ABSTRACT
RATIONALE: Since the last published Canadian Thoracic Society (CTS) COPD guideline in 2007 and the 2008 update – highlights for primary care, many new clinical trials have challenged COPD treatment practices. The current Canadian position statement provides the reader with an update on pharmacotherapy of patients with COPD as reviewed by the CTS.OBJECTIVES: The objectives of this position statement are: 1) to summarize the literature on topics relevant to the pharmacological therapy of patients with stable COPD; and 2) to provide clinical guidance with evidence-based recommendations and expert-informed key messages for the pharmacological therapy for patients with stable COPD.METHODS: The authors systematically reviewed the relevant literature focusing on randomized controlled trials and when available, systematic reviews of randomized controlled trials. The proposed key messages, based on scientific evidence and expert-informed opinion, were agreed upon by a majority consensus.MAIN RESULTS: There is typically a significant delay in seeking medical care by patients with dyspnea, often waiting until symptoms affect the performance of activities of daily living. The diagnosis of COPD requires spirometry to confirm the presence of airflow obstruction in any patient presenting with symptoms and/or risk factors of COPD. An effective management plan for individuals with COPD should include: smoking cessation, vaccination and education. A number of non-pharmacological treatments are available for COPD patients with symptoms to improve outcomes such as self-management with coaching from a health care professional; pulmonary rehabilitation; supplemental oxygen in selected patients; and surgery.
Current pharmacotherapy for COPD has been shown to alleviate symptoms and prevent exacerbations and related complications such as hospital admissions. In symptomatic patients with stable COPD not having or having infrequent exacerbation, treatment should be started with inhaled LAMA or LABA monotherapy, and if experiencing persistent or increased dyspnea, exercise intolerance, and/or reduced health status despite use of monotherapy, patients should be considered for treatment “step up” with an inhaled LAMA plus LABA dual therapy. In this situation, the use of a single inhaler would be preferred to simplify the treatment regimen and minimize the cost. In patients with stable COPD experiencing exacerbations despite the use of LAMA or LABA monotherapy, treatment “step up” with inhaled LAMA plus LABA dual therapy should be considered unless a patient has concomitant asthma (Asthma/COPD overlap (ACO)). There has been recent interest in using biomarkers to identify patients who are more likely to respond to ICS. Most of the studies have demonstrated that high blood eosinophils could be valuable to predict an increase response in terms of reduction of exacerbation rate when treated with combination ICS/LABA; there is still uncertainty about the exact cut-off level of blood eosinophils having potential therapeutic value. If a patient is still experiencing exacerbations despite the use of LAMA and LABA dual therapy, treatment “step up” with LAMA plus ICS/LABA triple therapy can be considered. Because the superiority of inhaled triple or dual therapy may not be achieved in every patient, the notion of treatment “step down” may be a consideration in some patients. These patients would be those not demonstrating expected benefits or having side effects exceeding benefits. In any circumstance, when a physician decides using a treatment “step down”, this approach should be undertaken under close medical supervision.
Individuals with ACO are a population of medical interest, however, the paucity of original studies precluded evidence-based recommendations. The position statement, therefore, presents key messages from a survey which at best reflects the practice in our Canadian community and academic respirologists on assessment, diagnosis, and pharmacotherapy of ACO patients.CONCLUSIONS: This position statement is an evolution towards personalized treatment, compared to the previous published CTS COPD guideline. It promotes approaches to match treatment decisions based on symptom burden and risk of future exacerbations. Personalized medicine becomes increasingly possible, but to make future progress, we will need clinical research to be more specific, including greater focus on or defining better subsets of patients that are characterized by specific biomarkers and disease severity.
RÉSUMÉ
JUSTIFICATION: Depuis les dernières lignes directrices publiées par la Société canadienne de thoracologie (SCT) en 2007 et actualisées pour le médecin de famille en 2008, de nombreux essais cliniques ont remis en question les pratiques relatives au traitement de la MPOC. Cet énoncé de position canadien présente au lecteur les plus récentes informations concernant la pharmacothérapie destinée aux patients souffrant de MPOC, telle que revue par la SCT.OBJECTIFS: Les objectifs de cet énoncé de position sont : 1) résumer la littérature sur les sujets pertinents au traitement pharmacologique des patients souffrant d'une MPOC stable; et 2) présenter des directives comprenant des recommandations fondées sur les données probantes et des messages-clés fondés sur l'avis d'experts pour le traitement pharmacologique des patients souffrant d'une MPOC stable.MÉTHODES: Les auteurs ont revu la littérature pertinente de manière systématique en privilégiant les essais contrôlés randomisés et, lorsque disponibles, les revues systématiques d'essais randomisés. Les messages-clés proposés, qui se fondent sur les données probantes et sur l'opinion d'experts, ont été approuvés par consensus obtenu à la majorité.PRINCIPAUX RÉSULTATS: Les patients souffrant de dyspnée retardent généralement leur quête de soins médicaux jusqu'au moment où les symptômes commencent à affecter leurs activités quotidiennes. Le diagnostic de MPOC nécessite une spirométrie pour confirmer la présence d'une obstruction des voies respiratoires chez un patient qui présente des symptômes ou des facteurs de risque de MPOC. Un plan de prise en charge efficace pour les individus souffrant de MPOC devrait comprendre : la cessation du tabagisme, la vaccination et l’éducation. Un certain nombre de traitements non pharmacologiques sont disponibles pour les patients atteints de MPOC symptomatique afin d'optimiser les résultats, dont l'auto-prise en charge accompagnée des conseils d'un professionnel de la santé; la réadaptation pulmonaire; l'oxygène d'appoint chez certains patients; et la chirurgie.
Il a été démontré que la pharmacothérapie actuelle pour la MPOC soulage les symptômes et prévient les exacerbations ainsi que les complications qui en découlent, comme les hospitalisations. Chez les patients atteints de MPOC symptomatique stable avec ou sans exacerbations occasionnelles, le traitement devrait commencer par l'inhalation d'un anticholinergique à longue durée d'action (ACLA) ou d'un Beta2 agoniste à longue durée d'action (BALA) en monothérapie, et dans les cas où la dyspnée, l'intolérance à l'exercice et la détérioration de l’état de santé persistent ou augmentent, une intensification du traitement devrait être envisagée pour ces patients en combinant l'inhalation d'un ACLA à celle d'un BALA en double thérapie. Dans une telle situation, l'usage d'un seul inhalateur devrait être privilégié afin de simplifier le régime de traitement et minimiser les coûts. Chez les patients atteints de MPOC stable qui ont des exacerbations malgré l'utilisation d'un ACLA ou d'un BALA en monothérapie, l'intensification du traitement par l'inhalation d'un ACLA associé au BALA en double thérapie devrait être envisagée, sauf dans les cas où le patient souffre d'asthme concomitant (chevauchement asthme/MPOC). Récemment, on assiste à un accroissement de l'intérêt pour l'utilisation de biomarqueurs afin de déterminer les patients qui sont les plus susceptibles de répondre aux CSI. La plupart des études ont démontré qu'un nombre élevé d’éosinophiles dans le sang pourrait être utile pour prédire une meilleure réponse en ce qui a trait à la réduction du taux d'exacerbation lorsque traité par une combinaison de CSI et de BALA; toutefois, l'incertitude demeure quant au seuil d’éosinophiles dans le sang pouvant avoir une valeur thérapeutique. Si le patient connaît encore des exacerbations malgré un double traitement par ACLA et BALA, l'intensification du traitement par l'ajout d'un ACLA au traitement CSI/BALA en trithérapie peut être envisagé. Puisque la supériorité du traitement par inhalation double ou triple pourrait ne pas être réalisable pour chaque patient, la notion de « diminution graduelle » du traitement pourrait être envisagée pour certains patients. Ces patients seraient ceux qui ne démontrent pas les bénéfices attendus ou qui ont des effets secondaires qui dépassent les bénéfices. Dans toute circonstance, lorsqu'un médecin décide d'avoir recours à une « diminution graduelle » du traitement, cette approche devrait être entreprise sous supervision médicale étroite.
Les personnes souffrants de chevauchement asthme/MPOC sont une population de grand intéretê médical. Toutefois, le nombre limité d’études originales empêche la formulation de recommandations fondées sur des données probantes. Cet énoncé de position présente donc des messages-clés issus d'une enquête qui, au mieux, reflète la pratique dans notre communauté canadienne et chez les pneumologues universitaires en ce qui concerne l'evaluation, le diagnostic et la pharmacothérapie dans le cas des patients souffrant de chevauchement asthma/MPOC.CONCLUSION: Cet énoncé de position constitue une évolution vers le traitement personnalisé, comparativement aux lignes directrices de la SCT relatives à la MPOC publiées précédemment. Il favorise les approches qui visent à établir une correspondance entre les décisions thérapeutiques fondées sur le fardeau des symptômes et le risque d'exacerbations futures. La médecine personnalisée devient de plus en plus possible, mais pour progresser, la recherche clinique doit être plus spécifique, notamment en ciblant ou en définissant plus clairement des sous-ensembles de patients caractérisés par des biomarqueurs précis et par le degré de gravité de leur maladie.
Introduction
Since the last published Canadian Thoracic Society (CTS) COPD guideline in 20071 and the 2008 update – highlights for primary care,Citation2 many new clinical trials have challenged COPD treatment practices. Identifying important new evidence and assessing whether these findings warrant change in current practice is needed. Furthermore, a new Canadian statement and position on the pharmacotherapy of COPD is timely considering the recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report 2017.Citation3 In the GOLD Report 2017, as in the previous CTS COPD guideline, severity of airflow limitation is no longer considered in the treatment algorithm. Rather, pharmacotherapy is modified according to symptom burden and risk of COPD exacerbations. The current Canadian position paper provides the reader with an update on pharmacotherapy of patients with COPD as reviewed by the CTS.
Epidemiology and burden of COPD
From the Global Burden of Disease Study 2015,Citation4 the Chronic Respiratory Disease Collaborators concluded: « COPD and asthma are important contributors to the burden of non-communicable disease. Although much of the burden is either preventable or treatable with affordable interventions, these diseases have received less attention than other prominent non-communicable diseases like cardiovascular disease, cancer, or diabetes. » Although we may have made progress in the adoption of some evidence-based practice in the last decades, there remains a degree of nihilism about COPD and its treatment.
COPD is the fourth leading cause of death worldwide, and twice as many individuals die from COPD than from lung cancer.Citation5 Although historically, more men than women died from COPD, the gender gap in mortality has virtually disappeared.Citation6 While 4% of Canadians aged 35 to 79 self-reported with a doctor diagnosis of COPD, direct measurements of lung function from the Canadian Health Measures Survey (CHMS) indicate that 13% of Canadians demonstrated airflow limitation consistent with the diagnosis of COPD.Citation7 In the recent Canadian Cohort Obstructive Lung Disease (CanCOLD), a population-based study among randomly chosen adult Canadians 40 years of age and older, the prevalence of COPD, defined spirometrically, was 2.5 times higher than the rate of self-reported diagnosis and four-fold higher than self-reported rates from annual community surveys.Citation8 Smoking is the largest contributor to the COPD burden in countries at the higher end of the sociodemographic index (SDI) such as Canada (69.4% of COPD burden in high-SDI quintile countries) although a considerable proportion of COPD still remains unexplained.Citation4 COPD can have a devastating impact on patient wellbeing, family members, health care system, and society. It accounts for the highest rate of hospital admissions among major chronic illnesses in Canada.Citation9 The Conference Board of Canada has estimated that with the population aging, the combined direct and indirect costs of COPD will increase from $4 billion in 2010 to $9.5 billion by 2030—an increase of 140 percent.Citation10 This is an average compounding annual growth in the economic burden associated with COPD of 4.5 percent over the 2010–2030 period, a significant social and economic toll on Canadians.
