ABSTRACT
Objective: The objective of this study is to analyze whether sociodemographic characteristics or cardiovascular risk factors differ in children and adolescents at the beginning of a lifestyle intervention (LI) for obesity within Germany.
Methods: A total of 40,942 children and adolescents with German residence from the APV-registry were included. Subjects were assigned to the 16 federal states of Germany according to their postal code. Sociodemographic and cardiovascular risk factors at the beginning of a LI for obesity were compared between the federal states. Logistic models were implemented for the prevalence of extreme obesity, hypertension, dyslipidemia, abnormal carbohydrate metabolism, nonalcoholic fatty liver disease (NAFLD), and treatment modality (inpatient vs. outpatient).
Results: Age at the beginning of a LI ranged from 11.5 to 13.5 years. Proportion with a migration background was between 5.8% and 49.7%. Within Germany, extreme obesity in children and adolescents initiating a LI strongly differed between 35.6% and 50.8%. Regional differences were also found for obesity-related risk factors: hypertension (39.0–68.1%), dyslipidemia (24.9–44.6%), NAFLD (9.4–20.4%), abnormal carbohydrate metabolism (0.7–6.2%) (all p < 0.0001). Inpatient treatment varied between 11.2% and 88.2%. Overall, no clear regional pattern was observed.
Conclusion: Several factors as individual socioeconomic status, personal attitude, treatment accessibility, or regional differences in reimbursement decisions might have contributed to the disparities.
Introduction
Childhood obesity is a serious public health problem which increases the risk for physical and psychological comorbidities [Citation1]. Worldwide but also within one continent, there are large differences in the prevalence of overweight and obesity [Citation2–Citation4]. In northern or central European countries (e.g. Sweden, Germany), 10–20% of the pediatric population is at least overweight [Citation2,Citation5] whereas in some southern European countries, 30% up to 40% can be classified as overweight (e.g. Portugal, Spain, Greece) [Citation2,Citation4,Citation6]. Differences in the prevalence of overweight or obesity are not only present between countries but also within countries as for example reported for the USA or some European countries [Citation7–Citation9]. A German analysis of children starting school indicated differences between the 16 federal states. Prevalence for overweight ranged from 8.4% to 11.9% and for obesity between 3.3% and 5.4% [Citation9].
For children and adolescents who are already overweight or obese, several lifestyle interventions (LIs) are offered. In Germany, inpatient as well as outpatient intervention programs are available [Citation10,Citation11]. In a study analyzing 1916 children (8 to <16 years of age) from Germany participating at a LI for obesity, therapy was more successful in less obese and in younger children [Citation12]. Similar results were reported by other studies [Citation10,Citation13] which emphasizes the importance of an early and effective intervention in childhood obesity. However, a recently published trend analysis indicated that during the last decade (from 2005 to 2015), children and adolescents from Germany became more obese at the onset of a LI and obesity-related comorbidities became more frequent [Citation11]. Aside from this temporal perspective, information on potential regional differences is of importance. Participation in LIs depends on motivation and acceptance in both children and family [Citation14], on local availability [Citation15] as well as on financing by insurance providers. All these determinants may vary regionally within one country and could lead to differences in baseline characteristics at the beginning of a LI.
We therefore aimed to investigate sociodemographic and clinical differences between children and adolescents starting a LI for obesity comparing the 16 federal states of Germany.
Materials and methods
Patients and data documentation
Data from the German/Austrian/Switzerland Adiposity Patients Registry (APV) were used for the present analysis. APV is a standardized multicenter database for prospective documentation of anthropometric and metabolic parameters in overweight or obese children and adolescents and is used by centers specialized in pediatric obesity care (inpatient rehabilitation or outpatient). Anonymized data are transmitted from participating APV centers to Ulm, Germany, and aggregated into a cumulative database for clinical research and quality assurance [Citation11]. Twice a year, implausible and inconsistent data are reported back to the centers for verification or correction. The APV initiative is authorized by the Ethics Committee of the University of Ulm, Germany.
