Abstract
Promoting sexual health is a World Health Organization (WHO) priority. Lubricants are widely available and used to improve sexual pleasure and reduce pain during intercourse. To inform WHO’s self-care interventions guideline, we conducted a systematic review of the peer-reviewed literature to answer the question: does use of lubricants during or prior to sex result in improved sexual health and well-being. We searched PubMed, CINAHL, LILACS and EMBASE on 8 July 2020 for effectiveness, values and preferences, and cost data related to commercially available vaginal and anal lubricants. Data were systematically extracted and qualitatively synthesised. Effectiveness evidence was summarised in GRADE evidence profiles. Seven studies met the effectiveness review criteria. Two randomised trials found lubricant use led to improved female sexual well-being and had no impact on incidence of human papillomavirus (moderate certainty evidence). One observational study with gay and bisexual men showed lubricants were associated with increased reports of pain during receptive intercourse and no difference in pain during insertive intercourse, but a reduced degree of pain in both types of intercourse (low/very low certainty evidence). One observational study with female breast cancer survivors found better outcomes of vaginal dryness and dyspareunia with lubricant use (very low certainty evidence). Twenty-one values and preferences studies from diverse populations globally found that most individuals supported lubricant use for reasons of comfort/reduced pain and sexual pleasure. No cost studies were identified. Although evidence is limited, lubricants appear to offer an acceptable approach to improving sexual health and well-being.
Résumé
Promouvoir la santé sexuelle est une priorité de l’Organisation mondiale de la santé (OMS). Les lubrifiants sont largement disponibles et utilisés pour améliorer le plaisir sexuel et réduire les douleurs pendant les rapports. Pour guider les principes directeurs de l’OMS sur les interventions d’auto-prise en charge, nous avons examiné systématiquement les publications à comité de lecture afin de répondre à la question: l’utilisation de lubrifiants pendant ou avant les rapports sexuels aboutit-elle à une meilleure santé sexuelle et un bien-être accru? Le 8 juillet 2020, nous avons recherché dans PubMed, CINAHL, LILACS et EMBASE des données sur l’efficacité, les valeurs et préférences, ainsi que sur les coûts, relatives aux lubrifiants vaginaux et anaux disponibles dans le commerce. Les données ont été extraites de manière systématique puis synthétisées qualitativement. Les données sur l’efficacité ont été résumées dans les profils de données de GRADE. Sept études réunissaient les critères de l’examen de l’efficacité. Deux essais randomisés ont montré que l’utilisation de lubrifiants améliorait le bien-être des femmes et n’avait pas d’impact sur l’incidence du papillomavirus humain (certitude modérée des données). Une étude observationnelle portant sur des hommes homosexuels et bisexuels a révélé que les lubrifiants étaient associés à des notifications accrues de douleurs pour le partenaire passif et à aucune différence de douleur pour le partenaire actif, mais à un degré de douleur moindre dans les deux types de rapports (certitude faible/très faible des données). Une étude observationnelle auprès de femmes guéries d’un cancer du sein a trouvé de meilleurs résultats en matière de sécheresse vaginale et de dyspareunie avec l’emploi d’un lubrifiant (très faible certitude des données). Vingt et une études sur les valeurs et les préférences auprès de diverses populations ont montré en général que la plupart des individus soutenaient l’utilisation de lubrifiants pour des raisons de confort/réduction de la douleur et plaisir sexuel. Aucune étude de coût n’a été identifiée. Même si les données sont limitées, les lubrifiants semblent offrir une approche acceptable pour l’amélioration de la santé sexuelle et du bien-être.
Resumen
La promoción de la salud sexual es una prioridad de la Organización Mundial de la Salud (OMS). Los lubricantes están ampliamente disponibles y son utilizados de manera extendida para mejorar el placer sexual y disminuir el dolor durante el coito sexual. Con el fin de informar la guía de la OMS sobre intervenciones en autocuidado, realizamos una revisión sistemática de la literatura revisada por pares para contestar la pregunta ¿el uso de lubricantes durante o antes del sexo contribuye al mejoramiento de la salud y el bienestar sexuales? El 8 de julio de 2020, realizamos una búsqueda en PubMed, CINAHL, LILACS y EMBASE de eficacia, valores y preferencias, y datos de costo relacionados con lubricantes vaginales y anales disponibles comercialmente. Los datos fueron extraídos sistemáticamente y sintetizados cualitativamente. La evidencia sobre la eficacia se resumió en perfiles de evidencia GRADE. Siete estudios reunieron los criterios de la revisión de eficacia. Dos ensayos clínicos aleatorizados encontraron que el uso de lubricantes contribuye al mejoramiento del bienestar sexual de las mujeres y que no tuvo ningún impacto en la incidencia del virus del papiloma humano (certeza de evidencia moderada). Un estudio observacional con hombres homosexuales y bisexuales mostró que los lubricantes estaban asociados con un mayor número de informes de dolor durante el coito receptivo y ninguna diferencia en dolor durante el coito insertivo, pero con menor grado de dolor en ambos tipos de coito (certeza de evidencia baja o muy baja). Un estudio observacional con sobrevivientes de cáncer de mama encontró mejores resultados del uso de lubricantes con relación a la sequedad vaginal y la dispareunia (certeza de evidencia muy baja). En 21 estudios de valores y preferencias de diversas poblaciones mundialmente, se encontró que la mayoría de las personas apoyan el uso de lubricantes por razones de comodidad/disminución del dolor y placer sexual. No se identificó ningún estudio de costo. Aunque existe evidencia limitada, al parecer, los lubricantes ofrecen un enfoque aceptable para mejorar la salud y el bienestar sexuales.
