?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Printing of electronics has rapidly gained ground on both laboratory and industrial level, showcasing the potential of additive microfabrication techniques and functional nanotechnology-based materials. Laser-induced forward transfer (LIFT) follows the trends of microelectronics towards miniaturized electronic components, by increasing the printing speed and accuracy. The study focuses on the preparation of highly loaded copper(II) oxide nanoparticle – based inks for LIFT applications. Suspensions of 25% w/w solids loading have been prepared in ethylene glycol – water, 1,3-propylene glycol – water and 1,2-propylene glycol – water co-solvent media and characterized via UV–Visible Spectroscopy, Transmission Electron Microscopy, rheological measurements, Thermogravimetric Analysis, and Differential Scanning Calorimetry. The samples showed good stability in the course of two months and the nanoparticles were spherical, with diameters of 14, 9, and 8 nm, forming small, flower-like clusters of 25, 21, and 23 nm, respectively. In addition to the above, their non-Newtonian, shear-thinning behavior and zero shear viscosities of 6.12·× 103, 1.60·× 102, and 5.25 Pa·s, as well as the smooth evaporation of their media during heating showcased their potential for LIFT applications.
Introduction
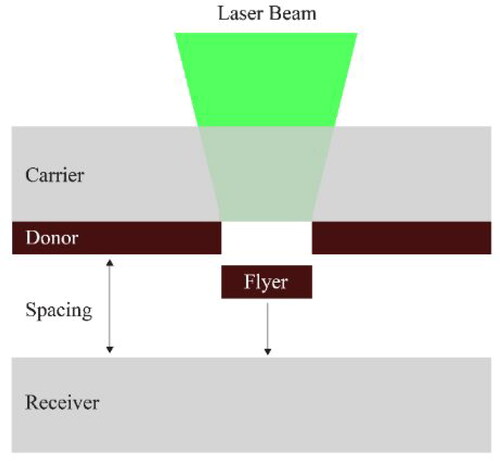
In recent years, substantial advances have been made in additive microfabrication of electronic components, transforming laboratory-scale proofs-of-concept into industrial manufacturing processes. As the demand for miniaturization of electronics rises, so does the need for high-throughput patterning techniques and novel nanotechnology-based materials (Hon, Li, & Hutchings, Citation2008; Koritsoglou et al., Citation2019). Laser-induced forward transfer (LIFT) responds to this need by increasing the printing speed (>1 m/s) and resolution (<50 μm) and minimizing the heat affected zone (Koritsoglou et al., Citation2019). LIFT is based on a pulsed laser beam which projects material from a donor thin film towards a receiver substrate according to a digitally defined preset () (Serra & Piqué, Citation2019). The printing process is typically followed by selective laser sintering of the conductive components of the printed pattern (Koritsoglou et al., Citation2019). The authors have previously reviewed a wide range of printing techniques employed for the fabrication of electronics and recent progresses in different printing approaches (Dimitriou & Michailidis, Citation2021).
Functional nanotechnology-based conductive inks are the most promising materials in the fabrication of micron-sized conductive patterns (Rajan et al., Citation2016). Typically, conductive inks are suspensions of nanoparticles of metallic species stabilized by surfactants and ligand shells, dispersed in an aqueous or organic media (Cruz, Rocha, & Viana, Citation2018; Rajan et al., Citation2016; Zenou & Grainger, Citation2018). The ligands are called capping agents and protect the particles from agglomerating. The capping agents can be removed during sintering of the printed parts, allowing physical contact between the particles, thus, the formation of a conductive path (Cruz et al., Citation2018). Silver and gold nanoparticles dominate in terms of abundancy due to their excellent chemical stability and electrical conductivity (Zenou & Grainger, Citation2018). Although copper nanoinks are a great candidate for replacing expensive inks based on noble metals, copper nanoparticles are easily oxidized (Magdassi, Grouchko, & Kamyshny, Citation2010). Therefore, efforts are focusing on ensuring the chemical stability of copper, either by controlling the oxygen content in the processing atmosphere or by using copper precursor nanoparticles, such as copper oxide nanoparticles, in printable inks instead of copper nanoparticles (Zenou & Grainger, Citation2018).