Until July 2017, 109,968 patients from 206 centers were registered in APV. Overweight patients (body mass index [BMI] ≥90th percentile) under the age of 20 years and documentation between the years 2010 and 2017 were included in this analysis. Only children and adolescents with German residence were included, resulting in 40,942 subjects from 152 APV centers (Supplement Fig. S1). Subjects were assigned to the respective federal state of Germany corresponding to the postcodes of their current residence.
Anthropometric measurements and biochemical parameters
BMI was given as standard deviation score, using nationally representative reference values [Citation16]. Overweight was defined as a BMI ≥90th sex and age-specific percentile, obesity as a BMI ≥97th, and extreme obesity as a BMI ≥99.5th percentile [Citation16]. To define hypertension, the 95th age- and sex-specific percentile of the KiGGS study was used [Citation3]. Dyslipidemia was defined by at least one abnormal lipid value (total cholesterol [C] ≥ 200 mg/dl, LDL-C ≥ 130 mg/dl, HDL-C ≤ 35 mg/dl, triglycerides ≥ 150/500 mg/dl) (fasting/non-fasting) [Citation17]. A nonalcoholic fatty liver disease (NAFLD) was assumed by at least one increased value for GOT, GPT, or γGT (>50 U/l, respectively) [Citation18] and abnormal carbohydrate metabolism as fasting glucose level ≥5.6 mmol/l (100 mg/dl) or 2 h glucose level ≥7.8 mmol/l (≥140 mg/dl) [Citation15]. Clinical characteristics were measured in local laboratories according to national guidelines [Citation19]. For each patient, the records during the first 6 weeks after their initial visit were aggregated and analyzed.
Statistical analysis
Descriptive statistics were implemented for the whole study population. Sociodemographic and clinical characteristics were presented as median (Q1; Q3) or as percentage. The percentage of patients with comorbidities refers to the number of patients with documented examinations and not to the total number of subjects included. Sociodemographic data were also stratified for the 16 federal states of Germany.
Logistic regression models, adjusted for age, sex, and migration background (defined when the child or at least one parent was born outside of Germany) were applied for dichotomous variables (prevalence of extreme obesity, hypertension, dyslipidemia, abnormal carbohydrate metabolism, NAFLD, treatment modality [inpatient or outpatient care]). Logistic models for comorbidities were additionally adjusted for weight category. Federal state was included as predictor. For each variable, regional differences were illustrated in tertile-based choropleth maps using traffic light colors for cardiovascular risk factors and three different shades of blue for the type of treatment (inpatient or outpatient care). Since patients’ characteristics can differ between outpatient and inpatient care [Citation11], a further regression model for obesity and obesity-related comorbidities was calculated including inpatient treatment as additional confounder.
A two-sided p-value <0.05 was considered significant. All statistical analyses were implemented with SAS 9.4 (Statistical Analysis Software, SAS Institute, Cary, NC, USA).
Results
The study population comprised 40,942 children and adolescents with a median age of 12.7 years (Q1: 10.6; Q3: 14.4) and a slightly higher proportion of girls (53.2%). Among all subjects included, a migration background was documented in 18.9%. Between the 16 federal states of Germany, median age at the start of obesity treatment varied between 11.5 (9.0; 13.7) and 13.5 (11.7; 15.1) years (p < 0.0001). Differences were also present in the number of subjects with a migration background (5.8% vs. 49.7%; p < 0001). More detailed information on sociodemographic differences stratified by federal states of Germany can be seen in Table S1 (Supplement).
Cardiovascular risk factors at the beginning of a LI program
Unadjusted data indicated a high number of children and adolescents with extreme obesity at the beginning of therapy with a high prevalence of obesity-related comorbidities ().
Table 1. Cardiovascular risk factors of the whole APV study population.
