?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The study investigated the corrosion-inhibitory properties of three synthetic triazole Mannich bases on C1018 carbon steel using various electrochemical techniques. The inhibitors, namely 4-amino-2-[(4-methylpiperazin-1-yl)methyl]-5-(pyridin-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (4MPT), 4-amino-2-[(piperidin-1-yl)methyl]-5-(pyridin-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (PPT) and 4-amino-2-[(morpholin-4-yl)methyl]-5-(pyridin-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (MPT), demonstrated the high inhibition efficiencies of 92.8%, 93.3% and 92.6%, respectively, and adhered to the Langmuir adsorption isotherm. The inhibitors also functioned as mixed-type inhibitors and improved the protective layer on the metal surface. The study also utilized scanning electron microscopy - energy dispersive x-ray (SEM-EDX) analysis to examine the surface morphology of the uninhibited and inhibited carbon steel specimens. Furthermore, density functional theory (DFT) was utilized for theoretical calculations, which corroborated with experimental measurements.
1. Introduction
One of the low-carbon steels that are frequently utilized is C1018 steel. It has superior machinability, higher mechanical qualities, strong surface hardening quality, and good weldability; therefore, these are highly preferred in industries (Cui, Yu, & Zheng, Citation2021; Jafari, Akbarzade, & Danaee, Citation2019). Further, C1018 carbon steel has a low cost, which increases its application in industries (Paul & Koley, Citation2016). However, carbon steel’s drawback is its susceptibility to acid corrosion. The use of acids in industries is a normal process for removing mineral oxides and mineral scale depositions (Molchan et al., Citation2015). Corrosion of carbon steel is also common, unavoidable process, but of course, it can be controlled.
The most widely used method for limiting metal dissolution is the use of corrosion inhibitors (Singh, Ebenso, & Quraishi, Citation2012). The organic corrosion inhibitors work by adhering to the metal surface and forming a barrier against corrosion. According to the research, organic molecules with many bonds and high electron density atoms (N, O, S or P) are good adsorption sites and work especially well to stop corrosion (Aoun, Citation2017). As per the literature, compounds with nitrogen atoms such as amine, amino acids, amides, pyridine, pyrimidine, triazole, pyrrole, etc., were found to be effective. Similarly, inhibitors bearing sulphur atoms such as thiol or mercapto compounds showed elevated inhibition efficiencies in the acid medium (Ibrahim, Yunus, & Hashim, Citation2013; Phadke Swathi, Alva, & Samshuddin, Citation2017).
In previous studies, many researchers studied the triazole derivatives containing sulphur for inhibiting metal corrosion in an acidic medium (). AitHaddou et al. (Citation2016) have assessed the inhibition efficiency of two synthesized 1,2,4 triazole thiol derivatives and found good inhibition efficacy. Nahlé et al. (Citation2021) synthesized and studied the inhibition efficacy of two 1,2,3-triazole derivatives and observed a high-efficiency percentage. Al-Amiery et al. (Citation2020) prepared a bromo containing 1,2,4 triazole derivative and observed 95% inhibition efficiency on mild steel.
Table 1. Various triazole and Mannich base derivatives were found effective against corrosion.
In addition, a lot of researchers are concentrating on examining the effectiveness of Mannich bases in preventing metal corrosion (). Using secondary amine, morpholine, Maithili, Shetty, Kumari, & Kagatikar (Citation2022) synthesized and studied corrosion inhibition of Aluminium alloy and found effective corrosion inhibitor. Sharma, Upadhyay, & Chaturvedi (Citation2014) discovered that some synthetic Mannich bases in nitric acid inhibited corrosion on mild steel.
Even though many triazole derivatives have been reported as corrosion inhibitors, no single corrosion inhibitor is treated as ideal. All the investigated inhibitors have their own merits and demerits. Despite very good anticorrosion properties of the reported 1,2,4-triazole derivatives, the potential of these derivatives was varied due to the substitution effect. Moreover, the solubility of inhibitor in corrosive media determines its applicability in usage. On the basis of the literature review and the increase in the scope for the investigation of new corrosion inhibitors, it is decided to synthesize some triazole Mannich bases containing a pyridine moiety.
Thus, some triazole Mannich bases containing a pyridine moiety were synthesized. Mannich bases are beta-amino ketone-carrying compounds synthesized by the Mannich reaction (Bala, Sharma, Kajal, Kamboj, & Saini, Citation2014). The Mannich reaction uses formaldehyde and a primary or secondary amine to amino alkylate, an acidic proton adjacent to a carbonyl group (Zhang, Zhang, Su, Wang, & Lv, Citation2022). Because of the presence of aromatic rings, heteroatoms and strong acid solubility, Mannich base compounds have gained much attention. Thus, Mannich base compounds may effectively adsorb on metal surfaces and reduce the rate of metal corrosion due to the lone pairs of electrons in heteroatoms.
2. Experimental
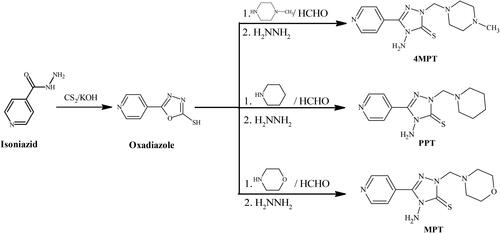
Isoniazid was used to prepare Mannich bases in two phases. The initial stage was adding isoniazid to alcoholic KOH at ambient temperature. After that, carbon disulphide was added, and the mixture was then refluxed for 12 h. After the reaction was finished, the mixture was put on crushed ice and acidified with dilute HCl before being washed with distilled water and the obtained product was dried (Banik et al., Citation2021). In the second step, the Mannich reaction was carried out. Oxadiazole and formaldehyde were stirred in ethanol (EtOH) at ambient temperature. Secondary amine was added to the reaction mixture, and the mixture was stirred continuously for roughly 12 h at an ambient temperature to produce the products (Scheme 1) (Hozien, EL-Mahdy, Abo Markeb, Ali, & El-Sherief, Citation2020). The formed products were filtered, washed and recrystallized from dimethyl formamide (DMF).
2.1. Synthesis of 4-Amino-2-[(4-methylpiperazin-1-yl)methyl]-5-(pyridin-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (4MPT)
Mannich base, 4MPT was prepared by refluxing a solution of oxadiazole (0.005 mol) in 10 ml ethanol, 0.005 mol of methylpiperazine and 0.005 mol of formaldehyde for 8 h. The product formed was filtered, dried and recrystallized from dimethyl formamide (DMF).
Yield: 76%; Melting point: 231–233 °C. Molecular weight: 305.4. IR (KBr, νmax in cm−1): 3157 (NH stretching), 3049 (Aromatic CH stretching), 2937 (Aliphatic CH stretching), 534 (C–S). 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.27 (m, 8H, 4CH2 of methyl piperazine), 2.99 (s, 3H, N–CH3), 4.77 (s, 2H, N–CH2–N), 5.56 (s, 2H, NH2), 7.83 (d, 2H, Pyridine-H, J = 9 Hz), 8.68 (d, 2H, Pyridine-H, J = 11 Hz).
2.2. Synthesis of 4-Amino-2-[(piperidin-1-yl)methyl]-5-(pyridin-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (PPT)
Mannich base, PPT was prepared by refluxing a solution of oxadiazole (0.005 mol) in 10 ml ethanol, 0.005 mol of piperidine, and 0.005 mol of formaldehyde for 8 h. The product formed was filtered, dried and recrystallized from dimethyl formamide (DMF).