Although LIFT has garnered significant attention over the past, the same cannot be said for the inks employed with LIFT (Kalaitzis et al., Citation2019). Most researchers use LIFT with commercial inks that have been designed for other printing techniques, such as screen printing (Piqué, Kim, Auyeung, Beniam, & Breckenfeld, Citation2016; Sopeña, Fernández-Pradas, & Serra, Citation2019).
Since LIFT is a non-contact, nozzle-free printing technique, it is more tolerant of anisotropic nanostructures, such as nanotubes, nanofibers and nanorods, as well as large particle sizes, ranging from a few nanometers up to few hundreds of microns (Fernández-Pradas et al., Citation2018; Serra & Piqué, Citation2019; Sopeña et al., Citation2019). However, nanoparticles used in conductive inks usually have a spherical shape (Rajan et al., Citation2016). Spherical particles possess the lowest energy shape, thus, forming suspensions of long-term colloidal stability. Furthermore, anisotropic particles exhibit greater changes in their rheological behavior under stress in comparison to isotropic particles. For the abovementioned reasons, spherical particles are favorable for inks with high solids loadings and provide easier control of the ink rheology (Cui, Citation2016; Nayak, Mohanty, Nayak, & Ramadoss, Citation2019; Piqué & Chrisey, Citation2002). The particles of conductive inks typically have diameters ranging from 5 to 100 nm, with narrow size distribution (Rajan et al., Citation2016). In general, small sizes are favorable for better colloidal stability and lower sintering temperatures (Hon et al., Citation2008). Additionally, small particles of uniform or bimodal size distribution are more promising for the formation of dense printed patterns and form more viscous inks as compared with large particles, if other parameters are the same (Cui, Citation2016; Piqué & Chrisey, Citation2002).
LIFT prefers high viscosity (up to 103 Pa·s), shear-thinning inks, offering homogeneous material deposition onto the donor and the formation of stable and reproducible printed patterns (Koritsoglou et al., Citation2019; Sopeña et al., Citation2019). Viscous inks allow for the size and shape of the printed voxel to be determined by the spatial distribution of the laser pulse, thus increasing the printing speed and resolution of the LIFT process (Piqué, Kim, Auyeung, & Smith, Citation2013; Piqué et al., Citation2016). Furthermore, LIFT prefers non-Newtonian inks, since the laser pulse induces significant shear stress, resulting in high shear rate (Theodorakos et al., Citation2019). Under these conditions, the viscosity of non-Newtonian, shear-thinning inks will adapt to the shear stress, ensuring smooth deposition of the material onto the receiver substrate. Absent any shear forces after printing of the material, the high zero shear viscosity of the inks will suppress wet-spreading effects, providing fine, high-quality printed patterns (Kalaitzis et al., Citation2019; Serra, Fernández-Pradas, & Sopeña, Citation2017; Theodorakos et al., Citation2019).
The tolerance of LIFT for viscous inks allows printing of highly loaded inks, which is preferred for high conductivity requirements (Serra & Piqué, Citation2019). The solids loading can be tailored to control the ink viscosity and minimize the variability of the printed result due to wetting effects and shrinkage during sintering (Piqué et al., Citation2013). Therefore, high solids loadings facilitate control of shape and size of the printed pattern (Piqué et al., Citation2016). LIFT has been successfully employed with screen printing inks containing solids in contents higher than 70% w/w (Kalaitzis et al., Citation2019; Piqué et al., Citation2013; Sopeña et al., Citation2019; Theodorakos et al., Citation2019).