Introduction
Promoting sexual health is one of the five priority areas of the World Health Organization (WHO) reproductive health strategy.Citation1 WHO’s working definition of sexual health is the “state of physical, emotional, mental and social well-being in relation to sexuality; it is not merely the absence of disease, dysfunction or infirmity. Sexual health requires a positive and respectful approach to sexuality and sexual relationships, as well as the possibility of having pleasurable and safe sexual experiences, free of coercion, discrimination and violence”.Citation2
Use of lubricants during sex may result in improved sexual health and well-being and may be particularly helpful for individuals experiencing vaginal dryness associated with menopause,Citation3 individuals experiencing dyspareunia (pain during sexual intercourse or other sexual activity that involves vaginal penetration),Citation4 or people engaging in anal sex.Citation5 Lubricants may also facilitate optimal sexual function, pleasure, and enjoyment for sexually active individuals, across genders, regardless of specific health conditions, and improve sexual relationships. Lubricants have also been recommended for use in conjunction with condoms to reduce condom breakage and therefore provide better protection against sexually transmitted infections (STIs), including HIV.Citation6 There is a wide range of lubricant products available on the market globally, which are used for both anal and vaginal sexual activity. However, while lubricant use may be generally helpful, substandard products used as lubricants could also result in adverse health outcomes.
We sought to systematically review the evidence for the use of lubricants during or prior to sex to improve sexual health and well-being. We conducted this review in the context of expanding the evidence base of WHO’s normative guidance on self-care interventionsCitation7 to interventions that promote sexual health. Lubricants can be used in isolation or with other products, such as condoms, but are generally over-the-counter products used by individuals and their partners without prescription or involvement of health workers; they are thus a form of self-care, which WHO defines as “the ability of individuals, families and communities to promote health, prevent disease, maintain health and cope with illness and disability with or without the support of a health worker”.Citation7 Current WHO guidance regarding self-care interventions and lubricants includes a good practice statement that “people from underserved populations should be able to experience full, pleasurable sex lives and have access to a range and choice of reproductive health options”Citation7 and an advisory note recommending procurement of additional lubricants for male and female condoms, with specific considerations for certain lubricant characteristics, such as osmolality and pH.Citation6 This review was also conducted as part of a response to the COVID-19 pandemic during which self-care interventions for sexual and reproductive health have been prioritised.Citation8 Finally, this review supports the move to improve universal health coverage for all, as implementation of self-care interventions within the context of human rights, gender equality, and a life course approach can promote comprehensive, integrated, and people-centred approaches to health service delivery.Citation9
Methods
This review addressed the question: Does use of lubricants during or prior to sex result in improved sexual health and well-being? We reviewed the extant literature in three areas relevant to answering these questions and developing WHO guidance: the effectiveness of the intervention, the values and preferences of end users and health workers, and cost information. The review followed PRISMA guidelines,Citation10 and the protocol was published on PROSPERO (registration number CRD42020208976).
For the purposes of this review, we focused on vaginal and anal lubricants (used immediately prior to or during sexual activity) as opposed to vaginal moisturisers (used daily over a longer term). A recent reviewCitation11 describes the difference between vaginal lubricants and vaginal moisturisers as follows:
“Lubricants may relieve vaginal dryness and discomfort during sexual activity, providing short-term relief from vaginal dryness and dyspareunia. Vaginal moisturisers are intended to be used primarily for the relief of vaginal dryness on a day-to-day basis, to provide comfort and offer long-term benefits. Vaginal moisturisers are classified as Class IIa Medical Devices by the Medicines and Healthcare products Regulatory Agency, based on the intended duration of their use (vaginal moisturisers are intended to be present in the body for longer than 60 min, but a single application should not last longer than 30 days). Lubricants may or may not be classified as medical devices, depending on their individual claims.”
