Abstract
In this guideline update, we highlight important and new findings related to pharmacological therapy of chronic obstructive pulmonary disease (COPD) that should change clinical practice and improve disease management. We present updated evidence, recommendations and expert clinical remarks on maintenance pharmacotherapy in patients with stable COPD. The diagnosis and nonpharmacological therapy of COPD are out-of-scope for this update.
In patients with COPD who have persistent shortness of breath, exercise intolerance and/or poor health status despite using inhaled LAMA or LABA monotherapy, we recommend augmenting treatment to LAMA/LABA dual therapy. In patients with high-risk exacerbations, LAMA/LABA is the preferred choice to ICS/LABA except in patients with previous exacerbations who have higher peripheral eosinophilia. There is no role for ICS monotherapy; when indicated, ICS should only be used in combination with bronchodilators. Treatment “step up” in COPD is proposed as a practical construct supported by evidence that inhaled combined therapy is superior to monotherapy and triple therapy to dual therapy in certain patient populations. Because the superiority of inhaled triple or dual bronchodilator therapy may not be achieved in every patient, “step down” may be considered for some patients (not at high risk for future exacerbations), but should be done with close medical supervision, as the risk of clinical deterioration is real and continues to exist. The decision of changing a therapy should always occur after a complete evaluation of the patient and the potential benefit to a change in therapy; as well as an assessment of any adverse effects of the therapy, and with a review of patient adherence, inhaler technique and patient preferences.
Pharmacological therapy plays a foundational role in therapy, but it should never be the sole treatment in managing COPD patients. Clinicians should always combine and optimize pharmacological and nonpharmacological therapies with the dual goals of reducing symptoms and preventing acute exacerbations of COPD (AECOPD).
RÉSUMÉ
La présente mise à jour des lignes directrices met de l’avant de nouveaux résultats importants sur le traitement pharmacologique de la maladie pulmonaire obstructive chronique (MPOC) qui devraient modifier la pratique clinique et améliorer la prise en charge de la maladie. Nous présentons une mise à jour des données probantes et des recommandations, et des observations cliniques d’experts sur la pharmacothérapie d’entretien pour les patients dont la MPOC est stable. La présente mise à jour ne porte pas sur le diagnostic et le traitement non pharmacologique de la MPOC.
Chez les patients ayant une MPOC et dont l’essoufflement, l’intolérance à l’effort et la détérioration de l’état de santé persistent malgré une monothérapie d’antimuscarinique à longue durée d’action (AMLA) ou de bêta2-agoniste à longue durée d’action (BALA), nous recommandons une progression du traitement vers une bithérapie AMLA/BALA. Chez les patients présentant un risque élevé d’exacerbations, il faut privilégier une bithérapie AMLA/BALA plutôt qu’une association de corticostéroïde en inhalation (CSI)/BALA, sauf chez les patients ayant déjà subi des exacerbations et dont le nombre d’éosinophiles de sang périphérique est élevé. Il n’y a pas lieu de recourir à la monothérapie de CSI; lorsqu’ils sont indiqués, les CSI doivent être utilisés uniquement en association avec des bronchodilatateurs. Pour la mise en place du traitement dans les cas de MPOC, il est proposé d’adopter une approche pratique et fondée sur les données probantes selon laquelle une inhalothérapie combinée est supérieure à la monothérapie et une trithérapie est supérieure à une bithérapie chez certaines populations de patients. Étant donné que la supériorité de la trithérapie ou de la bithérapie à l’aide d’un bronchodilatateur pourrait ne pas être observée chez tous les patients, il faudra envisager une dégression de traitement pour certains patients (qui ne présentent pas de risque élevé d’exacerbations futures), mais sous une étroite supervision médicale, car le risque de détérioration clinique est réel et persiste. La décision de modifier un traitement doit toujours être prise après une évaluation complète du patient et des avantages possibles d’une modification du traitement; ainsi qu’une évaluation de tous les effets indésirables du traitement et une vérification de l’observance du patient, de sa technique d’inhalation et de ses préférences.
La pharmacothérapie joue un rôle fondamental, mais elle ne doit jamais être utilisée comme seul traitement pour la prise en charge des patients ayant une MPOC. Les cliniciens doivent toujours associer et optimizer les traitements pharmacologiques et non pharmacologiques en ayant le double objectif de soulager les symptômes et de prévenir les exacerbations aiguës de la MPOC (EAMPOC).
Introduction
Since the last published Canadian Thoracic Society (CTS) position statement on the pharmacotherapy in patients with chronic obstructive pulmonary disease (COPD) in 2017,Citation1 several important publications have necessitated an update to the current approach. This document is intended to guide best practice in light of recent research.
In clinical practice, an integrated, comprehensive approach to care should include:
a diagnosis of COPD confirmed with spirometry;
clinical evaluation of the patient; and
comprehensive management, which includes nonpharmacological and pharmacological interventions ().
Figure 1. Comprehensive management of COPD.
Integrated approach to care that includes confirming COPD diagnosis with spirometry, evaluation of symptom burden and risk of exacerbations with on-going monitoring, assessment for features of asthma, and comprehensive management, both non-pharmacologic and pharmacologic.
* = Self-Management Education includes appropriate inhaler device technique and review, breathing techniques and review, early recognition of AECOPD, written action plan development and implementation (if appropriate).
mMRC is a modified (0-4 scale) version of the MRC breathlessness scale which was used in previous CTS guidelines. The mMRC aligns with the Global Initiative for Chronic Obstructive Airways Disease (GOLD) 2019 report.
Abbreviations: CAT = COPD assessment test; mMRC = Modified Medical Research Council; prn = as-needed; AECOPD = acute exacerbation of COPD; Inhaled Long-Acting Therapies = long-acting muscarinic antagonist and/or long-acting ẞ2-agonist and/or inhaled corticosteroid; NIV = non-invasive ventilation.
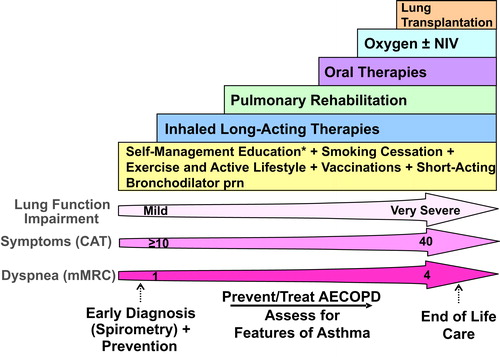
The diagnosis of COPD should be considered in patients at risk of developing this disease. Patients’ smoking history should be the main focus as it remains the most important risk factor. However, clinicians should be aware of an increased risk of COPD in individuals reporting a past medical history of asthma and/or severe childhood respiratory disease. Additionally, patients who have been exposed to passive smoke and/or to indoor biomass fuel are also at increased risk for the development of COPD.Citation2 This includes individuals from developing countries where indoor biomass exposure is the leading cause of COPD. Physicians should also be attentive to patients presenting with “exacerbation-like respiratory events” in the office or emergency setting, which may be an initial presentation of previously undiagnosed COPD. These events are common in undiagnosed COPD (22% in undiagnosed compared to 40% in diagnosed COPD) and have substantial impact on health service utilization, such as emergency department (ED) visits and hospital admissions.Citation3 An exacerbation-like respiratory event can be a trigger (opportunity) for patients to come to the attention of the healthcare system and for clinicians to consider the diagnosis of COPD and/or to optimize disease management.
Recent robust population data have confirmed that many individuals with COPD remain undiagnosed, but symptomatic, with an increased risk of exacerbations, pneumonia and death.Citation4 However, undiagnosed COPD, but asymptomatic, can also have exacerbations and pneumonia.Citation4 These patients who are “asymptomatic” may have adapted their lives to the limitations associated with their disease and may not want to reflect upon changes that occur to them as being a problem, that is, denial. If the physician uses probing questions, then symptoms may be better determined. This reality calls into question the validity of current recommendations for diagnosis of COPD that suggest targeted testing with spirometry only for symptomatic individuals.
It is important to remind physicians that spirometry is essential for the diagnosis of COPD, that is, a fixed postbronchodilator ratio of the FEV1/FVC of <0.70 or < the lower limit of normal (LLN) ratio (i.e., less than the lower fifth percentile of the reference value from a healthy population). Recent results support the use of fixed ratio less than 0.70 as appropriate to identify individuals at risk of clinically significant COPD.Citation5,Citation6 However, more than a single post-bronchodilator spirometric assessment may be necessary for diagnosing COPD for patients with mild airway obstruction at baseline.Citation7 We suggest post-bronchodilator FEV1/FVC ratio should be confirmed by a repeat spirometry on a separate occasion if the value is between 0.6 and 0.8, because the ratio may change as a result of biological variation. Findings, however, indicate that if the initial post-bronchodilator FEV1/FVC ratio is less than 0.6 it is very unlikely to rise above 0.7 spontaneously. While the diagnosis of COPD is confirmed by a reduced FEV1/FVC ratio < 0.7, the severity of airflow obstruction in COPD should be assessed by the degree of reduction in the post-bronchodilator FEV1 (% predicted).