Key messages from the last CTS COPD guideline that remain relevant
Since the last published CTS COPD Guidelines in 2007 and 2008,Citation1,2 key messages regarding disease management remain at the forefront:
| 1) | Use spirometry in targeted populations for case finding of “early” and undiagnosed COPD for prompt intervention (including enhancing effectiveness of smoking cessation interventions); | ||||
| 2) | Improve the treatment algorithms by incorporating accurate assessment of dyspnea and activity limitation and; | ||||
| 3) | Use strategies to prevent and manage acute exacerbations, particularly in moderate to severe disease. | ||||
Spirometry measurements in targeted populations for case finding
The presence of symptoms is not a reliable indicator to make the diagnosis; spirometry is a prerequisite for the diagnosis of COPD. Spirometry testing should focus on patients at risk, particularly from smoking, and those having respiratory-relevant symptoms (i.e. dyspnea). However, patients with potential chronic respiratory disease may not raise respiratory symptoms with their general practitioner; they may attribute their symptoms to ageing and/or multi-causal explanations that lessen the importance of obtaining a diagnosis. Fifty percent or more of patients with COPD are still undiagnosed in Canadian family medicine practices.Citation11,12 Early diagnosis is a contentious issue, but it optimizes the opportunities to prevent worsening of disease and prevention of exacerbations. The population-based cohort study CanCOLD has shed light on the question of undiagnosed COPD. The study has shown for the first time that despite experiencing fewer overall exacerbations, the rate of moderate to severe exacerbations (i.e. those requiring health care utilization including emergency department (ED) visits and/or hospital admissions) among undiagnosed COPD patients is similar to that among diagnosed individuals.Citation12 These findings further support the need to diagnose COPD using objective measures when symptoms are present, in order to prevent this morbidity by providing proper therapy.
Spirometry is a safe, practical and reproducible breathing test that can be used in primary care to objectively determine lung function. However, it is important to appreciate that the clinical value of spirometry is critically dependent on the correct operation and accuracy of the spirometer, performance of the correct maximal breathing maneuver, selection of the best test results to use and correct interpretation. A recent systematic scoping review found that misdiagnosis of COPD most commonly occurred due to an incorrect spirometric threshold used for defining COPD; errors linked to the spirometry test itself; errors in differentiating COPD from other diseases; and finally, patient-related factors affecting spirometry interpretation (e.g. obesity).Citation13 Most of these errors were noted to take place predominantly in the primary care setting. These findings support early diagnosis of COPD using objective measures with spirometry testing.
Improving symptom burden and preventing exacerbations in COPD patients
With the right treatment and support, people diagnosed with COPD can improve their health and reduce hospital admissions.Citation3 Current therapies are effective in reducing dyspnea and activity limitation, two of the most burdensome manifestations of COPD. Exacerbations can also be prevented with appropriate management. Management of COPD typically involves multiple interventions, including non-pharmacological and pharmacological therapies. presents an update from the last published CTS COPD Guideline 2007 and 20081,2 with an organized approach that includes COPD diagnosis with spirometry, evaluation of symptom burden and exacerbations with ongoing monitoring, assessment for concomitant asthma, and comprehensive management, i.e., pharmacological and non-pharmacological therapies.
Figure 1. Comprehensive Management of COPD. Integrated approach of care that includes COPD diagnosis with spirometry, evaluation of symptom burden and risk for future exacerbations with on-going monitoring, assessment for features of asthma, and comprehensive management, both non pharmacologic and pharmacologic. CAT = COPD assessment test; MRC = Medical Research Council; SABD PRN = short-acting bronchodilator as needed; AECOPD = acute exacerbation of COPD; Inhaled Long-Acting Therapies = long-acting muscarinic antagonist and/or long-acting B2-agonist and/or inhaled corticosteroid; LTOT = long-term oxygen therapy.
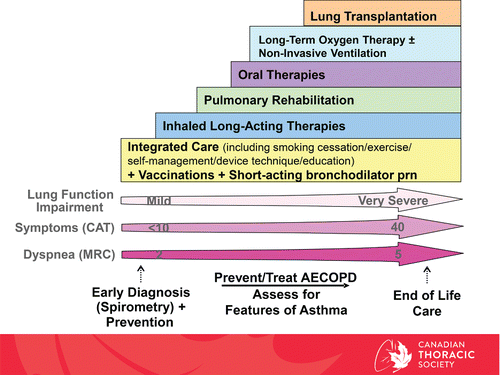
Non-pharmacological management of COPD includes smoking cessation strategies, vaccination, self-management education, pulmonary rehabilitation and supplemental oxygen. Smoking cessation and supplemental oxygen in those with persistent resting hypoxemia, remain the only interventions with a proven mortality benefit in COPD. Non-pharmacological treatment for a smaller number of patients may also include ventilatory support, in particular non-invasive positive pressure ventilation, various forms of lung volume reduction (bronchoscopic and surgical interventions) and lung transplantation. Palliative care in patients with advanced COPD is another area that requires special attention. It encompasses approaches to symptom control to management of patients with refractory dyspnea and also patients with terminal disease approaching death. Managing dyspnea in patients with advanced COPD has been addressed in a separate CTS clinical practice guideline.Citation14
Non-pharmacological management is an often neglected area of chronic respiratory care.
It is well known that emphasis should be given in practice to education and training in inhaler device technique. According to a systematic review on errors in inhaler use,Citation15 incorrect inhaler use remains unacceptably high outside of clinical trials. This may be a major obstacle to achieving good COPD control. As recommended in the GOLD report 2017,Citation3 instruction should be provided on inhalation technique and patient technique should be re-checked at each visit. Furthermore, the choice of inhalers should be individually tailored and will depend on access, cost, prescriber and most importantly, patient ability and preference. Another example of non-pharmacological management being neglected is access and referral to pulmonary rehabilitation (PR). According to a 2015 survey of PR in Canada,Citation16 despite an increase in the number of programs and patients enrolled since the previous survey in 2005, PR capacity has not kept pace with demand, with only 0.4% of eligible Canadians with COPD having access.Citation16 When asked, physicians who are regularly seeing COPD patients say that only 16% of their patients are referred to PR.Citation17 This is in contrast with the 34% of high-risk cardiac patients who have been referred to cardiac rehabilitation programs in Ontario.Citation18 Finally, an often forgotten non-pharmacological management principle in patients with advanced COPD is referral for end-of-life care services (e.g. palliative care, opiates for dyspnea relief, etc.).
New review and CTS COPD position statement
We did not review non-pharmacological therapies in this position statement, as recommendations have been promulgated through recent national and international guidelines.Citation3,19 For example, pulmonary rehabilitation has been addressed in the CTS clinical practice guideline: “Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease – practical issues”.Citation20 We did not review, COPD related to α1-antitrypsin deficiency, as recommendations on testing and treatment have been addressed in a separate CTS clinical practice guideline.Citation21
This position statement focuses on the pharmacological therapy of COPD. This position paper will be updated in accordance with the CTS Living Guideline Model www.cts-sct.ca/guidelines which includes a process for guideline review at least every 3 years to determine whether guideline updates are required. However, based on new evidence post March 2017 (literature search cut-off date), an update will be provided in 2018 and in following years.
The overall goal of the CTS COPD position statement is to help clinicians to appropriately match therapeutic decision to the clinical status of each individual patient. This is a first step towards personalizing therapy based on increasing individual characterization, i.e., COPD patient phenotypes.
The specific objectives are: 1) to summarize the literature on topics relevant to the pharmacological therapy of patients with stable COPD; and 2) to provide clinical guidance with evidence-based recommendations and expert-informed key messages for the pharmacological therapy for patients with stable COPD.
Target patient population
Patients with stable COPD, including those who have concomitant asthma (Asthma/COPD overlap).
Target users
The recommendations provided herein are intended for use primarily by respirologists, internists, primary care physicians, pharmacists, nurse practitioners, respiratory educators and certified respiratory educators (CRE). This document should also be useful to patients and patient advocates. Finally, health care decision makers may also use this in policy processes to inform coverage decisions.
Methodology
The position statement was developed in accordance with CTS requirements for a position statement (www.cts-sct.ca/guidelines), which is derived from the CTS guideline production methodology.Citation22 A working group was created within the CTS COPD Clinical Assembly. The lead authors utilized the AGREE II checklist to guide the development of this position statement. The COPD working group identified two relevant questions pertaining to the pharmacotherapy of COPD and one question to the specific diagnosis and pharmacotherapy of ACO (see subsection “Selection and Formulation of Key Questions”). These questions were selected based on the needs to assess new evidence and whether these findings warrant change in current practice. Each section provides a literature summary, recommendations from a systematic review that informs key messages for each question vetted by the working group members. The recommendations were classified using the GRADE system, as adapted for use by the American College of Chest Physicians for the level of evidence and were agreed upon by a majority consensus. The key messages provide a summary of the most important recommendations and include a step-by-step approach that will be useful in guiding practical choices by clinicians.
In accordance with the CTS guideline production methodology, this position statement underwent both internal and external review. The external review was conducted by two international COPD experts who were not part of the CTS. The internal review was conducted by three Certified Respiratory Educators (members of the Canadian Respiratory Health Professionals Assembly); one respirologist from another CTS Clinical Assembly who completed an AGREE II score sheet; and three Executive Members of the Canadian Respiratory Guidelines Committee. Original reviews and responses to reviews are posted along with the position statement and all authors' conflicts of interest at www.cts-sct.ca/guidelines. The CTS Executive approved the final document for publication.
Representation on the COPD position statement committee
The COPD working group was constituted of ten respirologists, two primary care physicians appointed by the College of Family Physicians of Canada, one pharmacist and one patient with COPD. These healthcare professionals are all directly involved in the clinical management of COPD patients in different health care settings. They had expressed interest in being part of the working group or were actively invited based on their expertise. Members of the working group were assigned to 1 of 3 writing groups that addressed each question. All members reviewed and agreed on the overall content of this position statement.
Selection and formulation of key questions
The COPD working group developed key clinical questions using the Problem/population Intervention/prognostic factor/exposure, Comparison, Outcome (PICO) format, which were then reviewed, revised and agreed upon by members from each writing group (details in ). The PICO questions were selected to address the concept that personalized treatment of COPD in clinical practice should be guided by recognized treatable and/or preventable clinical features such as symptom burden, health status, and risk of exacerbations.
Table 1. CTS COPD Position Statement PICO questions.
The members also agreed that dyspnea/exercise tolerance/health status (but not disease progression and/or mortality) would serve as the most relevant outcomes for PICO 1 and exacerbations would serve as the most relevant outcome for PICO 2.
Literature searches
Systematic reviews in Medline OVID were conducted for interventions identified in each PICO question starting with a search for guidelines and systematic reviews ( and ). The search for the ACO PICO question yielded primarily opinion papers and, for this reason, data were collected and expert-informed key messages formulated using features of a Delphi technique.Citation23 The review was limited to publications between January 2008 to March 6, 2017 for PICO questions 1 and 3 (starting from the review date of the prior CTS guideline) and between February 2014 and March 6, 2017 for PICO question 2 (starting from the review date of the “Prevention of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: American College of Chest Physicians and Canadian Thoracic Society Guideline”). Literature search results informing recommendations for each question are presented in .
Table 2. Literature search results informing recommendations.
Study selection and data extraction
Studies were excluded if not related to COPD. Only randomized clinical trials (RCTs) were kept for further review and inclusion into the evidence review. The inclusion/exclusion exercise and the review of full text articles for data extraction were completed by pairs of reviewers to ensure the selection criteria were met and agreed upon. The evidence tables are posted along with the position statement at www.cts-sct.ca/guidelines.