We included products that had no known harmful effects for use as lubricants (e.g. olive oil) but excluded biological lubricants (e.g. saliva, pre-seminal fluid) and microbicide gels. We did not focus on the range of vaginal drying products, bleaching products, or other topical anal or vaginal products that are available across settings.
Effectiveness review
The effectiveness review was designed according to the PICO format as follows:
Population: Sexually active individuals (with attention to specific subpopulations in the stratifications noted below)
Intervention: Use of lubricant during sexual activity (defined as any penetration, including vaginal/anal, with/without a partner, and with an object)
Comparison: Sexual activity without lubricant
Outcomes:
Vaginal dryness or pain during vaginal/anal penetration.
Sexual arousal dysfunctions (female sexual arousal dysfunction, male erectile dysfunction).
Sexual desire, arousal, lubrication, orgasm, satisfaction, and pleasure.
Vaginal discharge and bacterial vaginosis.
Side effects (irritation, infections [yeast; reproductive tract infection (RTI); STIs; urinary tract infection (UTI)]).
STIs/HIV (incidence, prevalence, transmission, etc.).
Self-efficacy, self-determination, autonomy, and empowerment around sexual health, and sexuality (confidence, communication with partners, self-esteem).
Other side effects or adverse events, or social harms (e.g. coercion, violence [including intimate-partner violence, violence from family members or community members, etc.], psycho-social harm, self-harm, etc.), and whether these harms were corrected/had redress available.
To be included in the review, an article had to meet the following criteria:
A study design that compared self-use of lubricants during sexual activity to sexual activity without lubricants. Comparative study designs included randomised controlled trials, non-randomised analytical trials, and comparative observational studies (including prospective analytical cohort studies, cross-sectional studies, analytical before-after studies and interrupted time series) that compared individuals who received the intervention to those who did not.
Measured one or more of the outcomes listed above.
Published in a peer-reviewed journal.
No restrictions were placed based on location of the intervention. No language restrictions were used on the search. Articles in English, French, Spanish, and Chinese were coded directly; articles in other languages were translated. No restrictions were placed on the date of publication, other than the search cut-off date.
The search strategy, designed for PubMed and adapted for other databases, combined search terms for two concepts: lubricants and sex (see Supplementary material). These search terms were used both for the main systematic review (PICO question) and for the values and preferences and cost reviews (described below).
The following electronic databases were searched through the search date of July 8, 2020: PubMed, CINAHL, LILACS, and EMBASE. Secondary reference searching was also conducted on all studies included in the review. We searched for ongoing randomised controlled trials (RCTs) through clinicaltrials.gov, the WHO International Clinical Trials Registry Platform, the Pan-African Clinical Trials Registry, and the Australian New Zealand Clinical Trials Registry. We also conducted a handsearch on Google Scholar and the Cochrane Library. Finally, selected experts in the field were contacted to identify additional articles not identified through other search methods.
Titles, abstracts, citation information, and descriptor terms of citations identified through the search strategy were screened by a member of the study staff. Full-text articles were obtained of all selected abstracts, and two independent reviewers assessed all full-text articles for eligibility to determine final study selection. Differences were resolved through consensus. Data were extracted independently by two reviewers using standardised data extraction forms. Differences in data extraction were resolved through consensus and referral to a senior study team member from WHO when necessary.
The following information was gathered from each included study:
Study identification: author(s); type of citation; year of publication.
Study description: study objectives; location; population characteristics; type of lubricant; study design; sample size; follow-up periods and loss to follow-up.
Outcomes: analytic approach; outcome measures; comparison groups; effect sizes; confidence intervals; significance levels; conclusions; limitations.
For randomised trials, risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias.Citation12 For non-randomised trials but comparative studies, risk of bias was assessed using the Evidence Project 8-item checklist for intervention evaluations.Citation13
Data were analysed according to coding categories and outcomes. If we had identified multiple studies reporting the same outcome measured in the same way, meta-analysis would have been conducted using random-effects models to combine risk ratios with Comprehensive Meta-Analysis (CMA).
We planned to stratify all analyses by the following categories, where data were available:
Condom use
Point of access (e.g. stores, pharmacies, online/telehealth, etc.)
Type of lubricant
Frequency of sex
Populations (e.g. adults/adolescents, individuals with specific medical conditions or on specific medications, perimenopausal/menopausal persons, persons with disabilities, postpartum, sex workers, sexual and gender minorities, race/ethnicity, etc.)
Vaginal vs. anal sex
Type of partner (transactional or not, steady vs. casual)
Vulnerabilities (e.g. poverty, disability, literacy/educational level)
High-income versus low or middle-income countries
Data were summarised in GRADE Evidence Profile tables using GRADEPro.