Non-pharmacological therapy is complementary to inhaled or oral medication and should be a foundational aspect of the comprehensive management of COPD. Physicians must ensure patients have the proper support to live in a smoke free environment, receive appropriate vaccinations, adhere to prescribed medication (including using proper inhaler technique), receive self-management education and coaching, remain physically active and be referred to and complete pulmonary rehabilitation.Citation1,Citation8,Citation9 In a recent survey by the COPD Foundation,Citation10 patients reported gaps such as not receiving information after diagnosis of COPD, and receiving almost no education on self-management skills. Patients wished they had mastered these skills sooner to recognize early signs of an exacerbation and what to do about it, to stay active, and to cope with episodes of anxiety and dyspnea.
In this guideline update, we highlight important and new findings related to pharmacological therapy that should change clinical practice and improve disease management. We present updated evidence and recommendations, and expert clinical remarks on maintenance pharmacotherapy in patients with stable COPD. The diagnosis and non-pharmacological therapy of COPD are out-of-scope for this update.
Objectives
The overall objective of this CTS clinical practice guideline is to help clinicians to match their therapeutic decisions to the clinical status of each patient. This is a step toward personalizing therapy based on increasing individual characterization.
The specific objective is to provide clinical guidance with evidence-based recommendations and expert-informed clinical remarks to optimize maintenance pharmacological therapy for patients with COPD.
Target patient population
The update applies to all individuals with stable COPD.
Key definitions
Bronchodilators open up the airways in the lungs by relaxing airway smooth muscle. They also reduce lung hyperinflation. Bronchodilator medications can be short- or long-acting. Different types of short- or long-acting bronchodilators work in different ways.
Short-acting bronchodilators can be either SABAs (short-acting beta agonists) or SAMAs (short-acting muscarinic antagonists).
Long-acting bronchodilators can be either LABAs (long-acting beta2 agonists) or LAMAs (long-acting muscarinic antagonists).
Acute Exacerbations of COPD (AECOPD): Exacerbations are “event-based” occurrences; that is, respiratory symptom(s) that worsen beyond the normal day-to-day variability and may require the use of antibiotics and/or systemic corticosteroids and/or healthcare services. The varying levels of exacerbation severity are:
mild (worsening or new respiratory symptoms without a change in prescribed medications);
moderate (prescribed antibiotic and/or oral corticosteroids); and
severe (requiring a hospital admission or ED visit).
We have chosen to reconsider the classification of exacerbations into low- and high-risk of future exacerbations to align with patients enrolled in recently published randomized clinical trials. This was a necessary decision considering that the recommendations made in the guideline are evidence based.
Low- and high-risk of future exacerbations: Patients are considered to be at:
low-risk of exacerbations if they had ≤1 moderate exacerbation in the last year and did not require an ED visit or hospitalization
high-risk of exacerbations if they had ≥2 moderate or ≥1 severe exacerbation in the last year requiring a hospital admission/ED visit.
Stable COPD: Patients are considered to have “stable COPD” in all clinical states other than during the period of an AECOPD. However, patients with “stable COPD” may have progressive symptoms and/or have experienced an exacerbation.
Symbol “/” for combination therapy: The symbol “/” refers to: combination products (in the same device) and combination regimens (in separate devices). Single or multiple inhalers for combination therapy represent the clinical reality of different approaches to manage patients for a variety of considerations, such as access to medication, response to treatment, medical conditions other than COPD and patient preference.
Methodology
This guideline was developed in accordance with the CTS guideline development process.Citation11 The panel used the AGREE II checklist to guide the development of the guideline.Citation12
Guideline panel composition
The COPD guideline panel comprised 12 experts: six respirologists with experience in COPD management, research and research methodology including three clinicians/epidemiologists; two primary care physicians appointed by the College of Family Physicians of Canada; and one pharmacist. All author conflicts of interests are available at https://cts-sct.ca/guideline-library/.
Key clinical questions
The key clinical questions were developed using the Patient/population; Intervention or interventions; Comparison groups; Outcome or outcomes of interest (PICO) method. The PICO questions were based on the last published CTS position statement on the pharmacotherapy in patients with COPD in 2017.Citation1 We identified new evidence for PICO questions 1 and 2 but no new evidence to support an update of PICO 3 on Asthma COPD overlap (ACO). We did not include new interventions or de novo clinical questions in this review.
Literature search and screening of abstracts
This update includes all new research publications from the end-date of the literature search for the 2017 CTS position statement on pharmacotherapy in patients with chronic obstructive pulmonary disease.Citation1 An initial search was conducted through the CTS/McMaster Plus database with selected relevant manuscripts included with publication dates through October 31, 2018. A dedicated literature search and additional articles were found by reviewing the references in included articles and based on authors’ knowledge of other relevant publications. See Appendix 1 for details of the search strategy and a flow chart of search results and articles reviewed. We indexed the studies according to the PICO questions and made them available to the guideline panel on a dedicated software platform for manual assignment to individual reviewers.
For each PICO question, two panel members scrutinized titles and abstracts to decide whether the article was relevant (JB/PH-PICO 1; MB/DM-PICO 2). Where opinions differed, the two panel members resolved the conflict by discussion. Upon reaching consensus on the list of relevant abstracts, we obtained and reviewed copies of the full articles of all relevant and possibly relevant articles. The chosen inclusion and exclusion criteria (Appendix 1) were documented at both the abstract and full-text review stages.
Study selection criteria
We excluded studies if they were not related to maintenance pharmacotherapy in patients with stable, moderate to very-severe COPD. We included only randomized clinical trials and systematic reviews for further review and inclusion. The same pairs of reviewers who scrutinized titles and abstracts also assessed inclusion/exclusion criteria (Appendix 1) for full-text articles. The Cochrane Risk of Bias Tool for randomized clinical trials was used to assess the risk of bias in individual studies. The Documentation and Appraisal Review (DART) tool was used to assess the quality of systematic reviews addressing a variety of research designs.
Critical appraisal of identified studies
We compiled data from all articles relevant to each PICO question into evidence tables (available at: https://cts-sct.ca/guideline-library/). The entire panel discussed each PICO question via webinars in June 2019, at which time, all evidence tables were reviewed. Accordingly, we established group consensus on the quality and strength of the evidence addressing each clinical question according to the GRADE criteria (Appendix 1).Citation13 In instances where there was insufficient evidence but a recommendation was still warranted, a suggestion was developed and “consensus-based (CB)” replaced the grade.
Synthesis of evidence-base and clinical judgment of risk-versus-benefit
For each clinical question, we considered the strength and directness of the evidence supporting an intervention or treatment approach. For each therapeutic approach, we also considered: the potential health benefit to the patient; the morbidity and mortality impact on the overall COPD population; risks/harms; the burden placed on the patient; and the cost-effectiveness (these are the factors categorized under the “Contextualization and Deliberations” domain of the guidelines).Citation14
We also included informed clinical remarks with PICO clinical questions and recommendations, in an effort to compliment recommendations with practical clinical advice. Some of these remarks are not based on strong evidence, but represent the consensus opinions of panel members based on expertise.
Update of recommendations and classification
We used recommendations in the 2015 Prevention of Acute Exacerbations of COPD – American College of Chest Physicians, the Canadian Thoracic Society Guideline documentCitation15 for PICO 2, and from the 2017 CTS Position Statement: Pharmacotherapy in patients with COPD — An UpdateCitation1 for PICOs 1 and 2.
Following open and extensive discussions and evidence review for each PICO question, the entire panel proposed wording updates to each prior recommendation pertaining to that PICO question, and where applicable, a change to the strength of the recommendation to reflect newly published literature. We based strength of the recommendation on the GRADE quality of evidenceCitation13 (Appendix 1), and our synthesis of clinical judgment. The CTS Canadian Respiratory Guidelines Committee (CRGC) Chair then vetted the recommendations to optimize language with a view to improving likelihood of uptake.Citation16,Citation17 Recommendations were then voted upon by electronic survey using a six-point voting scale, whereby it was defined a priori that a recommendation would only be accepted if each panel member voted for option 1, 2 or 3 (wholeheartedly agree, agree or can support). For a recommendation to be accepted, it had to be voted on by 75% of the eligible panel members and achieve ratings 1, 2 or 3 by 80% of the voting panelists. In the event of a failure to reach 80% of votes with ratings 1, 2 or 3, another period of discussion ensued, whereby dissenting opinions were heard and considered. The recommendation was revised and followed by a second round of voting by electronic survey using a three-point scale, for which acceptance of a recommendation required a majority (80%) of panelists to choose option 1 or 2 (Appendix 1). Throughout this process all recommendations achieved acceptance, with no recommendation requiring a second round of voting.
Review and approval process
The CTS independently invited formal review of the update by an external (non-CTS) content expert. The lead author responded to the comments and made corresponding changes. Two members of the CRGC then completed their own review and provided further feedback for consideration. Upon acceptance, the Committee recommended approval of the guideline to the CTS Executive Committee.
Living guideline/future updates
The guideline will be formally reviewed every three years or sooner to determine the need for and nature of any updates, in accordance with the CTS Living Guideline Model (details available at https://cts-sct.ca/guideline-library/methodology/). Authors and/or the CTS COPD Assembly Steering Committee members will also use the continuously updated McMaster Plus database, whereby they will receive alerts when new articles pertaining to these PICO questions are published (starting from the last date of the literature search conducted for this guideline). This will serve to prompt members to consider timely guideline updates with evolving evidence and will facilitate formal literature reviews.
