-X. PMID:22901886.
- Consult E-BM. Lab test: Eosinophil count. https://www.ebmconsult.com/articles/lab-test-eosinophil-count.
- Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–9. doi:10.1136/thoraxjnl-2012-202698. PMID:23291349.
- Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunology. 2013;132(2):485–6.e11. doi:10.1016/j.jaci.2013.02.032. PMID:23591271.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. New England J Med. 2014;371(13):1198–207. doi:10.1056/NEJMoa1403290. PMID:25199059.
- Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respiratory Med. 2016;4(7):549–56. doi:10.1016/S2213-2600(16)30031-5. PMID:27177493.
- Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125–32. doi:10.1164/rccm.201103-0396OC. PMID:21852542.
- Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of Reslizumab in patients with poorly controlled Asthma: Effects across a broad range of Eosinophil counts. Chest. 2016;150(4):799–810. doi:10.1016/j.chest.2016.03.018. PMID:27018175.
- Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled Asthma with elevated blood Eosinophil levels: A Randomized Phase 3 study. Chest. 2016;150:789–98. doi:10.1016/j.chest.2016.03.032. PMID:27056586.
- Nowak RM, Parker JM, Silverman RA, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, after acute asthma. Am J Emergency Med. 2015;33(1):14–20. doi:10.1016/j.ajem.2014.09.036. PMID:25445859.
- Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respiratory Med. 2014;2(11):879–90. doi:10.1016/S2213-2600(14)70201-2. PMID:25306557.
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet (London, England). 2016;388(10056):2115–27. doi:10.1016/S0140-6736(16)31324-1. PMID:27609408.
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England). 2016;388(10056):2128–41. doi:10.1016/S0140-6736(16)31322-8. PMID:27609406.
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet (London, England). 2016;388(10056):2115–27. doi:10.1016/S0140-6736(16)31324-1. PMID:27609408.
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–45. doi:10.1164/ajrccm.161.5.9903102. PMID:10806180.
- Bousquet J, Rabe K, Humbert M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respiratory Med. 2007;101(7):1483–92. doi:10.1016/j.rmed.2007.01.011. PMID:17339107.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16. doi:10.1111/j.1398-9995.2004.00772.x. PMID:15679715.
- Izuhara K, Ohta S, Ono J. Using Periostin as a biomarker in the treatment of Asthma. Allergy Asthma Immunol Res. 2016.;8(6):491–8. doi:10.4168/aair.2016.8.6.491. PMID:27582399.
- Inoue Y, Izuhara K, Ohta S, Ono J, Shimojo N. No increase in the serum periostin level is detected in elementary school-age children with allergic diseases. Allergol Int. 2015;64(3):289–90. doi:10.1016/j.alit.2015.04.001. PMID:26117266.
- Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respiratory Critical Care Med. 2008;177(2):148–55. doi:10.1164/rccm.200707-1134OC. PMID:17947611.
- (NICE) NIfHCE. Chronic obstructive pulmonary disease in over 16s: Diagnosis and maangement 2010. nice.org.uk/guidance/cg101.
- Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/.ICS for adults with asthma. Cochrane Database Syst Rev. 2016;(1):CD011721. PMID:26798035.
- Rodrigo GJ, Castro-Rodriguez JA. What is the role of tiotropium in asthma?: a systematic review with meta-analysis. Chest. 2015;147(2):388–96. doi:10.1378/chest.14-1698. PMID:25322075.
- Rodrigo GJ, Castro-Rodriguez JA. Tiotropium for the treatment of adolescents with moderate to severe symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2015;115(3):211–6. doi:10.1016/j.anai.2015.06.029. PMID:26231467.
- Szefler SJ, Murphy K, Harper T, 3rd, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017. In press. doi:10.1016/j.jaci.2017.01.014.
- Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, et al. A randomised dose-ranging study of tiotropium Respimat(R.) in children with symptomatic asthma despite inhaled corticosteroids. Respir Res. 2015;16:20. doi:10.1186/s12931-015-0175-9. PMID:25851298.
