 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was designed to investigate the protective effects of propolis and vitamin E against time-related metal (aluminum chloride)-induced toxicity on the male reproductive system in albino mice (n = 63). Animals were randomly divided into seven groups (n = 9 each). Group I (control) received 0.9% saline solution; group II was given 50 mg/kg of aluminum chloride (AlCl3); groups III and IV were administered vitamin E (150 mg/kg) and propolis (50 mg/kg), respectively; and groups V, VI and VII were administered AlCl3 + vitamin E, AlCl3 + propolis, and AlCl3 + vitamin E + propolis, respectively, for 45 days. Animals were sacrificed; blood and tissue samples were collected (thrice; fortnightly) for the estimation of hormones i.e., Follicle stimulating hormone (FSH), Luteinizing hormone (LH) and testosterone using Enzyme linked immunosorbent assay (ELISA). Testicular histology, testicular weight, sperm count and motility were estimated. Group II revealed a highly significant decrease in LH, FSH, testosterone, testis weight, sperm count and motility. Groups III–VII showed a significant increase in LH, FSH, testosterone, organ weight, sperm count and motility in the 3rd sampling, as compared to group II. Hence, it is concluded that vitamin E and propolis, acting individually as well as synergistically, mitigate metal-induced time-related toxicity on the reproductive system and testicular histology of male mice.
Introduction
Aluminum is used extensively nowadays, in industries, pharmaceutical products containing phosphate binders, food additives, and certain antacids, causing detrimental effects to human beings (Proudfoot Citation2009). Aluminum provokes neurotoxicity, cardiotoxicity, hepatotoxicity and nephrotoxicity by inducing oxidative stress, due to its capacity to produce enormous amounts of free radicals (Sushma & Rao Citation2007). Moreover, metals affect spermatogenesis in rodents as well as in humans, leading to lowering of sperm count, abnormal sperm morphology and poor semen quality (Pizent et al. Citation2012). Different antioxidants are known to attenuate aluminum-induced toxicity; for example, it was reported in a previous study that Allium cepa mitigated aluminum-induced hepatotoxicity (Ige et al. Citation2017). In another study, treatment with açai and vitamin C improved the adverse effects resulting from aluminum chloride-induced hepatic damage (Ahmed & Hamaad Citation2018). Propolis mitigates concanavalin A-induced hepatitis by modulating cytokine secretion and inhibiting reactive oxygen species (ROS) (Mounieb et al. Citation2017). Propolis exhibits a prophylactic role against cyclophosphamide-induced hepatic and renal toxicity in mice, and it produced an improvement in biochemical parameters and histological findings as well (El-Naggar et al. Citation2015). Brazilian green propolis displayed anti-tumor activity by acting synergistically with protoporphyrin IX (PpIX) to attenuate the expression of p-IKKα/β, NF-κB and COX-2 (Wang et al. Citation2017). Vitamin E (tocopherol) works as a biological antioxidant to protect cellular macromolecules (DNA, protein, lipids) from uncontrolled oxidation by free radicals (Huang & Huang Citation2004). Vitamin E, vitamin C and Omega-3 were shown to protect against aluminum chloride-induced liver and kidney toxicity in female albino mice (Gorgees et al. Citation2016). Recent studies have reported that Vitamin E and metallothionein alleviate Cd-induced hepatotoxicity through their antioxidative and antiapoptotic effects (Duan et al. Citation2018). The present study was designed to investigate the effects of propolis and vitamin E against metal-induced reproductive toxicity, in a time-dependent manner.
Materials and methods
Chemicals used
AlCl3 from AnalaR, BDH Laboratory Supplies Poole, BH151TD, England; Lot No. 2918300;
Vitamin E capsules, 200 mg (Martin Dow Markers Ltd; B # 7373);
Sunflower oil, Hamza vegetable oil refinery, Pvt, Ltd. B # 164 H-2; License No. CM/L-907/1997 (R);
0.9% saline (sodium chloride), Otsuka Pakistan Ltd. F/4-9, Balochistan, Pakistan.
