Abstract
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterised by diverse organ damages resulting from various autoantibodies, such as antinuclear or anti-DNA antibodies. Neuropsychiatric lupus (NPSLE) refers to the neurological and psychiatric disorders complicated with SLE and can be challenging for physicians to manage. NPSLE has a broad spectrum and high heterogeneity of clinical phenotypes, including headaches, psychiatric symptoms and peripheral neuropathy. Additionally, various immune effectors have been reported to contribute to the pathogenesis, including cytokines, cell-mediated inflammation and brain-reactive autoantibodies. In some patients with SLE, neuropsychiatric symptoms develop for the first time after the initiation of the steroid treatment, hindering the differentiation from steroid psychosis. The administration of high doses of steroids in patients with SLE is believed to trigger psychiatric symptoms. No clear evidence has yet been found regarding the treatment of NPSLE. Therefore, NPSLE-specific markers need to be developed, and treatment guidelines should be established. This article provides an overview of NPSLE as well as its pathogenesis and treatment.
1. Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterised by the production of various autoantibodies, such as antinuclear or anti-DNA antibodies [Citation1]. The management of the neuropsychiatric manifestation of SLE (NPSLE) is especially challenging. NPSLE has diverse and highly heterogeneous clinical phenotypes, including headaches, psychiatric symptoms and peripheral neuropathy. In addition, many other SLE complications, such as infections of the central nervous system (CNS), drug-induced diseases and metabolic disorders, cause neuropsychiatric symptoms. Various immune effectors, including brain-reactive autoantibodies, cytokines and cell-mediated inflammation have been reported as the contributors of SLE pathogenesis. However, patients with NPSLE are often weakly responsive to immunosuppressive therapy. Therefore, NPSLE-specific markers need to be developed, and specific treatment guidelines should be established. This review article describes the clinical features, pathogenic mechanisms and management of patients with NPSLE.
2. Classification of NPSLE
NPSLE refers to neurological and psychiatric disorders complicated with SLE. The symptoms of NPSLE have been classified into 19 neuropsychiatric (NP) manifestations by the American College of Rheumatology (ACR) [Citation2], leading to the elucidation of the incidence and features of the disease through clinical studies. The defined 19 NP manifestations comprised of two major classifications such as CNS and peripheral nervous system. CNS manifestations are further divided into ‘focal’ and ‘diffuse’ (). The prevalence of NPSLE in patients with SLE is 30–40%. Additionally, 50–60% of these develop NPSLE within one year of the SLE onset [Citation3]. The prevalence of each type of NPSLE significantly differs according to the study design followed. These discrepancies might be due to the ambiguity in the diagnosis of NP symptoms, especially of those non-specific to SLE, such as headache or mood disorders. Cerebrovascular disorders and epileptic seizures are found in 5–15% of patients with NPSLE. Cognitive impairment, mood disorders, acute confusional state or peripheral neuropathy are found in only 1–5% patients, whereas psychosis, myelitis, involuntary movements of the limbs and facial muscles, and aseptic meningitis are extremely rare. In Systemic Lupus International Collaborating Clinic Criteria (SLICC) [Citation4] proposed in 2012 for the classification of SLE, epileptic seizures, psychosis, mononeuritis multiplex, myelitis, peripheral neuropathy, cranial nerve disorders and acute confusional state are defined as the NP manifestations of SLE, whereas other types of NP symptoms, such as headaches, mood disorders and cerebrovascular disorders are excluded due to their low specificity. In 2019, new classification criteria for SLE were proposed by the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) [Citation5,Citation6]. In the new criteria, only delirium, psychosis and seizure are defined as the NP manifestations to improve specificity for diagnosis with SLE. Diagnosis with the new criteria will be subjected to verification with time.
Table 1. Neuropsychiatric manifestations in systemic lupus erythematosus.