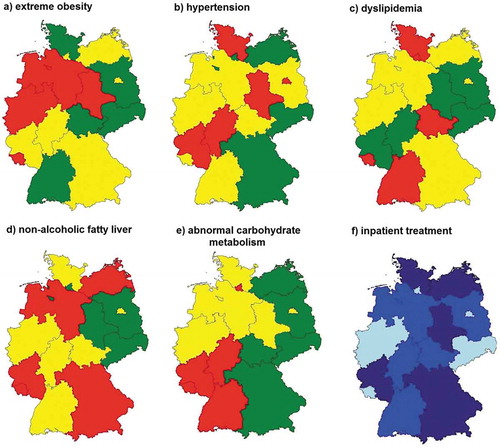
Logistic regression models (adjusted for age, sex, and weight-category) revealed significant differences in the prevalence of cardiovascular risk factors at the initiation of an obesity intervention between the 16 federal states of Germany (all p < 0.01). The proportion of subjects with extreme obesity differed between 35.6% and 50.8%. The prevalence of hypertension (39.0% up to 68.1%), dyslipidemia (24.9% up to 44.6%), or NAFLD (9.4% up to 20.4%) also varied between the 16 federal states of Germany. An abnormal carbohydrate-metabolism was less frequent compared to other cardiovascular risk factors and ranged between 0.7% and 6.2%. The proportion of children and adolescents with inpatient treatment varied between 11.2% and 88.2%. Overall, no clear regional pattern (e.g. east–west or north–south gradient) was found. Only the prevalence of an abnormal carbohydrate metabolism tended to be higher in the Southwestern part of Germany. The regional distribution of each variable is illustrated in and .
Table 2. Prevalence of cardiovascular risk factors and treatment modality, stratified by 16 federal states of Germany.
In most cases, only small changes in the proportion of subjects with obesity or extreme obesity and obesity-related comorbidities were observed after additional adjustment for inpatient care. Results were presented in (values in parentheses).
Discussion
Between the 16 federal states of Germany, this multicenter APV analysis revealed in children and adolescents initiating a LI for obesity significant differences in sociodemographic characteristics and cardiovascular risk factors. Large differences were also observed for the choice of treatment modality (inpatient rehabilitation vs. outpatient treatment).
Within Germany, the proportion of starting a LI for extreme obese children and adolescents varied from 35% to 50% ()). Given the fact that a lower BMI at the beginning of a LI is one predictor for therapy success [Citation9,Citation12], this is an alarming difference. Aside from weight differences at baseline, the prevalence of cardiovascular risk factors partly varies substantially – despite adjustment for weight categories. Disparities between the 16 federal states of Germany amounted up to 20% (dyslipidemia) or 30% (hypertension) without a clear regional pattern (e.g. north–south gradient) (; ). There are some potential explanations for the differences at the beginning of an intervention. Maybe, there is a regional variation in family’s or the child’s motivation or acceptance to participate in specific LIs [Citation14]. It is also possible that problem awareness differs within the APV study population. A lower problem awareness of obesity and its consequences could lead to a later start of LIs when the child is more obese and/or cardiovascular risk factors are more present. In addition, local availability of therapy options could be a contributing factor [Citation15]. In rural areas, the number of treatment centers can be limited and, in some cases, long distances have to be travelled which could also lead to the decision to participate in a LI at a later stage or in an inpatient center. Moreover, studies indicated that high health literacy (a proxy for socioeconomic status [SES]) in adults is associated with better cardiovascular risk profiles and that children of parents with higher health literacy are more likely to have better outcomes in child health promotion and disease prevention [Citation20,Citation21]. Unfortunately, data for SES or health literacy are not available in the APV database to confirm this assumption. Aside from individual SES/health literacy, disparities could be partly explained by regional deprivation (including e.g. unemployment rate, disposable income, physician density). Differences in living conditions are related to different capabilities and participation opportunities and might therefore influence health outcomes and health care decisions. A recently published study reported that more deprived patients with obesity have a more serious disease compared to less deprived patients [Citation22].
Figure 1. Regional distribution of the prevalence of (a) extreme obesity, (b) hypertension, (c) dyslipidemia, (d) nonalcoholic fatty liver, (e) abnormal carbohydrate metabolism, (f) and the proportion with inpatient treatment. Adjusted estimates from logistic regression models. The limit of significance of two-sided tests was set at p < 0.05. The different colors reflect the lower, middle, and highest tertile.