Ethanolic extraction of propolis
Propolis was extracted with 100 mL of ethanol 70% at ambient temperature, in the absence of bright light and with moderate shaking for 1 week. Extracts were filtered and concentrated to dryness with a rotary evaporator at 50 ± 1°C to give solid residues (Sforcin et al. Citation2002).
Experimental design
Healthy male albino mice (n = 63) 3–4 months of age and weighing 23–35 g were obtained from the University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan. Animals were kept under the standard laboratory conditions (22–26°C and 12 h light:dark cycle), and fed on commercial pelleted food. Water was available ad libitum. The experiment was performed in compliance with the guidelines of the Research Ethical Review Committee of Lahore College for Woman University (Memo number: RERC/Zoo/2018). Mice were acclimatized for 2 weeks and randomly divided into seven groups (n = 9 each). Group I (control) received 0.9% saline solution, group II received AlCl3 (50 mg/kg of body weight; Shahraki et al. Citation2008), group III was treated with vitamin E (150 mg/kg of body weight) administered using sunflower oil as a vehicle, group IV was administered ethanolic extract of propolis (50 mg/kg of body weight; Al-Qayim et al. Citation2014), group V received AlCl3 and vitamin E (Sforcin et al. Citation2002; Abdel-Hamid Citation2013), group VI was administered AlCl3 and propolis, and group VII was treated with AlCl3, vitamin E and propolis.
Sampling protocol
Three animals were euthanized, and samples of their blood and tissue were collected fortnightly, every 15th day, over a period of 45 days for the estimation of hormones i.e., Follicle stimulating hormone (FSH), Luteinizing hormone (LH) and testosterone using Enzyme linked immunosorbent assay (ELISA), and for testicular histology. Blood was collected by cardiac puncture and animals were dissected for the collection of testicular tissues. Serum was separated using centrifugation at 3500–4000 rpm for 10–15 min and stored below −10°C until it was used for the estimation of hormonal parameters with commercially available ELISA kits (PISHTAZ TEB DIAGNOSTICS kit, Cat No. PT-FSH-96, DiaMetra diagnostic kits, DCM 009–11. Via Pozzuolo 14, 06038 SPELLO8 SPELLO (PG) Italy and Human Diagnostics Worldwide kit, REF 55010. Human Gesellschaft fur Biochemica und Diagnostica mbH. Max-Plank-Ring 21.65205 Wisebaden, Germany). Tissues of testis were rinsed in 0.9% saline solution (Ali et al. Citation2019) and fixed to process for histology through microtomy.
Histopathological examination
Testicular tissues were processed for histological examination using the standard protocol of fixation, embedding and staining using hematoxylin and eosin stains (H&E; Drury & Wallington Citation1980; Srivastava & Yadav Citation2007). The slides, prepared to a thickness of 5 µm, were studied and photographed under a Trinocular camera-fitted microscope (E- 200, digital microscopic camera- Nikon Japan Ei1-L2).
Organ weight analysis
Reproductive organs (testis, epididymis, vas deferens and seminal vesicles) were collected from control and experimental groups fortnightly during the 45-day sampling period. Organ fat was removed for determining absolute organ weight. Relative organ weight was calculated using the following formula (Abdel-Azim et al. Citation2015):
Sperm count and motility
Sperm count and motility were determined following the previously described protocol (Geng et al. Citation2015). For this purpose the epididymis of mice was homogenized in 2 mL of warm Phosphate buffered saline solution (PBS; 37°C). This solution (20 mL) was then used for measuring sperm count and motility using a hemocytometer. Sperm motility was evaluated as motile sperm percentage in the total spermatozoa, according to the previously described protocol (Zhao et al. Citation2015).
Statistical analysis
Data were statistically analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s T3 post hoc test for estimating significant difference between different groups, and Pearson’s correlation test was used to find the correlation among LH, FSH and testosterone using SPSS v. 19 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2007. A value of p < 0.05 was considered significant, and p < 0.01 was considered highly significant.