Recommendations
All of the studies included in the evidence review informed the recommendations and their associated grades. The recommendations were devised using recognized document evaluation tools to assess and choose the most appropriate studies and evidence to extract meaningful data in a balanced and unbiased fashion. All recommendations were classified using the GRADE system, as adapted for use by the American College of Chest Physicians (CHEST).Citation24 In instances where there was insufficient evidence, but a recommendation was still warranted, a weak suggestion was developed and consensus-based (CB) replaced the grade. Completed recommendations and supporting text were reviewed by each writing group and revised before they were shared with the entire working group. Recommendations and supporting text were sent out to all the members of the working group asking them to identify any recommendations deemed controversial based on their wording and/or grade. Members of the working group were then sent revised statements and were asked to vote on the recommendations. Any recommendations that were not agreed upon by a majority were revised based on feedback and included in the second round of voting. For the purposes of this document, when the comparison treatment arm refers to “placebo therapy,” it applies to the use of a prn short-acting beta-2-agonist only.
Summary of evidence
Section 1 – Improving symptom, exercise tolerance and health status in stable COPD
PICO 1: How does a clinician choose appropriate maintenance pharmacotherapies in patients with stable COPD to improve dyspnea, exercise tolerance, physical activity and health status?
Alleviation of symptom burden (e.g. dyspnea), improving exercise tolerance, physical activity and health status are key goals of COPD management.Citation1,3 This section of the COPD position statement specifically addresses the use of pharmacological maintenance therapies and their impact on patient-related outcomes (PRO) in patients with stable COPD. The importance of effective non-pharmacological therapies that target these PRO in COPD have been discussed previously and reviewed elsewhere.Citation1,3
Dyspnea, a subjective experience of unpleasant, discomfort with breathing, is a cardinal symptom of COPD.Citation1,3 Dyspnea is associated with decreased exercise intolerance, reduced health status and poor prognosis in COPD. Pathophysiological mechanisms for dyspnea and exercise intolerance in COPD have been well described elsewhere.Citation25 Dyspnea measurement in clinical practice using a validated tool such as the Medical Research Council (MRC) dyspnea scale is important in determining COPD disease severity; moderate to severe COPD is associated with MRC scores 3–5.Citation1 Overall symptom burden and health status can be assessed clinically with a validated and simple to use measurement tool such as the COPD Assessment Test (CAT); a CAT score of ≥10 (out of possible maximum score of 40) suggests significant symptom burden and impairment in health status.Citation3,26
Bronchodilators are the mainstay of maintenance pharmacotherapy in stable COPD. Bronchodilators reduced dyspnea that is a consequence of improving lung function (FEV1), reducing resting hyperinflation and gas trapping, and reducing dynamic hyperinflation during exercise. Due to better efficacy and safety profiles, inhaled bronchodilators are preferred over oral bronchodilators (e.g. theophyllines). The two main classes of inhaled bronchodilator drugs are beta-2-agonists and antimuscarinics (or anticholinergics). Both classes of inhaled medications are available in short-acting (taken on a prn basis or 4 times or more daily) beta-2-agonists (SABA) and antimuscarinics (SAMA) and long-acting (taken once or twice daily) beta-2-agonists (LABA) and antimuscarinics (LAMA). Inhaled long-acting bronchodilators are preferred over short-acting bronchodilators (SABD) for regular maintenance use except in less symptomatic individuals (e.g. MRC score 1–2, CAT score < 10) or for those with intermittent symptoms.
Conclusions from the systematic literature review (for Key Messages, see Box 1)
For maintenance therapy in patients with stable COPD, and with the intention of alleviating symptom burden (e.g. dyspnea), improving exercise tolerance, physical activity, and health status:
| 1. | We recommend the use of an inhaled long-acting bronchodilator, either LAMA or LABA monotherapy, to reduce dyspnea, improve exercise tolerance, and improve health status in stable COPD patientsCitation27–48,32,33,37,38,40,44,49–60 (Grade 1A). | ||||
| 2. | We suggest that in stable COPD patients who experience persistent dyspnea, exercise intolerance, and/or poor health status despite use of inhaled LAMA or LABA monotherapy that they be considered for treatment “step up” with LAMA plus LABA dual therapyCitation32,61–71, 70,72–76 (Grade 2A). | ||||
| 3. | We suggest the use of a treatment with an inhaled long-acting bronchodilator(s), i.e. LAMA, LABA, or LAMA plus LABA dual therapy, to improve physical activity levels in stable COPD patientsCitation30,43,77–80 (Grade 2A). | ||||
| 4. | We suggest in stable COPD patients without ACO who have persistently poor health status despite the regular use of a LABA, to “step up” therapy to an inhaled LAMA plus LABA dual therapy rather than to inhaled ICS/LABA combinationCitation81,82 (Grade 2B). | ||||
| 5. | There is insufficient evidence in stable COPD patients to determine whether inhaled LAMA plus ICS/LABA triple therapy confers additional benefit to inhaled LAMA plus LABA dual therapy in reducing dyspnea, improving exercise tolerance and activity levels, or improving health status.Citation83,84 However, in stable COPD patients with high symptom burden and poor health status despite the use of inhaled LAMA plus LABA dual therapy, “step up” of treatment to LAMA plus ICS/LABA triple therapy may be considered (CB). | ||||
| 6. | There is insufficient evidence in stable COPD patients to determine whether treatment “step down”, i.e., inhaled triple therapy to inhaled LAMA plus LABA dual therapy or inhaled LAMA plus LABA dual therapy to LAMA or LABA monotherapy can be safe and/or without reducing patient benefits (i.e., dyspnea, exercise tolerance and health status). However, in stable COPD patients with no improvement of dyspnea, exercise tolerance or health status despite the use of triple inhaled therapy or inhaled LAMA plus LABA dual therapy, treatment “step down” may be considered (CB). | ||||
| 7. | There is insufficient or equivocal evidence in stable COPD patients to determine whether the addition of an oral therapy, such as theophyllines,Citation85,86 phosphodiesterase-4-inhibitors,Citation87–89 mucolytics,Citation90 statins,Citation91–93 anabolic steroids,Citation94,95 oral Chinese herbal medicines,Citation65,96–98 or phosphodiesterase-5-inhibitors,Citation99–102 confers additional benefit to inhaled LAMA or LABA monotherapy, or LAMA plus LABA dual therapy in reducing dyspnea, improving exercise tolerance and activity levels, or improving health status (Grade 2C). | ||||
| 8. | We recommend against treatment with ICS monotherapy in stable COPD patients (CB). | ||||
Box 1. Improving symptom burden, exercise tolerance and health status in stable COPD
PICO 1: How does a clinician choose appropriate maintenance pharmacotherapies in patients with stable COPD to improve dyspnea, exercise tolerance, physical activity and health status?
Key Messages:
| 1) | In symptomatic patients with stable COPD, treatment should be started with inhaled LAMA or LABA monotherapy, and if experiencing persistent or increased dyspnea, exercise intolerance, and/or poor health status despite use of monotherapy, patients should be considered for treatment “step up” with an inhaled LAMA plus LABA dual therapy. In this situation, the use of a single inhaler would be preferred to simplify the treatment regimen and minimize the cost. Recommendations #1, 2, 3, 4 | ||||
| 2) | In stable COPD patients with increasing symptom burden, exercise intolerance, and/or reduced health status despite the use of an inhaled LAMA plus LABA dual therapy, treatment “step up” to LAMA plus ICS/LABA triple therapy may be considered (the main indication is exacerbation prevention). Recommendation #5 | ||||
| 3) | In stable COPD patients with no improvement of dyspnea, exercise tolerance, physical activity or health status despite the use of inhaled triple therapy or inhaled LAMA plus LABA dual therapy, treatment “step down” may be considered, but patients will require careful follow up for any evidence of clinical deterioration. Recommendation #6 | ||||
| 4) | ICS monotherapy should not be used in stable COPD patients. Recommendation #8 | ||||
The summary of the most important evidence-based recommendations on the pharmacotherapy treatment and a step-by-step approach to guide practical choices is shown in .
Figure 2. COPD Pharmacotherapy. Suggested COPD pharmacotherapy promoting an approach that matches treatment decisions with symptom burden and risk of future exacerbations. Solid arrows indicate step up therapy to optimally manage symptoms of dyspnea and/or activity limitation, as well as the prevention of AECOPD where appropriate. Dashed arrows indicate potential step down of therapy, with caution, and with close monitoring of the patient symptoms, exacerbations and lung function. Frequent AECOPD is ≥2 events requiring antibiotics ± systemic corticosteroids over 2 years; or ≥1 Severe AECOPD requiring hospitalization. As-needed (prn) use of short-acting bronchodilator should accompany all recommended therapies. CAT = COPD assessment test; MRC = Medical Research Council; SABD prn = short-acting bronchodilator as needed; AECOPD = acute exacerbation of COPD; LAMA = long-acting muscarinic antagonist; LABA = long-acting B2-agonists; ICS = inhaled corticosteroid; PDE4 = phosphodiesterase-4.
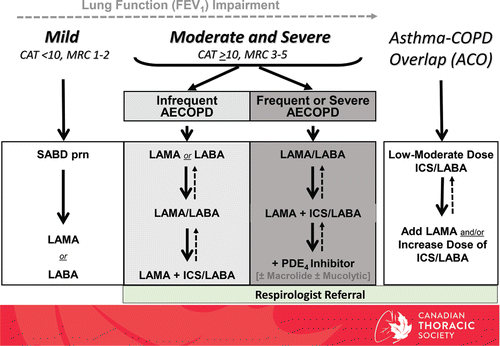
Section 2 – Preventing acute exacerbation in stable COPD
PICO 2: How does a clinician choose appropriate maintenance pharmacotherapies in patients with stable COPD to reduce the risk of frequent exacerbations?
Preventing acute exacerbations of COPD (AECOPD) is a key goal of COPD management.Citation1,3 An AECOPD is defined as an acute worsening of respiratory symptoms that results in a requirement for additional therapy.Citation3 This section of the position statement specifically addresses the use of inhaled and oral pharmacologic maintenance therapies demonstrated to be effective in preventing AECOPD in patients with stable COPD. These current recommendations have been revised and updated from the 2015 Prevention of Acute Exacerbations of COPD – American College of Chest Physicians and Canadian Thoracic Society Guideline document,Citation19 i.e. to include new publications from February 2014 to March 6, 2017. Some recommendations have been revised to account for additional research findings or have had their strength updated to reflect more recently published literature. The number of recommendations for the specific purpose of preventing AECOPD in patients with stable COPD has been reduced to focus on recommendations with the highest clinical impact. The importance of effective non-pharmacological therapies that target preventing exacerbations, and complications such as ED visits and hospital admissions in COPD have been discussed previously and reviewed elsewhere.Citation1,3
Exacerbations are to COPD what myocardial infarctions are to coronary artery disease: they are acute, trajectory changing and often deadly manifestations of a chronic disease.Citation19 Exacerbations cause frequent hospital admissions, relapses, and readmissions; contribute to death during hospitalization or shortly thereafter; dramatically reduce quality of life, and hasten a progressive decline in pulmonary function, a cardinal feature of COPD. Admission to hospital due to AECOPD is the most frequent cause for acute hospitalization in adult in Canada.Citation9
The best way to identify COPD patients susceptible to exacerbations is through their exacerbation history, where frequent exacerbations predict risk of future events.Citation103 An exacerbation-like respiratory event is a trigger for patients to come to the attention of the health care system and for a physician to consider the diagnosis of COPD. The severity of AECOPD is stratified as mild – when the changes in clinical symptoms require an increased use of as-needed bronchodilators but no changes in maintenance treatment; moderate – when changes in medication such as the use of antibiotics and/or systemic corticosteroids are required; and severe – when ED visit or hospitalization is necessary.Citation19 In the CanCOLD study, individuals with diagnosed COPD reported 0.63 exacerbations per person-year.Citation12 Overall, exacerbations of any severity were reported by 40%, and moderate to severe exacerbations by 32% of individuals with diagnosed COPD. An important and achievable goal of therapy in the management of stable COPD is to decrease the frequency and reduce the severity of AECOPD. Furthermore, providing proper preventive therapy in patients at increased risk of exacerbation will reduce and prevent ED visits and hospital admissions.