3. Autoantibodies and NPSLE
SLE is characterised by multiple types of autoantibodies with multi-organ involvement. Several antibodies identified in patients with NPSLE are believed to contribute to the pathogenesis of the diseases, and various manifestations of NPSLE are now explained according to the antibodies present. Most cerebrovascular disorders in NPSLE are due to antiphospholipid antibodies (aPL) associated with the development of thrombosis. In addition, aPL has been reported to be associated with headaches, chorea, transverse myelitis and epileptic seizures [Citation7,Citation8]. Previous reports have shown that the anti-ribosomal protein P antibody is associated with psychiatric symptoms [Citation9,Citation10]. Additionally, the antibodies against the subunits of the N-methyl-D-aspartate (NMDA) receptor (anti-DNA/NR2 antibodies) are associated with diffuse NPSLE [Citation11,Citation12]. Increased levels of anti-DNA/NR2 antibodies in the spinal fluid are triggered by a failure of the blood–brain barrier (BBB) and can predict a recurrence of NPSLE. A systematic review of the association between NPSLE and autoantibodies has previously been reported. It shows that only aPL is associated with the onset of NPSLE and that there is no correlation with other antibodies [Citation13]. However, since the symptoms of NPSLE vary and overlap, the possible roles of other autoantibodies cannot be excluded.
4. Autoantibodies and the mechanism of NPSLE
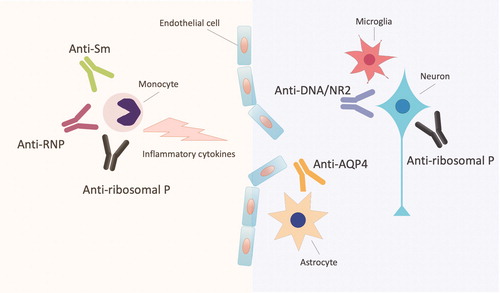
The graphical summary was provided regarding autoantibodies and mechanism of NPSLE (). Anti-DNA/NR2 and anti-ribosomal P antibodies are considered to target specific parenchymal structures in the brain and underly the onset of NP manifestations. Anti-Sm antibodies (anti-Sm) and anti-U1-ribonucleoprotein antibodies (anti-RNP) are frequently found in patients with NPSLE. In addition, anti-aquaporin 4 antibodies (anti-AQP4) which are diagnostic marker for neuromyelitis optica spectrum disorder (NMOSD) are contribute to NPSLE.
Figure 1. Autoantibodies and the mechanism of neuropsychiatric systemic lupus erythematosus. Anti-DNA/NR2 and anti-ribosomal P antibodies (anti-ribosomal P) are considered to target specific parenchymal structures in the brain and underly the onset of NP manifestations. Anti-Sm antibodies (anti-Sm), anti-U1-ribonucleoprotein antibodies (anti-RNP) and anti-ribosomal P enhance the production of inflammatory cytokines in human monocytes. Anti-aquaporin 4 antibodies (anti-AQP4) which are diagnostic marker for neuromyelitis optica spectrum disorder are contribute to NPSLE. Anti-DNA/NR2: the antibodies against the subunits of the N-methyl-D-aspartate (NMDA) receptor.
4.1. Anti-DNA/NR2 antibodies
Anti-DNA/NR2 antibodies are a subset of anti-DNA antibodies that cross-reacts with the GluN2(NR2) subunit of the NMDA receptor (NMDAR). NMDA receptor-mediated excitotoxicity has been reported to induce neuronal cell death [Citation14]. NMDAR is one of the ionotropic receptors of glutamate, an excitatory neurotransmitter in the nervous system, and plays a central role in learning and memory. NMDAR is a tetramer expressed on the cell membrane and composed of GluN1 (NR1) and GluN2 (NR2) subunits. Anti-DNA/NR2 antibodies bind to NR2, assemble the channel and induce cell death by a high influx of calcium into the cell [Citation15]. Notably, anti-DNA/NR2 antibodies differ from other anti-NMDAR antibodies found in patients who have ovarian teratoma. The anti-NMDAR antibodies are targeted against NR1 leading to the endocytosis of NMDAR [Citation16]. Although anti-DNA/NR2 antibodies have been detected in a mouse model immunized with the peptide antigens [Citation14], the phenotype could not be determined. In this model, a breach in the integrity of the BBB is required for the antibodies to access the brain tissue and affect the neuronal function and viability. Lipopolysaccharide (LPS) injection as a surrogate for infection causes antibody influx into the hippocampus, and epinephrine injection as a surrogate for stress allows the antibody to penetrate the amygdala. In a previous study that used LPS, hippocampal neurons decreased in number, and the mice experienced memory loss [Citation17]. In another study, which used epinephrine, the neurons in the amygdala decreased in number, and the mice displayed increased anxiety [Citation18]. These findings suggest that the regional disruption of the BBB depends on the agents used to modify the BBB and the same antibody can cause diverse behavioural changes. BBB breach causes antibody flow into the brain with a peak influx 48 h after the breach and disappear