Results
Animals treated with AlCl3 (Group II) showed a highly significant decrease in serum FSH, LH and testosterone levels at the 3rd sampling as compared to the 1st and 2nd samplings (). All groups showed non-significant changes in serum FSH, LH and testosterone level at the 2nd sampling as compared to the 1st sampling. Groups III–VII showed a significant increase in LH, FSH and testosterone as compared to group II at the 3rd sampling (). The data obtained showed a highly significant positive correlation between LH and FSH concentration during the 1st sampling (r = 0.469**). FSH and testosterone showed no correlation during week 1 (r = 0.074). LH and FSH showed highly significant positive correlation (r = 0.698**) in the treated group VII during the 3rd sampling. FSH and testosterone showed a highly significant positive correlation (r = 0.523**) during the 3rd sampling. Group II showed a significant reduction in absolute and relative weight of testis, epididymis, vas deferens and seminal vesicle at the 3rd sampling ( and ). Moreover, sperm count and motility were significantly decreased in group II. On the other hand, Group VII showed a highly significant increase in the aforementioned parameters (organ weight, sperm count and motility) at the 3rd sampling in the present study ( and ).
Table I. Serum Follicle stimulating hormone (FSH ng/mL), Luteinizing hormone (LH ng/mL) and testosterone (ng/mL) of male albino mice in Control (I), Al (II), vitamin E (III), propolis (IV), Al+vitamin E (V), Al+propolis (VI), Al+vitamin E+propolis (VII) groups after 1st, 2nd and 3rd samplings
Table II. Time-related effects of different treatments on absolute and relative weight of male reproductive organs after 1st, 2nd and 3rd samplings
Table III. Comparison of sperm count and motility between the control and treatment groups after 1st, 2nd and 3rd samplings
Histological findings in group I showed normal testicular anatomy, including seminiferous tubules with smooth outlines surrounded by thin basement membrane. Group II showed a deterioration in the testicular structure as compared to the control group at the 1st sampling (). Group III showed severe degeneration of seminiferous tubules, with necrotic spermatogenic cells and congestion of the blood vessels, at the 3rd sampling (). Groups III and IV showed normal structure of spermatocytes at the 1st and 3rd samplings (). Groups V and VI showed damage to interstitial cells at the 1st sampling, whereas tissues at the 3rd sampling showed normal structure of seminiferous tubules (). Group VII displayed a more or less normal histological appearance of testis at the 3rd sampling ().
Figure 1. (a) Control group during 1st and 3rd week of treatment showing normal structure at (400x)Spermatid population (Sp P), lumen (L), seminiferous tubules (S T), basement membrane (BM),spermatocytes (Sp), interstitial cells (I C), interstitial spaces (I S), tunica albuginea (T A). (b) Testes ofmice treated with AlCl3 for 1 week showing damaged testicular tissues at (100x) Interstitial spaces (I S),lumen (L), blood vessels (B V), seminiferous tubules (S T), shrunken interstitial cells (S I C), damagedbasement membrane (D B M). (c) Cross section of testis of male albino mice treated with AlCl3 for twoweeks showing damaged testicular tissues at (100x), (H & E). Tunica albuginea (T A), irregular shapedseminiferous tubules (Ir S T), interstitial spaces (I S), basement membrane (B M), interstitial cells (I C),Information Classification: Generaland spermatocytes (Sp). (d) Testes of mice treated with AlCl3 for three weeks (400x) Vacuolation (V),damaged spermatocytes (Sp), lumen (L), interstitial cells (I C), irregular