Summary PICO 1: Improving symptoms, exercise tolerance, physical activity and health status in stable COPD patients
Among respiratory symptoms, shortness of breath (dyspnea) on exertion is the most debilitating symptom COPD patients experience.Citation18 Since disease progression reduces patients’ capacity to exercise and, therefore, affects the ability to perform the activities of daily life,Citation19,Citation20 they consider relief of this symptom to be one of the most important outcomes in the management of their disease.Citation21 Shortness of breath also contributes to the established extra-pulmonary manifestations of COPD, including anxiety, depression,Citation22 cardiovascular diseaseCitation23 and peripheral locomotor muscle deconditioning.Citation24 Importantly, it is strongly associated with increased morbidity and mortality in adults with COPD.Citation25,Citation26 Persistent shortness of breath is associated with increased exacerbation risk.Citation26,Citation27 Dyspnea and exacerbation are not independent or dichotomous outcomes and they are often present in the same patient. Alleviating shortness of breath is a key goal of COPD management.
Inhaled bronchodilators are the mainstay medications in the pharmacologic management of COPD. There are two main classes of bronchodilators: B2-adrenoreceptor agonists and muscarinic antagonists, both in long- and short-acting forms. They can be used as monotherapy, combined as dual bronchodilators or combined with ICS for maintenance treatment. Bronchodilators enhance the neuromechanical coupling of the respiratory system and delay the onset of mechanical constraints, providing relief from exertional shortness of breath with concomitant improvement in exercise tolerance in patients with COPD.Citation28
This section discusses the optimal use of inhaled and oral pharmacologic maintenance therapies shown to improve shortness of breath, exercise tolerance, physical activity and health status in stable COPD patients.
Key evidence
Based on this review for PICO 1, recommendations 1, 3, 7 and 8 remain unchanged, while recommendations 2, 4 and 5 have a change in GRADE assessment due to new research findings, and 2, 5 and 6 are revised based on evidence from published literature. See Appendix 2 detailing the upgrades and revisions from 2017.
As stated in the previous position statement, use of LAMA or LABA monotherapy is endorsed to reduce shortness of breath, improve exercise tolerance and improve health status in patients with stable COPD. Although LAMA is often preferred to LABA in monotherapy, the evidence of its superiority comes primarily from studies in which the main outcome was to prevent COPD exacerbations. In patients with COPD who have persistent shortness of breath, exercise intolerance and/or poor health status despite using inhaled LAMA or LABA monotherapy, we recommend augmenting treatment to LAMA/LABA dual therapy. Patients should be routinely monitored and evaluated for their response after any change in their therapy, as many have persisting symptoms with an impact on their well-being.Citation29
In terms of improving physical activity, the evidence suggests that combining a self-management behavioral intervention with exercise and pharmacologic interventions has the largest effect on physical activity and symptom improvement. A self-management behavioral intervention is more likely to help patients change their behavior and can lead to long-term adoption of a more physically active lifestyle.Citation30,Citation31 For patients who remain symptomatic and have poor exercise tolerance or health status despite being on LAMA/LABA combination therapy, the evidence supports patients enrolling in a pulmonary rehabilitation program.Citation32 For patients who remain symptomatic and have poor health status despite these interventions, a clinician should consider “step up” to triple therapy (LAMA/LABA/ICS), although each individual should be evaluated for risk/benefit of adding ICS in these circumstances. Evidence for its benefit has been demonstrated primarily in patients who have a high risk of exacerbations.Citation33,Citation34
For PICO 1, data is lacking with respect to withdrawal or “step down” from LAMA/LABA/ICS to LAMA/LABA dual therapy or from dual therapy to monotherapy. We continue to support guidance from the previous position statement.Citation1 The consensus was that, in patients with COPD with no improvement in shortness of breath, exercise tolerance or health status despite the use of triple inhaled therapy or inhaled LAMA/LABA dual therapy, clinicians may cautiously consider “step down” treatment for some patients. These patients need to be monitored carefully with close clinical follow-ups to detect any signs of clinical deterioration after medication “step down.”
As per previous guidelines, oral therapies such as theophylline, phosphodiesterase-4-inhibitor, mucolytics, statins, anabolic steroids, oral Chinese herbal medicines or phosphodiesterase-5-inhibitor demonstrate no evidence of conferring additional benefit in patients already on combination long-acting bronchodilators. We reiterate that ICS monotherapy, as a lone intervention, has no place in treating COPD patients. If there is an indication for ICS therapy or the patient has asthma in addition to COPD, then ICS should be prescribed in a combination inhaler with long-acting bronchodilator(s).
Summary PICO 2: Preventing acute exacerbations in stable COPD patients
In Canada, AECOPD continues to be the most frequent cause of acute hospitalization in adults,Citation120 and is associated with the highest total hospital cost of care (2016–2017). The cost of hospitalizations related to COPD is more than 30% higher than that of the next most expensive health condition (heart failure).Citation121 AECOPDs are a gateway to poor outcomes and adverse consequences.Citation122–124 They accelerate lung function decline, dramatically reduce quality of life, and are strong predictors of future AECOPDs. They are acute, trajectory-changing manifestations of a chronic disease associated with increased mortality. COPD is the third leading cause of death worldwide.Citation125,Citation126
A fundamental and achievable goal of therapy in managing stable COPD is to reduce the occurrence and severity of AECOPDs. Furthermore, providing appropriate preventive therapy for patients at increased risk of exacerbation increases the likelihood of reducing and preventing ED visits and hospital admissions. In patients with severe COPD, reducing AECOPD may also reduce mortality.Citation33
This section discusses the optimal use of inhaled and oral pharmacologic maintenance therapies shown to prevent AECOPD in patients with stable COPD, not the treatment of acute exacerbations.
Key evidence
Based on this review for PICO 2, recommendations 3 and 10 remain unchanged, while recommendations 1, 2, 4, 5, 6, 7, 8 and 9 have a change in GRADE assessment due to new research findings. Recommendations 5, 6, 7, 9 and 11 are revised based on evidence from published literature. See Appendix 2 detailing the upgrades and revisions from 2017.
A significant change is the use of either an ICS/LABA or LAMA/LABA as a first step in patients with high risk of AECOPD. Although ICS/LABA or LAMA/LABA is a viable inhaled therapeutic option in this setting, LAMA/LABA is the preferred choice except in patients with previous exacerbations who have higher peripheral eosinophilia (). In this case, ICS/LABA could be the favored therapy as stated by the evidence described in the Discussion section of this guideline. Monotherapy with either LAMA or LABA is not the optimal initial maintenance treatment for patients who experience or are at high risk of AECOPD. Acknowledging the significant adverse consequences of AECOPD, consider inhaled therapy with either ICS/LABA or LAMA/LABA as the acceptable minimum maintenance therapy for this high risk population. It is important to remember that in real life practice, it is the exception to treat a patient only to prevent exacerbations; the vast majority of time we optimize bronchodilator therapy to improve a patient’s dyspnea.
Figure 2. COPD Pharmacotherapy.
COPD pharmacotherapy promoting an approach that aligns treatment decisions with symptom burden and risk of future exacerbations. To learn more about the Asthma-COPD Overlap (ACO) treatment algorithm, refer to the CTS position statement on the pharmacotherapy in patients with COPD in 2017.Citation1
mMRC is a modified (0-4 scale) version of the MRC breathlessness scale which was used in previous CTS guidelines. The mMRC aligns with the Global Initiative for Chronic Obstructive Airways Disease (GOLD) 2019 report.
SABD prn (as needed) should accompany all recommended therapies. Solid arrows indicate step up therapy to optimally manage symptoms of dyspnea and/or activity limitation, as well as prevention of AECOPD where appropriate. Dashed arrows indicate potential step down of therapy, with caution and with close monitoring of patient symptoms, exacerbations and lung function. Symbol “/” refers to combination products (in the same device) and combination regimens (in separate devices). ICS should ideally be administered in a combination inhaler.
†Patients are considered at Low Risk of AECOPD with ≤1 moderate AECOPD in the last year (moderate AECOPD is an event with prescribed antibiotic and/or oral corticosteroids), and did not require hospital admission/ED visit; or at High Risk of AECOPD with ≥2 moderate AECOPD or ≥1 severe exacerbation in the last year (severe AECOPD is an event requiring hospitalization or ED visit).
*Blood eosinophil ≥300/µL in patients with previous AECOPD may be useful to predict a favorable response to ICS combination inhaler.
‡Oral Therapies = Roflumilast, N-acetylcysteine, daily dose Azithromycin could be considered with patients with high risk AECOPD despite on optimal long-acting inhaled therapy. Oral corticosteroids as maintenance therapy are not indicated in COPD.
Abbreviations: CAT = COPD assessment test; mMRC = Modified Medical Research Council; SABD prn = short-acting bronchodilator as needed; AECOPD = acute exacerbation of COPD; LAMA = long-acting muscarinic antagonist; LABA = long-acting ẞ2-agonist; ICS = inhaled corticosteroid.