from the brain in two weeks. The evaluation of the nerve damage using 18F-fluorodeoxyglucose positron emission tomography in mice showed low glucose uptake in the brain two weeks after the BBB breach [Citation19]. At 4 weeks post-BBB breach, the mice exhibit increased glucose uptake in the affected region. Interestingly, although metabolism returned to normal, the numbers of neurons and dendritic spines, as well as dendritic complexity sustained to decrease after 8 weeks [Citation20]. The mice also exhibit behavioural disorders from 8 weeks after subjected to an anti-DNA/NR2 antibody-mediated insult. Taken together, the anti-DNA/NR2 antibody-induced brain pathology proceeds through an acute phase of excitotoxic neuronal loss, followed by permanent alteration in neuronal integrity and by spatial memory impairment. However, these findings are found in only experimental mice. Since some NPSLE patients with severe diffuse symptoms recovered completely after treatment, further research is needed to clarify the mechanism in human. Arinuma et al. [Citation21] have revealed the mechanism of long-term neuronal dysfunction and shown that activated microglia and C1q are critical mediators of the neuronal damage. The involvement of microglia and complements is a possible explanation for the changes occurring during both the acute and chronic phases [Citation21,Citation22]. They have also shown that angiotensin-converting enzyme (ACE) inhibitor can prevent the microglia activation and preserve neuronal function and cognitive performance. Accordingly, ACE inhibitors may be a promising class of therapeutics against the cognitive impairment in SLE.
4.2. Anti-ribosomal P protein antibodies
Ribosomes are organelles of protein synthesis and are composed of ribosomal protein–RNA complexes. Ribosomal P protein refers to three types of phosphorylated proteins present on the 60S subunit of eukaryotic ribosomes. It is also known as the neuronal surface P antigen (NSPA) due to their expression on the neuronal cell surface in the cerebral cortex, hippocampus and amygdala [Citation23]. P antigen consists of the highly conserved carboxy-terminal residues of three ribosomal phosphoproteins, P0 (38 kDa), P1 (19 kDa) and P2 (17 kDa) [Citation24]. Anti-ribosomal P protein antibodies recognize all these proteins [Citation25], increase cellular calcium influx and induce cell death [Citation26]. Passive transfer experiments in mice have shown that anti-ribosomal P protein antibodies isolated from SLE patients induce olfactory abnormalities [Citation27], depression-like manifestations [Citation28] and memory impairment [Citation29]. In addition, ribosomal P proteins are expressed on the surfaces of peripheral blood monocytes. Binding of anti-ribosomal P protein antibody to monocytes increases the production of proinflammatory cytokines, such as tumour necrosis factor-α and interleukin (IL)-6 from monocytes [Citation30]. Since these cytokines contribute to the BBB breach, the association between anti-ribosomal P antibodies and BBB breach has recently been considered.
4.3. Anti-Sm antibodies and anti-U1-ribonucleoprotein antibodies
Anti-Sm recognize the U1, U2, U4/U6 and U5 small nuclear RNPs (snRNPs), and anti-RNP recognize the U1snRNPs, respectively [Citation31]. The presence of serum anti-Sm correlates with the prevalence of diffuse NPSLE [Citation32], and high mortality in patients with NPSLE [Citation33]. High anti-RNP levels were also found in patients with NPSLE in both serum and cerebrospinal fluid [Citation34]. Matsueda et al. showed that anti-Sm and anti-RNP bind on the cell surface of human monocyte. In addition, anti-Sm and anti-RNP synergistically enhance the production of IL-6 by human monocytes [Citation35]. Since anti-Sm and anti-RNP often coexist, the synergistic effects by the two antibodies may explain the mechanism of the BBB breach in patients with NPSLE.
4.4. Anti-aquaporin 4 antibodies
NMOSD is an autoimmune disease characterised by damage to CNS, and the presence of anti-AQP4 [Citation36]. NMOSD commonly affects areas with high levels of AQP-4 expression, such as demyelinating spinal cord lesions and/or optic neuritis. Demyelination is also part of the symptoms in NPSLE. Since it was unclear whether the autoantibodies found in NPSLE with demyelinating symptoms are common to those in NMOSD, Mader et al. clarified the prevalence of anti-AQP4 in patients with NPSLE [Citation37]. Anti-AQP4 was found in 27% of NPSLE with demyelinating symptoms. Notably, NPSLE without demyelinating symptoms was negative of anti-AQP4, suggesting that anti-AQP4 may be associated with demyelination in NPSLE.