shaped seminiferous tubules (ir ST), basement membranes (B M). (e) Testes of mice treated with Vitamin E for 1 week (400x)Seminiferous tubules (S T), lumen (L), interstitial cells (I C), spermatocytes (Sp). (f) Cross section oftestes of vitamin E treated male albino mice for two weeks showing normal structure at (400x), (H & E).Spermatid population (Sp P), basement membrane (B M), interstitial cells (I C), lumen (L), spermatocytes(Sp). (g) Testes of mice treated with vitamin E for three weeks (400x), (H & E) Basement membranes(BM), interstitial cells (I C), lumen (L), spermatid population (Sp P). (h) Testes of mice treated for oneweek with propolis (400x)
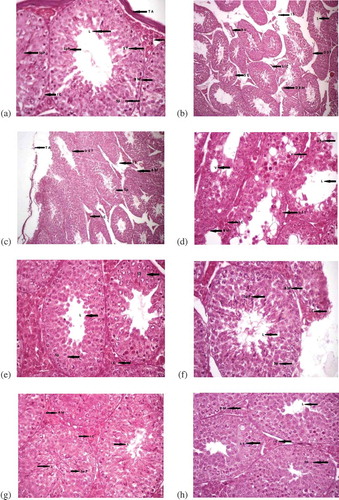
Figure 1. (Continued). (i) Cross section of testes of male albino mice treated with propolis for twoweeks showing normal structure at (400x), (H & E). Spermatocytes (Sp), interstitial cells (I C), basementmembranes (B M), seminiferous tubules (S T). (j) Testes of mice treated with propolis for three weeks(400x), (H & E). Lumen (L), basement membrane (B M), interstitial cells (I C), interstitial spaces (I S),seminiferous tubules. (k) Testes of mice treated with AlCl3 + vitamin E after 1 week (100x). Interstitialcells (I C), irregular shaped seminiferous tubules (Ir S T), damaged seminiferous tubules (D S T), bloodvessels (B V), interstitial spaces (I S), seminiferous tubules regular in shape (R S T), leydig cells (L C). (l)Cross section of testes of male albino mice showing effects after treatment with AlCl3 and vitamin E fortwo weeks at (100x), (H & E). The structure of seminiferous tubules (S T), lumen of the S T (L),interstitial spaces (I S). (m) Testes of mice showing effects after treatment with AlCl3 + vitamin E forthree weeks (100x). Seminiferous tubules (S T), basement membranes (B M), lumen having cells in it(L(c)), interstitial spaces (I S), interstitial cells (I C). (n) Testes of mice treated with AlCl3 and propolisfor 1 week (100x). Intercellular spaces (I S), decreased lumen (De L), basement membranes (B M),=irregular seminiferous tubules (Ir S T), contracted interstitial cells (I C). (o) Cross section of testes ofmale albino mice showing effects after treatment with AlCl3 and propolis for two weeks at (100x), (H & E). Tunica albuginea (T A), damaged seminiferous tubules (D S T), interstitial cells (I C), blood vessels(B V). (p) Testes of mice treated with AlCl3 and propolis for three weeks (100x), (H & E). Seminiferoustubules (S T), interstitial cells (I C), blood vessels (B V), leydig Cells (L C)
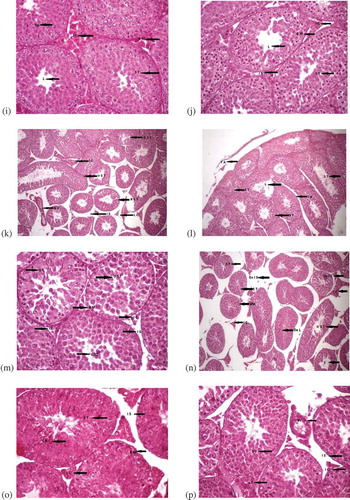
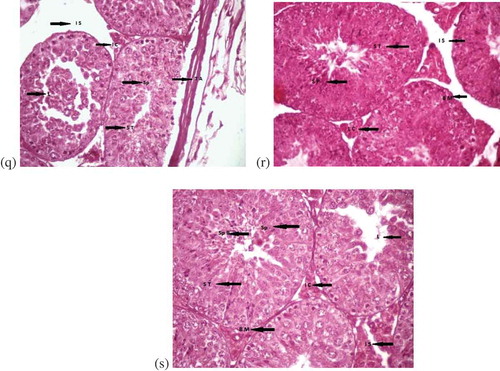
Figure 1. (Continued). (q) Testes of mice treated with AlCl3, vitamin E and propolis for one week at (400x). Interstitial spaces (I S), spermatocytes (Sp), seminiferous tubules (S T), tunica albuginea (T A). (r) Cross section of testes of male albino mice showing effects after treatment with AlCl3, vitamin E and propolis for two weeks at (400x), (H & E). Spermatocytes (Sp), interstitial spaces (I S), leydig cells (L C), basement membranes (B M), seminiferous tubules (S T). (s) Testes of mice with AlCl3, vitamin E and treated with propolis for three weeks (400x). Spermatid population (Sp P), spermatocytes (Sp), interstitial cells (I C), interstitial spaces (I S), basement membranes (B M), lumen (L), seminiferous tubules (S T)
Discussion