Yield: 75%; Melting point: 233–235 °C. Molecular weight: 290.3. IR (KBr, νmax in cm−1): 3224 (NH Stretching), 3051 (Aromatic CH stretching), 2939 (Aliphatic CH stretching). 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.15 (m, 6H, 3CH2), 1.58 (m, 6H, 3CH2), 5.08 (s, 2H, N–CH2–N), 5.66 (s, 2H, NH2), 7.85 (d, 2H, Pyridine-H, J = 11 Hz), 8.63 (d, 2H, Pyridine-H, J = 9 Hz).
2.3. Synthesis of 4-Amino-2-[(morpholin-4-yl)methyl]-5-(pyridin-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (MPT)
Mannich base, MPT was prepared by refluxing a solution of oxadiazole (0.005 mol) in 10 ml ethanol, 0.005 mol of morpholine and 0.005 mol of formaldehyde for 8 h. The product formed was filtered, dried and recrystallized from dimethyl formamide (DMF).
Yield: 73%; Melting point: 234–236 °C. Molecular weight: 292.3. IR (KBr, νmax in cm−1): 3454 (NH stretching), 3051 (Aromatic CH stretching), 2951 (Aliphatic CH stretching), 1602 (C=N stretching). 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.30 (t, 4H, N–CH2 of morpholine), 3.52 (t, 4H, O–CH2 of morpholine), 4.98 (s, 2H, N–CH2–N), 5.51 (s, 2H, NH2), 7.85 (d, 2H, Pyridine-H, J = 9 Hz), 8.65 (d, 2H, Pyridine-H, J = 10 Hz).
2.4. Electrochemical measurements
Hydrochloric acid (1M) was chosen as the corrosive medium for this study. The solutions of inhibitors with various concentrations (50, 100, 250, 500 and 750 ppm) were prepared by dissolving the appropriate amount of inhibitor in 1 M HCl. After soaking the metal for nearly an hour in an acid solution with various inhibitor concentrations, electrochemical parameters were determined. Different concentrations (50–750 ppm) of the Mannich bases abbreviated as 4MPT, PPT and MPT were prepared directly in 1 M HCl. The measurements were conducted in a stagnant aerated solution. The experiments were performed using a Gamry Potentiostat (Model: 1010E) with a three-electrode configuration (Reference electrode: Ag/AgCl, Working electrode: C1018 carbon steel, Counter electrode: cylindrical graphite rod). The voltage was scanned at a rate of 0.5 mVs−1 from -0.25 V to +0.25 V to record the potentiodynamic polarization data. Impedance data analysis was performed using the Gamry Echem Analyst software suite (Version:7.8.2) (Shahzad et al., Citation2020). Duplicate experiments were conducted and the errors were not more than 5%.
2.5. Surface characterization
Observing the morphology of the metal surface after submerging it in an inhibited corrosive medium makes it possible to see how well the inhibitors act as anticorrosive agents (Ouakki, Galai, Benzekri, Aribou, et al., Citation2021; Singh, Ebenso, Olasunkanmi, Obot, & Quraishi, Citation2016). Before the examination, the metal samples in this investigation were submerged in inhibited and uninhibited 1 M HCl for 12 h each. For SEM with energy dispersive x-ray (EDX) (VEGA 3 TESCAN and AMETEK EDX), a 20 kV working voltage and a 10 mm working distance were used to find out how the metal surface looked.
2.6. Quantum chemical calculation
Theoretical computations were used to verify the theoretical findings. DFT is a popular quantum chemical technique for figuring out a molecule’s structural characteristics (Ju, Ding, Sun, & Chen, Citation2015). To conduct atomistic simulations and DFT computations, this study used the COMPASS force field and Material Studio 7.0 (Accelrys Inc., San Diego, CA) (Sun, Citation1998). According to our earlier research, various quantum chemical parameters were identified (Rasheeda et al. Citation2022).
An adsorption locator module was used in MC simulations for ascertaining the metal-inhibitor relationship. The crystal plane of Fe (110) was considered for all adsorption characteristic calculations, which were done using a force field COMPASS II (Akkermans, Spenley, & Robertson, Citation2013).
3. Results and discussions
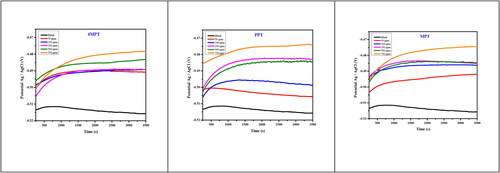
3.1. Open circuit potentials
The OCP plots for the C1018 electrode in the presence and the absence of 4MPT, PPT and MPT are represented in . From the plots, it is observed that a steady open circuit potential was reached for the solution in the absence of Mannich bases. But more time was required to get a stable OCP by the solution in the presence of Mannich bases. It can also be noted that there is movement in the potential towards the positive direction by adding Mannich bases. This happens when adsorbed molecules are present on the metal surface (Al-Amiery, Kadhum, Alobaidy, Mohamad, & Hoon, Citation2014; Kamble & Dubey, Citation2021).
3.2. Electrochemical impedance spectroscopy (EIS) measurements
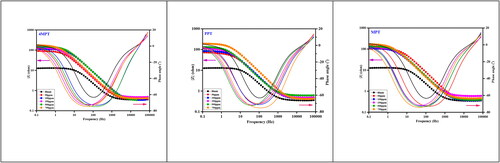
To study the corrosion inhibition efficacy of synthesized Mannich bases, electrochemical impedance spectroscopy (EIS) was employed (Idris, Daud, & Othman, Citation2013). The obtained Nyquist and Bode plots at different concentrations of 4MPT, PPT and MPT are depicted in and . A single charge transfer process during the dissolution is suggested by the semi-circular shape and is unaffected by the presence of inhibitors. The large capacitive loop shows how inhibitor molecules stick to carbon steel’s surface (Raghavendra & Ishwara Bhat, Citation2019).
Figure 2. Nyquist plots for the corrosion control of carbon steel in 1 M HCl in the presence and absence of 4MPT, PPT and MPT.

Figure 3. Bode plots for the corrosion control of carbon steel in 1 M HCl in the presence and absence of 4MPT, PPT and MPT.
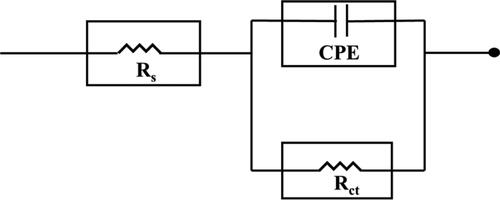
The Randles equivalent circuit was used for fitting the Nyquist plots and represented in . This circuit contains polarization resistance Rp, electrolyte resistance Rs, and CPE. The Nyquist graph shows a semi-circle nature because ideal double-layer capacitor Cdl was replaced by the constant phase element CPE (Visser, Terryn, & Mol, Citation2019). The CPE impedance (ZCPE) was obtained from the values of n (CPE exponent), angular frequency (ω) and Yo (CPE constant), as per the following EquationEquation 1(1)
(1) .
(1)
(1)
The value of CPE exponent (n), for the majority of organic inhibitors, is close to 1. This constant n helps to assess the surface heterogeneity. This circuit behaves as an ideal capacitor if the value equals 1 (Gadow & Fakeeh, Citation2022).