Conclusions from the systematic literature review (for Key Messages, see Box 2)
For maintenance therapy in patients with moderate to very-severe COPD, and with the intention of preventing moderate to severe AECOPD:
| 1. | We recommend the use of an inhaled LAMA (Grade 1A) or an inhaled LABA (Grade 1B) compared with placebo (i.e. SABD prn) to prevent AECOPD.Citation19,59,104–109 | ||||
| 2. | We recommend the use of an inhaled LAMA as a preferred choice compared with an inhaled LABA to prevent AECOPD (Grade 1B).Citation19,110 | ||||
| 3. | We recommend an inhaled LAMA (Grade 1A) or suggest an inhaled LABA (Grade 2C) compared with an inhaled short-acting muscarinic antagonist to prevent AECOPD.Citation19,51,108 | ||||
| 4. | We recommend inhaled LAMA plus LABA dual therapy for patients experiencing AECOPD despite the use of inhaled LAMA or LABA monotherapy (Grade 1C).Citation19,74,111–118 | ||||
| 5. | We recommend combination ICS/LABA compared to placebo (Grade 1B) or an inhaled LABA (Grade 1C) to prevent AECOPD.Citation19,119–125 | ||||
| 6. | We recommend inhaled LAMA plus LABA dual therapy as a preferred choice compared with ICS/LABA combination therapy to prevent AECOPD (Grade 1C).Citation19,116,117,126–131 | ||||
| 7. | We recommend LAMA plus LABA/ICS triple therapy to prevent AECOPD for patients experiencing AECOPD despite the use of inhaled LAMA (Grade 1B) or ICS/LABA (Grade 1C).Citation19,106,132–135 | ||||
| 8. | We recommend the use of oral roflumilast to prevent AECOPD for patients with chronic bronchitis and a history of at least one exacerbation in the previous year despite long-acting inhaled therapy (Grade 1B).Citation19,136–141 | ||||
| 9. | We suggest treatment with oral N-acetylcysteine (600 mg po BID) to prevent AECOPD for patients with chronic bronchitis, a history of at least one exacerbation in the previous year, and on long-acting inhaled therapy (Grade 2B).Citation19,142–147 | ||||
| 10. | We suggest a macrolide as maintenance therapy to prevent AECOPD for patients with a history of recurrent moderate or severe COPD exacerbations in the previous year despite long-acting inhaled therapy (Grade 2A).Citation19,126,148–151 | ||||
| 11. | We suggest treatment with oral slow-release theophylline* to prevent AECOPD for patients on long-acting inhaled therapy (Grade 2B).Citation19 | ||||
Although recent evidence supports the benefit of inhaled LAMA plus LABA dual therapy in preventing AECOPDCitation130 compared to a combination of ICS/LABA, further prospective comparisons are necessary, particularly in patient subgroups with 2 or more exacerbations per year or those with very severe airflow obstruction. These new data would suggest that for COPD patients who don't have concomitant asthma, step up from LAMA or LABA monotherapy to a LAMA plus LABA dual therapy is preferred over step up to combination ICS/LABA. Regardless, it is important to emphasize that ICS should not be used as monotherapy in COPD to prevent exacerbations and when used should only be combined with an inhaled LABA i.e. ICS/LABA. This has been highlighted in previous CTSCitation1 and joint CHEST/CTS guidelines.Citation19 Furthermore, when a combination of ICS/LABA is used, high doses of ICSFootnote1 are typically not needed to achieve optimum benefit in COPD, as recently shown by a relatively flat dose-response curve.Citation152 More recently, considering the potential for ICS side effects (pneumonia, cataract, osteoporosis, etc.) that have been demonstrated in many RCTsCitation152,153 and administrative database studies,Citation154 step down ICS or stepwise ICS withdrawal has been proposed in COPD patients on ICS treatment. In one recent large trial, patients with COPD receiving combined inhaled treatment with a LAMA plus ICS/LABA who underwent stepwise ICS withdrawal did not experience significantly increased exacerbation rates.Citation128 The overall exacerbation rate in both groups was quite low (about 0·5 exacerbations per patient per year) and 1/3 of participants were not on ICS at screening (before the study). Finally, there was a statistically significant reduction in FEV1 of 43 ml after ICS withdrawal at the end of the study and the number of deaths was numerically higher in the ICS withdrawal group (n = 40) compared to the ICS continuation group (n = 34).
If inappropriate therapy was previously initiated, step down should be undertaken. However, if appropriate therapy was initiated and treatment was shown to be effective, step down may not be appropriate. Given the significantly negative consequences of AECOPD, including hospitalization and death, the panel felt this could only be considered in patients at low risk of morbidity and mortality, and after a period of considerable stability. Moreover, while awaiting further objective proof supporting the safety of this approach, close supervision would be mandatory if the decision is made to step down, including monitoring of lung function and the re-occurrence of AECOPD.
There has been recent interest in using biomarkers to identify patients who are more likely to respond to ICS, and it has been suggested that blood eosinophils could predict benefit with ICS therapy in COPD. Most of the studiesCitation155–157 except oneCitation158 have demonstrated that high blood eosinophils could be valuable to predict an increase response in terms of reduction of exacerbation rate when treated with combination ICS/LABA. However, all these studies were secondary analyses or post hoc subgroup analyses. Furthermore, there is still uncertainty about the exact cut-off level of blood eosinophils having potential therapeutic value. Some studiesCitation155,157 have shown a better response in patients with blood eosinophils ≥2% while in other studiesCitation159 a cut-off of 4% or eosinophil count >300 cells/μL has been suggested. Data suggests that in COPD, blood eosinophil levels >340 cells/μL is associated with a 1.76 fold increased risk of severe exacerbationsCitation160; however using the 2% cut-off in this study did not predict increased risk of exacerbations. Finally, blood eosinophils demonstrated reasonable repeatability over 1 year in a population-based cohort of COPD patients in primary care.Citation161
Box 2. Preventing acute exacerbation in stable COPD
PICO 2: How does a clinician choose appropriate maintenance pharmacotherapies in patients with stable COPD to reduce the risk of frequent exacerbations?
Key messages:
| 1) | In stable COPD patients susceptible to exacerbations, LAMA or LABA inhaled monotherapy can be used to prevent moderate to severe exacerbations
| ||||||||||||||||
| 2) | In patients with stable COPD experiencing exacerbations despite the use of LAMA or LABA monotherapy, treatment “step up” with inhaled LAMA plus LABA dual therapy should be considered
| ||||||||||||||||
| 3) | In patients with stable COPD experiencing exacerbations despite the use of LAMA and LABA dual therapy, treatment “step up” with LAMA plus ICS/LABA triple therapy can be considered (unknown if triple therapy superior to LAMA plus LABA dual therapy: ongoing trials). | ||||||||||||||||
| 4) | In stable COPD without exacerbation (none or exacerbation that has been both infrequent and exclusively mild), treatment “step down”, i.e., stepwise ICS withdrawal may be cautiously considered, but careful monitoring and close clinical follow-up for recurrent AECOPD and any deterioration is mandatory. | ||||||||||||||||
| 5) | In stable COPD susceptible to exacerbations despite being on optimal inhaled therapy, oral therapies that include 5.1) PDE4-inhibitors and mucolytics may be considered in patients who still have exacerbations. Recommendations #8, 9, 11 5.2) macrolides may be considered in patients who have recurrent exacerbations. Recommendations #10 | ||||||||||||||||
| 6) | Systemic corticosteroids should not be used for maintenance therapy. | ||||||||||||||||
The summary of the most important evidence-based recommendations on the pharmacotherapy treatment and a step-by-step approach to guide practical choices is shown in .
Section 3 –Asthma/COPD overlap (ACO)
PICO 3: How does a clinician approach the treatment of patients who have COPD and features of asthma?
Most of the evidence we have on the pharmacotherapy of COPD comes from RCTs that have excluded patients who have features of asthma. There is a growing recognition that many COPD patients may also present with features of asthma, thus making an accurate and singular diagnosis challenging. These patients are often labeled as having ACO. This section of the position statement specifically addresses diagnosis and pharmacotherapy in patients with ACO. The current recommendations could not be based on reviewed publications because only 2 articles were selected for data extraction, as most of the studies published are opinion papers. For this reason, key messages in our position statement were determined using a survey with features of a Delphi technique.Citation23
Although the terminology and discussion surrounding ACO is relatively new, the concept of asthma and COPD overlapping is not. Fletcher coined the term “the Dutch Hypothesis” in the 1960's and ever since, there has been ongoing debate regarding the validity of this explanation for the pathophysiology of obstructive lung disease.Citation162–164 In recent years, there has been a greater push to further our understanding of ACO and assessing its impact on clinical practice, largely driven by the evolution of the inhaled medications available for both asthma and COPD, and more specifically the role of ICS in COPD. Clinicians now more than ever need clarity on the definition and diagnosis of ACO as the approach to the inhaled medication treatment for an ACO patient may differ from that of a patient with asthma or COPD alone. Unfortunately, despite this need and the plethora of articles published on this topic, many significant gaps in knowledge around ACO currently exist.
This knowledge gap presented several challenges in the preparation of this section regarding the diagnosis and pharmacotherapy of ACO. First, there is no universally accepted definition or diagnostic criteria for ACO. The Spanish consensus paperCitation165 agreed upon a combination of six major and minor criteria, which included both clinical and physiologic components. These major and minor criteria differ slightly from the ones proposed by Sin et al.Citation166 Both of those reports differ from the approach recommended in the 2015 Global Initiative for Asthma (GINA) and GOLD recommendations. Several other articles have been written on diagnostic criteria,Citation165–167 all with differing opinions. This lack of consensus affects our understanding of the impact, management and outcomes of ACO. To highlight the challenges faced as it pertains to ACO, its prevalence has been reported to vary from 15–45% depending on the definition used.Citation168,169 Although both a recent systematic review and a meta-analysis report higher health care utilization and worse outcomes for ACO patients when compared to asthma or COPD alone, both articles highlight that without a consensus definition or diagnostic criteria, there are significant limitations to the reliability of the data.Citation170,171
Secondly, there are no consistent recommendations regarding the evaluation of patients with suspected ACO. There remains no consistent agreement among experts as to which historical factors are required (e.g. patient-reported vs physician diagnosis of previous asthma); physiologic criteria on spirometry (e.g. the degree of reversibility, i.e., 12% and 200 ml or 15% and 400 ml, or frequency of demonstrating reversibility); the role of biomarkers (blood and/or sputum eosinophil levels, IgE, FeNO and allergy skin tests are all debated without consensus on cut-off levels or their significance if present).Citation172–174
Lastly, there are no randomized controlled pharmacotherapy studies in this patient population. The majority of asthma RCTs exclude smokers and the majority of COPD trials exclude patients with a history of asthma or who demonstrate significant bronchial reversibility. Sin et alCitation166 reviewed the inclusion and exclusion criteria of recent large phase 3 COPD and asthma clinical trials and found that the likely ACO population was systematically excluded from all of the trials. Thus, the majority of the pharmacotherapy recommendations for patients with ACO are consensus based.Citation166,167,175 The CTS COPD Clinical Assembly highlights the urgent need for well-designed RCTs to support clinical decision making for these patients.