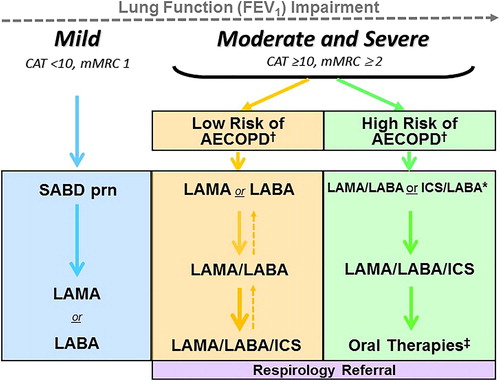
Similar to our analysis in PICO 1, we conclude that there is no role for ICS monotherapy and ICS should only be used in combination with bronchodilators. ICS/LABA and LAMA/LABA/ICS are the only current single inhaler options. Health Canada has not approved an inhaled ICS for monotherapy use in COPD. Furthermore, in prior studies, the monotherapy ICS arm underperformed compared to combination ICS/LABA, and post-hoc analysis suggested increased mortality in the ICS monotherapy participants.Citation127 Administering ICS with LAMA/LABA in separate inhalers has not been studied in COPD.Citation120,Citation122,Citation123 When combination ICS/LABA or LAMA/LABA/ICS is used, high doses of ICSCitation122 are not typically necessary to achieve optimum benefit in COPD, as shown by a relatively flat dose-response curveCitation128 and greater incidence of adverse effect with higher inhaled ICS doses.Citation129
There remains clinical uncertainty regarding potential “step down” of therapyCitation90,Citation123 in patients with a history of a high risk of future AECOPD. Evidence from a randomized clinical trial involving participants with COPD receiving combination ICS/LABA with LAMA in separate inhalers who underwent stepwise ICS withdrawal suggests the intervention is not associated with a significantly increased risk of exacerbation over a short term of follow up.Citation130 However, in this study the initial baseline exacerbation rate was low, about one-third of participants had not previously required inhaled triple therapy before recruitment, there was a statistically significant reduction in FEV1 (43 mL, p = 0.001) after ICS withdrawal, and the number of deaths was numerically small but higher in the ICS withdrawal group (n = 40) compared to the ICS continuation group (n = 34). More recently, another randomized clinical trial of patients with COPD with low risk of AECOPD on long-term triple inhaled therapy with direct de-escalation to LAMA/LABA led to a small decrease in lung function as a primary endpoint, with no difference in exacerbation rate. However, the primary study endpoint was not met, with confidence limits for trough FEV1 exceeding the non-inferiority margin of −50 mL. Further analysis of these studies revealed a higher rate of exacerbation in patients with ≥300 blood eosinophils/μL, suggesting that, as a biomarker, blood eosinophil at ≥300/μL in patients with previous AECOPD could be useful to predict a favorable response to ICS when combined with long-acting bronchodilator(s). However, no RCT has compared ICS/LABA versus LABA/LAMA in patients with high risk of and blood eosinophil at ≥300/μL, with exacerbation as a primary endpoint.
Given these findings and acknowledging the negative impact of AECOPD, reductions in lung function and the potential adverse consequences of therapy, we continue to support guidance from the previous CTS guidance documentCitation123 that the clinical phenotype should drive pharmacotherapy for patients with COPD. If therapy was started without a clear indication (such as the use of an ICS in a patient with no history of exacerbations), you may consider initiating a “step down.” However, if therapy was started according to recommendations (such as the use of LAMA/LABA/ICS in a patient with moderate-severe COPD with poor quality of life and history of frequent and/or severe AECOPD) and treatment has been effective, a “step down” is NOT recommended. Given the potential for serious negative consequences of AECOPD, including hospitalization and death, we believe that de-escalation should only be considered in patients at low risk of morbidity and mortality, and this after a period of considerable stability. Moreover, while awaiting objective documentation supporting the safety of this approach, if you decide to “step down,” we highly recommend monitoring your patients carefully with regular clinical assessments that includes the monitoring of lung function and re-occurrence of AECOPD.
As noted, the incidence of pneumonia is higher with maintenance therapy of ICS-containing inhaled medicines, especially in COPD patients with severe and very severe disease. However, these are also the patients who benefit most from an ICS-containing regimen. Debate still ensues as to an intra-class difference between fixed combinations of inhaled corticosteroid/long acting β2 agonist regarding the risk of pneumonia and pneumonia-related events in treating patients with COPD.Citation131 However, the clinical significance of increased pneumonia in COPD patients who use ICS remains unclear, since there is no concurrent documented increase risk of mortality in this group.Citation131 Results from a large clinical trialCitation33 confirmed a higher incidence of pneumonia with ICS-combination therapy, but this was accompanied by significant improvements in lung function and quality of life and significant reductions in exacerbations and mortality, which are endpoints of significant importance.
As stated in the previous position statement, if patients with COPD continue to experience exacerbations despite being on optimal long-acting inhaled therapy, consider adding a daily macrolide (e.g. Azithromycin) as maintenance therapy in appropriate patients who have normal QT interval on ECG and no evidence of either colonization or acute infection with atypical mycobacterium. Also consider oral Roflumilast or oral N-acetylcysteine (600 mg po BID) in those having a clinical phenotype by history in keeping with chronic bronchitis. In recommendation 2.11 in this update, recent evidence supports not using theophylline in patients who are on long-acting inhaled therapy. We reiterate that systemic corticosteroids should not be used for maintenance pharmacotherapy in stable COPD.
Discussion
Since the 2017 CTS pharmacotherapy position statement, there have been several important clinical trials that have necessitated an update. In this guideline we have incorporated new evidence from published large multicenter clinical trials and systematic reviews that have an impact on clinicians’ approach to the medical management of patients living with COPD. We have summarized our updated recommendations in and and included a comparison of 2017 and 2019 recommendations in Appendix 2. An updated COPD pharmacologic algorithm () that reflects these updates was also derived.
Table 1. 2019 Recommendations on improving symptoms, exercise tolerance, physical activity and health status in stable COPD patients.
Table 2. 2019 Recommendations on preventing acute exacerbation in stable COPD.
The treatment propositions presented in this updated Guideline, in particular the approach of a treatment “step up” and “step down” are pragmatic and intended to provide meaningful guidance for clinicians. Most research trials were not strictly designed to assess such a therapeutic approach. However, treatment “step up” in COPD is a practical construct with wide appeal that is supported by evidence that inhaled combined therapy is superior to monotherapy and triple therapy to dual therapy in certain patient populations. Because the superiority of inhaled triple or dual bronchodilator therapy may not be achieved in every patient, “step down” may be considered for some patients, but should only be done with close medical supervision, as the risk of clinical deterioration is real and continues to exist.
There are several important considerations in the management of COPD that are not addressed by the PICO questions in this guideline document. We have therefore provided a commentary of selected topics in this discussion. A full review of these clinical issues may be undertaken in subsequent guideline development.
Choice of inhaler device
The choice of the inhaler device and/or the decision to use single or multiple devices for combination therapy remains a subject of clinical interest and controversy. Very few studies have compared combination products in the same device compared to separate devices. In the only randomized clinical trial examining this issue, a single-inhaler LAMA/LABA/ICS was compared to an ICS/LABA and LAMA in separate devices; this study demonstrated non-inferiority between the two treatment strategies.Citation191 There is no study that has been performed in COPD comparing LABA or combined LAMA/LABA with ICS in single and separate devices. Although the use of single or multiple devices for combination therapy is a clinical reality, properly designed trials or real-life data are lacking in COPD.
Choice of bronchodilator combination
Another topic is the question clinicians may have with respect to the equivalence or superiority of the various combined long-acting bronchodilators (LAMA/LABA). Few comparative efficacy trials compared combination long-acting bronchodilator therapy; those that did showed between-treatment differences in FEV1 that were small and of uncertain clinical significance.Citation192,Citation193
Interval for changing inhaler therapy
The decision to change a therapy should always occur after a complete evaluation of the patient and the potential benefit of a change in therapy; as well as an assessment of any adverse effects of the therapy, and with a review of patient adherence, inhaler technique and preferences. Although there is no absolute interval time at which the evaluation should be performed following a change in therapy, 6 months after initiating a long acting bronchodilator and 12 months after initiating a combination regimen with an ICS are suggested timeframes.
Peripheral blood eosinophils in COPD
While peripheral blood eosinophil counts have demonstrated reasonable repeatability over a year in a population-based cohort of COPD patients in primary care,Citation194 practical uncertainty remains regarding the exact cut-off level of sputum or blood eosinophils for predicting therapeutic response in COPD. Despite this uncertainty, peripheral blood eosinophil counts may play a role in certain clinical settings. New information is incorporated into this update with respect to blood eosinophils as a potential biomarker for use in COPD patients known to have exacerbations to prevent future exacerbations. A consistent pattern of results from randomized clinical trials conducted in COPD patients at risk of exacerbations has emerged. Lower eosinophil counts (<100 eosinophils/µL) predict a lower or no response to ICS containing regimens in terms of preventing exacerbation. ICS containing regimens will benefit in reducing the likelihood of exacerbations in the magnitude of effect being greater at higher eosinophil counts, particularly ≥300 eosinophils/μL.Citation156 This provides a measure of probability of response to ICS containing regimen in patients who had previous exacerbations, aligning with a more personalized approach.