Values and preferences review
Values and preferences have been defined as the “collection of goals, expectations, predispositions, and beliefs that individuals have for certain decisions and their potential outcomes”Citation14 and are a required part of the WHO guideline development process.Citation15 The same search terms were used to search and screen for studies to be included in the values and preferences review. Studies were included in this review if they presented primary data examining preferences of lubricant users, or individuals who might be candidates for lubricant use. We focused on studies examining the values and preferences of end users, but also included studies examining the values and preferences of health workers. We considered issues related to age of availability, informed decision-making, coercion, and seeking redress in this section. These studies could be qualitative or quantitative in nature, but had to present primary data collection – think pieces and review articles were not be included. Values and preferences literature was summarised qualitatively and was organised by study design and methodology, location, and population.
Cost review
Consideration of costs and resource use is also a required component of the WHO guideline development process.Citation15 The same search terms were used to search and screen for studies to be included in the cost review. Studies were included in this review if they presented primary data comparing costing, cost-effectiveness, cost-utility, or cost-benefit of the intervention and comparison listed in the PICO above, or if they presented cost-effectiveness of the intervention as it relates to the PICO outcomes listed above. We planned to summarise cost literature qualitatively. We planned to classify cost literature into four categories (health sector costs, other sector costs, patient/family costs, and productivity impacts) and within each category organise by study design/methodology, location, and population.
Results
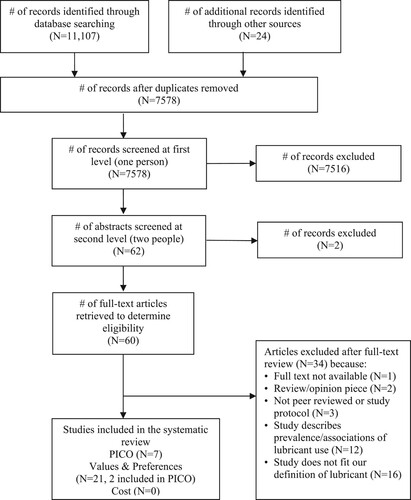
Our search yielded 7578 unique references, of which 60 were retained for full-text review (). Ultimately, we identified seven that met the inclusion criteria for the effectiveness review,Citation4,Citation16–21 twenty-one values and preferences studies,Citation4,Citation17,Citation22–40 and no cost studies. A table of excluded studies is provided in Supplementary Table A.
Figure 1. PRISMA flow chart showing disposition of citations through the search and screening process.
Effectiveness review
Overall, seven studies met the inclusion criteria for the effectiveness review.Citation4,Citation16–21 This included two RCTs and five observational studies. presents descriptive data from the two RCTs and the two observational studies presented in the GRADE evidence profileCitation4,Citation17,Citation20,Citation21; we included RCT data in GRADE for each outcome category when available, and where RCT data were not available, we included data from observational studies. presents a GRADE Evidence Profile for these four studies. Overall, the quality of evidence for each outcome rated from moderate (three outcomes) to very low (six outcomes) in the GRADE system. Given the small number of studies presenting outcome data, no further stratifications from our a priori list were possible, and meta-analysis was not conducted. provides a summary of findings from all seven included studies, which are described in the text below.
Table 1. Description of studies included in GRADE.
Table 2. GRADE Evidence Profile.
Table 3. Comparative findings from studies included in the effectiveness review
Sexual desire, arousal, lubrication, orgasm, satisfaction, and pleasure
One RCTCitation20 among sexually active adult women in stable heterosexual partnerships in the United States reported on female sexual well-being. This study was graded as moderate certainty evidence due to risk of bias: blinding participants to lubricant use was not possible given the nature of the intervention, and participant reports of sexual well-being may have been affected by their knowledge of lubricant use. Findings indicated that lubricant use was associated with improved female sexual well-being (FSWB scale overall score: Couple lubricant vs no lubricant: 6.35 vs 1.94; Female lubricant vs no lubricant: 3.99 vs 1.94).
STIs/HIV
One large RCTCitation21 among sexually active women in Zimbabwe measured HPV outcomes. This study was graded as moderate certainty evidence due to imprecision, as the 95% confidence interval (CI) for relative risk (RR) crossed 1 and included the potential for both appreciable benefit and appreciable harm. This trial found that lubricant use did not affect the incidence of HPV (any HPV: RR: 0.91, 95% CI: 0.73–1.13; any oncogenic HPV: RR: 1.09, 95% CI: 0.76–1.56).
Vaginal dryness or pain during vaginal/anal penetration
One observational studyCitation17 reported the relationship between lubricant use and pain among self-identified gay and bisexual men in the United States. This study was graded as very low certainty of evidence due to potential self-report bias, reverse causality, and imprecision, as the 95% CI crossed 1 and included both appreciable benefit and harm. Pain was assessed through several questions, including whether pain was experienced (yes/no) and degree of pain. Using lubricants was not associated with self-reported pain at last insertive sex (RR: 1.26, 95% CI: 0.39–4.09), but men using lubricants were more likely to report experiencing pain during their last receptive partnered sexual event (RR: 3.59, 95% CI: 1.27–10.18). However, lubricant use was associated with a lower degree of pain reported during both insertive and receptive sex (mean difference: 0.6 lower for insertive sex; 0.8 lower for receptive sex).