As a result of the limitations in the literature regarding the definition, assessment, diagnosis and treatment of patients with ACO, the authors of this position statement sought this opportunity to provide readers of this statement with a Canadian perspective on the evaluation, diagnosis and treatment of patients with ACO. The position statement process included two e-surveys with features of a Delphi technique, and after each round, results were discussed and finalized within the ACO author group. The key messages were approved by all authors of this position statement.
In order to achieve a broader Canadian perspective, a decision was made by the authors to include CTS respirologist members from across Canada (both academic and community) to participate in this two-part survey, to gain insights on ACO. In total, 93 respirologists from across Canada were invited to participate in the survey. In the first round, we received 34 responses, and we were able to gain consensus (>80% agreement) on the components of the assessment and treatment of patients with ACO. However, there was disagreement on the specific diagnostic criteria and definition of reversibility on spirometry that would be consistent with ACO. Thus, a second survey with features of a Delphi technique was conducted, focusing on these two topics. We received 23 responses and an agreement was achieved on a revised diagnostic criteria but a consensus was not achieved on the definition of reversibility. The full summary of the questions and results of the full survey is available separately online at www.cts-sct.ca/guidelines.
Based on information from this survey and further input by the authors of the position statement, we submit the following as the Canadian perspective and recommendations on ACO. The proposed definition of ACO is the following: “ACO is characterized by post bronchodilator airflow limitation that is not fully reversible, in symptomatic patients with risk factors for COPD and who have clinical features of both asthma and COPD.” The proposed assessment of patients suspected of having ACO and the diagnostic criteria for ACO are presented in . There was strong agreement amongst all participants that there is no high quality RCTs in this patient population and no evidence-based recommendations can be formulated. A majority of respondents (more than 80%) indicated that they would initiate maintenance therapy for patients with ACO with a low/moderate dose ICS/LABA (i.e. maximum fluticasone propionate 500 mcg/day or equivalent). If a patient reaches maximal inhaled therapy (high dose ICS/LABA and LAMA), and after insuring that related factors such as patient adherence and inhaler technique were addressed, clinicians might consider the addition of biologics that are currently indicated for asthma, if specific indication criteria are met (e.g. significant blood eosinophils). This would particularly be a consideration in patients experiencing recurrent exacerbations and/or requiring repeated use of systemic corticosteroids. Although studies examining the use of these biologics in COPD are ongoing, there was strong agreement that the use of these medications would be considered if the patient met the criteria from the individual product monographs. However, the group agreed that such patients would require referral to a respirologist. Furthermore, it is also important to evaluate if the patient would be a candidate for a third line pharmacotherapy such as a prescription of a PDE4 antagonist (roflumilast) and/or macrolide prophylaxis.
Table 3. Proposed assessment and diagnostic criteria of patients suspected of having ACO.
Despite our endeavors to add further perspective to the questions that surround ACO, we recognize that limitations and gaps from the definition to treatment continue to exist. We further recognize that the concepts put forward in this position paper on ACO require further validation and may change depending on research and further understanding of this disease entity. We make a strong call to action to all partners in the respiratory field to engage in collaborative efforts to further our knowledge and understanding of ACO in order to provide optimal care to our patients.
Box 3. Asthma/COPD Overlap (ACO)
PICO 3: How does a clinician approach the treatment of patients who have COPD and features of Asthma (ACO)?
Key messages:
| 1) | ACO represents a significant challenge both in terms of diagnosis and treatment. For ACO, scientific consensus regarding diagnostic criteria and management is currently lacking. | ||||||||||||||||||||||
| 2) | Based on a survey among Canadian respirologists and the authors, we propose 3 diagnostic criteria that are required to support ACO (this will require future validation and may change, depending on research and progress in this area):
| ||||||||||||||||||||||
| 3) | Treatment for COPD patients who have ACO: | ||||||||||||||||||||||
| 3.1) | Initiate maintenance therapy with a ICS/LABA (low/moderate dose), and consider step up treatment to high dose ICS/LABA (high dose), addition of a LAMA, or both based on symptoms and exacerbation frequency; | ||||||||||||||||||||||
| 3.2) | Refer patient to a respirologist after insuring that related factors such as patient's compliance and inhaler technique were addressed; consider addition of biologics that are currently indicated for asthma when indication criteria are met (particularly in patients experiencing recurrent exacerbations and requiring repeated or long-term use of systemic corticosteroids). | ||||||||||||||||||||||
The summary of the recommendations on the pharmacotherapy treatment of ACO and a step-by-step approach to guide practical choices is shown in .
Discussion
This position statement is an evolution towards personalized treatment, compared to the last published CTS COPD guideline in 2007Citation1 and the 2008 update – highlights for primary care.Citation2 Although the position statement emphasizes the importance of patient characteristics in treatment choice, most of the evidence coming from large clinical trials investigating COPD pharmacological treatments have not clearly identified predictors of response to treatment or patients at increased risk of side-effects.
This position statement promotes approaches that are intended to reduce both symptom burden and the risk of future exacerbations. This is an important step forward acknowledging the importance of making decisions on treatment options based not only on the degree of airflow obstruction and/or the FEV1, but also on the clinical assessment of symptom burden (MRC or CAT) and risk of exacerbations. Other groups have proposed conceptually similar approaches,Citation176,177 including GOLD in 2013.Citation178 GOLD proposed treatment objectives to be matched not only on the degree of airflow obstruction (FEV1) but to be reorganized into a four-quadrant assessment system (GOLD ABCD) characterizing patients on symptom burden and risk of exacerbations. In 2017, GOLDCitation3 adopted an approach even more conceptually similar to the CTS COPD 2007 guideline,Citation1 matching treatment decisions exclusively on symptom burden and risk of COPD exacerbations and not taking into account airflow obstruction. GOLD 2017 maintains spirometry as a requirement for the diagnosis of COPD and for classifying the degree of airflow obstruction (GOLD1–4).
This position statement identifies new data and proposes new evidence-based recommendations on pharmacotherapy (PICO 1 and 2) using the CHEST grading system.Citation24 However, this position statement is different from a guideline. Where there was a lack of data and/or insufficient evidence, but a recommendation was still warranted for guiding clinical practice, a suggestion was developed and designated as “consensus-based” instead of with a conventional “grade.” Furthermore, for each PICO question, key messages are presented to better assist physician practice in managing COPD patients with different phenotypes. Some treatment propositions from this new CTS position statement, in particular the “step up” and “step down” treatment approaches could be criticized for not being evidence-based, as no therapeutic trial strictly assessed such therapeutic approaches. The notion to add or to stop a drug according to the evaluation of the prescribing physician has always been around, in any medical field, and it is considered as a good clinical practice. Treatment “step up” in COPD comes from the evidence that inhaled combined therapy is superior to monotherapy. Based on more limited data, the evidence of treatment “step up” to triple therapy (the combination of LAMA, ICS and LABA) is also suggested. The data are even more scattered to support the notion of treatment “step down,” except for some ICS withdrawal trials in COPD.Citation128 Because the superiority of inhaled triple or dual therapy may not be achieved in every patient, the notion of treatment “step down” may be a consideration in some patients. However, this is a challenge to assess in an individual patient since medication may have limited benefit in some patients and the disease may continue to progress in others. Patients in whom to consider treatment “step down” would be those not demonstrating expected benefits or having side effects exceeding benefits. In any circumstance, when a physician decides using a treatment “step down” for a given patient, this approach should be undertaken under close medical supervision.
Any decision physicians are taking should be in consideration of the known risk – benefit rather than an approach solely based on effectiveness. For example, for COPD patients not having concomitant asthma (ACO), we should favor the combination of inhaled long-acting bronchodilators instead of ICS and LABA. There are growing concerns that ICS are associated with an increased risk of pneumonia and systemic side effects, especially when high doses are usedCitation154,179 Yet, these risks should be balanced with an apparent reduction of a composite outcome of death and admissions to hospital for COPD, as described in patients who received LABA and inhaled corticosteroids compared with those given LABA alone.Citation180–182 More studies are needed to support a risk-benefit approach based on the specific therapy and patient phenotypes.
More recently, there have been studies pointing to blood biomarkers that might be clinically useful to individualize the treatment regimen. Biomarker-directed pharmacotherapy approaches are starting to gain interest in practice and research. For example, it has been suggested that blood eosinophils could be useful clinically to predict COPD phenotype more likely to respond to ICS.Citation155–159 Another example is the bacterial colonization in some COPD patients that could contribute to the biological mechanisms of recurrent acute exacerbations. Under these circumstances, macrolides may reduce the risk for AECOPD although patients should be selected carefully considering the risk of bacterial resistance and side effects.Citation183–185 Whether this benefit is due to the antibacterial effect or more from direct anti- inflammatory effects is uncertain, but biomarkers of bacterial colonization in COPD could be valuable to target azithromycin or other emerging antibacterial approaches. However, we need studies to support or challenge these new pharmacotherapy approaches and to weigh the benefits and risks. There is currently a paucity of data and/or well-designed trials to inform evidence based recommendations.
This position statement also focuses on the phenotype ACO (PICO 3), a subgroup of patients which has been given much attention. Several national and international societies have suggested diagnostic criteria for the diagnosis of ACO.Citation119,176,182,186 Recently, the joint consensus GOLD-GINA proposed some characteristics of asthma and COPD to help to identify ACO individuals,Citation119 although the same weight was given for each particular characteristic and no further specifications were provided to reach the diagnosis. The paucity of original studies on ACO versus patients with only COPD made any evidence-based recommendations impossible. All the drug trials have excluded patients with features of asthma in COPD studies and COPD patients in studies on asthma. For this reason, the position taken in this position statement on ACO comes from a pan Canadian survey with features of Delphi technique and, at best, reflects the practice of Canadian community and academic respirologists on the assessment, diagnosis and pharmacotherapy of ACO patients.
Knowledge transfer and tools for practice
| · | The present document is available for download at www.cts-sct.ca/guidelines and in the new CTS Journal, Respiratory, Critical Care, and Sleep Medicine www.tandfonline.com | ||||
| · | A slide deck for teaching and self-learning as well as a handout for health care professionals and students is available at www.cts-sct.ca/guidelines | ||||
| · | The CTS COPD Clinical Assembly welcomes the opportunity to partner with other organizations and stakeholders in the development of educational tools and resources that support the implementation of the key messages described herein, with various targeted groups. | ||||
Future research directions
In order to generate evidence supporting, complementing or challenging current treatment recommendations, future studies are required in which patients are assigned specific pharmacotherapy based on symptom burden and exacerbation risk. A priori sub groups of patients defined by specific features rather than the current practice of a posteriori extensive subgroup analyses. Important questions remain such as to in whom and when to use combination therapies – inhaled LABA plus LAMA, ICS plus LABA, and LAMA plus ICS plus LABA triple therapy. It remains uncertain whether inhaled therapy should be initiated early with the hope of preserving lung function or only in the presence of COPD-related symptoms. Future studies should also encourage pragmatic trial designs that strike a good balance between internal and external validity, including analyses which highlight both numbers needed to treat and to harm among populations that are representative of COPD patients not typically included RCTs.
Personalized medicine becomes increasingly possible as clinical research becomes more specific, including greater focus on or defining better subsets of patients that are characterized by specific biomarkers and disease severity. Existing longitudinal COPD cohorts in which detailed phenotype data are available through biologic specimen and a data repository, should be supported as these cohorts offer opportunities to evaluate new scientific hypotheses that translate bench research to disease prevention and treatment. They also provide insight into the complex interaction of environment, behaviors, and genetics on chronic obstructive pulmonary diseases. This is a very promising way for the clinical and research communities to improve the evaluation of personal risk, identify mechanisms of disease and direct potential targets for medical interventions.
Disclosures
The authors declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted at www.cts-sct.ca/guidelines.