5. Cytokines in the cerebrospinal fluid (CSF)
Previous reports showed elevated levels of several cytokines, such as fractalkine [Citation38], IL-6 [Citation39,Citation40], IL-8 [Citation41], interferon-α (IFN-α) [Citation42,Citation43], IL-1, BAFF (B cell-activating factor) and APRIL (a proliferation-inducing ligand) [Citation44] in the CSF of patients with NPSLE. Hirohata et al. showed that the presentation of high IL-6 levels in the CSF was the most sensitive and abnormal laboratory test results in NPSLE [Citation45]. Santer et al. showed that the CSF from SLE patients contains abnormally high levels of IFN-α-inducing activity. In the CSF, immune complexes bound to FCγRII on plasmacytoid dendritic cells leading to cytokine production via TLR7 activation [Citation42]. Therefore, autoantibodies entering the brain and reacting with neurons and glial cells may increase the levels of cytokines in the CSF.
6. Post-steroid NP manifestation in SLE
In some patients with SLE, NP symptoms develop for the first time after the initiation of the steroid treatment, hindering the differentiation from steroid psychosis. Interestingly, the incidence of ‘post-steroid NP manifestation’ (PSNP) is significantly higher in patients with SLE than in those with other autoimmune diseases. To clarify the characteristics of PSNP in SLE, we defined the NP symptoms that occur after the steroid administration as PSNP-SLE, and the NPSLE with NP symptoms that develop before high-dose steroid treatments as ‘de novo NPSLE’ [Citation46]. Subsequently, we compared the characteristics including the complications of the APS and previous medical history of psychiatric disorders in PSNP-SLE and de novo NPSLE and revealed the risk factors for PSNP-SLE. Most of the PSNP-SLE cases show diffuse manifestations, whereas de novo NPSLE shows focal manifestation according to the ACR classification. Further, we have evaluated the clinical significance of anti-DNA/NR2 antibodies in both de novo NSPLE and PSNP-SLE [Citation47]. Anti-DNA/NR2 antibody levels in PSNP-SLE are similar with the levels in de novo NPSLE, and they show a strong correlation with the levels of anti-DNA antibodies. Thus, anti-DNA/NR2 antibodies in PSNP-SLE are a dominant subset of anti-DNA antibodies. Our findings suggest that anti-DNA/NR2 antibodies may be a predictive factor not only in de novo NPSLE but also in PSNP-SLE. The administration of high doses of steroids in patients with SLE is believed to trigger psychiatric symptoms.
7. Treatment of NPSLE
No clear evidence has yet been found regarding the treatment of NPSLE. Various immunosuppressive treatments have been introduced for a variety of NP symptoms in patients with SLE (e.g. high-dose corticosteroids, methylprednisolone pulse therapy, intravenous immunoglobulins (IVIG), plasma exchange, immunosuppressants, including cyclophosphamide, azathioprine, mycophenolate mofetil (MMF) and biologics represented by rituximab).
Cyclophosphamide is the only drug that has been compared with steroid pulse therapy in a randomized controlled trial and has been found more useful than steroid pulse therapy [Citation48]. However, the evidence from this study is considered insufficient due to the characteristics of the patients. For instance, most patients had epilepsy or peripheral neuropathy, and some had optic neuritis. Azathioprine can be used as a maintenance therapy or to reduce the steroid dosage applied. Azathioprine used as a maintenance therapy has been shown to suppress the recurrence of NP manifestations in patients with NPSLE [Citation49]. MMF is widely used as the first-line option for both the induction and maintenance therapies for lupus nephritis. Several observational studies have suggested the potential benefit of MMF in the non-renal manifestations of SLE [Citation50]. However, it is difficult to make any definite conclusion. Rituximab is anti-CD20 IgG1 type 1 chimeric monoclonal antibody that destroys B cells by specifically targeting the CD20 molecules on their surfaces [Citation51]. The effectiveness of rituximab against refractory NPSLE has previously been reported by Tokunaga et al. [Citation52]. In a systematic review, clinical response to rituximab has been detected in 85% of patients with NPSLE [Citation53]. However, two SLE patients under rituximab treatment have died due to progressive leukoencephalopathy, and thus the clinical trial has been aborted. The evidence on the efficacy of IVIG is limited. Case reports and retrospective studies indicate that IVIG successfully treats SLE patients with a broad spectrum of NP symptoms [Citation54]. Hydroxychloroquine is used as a first-line drug in SLE patients who do not display major organ disorders. This drug was finally approved in Japan in 2015 and can now be administered to patients with cutaneous SLE or those showing rash, arthritis, myalgia, malaise or fever. It has also been proposed for the primary prevention of NPSLE, especially for cerebrovascular disorders [Citation3,Citation55] and epileptic seizures [Citation56].