A second, small observational study among female breast cancer survivors in AustraliaCitation4 was graded very low certainty evidence due to potential self-report bias and imprecision due to a very small sample size (n = 25). This study found lubricant use (specifically olive oil, along with pelvic floor muscle relaxation exercises and vaginal moisturiser) was associated with lower dyspareunia scores (mean difference: 4.3 lower) and lower sexual discomfort scores (mean difference: 2.1 higher in comfort score).
Other outcomes of interest
No quantitative comparative data were identified from either RCTs or from observational studies related to sexual arousal dysfunctions, vaginal discharge, and bacterial vaginosis, side effects like irritation or infections (yeast, RTI, UTI), and other side effects, adverse events, or social harms.
Values and preferences review
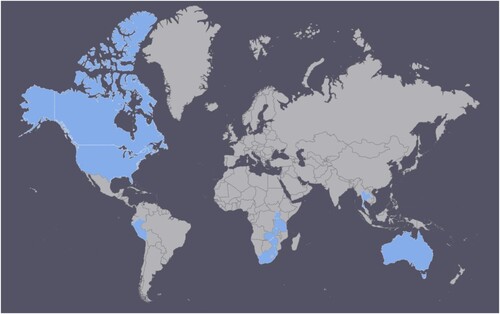
Overall, 21 studies were included in the values and preferences review ().Citation4,Citation17,Citation22–40 The studies were primarily quantitative (n = 16, 9 of which were cross-sectional), although there were several qualitative studies (n = 4) and a multi-method study (n = 1). Twelve were conducted in high-income countries, but others took place in upper-middle (n = 6), lower-middle (n = 5), and low-income (n = 1) countries. presents a map showing the distribution of values and preferences studies globally. The country with the most studies was the USA (n = 9), followed by South Africa (n = 4), Zimbabwe (n = 3), and Australia (n = 2). One study each was conducted in Canada, Peru, Tanzania, Thailand, Uganda, and Zambia. One global internet survey was conducted with respondents primarily from North America (also from Europe, Latin America/Caribbean, Asia, Oceania, and other regions). Populations also varied widely, including heterosexuals, men who have sex with men, HIV-infected and HIV-uninfected individuals, individuals with dyspareunia, and clients of STI services.
Table 4. Description of studies included in the values and preferences review1
Support for lubricant use ranged from 55-100% across studies, as assessed with a wide range of measures across populations and settings, including whether participants would be “willing to experiment”, “would use again”, “would use on a regular basis”, “would recommend to others”, “acceptable”, and “liked very much”.
In three studies that compared water-based lubricant to either no lubricant or an oil-based lubricant, individuals generally preferred water-based lubricants.Citation25,Citation39,Citation40 One study found that participants preferred odourless and tasteless lubricants, while another found that lubricant taste or smell did not matter, or participants preferred lubricants without flavour, colour, or smell.Citation22,Citation28
Reasons why individuals liked lubricants or would choose to use them ranged widely, and included comfort, reduced dryness/pain/discomfort, increased pleasure (for themselves or their partners), their partner’s preference, ease of orgasm (e.g. ability to orgasm, time needed to orgasm, quality of orgasm), preference for sex to feel more wet, more fun, curiosity, enhanced foreplay, clean, fast, easy insertion, reduced risk of tearing the vulva/vagina/anus, easier to feel aroused, increased readiness for sex, reciprocity, reduced chance of condoms drying out/breaking, and making condom use more enjoyable.
Reasons why individuals disliked lubricants or would choose not to use them also ranged widely, and included that lubricants were perceived as sticky, slippery, wet, messy, runny, gooey, burning, itchy, or leaky (nuisance); that lubricants were expensive, unavailable, or inaccessible; that individuals were not prepared when “in the heat of the moment” to quickly try using it, or that lubricant use interrupted sexual interaction; that individuals or their partners preferred dry sex or preferred to use non-commercial products (e.g. saliva, pre-seminal fluid) instead; or that participants perceived that lubricants were “only for older people”, or that they did not think they needed to use lubricant.
We identified no studies on values and preferences of health workers.
Cost review
No studies presented primary data examining cost-effectiveness, cost-utility, or cost–benefit for lubricants.