The Cdl and Rp are related as per EquationEquation 2(2)
(2) :
(2)
(2)
Using EquationEquation 3(3)
(3) , the value of θ (fractional surface coverage) was calculated to find out how many inhibitors were absorbed onto the metal surface.
(3)
(3)
Where the polarization resistances Rp(Inhi) and Rp(Blank) refer to the presence and lack of inhibitors, respectively. Furthermore, by entering the Rp values into EquationEquation 4(4)
(4) , it was possible to get the percentage inhibition efficiency:
(4)
(4)
The Bode figure shows that the phase angle rises with increasing inhibitor concentrations until it reaches an ideal level. The mechanism of surface adsorption is what causes the phase angle to grow (Ouakki et al., Citation2019).
Indicative of the corrosion behaviour of carbon steel in the existence of various inhibitor concentrations are the data gained from fitted EIS plots, which are presented in . The table clearly shows that as the inhibitor concentration rises, the Cdl value is reduced. In addition, the Rp values significantly increased in comparison to Rs when various inhibitor doses were present. Reduced capacitance indicates that the local dielectric constant has decreased as a result of the thickening of the electrical double layer (Ebadi, Basirun, Khaledi, & Ali, Citation2012). It is well-known that capacitance and Cdl thickness have an inverse relationship.
Table 2. The data obtained from fitted EIS curves for corrosion control of carbon steel with the presence and absence of 4MPT, PPT and MPT.
3.3. Potentiodynamic polarization studies (PDP)
The kinetics of the anode and cathode reactions can be studied, as well as the effectiveness of the inhibitor, using the PDP approach.
An overview of all major electrochemical parameters derived from Tafel plots () is given in , including the corrosion potential (Ecorr), corrosion current density (icorr), corrosion rate (CR), anodic and cathodic Tafel slopes (βa and βc) and inhibition efficiency (IE%) (Kuriakose, Kakkassery, Raphael, & Shanmughan, Citation2014). According to the theory that 4MPT, PPT and MPT stop both anodic and cathodic reactions, adding these compounds to a corrosive medium lowers the anodic and cathodic current densities (Guo et al., Citation2022).
Figure 5. Potentiodynamic polarization plot for the corrosion control of carbon steel in 1 M HCl by different concentration of inhibitors.
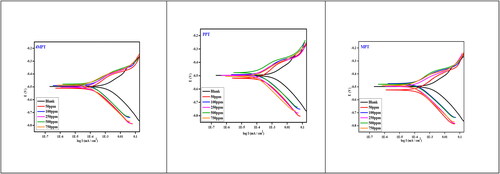
Table 3. Results for the inhibitory effect of 4MPT, PPT and MPT on carbon steel by potentiodynamic polarization and linear polarization resistance experiments.
The Tafel plot’s cathodic (βc) and anodic (βa) portions provide evidence for the pathway of corrosion reactions, i.e. whether it proceeded by hydrogen evolution by removing electrons from metal (cathodic type) or metal dissolution (anodic type). The addition of 4MPT, PPT and MPT has not altered the anodic cathodic curves’ performances, indicating that the studied inhibitors affect both cathodic and anodic reactions (mixed type) (Zulfareen, Venugopal, & Kannan, Citation2018).
Further, from the icorr(Blank) (current density for blank) and icorr(Inh) (current density for inhibitors) values, the inhibition efficiencies of 4MPT, PPT and MPT have been calculated using EquationEquation 5(5)
(5)
(5)
(5)
If the change in corrosion potential value (Ecorr) after adding the inhibitor reaches 85 mV, depending on the sign, the literature classifies an inhibitor as either anodic or cathodic; otherwise, it is categorized as a mixed inhibitor (Lai et al., Citation2017). For every inhibitor tested in the current work, the Ecorr difference is less than 85 mV for every concentration. So, it’s possible to say that these inhibitors have a mixed type of effect.
3.4. Linear polarization measurements (LPR)
The anticorrosive nature of 4MPT, PPT and MPT on C1018 metal in 1 M HCl was evaluated by the LPR method (Faritov, Rozhdestvenskii, Yamshchikova, Minnikhanova, & Tyusenkov, Citation2016). The important parameter, polarization resistance (Rp), derived from LPR measurement for all the inhibitors, is tabulated in . The inhibition efficacy is determined using the Rp values.
Among the three, PPT has a little higher inhibition efficacy (94.2%) compared to that of 4MPT (93.6) and MPT (93.4%). All of these inhibitors work well at stopping the reaction. This may be because the triazole part has heteroatoms.
3.5. Electrochemical frequency modulation (EFM)
EFM is a nondestructive corrosion measurement method that provides corrosion current estimates directly without the need for a prior understanding of Tafel constants (El-Aziz, Fouda, Al-Bonayan, Eissa, & Eid, Citation2022). Supporting file Figure S1 illustrates the intermodulation spectra from EFM observations of metal in 1 M HCl at various inhibitor doses. Electrochemical parameters were derived from the EFM data and are listed in . Here, βc and βa are Tafel constants, and CF2 and CF3 are causality factors. The causation factors, which act as an internal review of the EFM measurement’s validity, are an essential application of EFM (Al-Mobarak, Khaled, Hamed, & Abdel-Azim, Citation2011). The EFM results are generally accepted if CF2 and CF3 have values of 2 and 3, respectively (Li et al., Citation2021). Here, the CF2 and CF3 values are discovered to be in the range of 0 to 2 and 0 to 3, respectively; as a result, the EFM data has been validated. From the corrosion rate values, EquationEquation 6(6)
(6) is used to figure out how well the investigated inhibitors stop corrosion:
Table 4. Results for the inhibitory effect of 4MPT, PPT and MPT on carbon steel by electrochemical frequency modulation method.
A nondestructive corrosion measurement method that directly gives corrosion current values without prior knowledge of Tafel constants is EFM (El-Aziz et al., Citation2022). The intermodulation spectra obtained from EFM measurements of metal in 1 M HCl at different inhibitor concentrations are depicted in Supporting file Figure S1. From the EFM results, electrochemical parameters were obtained and are tabulated in . Here, βc and βa are the Tafel constants, CF2 and CF3 are causality factors. The important application of EFM is the causality factors which serve as an internal check on the validity of the EFM measurement (Al-Mobarak et al., Citation2011). In general, if the values of CF2 and CF3 are 2 and 3, respectively, then the EFM data are accepted (Li et al., Citation2021). Here the CF2 and CF3 values are found to be in the range of 0 to 2 and 0 to 3, respectively; thus, the EFM data has got validation. From the corrosion rate values, the inhibition efficiency of studied inhibitors is calculated using EquationEquation 6(6)
(6) :
(6)
(6)
where CRBlank is the corrosion rate of blank and CRInh is the corrosion rate of inhibitors.