Selection of the ink medium can determine its colloidal stability. The viscosity and boiling point of the vehicle are important parameters that need to be considered. Humectants, such as glycols, are sometimes added to the ink as co-solvent in order to tailor the evaporation rate of the ink and avoid ‘coffee ring’ formation due to nonuniform solvent evaporation during drying of the printed pattern (Rajan et al., Citation2016; Zeng & Zhang, Citation2019). Selection of the ink carrier can also influence the stability of the ink during storage (Magdassi & Kamyshny, Citation2017). Previous studies have reported successful printing of inks containing diethylene/triethylene glycol monobutyl ether and tripropylene glycol monomethyl ether in LIFT applications, which has been attributed to their high boiling point (171.0, 279.7, and 243.0 °C at 760 mm Hg, respectively), enabling smooth solvent evaporation and elimination of the coffee ring effect (Kalaitzis et al., Citation2019; Koritsoglou et al., Citation2019; Theodorakos et al., Citation2019).
This work focuses on the development of high-content inks of copper(II) oxide nanoparticles (CuONPs) with properties corresponding to the processing parameters of LIFT. These properties include the size and shape of the nanoparticles, the rheology of the inks and their chemical stability in terms of nanoparticle dispersion and fit of the ink medium.
Materials and methods
The CuONPs used in this study were synthesized by PLiN Nanotechnology SA. The particles were available in the form of aqueous suspensions, their average diameter was approximately 4 nm with narrow distribution and their concentration was 1450 ± 50 ppm. Additionally, they were spherical and covered by an animal protein with a molecular mass between 20,000 and 25,000 g/mol and isoelectric point between 4.7 and 5.4, acting as stabilizing agent (Ntasiou et al., Citation2021; Varympopi et al., Citation2020). The main volume of the particles was uniformly composed of copper(II) oxide. The pH of the suspensions was 10. Sodium hydroxide (NaOH) of ≥98% purity and deionized water were used for the preparation of an aqueous 6 M NaOH solution. Hydrochloric acid (HCl) solution, 37% a.r., acetone of ≥99% purity and ethylene glycol, 1,3-propylene glycol and 1,2-propylene glycol of analytical grade were used as received. The glycols were selected as the main solvent of the inks prepared, due to their high viscosity and boiling point.
In a typical procedure, a few milliliters of the HCl solution were added to 1 L of the CuONPs suspension in a 5 L beaker under mild mechanical stirring, until the pH of the suspension was approximately 5.5. Under these pH conditions, the particles began to coalesce. Then, 4 L of acetone were added to the destabilized colloid. The beaker was sealed with membrane and the suspension was stored at rest for the particles to precipitate overnight. The supernatant was discarded and the sediment was collected with a spatula and dried for 3 h in a plastic Petri dish in a drying oven set at 60 °C. Every 30 min, it was stirred with a spatula to ensure homogeneous evaporation of the remaining supernatant. Next, it was pulverized with a mortar. Suspensions of 25% w/w solids loading were prepared by redispersion of weighed sediment quantities into a mixture of NaOH solution and the respective glycol in a 1:10 mass ratio under overnight mechanical stirring. The presence of NaOH serves the purpose of restoring the pH of the final suspensions to alkaline values. The sample media will be referred to as EG/W (ethylene glycol and water), 1,3PrG/W (1,3-propylene glycol and water) and 1,2PrG/W (1,2-propylene glycol and water), respectively. The suspensions prepared were stored in a cooler set at 15 °C for characterization.
The optical properties of the suspensions were analyzed via UV–Visible Spectroscopy. Absorbance spectra were collected for each sample, on the day of preparation, after 1 month and after 2 months, using a double beam Shimadzu UV-1800 spectrophotometer and quartz cuvettes with a path length of 10 mm. The spectral bandwidth ranged from 200 to 800 nm at a wavelength resolution of 2 nm. The instrument was controlled using the UV Probe software package. The samples were prepared by dilution of 1 g of each suspension with 70 g of their respective media and regulation of the pH of the dilute suspensions to 10. The medium of each sample was used as blank.