Discussion
Improving sexual health and well-being is an important but often neglected element of the WHO’s reproductive health strategy. Our systematic literature review highlights the limited evidence for this relatively low-cost and simple intervention. In our effectiveness review, we identified just one RCT that looked at sexual pleasure or well-being, finding that lubricants increased female sexual well-being. Though limited, this evidence does suggest that lubricants can be an important part of improving sexual health and well-being. However, more research is needed. In particular, sexual pleasure and well-being can only be measured subjectively and may be subject to self-report bias. This makes research challenging, particularly as it is difficult to blind participants to lubricant use. However, these are critical outcomes to measure when taking a positive approach to sexual health.
Our review found limited data on the association between lubricant use and STIs/HIV, except for one RCT showing that women who used lubricants were not more likely to acquire HPV. This finding is encouraging, particularly given the long-standing negative reverberations of evidence two decades ago that spermicides containing nonoxynol-9 did not protect against HIV infection and may even have increased HIV risk among women using these products frequently.Citation41 While there has been substantial interest in lubricant use to reduce the risk of condom breakage and thus reduce HIV/STI risk,Citation42 our review did not identify studies in this area. It is possible that many HIV trials provide condoms and lubricant to both study arms and thus do not provide comparative data on lubricant use versus no lubricant use, or that they measure outcomes such as condom slippage or breakage which were not in our a priori list of outcomes. It is also possible that our search string, which focused on studies with lubricant terms in the title or abstract, did not catch these trials.
Evidence on pain came from only cross-sectional studies and was of very low certainty for our questions of interest. One observational study showed lubricant use was associated with an increased proportion of gay and bisexual male participants reporting pain during receptive intercourse and no difference during insertive intercourse, but a reduced degree of pain in both types of intercourse. While this finding may raise concern, we interpret this as reverse causality in a cross-sectional survey. Participants who reported using lubricant during their last partnered event were asked to indicate their reasons for using lubricant; the most highly endorsed statement (89.3%) was that lubricant reduced their pain/discomfort. Men who experienced pain during receptive intercourse were more likely to use lubricants, but then experienced a reduced degree of pain, most likely because of the lubricant. A second observational study showed lubricant use was associated with better outcomes of vaginal dryness and dyspareunia for female breast cancer survivors. This study used olive oil as the lubricant of choice, along with pelvic floor muscle relaxation exercises and vaginal moisturiser, so the experience of participants and effectiveness of the regimen may be different from studies using other types of water- or petroleum-based lubricants. Although limited, these findings are overall encouraging for the impact of lubricants on pain and vaginal dryness.
While we identified 21 studies on values and preferences, these covered a range of country settings, methods, and population groups; additional evidence for specific populations and from lower-income settings is still needed. In particular, our findings on reasons why many individuals do not like lubricant may help product developers identify ways to improve current lubricant selection.
Although we identified no cost studies meeting our inclusion criteria, lubricants are available in many settings globally,Citation43 with prices within the range of other over-the-counter sexual and reproductive health products. Currently, while the global lubricants market is dominated by North America (with approximately 37% of market share in 2019), the Asia Pacific region is the fastest-growing market.Citation44,Citation45 In addition, lubricants are also provided by many national HIV programmes in high-prevalence settings,Citation46 and are thus within the realm of something that could feasibly be provided by many national health care services. However, further costing and cost-effectiveness research on lubricants for the outcomes we examined is needed.
A strength of this review is our inclusion of a range of research designs and our focus not only on effectiveness data, but also on values and preferences and cost data. We also conducted a comprehensive search and inclusion process, including multiple databases, secondary screening, and manual searches, with no exclusions based on language, location, or publication date. Furthermore, we followed best practices in systematic data extraction in duplicate. Limitations of our review include the focus on commercial or commonly available lubricants, excluding data on bodily fluids commonly used as lubricants (e.g. saliva, pre-seminal fluid), vaginal moisturisers, and microbicide gels. Given the range of body fluids, food products, and other items used as lubricants across settings, further study of non-commercial lubricant use is warranted. Further, our focus on peer-reviewed scientific articles may have limited inclusion of findings from market research or other relevant grey literature. As sexual health is a sensitive and private topic in many settings, findings may have been subject to social desirability bias, or individuals may not have felt fully comfortable expressing their true values and preferences. Finally, as noted above, many of our outcomes of interest are only measurable using self-report and it is difficult to blind participants to lubricant use; these factors may have introduced bias into the results of included studies.
Based on findings from this review as well as other inputs and discussion among diverse stakeholders, the World Health Organization self-care guideline made the following recommendation: “WHO recommends making lubricants available for optional use during sexual activity, among sexually active individuals”.Citation47
Conclusion
Overall, this systematic review found that lubricants may offer an accessible means to improve sexual wellbeing. The current reporting gives insights into values and preferences of end users and potentially important outcomes, but not the strength of evidence to reach conclusions on effectiveness or cost-effectiveness. Furthermore, although lubricants are a globally available product, study populations have lacked diversity. To fully inform guidelines, there is a need for improved research and reporting. Furthermore, publication bias is likely to have contributed to the fact that published studies tend to suggest effectiveness. Improving access to and availability of quality lubricants may contribute to the goals of respecting, protecting, and realising the right to health, and improving sexual health and well-being.