3.6. Adsorption isotherm
Adsorption isotherm research focuses on inhibitor molecule interactions with metal surfaces (Abd El Rehim, Sayyah, El-Deeb, Kamal, & Azooz, Citation2016). Several adsorption isotherms were used in this study to explain how the inhibitor binds to the surface. There are typically two different types of adsorption mechanisms: physisorption and chemisorption. Inhibitors bind to the active metal sites during physisorption. In chemisorption, coordination bonds are created by a lone pair of heteroatoms between the d-orbitals of Fe atoms and the inhibitors (El Ouadi et al., Citation2020). In this study, the Frumkin, Langmuir, Freundlich, El-Awady, Temkin, and Flory-Huggins isotherms were used to fit the experimental data. Among these models, the Langmuir model fits appropriately (Supporting file Figures S2–S4 for 4MPT, PPT and MPT, respectively). The Langmuir adsorption isotherm plot (C/θ vs. θ) was drawn using the data obtained from the PDP method based on EquationEquation 7(7)
(7) :
(7)
(7)
where, C is the inhibitor concentration, Kads, represents the adsorption equilibrium constant, and θ signifies the fractional surface area of the metal covered by the inhibitor. For all the studies inhibitors, a straight line was obtained from Langmuir adsorption isotherm having linear correlation coefficient, R2 value close to unity. This verifies that the studied inhibitors adsorb on the C1018 metal surface with the Langmuir adsorption model (Swathi, Samshuddin, Alamri, et al., Citation2022). Here, the inhibitors occupy certain sites on the surface of the metal to form a monolayer, which was signified by the slope value near unity. From the intercepts of adsorption isotherm, Kads (equilibrium constant) values were determined (EquationEquation 8
(8)
(8) ) and are tabulated in the Supporting file, Table S2.
(8)
(8)
Increased Kads values show that inhibitors have been effectively adsorbed to the C1018 metal surface. EquationEquation 9(9)
(9) is used to calculate the inhibitor’s free energy of adsorption (ΔGoads).
(9)
(9)
where T and R denote the temperature in Kelvin, and the universal gas constant as 8.314 JK−1mol−1, respectively. The 55.5 indicates the water concentration in molarity (Manimegalai & Manjula, Citation2015). The ΔGoads values obtained from various electrochemical methods are tabulated in . A negative ΔGoads value demonstrates the spontaneous adsorption of 4MPT, PPT and MPT on the surface of C1018 metal. Generally, the value of ΔGoads approaching −40 kJmol−1 or above implies chemisorption, while a value up to −20 kJmol−1 generally shows physisorption (Akinbulumo, Odejobi, & Odekanle, Citation2020). The calculated ΔGoads values of all the inhibitors lie between −34.86 to −37.11 kJmol−1, indicating the coordinate type of bond formation by charge transfer or electron sharing from the inhibitor to the C1018 metal surface.
Table 5. Calculated values of Kads and ΔGoads determined by electrochemical techniques for carbon steel with 4MPT, PPT and MPT.
3.7. Surface morphological analysis
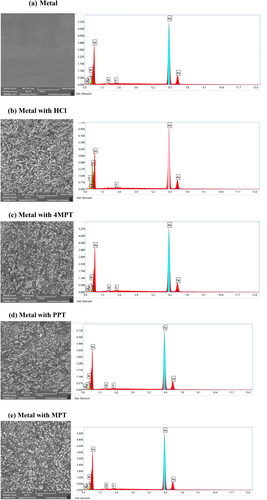
The SEM micrographs were studied to better understand the nature of the carbon steel surface and corrosion in the absence and presence of a synthesized inhibitor system (Ouakki et al. Citation2021). displays the SEM images of carbon steel specimens submerged in 1 M HCl for 12 h in the absence and presence of 4MPT, PPT and MPT. The monograph of the uncorroded C1018 metal is provided in . micrographs of the carbon steel surface in acid demonstrate the metal’s surface roughness, which denotes surface corrosion (Xavier Stango & Vijayalakshmi, Citation2018). The visible surface was quite rough and extensively corroded. However, the addition of inhibitors 4MPT, PPT and MPT significantly reduces the rate of corrosion and surface degradation, which are represented in .
3.8. Energy dispersive x-ray (EDX) analysis
EDX analysis compares the elemental composition of the metals before and after immersion in the inhibitors (Ech-Chihbi et al., Citation2020). displays the percentage atomic content as calculated from the EDX spectra, and displays the EDX spectra of carbon steel in the absence and presence of inhibitors. displays the oxygen-free EDX spectrum of uncorroded carbon steel. In contrast, depicts carbon steel corroding in 1 M HCl with an oxygen peak (Rehioui et al., Citation2021). However, the EDX spectrum of carbon steel shows separate peaks for carbon, sulphur and oxygen in the presence of inhibitors, suggesting that synthetic inhibitors coat the surface of carbon steel with a protective layer.
Table 6. The atomic percentage of metal in the presence and absence of 4MPT, PPT and MPT.
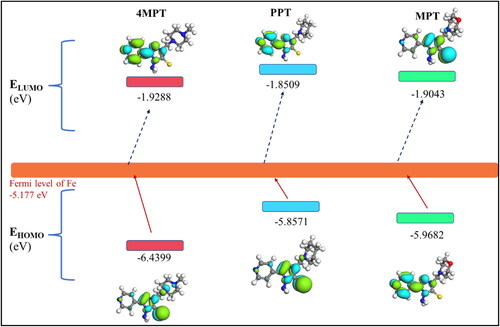
Density functional theory (DFT) was applied to 4MPT, PPT and MPT molecules to investigate the corrosion performance of C1018 metal. The HOMO and LUMO of molecular orbital for 4MPT, PPT and MPT are shown in . The values of EHOMO and ELUMO are highly significant and help to explain the adsorption ability of designed inhibitors on the metallic surface (Swathi et al. Citation2022). A greater or less negative value of EHOMO makes it easier for an inhibitor to donate electrons to an unoccupied d-orbital of iron and vice versa (Al-Amiery, Salman, et al., Citation2020). The lesser or more negative value ELUMO implies a good electron-accepting capability of inhibitors from the metal atom (Tüzün & Bhawsar, Citation2021). shows that PPT has a less negative value of EHOMO, indicating that it is a good electron donor than 4MPT and MPT.
Table 7. Calculated quantum chemical parameters for 4MPT, PPT and MPT by DFT computation study.
The location of frontier orbital energy levels of the inhibitors and the Fermi level of iron play a major role in the inhibition efficiency (). The DFT results show that EHOMO of 4MPT is −6.4399 eV, PPT is −5.8571 eV and MPT is −5.9682 eV while the value of the Fermi level of iron is −5.177 eV (Swathi, Samshuddin, Alamri, et al., Citation2022). Thus, the EHOMO of PPT is not so far from the Fermi level of iron, indicating that electron donation or acceptance from the HOMO orbital of the PPT molecule to the Fe surface is easier than that from 4MPT and MPT. Further, ELUMO of 4MPT (−1.9288 eV), PPT (−1.8509 eV) and MPT (−1.9043 eV) are comparatively away from the Fermi level of Fe. This suggests that the electron donation from inhibitor to metal is predominant rather than forming feedback bonds (Rodríguez, Cruz-Borbolla, Arizpe-Carreón, & Gutiérrez, Citation2020).
The values of EHOMO and ELUMO are associated with the ionization potential and electron affinity, respectively, according to EquationEquations 10(10)
(10) and Equation11
(11)
(11) ,
(10)
(10)
(11)
(11)
Ionization potential is essential to understand the chemical reactivity of the inhibitors, and it is the amount of energy necessary to remove an electron from a molecule (Oyeneyin, Akerele, Ojo, & Oderinlo, Citation2021). Generally, inhibitors with high ionization potential are highly stable and chemically inert, whereas those with low ionization potential are highly reactive (Gad, Azzam, & Halim, Citation2018). Among the three inhibitors, PPT has low ionization energy (5.8571 eV) compared to that of 4MPT (6.4399 eV) and MPT (5.9682 eV), indicating the high inhibition efficiency of PPT. Electron affinity is related to the neutral molecule’s liberated energy when an electron is added to it (Rasheeda et al. Citation2022). Thus, the higher the electron affinity value, the higher the inhibitor’s tendency to receive the electrons. In other words, more possibility of the formation of feedback bonds.