Nanoscale investigation was performed with a high resolution JEOL JEM-2100 LaB6 transmission electron microscope (HRTEM), operating at 200 kV. The samples under investigation (∼0.2 mL) were suspended in deionized water and treated with ultrasound to disaggregate the agglomerated particles. A drop from the suspension was then placed on a 300-mesh carbon coated copper grid and air-dried overnight. Rheological measurements were carried out using a rotational TA Instruments Discovery Hybrid Rheometer HR 20, with a parallel plate setup in an Environmental Test Chamber (ETC) with a temperature range of –160 °C to 600 °C and heating rates up to 60 °C/min. The measurement temperature was 25 °C. The measurement parameters were controlled using the TRIOS software package. Viscosity values were collected as a function of shear rate in a 10−2–105 μN·m torque range. Steady state sensing was performed with 5 points per decade of torque values, maximum equilibration time of 90 s, sample period of 10 s and 5% tolerance.
Since laser-printing and -sintering are thermal processes, it was of utmost importance to understand the thermal behavior of the suspensions. Thermogravimetric analysis was performed using the TA Instruments Discovery Simultaneous Thermal Analyzer SDT 650, with a temperature range from ambient temperature to 1500 °C and heating rate from 0.1 to 100 °C/min, weighing accuracy of ±0.5% and precision of ±0.1%. The measurements were carried out on approximately 10 mg of each sample in an alumina pan, under nitrogen atmosphere. Under these conditions, the CuONPs do not react with the alumina pan (Hu, Donat, Scott, & Dennis, Citation2016). The weight percentage was recorded as a function of temperature, in a temperature range of 50–250 °C, with a heating rate of 50 °C/min. The instrument was controlled using the TRIOS software package.
The thermal properties of the suspensions were further analyzed via Differential Scanning Calorimetry. The instrument used was the TA Instruments Differential Scanning Calorimeter DSC 25, equipped with the Refrigerated Cooling System RSC 90, allowing a temperature range from –90 to 725 °C and heating rates from 0.01 up to 100 °C/min. Approximately 3 mg of each sample was tested, in aluminum pans hermetically sealed by aluminum lids, under nitrogen atmosphere. The samples were heated from 25 to 275 °C with a heating rate of 15 °C/min. Under these conditions, the CuONPs do not react with the aluminum surface (Marín et al., Citation2015). The measurement parameters were set up using the TRIOS software package.
Results
shows the UV–Visible spectra collected. In the samples in EG/W and 1,3PrG/W, small variations were observed among the absorbance data collected on the day of preparation, after 1 month and after 2 months.
Figure 2. UV–Visible absorption spectra of the CuONPs suspension EG/W (a), 1,3PrG/W (b), and in 1,2PrG/W (c).
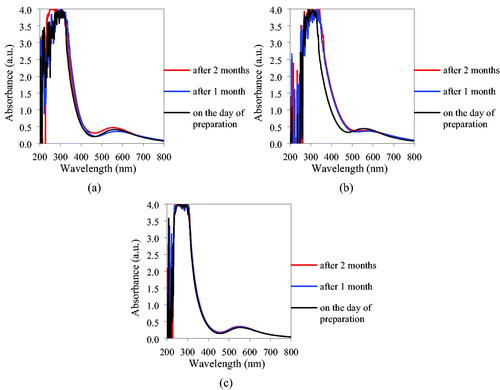
The sample in EG/W exhibited its maxima at 564, 576, and 554 nm and the one in 1,3PrG/W at 556, 576, and 566 nm, respectively. In the case of the sample in 1,2PrG/W the spectra almost overlapped, with the absorption maxima appearing at wavelengths of 554, 552, and 550 nm, respectively. The absorption properties of the inks facilitate appropriate laser selection. Specifically, the laser should have a wavelength that matches the absorption peak of the ink to ensure efficient material transfer onto the substrate. Most researchers employ LIFT with an Nd:YAG laser (Kalaitzis et al., Citation2019; Koritsoglou et al., Citation2019; Serra & Piqué, Citation2019; Theodorakos et al., Citation2019).