Mortality
Reducing mortality has been a long-standing goal of therapy in COPD. Older studies have revealed mortality-reducing trends with inhaled therapyCitation127,Citation195 but statistical significance was not achieved. Although there is still no definitive answer, more recent evidence from a large randomized controlled trial study demonstrated significant relative reduction in all-cause mortality during treatment with regimens that included inhaled ICS/LABA or triple therapy (LAMA/LABA/ICS) compared to LAMA/LABA for COPD patients with high risk of exacerbations. Despite a higher incidence of study-reported pneumonia in the ICS-containing treatment regimens, mortality was reduced by 42% in favor of LAMA/LABA/ICS vs. LAMA/LABA (unadjusted p = 0.01), and 39% in favor of ICS/LABA vs LAMA/LABA (unadjusted p = 0.02). An analysis of adjudicated cause-specific death during treatment demonstrated fewer deaths from both respiratory and cardiovascular etiologies in the ICS-containing regimens. This topic requires further attention, but mortality is an important outcome that should have our consideration in clinical decision.
Dissemination and implementation
Our guideline will be disseminated through traditional channels including this publication, through the CTS website and social media channels, and through an accompanying slide deck that will be used to present this content to various target groups across the country. It is also anticipated that we will produce a separate implementation document that will include key indicators of appropriate care and practical guidance for healthcare system change. Our goal is to monitor the impact of these actionable recommendations through their ability to correct knowledge gaps and improve actual behaviors within the target user groups. On a population level, we also believe that monitoring the frequency of COPD ED visits, hospital admissions and re-admissions would be relevant metrics to assess the success of this guideline. For messages targeting nonexperts, we will seek to tailor messages and produce corresponding educational content, in collaboration with key stakeholders such as provincial lung associations, RESPIPLUS and RESPTREC.
Conclusion
This update is an important step toward optimizing the pharmacologic management of COPD. Recommendations enable better-personalized therapy based on more specific individual characteristics. The update further highlights the intent and benefit of therapy in both improving symptoms and activity limitations, and reducing the risk of AECOPD. Areas of debate still require further study, including approach to treatment “step down” targeting specific group of patients, blood eosinophil use and its cut off, and use of single or multiple inhalers for combination therapy, but recent evidence has strengthened many recommendations to optimize management of COPD. In summary, pharmacological therapy plays a foundational role in therapy, but it should never be the sole treatment in managing COPD patients. Clinicians should always combine and optimize pharmacological and non-pharmacological therapies with the dual goals of reducing symptoms and preventing AECOPD.
Acknowledgments
The authors would like to thank Anne Van Dam from CTS and Samir Gupta and Christopher Licskai, Executive members of the CTS CRGC for their input and guidance. We would like to acknowledge with sincere appreciation our expert reviewer, Marc Miravitlles from the Pneumonlogy Department, University Hospital Vall d’Hebron, Ciber de Enfermedales Respiratorias (CIBERES), Barcelona, Spain.
Editorial independence
The CTS COPD guideline panel is accountable to the CTS Canadian Respiratory Guidelines Committee and the CTS Board of Directors. The CTS COPD guideline panel is functionally and editorially independent from any funding sources of the CTS and does not receive any direct funding from external sources. The CTS receives unrestricted grants that are combined into a central operating account to facilitate the knowledge translation activities of the CTS Assemblies and its guideline panels. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the key messages presented in this document.
Disclosures
Members of the CTS COPD Guideline Panel declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted at https://cts-sct.ca/guideline-library/.
References
- Bourbeau J, Bhutani M, Hernandez P, et al. CTS position statement: pharmacotherapy in patients with COPD—an update. Can J Respir Crit Care Sleep Med. 2017;1(4):222–241. doi:10.1080/24745332.2017.1395588.
- Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822–829. doi:10.1136/thoraxjnl-2015-206938.
- Labonté LE, Tan WC, Li PZ, et al. Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use. Data from the CanCOLD study. Am J Respir Crit Care Med. 2016;194(3):285–298. doi:10.1164/rccm.201509-1795OC.
- Colak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434. doi:10.1016/S2213-2600(17)30119-4.
- Bhatt SP, Balte PP, Schwartz JE, et al. Discriminative Accuracy of FEV1: FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321(24):2438–2447. doi:10.1001/jama.2019.7233.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214.
- Aaron SD, Tan WC, Bourbeau J, et al. Diagnostic instability and reversals of chronic obstructive pulmonary disease diagnosis in individuals with mild to moderate airflow obstruction. Am J Respir Crit Care Med. 2017;196(3):306–314. doi:10.1164/rccm.201612-2531OC.
- Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: a report from the Canadian Thoracic Society COPD Clinical Assembly. Can Respir J. 2015;22(3):147–152. doi:10.1155/2015/369851.
- Marciniuk DD, Brooks D, Butcher S, et al. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease–practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J. 2010;17(4):159–168. doi:10.1155/2010/425975.
- COPD Foundation. COPE survey results: low patient awareness about COPD exacerbations poses barrier to effective management. Published June 17, 2014. http://www.copdfoundation.org/About-Us/Press-Room/Press-Releases/ID/256/COPD-Foundation-Releases-Groundbreaking-COPE. Accessed December 6, 2018.
- Canadian Thoracic Society Guideline Development Process and Methodology. 2018. https://cts-sct.ca/guideline-library/methodology/. Accessed November 27, 2018.
- Brouwers M, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2010;182(18):E839–E842.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129(1):174–181. doi:10.1378/chest.129.1.174.
- Brouwers M, Makarski J, Kastner M, et al. The Guideline Implementability Decision Excellence Model (GUIDE-M): a mixed methods approach to create an international resource to advance the practice guideline field. Implement Sci. 2015;10:36.
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society guideline. Chest. 2015;147(4):894–942. doi:10.1378/chest.14-1676.
- Kastner M, Bhattacharyya O, Hayden L, et al. Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: a realist review. J Clin Epidemiol. 2015;68(5):498–509. doi:10.1016/j.jclinepi.2014.12.013.
- Gupta S, Rai N, Bhattacharrya O, et al. Optimizing the language and format of guidelines to improve guideline uptake. Can Med Am J. 2016;188(14):E362–E368. doi:10.1503/cmaj.151102.
- Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi:10.1164/rccm.201111-2042ST.
- Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi:10.1164/rccm.200407-855OC.
- Johnson-Warrington V, Harrison S, Mitchell K, et al. Exercise capacity and physical activity in patients with COPD and healthy subjects classified as Medical Research Council dyspnea scale grade 2. J Cardiopulm Rehabil Prev. 2014;34(2):150–154. doi:10.1097/HCR.0000000000000038.
- Zhang Y, Morgan RL, Alonso-Coello P, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52(1):1800222. doi:10.1183/13993003.00222-2018.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–349. doi:10.1183/09059180.00007813.
- Müllerová H, Agusti A, Erqou S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):163–1178. doi:10.1378/chest.12-2847.
- Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. doi:10.1164/rccm.201402-0373ST.
- Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi:10.1378/chest.121.5.1434.
- Müllerová H, Lu C, Li H, et al. Prevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary care. PLos One. 2014;9(1):e85540. doi:10.1371/journal.pone.0085540.
- Calverley PM, Tetzlaff K, Dusser D, et al. Determinants of exacerbation risk in patients with COPD in the TIOSPIR study. Int J Chron Obstruct Pulm Dis. 2017;12:3391–3405. doi:10.2147/COPD.S145814.
- O’Donnell DE, Hamilton AL, Webb KA. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol. 2006;101(4):1025–1035. doi:10.1152/japplphysiol.01470.2005.
- Dransfield MT, Bailey W, Crater G, et al. Disease severity and symptoms among patients receiving monotherapy for COPD. Prim Care Respir J. 2010;20(1):46–53. doi:10.4104/pcrj.2010.00059.
- Troosters T, Maltais F, Leidy N, et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(8):1021–1032. doi:10.1164/rccm.201706-1288OC.
- Bourbeau J, Lavoie KL, Sedeno M, et al. Behaviour-change intervention in a multicentre, randomised, placebo-controlled COPD study: methodological considerations and implementation. BMJ Open. 2016;6(4):e010109. doi:10.1136/bmjopen-2015-010109.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:CD003793.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901.
- Ferguson GT, Rabe KF, Martinez FJ. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;10:747–758. doi:10.1016/S2213-2600(18)30327-8.
- Bedard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Respir J. 2012;39(2):265–271. doi:10.1183/09031936.00059511.
- Beeh KM, Singh D, Di Scala L, et al. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulm Dis. 2012; 7:503–513.
- Beeh KM, Wagner F, Khindri S, et al. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD J Chron Obstruct Pulm Dis. 2011;8(5):340–345. doi:10.3109/15412555.2011.594464.
- Beeh KM, Watz H, Puente-Maestu L, et al. Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: a randomized, placebo-controlled, crossover trial. BMC Pulm Med. 2014;14(1):209. doi:10.1186/1471-2466-14-209.
- Brouillard C, Pepin V, Milot J, et al. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J. 2008;31(3):579–584. doi:10.1183/09031936.00119007.