Chemical softness (σ) refers to the inhibitor’s propensity to accept electrons, while chemical hardness (η) measures the inhibitor’s resistance to the transfer of charge (Kabanda et al., Citation2012). A soft molecule has high reactivity, whereas a hard molecule has lower reactivity. In addition, a hard molecule has a larger energy gap than a soft one. An inhibitor is typically considered to have good inhibitory efficacy if its chemical hardness and chemical softness values are low and high, respectively. In the present study, PPT has low η and high σ values (η = 2.0031 eV and σ = 0.4992 eV−1) compared to that of 4MPT (η = 2.2556 eV and σ = 0.4433 eV−1) and MPT (η = 2.0320 eV and σ = 0.4921 eV−1). Following EquationEquations 12(12)
(12) and Equation13
(13)
(13) are used to calculate η and σ,
(12)
(12)
(13)
(13)
EquationEquation 14(14)
(14) can be used to figure out a molecule’s electronegativity (χ), which measures how well it can take in electrons (Al-Amiery et al., Citation2022).
(14)
(14)
The factor ΔN, the fraction of transferred electrons, determines a molecule’s ability to donate electrons. The molecule’s electron-donating property is shown by a positive value for ΔN, whereas a negative value indicates its electron-accepting property (Anusuya, Sounthari, Saranya, Parameswari, & Chitra, Citation2015). In the present work, positive values are shown by 4MPT (ΔN = 0.1409), PPT (ΔN = 0.2411 and MPT (ΔN = 0.175), which proves the electron-donating ability.
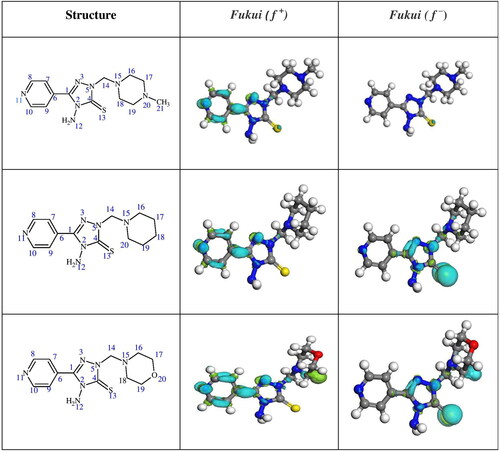
3.9. Fukui index analysis
This evaluation aids in determining the inhibitor’s site of potential electron donation or acceptance (Oyeneyin, Ojo, Ipinloju, Agbaffa, & Emmanuel, Citation2022). The feedback bond is created by the inhibitor’s electron-accepting site, which is known as an electrophilic site (f +). The nucleophilic site (f –), contributes electrons to form a coordinate bond with the metal. Higher values of f + and f – denote a higher degree of electron acceptance and donation skills, respectively. gives the density distribution of 4MPT, PPT and MPT, and the Supporting files; Tables S4–S6 give the calculated Fukui indices of 4MPT, PPT and MPT, respectively. Thus, 4MPT has C1, N3, C6, C8, N11 and S13 sites that favour electron acceptance and C1, N3, N5, N11, S13, N9 and N15 sites for electron donation. Further, for PPT, C1, N3, C6, C7, C8, N11 and S13 tend towards electron acceptance, and sites favour electron donation by C1, N3, N5, N11 and S13. Furthermore, C1, N3, N5, N11 and S13 favour accepting an electron, and C1, N3, N5, N11, S13, and O20 have for donating an electron. Thus, triazole Mannich bases enhance the electron donor-acceptance interaction, thus increases the adsorbing abilities of 4MPT, PPT and MPT on the C1018 metal surface.
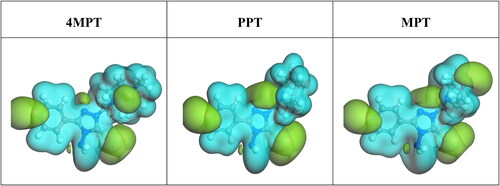
3.10. Electrostatic potential maps (ESP)
The ESP approach is used to analyze the nucleophilic (negative region) and electrophilic (positive region) sites of inhibitor compounds (Shenoy, Venugopal, Reena Kumari, & Chakraborty, Citation2022). Here, the negative site is represented by the dark region, and the other regions represent the positive sites. In , the dark areas of 4MPT, PPT and MPT denote the presence of heteroatoms such as O, N and S. Thus, reaction sites of 4MPT, PPT and MPT were visible on the ESP maps, through which the synthesized compounds could decrease the corrosion of carbon steel.
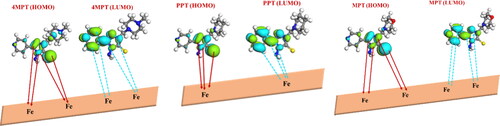
3.11. Monte Carlo simulation
The 4MPT, PPT and MPT adsorption capacity on Fe (110) were examined using the Monte Carlo simulations method (Sasikumar et al., Citation2015). Fe (110) surface is favoured among the various Fe surfaces due to its highly stable density-packed surface. signifies the possible interaction of inhibitors with Fe (110). The figure shows that inhibitors are inclined parallel to the metal surface, thus getting adsorbed efficiently on the iron (110) facet. The parameters determined by the MC simulations are presented in . Adsorption energy is the energy that is either released or needed when an adsorbate component relaxes on a substrate (Sliem, El Basiony, Zaki, Sharaf, & Abdullah, Citation2020). Similar to this, rigid adsorption energy refers to the energy associated with the adsorption of the unrelaxed adsorbate component. Further, the value of ΔEad/ΔNi indicates the energy of substrate–adsorbate configurations, which works only when one of the adsorbate components has been eliminated (Saadouni et al., Citation2018). The adsorption energies of 4MPT, PPT and MPT in the current investigation were higher than those of the water molecule (−74.08 kJmol−1). As a result, the synthesized inhibitors would likely take the place of the water molecules bonded to the C1018 steel, avoiding corrosion.
Figure 10. Top and side views of the most stable low energy configurations for the adsorption of 4MPT, PPT and MPT molecules on Fe (110)/100 H2O interface obtained using Monte Carlo simulations.
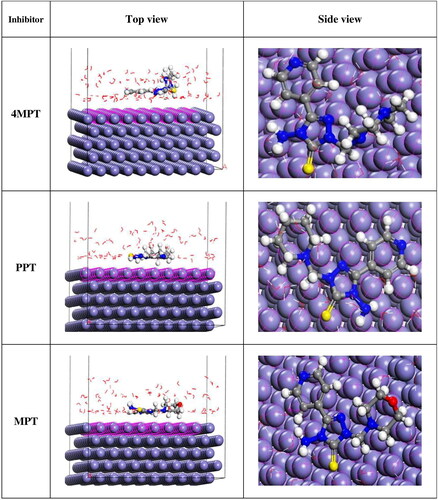
Table 8. The parameters calculated by the Monte Carlo simulation for the most stable adsorption configurations of inhibitor molecules on Fe (110)/100 H2O system (all units in kcal mol−1).