Acknowledgments
The authors would like to thank Anne Van Dam from the CTS, María Fernanda Sedeño from RESPIPLUS, and Louise Auclair from the Respiratory Epidemiology and Clinical Research Unit (RECRU), Research Institute of the McGill University Health Centre for their tremendous support. We recognize and thank the Executive Committee of the Canadian Respiratory Guidelines Committee for their guidance throughout the process: Louis-Philippe Boulet, Samir Gupta, and Christopher Licskai. We would also like to acknowledge with deep appreciation our Expert Peer Reviewers who made valuable contributions to the manuscript: Dr. Marc Miravitlles, Pneumology Department, University Hospital Vall d'Hebron, Ciber de Enfermedades Respiratorias (CIBERES), Barcelona, Spain; Dr. Miriam Barrecheguren Fernandez, Pneumology Department, University Hospital Vall d'Hebron, Ciber de Enfermedades Respiratorias (CIBERES), Barcelona, Spain; Dr. George A. Fox, Professor of Medicine (Respirology), Memorial University, St. John's, Newfounland & Labrador, Canada; Margot F. Underwood, Assistant Professor, School of Nursing and Midwifery, Mount Royal University, Calgary, Alberta; and Trent Litzenberger, & Jan Neumann, Certified Respiratory Educators, Lung Association, Saskatchewan, Saskatoon, Saskatchewan. Finally, we would like to express our warmest thanks to Mr. William Nash for his feedback on the position statement objectives and key messages. His insight as a person living with COPD was extremely helpful.
Funding
The CTS COPD Clinical Assembly is accountable to the CTS Respiratory Guidelines Committee and the CTS Board of Directors. The CTS COPD Clinical Assembly is functionally and editorially independent from any funding sources of the CTS and does not receive any direct funding from external sources. The CTS receives unrestricted grants which are combined into a central operating account to facilitate the knowledge translation activities of the CTS Clinical Assemblies. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the recommendations and key messages presented in this document.
Notes
1 Low to moderate dose ICS <500 ugs fluticasone propionate or <1000 ugs budesonide; high dose ICS >=500 ugs fluticasone propionate or >=1000 ugs budesonide.
*Clinical remark: Theophylline has a relatively narrow therapeutic window, and the lowest effective dose should be used in prescribing theophylline to avoid adverse effects. Theophylline use requires close vigilance to avoid serious drug interactions which could lead to significant changes in serum theophylline levels and serious toxicity.
References
- O'Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J. 2007;14(Suppl B):5B–32B. doi:10.1155/2007/830570. PMID:17885691.
- O'Donnell DE, Hernandez P, Kaplan A, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2008 update – highlights for primary care. Can Respir J. 2008;15(Suppl A):1 A–8A.
- From the Global Strategy for the Diagnosis MaPoC, Global Initiative for Chronic Obsctructive Lung Disease (GOLD) 2017. http://goldcopd.org.
- Collaborators GCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma,1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691. doi:10.1016/S2213-2600(17)30293-X. PMID:28822787.
- Organization WH. Noncommunicable diseases progress monitor, 2015, 2015
- Bryan S NT. Deaths from Chronic Obstructive Pulmonary Disease in Canada, 1950 to 2011: Statistics Canada. CIHI (Canadain Institute for Health Information); 2015.
- Chronic obstructive pulmonary disease in Canadians, 2009 to 2011: Statistic Canada, 2013; http://www.statcan.gc.ca/pub/82-625-x/2012001/article/2011709-eng.htm
- Tan WC, Bourbeau J, FitzGerald JM, et al. Can age and sex explain the variation in COPD rates across large urban cities? A population study in Canada. Int J Tuberc Lung Dis. 2011;15:1691–1698. doi:10.5588/ijtld.11.0211. PMID:22118181.
- Information CIfH. Health Indicators. Ottawa: CIHI; 2008, 2008
- Hermus G SC, Goldfarb D, Theriault L, Bounajm F. Cost Risk Analysis for Chronic Lung Disease in Canada The Conference Board of Canada, 2012
- Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182:673–678. doi:10.1503/cmaj.091784. PMID:20371646.
- Labonte LE, Tan WC, Li PZ, et al. Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use. Data from the CanCOLD Study. Am J Respir Crit Care Med. 2016;194:285–298. doi:10.1164/rccm.201509-1795OC. PMID:26836958.
- Hangaard S, Helle T, Nielsen C, et al. Causes of misdiagnosis of chronic obstructive pulmonary disease: A systematic scoping review. Respir Med. 2017;129:63–84. doi:10.1016/j.rmed.2017.05.015. PMID:28732838.
- Marciniuk DD, Goodridge D, Hernandez P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Canadian Respiratory J. 2011;18:69–78. doi:10.1155/2011/745047.
- Sanchis J, Gich I, Pedersen S, et al. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest. 2016;150:394–406. doi:10.1016/j.chest.2016.03.041. PMID:27060726.
- Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: A report from the Canadian Thoracic Society COPD Clinical Assembly. Can Respir J. 2015;22:147–152. doi:10.1155/2015/369851. PMID:25848802.
- Hernandez P, Balter M, Bourbeau J, et al. Living with chronic obstructive pulmonary disease: a survey of patients' knowledge and attitudes. Respir Med. 2009;103:1004–1012. doi:10.1016/j.rmed.2009.01.018. PMID:19269150.
- Grace SL, Chessex C, Arthur H, et al. Systematizing inpatient referral to cardiac rehabilitation 2010: Canadian Association of Cardiac Rehabilitation and Canadian Cardiovascular Society joint position paper endorsed by the Cardiac Care Network of Ontario. Can J Cardiol. 2011;27:192–199. doi:10.1016/j.cjca.2010.12.007. PMID:21459268.
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894–942. doi:10.1378/chest.14-1676. PMID:25321320.
- Marciniuk DD, Brooks D, Butcher S, et al. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease–practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J. 2010;17:159–168. doi:10.1155/2010/425975. PMID:20808973.
- Marciniuk DD, Hernandez P, Balter M, et al. Alpha-1 antitrypsin deficiency targeted testing and augmentation therapy: a Canadian Thoracic Society clinical practice guideline.[Erratum appears in Can Respir J. 2012 Jul-Aug;19(4):272]. Canadian Respiratory Journal. 2012;19:109–116. doi:10.1155/2012/920918. PMID:22536580.
- Gupta S, Bhattacharyya OK, Brouwers MC, et al. Canadian Thoracic Society: Presenting a new process for clinical practice guideline production. Can Respir J. 2009;16:e62–68. doi:10.1155/2009/397818. PMID:20011719.
- Hsu CC SB. The Delphi technique: making sense of consensus. Practical Assessment Research & Evaluation. 2007;12:1–8
- Lewis SZ, Diekemper R, Ornelas J, et al. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146:182–192. doi:10.1378/chest.14-0824. PMID:25010961.
- O'Donnell DE, Laveneziana P, Webb K, et al. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med. 2014;35:51–69. doi:10.1016/j.ccm.2013.09.008. PMID:24507837.
- Gupta N, Pinto LM, Morogan A, et al. The COPD assessment test: a systematic review. Eur Respir J. 2014;44:873–884. doi:10.1183/09031936.00025214. PMID:24993906.
- Bedard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Resp J. 2012;39:265–271. doi:10.1183/09031936.00059511.
- Beeh KM, Singh D, Di Scala L, et al. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Inter J Copd. 2012;7:503–513. doi:10.2147/COPD.S32451.
- Beeh KM, Wagner F, Khindri S, et al. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. Copd J Chro Obstr Pulm Dise. 2011;8:340–345. doi:10.3109/15412555.2011.594464.
- Beeh KM, Watz H, Puente-Maestu L, et al. Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: a randomized, placebo-controlled, crossover trial. BMC Pulm Medi. 2014;14:209. doi:10.1186/1471-2466-14-209. PMID:25539654.
- Brouillard C, Pepin V, Milot J, et al. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Resp J. 2008;31:579–584. doi:10.1183/09031936.00119007.
- Cooper CB, Celli BR, Jardim JR, et al. Treadmill endurance during 2-year treatment with tiotropium in patients with COPD: a randomized trial. Chest. 2013;144:490–497. doi:10.1378/chest.12-2613. PMID:23558890.
- Gotfried MH, Kerwin EM, Lawrence D, et al. Efficacy of indacaterol 75 mug once-daily on dyspnea and health status: results of two double-blind, placebo-controlled 12-week studies. C J Chro Obstr Pulm Dise. 2012;9:629–636. doi:10.3109/15412555.2012.729623.
- Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized placebo-controlled trials. BMC Pulm Medi. 2013;13:26. doi:10.1186/1471-2466-13-26. PMID:23617268.
- Jiang FM, Liang ZA, Zheng QL, et al. Safety and efficacy of 12-week or longer indacaterol treatment in moderate-to-severe COPD patients: a systematic review. Lung. 2013;191:135–146. doi:10.1007/s00408-012-9444-2. PMID:23306410.
- Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Resp J. 2012;40:830–836. doi:10.1183/09031936.00225511.
- Kaplan A. Effect of tiotropium on quality of life in COPD: A systematic review. Prim Care Resp J. 2010;19:315–325. doi:10.4104/pcrj.2010.00067.
- Kerwin EM, D'Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). Copd J Chro Obstr Pulm Dise. 2012;9:90–101. doi:10.3109/15412555.2012.661492.
- Kerwin EM, Gotfried MH, Lawrence D, et al. Efficacy and tolerability of indacaterol 75 mug once daily in patients aged >40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studies. Clin Therap. 2011;33:1974–1984. doi:10.1016/j.clinthera.2011.11.009. PMID:22177371.
- Kinoshita M, Lee SH, Hang LW, et al. Efficacy and safety of indacaterol 150 and 300 micro g in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12-week, placebo-controlled study. Respirology. 2012;17:379–389. doi:10.1111/j.1440-1843.2011.02107.x. PMID:22122202.
- Maltais F, Celli B, Casaburi R, et al. Aclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPD. Resp Medi. 2011;105:580–587. doi:10.1016/j.rmed.2010.11.019. PMID:21183326.
- Mroz RM, Minarowski L, Chyczewska E. Indacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patients. Adva Experi Medi Biol. 2013;756:23–28. doi:10.1007/978-94-007-4549-0_4.
- O'Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Medi. 2011;105:1030–1036. doi:10.1016/j.rmed.2011.03.014. PMID:21498063.
- Rennard SI, Scanlon PD, Ferguson GT, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clin Drug Invest. 2013;33:893–904. doi:10.1007/s40261-013-0138-1. PMID:24085591.
- Santus P, Radovanovic D, Di Marco F, et al. Faster reduction in hyperinflation and improvement in lung ventilation inhomogeneity promoted by aclidinium compared to glycopyrronium in severe stable COPD patients. A randomized crossover study. Pulm Pharm Therap. 2015;35:42–49. doi:10.1016/j.pupt.2015.11.001.
- Satake M, Takahashi H, Sugawara K, et al. Inhibitory effect of procaterol on exercise dynamic lung hyperinflation during the 6-min walk test in stable patients with chronic obstructive pulmonary disease. Arzneim-Forsch. 2011;61:8–13. doi:10.1055/s-0031-1296162.
- Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study.[Erratum appears in Eur Respir J. 2014 Aug;44(2):555 Note: Dosage error in published abstract; MEDLINE/PubMed abstract corrected; Dosage error in article text]. Eur Respir J.2014;43:72–81. doi:10.1183/09031936.00033213.
- Yao W, Wang C, Zhong N, et al. Effect of once-daily indacaterol in a predominantly Chinese population with chronic obstructive pulmonary disease: a 26-week Asia-Pacific study. Respirology. 2014;19:231–238. doi:10.1111/resp.12211. PMID:24383720.