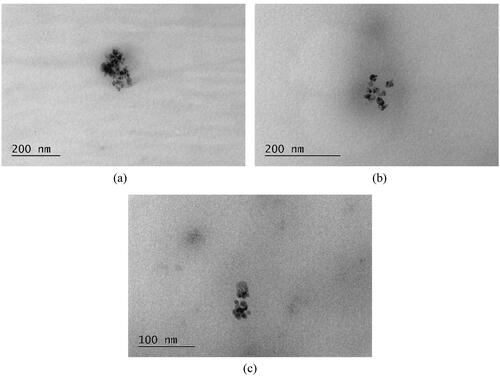
The TEM images () showed spherical particles with diameters of a few nanometers. More specifically, the particles had an average diameter of approximately 14, 9, and 8 nm and tended to overlap each other and form flower-like clusters of 25, 21, and 23 nm, respectively. Similar aggregation behavior has been reported in the literature (Ahamed, Alhadlaq, Majeed Khan, Karuppiah, & Al-Dhabi, Citation2014; Alagarasan et al., Citation2023; Buazar, Sweidi, Badri, & Kroushawi, Citation2019; Etefagh, Azhir, & Shahtahmasebi, Citation2013; Jeong & Kim, Citation2009; Khaldari, Naghavi, & Motamedi, Citation2021; Ren et al., Citation2009; Tamuly, Saikia, Hazarika, & Das, Citation2014). However, the aggregation can be attributed to the limitations of the TEM technique in the preparation of the samples (Buazar et al., Citation2019). The decreasing particle size from the first to the third sample is consistent with the UV–Vis spectroscopy results: the blue shift in the absorption maxima of the first to the third sample, following the decrease of the particle diameter can provide evidence for a possible quantum confinement event (Jillani, Jelani, Hassan, Ahmad, & Hafeez, Citation2018; Neikov & Yefimov, Citation2019; Yin et al., Citation2005; Zayyoun, Jaber, Laânab, Ntsoenzok, & Bekkari, Citation2016).
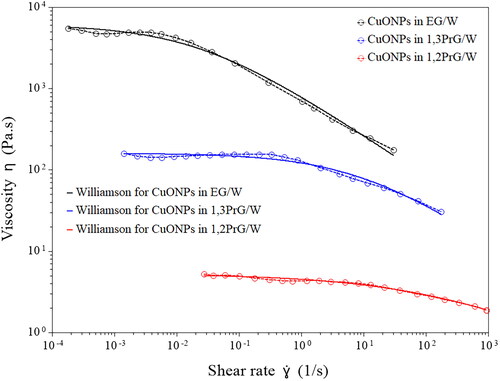
The results of the rheological measurements () demonstrated the non-Newtonian, shear thinning behavior of all three inks. The CuONPs suspension in EG/W appeared to have the highest viscosity values and the strongest shear thinning behavior, while the suspension in 1,2PrG/W exhibited the lowest viscosity values and the mildest shear thinning behavior. The zero-shear viscosity was determined with the analysis tools of the TRIOS software package, using the Williamson model, at 6.12·× 103, 1.60·× 102, and 5.25 Pa·s, respectively. The mathematical expression of the Williamson model is that of EquationEq. (1)(1)
(1) , in which η is the viscosity, η0 is the zero-shear viscosity, kγ. is the consistency, and n is the power law index. summarizes the parameters of the Williamson model for the three samples, as well as the R2 coefficient of the fitting. The different extent of shear thinning could be attributed to various factors. The surface chemistry of the nanoparticles could affect the interaction between the particles and the solvents, leading to different levels of stability and rheological behavior. Another possible reason could be differences in the solvent properties. The ink media used have different viscosities and intermolecular interactions, which can influence the interactions between the nanoparticles and the solvents, as well as the shear thinning behavior of the suspension.
(1)
(1)
Table 1. Parameters of the Williamson model for the three samples.