3.12. Possible adsorption mechanism
The cumulative results of experimental and theoretical studies revealed that all the synthesized 1,2,4-triazole derivatives inhibit the corrosion of the carbon steel surface in 1 M HCl by adsorption mechanism. The presence of numerous hereto atoms in the triazole moiety may cause the significant inhibitory efficiencies of triazole Mannich base derivatives. The donation of electrons to the unoccupied d-orbitals of iron, which form the coordinate bonds with metal, is mostly facilitated by the three nitrogen atoms of the triazole ring, the sulphur linked to the triazole ring, and the π-electrons (). In addition, by accepting the d-electrons from iron, heteroatoms, π-electrons of pyridine, and secondary amino groups can form feedback bonds. As 4MPT, PPT and MPT can more easily bind to metal surfaces to form coordinate and feedback bonds, they are effective corrosion inhibitors.
4. Conclusions
The inhibitory efficacy of three novel triazole Mannich bases, 4MPT, PPT and MPT, was investigated for the corrosion of C1018 steel in 1 M HCl using electrochemical testing, surface characterization and quantum chemical calculations. Electrochemical tests demonstrated the effectiveness of these triazole derivatives in halting metallic corrosion. The mixed-type character of the examined inhibitors was clearly shown by the findings of the polarization measurements from corrosion potential. Furthermore, it was found that the adsorption of these triazole derivatives on metallic surfaces followed the Langmuir isotherm. The metal inhibition by these chemicals was observed using SEM-EDX analysis. The experimental results were supported by quantum chemical computations. The results of DFT and MC simulations were used to figure out if these triazole Mannich bases could strongly stick to the surface of metal and then form a protective barrier between the metal and the corrosive medium.
Supplemental Material
Download MS Word (805 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd El Rehim, S. S., Sayyah, S. M., El-Deeb, M. M., Kamal, S. M., & Azooz, R. E. (2016). Adsorption and corrosion inhibitive properties of P(2-aminobenzothiazole) on mild steel in hydrochloric acid media. International Journal of Industrial Chemistry, 7(1), 39–52. doi:10.1007/s40090-015-0065-5
- AitHaddou, B., Chebabe, D., Dermaj, A., Benassaoui, H., Assyry, A., Hajjaji, N., … Srhiri, A. (2016). Comparative study of low carbon steel corrosion inhibition in 1M HCl by 1,2,4-triazole-5-thione derivatives. Journal of Materials and Environmental Science, 7, 2000–2191.
- Akinbulumo, O. A., Odejobi, O. J., & Odekanle, E. L. (2020). Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results in Materials, 5, 100074. doi:10.1016/j.rinma.2020.100074
- Akkermans, R. L. C., Spenley, N. A., & Robertson, S. H. (2013). Monte Carlo methods in materials studio. Molecular Simulation, 39(14-15), 1153–1164. doi:10.1080/08927022.2013.843775
- Al-Amiery, A., Kadhum, A., Alobaidy, A. H., Mohamad, A., & Hoon, P. (2014). Novel corrosion inhibitor for mild steel in HCl. Materials (Basel, Switzerland), 7(2), 662–672. doi:10.3390/ma7020662
- Al-Amiery, A. A., Mohamad, A. B., Kadhum, A. A. H., Shaker, L. M., Isahak, W. N. R. W., & Takriff, M. S. (2022). Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution. Scientific Reports, 12(1), 4705. doi:10.1038/s41598-022-08146-8
- Al-Amiery, A., Salman, T. A., Alazawi, K. F., Shaker, L. M., Kadhum, A. A. H., & Takriff, M. S. (2020). Quantum chemical elucidation on corrosion inhibition efficiency of Schiff base: DFT investigations supported by weight loss and SEM techniques. International Journal of Low-Carbon Technologies, 15(2), 202–209. doi:10.1093/ijlct/ctz074
- Al-Amiery, A., Shaker, L. M., Kadhum, A. A. H., & Takriff, M. S. (2020). Synthesis, characterization and gravimetric studies of novel triazole-based compound. International Journal of Low-Carbon Technologies, 15(2), 164–170. doi:10.1093/ijlct/ctz067
- Allangawi, A., Sajid, H., Ayub, K., Gilani, M. A., Akhter, M. S., & Mahmood, T. (2023). High drug carrying efficiency of boron-doped Triazine based covalent organic framework toward anti-cancer tegafur; a theoretical perspective. Computational and Theoretical Chemistry, 1220, 113990. doi:10.1016/j.comptc.2022.113990
- Al-Mobarak, N. A., Khaled, K. F., Hamed, M. N. H., & Abdel-Azim, K. M. (2011). Employing electrochemical frequency modulation for studying corrosion and corrosion inhibition of copper in sodium chloride solutions. Arabian Journal of Chemistry, 4(2), 185–193. doi:10.1016/j.arabjc.2010.06.036
- Anusuya, N., Sounthari, P., Saranya, J., Parameswari, K., & Chitra, S. (2015). Quantum chemical study on the corrosion inhibition property of some heterocyclic azole derivatives. Oriental Journal of Chemistry, 31(3), 1741–1750. http://www.orientjchem.org/vol31no3/quantum-chemical-study-on-the-corrosion-inhibition-property-of-some-heterocyclic-azole-derivatives/. (accessed October 24, 2022). doi:10.13005/ojc/310355
- Aoun, S. B. (2017). On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium-ionic liquid: Chemical, thermodynamic, kinetic and electrochemical studies. RSC Advances, 7, 36688–36696. doi:10.1039/C7RA04084A
- Bala, S., Sharma, N., Kajal, A., Kamboj, S., & Saini, V. (2014). Mannich bases: An important pharmacophore in present scenario. International Journal of Medicinal Chemistry, 2014, 191072. () doi:10.1155/2014/191072
- Banik, B. K., Sahoo, B. M., Kumar, B. V. V. R., Panda, K. C., Jena, J., Mahapatra, M. K., & Borah, P. (2021). Green synthetic approach: An efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules, 26, 1163. doi:10.3390/molecules26041163
- Cui, M., Yu, Y., & Zheng, Y. (2021). Effective corrosion inhibition of carbon steel in hydrochloric acid by dopamine-produced carbon dots. Polymers, 13, 1923. doi:10.3390/polym13121923
- Ebadi, M., Basirun, W. J., Khaledi, H., & Ali, H. M. (2012). Corrosion inhibition properties of pyrazolylindolenine compounds on copper surface in acidic media. Chemistry Central Journal, 6(1), 163. doi:10.1186/1752-153X-6-163
- Ech-Chihbi, E., Nahlé, A., Salim, R., Benhiba, F., Moussaif, A., El-Hajjaji, F., … Zarrouk, A. (2020). Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 1.0 M HCl solution. Journal of Alloys and Compounds, 844, 155842. doi:10.1016/j.jallcom.2020.155842
- El Ouadi, Y., El Fal, M., Hafez, B., Manssouri, M., Ansari, A., Elmsellem, H., … Bendaif, H. (2020). Physisorption and corrosion inhibition of mild steel in 1 M HCl using a new pyrazolic compound: Experimental data & quantum chemical calculations. Materials Today: Proceedings, 27, 3010–3016. doi:10.1016/j.matpr.2020.03.340
- El-Aziz, A., Fouda, S., Al-Bonayan, A. M., Eissa, M., & Eid, D. M. (2022). Electrochemical and quantum chemical studies on the corrosion inhibition of 1037 carbon steel by different types of surfactants. RSC Advances, 12(6), 3253–3273. doi:10.1039/D1RA07983B
- Faritov, A. T., Rozhdestvenskii, Y., Yamshchikova, S. A., Minnikhanova, E. R., & Tyusenkov, A. S. (2016). Improvement of the linear polarization resistance method for testing steel corrosion inhibitors. Russian Metallurgy (Metally), 2016(11), 1035–1041. doi:10.1134/S0036029516110070
- Gad, E. A. M., Azzam, E. M. S., & Halim, S. A. (2018). Theoretical approach for the performance of 4-mercapto-1-alkylpyridin-1-ium bromide as corrosion inhibitors using DFT. Egyptian Journal of Petroleum, 27(4), 695–699. doi:10.1016/j.ejpe.2017.10.005
- Gadow, H. S., & Fakeeh, M. (2022). Green inhibitor of carbon steel corrosion in 1 M hydrochloric acid: Eruca sativa seed extract (Experimental and theoretical studies. RSC Advances, 12(15), 8953–8986.) doi:10.1039/D2RA01296K
- Guo, L., Luo, Y., Huang, Y., Yang, W., Zheng, X., Lin, Y., & Marzouki, R. (2022). Imidazolidiny urea as a potential corrosion inhibitor for mild steel in HCl medium: Experimental and density-functional based tight-binding methods. International Journal of Electrochemical Science, 17(7), 220748. doi:10.20964/2022.07.34
- Hozien, Z. A., EL-Mahdy, A. F. M., Abo Markeb, A., Ali, L. S. A., & El-Sherief, H. A. H. (2020). Synthesis of Schiff and Mannich bases of new s -triazole derivatives and their potential applications for removal of heavy metals from aqueous solution and as antimicrobial agents. RSC Advances, 10(34), 20184–20194. doi:10.1039/D0RA02872J
- Ibrahim, I. M., Yunus, S., & Hashim, M. A. (2013). Relative performance of isopropylamine, pyrrole and pyridine as corrosion inhibitors for carbon steels in saline water at mildly elevated temperatures. IJSER, 4, 1–12.