A previous APV analysis indicated that not all obese individuals demonstrate cardiovascular risk factors [Citation23]. This phenomenon is also known as metabolically healthy obesity (MHO). In obese children and adolescents, the proportion of MHO is estimated between 6% and 36% (depending on the definition of MHO) [Citation23,Citation24]. Aside from lower waist circumference or healthier lifestyle behavior (physical activity, lower energy intake), a strong association between prepubertal stage (independent from age) and MHO was reported [Citation23,Citation24]. An unequal distribution of MHO determinants within the APV study population might be therefore another explanation.
In Germany, reimbursement for an obesity LI program is usually covered by health insurance providers (outpatient programs) or pension funds (inpatient rehabilitation) and depends on medical, mental, and social conditions [Citation25]. According to criteria given in a consensus paper, a LI is only indicated in children and adolescents with overweight and at least one additional comorbidity (e.g. type 2 diabetes), with obesity and at least one cardiovascular risk factor (e.g. dyslipidemia), or with extreme obesity [Citation25,Citation26]. On the other hand, the pension fund will pay for inpatient rehabilitation for children or adolescence with obesity without additional risk factors or complications. For another part of the patients, the fulfillment of those criteria is a precondition for potential reimbursement by health insurance providers. Hence, being overweight or obese without any further complication is not enough to be reimbursed after participation at a LI for the health insurance companies. However, there are over 100 German health insurance funds and 16 different pension funds with a different regional distribution. It cannot be excluded that some insurance providers are more accommodating and stretch recommendations for reimbursement which in turn might have led to regional differences in clinical characteristics at the beginning of a LI.
Some methodological factors could have biased the findings as well. Although over 200 centers all over Germany participate at the APV initiative, the database is not representative for all German children and adolescents with obesity initiating a LI program. Moreover, the number of patients differed widely between the federal states of Germany. The number of children and adolescents ranged from <1000 (e.g. Bremen, Mecklenburg-Pomerania) to >5000–7000 (North Rhine-Westphalia, Bavaria). Resident nutrition counseling practices offering LI programs for obesity are often not supposed to take blood samples to analyze cardiovascular risk factors. Children and adolescents (or their parents) need to arrange an additional appointment by a physician. Results of the blood tests have then to be send to the nutritionist for documentation in the APV software. This somewhat circuitous procedure might explain that data about serum lipids, liver enzymes, or carbohydrate metabolism were only available for about 50–60% of all subjects included. Regional variation in data completeness could be also attributed to the differences between the federal states of Germany.
Aside from clinical differences at baseline, we further analyzed whether there is a difference in the choice of treatment modalities. In Germany, there are two types of obesity LIs: long-term therapy in outpatient practices and inpatient rehabilitation (average duration of a stay is 4–6 weeks). The proportion of children and adolescents who participated in inpatient rehabilitation ranged from 11.2% in Berlin to 88.2% in Mecklenburg-Pomerania (), ). In APV, there is a preponderance of outpatient centers (n = 127) compared to inpatient rehabilitation clinics (n = 27) (Fig. S2), but 56% of all patients are treated in inpatient rehabilitation clinics. Furthermore, the regional distribution of inpatient rehabilitation centers is very heterogeneous. The array of inpatient treatments is concentrated in only six federal states (Schleswig-Holstein and Mecklenburg-Pomerania in the north, Saxony-Anhalt and North Rhine-Westphalia in the midth; Bavaria and Baden-Württemberg in the south) which partly explains the higher proportion of children who decided to participate at an inpatient rehabilitation program in those regions. In contrast, a large number of outpatient centers are present in metropolitan areas as Berlin or Hamburg where a very low number of inpatient rehabilitation was observed. Regional differences in cultural or language barriers for inpatient rehabilitation or the lack of willingness for a long stay away from home/the family might be further explanations for the heterogeneity in the choice of treatment modalities.
Strengths and limitations
The main strength of this APV multicenter analysis is the large number of children and adolescents included. Furthermore, detailed information on sociodemographic characteristics is documented which allows controlling for potential confounders. However, due to the multicenter nature of data collection, variability in the measurements of body weight and body height as well as of biochemical parameters cannot be excluded despite standardized procedures. A further limitation is that complete data on SES and on lifestyle behavior (physical activity, eating habits) before starting a LI were not available in the database. Another pitfall is that some clinical characteristics were not completely documented in all patients and completeness may also differ regionally.