- Huang J, Chen Y, Long Z, Zhou X, Shu J. Clinical efficacy of tiotropium in children with asthma. Pak J Med Sci. 2016;32(2):462–5. PMID:27182262.
- Dahl R, Engel M, Dusser D, et al. Safety and tolerability of once-daily tiotropium Respimat((R)) as add-on to at least inhaled corticosteroids in adult patients with symptomatic asthma: A pooled safety analysis. Respiratory Med. 2016;118:102–11. doi:10.1016/j.rmed.2016.07.001. PMID:27578478.
- Lee SW, Kim HJ, Yoo KH, et al. Long-acting anticholinergic agents in patients with uncontrolled asthma: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18(12):1421–30. doi:10.5588/ijtld.14.0275. PMID:25517806.
- Tian JW, Chen JW, Chen R, Chen X. Tiotropium versus placebo for inadequately controlled asthma: a meta-analysis. Respir Care. 2014;59(5):654–66. doi:10.4187/respcare.02703. PMID:24170916.
- Anderson DE, Kew KM, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma. Cochrane Database Syst Rev. 2015;(8):CD011397. PMID:26301488Please provide missing [Volume number] for [Reference 69]..
- Kew KM, Evans DJ, Allison DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database Syst Rev. 2015;(6):CD011438. PMID:26031392Please provide missing [Volume number] for [Reference 70]..
- Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015;(9):CD002997. PMID:26371536Please provide missing [Volume number] for [Reference 71]..
- Kamada AK, Hill MR, Iklé DN, Brenner AM, Szefler SJ. Efficacy and safety of low-dose troleandomycin therapy in children with severe, steroid-requiring asthma. J Allergy Clin Immunol. 1993;91(4):873–82. doi:10.1016/0091-6749(93)90345-G. PMID:8473676.
- Strunk RC, Bacharier LB, Phillips BR, et al. Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study. J Allergy Clin Immunol. 2008;122(6):1138–44.e4. doi:10.1016/j.jaci.2008.09.028. PMID:18951618.
- Nelson HS, Hamilos DL, Corsello PR, Levesque NV, Buchmeier AD, Bucher BL. A double-blind study of troleandomycin and methylprednisolone in asthmatic subjects who require daily corticosteroids. Am Rev Respir Dis. 1993;147(2):398–404. doi:10.1164/ajrccm/147.2.398. PMID:8430965.
- Piacentini GL, Peroni DG, Bodini A, et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc. 2007;28(2):194–8. doi:10.2500/aap.2007.28.2958. PMID:17479604.
- Mosholder AD, Mathew J, Alexander JJ, Smith H, Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. New England J Med. 2013;368(18):1665–8. doi:10.1056/NEJMp1302726. PMID:23635046.
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. New England J Med. 2011;365(8):689–98. doi:10.1056/NEJMoa1104623. PMID:21864166.
- Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977–82. doi:10.1164/rccm.201003-0503OC. PMID:20508209.
- Presta LG, Lahr SJ, Shields RL, et al. Humanization of an antibody directed against IgE. J Immunology (Baltimore, Md : 1950). 1993;151(5):2623–32. PMID:8360482.
- Canada NP. Product Monograph: Xolair. 2017. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=94712.
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1): Cd003559. PMID:24414989Please provide missing [Volume number] for [Reference 81]..
- Holgate ST, Chuchalin AG, Hebert J, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy : J Br Soc Allergy Clin Immunol. 2004;34(4):632–8. doi:10.1111/j.1365-2222.2004.1916.x. PMID:15080818.
- Bardelas J, Figliomeni M, Kianifard F, Meng X. A 26-week, randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma. 2012;49(2):144–52. doi:10.3109/02770903.2011.648296. PMID:22277052.
- Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol. 2003;91(2):154–9. doi:10.1016/S1081-1206(10)62170-9. PMID:12952109.
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–90. doi:10.1067/mai.2001.117880. PMID:11496232.
- Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–82. doi:10.7326/0003-4819-154-9-201105030-00002. PMID:21536936.
- Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med. 2008;102(10):1371–8. doi:10.1016/j.rmed.2008.06.002. PMID:18657960.
- Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124(6):1210–6. doi:10.1016/j.jaci.2009.09.021. PMID:19910033.
- Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. The Eur Respir J. 2001;18(2):254–61. doi:10.1183/09031936.01.00092101. PMID:11529281.
- Milgrom H, Fick RB, Jr., Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. The New Engl J Med. 1999;341(26):1966–73. doi:10.1056/NEJM199912233412603. PMID:10607813.
- Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59(7):709–17. doi:10.1111/j.1398-9995.2004.00550.x. PMID:15180757.
- Rubin AS, Souza-Machado A, Andradre-Lima M, Ferreira F, Honda A, Matozo TM. Effect of omalizumab as add-on therapy on asthma-related quality of life in severe allergic asthma: a Brazilian study (QUALITX). J Asthma. 2012;49(3):288–93. doi:10.3109/02770903.2012.660297. PMID:22356355.
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59(7):701–8. doi:10.1111/j.1398-9995.2004.00533.x. PMID:15180756.
- Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New Engl J Med. 2011;364(11):1005–15. doi:10.1056/NEJMoa1009705. PMID:21410369.
- Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108(2):E36. doi:10.1542/peds.108.2.e36. PMID:11483846.
- Karpel J, Massanari M, Geba GP, Kianifard F, Inhaber N, Zeldin RK. Effectiveness of omalizumab in reducing corticosteroid burden in patients with moderate to severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105(6):465–70. doi:10.1016/j.anai.2010.09.011. PMID:21130385.
- Kulus M, Hebert J, Garcia E, Fowler Taylor A, Fernandez Vidaurre C, Blogg M. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Curr Med Res Opin. 2010;26(6):1285–93. doi:10.1185/03007991003771338. PMID:20377320.
- Busse WW, Morgan WJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. New England Journal of Medicine. 2011;364(11):1005–1015.
- Garcia G, Magnan A, Chiron R, et al. A proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthma. Chest. 2013;144(2):411–9. doi:10.1378/chest.12-1961. PMID:23579324.
- de Llano LP, Vennera Mdel C, Alvarez FJ, et al. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. J Asthma. 2013;50(3):296–301. doi:10.3109/02770903.2012.757780. PMID:23350994.
- Maselli DJ, Singh H, Diaz J, Peters JI. Efficacy of omalizumab in asthmatic patients with IgE levels above 700 IU/mL: a retrospective study. Ann Allergy Asthma Immunol. 2013;110(6):457–61. doi:10.1016/j.anai.2013.04.011. PMID:23706716.
- Kornmann O, Watz H, Fuhr R, Krug N, Erpenbeck VJ, Kaiser G. Omalizumab in patients with allergic (IgE-mediated) asthma and IgE/bodyweight combinations above those in the initially approved dosing table. Pulmonary Pharmacol Ther. 2014;28(2):149–53. doi:10.1016/j.pupt.2014.03.003.
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;13. doi:10.1002/14651858.CD003559.pub4.
- Kim HL, Leigh R, Becker A. Omalizumab: Practical considerations regarding the risk of anaphylaxis. Allergy, Asthma, Clin Immunology: Official J Canadian Soc Allergy Clin Immunology. 2010;6(1):32. doi:10.1186/1710-1492-6-32. PMID:21129189.
- Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV. American academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol. 2007;120(6):1373–7. doi:10.1016/j.jaci.2007.09.032. PMID:17996286.
- Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560–7.e4. doi:10.1016/j.jaci.2014.02.007. PMID:24679845.
- Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983–9.e6. doi:10.1016/j.jaci.2012.01.033. PMID:22365654.
- Corren J, Casale TB, Lanier B, Buhl R, Holgate S, Jimenez P. Safety and tolerability of omalizumab. Clin Exp Allergy : J British Soc Allergy Clin Immunology. 2009;39(6):788–97. doi:10.1111/j.1365-2222.2009.03214.x. PMID:19302249.
- Mepolizumab (Nucala). Ottawa (ON): Product Monograph Nucala; 2016. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=93562.
- Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. The New Engl J Med. 2009;360(10):973–84. doi:10.1056/NEJMoa0808991. PMID:19264686.
- Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–71. doi:10.1164/rccm.200701.-085OC. PMID:17872493.
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. The New Engl J Med. 2014;371(13):1189–97. doi:10.1056/NEJMoa1403291. PMID:25199060.
- Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol. 2014;133(3):921–3. doi:10.1016/j.jaci.2013.11.026. PMID:24418480.
- Liu Y, Zhang S, Li DW, Jiang SJ. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PLoS One. 2013;8(3):e59872. doi:10.1371/journal.pone.0059872. PMID:23544105.
- Powell C, Milan SJ, Dwan K, Bax L, Walters N. Mepolizumab versus placebo for asthma. Cochrane Database Syst Rev. 2015;(7):Cd010834. PMID:26214266.
- Li J, Wang F, Lin C, et al. The efficacy and safety of reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: A systematic review and meta-analysis. J Asthma. 2017;54(3):300–7. doi:10.1080/02770903.2016.1212371. PMID:27435534.
- Nair P, Wenzel S, Rabe KF, et al. Oral Glucocorticoid-Sparing effect of Benralizumab in Severe Asthma. New Engl J Med. 2017;376(25):2448–58. doi:10.1056/NEJMoa1703501. PMID:28530840.
- Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2017;47(1):129–38. doi:10.1111/cea.12853. PMID:27859832.
- Wang FP, Liu T, Lan Z, Li SY, Mao H. Efficacy and safety of Anti-Interleukin-5 therapy in patients with Asthma: A systematic review and meta-analysis. PLoS One. 2016;11(11):e0166833. doi:10.1371/journal.pone.0166833. PMID:27875559.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127(6):1999–2006. doi:10.1378/chest.127.6.1999. PMID:15947312.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med. 2006;173(9):965–9. doi:10.1164/rccm.200507-1162OC. PMID:16456145.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol (Bethesda, Md : 1985). 2004;97(5):1946–53. doi:10.1152/japplphysiol.01282.2003. PMID:15258133.
- Chakir J, Haj-Salem I, Gras D, et al. Effects of Bronchial Thermoplasty on airway smooth muscle and collagen deposition in Asthma. Ann Am Thoracic Soc. 2015;12.(11):1612–8. PMID:26325484.
- Denner DR, Doeing DC, Hogarth DK, Dugan K, Naureckas ET, White SR. Airway Inflammation after Bronchial Thermoplasty for Severe Asthma. Ann Am Thoracic Soc. 2015;12(9):1302–9. doi:10.1513/AnnalsATS.201502-082OC. PMID:26230374.
- Pretolani M, Dombret MC, Thabut G, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014;190(12):1452–4. doi:10.1164/rccm.201407-1374LE. PMID:25496106.
- Pretolani M, Bergqvist A, Thabut G, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: Clinical and histopathologic correlations. J Allergy Clin Immunol. 2017;139(4):1176–85. doi:10.1016/j.jaci.2016.08.009. PMID:27609656.
- Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. New England J Med. 2007;356(13):1327–37. doi:10.1056/NEJMoa064707. PMID:17392302.
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–24. doi:10.1164/rccm.200903-0354OC. PMID:19815809.
- Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185–91. doi:10.1164/rccm.200704-571OC. PMID:17901415.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol. 2011;107(1):65–70. doi:10.1016/j.anai.2011.03.005. PMID:21704887.
- Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulmonary Med. 2011;11:8. doi:10.1186/1471-2466-11-8. PMID:21314924.
- Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295–302. doi:10.1016/j.jaci.2013.08.009. PMID:23998657.
- Doeing DC, Mahajan AK, White SR, Naureckas ET, Krishnan JA, Hogarth DK. Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma. 2013;50(2):215–8. doi:10.3109/02770903.2012.751997. PMID:23252954.
- Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 2015;136(3):531–45. doi:10.1016/j.jaci.2015.05.052. PMID:26343937.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. GIfAGsfama. Updated 2015. http://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdf.