- Idris, M. N., Daud, A. R., & Othman, N. K. (2013). Electrochemical impedance spectroscopy study on corrosion inhibition of benzyltriethylammonium chloride, in. Selangor, Malaysia. 23–28. doi:10.1063/1.4858624
- Jafari, H., Akbarzade, K., & Danaee, I. (2019). Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives. Arabian Journal of Chemistry, 12(7), 1387–1394. doi:10.1016/j.arabjc.2014.11.018
- Ju, H., Ding, L., Sun, C., & Chen, J. (2015). Quantum chemical study on the corrosion inhibition of some oxadiazoles. Advances in Materials Science and Engineering, 2015, 1–5. doi:10.1155/2015/519606
- Kabanda, M. M., Murulana, L. C., Ozcan, M., Karadag, F., Dehri, I., Obot, I. B., & Ebenso, E. E. (2012). Quantum chemical studies on the corrosion inhibition of mild steel by some triazoles and benzimidazole derivatives in acidic medium. International Journal of Electrochemical Science, 7(6), 5035–5056. doi:10.1016/S1452-3981(23)19602-7
- Kamble, P. P., & Dubey, R. S. (2021). Effect of 1-acetyl-1H-benzotriazole on corrosion of mild steel in 1M HCl. Journal of Scientific Research, 13(3), 979–988. doi:10.3329/jsr.v13i3.52725
- Kuriakose, N., Kakkassery, J. T., Raphael, V. P., & Shanmughan, S. K. (2014). Electrochemical impedance spectroscopy and potentiodynamic polarization analysis on anticorrosive activity of thiophene-2-carbaldehyde derivative in acid medium. Indian Journal of Engineering and Materials Science, 20141–6. doi:10.1155/2014/124065
- Lai, C., Xie, B., Zou, L., Zheng, X., Ma, X., & Zhu, S. (2017). Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by S-allyl-O,O′-dialkyldithiophosphates. Results in Physics, 7, 3434–3443. doi:10.1016/j.rinp.2017.09.012
- Li, D., Zhao, X., Liu, Z., Liu, H., Fan, B., Yang, B., … Zou, H. (2021). Synergetic anticorrosion mechanism of main constituents in Chinese yam peel for copper in artificial seawater. ACS Omega, 6(44), 29965–29981. doi:10.1021/acsomega.1c04500
- Maithili, K., Shetty, P., Kumari, P. P., & Kagatikar, S. (2022). Mannich base as an efficient corrosion inhibitor of AA6061 in 0.5 M HCl: Electrochemical, surface morphological and theoretical investigations. Arabian Journal for Science and Engineering, 47(6), 7053–7067. doi:10.1007/s13369-021-06302-2
- Manimegalai, S., & Manjula, P. (2015). Thermodynamic and adsorption studies for corrosion inhibition of mild steel in aqueous media by Sargasam swartzii (Brown algae). Journal of Materials and Environmental Science. 6, 1629–1637.
- Molchan, I. S., Thompson, G. E., Skeldon, P., Lindsay, R., Walton, J., Kouvelos, E., … Schubert, T. J. S. (2015). Microscopic study of the corrosion behaviour of mild steel in ionic liquids for CO 2 capture applications. RSC Advances, 5(44), 35181–35194. doi:10.1039/C5RA01097G
- Nahlé, A., Salim, R., El Hajjaji, F., Aouad, M. R., Messali, M., Ech-Chihbi, E., … Taleb, M. (2021). Novel triazole derivatives as ecological corrosion inhibitors for mild steel in 1.0 M HCl: Experimental & theoretical approach. RSC Advances, 11(7), 4147–4162. doi:10.1039/D0RA09679B
- Ouakki, M., Galai, M., Benzekri, Z., Aribou, Z., Ech-Chihbi, E., Guo, L., … Cherkaoui, M. (2021). A detailed investigation on the corrosion inhibition effect of newly synthesized pyran derivative on mild steel in 1.0 M HCl: Experimental, surface morphological (SEM-EDS, DRX& AFM) and computational analysis (DFT & MD simulation. Journal of Molecular Liquids. 344, 117777. doi:10.1016/j.molliq.2021.117777
- Ouakki, M., Galai, M., Benzekri, Z., Verma, C., Ech-Chihbi, E., Kaya, S., … Cherkaoui, M. (2021). Insights into corrosion inhibition mechanism of mild steel in 1 M HCl solution by quinoxaline derivatives: Electrochemical, SEM/EDAX, UV-visible, FT-IR and theoretical approaches. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 611, 125810. doi:10.1016/j.colsurfa.2020.125810
- Ouakki, M., Galai, M., Rbaa, M., Abousalem, A. S., Lakhrissi, B., Rifi, E. H., & Cherkaoui, M. (2019). Quantum chemical and experimental evaluation of the inhibitory action of two imidazole derivatives on mild steel corrosion in sulphuric acid medium. Heliyon, 5(11), e02759. doi:10.1016/j.heliyon.2019.e02759
- Oyeneyin, O., Akerele, D., Ojo, N., & Oderinlo, O. (2021). Corrosion inhibitive potentials of some 2H-1-benzopyran-2-one derivatives- DFT calculations. Biointerface Research in Applied Chemistry, 11, 13968–13981. doi:10.33263/BRIAC116.1396813981
- Oyeneyin, O. E., Ojo, N. D., Ipinloju, N., Agbaffa, E. B., & Emmanuel, A. V. (2022). Investigation of the corrosion inhibition potentials of some 2-(4-(substituted)arylidene)-1H-indene-1,3-dione derivatives: Density functional theory and molecular dynamics simulation. Beni-Suef University Journal of Basic and Applied Sciences, 11(1), 132. doi:10.1186/s43088-022-00313-0
- Paul, S., & Koley, I. (2016). Corrosion inhibition of carbon steel in acidic environment by papaya seed as green inhibitor. Journal of Bio- and Tribo-Corrosion, 2(2), 6. doi:10.1007/s40735-016-0035-2
- Phadke Swathi, N., Alva, V. D. P., & Samshuddin, S. (2017). A Review on 1,2,4-triazole derivatives as corrosion inhibitors. Journal of Bio- and Tribo-Corrosion, 3(4), 42. doi:10.1007/s40735-017-0102-3
- Raghavendra, N., & Ishwara Bhat, J. (2019). Inhibition of Al corrosion in 0.5 M HCl solution by Areca flower extract. JKSUES, 31(3), 202–208. doi:10.1016/j.jksues.2017.06.003