Supplementary Table A
Download MS Word (46.1 KB)Supplemental Material - Search Terms
Download MS Word (15.4 KB)Acknowledgements
We thank Johns Hopkins graduate students Holly Nishimura, Vagarshak Avetisyan, and Xuhao Yang for their help screening citations and coding articles for this review, and Chris Purdy, Laura Ferguson, and Nandi Siegfried for their thoughtful comments on the protocol. Authors' contribution: MN conceptualised the study. CK and PTY designed the protocol, with feedback from MN and LG. PTY ran the database search and oversaw the search, screening, full text review, and data extraction process, with assistance from JL and CK. CK drafted the manuscript; PTY and JL drafted several tables. CK and PTY conducted the GRADE analysis. All authors reviewed the draft, provided critical review, and read and approved the final manuscript. The corresponding author, as guarantor, accepts full responsibility for the finished article has access to any data and controlled the decision to publish. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data come from published articles. Extracted data are available on request to the corresponding author.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/26410397.2022.2044198.
Additional information
Funding
References
- World Health Organization. Reproductive health strategy to accelerate progress towards the attainment of international development goals and targets. Geneva: WHO; 2004.
- World Health Organization. Sexual and reproductive health: defining sexual health. Geneva: WHO; 2006.
- Lev-Sagie A. Vulvar and vaginal atrophy: physiology, clinical presentation, and treatment considerations. Clin Obstet Gynecol. 2015;58(3):476–491.
- Juraskova I, Jarvis S, Mok K, et al. The acceptability, feasibility, and efficacy (phase I/II study) of the OVERcome (olive oil, vaginal exercise, and MoisturizeR) intervention to improve dyspareunia and alleviate sexual problems in women with breast cancer. J Sex Med. 2013;10(10):2549–2558.
- Andelloux M. Products for sexual lubrication: understanding and addressing options with your patients. Nurs Womens Health. 2011;15(3):253–257.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI360: advisory note. Geneva: WHO; 2012.
- World Health Organization. Consolidated guideline on self-care interventions for health: sexual and reproductive health and rights. Geneva: WHO; 2019.
- World Health Organization. Maintaining essential health services: operational guidance for the COVID-19 context: interim guidance, 1 June 2020. Geneva: WHO; 2020.
- Narasimhan M, Logie CH, Gauntley A, et al. Self-care interventions for sexual and reproductive health and rights for advancing universal health coverage. Sex Reprod Health Matters. 2020;28(2):1778610.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19(2):151–161.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Vol. 6.0 (updated July 2019); 2019.
- Kennedy CE, Fonner VA, Armstrong KA, et al. The evidence Project risk of bias tool: assessing study rigor for both randomized and non-randomized intervention studies. Syst Rev. 2019;8(1):3.
- Guyatt G, Jaeschke R, Wilson MC, et al. What is evidence-based medicine? In: Guyatt G, Rennie D, Meade MO, Cook DJ, editor. Users’ guides to the medical literature: a manual for evidence-based clinical practice (3rd ed). New York: McGraw Hill Edication; 2015. p. 7–14.
- World Health Organization. WHO Handbook for guideline development, 2nd edn. Geneva: WHO; 2014.
- Blair CS, Javanbakht M, Comulada WS, et al. Lubricants and rectal douching: associations with rectal gonorrhea, chlamydia, and/or syphilis infection among men who have sex with men. Int J STD AIDS. 2020;31(11):1040–1046.
- Dodge B, Hubach RD, Schick V, et al. Lubricant use at last sexual encounter with a male partner: findings from a nationally representative sample of self-identified gay and bisexual men in the United States. Sex Health. 2015;12(4):300–307.
- Gorbach PM, Weiss RE, Fuchs E, et al. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sex Transm Dis. 2011;39(1):59–64.
- Maierhofer C, Rice CE, Shu-Hua W, et al. Lubricant use and rectal chlamydial and gonococcal infections among men who engage in receptive anal intercourse. Sex Transm Dis. 2016;43(7):423–428.
- Rosen RC, Althof SE, Barbach LG, et al. Female sexual well-being ScaleTM: responsiveness to interventional product use by sexually functional women. J Sex Med. 2010;7(7):2479–2486.
- Sawaya GF, Chirenje MZ, Magure MT, et al. Effect of diaphragm and lubricant gel provision on human papillomavirus infection among women provided with condoms: a randomized controlled trial. Obstet Gynecol. 2008;112(5):990–997.