- Cooper CB, Celli BR, Jardim JR, et al. Treadmill endurance during 2-year treatment with tiotropium in patients with COPD: a randomized trial. Chest. 2013;144(2):490–497. doi:10.1378/chest.12-2613.
- Gotfried MH, Kerwin EM, Lawrence D, et al. Efficacy of indacaterol 75 mug once-daily on dyspnea and health status: results of two double-blind, placebo-controlled 12-week studies. COPD J Chron Obstruct Pulm Dis. 2012;9(6):629–636. doi:10.3109/15412555.2012.729623.
- Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized placebo-controlled trials. BMC Pulm Med. 2013;13(1):26. doi:10.1186/1471-2466-13-26.
- Jiang FM, Liang ZA, Zheng QL, et al. Safety and efficacy of 12-week or longer indacaterol treatment in moderate-to-severe COPD patients: a systematic review. Lung. 2013;191(2):135–146. doi:10.1007/s00408-012-9444-2.
- Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–836. doi:10.1183/09031936.00225511.
- Kaplan A. Effect of tiotropium on quality of life in COPD: a systematic review. Primary Care Respir J. 2010;19(4):315–325. doi:10.4104/pcrj.2010.00067.
- Kerwin EM, D’Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD J Chron Obstruct Pulm Dis. 2012;9(2):90–101. doi:10.3109/15412555.2012.661492.
- Kerwin EM, Gotfried MH, Lawrence D, et al. Efficacy and tolerability of indacaterol 75 mug once daily in patients aged >40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studies. Clin Ther. 2011;33(12):1974–1984. doi:10.1016/j.clinthera.2011.11.009.
- Kinoshita M, Lee SH, Hang LW, et al. Efficacy and safety of indacaterol 150 and 300 micro g in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12-week, placebo-controlled study. Respirology. 2012;17(2):379–389. doi:10.1111/j.1440-1843.2011.02107.x.
- Maltais F, Celli B, Casaburi R, et al. Aclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPD. Respir Med. 2011;105(4):580–587. doi:10.1016/j.rmed.2010.11.019.
- Mroz RM, Minarowski L, Chyczewska E. Indacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patients. Adv Exp Med Biol. 2013;756:23–28. doi:10.1007/978-94-007-4549-0_4.
- O’Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105(7):1030–1036. doi:10.1016/j.rmed.2011.03.014.
- Rennard SI, Scanlon PD, Ferguson GT, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clin Drug Invest. 2013;33(12):893–904. doi:10.1007/s40261-013-0138-1.
- Santus P, Radovanovic D, Di Marco F, et al. Faster reduction in hyperinflation and improvement in lung ventilation inhomogeneity promoted by aclidinium compared to glycopyrronium in severe stable COPD patients. A randomized crossover study. Pulm Pharmacol Ther. 2015;35:42–49. doi:10.1016/j.pupt.2015.11.001.
- Satake M, Takahashi H, Sugawara K, et al. Inhibitory effect of procaterol on exercise dynamic lung hyperinflation during the 6-min walk test in stable patients with chronic obstructive pulmonary disease. Arzneim Forsch. 2011;61:8–13. doi:10.1055/s-0031-1296162.
- Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. [Erratum appears in Eur Respir J. 2014 Aug;44(2):555 Note: dosage error in published abstract; MEDLINE/PubMed abstract corrected; dosage error in article text]. Eur Respir J. 2014;43(1):72–81. doi:10.1183/09031936.00033213.
- Yao W, Wang C, Zhong N, et al. Effect of once-daily indacaterol in a predominantly Chinese population with chronic obstructive pulmonary disease: a 26-week Asia-Pacific study. Respirology. 2014;19(2):231–238. doi:10.1111/resp.12211.
- Bogdan MA, Aizawa H, Fukuchi Y, et al. Efficacy and safety of inhaled formoterol 4.5 and 9 mug twice daily in Japanese and European COPD patients: phase III study results. BMC Pulm Med. 2011;11(1):51. doi:10.1186/1471-2466-11-51.
- Braido F, Baiardini I, Cazzola M, et al. Long-acting bronchodilators improve health related quality of life in patients with COPD. Respir Med. 2013;107(10):1465–1480. doi:10.1016/j.rmed.2013.08.007.
- Cheyne L, Irvin-Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; Sep 16(9):CD009552.
- Geake JB, Dabscheck EJ, Wood-Baker R, et al. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta(2)-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;1:CD010139. doi:10.1002/14651858.CD010139.pub2.
- Gross NJ, Nelson HS, Lapidus RJ, et al. Efficacy and safety of formoterol fumarate delivered by nebulization to COPD patients. Respir Med. 2008;102(2):189–197. doi:10.1016/j.rmed.2007.10.007.
- Jones PW, Mahler DA, Gale R, et al. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respir Med. 2011;05:892–899. doi:10.1016/j.rmed.2011.02.013.
- Karner C, Cates CJ. Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;Apr 18(4):CD008989.
- Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;Oct 15(10):CD010177.
- Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulm Dis. 2014;9:697–714.
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–279. doi:10.1183/09031936.00045810.
- Ni H, Soe Z, Moe S. Aclidinium bromide for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;Sep 19(9):CD010509.
- Park J, Lee JS, Rhee C, et al. Effect of indacaterol on cough and phlegm in chronic obstructive pulmonary disease patients: a meta-analysis of five randomized controlled trials. J Korean Med Sci. 2015;30(10):1453–1458. doi:10.3346/jkms.2015.30.10.1453.
- Chen WC, Huang CH, Sheu CC, et al. Long-acting beta2-agonists versus long-acting muscarinic antagonists in patients with stable COPD: a systematic review and meta-analysis of randomized controlled trials. Respirology. 2017;22(7):1313–1319. doi:10.1111/resp.13100.
- Di Marco F, Sotgiu G, Santus P, et al. Long-acting bronchodilators improve exercise capacity in COPD patients: a systematic review and meta-analysis. Respir Res. 2018;19(1):18. doi:10.1186/s12931-018-0721-3.
- Ni H, Htet A, Moe S. Umeclidinium bromide versus placebo for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;6:CD011897. doi:10.1002/14651858.CD011897.pub2.
- Beeh KM, Korn S, Beier J, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respir Med. 2014;108(4):584–592. doi:10.1016/j.rmed.2014.01.006.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288–1296. doi:10.1016/j.rmed.2010.05.017.
- Calzetta L, Ciaprini C, Puxeddu E, et al. Olodaterol + tiotropium bromide for the treatment of COPD. Expert Rev Respir Med. 2016;10(4):379–386.
- Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145(5):981–991. doi:10.1378/chest.13-1579.
- Chen X, May B, Di YM, et al. Oral Chinese herbal medicine combined with pharmacotherapy for stable COPD: a systematic review of effect on BODE index and six-minute walk test. PLoS One. 2014;9(3):e91830. doi:10.1371/journal.pone.0091830.
- Jayaram L, Wong C, McAuley S, et al. Combined therapy with tiotropium and formoterol in chronic obstructive pulmonary disease: effect on the 6-minute walk test. COPD J Chron Obstruct Pulm Dis. 2013;10(4):466–472. doi:10.3109/15412555.2013.771162.
- Mahler DA, Decramer M, D’Urzo A, et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014;43(6):1599–1609. doi:10.1183/09031936.00124013.
- Maltais F, Singh S, Donald AC, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. [Erratum appears in Ther Adv Respir Dis. 2016 Jun;10(3):289; PMID: 27255756]. Ther Adv Respir. 2014;8(6):169–181. doi:10.1177/1753465814559209.
- Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting β-agonists for stable COPD: a systematic review . Chest. 2012;142(5):1104–1110. doi:10.1378/chest.11-2252.
- Vincken W, Aumann J, Chen H, et al. Efficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study. Int J Chron Obstruct Pulm Dis. 2014; 9:215–228.
- Wang L, Zhai CJ, Liu Y, et al. Umeclidinium plus vilanterol versus placebo, umeclidinium, or vilanterol monotherapies for chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Clin Drug Invest. 2016;36(11):865–875. doi:10.1007/s40261-016-0449-0.
- Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). [Erratum appears in Eur Respir J. 2015 Jun;45(6):1763; PMID: 26028626]. Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014.
- Calzetta L, Rogliani P, Matera MG, et al. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149(5):1181–1196. doi:10.1016/j.chest.2016.02.646.
- Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;Oct 22(10):CD008989.
- Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med. 2014;108(12):1752–1760. doi:10.1016/j.rmed.2014.10.002.
- ZuWallack R, Allen L, Hernandez G, et al. Efficacy and safety of combining olodaterol Respimat® and tiotropium HandiHaler® in patients with COPD: results of two randomized, double-blind, active-controlled studies. Int J Chron Obstruct Pulm Dis. 2014;9:1133–1144.
- Kardos P, Hagedorn-Peinz I. The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study–the FAVOR study. Int J Chron Obstruct Pulm Dis. 2018;13:69–77. doi:10.2147/COPD.S146189.
- Vogelmeier C, Gaga M, Aalamian-Mattheis M, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18(1):140. doi:10.1186/s12931-017-0622-x.