Conclusion
Sociodemographic characteristics and cardiovascular risk factors in children and adolescents starting a LI for obesity strongly differ between the 16 federal states of Germany. We further observed large differences in the choice of treatment modality. Several factors as individual SES, personal attitudes, treatment accessibility or local availability, as well as regional differences in reimbursement decisions might have contributed to the disparities.
Dataset availability statement
All data relevant to the given manuscript have been stored in a separate file which can be made freely available to external investigators upon request.
Supplemental Material
Download MS Word (17.3 KB)Acknowledgments
Special thanks to A. Hungele and R. Ranz for support and the development of the APV documentation software and K. Fink and E. Bollow for the APV data management (in each case clinical data manager, Ulm University). We also thank E. Bollow for statistical analysis. Furthermore, we thank all participating centers of the APV initiative, especially the collaborating centers: Gaissach Fachklinik Deutsche Rentenversicherung Bayern-Süd, Bad Kreuznach Viktoriastift, Scheidegg Prinzregent Luitpold Reha, Berlin Charite Kinderklinik, Westerland/Sylt, Haus Quickborn, Westerland/Sylt, Kinder-Reha, Wyk auf Föhr - AOK Kinderkurheim, Berchtesgaden Klinik Schönsicht Kinder-Reha, Wangen Kinder-Rehaklinik, Hamburg Wilhelmstift, Bad Kösen Kinder-Reha, Berlin Lichtenberg Kinderklinik, Amrum Satteldüne Kinder-Reha, Leipzig Uni-Kinderklinik, Datteln Vestische Kinderklinik, Ulm Uni-Kinderklinik, Garz Fachklinik CJD, Bad Frankenhausen Kinder-Reha, Darmstadt Kinderklinik, Seebad Heringsdorf - Kinder-Reha, Oy-Mittelberg Reha, Bischofswiesen/Strub, INSULA, Osnabrück christliches Kinderhospital, Düren sozialpäd. Zentrum Marienhospital, Kreischa Klinikum Bavaria Zscheckwitz, Bad Orb Spessartklinik - Kinder-Reha, Tübingen Universitäts-Kinderklinik, Mühlhausen Präventionspraxis Scherf, Oberhausen Adipositaszentrum, Saarbrücken Moby Kids, Bad Segeberg/Neumünster junior marvelesse, Göttingen Uni-Kinderklinik, Buchholz Ernährungsberatung, Untergruppenbach Ernährungsberatung, Hannover Kinderklinik Bult, Herrenberg JumboKids, Gauting, Kinderarztpraxis, Kölpinsee, Seebad Klaus Störtebecker Kinder-Reha, Freiburg - Fitoc, Bensheim Ernährungspraxis, Leipzig - Klaks e. V., Bruchweiler Kinder-Reha, Bad Salzungen Reha-Klinik Charlottenhall, Siegen DRK Kinderklinik, Bad Neuenahr - DRK Institutsambulanz, Wiesmoor KIDS Schulungsprogramm, Köln Sporthochschule, Friedrichsdorf Ernährungsberatung, Berchtesgaden CJD, Wustrow Ostseebad Fischland, München Adieupositas, Hamburg Rallye Energy, Feldberg ITZ Caritas-Haus, Hamburg Moby Kids Partner Konopka, Bad Frankenhausen Kinder-Reha, St. Augustin Kinderklinik, Lübeck Uni-Kinderklinik, Hagen Kinderklinik, Berlin Vivantes Beh.Zentrum SPZ, Flensburg Fördekids, Würzburg ambulantes Schulungszentrum, Villingen Kinder-leicht-Programm, Regensburg Kinderarztpraxis, Mönchengladbach Städt. Kinderklinik, Mahlow Programm TRI FIT junior, München Ernährungsinstitut Kinderleicht, Magdeburg VSB 1980 - bärenstark abnehmen, Frankfurt Päd. Endokrinologie, Oberstenfeld Ernährungspraxis, Wiesbaden DKD Kinderklinik, Bremen - ZABS, Menden BIG, Kiel städt. Krankenhaus Fördekids, Euskirchen