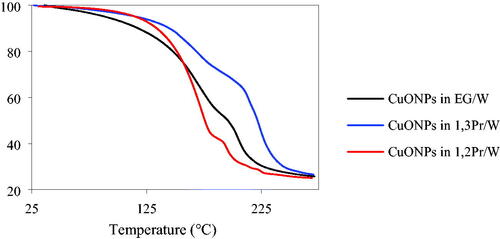
and show the TGA and DSC results, respectively. The sample in EG/W appeared to exhibit the smoothest evaporation of solvents. The weight percentage decreased mildly at temperatures up until 125 °C, and then more rapidly until 225 °C. The suspension in 1,3PrG/W exhibited smooth weight decrease until approximately 150 °C and a strong decrease until 250 °C. In the case of the sample in 1,2PrG/W, evaporation was slow until 125 °C and then dramatic until 225 °C. For the first two samples, two-stage evaporation was observed, corresponding to the evaporation of the two sovents in each sample. For the third sample, two more stages were observed towards the end of the heating process, which were attributed to the decomposition of the organic capping agent of the sample. In other words, the medium of the third sample evaporated sooner than those of the first two samples, leaving the particles exposed to heating, thus resulting in the rapid decomposition of their capping agent. The DSC results showed a mild endothermic response between 160 °C and 170 °C and a strong endothermic response between 200 °C and 212 °C, corresponding to the evaporation of the solvents. The sample in 1,2PrG/W exhibited a third endothermic response, with a peak temperature at 235.84 °C, corresponding to the decomposition of the capping agent. These observations showed good consistency with the TGA results. In all three cases, the solvents had evaporated by the temperature of 275 °C and the total weight loss was approximately 75%, which confirmed that the weight percentage of the CuONPs in the samples was 25% (Rahman, Lu, & Kwon, Citation2018). The DSC results of the samples were compared to those of the pure media (, respectively). summarizes the peak temperatures, as well as the enthalpies calculated from the peak data, for both the suspensions and the pure media. Although the difference in the peak temperatures between the suspensions and the pure media was small, the enthalpies measured for the suspensions were lower than those of the media. This could be attributed to various factors. First, the nanoparticles in the suspension can increase the surface area of the medium. This increased surface area results in increased surface energy and, as a result, a decrease in the enthalpy of the medium. Second, the presence of nanoparticles can lead to the formation of a depletion or boundary layer at the surface of the medium. This layer contains fewer solvent molecules than the bulk of the medium, which can alter the thermodynamic properties of the system, including the evaporation enthalpy. Finally, the adsorption of nanoparticles on the surface of the medium can change the intermolecular interactions between the solvent molecules, which can also affect the evaporation enthalpy.
Figure 6. Heat flow as a function of temperature as obtained from the DSC tests on the samples (a) and the pure media (b), respectively.
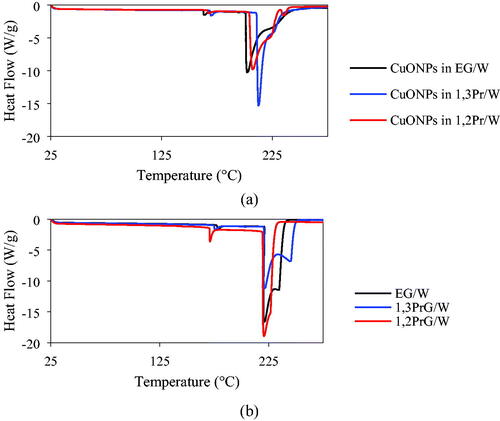
Table 2. Peak data obtained from the DSC results for the samples and the pure media.
Discussion
In this work, copper oxide nanoparticle suspensions of high solids loading, intended for LIFT-based microfabrication of electronics, were developed and characterized in terms of stability, shape, and size of the nanoparticles, rheology, and thermal behavior.
UV–Visible Spectroscopy provided valuable information about the colloidal stability of the samples in the time frame of two months. While the absorption data of the samples in ethylene glycol – water and 1,3-propylene glycol – water after 1 and 2 months showed some variations as compared with the spectrum collected on the day of their preparation, the absorption profile of the sample in 1,2-propylene glycol – water was consistent. However, further experiments are needed to confirm the stability of the suspensions, such as zeta potential analysis or TEM after specific time periods.