- Calzetta L, Ora J, Cavalli F, et al. Impact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: a pair-wise and network meta-analysis. Respir Med. 2017;129:189–198. doi:10.1016/j.rmed.2017.06.020.
- Watz H, Krippner F, Kirsten A, et al. Indacaterol improves lung hyperinflation and physical activity in patients with moderate chronic obstructive pulmonary disease–a randomized, multicenter, double-blind, placebo-controlled study. BMC Pulm Med. 2014;14(1):158. doi:10.1186/1471-2466-14-158.
- Watz H, Mailander C, Baier M, et al. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (the MOVE study). BMC Pulm Med. 2016;16(1):95. doi:10.1186/s12890-016-0256-7.
- Watz H, Beeh KM, Garcia-Aymerich J, et al. ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int J Chron Obstruct Pulm Dis. 2017;12:2545–2558.
- Troosters T, Sciurba FC, Decramer M, et al. Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial. NPJ Prim Care Respir Med. 2014;24:14003.
- Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD) (Review). Cochrane Database Syst Rev. 2017;2:CD012066.
- Hoshino M, Ohtawa J, Akitsu K. Comparison of airway dimensions with once daily tiotropium plus indacaterol versus twice daily Advair® in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;30:128–133. doi:10.1016/j.pupt.2014.08.002.
- Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. doi:10.7326/0003-4819-146-8-200704170-00152.
- Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;Mar 16(3):CD008532.
- Calzetta L, Matera MG, Braido F, et al. Withdrawal of inhaled corticosteroids in COPD: a meta-analysis. Pulm Pharmacol Ther. 2017;45:148–158. doi:10.1016/j.pupt.2017.06.002.
- Kawayama T, Hoshino T, Ichiki M, et al. Effect of add-on therapy of tiotropium in COPD treated with theophylline. Int J Chron Obstruct Pulm Dis. 2008;3(1):137–147.
- Voduc N, Alvarez GG, Amjadi K, et al. Effect of theophylline on exercise capacity in COPD patients treated with combination long-acting bronchodilator therapy: a pilot study. Int J Chron Obstruct Pulm Dis. 2012;7:245–252.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi:10.1016/S0140-6736(09)61252-6.
- O’Donnell DE, Bredenbroker D, Brose M, et al. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J. 2012;39(5):1104–1112. doi:10.1183/09031936.00096511.
- Pan L, Guo YZ, Zhang B, et al. Does roflumilast improve dyspnea in patients with chronic obstructive pulmonary disease? A meta-analysis. J Thorac Dis. 2013;5(4):422–429.
- Johnson K, McEvoy CE, Naqvi S, et al. High-dose oral N-acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: a randomized, placebo-controlled trial. Int J Chron Obstruct Pulm Dis. 2016;11:799–807.
- Lee TM, Chen CC, Shen HN, et al. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci. 2009;116(6):497–505. doi:10.1042/CS20080241.
- Maneechotesuwan K, Wongkajornsilp A, Adcock IM, et al. Simvastatin suppresses airway IL-17 and upregulates IL-10 in patients with stable COPD. Chest. 2015;148(5):1164–1176. doi:10.1378/chest.14-3138.
- Mroz RM, Lisowski P, Tycinska A, et al. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. A controlled pilot study. J Physiol Pharmacol. 2015;66(1):111–128.
- Daga MK, Khan NA, Malhotra V, et al. Study of body composition, lung function, and quality of life following use of anabolic steroids in patients with chronic obstructive pulmonary disease. Nutr Clin Pract. 2014;29(2):238–245. doi:10.1177/0884533614522832.
- Pan L, Wang M, Xie X, et al. Effects of anabolic steroids on chronic obstructive pulmonary disease: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(1):e84855. doi:10.1371/journal.pone.0084855.
- An X, Zhang AL, May BH, et al. Oral Chinese herbal medicine for improvement of quality of life in patients with stable chronic obstructive pulmonary disease: a systematic review. J Altern Complementary Med. 2012;18:731–743. doi:10.1089/acm.2011.0389.
- An X, Zhang AL, Yang AW, et al. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respir Med. 2011;105(2):165–176. doi:10.1016/j.rmed.2010.11.007.
- Liu J, Gao F, Li Z. Effect of yiqibushenhuoxue decoction on chronic obstructive pulmonary disease measured by St. George’s respiratory disease questionnaire scores and forced expiratory volume. J Tradit Chin Med. 2014;34(4):445–449. doi:10.1016/S0254-6272(15)30044-3.
- Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J. 2013;42(4):982–992. doi:10.1183/09031936.00176312.
- Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2014;2(4):293–300. doi:10.1016/S2213-2600(14)70013-X.
- Holverda S, Rietema H, Bogaard HJ, et al. Acute effects of sildenafil on exercise pulmonary hemodynamics and capacity in patients with COPD. Pulm Pharmacol Ther. 2008;21:558–564. doi:10.1016/j.pupt.2008.01.012.
- Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci. 2011;53(2):81–85.
- Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease (review). Cochrane Database Syst Rev. 2017;9:CD002309.
- Zeng Z, Yang D, Huang X, et al. Effect of carbocisteine on patients with COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulm Dis. 2017;12:2277–2283. doi:10.2147/COPD.S140603.
- Canadian Institute for Health Information. Inpatient Hospitalizations, Surgeries and Childbirth Indicators in 2013–14. Hospital Morbidity Database and Ontario Mental Health Reporting System. Canadian Institute for Health Information. 2015. https://secure.cihi.ca/free_products/CAD_Hospitalization%20and%20Childbirth_Infosheet_ENrev-web.pdf. Accessed June 27, 2019.
- Canadian Institute for Health Information. Which health conditions are the most expensive in 2016-17? Canadian MIS Database and Discharge Abstract Database, Canadian Institute for Health Information. 2019. https://www.cihi.ca/en/which-health-conditions-are-the-most-expensive. Accessed March 18, 2019.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease–2007 update. Can Respir J. 2007;14(Suppl B):5B–32B. doi:10.1155/2007/926421.
- O’Donnell DE, Hernandez P, Kaplan A, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease–2008 update–highlights for primary care. Can Respir J. 2008;15(Suppl A):1A–8A. doi:10.1155/2008/641965.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). The Global Strategy for Diagnosis, Management and Prevention of COPD (updated 2019). 2019. https://goldcopd.org/gold-reports/. Accessed June 27, 2019.
- Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239–1247. doi:10.1183/09031936.00142414.
- Institute for Health Metrics and Evaluation. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Institute for Health Metrics and Evaluation. The Lancet. 2017;390:1151–1210.
- Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070.
- Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/S2213-2600(13)70040-7.
- Izquierdo JL, Cosio BG. The dose of inhaled corticosteroids in patients with COPD: when less is better. Int J Chron Obstruct Pulm Dis. 2018;13:3539–3547. doi:10.2147/COPD.S175047.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154.
- Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS). BMJ. 2013;346:f3306. doi:10.1136/bmj.f3306.
- D’Urzo A, Kerwin E, Overend T, et al. Once daily glycopyrronium for the treatment of COPD: pooled analysis of the GLOW1 and GLOW2 studies. Curr Med Res Opin. 2014;30(3):493–508. doi:10.1185/03007995.2013.858618.
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;Jul 11(7):CD009285.
- Lee SH, Lee J, Yoo KH, et al. Efficacy and safety of aclidinium bromide in patients with COPD: a phase 3 randomized clinical trial in a Korean population. Respirology. 2015;20(8):1222–1228. doi:10.1111/resp.12641.
- Matera MG, Rogliani P, Cazzola M. Indacaterol for the treatment of chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2015;16(1):107–115. doi:10.1517/14656566.2015.983076.
- Oba Y, Lone NA. Comparative efficacy of long-acting muscarinic antagonists in preventing COPD exacerbations: a network metaanalysis and meta-regression. Ther Adv Respir. 2015;9(1):3–15. doi:10.1177/1753465814565624.
- Pleasants RA, Wang T, Gao J, et al. Inhaled umeclidinium in COPD patients: a review and meta-analysis. Drugs. 2016;76(3):343–361. doi:10.1007/s40265-015-0532-5.
- Maia IS, Pincelli MP, Leite VF, et al. Long-acting muscarinic antagonists vs. long-acting β2 agonists in COPD exacerbations: a systematic review and meta-analysis. J Bras Pneumol. 2017;43(4):302–312. doi:10.1590/s1806-37562016000000287.
- Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest. 2017; 151(2):340–357. doi:10.1016/j.chest.2016.11.028.
- Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res. 2015;16(1):92. doi:10.1186/s12931-015-0250-2.
- Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–486. doi:10.1016/S2213-2600(14)70065-7.
- Donohue JF, Singh D, Munzu C, et al. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respir Med. 2016;112:65–74. doi:10.1016/j.rmed.2016.01.001.
- Horita N, Kaneko T. Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan. Int J Chron Obstruct Pulm Dis. 2015;10:813–822.
- Kalberg C, O’Dell D, Galkin D, et al. Dual bronchodilator therapy with umeclidinium/vilanterol versus tiotropium plus indacaterol in chronic obstructive pulmonary disease: a randomized controlled trial. Drugs R D. 2016;16(2):217–227. doi:10.1007/s40268-016-0131-2.