Kinderarztpraxis, Neunkirchen Kinderklinik, Dinslaken Kinderklinik, Gotha Helios Kinderklinik, Greifswald Neuropädiatrie/Stoffwechsel, Oldenburg Kids-Schulungsprogramm, Rosenheim Lufti-Team, Bremen-Nord Kinderklinik, Pocking Kinderarztpraxis, Pforzheim Adipositas Training, Rickert Ernährungsberatung, Münster ADI MOBIL, Bad Bodenteich Moby Kids Seeparkklinik, Eppingen Kinderarztpraxis Schulze, Homburg CJD, Herdecke Kinderklinik, Neuss Lukaskrankenhaus, Paderborn Ernährungspraxis, Freiburg Uni-Kinderklinik, Berghaupten TOP-LIFE, Potsdam Patienten Trainings Zentrum, Ravensburg Ernährung und Diät, Hirschberg Praxis Maurer, Oberstenfeld Ernährungspraxis2, Pönitz FiFaFu KIDS-Programm, Rottweil Kinder-Leicht, Delmenhorst Kinderklinik, Senden Ernährungsberatung, Simonswald Klinik Eichhof, Fürth Kinderklinik, Berliner Jugendrotkreuz (JRK), Köln - Amsterdamerstrasse, Power Pänz, Ettenheim Kinderarztpraxis, Ronneburg Ernährungsberatung, Halle Universitäts-Kinderklinik, Korbach Ernährungsberatung, Hamburg Moby Kids, Düsseldorf Ernährungsberatung “richtig essen”, Siegburg KIDS Schulungsprogramm, Eschede Adipositastraining KIDS, Lingen Bonifatius-Hospital, Leverkusen Kinderklinik, Passau Kinderklinik, Solingen Ernährungsberatung, Niederkassel Kinderarztpraxis Sprenker, Overath KIDS-Schulungsprogramm, Sonneberg KIDS Ernährungspraxis, Essen Kinder und Jugendpsychiatrie, Krefeld Kinderklinik, Poppenricht Ernährungsberatung, Augsburg Bunter Kreis, Dresden Moby Kids, Rüsselsheim Gesundheits- und Pflegezentrum, Haßfurt Adipositasschulung Haßberge, Köln MeLo KIDS Schulungsprogramm, Lindau Forum Adipositas e.V., Rendsburg Villa Schwensen Praxisgemeinschaft KJPP, Bonn Ernährungsberatung KIDS Schulung, Tholey/SPZ Neunkirchen, Oberstaufen Ernährungsmedizin, Gera Waldklinikum, Hagen Kinderarztpraxis, Berlin Pfundskinder, Köln - Prävention UniReha GmbH, Lörrach VPS, Dieburg Ernährungsberatung KIDS Schulung, Detmold Kinderklinik, Magdeburg Uni-Kinderklinik, Reiskirchen Ernährungspraxis, Bad Hersfeld Kinderklinik, Göttingen, KIDS Schulungsprogramm, Waldbröl Gemeinschaftspraxis, Hamburg-Sprungbrett, Viersen Kinderklinik Nikolaus, Gelnhausen Ernährungsberatung, Gera SRH Wald-Klinikum, Hagen Allgemeines Krankenhaus, Göttingen interdis. Adipositaszentrum, Munster Ernährungs- & Bewegungsschulung für K&J.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary material can be accessed here.
Additional information
Funding
References
- Sanders RH, Han A, Baker JS, et al. Childhood obesity and its physical and psychological co-morbidities: a systematic review of Australian children and adolescents. Eur J Pediatr. 2015;174:715–746.
- Yngve A, de Bourdeaudhuij I, Wolf A, et al. Differences in prevalence of overweight and stunting in 11-year olds across Europe: the pro children study. Eur J Public Health. 2008;18:126–130.
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25.
- Pigeot I, Barba G, Chadjigeorgiou C, et al. Prevalence and determinants of childhood overweight and obesity in European countries: pooled analysis of the existing surveys within the IDEFICS Consortium. Int J Obes (Lond). 2009;33:1103–1110.
- Kurth BM, Schaffrath Rosario A. Overweight and obesity in children and adolescents in Germany [Article in Gemran]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53:643–652.