The results reveal that AlCl3 decreased the serum FSH and LH levels in a time-related order. The decrease in serum FSH and LH might be due to AlCl3-induced damage to interstitial cells of Leydig (Shahraki et al. Citation2008). AlCl3 intake affects the release of LH from the pituitary, which further decreases the level of testosterone. Moreover, a previous study also reported that AlCl3 predisposes its toxic effects by inhibiting Ca2+ ions release. Ca2+ ions were not measured in the current study, but previous studies indicate that aluminum decreases ionized calcium and increases bound calcium (along with anions or albumin; Llach et al. Citation1986). Ca2+ ions are responsible for gonadotropin (GnRH) exocytosis via synaptotagmin secretory vesicle (Stojilkovic Citation2005). Moreover, a previous study reported that the aforementioned mechanism is important for FSH and LH secretion via granin-independent and -dependent pathways, respectively (Nicol et al. Citation2004; Shahraki et al. Citation2008). Hence, it is evident from previous results that the decrease in FSH and LH might be related to a deficiency of ionized calcium. Furthermore, Ca2+ deficiency might lead to aluminum accumulation in different tissues, because Ca2+ deficiency cause excess parathyroid hormone (PTH) secretion and PTH absorbs aluminum along with Ca2+ from renal tubules (Provan & Yokel Citation1990). The current study demonstrates that co-administration of propolis and vitamin E mitigates the toxic effects of AlCl3 in a time-dependent manner, supporting the results of a previous study in which propolis ameliorated the toxic effect of AlCl3 on renal histology of wistar albino rats (El-Kenawy et al. Citation2014). According to another study, propolis showed marked protection against AlCl3-induced reproductive toxicity (Yousef & Salama Citation2009). Moreover, the present study revealed that co-administration of propolis and vitamin E along with AlCl3 produced more protective effects on FSH, LH and testosterone levels as compared to their individual effects. A previous study reported that administration of either vitamin C or vitamin E along with AlCl3 resulted in evident protection against AlCl3-induced cytotoxic effects, by decreasing thiobarbituric acid-reactive substances (TBARS) that are responsible for increasing ROS production, causing further oxidative damage (Yousef et al. Citation2007; Mondal et al. Citation2016). The decreased organ weight, sperm count and motility observed in the present study might be due to testicular damage, as it was earlier reported that AlCl3 exposure resulted in degeneration of seminiferous tubules, changes in the cytoplasm, damaged sertoli cells and disruption of spermatozoa (Khattab Citation2007). In the current study, vitamin E and propolis showed normal structure and improved toxic alterations when concomitantly administered with AlCl3 in the last week of treatment. A previous study reported that propolis provides protection against AlCl3-induced testicular damage, as indicated by increased male reproductive organ weight, sperm count and motility, and by the normal structure of the seminiferous tubules (Yousef & Salama Citation2009).
Conclusion
Being antioxidants, propolis and vitamin E effectively reduce the degenerative alterations in testicular tissues caused by aluminum chloride. A combination of propolis and vitamin E acted synergistically and therefore dramatically restored the alterations in organ weight, sperm count and motility caused by metal-induced toxicity in testicular tissues. It is evident from the present study that the severity of AlCl3 damage is time dependent, and more pronounced mitigating effects of both vitamin E and propolis were observed in the last sampling run of the experimental protocol. Hence, this study suggests that long-term treatment with natural antioxidants is effective in attenuating metal-induced reproductive damage, which might be due to its free radical scavenging activity.