- Bogdan MA, Aizawa H, Fukuchi Y, et al. Efficacy and safety of inhaled formoterol 4.5 and 9 mug twice daily in Japanese and European COPD patients: phase III study results. BMC Pulm Medi. 2011;11:51. doi:10.1186/1471-2466-11-51. PMID:22085439.
- Braido F, Baiardini I, Cazzola M, et al. Long-acting bronchodilators improve health related quality of life in patients with COPD. Respir Med. 2013;107:1465–1480. doi:10.1016/j.rmed.2013.08.007. PMID:24001507.
- Cheyne L, Irvin-Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD009552 PMID:26391969.
- Geake JB, Dabscheck EJ, Wood-Baker R, et al. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta(2)-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;1:CD010139 PMID:25575340.
- Gross NJ, Nelson HS, Lapidus RJ, et al. Efficacy and safety of formoterol fumarate delivered by nebulization to COPD patients. Respir Med. 2008;102:189–197. doi:10.1016/j.rmed.2007.10.007. PMID:18363201.
- Jones PW, Mahler DA, Gale R, et al. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respir Med. 2011;105:892–899. doi:10.1016/j.rmed.2011.02.013. PMID:21397482.
- Karner C, Cates CJ. Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012:CD008989 PMID:22513969.
- Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013:CD010177 PMID:24127118.
- Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Intern J Copd. 2014;9:697–714. doi:10.2147/COPD.S62502.
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37:273–279. doi:10.1183/09031936.00045810. PMID:20693243.
- Ni H, Soe Z, Moe S. Aclidinium bromide for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014:CD010509 PMID:25234126.
- Park J, Lee JS, Rhee C, et al. Effect of Indacaterol on Cough and Phlegm in Chronic Obstructive Pulmonary Disease Patients: A Meta-Analysis of Five Randomized Controlled Trials. J Korean Med Sci. 2015;30:1453–1458. doi:10.3346/jkms.2015.30.10.1453. PMID:26425042.
- Beeh KM, Korn S, Beier J, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respir Med. 2014;108:584–592. doi:10.1016/j.rmed.2014.01.006. PMID:24534204.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104:1288–1296. doi:10.1016/j.rmed.2010.05.017. PMID:20580216.
- Calzetta L, Ciaprini C, Puxeddu E, et al. Olodaterol + tiotropium bromide for the treatment of COPD. Expert Rev Respir Med. 2016;6:1–8 PMID:26894830.
- Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145:981–991. doi:10.1378/chest.13-1579. PMID:24385182.
- Chen X, May B, Di YM, et al. Oral Chinese herbal medicine combined with pharmacotherapy for stable COPD: a systematic review of effect on BODE index and six minute walk test. PLoS ONE [Electronic Resource]. 2014;9:e91830. doi:10.1371/journal.pone.0091830.
- Jayaram L, Wong C, McAuley S, et al. Combined therapy with tiotropium and formoterol in chronic obstructive pulmonary disease: effect on the 6-minute walk test. Copd J Chro Obstr Pulm Dise. 2013;10:466–472. doi:10.3109/15412555.2013.771162.
- Mahler DA, Decramer M, D'Urzo A, et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014;43:1599–1609. doi:10.1183/09031936.00124013. PMID:24176997.
- Maltais F, Singh S, Donald AC, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. [Erratum appears in Ther Adv Respir Dis. 2016 Jun;10(3):289;PMID:27255756]. Therap Adva Respir Dise. 2014;8:169–181. doi:10.1177/1753465814559209. PMID:25452426.
- Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting beta -agonists for stable COPD: a systematic review. Chest. 2012;142:1104–1110. doi:10.1378/chest.11-2252. PMID:22383666.
- Vincken W, Aumann J, Chen H, et al. Efficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study. Inter J Copd. 2014;9:215–228. doi:10.2147/COPD.S51592.
- Wang L, Zhai CJ, Liu Y, et al. Umeclidinium plus vilanterol versus Placebo, Umeclidinium, or Vilanterol Monotherapies for chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Clin Drug Investi. 2016;36:865–875. doi:10.1007/s40261-016-0449-0. PMID:27539612.
- Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4).[Erratum appears in Eur Respir J. 2015 Jun;45(6):1763;PMID: 26028626]. Eur Respir J 2015;45:969–979. doi:10.1183/09031936.00136014. PMID:25573406.
- Calzetta L, Rogliani P, Matera MG, et al. A Systematic Review With Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest 2016;149:1181–1196. doi:10.1016/j.chest.2016.02.646. PMID:26923629.
- Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015:CD008989 PMID:26490945.
- Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med. 2014;108:1752–1760. doi:10.1016/j.rmed.2014.10.002. PMID:25458157.
- ZuWallack R, Allen L, Hernandez G, et al. Efficacy and safety of combining olodaterol Respimat() and tiotropium HandiHaler() in patients with COPD: results of two randomized, double-blind, active-controlled studies. Inter J Copd. 2014;9:1133–1144. doi:10.2147/COPD.S72482.
- Watz H, Krippner F, Kirsten A, et al. Indacaterol improves lung hyperinflation and physical activity in patients with moderate chronic obstructive pulmonary disease–a randomized, multicenter, double-blind, placebo-controlled study. BMC Pulm Medi. 2014;14:158. doi:10.1186/1471-2466-14-158. PMID:25280934.
- Watz H, Mailander C, Baier M, et al. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (The MOVE Study). BMC Pulm Medi. 2016;16:95. doi:10.1186/s12890-016-0256-7. PMID:27301417.
- Watz HTT, Beeh KM, Garcia-Aymerich J, Paggiaro P, Molins E, Notari M, Zapata A, Jarreta D, Garcia Gil E. ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2545–2558.
- Troosters T, Sciurba FC, Decramer M, et al. Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial. NPJ Prim Care Respir Med. 2014;24:14003. doi:10.1038/npjpcrm.2014.3. PMID:24841833.
- Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066 PMID:28185242.
- Hoshino M, Ohtawa J, Akitsu K. Comparison of airway dimensions with once daily tiotropium plus indacaterol versus twice daily Advair() in chronic obstructive pulmonary disease. Pulm Pharmacol Therap. 2015;30:128–133. doi:10.1016/j.pupt.2014.08.002.
- Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–555. doi:10.7326/0003-4819-146-8-200704170-00152. PMID:17310045.
- Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011:CD008532 PMID:21412920.
- Kawayama T, Hoshino T, Ichiki M, et al. Effect of add-on therapy of tiotropium in COPD treated with theophylline. Inter J Copd. 2008;3:137–147. doi:10.2147/COPD.S2103.
- Voduc N, Alvarez GG, Amjadi K, et al. Effect of theophylline on exercise capacity in COPD patients treated with combination long-acting bronchodilator therapy: A pilot study. Inter J Copd. 2012;7:245–252. doi:10.2147/COPD.S29990.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695–703. doi:10.1016/S0140-6736(09)61252-6. PMID:19716961.
- O'Donnell DE, Bredenbroker D, Brose M, et al. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J. 2012;39:1104–1112. doi:10.1183/09031936.00096511. PMID:21965226.
- Pan L, Guo YZ, Zhang B, et al. Does roflumilast improve dyspnea in patients with chronic obstructive pulmonary disease? A meta-analysis. J Thor Dise. 2013;5:422–429 PMID:23991297.
- Johnson K, McEvoy CE, Naqvi S, et al. High-dose oral N-acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: a randomized, placebo-controlled trial. Inter J Copd. 2016;11:799–807.
- Lee TM, Chen CC, Shen HN, et al. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci. 2009;116:497–505. doi:10.1042/CS20080241. PMID:18831711.
- Maneechotesuwan K, Wongkajornsilp A, Adcock IM, et al. Simvastatin Suppresses Airway IL-17 and Upregulates IL-10 in Patients With Stable COPD. Chest. 2015;148:1164–1176. doi:10.1378/chest.14-3138. PMID:26043025.
- Mroz RM, Lisowski P, Tycinska A, et al. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. A controlled pilot study. J Physiol Pharmacol. 2015;66:111–128
- Daga MK, Khan NA, Malhotra V, et al. Study of body composition, lung function, and quality of life following use of anabolic steroids in patients with chronic obstructive pulmonary disease. Nutr Clin Pract. 2014;29:238–245. doi:10.1177/0884533614522832. PMID:24552826.
- Pan L, Wang M, Xie X, et al. Effects of anabolic steroids on chronic obstructive pulmonary disease: a meta-analysis of randomised controlled trials. PLoS ONE [Electronic Resource]. 2014;9:e84855. doi:10.1371/journal.pone.0084855.
- An X, Zhang AL, May BH, et al. Oral Chinese herbal medicine for improvement of quality of life in patients with stable chronic obstructive pulmonary disease: a systematic review. J Altern Complem Medi. 2012;18:731–743. doi:10.1089/acm.2011.0389.
- An X, Zhang AL, Yang AW, et al. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respir Med. 2011;105:165–176. doi:10.1016/j.rmed.2010.11.007. PMID:21146973.
- Liu J, Gao F, Li Z. Effect of yiqibushenhuoxue decoction on chronic obstructive pulmonary disease measured by St. George's respiratory disease questionnaire scores and forced expiratory volume. J Tradi Chin Medi. 2014;34:445–449. doi:10.1016/S0254-6272(15)30044-3.
- Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J. 2013;42:982–992. doi:10.1183/09031936.00176312. PMID:23429918.
- Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. The Lanc Resp Medi. 2014;2:293–300. doi:10.1016/S2213-2600(14)70013-X. PMID:24717626.
- Holverda S, Rietema H, Bogaard HJ, et al. Acute effects of sildenafil on exercise pulmonary hemodynamics and capacity in patients with COPD. Pulmo Pharmacol Therape. 2008;21:558–564. doi:10.1016/j.pupt.2008.01.012.
- Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Ind J Chest Dise Alli Sci. 2011;53:81–85
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi:10.1056/NEJMoa0909883. PMID:20843247.
- D'Urzo A, Kerwin E, Overend T, et al. Once daily glycopyrronium for the treatment of COPD: pooled analysis of the GLOW1 and GLOW2 studies. Curr Med Res Opin. 2014;30:493–508. doi:10.1185/03007995.2013.858618. PMID:24156566.
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012:CD009285 PMID:22786525.
- Lee SH, Lee J, Yoo KH, et al. Efficacy and safety of aclidinium bromide in patients with COPD: A phase 3 randomized clinical trial in a Korean population. Respirology. 2015;20:1222–1228. doi:10.1111/resp.12641. PMID:26370136.
- Matera MG, Rogliani P, Cazzola M. Indacaterol for the treatment of chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2015;16:107–115. doi:10.1517/14656566.2015.983076. PMID:25418284.
- Oba Y, Lone NA. Comparative efficacy of long-acting muscarinic antagonists in preventing COPD exacerbations: a network meta-analysis and meta-regression. Ther Adv Respir Dis. 2015;9:3–15. doi:10.1177/1753465814565624. PMID:25586493.
- Pleasants RA, Wang T, Gao J, et al. Inhaled Umeclidinium in COPD Patients: A Review and Meta-Analysis. Drugs. 2016;76:343–361. doi:10.1007/s40265-015-0532-5. PMID:26755180.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. 2017;49.
- Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res. 2015;16:92. doi:10.1186/s12931-015-0250-2. PMID:26233481.
- Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2:472–486. doi:10.1016/S2213-2600(14)70065-7. PMID:24835833.
- Donohue JF, Singh D, Munzu C, et al. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: Results from two randomised controlled trials. Respir Med. 2016;112:65–74. doi:10.1016/j.rmed.2016.01.001. PMID:26797016.
- Horita N, Kaneko T. Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan. Int J Chron Obstruct Pulmon Dis. 2015;10:813–822. doi:10.2147/COPD.S56067. PMID:25960646.