- Cadenas-Sanchez C, Nyström C, Sanchez-Delgado G, et al. Prevalence of overweight/obesity and fitness level in preschool children from the north compared with the south of Europe: an exploration with two countries. Pediatr Obes. 2016;11:403–410.
- Wang Y. Disparities in pediatric obesity in the United States. Adv Nutr. 2011;2:23–31.
- Grajda A, Kułaga Z, Gurzkowska B, et al. Regional differences in the prevalence of overweight, obesity and underweight among polish children and adolescents. Med Wieku Rozwoj. 2011;15:258–265.
- Moss A, Klenk J, Simon K, et al. Declining prevalence rates for overweight and obesity in German children starting school. Eur J Pediatr. 2012;171:289–299.
- Wiegand S, Keller KM, Lob-Corzilius T, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr. 2014;82:380–387.
- Bohn B, Wiegand S, Kiess W, et al. Changing characteristics of obese children and adolescents entering pediatric lifestyle intervention programs in Germany over the last 11 years: an Adiposity Patients Registry multicenter analysis of 65,453 children and adolescents. Obes Facts. 2017;10:517–530.
- Hoffmeister U, Bullinger M, van Egmond-Fröhlich A, et al. Overweight and obesity in childhood and adolescence. Evaluation of inpatient and outpatient care in Germany: the EvAKuJ study [article in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54:128–135.
- Reinehr T, Kleber M, Lass N, et al. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91:1165–1171.
- Gunnarsdottir T, Njardvik U, Olafsdottir AS, et al. The role of parental motivation in family-based treatment for childhood obesity. Obesity. 2011;19:1654–1662.
- World Health Organization, International diabetes federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation; 2006.
- Kromeyer-Hauschild K, Moss A, Wabitsch M. Referenzwerte für den Body-Mass-Index für Kinder, Jugendliche und Erwachsene in Deutschland: anpassung der AGA-BMI-Referenz im Altersbereich von 15 bis 18 Jahren. Adipositas Ursachen Folgeerkrankungen Ther. 2015;9:123–127.
- NCEP Expert. Panel on blood cholesterol levels in children and adolescents. national cholesterol education program (NCEP): highlights of the report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. 1992;89:495–501.
- Wiegand S, Keller KM, Röbl M, et al. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond). 2010;34:1468–1474.
- Bundesärztekammer (BÄK) Arbeitsgemeinschaft der Deutschen Ärztekammern. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen (Rili-BÄK). Dtsch Arztebl. 2014;111:A1583–A1618.
- Peters RJG. Health literacy skills and the benefits of cardiovascular disease prevention. Neth Heart J. 2017;25:407–408.
- Sanders LM, Shaw JS, Guez G, et al. Health literacy and child health promotion: implications for research, clinical care, and public policy. Pediatrics. 2009;124(Suppl 3):S306–14.
- Loddo C, Pupier E, Amour R, et al. Lifestyle intervention program in deprived obese adult patients and their non-deprived counterparts. PLoS ONE. 2017;12:e0188297.
- Reinehr T, Wolters B, Knop C, et al. Strong effect of pubertal status on metabolic health in obese children: a longitudinal study. J Clin Endocrinol Metab. 2015;100:301–308.
- Prince RL, Kuk JL, Ambler KA, et al. Predictors of metabolically healthy obesity in children. Diabetes Care. 2014;37:1462–1468.
- Goldapp C, Mann R, Shaw R. Qualitätsraster für Präventionsmaßnahmen für übergewichtige und adipöse Kinder und Jugendliche. In: Qualitätskriterienfür Programme zur Prävention und Therapie von Übergewicht undAdipositas bei Kindern und Jugendlichen. 2nd ed. Köln: Bundeszentrale für Gesundheitliche Aufklärung (BZgA); 2005.
- Böhler T, Wabitsch M, Winkler U: Konsensuspapier. Patientenschulungsprogramme für Kinder und Jugendliche mit Adipositas. In: Qualitätskriterienfür Programme zur Prävention und Therapie von Übergewicht undAdipositas bei Kindern und Jugendlichen. 2nd ed. Köln: Bundeszentrale für Gesundheitliche Aufklärung (BZgA); 2005.