PEAK_TABLE_DR_SUMERA_SAJJAD_.pdf
Download PDF (23.7 KB)LIBRARY_SEARCH_SUMERA_SAJJAD_.pdf
Download PDF (946.7 KB)CROMATOGRAM_x__y_.pdf
Download PDF (57 KB)europ_j_ethical_com.pdf
Download PDF (164.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemented data of this article can be accessed here.
References
- Abdel-Azim SA, Darwish HA, Rizk MZ, Ali SA, Kadry MO. 2015. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: Possible role of some antioxidants. Experimental and Molecular Pathology 67:305–314.
- Abdel-Hamid GA. 2013. Effect of vitamin E and selenium against aluminum induced nephrotoxicity in pregnant rats. Folia Histochemica et Cytobiologica 51:312–319. DOI: 10.5603/FHC.2013.0042.
- Ahmed NM, Hamaad FA. 2018. Protective effects of Açai in combination with vitamin C against aluminum-induced toxicity in rat liver. Journal of Biology and Life Science 9:1–16. DOI: 10.5296/jbls.v9i1.11670.
- Ali BH, Al-Salam S, Adham SA, Al Balushi K, Za’abi A, Beegam S, Yuvaraju P, Manoj P, Nemmar A. 2019. Testicular toxicity of water pipe smoke exposure in mice and the effect of treatment with nootkatone thereon. Oxidative Medicine and Cellular Longevity 2019: 1–10.
- Al-Qayim AJ, Ghali L, Al-Azwai T. 2014. Comparative effects of propolis and malic acid on hematological parameters of aluminum exposed male rats. Global Journal of Bio-Science and BioTechnology 3:6–11.
- Drury RAB, Wallington EA. 1980. Carltons Histopathologic technique. 5th ed. New York: Oxford University press.
- Duan Y, Duan J, Feng Y, Huang X, Fan W, Wang K, Ouyang P, Deng Y, Du Z, Chen D, Geng Y. 2018. Hepatoprotective activity of Vitamin E and metallothionein in cadmium-induced liver injury in Ctenopharyngodon idellus. Oxidative Medicine and Cellular Longevity 2018:1–12.
- El-Kenawy A-M, Hussein Osman HE, Daghestani MH. 2014. Role of propolis (bee glue) in improving histopathological changes of the kidney of rat treated with aluminum chloride. Environmental Toxicology 29:1000–1010. DOI: 10.1002/tox.21830.
- El-Naggar SA, Alm-Eldeen AA, Germoush MO, El-Boray KF, Elgebaly HA. 2015. Ameliorative effect of propolis against cyclophosphamide-induced toxicity in mice. Pharmaceutical Biology 53:235–241. DOI: 10.3109/13880209.2014.914230.
- Geng X, Shao H, Zhang Z, Ng JC, Peng C. 2015. Malathion-induced testicular toxicity is associated with spermatogenic apoptosis and alterations in testicular enzymes and hormone levels in male Wistar rats. Journal of Environmental Toxicology and Pharmacology 39:659–667. DOI: 10.1016/j.etap.2015.01.010.
- Gorgees N, Al-Habbib O, Hussein R. 2016. The protective role of certain antioxidants (vitamin C, E and Omega-3) against aluminum chloride induced histological changes in the liver and kidney of female albino rats (Rattus Rattus Norvegicus). Science Journal of University of Zakho 2:591–603.
- Huang CH, Huang SL. 2004. Effect of dietary vitamin E on growth, tissue lipid peroxidation and liver glutathione level of juvenile hybrid tilapia, Oreocromis niloticus x O. aureus fed oxidized oil. Aquaculture 237:381–389. DOI: 10.1016/j.aquaculture.2004.04.002.
- Ige SF, Adeniyi MJ, Iyalla GO. 2017. Allium cepa mitigates aluminum chloride-induced hepatotoxicity in male wistar rats. The Journal of Biomedical Science 6(4):27.
- Khattab FKI. 2007. Histological studies on the testis of rats after treatment with aluminium chloride. The Australian Journal of Basic and Applied Sciences 1:63–72.