- Rasheeda, K., Alamri, A. H., Krishnaprasad, P. A., Swathi, N. P., Alva, V. D. P., & Aljohani, T. A. (2022). Efficiency of a pyrimidine derivative for the corrosion inhibition of c1018 carbon steel in aqueous acidic medium: Experimental and theoretical approach. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 642, 128631. doi:10.1016/j.colsurfa.2022.128631
- Rasheeda, K., Swathi, N. P., Alva, V. D. P., Aljohani, T. A., Alomari, F. Y., & Alamri, A. H. (2022). Anticorrosive behavior of new pyrimidine derivatives on carbon steel in acidic medium: Experimental, theoretical, and surface studies. Journal of Bio- and Tribo-Corrosion, 8(4), 89. doi:10.1007/s40735-022-00688-8
- Rehioui, M., Abbout, S., Benzidia, B., Hammouch, H., Erramli, H., Daoud, N. A., … Hajjaji, N. (2021). Corrosion inhibiting effect of a green formulation based on Opuntia dillenii seed oil for iron in acid rain solution. Heliyon, 7(4), e06674. doi:10.1016/j.heliyon.2021.e06674
- Rodríguez, J. A., Cruz-Borbolla, J., Arizpe-Carreón, P. A., & Gutiérrez, E. (2020). Mathematical models generated for the prediction of corrosion inhibition using different theoretical chemistry simulations. Materials, 13(24), 5656. doi:10.3390/ma13245656
- Saadouni, M., Galai, M., Aoufir, Y. E., Skal, S., Boukhris, S., Hassikouz, A., … Souizi, A. (2018). Experimental, quantum chemical and Monte Carlo simulations studies on the corrosion inhibition of mild steel in 1 M HCl by two benzothiazine derivatives. Journal of Materials and Environmental Science, 9, 2493–2504.
- Sasikumar, Y., Adekunle, A. S., Olasunkanmi, L. O., Bahadur, I., Baskar, R., Kabanda, M. M., … Ebenso, E. E. (2015). Experimental, quantum chemical and Monte Carlo simulation studies on the corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on mild steel in acidic medium. Journal of Molecular Liquids, 211, 105–118. doi:10.1016/j.molliq.2015.06.052
- Shahzad, K., Sliem, M. H., Shakoor, R. A., Radwan, A. B., Kahraman, R., Umer, M. A., … Abdullah, A. M. (2020). Electrochemical and thermodynamic study on the corrosion performance of API X120 steel in 3.5% NaCl solution. Scientific Reports, 10(1), 4314. doi:10.1038/s41598-020-61139-3
- Sharma, P., Upadhyay, R. K., & Chaturvedi, A. (2014). Efficiency of some newly synthesized Mannich bases as corrosion inhibitor for mild steel in HNO3 solution. Asian Journal of Advance Basic Science, 3, 67–73.
- Shenoy, V. K., Venugopal, P. P., Reena Kumari, P. D., & Chakraborty, D. (2022). Anti-corrosion investigation of a new nitro veratraldehyde substituted imidazopyridine derivative Schiff base on mild steel surface in hydrochloric acid medium: Experimental, computational, surface morphological analysis. Materials Chemistry and Physics. 281, 125855. doi:10.1016/j.matchemphys.2022.125855
- Singh, P., Ebenso, E. E., Olasunkanmi, L. O., Obot, I. B., & Quraishi, M. A. (2016). Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. The Journal of Physical Chemistry C, 120(6), 3408–3419. doi:10.1021/acs.jpcc.5b11901
- Singh, A., Ebenso, E. E., & Quraishi, M. A. (2012). Corrosion inhibition of carbon steel in HCl solution by some plant extracts. International Journal of Corrosion. 2012, 1–20. doi:10.1155/2012/897430
- Sliem, M. H., El Basiony, N. M., Zaki, E. G., Sharaf, M. A., & Abdullah, A. M. (2020). Corrosion inhibition of mild steel in sulfuric acid by a newly synthesized schiff base: An electrochemical, DFT, and Monte Carlo Simulation study. Electroanalysis, 32(12), 3145–3158. doi:10.1002/elan.202060461
- Sun, H. (1998). COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. The Journal of Physical Chemistry B, 102(38), 7338–7364. doi:10.1021/jp980939v
- Swathi, N. P., Samshuddin, S., Alamri, A. H., Rasheeda, K., Alva, V. D. P., & Aljohani, T. A. (2022). Experimental and theoretical investigation of a new triazole derivative for the corrosion inhibition of carbon steel in acid medium. Egyptian Journal of Petroleum. 31(2), 15–21. doi:10.1016/j.ejpe.2022.04.002
- Swathi, N. P., Samshuddin, S., Aljohani, T. A., Rasheeda, K., Alva, V. D. P., Alomari, F. Y., & Alamri, A. H. (2022). A new 1,2,4-triazole derivative as an excellent corrosion inhibitor: Electrochemical experiments with theoretical validation. Materials Chemistry and Physics. 291, 126677. doi:10.1016/j.matchemphys.2022.126677
- Tüzün, B., & Bhawsar, J. (2021). Quantum chemical study of thiaozole derivatives as corrosion inhibitors based on density functional theory. Arabian Journal of Chemistry, 14(2), 102927. doi:10.1016/j.arabjc.2020.102927
- Visser, P., Terryn, H., & Mol, J. M. C. (2019). Active corrosion protection of various aluminium alloys by lithium‐leaching coatings. Surface and Interface Analysis, 51(12), 1276–1287. doi:10.1002/sia.6638
- Xavier Stango, S. A., & Vijayalakshmi, U. (2018). Studies on corrosion inhibitory effect and adsorption behavior of waste materials on mild steel in acidic medium. Journal of Asian Ceramic Societies, 6(1), 20–29. doi:10.1080/21870764.2018.1439608
- Zhang, X., Zhang, Y., Su, Y., Wang, X., & Lv, R. (2022). Synthesis and corrosion inhibition performance of Mannich bases on mild steel in lactic acid media. ACS Omega, 7(36), 32208–32224. doi:10.1021/acsomega.2c03545
- Zulfareen, N., Venugopal, T., & Kannan, K. (2018). Experimental and theoretical studies on the corrosion inhibition of brass in hydrochloric acid by n-(4-((4-benzhydryl piperazin-1-yl) methyl carbamoyl) phenyl) furan-2-carboxamide. International Journal of Corrosion, 2018, e9372804–18. doi:10.1155/2018/9372804