8. Conclusion
NPSLE is one of the severe manifestations in SLE. Because of the small patient number and severity of the disease, it is hard to conduct RCTs for patients with NPSLE. However, NPSLE was defined by the ACR in 1999, and the guidelines for the management and treatment of NPSLE were provided by EULAR in 2010. The clinical framework of NPSLE has gradually been established. In addition, promising research efforts for novel targeted therapies and improved diagnostic tools are in progress. We believe there will be a better way to treat patients for enhanced prognosis and quality of life.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Yasuda S. Emerging targets for the treatment of lupus erythematosus: there is no royal road to treating lupus. Mod Rheumatol. 2019;29(1):60–69.
- The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608.
- Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69(12):2074–2082.
- Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412.
- Afeltra A, Garzia P, Mitterhofer AP, et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology. 2003;61(1):108–110.
- Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30(5):985–992.
- Isshi K, Hirohata S. Association of anti-ribosomal P protein antibodies with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 1996;39(9):1483–1490.
- Bonfa E, Golombek SJ, Kaufman LD, et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317(5):265–271.
- Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;58(4):1130–1135.
- Hirohata S, Arinuma Y, Yanagida T, et al. Blood–brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther. 2014;16(2):R77.
- Sciascia S, Bertolaccini ML, Roccatello D, et al. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus: a systematic review. J Neurol. 2014;261(9):1706–1714.
- DeGiorgio LA, Konstantinov KN, Lee SC, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–1193.
- Faust TW, Chang EH, Kowal C, et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc Natl Acad Sci USA. 2010;107(43):18569–18574.
- Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098.
- Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179–188.
- Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103(3):678–683.
- Vo A, Volpe BT, Tang CC, et al. Regional brain metabolism in a murine systemic lupus erythematosus model. J Cereb Blood Flow Metab. 2014;34(8):1315–1320.
- Chang EH, Volpe BT, Mackay M, et al. Selective impairment of spatial cognition caused by autoantibodies to the N-methyl-D-aspartate receptor. EBioMedicine. 2015;2(7):755–764.
- Nestor J, Arinuma Y, Huerta TS, et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J Exp Med. 2018;215(10):2554–2566.
- Nestor J, Gata-Garcia A, Arinuma Y, et al. Immune-mediated brain pathology: from autoantibodies to microglia. Discov Med. 2016;22(121):201–207.
- Segovia-Miranda F, Serrano F, Dyrda A, et al. Pathogenicity of lupus anti-ribosomal P antibodies: role of cross-reacting neuronal surface P antigen in glutamatergic transmission and plasticity in a mouse model. Arthritis Rheumatol. 2015;67(6):1598–1610.
- Elkon K, Skelly S, Parnassa A, et al. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1986;83(19):7419–7423.
- Elkon KB, Parnassa AP, Foster CL. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985;162(2):459–471.
- Matus S, Burgos PV, Bravo-Zehnder M, et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J Exp Med. 2007;204(13):3221–3234.
- Katzav A, Ben-Ziv T, Chapman J, et al. Anti-P ribosomal antibodies induce defect in smell capability in a model of CNS-SLE (depression). J Autoimmun. 2008;31(4):393–398.
- Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56(3):938–948.
- Bravo-Zehnder M, Toledo EM, Segovia-Miranda F, et al. Anti-ribosomal P protein autoantibodies from patients with neuropsychiatric lupus impair memory in mice. Arthritis Rheumatol. 2015;67(1):204–214.
- Nagai T, Arinuma Y, Yanagida T, et al. Anti-ribosomal P protein antibody in human systemic lupus erythematosus up-regulates the expression of proinflammatory cytokines by human peripheral blood monocytes. Arthritis Rheum. 2005;52(3):847–855.
- Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151.
- Tikly M, Burgin S, Mohanlal P, et al. Autoantibodies in black South Africans with systemic lupus erythematosus: spectrum and clinical associations. Clin Rheumatol. 1996;15(2):143–147.
- Abe G, Kikuchi H, Arinuma Y, et al. Brain MRI in patients with acute confusional state of diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Mod Rheumatol. 2017;27(2):278–283.
- Sato T, Fujii T, Yokoyama T, et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum. 2010;62(12):3730–3740.
- Matsueda Y, Arinuma Y, Nagai T, et al. Synergistic enhancement of production of proinflammatory cytokines of human peripheral blood monocytes by anti-Sm and anti-RNP antibodies. PLoS One. 2018;13(12):e0209282.
- Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477.
- Mader S, Jeganathan V, Arinuma Y, et al. Understanding the antibody repertoire in neuropsychiatric systemic lupus erythematosus and neuromyelitis optica spectrum disorder: do they share common targets? Arthritis Rheumatol. 2018;70(2):277–286.
- Yajima N, Kasama T, Isozaki T, et al. Elevated levels of soluble fractalkine in active systemic lupus erythematosus: potential involvement in neuropsychiatric manifestations. Arthritis Rheum. 2005;52(6):1670–1675.
- Hirohata S, Miyamoto T. Elevated levels of interleukin-6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system involvement. Arthritis Rheum. 1990;33(5):644–649.
- Trysberg E, Carlsten H, Tarkowski A. Intrathecal cytokines in systemic lupus erythematosus with central nervous system involvement. Lupus. 2000;9(7):498–503.
- Katsumata Y, Harigai M, Kawaguchi Y, et al. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J Rheumatol. 2007;34(10):2010–2017.
- Santer DM, Yoshio T, Minota S, et al. Potent induction of IFN-alpha and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J Immunol. 2009;182(2):1192–1201.
- Shiozawa S, Kuroki Y, Kim M, et al. Interferon-alpha in lupus psychosis. Arthritis Rheum. 1992;35(4):417–422.
- George-Chandy A, Trysberg E, Eriksson K. Raised intrathecal levels of APRIL and BAFF in patients with systemic lupus erythematosus: relationship to neuropsychiatric symptoms. Arthritis Res Ther. 2008;10(4):R97.
- Hirohata S, Kanai Y, Mitsuo A,, et al. Accuracy of cerebrospinal fluid IL-6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin Rheumatol. 2009;28(11):1319–1323.
- Shimizu Y, Yasuda S, Kako Y, et al. Post-steroid neuropsychiatric manifestations are significantly more frequent in SLE compared with other systemic autoimmune diseases and predict better prognosis compared with de novo neuropsychiatric SLE. Autoimmun Rev. 2016;15(8):786–794.
- Fujieda Y, Mader S, Jeganathan V, et al. Clinical significance of anti-DNA/N-methyl-D-aspartate receptor 2 antibodies in de novo and post-steroid cases with neuropsychiatric systemic lupus erythematosus. Int J Rheum Dis. 2019;22(3):443–448.
- Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64(4):620–625.
- Ginzler E, Sharon E, Diamond H, et al. Long-term maintenance therapy with azathioprine in systemic lupus erythematosus. Arthritis Rheum. 1975;18(1):27–34.
- Mok CC. Mycophenolate mofetil for non-renal manifestations of systemic lupus erythematosus: a systematic review. Scand J Rheumatol. 2007;36(5):329–337.
- Lee WS, Amengual O. B cells targeting therapy in the management of systemic lupus erythematosus. Immunol Med. 2020;43(1):16–35.
- Tokunaga M, Saito K, Kawabata D, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–475.
- Narvaez J, Rios-Rodriguez V, de la Fuente D, et al. Rituximab therapy in refractory neuropsychiatric lupus: current clinical evidence. Semin Arthritis Rheum. 2011;41(3):364–372.
- Magro-Checa C, Zirkzee EJ, Huizinga TW, et al. Management of neuropsychiatric systemic lupus erythematosus: current approaches and future perspectives. Drugs. 2016;76(4):459–483.
- Petri M. Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep. 2011;13(1):77–80.
- Andrade RM, Alarcon GS, Gonzalez LA, et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV). Ann Rheum Dis. 2008;67(6):829–834.