- Carballo-Diéguez A, Stein Z, Sáez H, et al. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000;90(7):1117–1121.
- Clark J, Salvatierra H, Segura E, et al. Frequency, patterns, and preferences of lubricant use during anal intercourse within male sexual partnerships in Lima, Peru: implications for a rectal microbicide HIV prevention intervention. AIDS Care. 2013;25(5):579–585.
- Duby Z, Hartmann M, Montgomery E, et al. Condoms, lubricants and rectal cleansing: practices associated with heterosexual penile-anal intercourse amongst participants in an HIV prevention trial in South Africa, Uganda and Zimbabwe. AIDS Behav. 2016;20(4):754–762.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women's sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8(1):202–212.
- Herbenick D, Reece M, Schick V, et al. Women's use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11(3):642–652.
- Hickey M, Marino JL, Braat S, et al. A randomized, double-blind, crossover trial comparing a silicone- versus water-based lubricant for sexual discomfort after breast cancer. Breast Cancer Res Treat. 2016;158(1):79–90.
- Javanbakht M, Murphy R, Gorbach P, et al. Preference and practices relating to lubricant use during anal intercourse: implications for rectal microbicides. Sex Health. 2010;7(2):193–198.
- Jones DL, Weiss SM, Chitalu N, et al. Acceptability and use of sexual barrier products and lubricants among HIV-seropositive Zambian men. AIDS Patient Care STDS. 2008;22(12):1015–1020.
- Jozkowski KN, Herbenick D, Schick V, et al. Women's perceptions about lubricant use and vaginal wetness during sexual activities. J Sex Med. 2013;10(2):484–492.
- Lee M, Sandfort T, Collier K, et al. Breakage is the norm: use of condoms and lubrication in anal sex among Black South African men who have sex with men. Cult Health Sex. 2017;19(4):501–514.
- Montgomery ET, Blanchard K, Cheng H, et al. Diaphragm and lubricant gel acceptance, skills and patterns of use among women in an effectiveness trial in Southern Africa Montgomery et al. diaphragm gel patterns of use in Southern Africa. Eur J Contracept Reprod Health Care. 2009;14(6):410–419.
- Reece M, Herbenick D, Schick V, et al. Men's use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11(5):1125–1135.
- Rojanapithayakorn W, Goedken J. Lubrication use in condom promotion among commercial sex workers and their clients in Ratchaburi, Thailand. J Med Assoc Thai. 1995;78(7):350–354.
- Romijnders KA, Nyoni JE, Ross MW, et al. Lubricant use and condom use during anal sex in men who have sex with men in Tanzania. Int J STD AIDS. 2015;27(14):1289–1302.
- Sahin-Hodoglugil NN, Montgomery E, Kacanek D, et al. User experiences and acceptability attributes of the diaphragm and lubricant gel in an HIV prevention trial in Southern Africa. AIDS Care. 2011;23(8):1026–1034.
- Sanders SA, Crosby RA, Milhausen RR, et al. Women’s willingness to experiment with condoms and lubricants: a study of women residing in a high HIV seroprevalence area. Int J STD AIDS. 2018;29(4):367–374.
- Schick VR, Hensel D, Herbenick D, et al. Lesbian- and bisexually-identified women's use of lubricant during their most recent sexual event with a female partner: findings from a nationally representative study in the United States. LGBT Health. 2016;2(2):169–175.
- Steiner M, Piedrahita C, Glover L, et al. The impact of lubricants on latex condoms during vaginal intercourse. Int J STD AIDS. 1994;5(1):29–36.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9(1):240–250.
- WHO/CONRAD Technical Consultation on Nonoxynol-9 - Geneva, 9-10 October, 2001. https://www.who.int/reproductivehealth/publications/rtis/RHR_03_8/en/.
- Siegler AJ, Rosenthal EM, Sullivan PS, et al. Levels of clinical condom failure for anal sex: a randomized cross-over trial. EClinicalMedicine. 2019;17:100199.
- Potter N, Panay N. Vaginal lubricants and moisturizers: a review into use, efficacy, and safety. Climacteric. 2021;24(1):19–24.
- Personal Lubricant Market Analysis By Type (Water-Based, Silicone-Based, Oil-Based). By distribution channel (E-commerce, drug stores), by region, competitive insights, and segment forecasts, 2019-2026. Market Analysis Report. San Francisco (CA): Grand View Research; 2019.
- Personal Lubricants Market. Growth, trends, COVID-19 impact, and forecasts (2021-2026). Hyderabad: Mordor Intelligence; 2019.
- UNAIDS. Condom and lubricant programming in high HIV prevalence countries. Guidance Note. Geneva: Joint United Nations Programme on HIV/AIDS; 2014.
- World Health Organization. WHO guideline on self-care interventions for health and well-being. Geneva: WHO; 2021.