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25. doi:10.1136/thoraxjnl-2014-206732.
- Schlueter M, Gonzalez-Rojas N, Baldwin M, et al. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting beta2-agonists: a systematic review and network meta-analysis. Ther Adv Respir. 2016;10(2):89–104. doi:10.1177/1753465815624612.
- Wedzicha JA, Dahl R, Buhl R, et al. Pooled safety analysis of the fixed-dose combination of indacaterol and glycopyrronium (QVA149), its monocomponents, and tiotropium versus placebo in COPD patients. Respir Med. 2014;108(10):1498–1507. doi:10.1016/j.rmed.2014.07.011.
- Calverley PMA, Anderson JA, Brook RD, et al. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med. 2018;197(1):47–55. doi:10.1164/rccm.201610-2086OC.
- GINA/GOLD. Diagnosis of diseases of Chronic Airflow Limitation: asthma, COPD and asthma-COPD Overlap Syndrome (ACOS). Published 2015. https://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/. Accessed May 28, 2019.
- Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulm Dis. 2014;9:469–479.
- Singh D, Nicolini G, Bindi E, et al. Extrafine beclomethasone/formoterol compared to fluticasone/salmeterol combination therapy in COPD. BMC Pulm Med. 2014;14(1):43. doi:10.1186/1471-2466-14-43.
- Stynes G, Svedsater H, Wex J, et al. Once-daily fluticasone furoate/vilanterol 100/25 mcg versus twice daily combination therapies in COPD—mixed treatment comparisons of clinical efficacy. Respir Res. 2015;16(1):25. doi:10.1186/s12931-015-0184-8.
- Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–1260. doi:10.1056/NEJMoa1608033.
- Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108(8):1153–1162. doi:10.1016/j.rmed.2014.05.013.
- Singh D, Vezzoli S, Petruzzelli S, et al. The efficacy of extrafine beclomethasone dipropionate–formoterol fumarate in COPD patients who are not “frequent exacerbators”: a post hoc analysis of the FORWARD study. Int J Chron Obstruct Pulm Dis. 2017;12:3263–3271. doi:10.2147/COPD.S141416.
- Ohar JA, Crater GD, Emmett A, et al. Fluticasone propionate/salmeterol 250/50 mug versus salmeterol 50 mug after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15(1):105. doi:10.1186/s12931-014-0105-2.
- Ferguson GT, Tashkin DP, Skärby T, et al. Effect of budesonide/formoterol pressurized metered-dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6-month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) study. Respir Med. 2017;132:31–41. doi:10.1016/j.rmed.2017.09.002.
- Hedeker DG, Waternaux RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Stat. 1999;24(1):70–93. doi:10.3102/10769986024001070.
- Horita N, Miyazawa N, Morita S, et al. Long-acting beta-agonists reduce mortality of patients with severe and very severe chronic obstructive pulmonary disease: a propensity score matching study. Respir Res. 2013;14(1):62. doi:10.1186/1465-9921-14-62.
- Oba Y, Chandran AV, Devasahayam JV. Long-acting muscarinic antagonist versus inhaled corticosteroid when added to long-acting beta-agonist for COPD: a meta-analysis. COPD J Chron Obstruct Pulm Dis. 2016;13(6):677–685. doi:10.3109/15412555.2016.1170799.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385.
- Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulm Dis. 2015;10:1015–1026.
- Kwak MS, Kim E, Jang EJ, et al. The efficacy and safety of triple inhaled treatment in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis using Bayesian methods. Int J Chron Obstruct Pulm Dis. 2015;10:2365–2376.
- Liu Y, Shi H, Sun X, et al. Benefits of adding fluticasone propionate/salmeterol to tiotropium in COPD: a meta-analysis. Eur J Intern Med. 2014;25(5):491–495. doi:10.1016/j.ejim.2014.04.007.
- Rojas-Reyes MX, Garcia Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;Jun 6(6):CD008532.
- Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5.
- Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X.
- Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC.
- Carone M, Donner CF, Jones PW. Health status measurement: an increasingly important outcome evaluation in COPD patients. Monaldi Arch Chest Dis. 2001;56(4):297–298.
- Hanania NA, Calverley PM, Dransfield MT, et al. Pooled subpopulation analyses of the effects of roflumilast on exacerbations and lung function in COPD. Respir Med. 2014;108(2):366–375. doi:10.1016/j.rmed.2013.09.018.
- Luo J, Wang K, Liu D, et al. Can roflumilast, a phosphodiesterase-4 inhibitor, improve clinical outcomes in patients with moderate-to-severe chronic obstructive pulmonary disease? A metaanalysis. Respir Res. 2016;17(1):18. doi:10.1186/s12931-016-0330-y.
- Luo P, Li S, Chen Y, et al. Efficiency and safety of roflumilast combined with long-acting bronchodilators on moderate-to severe stable chronic obstructive pulmonary disease patients: a meta-analysis. J Thorac Dis. 2016;8(9):2638–2645. doi:10.21037/jtd.2016.09.12.
- Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. doi:10.1016/S0140-6736(14)62410-7.
- Yan JH, Gu WJ, Pan L. Efficacy and safety of roflumilast in patients with stable chronic obstructive pulmonary disease: a meta-analysis. Pulm Pharmacol Ther. 2014;27(1):83–89. doi:10.1016/j.pupt.2013.04.004.
- Martinez FJ, Rabe KF, Calverley MA, et al. Determinants of response to roflumilast in severe chronic obstructive pulmonary disease pooled analysis of two randomized trials. Am J Respir Crit Care Med. 2018;198(10):1268–1278. doi:10.1164/rccm.201712-2493OC.
- Shen LF, Lv XD, Chen WY, et al. Effect of roflumilast on chronic obstructive pulmonary disease: a systematic review and meta-analysis. Irish J Med Sci. 2018;187(3):731–738. doi:10.1007/s11845-018-1738-9.
- Ayfer Aytemur Z, Baysak A, Ozdemir O, et al. N-acetylcysteine in patients with COPD exacerbations associated with increased sputum. Wien Klin Wochenschr. 2015;127(7-8):256–261. doi:10.1007/s00508-014-0692-4.
- Cazzola M, Calzetta L, Page C, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015;24(137):451–461. doi:10.1183/16000617.00002215.
- Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;Jul 29(7):CD001287.
- Shen Y, Cai W, Lei S, et al. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD J Chron Obstruct Pulm Dis. 2014;11:351–358. doi:10.3109/15412555.2013.858315.
- Tse HN, Raiteri L, Wong KY, et al. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014;146(3):611–623. doi:10.1378/chest.13-2784.
- Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2(3):187–194. doi:10.1016/S2213-2600(13)70286-8.
- Dal Negro RW, Wedzicha JA, Iversen M, et al. Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study. Eur Respir J. 2017;50(4):1700711. doi:10.1183/13993003.00711-2017.
- Cazzola M, Rogliani P, Calzetta L, et al. Impact of mucolytic agents on COPD exacerbations: a pair-wise and network meta-analysis. COPD J Chron Obstruct Pulm Dis. 2017; 14(5):552–563. doi:10.1080/15412555.2017.1347918.
- Cazzola M, Calzetta L, Page C, et al. Impact of erdosteine on chronic bronchitis and COPD: a meta-analysis. Pulm Pharmacol Ther. 2018;48:185–194. doi:10.1016/j.pupt.2017.11.009.
- Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503–1508. doi:10.1164/rccm.201402-0207OC.
- Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One. 2015;10(3):e0121257. doi:10.1371/journal.pone.0121257.
- Simpson JL, Powell H, Baines KJ, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised placebo controlled trial. PLoS One. 2014;9(8):e105609. doi:10.1371/journal.pone.0105609.
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368. doi:10.1016/S2213-2600(14)70019-0.
- Devereux G, Cotton S, Fielding S, et al. Effect of theophylline as adjunct to inhaled corticosteroids on exacerbations in patients with COPD a randomized clinical trial. JAMA. 2018;320(15):1548–1559. doi:10.1001/jama.2018.14432.
- Bremner PR, Birk R, Brealey N, et al. Single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: a randomized noninferiority study. Respir Res. 2018;19(1):19. doi:10.1186/s12931-018-0724-0.
- Feldman GJ, Sousa AR, Lipson DA, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: a randomized study. Adv Ther. 2017;34(11):2518–2533. doi:10.1007/s12325-017-0626-4.
- Kerwin E, Ferguson GT, Sanjar S, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung. 2017;195(6):739–747.
- Landis SH, Suruki R, Hilton E, et al. Stability of blood eosinophil count in patients with COPD in the UK Clinical Practice Research Datalink. COPD J Chron Obstruct Pulm Dis. 2017;14(4):382–388. doi:10.1080/15412555.2017.1313827.
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800.
Appendix 1
A) Search strategy
B)Flow chart of search results: PRISMA diagram
Literature search — March 1, 2017 to August 31, 2018 (CTS/McMaster Plus database under each topic area) with selected relevant manuscripts included with publication dates up to October 31, 2018
Reference: Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement; 2009. PLoS Med. 6(7): e1000097. www.prisma-statement.org.
C) Strength of the recommendations grading system
D) Voting scales for assessing consensus on draft recommendations
Appendix 2: Summary of updates by section/recommendation