- Kalberg C, O'Dell D, Galkin D, et al. Dual Bronchodilator Therapy with Umeclidinium/Vilanterol Versus Tiotropium plus Indacaterol in Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Drugs R D. 2016;16:217–227. doi:10.1007/s40268-016-0131-2. PMID:27028749.
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71:15–25. doi:10.1136/thoraxjnl-2014-206732. PMID:26490732.
- Schlueter M, Gonzalez-Rojas N, Baldwin M, et al. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting beta2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis. 2016;10:89–104. doi:10.1177/1753465815624612. PMID:26746383.
- Wedzicha JA, Dahl R, Buhl R, et al. Pooled safety analysis of the fixed-dose combination of indacaterol and glycopyrronium (QVA149), its monocomponents, and tiotropium versus placebo in COPD patients. Respir Med. 2014;108:1498–1507. doi:10.1016/j.rmed.2014.07.011. PMID:25135743.
- GINA/GOLD. Diagnosis of diseases of Chronic Airflow Limitation: asthma, COPD and asthma-COPD Overlap Syndrome (ACOS), 2015
- Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:469–479. doi:10.2147/COPD.S48492. PMID:24872685.
- Ohar JA, Crater GD, Emmett A, et al. Fluticasone propionate/salmeterol 250/50 mug versus salmeterol 50 mug after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15:105. doi:10.1186/s12931-014-0105-2. PMID:25248764.
- Singh D, Nicolini G, Bindi E, et al. Extrafine beclomethasone/formoterol compared to fluticasone/salmeterol combination therapy in COPD. BMC Pulm Med. 2014;14:43. doi:10.1186/1471-2466-14-43. PMID:24621109.
- Stynes G, Svedsater H, Wex J, et al. Once-daily fluticasone furoate/vilanterol 100/25 mcg versus twice daily combination therapies in COPD – mixed treatment comparisons of clinical efficacy. Respir Res. 2015;16:25. doi:10.1186/s12931-015-0184-8. PMID:25849223.
- Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of Fluticasone Furoate-Vilanterol for COPD in Clinical Practice. N Engl J Med. 2016;375:1253–1260. doi:10.1056/NEJMoa1608033. PMID:27593504.
- Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108:1153–1162. doi:10.1016/j.rmed.2014.05.013. PMID:24953015.
- Hedeker DG, Waternaux RD. C. Sample size estimation for longitudinal designs with attrition: Comparing time-related contrasts between two groups. J Educ Behav Stat. 1999;24:70–93. doi:10.3102/10769986024001070.
- Horita N, Miyazawa N, Morita S, et al. Long-acting beta-agonists reduce mortality of patients with severe and very severe chronic obstructive pulmonary disease: a propensity score matching study. Respir Res. 2013;14:62. doi:10.1186/1465-9921-14-62. PMID:23725215.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–1294. doi:10.1056/NEJMoa1407154. PMID:25196117.
- Oba Y, Chandran AV, Devasahayam JV. Long-acting Muscarinic Antagonist Versus Inhaled Corticosteroid when Added to Long-acting beta-agonist for COPD: A Meta-analysis. COPD. 2016;13:677–685. doi:10.3109/15412555.2016.1170799. PMID:27148815.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. doi:10.1056/NEJMoa1516385. PMID:27181606.
- Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026 PMID:26082625.
- Kwak MS, Kim E, Jang EJ, et al. The efficacy and safety of triple inhaled treatment in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis using Bayesian methods. Int J Chron Obstruct Pulmon Dis. 2015;10:2365–2376 PMID:26604734.
- Liu Y, Shi H, Sun X, et al. Benefits of adding fluticasone propionate/salmeterol to tiotropium in COPD: a meta-analysis. Eur J Intern Med. 2014;25:491–495. doi:10.1016/j.ejim.2014.04.007. PMID:24816076.
- Rojas-Reyes MX, Garcia Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016:CD008532 PMID:27271056.
- Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–1929. doi:10.1016/S0140-6736(17)30188-5. PMID:28385353.
- Carone M, Donner CF, Jones PW. Health Status measurement: an increasingly important outcome evaluation in COPD patients. Monaldi Arch Chest Dis. 2001;56:297–298 PMID:11770207.
- Hanania NA, Calverley PM, Dransfield MT, et al. Pooled subpopulation analyses of the effects of roflumilast on exacerbations and lung function in COPD. Respir Med. 2014;108:366–375. doi:10.1016/j.rmed.2013.09.018. PMID:24120253.
- Luo J, Wang K, Liu D, et al. Can roflumilast, a phosphodiesterase-4 inhibitor, improve clinical outcomes in patients with moderate-to-severe chronic obstructive pulmonary disease? A meta-analysis. Respir Res. 2016;17:18. doi:10.1186/s12931-016-0330-y. PMID:26887407.
- Luo P, Li S, Chen Y, et al. Efficiency and safety of roflumilast combined with long-acting bronchodilators on moderate-to-severe stable chronic obstructive pulmonary disease patients: a meta-analysis. J Thorac Dis. 2016;8:2638–2645. doi:10.21037/jtd.2016.09.12. PMID:27747018.
- Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385:857–866. doi:10.1016/S0140-6736(14)62410-7. PMID:25684586.
- Yan JH, Gu WJ, Pan L. Efficacy and safety of roflumilast in patients with stable chronic obstructive pulmonary disease: a meta-analysis. Pulm Pharmacol Ther. 2014;27:83–89. doi:10.1016/j.pupt.2013.04.004. PMID:23624309.
- Ayfer Aytemur Z, Baysak A, Ozdemir O, et al. N-acetylcysteine in patients with COPD exacerbations associated with increased sputum. Wien Klin Wochenschr. 2015;127:256–261. doi:10.1007/s00508-014-0692-4. PMID:25595117.
- Cazzola M, Calzetta L, Page C, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015;24:451–461. doi:10.1183/16000617.00002215. PMID:26324807.
- Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015:CD001287 PMID:26222376.
- Shen Y, Cai W, Lei S, et al. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2014;11:351–358 PMID:24378052.
- Tse HN, Raiteri L, Wong KY, et al. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014;146:611–623. doi:10.1378/chest.13-2784. PMID:24833327.
- Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187–194. doi:10.1016/S2213-2600(13)70286-8. PMID:24621680.
- Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189:1503–1508. doi:10.1164/rccm.201402-0207OC. PMID:24779680.
- Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One. 2015;10:e0121257. doi:10.1371/journal.pone.0121257. PMID:25812085.
- Simpson JL, Powell H, Baines KJ, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLoS One. 2014;9:e105609. doi:10.1371/journal.pone.0105609. PMID:25148049.
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361–368. doi:10.1016/S2213-2600(14)70019-0. PMID:24746000.
- Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–223. doi:10.1016/S2213-2600(13)70040-7. PMID:24429127.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi:10.1056/NEJMoa063070. PMID:17314337.
- Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. doi:10.1136/thoraxjnl-2012-202872. PMID:24130228.
- Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–442. doi:10.1016/S2213-2600(15)00106-X. PMID:25878028.
- Pavord ID, Lettis S, Anzueto A, et al. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. The Lancet Respir Medi. 2016;4:731–741. doi:10.1016/S2213-2600(16)30148-5. PMID:27460163.
- Siddiqui SH, Guasconi A, Vestbo J, et al. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192:523–525. doi:10.1164/rccm.201502-0235LE. PMID:26051430.
- Roche N, Chapman KR, Vogelmeier CF, et al. Blood Eosinophils and Response to Maintenance Chronic Obstructive Pulmonary Disease Treatment. Data from the FLAME Trial. Am J Respir Crit Care Med. 2017;195:1189–1197. doi:10.1164/rccm.201701-0193OC.
- Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–398. doi:10.1016/S2213-2600(16)00100-4. PMID:27066739.
- Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood Eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193:965–974. doi:10.1164/rccm.201509-1869OC. PMID:26641631.
- Landis SH, Suruki R, Hilton E, et al. Stability of Blood Eosinophil Count in Patients with COPD in the UK Clinical Practice Research Datalink. COPD. 2017;14:382–388. doi:10.1080/15412555.2017.1313827. PMID:28569614.
- Fletcher CM, Pride NB. Definitions of emphysema, chronic bronchitis, asthma, and airflow obstruction: 25 years on from the Ciba symposium. Thorax. 1984;39:81–85. doi:10.1136/thx.39.2.81. PMID:6701830.
- Kraft M. Asthma and chronic obstructive pulmonary disease exhibit common origins in any country! Am J Respir Crit Care Med. 2006;174:238–240; discussion 243–234. doi:10.1164/rccm.2604007.
- Pride N. Smoking, allergy and airways obstruction: revival of the ‘Dutch hypothesis’. Clin Allergy. 1986;16:3–6. doi:10.1111/j.1365-2222.1986.tb01946.x. PMID:3485478.
- Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48:331–337. doi:10.1016/j.arbr.2012.06.017. PMID:22341911.
- Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48:664–673. doi:10.1183/13993003.00436-2016. PMID:27338195.
- Araujo D, Padrao E, Morais-Almeida M, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome – Literature review and contributions towards a Portuguese consensus. Rev Port Pneumol (2006) 2017;23:90–99 PMID:28089081.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–735. doi:10.1136/thx.2008.108027. PMID:19638566.
- de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8:e62985. doi:10.1371/journal.pone.0062985. PMID:23675448.
- Nielsen M, Barnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome–a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443–1454 PMID:26251584.
- Alshabanat A, Zafari Z, Albanyan O, et al. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS One. 2015;10:e0136065. doi:10.1371/journal.pone.0136065. PMID:26336076.
- Cosio BG, Soriano JB, Lopez-Campos JL, et al. Defining the Asthma-COPD Overlap Syndrome in a COPD Cohort. Chest. 2016;149:45–52. doi:10.1378/chest.15-1055. PMID:26291753.
- Tamada T, Sugiura H, Takahashi T, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176. doi:10.2147/COPD.S88274. PMID:26491283.
- Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax. 2016;71:118–125. doi:10.1136/thoraxjnl-2015-207021. PMID:26585525.
- Hines KL, Peebles RS, Jr. Management of the Asthma-COPD Overlap Syndrome (ACOS): a Review of the Evidence. Curr Allergy Asthma Rep. 2017;17:15. doi:10.1007/s11882-017-0683-4. PMID:28283854.
- Koblizek V, Chlumsky J, Zindr V, et al. Chronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:189–201 PMID:23733084.
- Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch Bronconeumol. 2017;53:324–335. doi:10.1016/j.arbres.2017.03.018. PMID:28477954.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi:10.1164/rccm.201204-0596PP. PMID:22878278.
- Chen D, Restrepo MI, Fine MJ, et al. Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am J Respir Crit Care Med. 2011;184:312–316. doi:10.1164/rccm.201012-2070OC. PMID:21512168.
- Bourbeau J, Aaron SD, Barnes NC, et al. Evaluating the risk of pneumonia with inhaled corticosteroids in COPD: Retrospective database studies have their limitations SA. Respir Med. 2017;123:94–97. doi:10.1016/j.rmed.2016.12.015. PMID:28137503.
- Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am J Respir Crit Care Med. 2015;191:141–148. doi:10.1164/rccm.201409-1654PP. PMID:25409118.
- Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47:625–637. doi:10.1183/13993003.01170-2015. PMID:26797035.
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi:10.1056/NEJMoa1104623. PMID:21864166.
- Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–1147. doi:10.1164/rccm.200801-145OC. PMID:18723437.
- Suresh Babu K, Kastelik J, Morjaria JB. Role of long term antibiotics in chronic respiratory diseases. Respir Med. 2013;107:800–815. doi:10.1016/j.rmed.2013.02.009. PMID:23522403.
- Miravitlles M. What was the impact of the Spanish COPD guidelines (GesEPOC) and how can they be improved? Arch Bronconeumol. 2016;52:1–2 doi:10.1016/j.arbres.2015.04.001. PMID:26026686.