- Llach F, Rodríguez M, Felsenfeld A. 1986. Aluminium effect on serum calcium concentration. Nefrología 6:63–65.
- Mondal R, Biswas S, Chatterjee A, Mishra R, Mukhopadhyay A, Bhadra RK, Mukhopadhyay PK. 2016. Protection against arsenic-induced hematological and hepatic anomalies by supplementation of vitamin C and vitamin E in adult male rats. The Journal of Basic and Clinical Physiology and Pharmacology 27:643–652. DOI: 10.1515/jbcpp-2016-0020.
- Mounieb F, Ramadan L, Akool ES, Balah A. 2017. Propolis alleviates concanavalin A-induced hepatitis by modulating cytokine secretion and inhibition of reactive oxygen species. Naunyn-Schmiedeberg’s Archives of Pharmacology 390:1105–1115. DOI: 10.1007/s00210-017-1410-3.
- Nicol L, McNeilly JR, Stridsberg M, McNeilly AS. 2004. Differential secretion of gonadotrophins: Investigation of the role of secretogranin II and chromogranin A in the release of LH and FSH in LbetaT2 cells. The Journal of Molecular Endocrinology 32:467–480. DOI: 10.1677/jme.0.0320467.
- Pizent A, Tariba B, Živković T. 2012. Reproductive toxicity of metals in men. Archives of Industrial Hygiene and Toxicology 63:35–46. DOI: 10.2478/10004-1254-63-2012-2151.
- Proudfoot AT. 2009. Aluminium and zinc phosphide poisoning. Clinical Toxicology 47:89–100. DOI: 10.1080/15563650802520675.
- Provan SD, Yokel RA. 1990. Reduced intestinal calcium and dietary calcium intake, increased aluminium absorption, and tissue concentration in the rat. Biological Trace Element Research 23:119–132. DOI: 10.1007/BF02917183.
- Sforcin JM, Novelli ELB, Funari SRC. 2002. Seasonal effect of Brazilian propolis on seric biochemical variables. Journal of Venomous Animals and Toxins 8:244–254. DOI: 10.1590/S0104-79302002000200005.
- Shahraki MR, Palan Mony EY, Zahed Asl S, Sarkaki AR, Shahraki AR. 2008. Effects of aluminium chloride injection in lateral ventricle on serum gonadothropines, testosterone and spermatogenesis in rats. The International Journal of Medical Sciences 8:410–414.
- Srivastava M, Yadav RS. 2007. Principles of laboratory techniques and methods. 1st ed. Lucknow: International Book Distributing Company. pp. 249.
- Stojilkovic SS. 2005. Ca2+ regulated exocytosis and SNARE function. Trends in Endocrinology and Metabolism 16:81–83. DOI: 10.1016/j.tem.2005.02.002.
- Sushma NJ, Rao KJ. 2007. Total ATPases activity in different tissues of albino mice exposed to aluminium acetate. The Journal of Environmental Biology 28:483–484.
- Wang CC, Wang YX, Yu NQ, Hu D, Wang XY, Chen XG, Liao YW, Yao J, Wang H, He L, Wu L. 2017. Brazilian green propolis extract synergizes with Protoporphyrin IX-mediated photodynamic therapy via enhancement of intracellular accumulation of protoporphyrin ix and attenuation of NF-κB and COX-2. Molecules 22:1–13.
- Yousef MI, Kamel KI, El-Guendi MI, El-Demerdash FM. 2007. An in vitro study on reproductive toxicity of aluminium chloride on rabbit sperm: The protective role of some antioxidants. Toxicology 239:213–223. DOI: 10.1016/j.tox.2007.07.011.
- Yousef MI, Salama AF. 2009. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food and Chemical Toxicology 47:1168–1175. DOI: 10.1016/j.fct.2009.02.006.
- Zhao H, Jin B, Zhang X, Cui Y, Sun D, Gao C, Gu Y, Cai B. 2015. Yangjing capsule ameliorates spermatogenesis in male mice exposed to cyclophosphamide. Evidence-Based Complementary and Alternative Medicine 2015:1–9.