Results obtained from TEM encouraged the employment of the inks prepared via LIFT. The spherical shape of the nanoparticles possesses the lowest energy, hence, easing long-term colloidal stability of the suspensions (El Badawy et al., Citation2010; Hon et al., Citation2008; Nayak et al., Citation2019). Furthermore, the spherical shape is promising for better control of the rheology of the ink during printing (El Badawy et al., Citation2010). Owing to their small size, thus their high surface to volume ratio, the particles are expected to require low sintering temperatures (Hon et al., Citation2008). Additionally, they are expected to facilitate the formation of dense printed films, thereby adding to the electrical conductivity of the printed patterns (Cui, Citation2016; El Badawy et al., Citation2010).
Besides the good dispersability of the particles in the ink vehicle, other benefits of the glycol-water co-solvent media was the high viscosity and boiling points achieved in the final suspensions. The rheological properties of the suspensions were compatible to the LIFT processing parameters. Finally, the thermal behavior of the samples further highlighted their potential for LIFT applications. The suspensions showed smooth drying of the solvents when heated, which is important to avoid quick drying during printing, as well as bubble and coffee ring formation after deposition of the material onto the receiver substrate.
Acknowledgments
The authors would like to acknowledge the work of the Physical Metallurgy Laboratory of the School of Mining and Metallurgical Engineering of the National Technical University of Athens in carrying out the TEM investigations and PLiN Nanotechnology SA for supplying the CuONPs used in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahamed, M., Alhadlaq, H., Majeed Khan, M., Karuppiah, P., & Al-Dhabi, N. (2014). Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. Journal of Nanomaterials, 2014, 1–15. doi:10.1155/2014/637858
- Alagarasan, D., Harikrishnan, A., Surendiran, M., Indira, K., Khalifa, A. S., & Elesawy, B. H. (2023). Synthesis and characterization of CuO nanoparticles and evaluation of their bactericidal and fungicidal activities in cotton fabrics. Applied Nanoscience, 13(3), 1797. doi:10.1007/s13204-021-02054-5
- Buazar, F., Sweidi, S., Badri, M., & Kroushawi, F. (2019). Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach. Green Processing and Synthesis, 8(1), 691–702. doi:10.1515/gps-2019-0040
- Cruz, S. M. F., Rocha, L. A., & Viana, J. C. (2018). Printing technologies on flexible substrates for printed electronics. Flexible Electron, 47–70. doi:10.5772/intechopen.76161
- Cui, Z. (2016). Printed electronics: Materials, technologies and applications (1st ed.). Singapore: Wiley.
- Dimitriou, E., & Michailidis, N. (2021). Printable conductive inks used for the fabrication of electronics: An overview. Nanotechnology, 32(50), 502009. doi:10.1088/1361-6528/abefff
- El Badawy, A. M., Luxton, T. P., Silva, R. G., Scheckel, K. G., Suidan, M. T., & Tolaymat, T. M. (2010). Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environmental Science & Technology, 44(4), 1260–1266. doi:10.1021/es902240k
- Etefagh, R., Azhir, E., & Shahtahmasebi, N. (2013). Synthesis of CuO nanoparticles and fabrication of nanostructural layer biosensors for detecting Aspergillus niger fungi. Scientia Iranica, 20(3), 1055–1058. doi:10.1016/j.scient.2013.05.015
- Fernández-Pradas, J. M., Sopeña, P., González-Torres, S., Arrese, J., Cirera, A., & Serra, P. (2018). Laser-induced forward transfer for printed electronics applications. Applied Physics A, 124(2), 1–8. doi:10.1007/s00339-018-1648-8
- Hon, K. K. B., Li, L., & Hutchings, I. M. (2008). Direct writing technology-Advances and developments. CIRP Annals, 57(2), 601–620. doi:10.1016/j.cirp.2008.09.006
- Hu, W., Donat, F., Scott, S. A., & Dennis, J. S. (2016). The interaction between CuO and Al2O3 and the reactivity of copper aluminates below 1000 °C and their implication on the use of the Cu-Al-O system for oxygen storage and production. RSC Advances, 6(114), 113016–113024. doi:10.1039/C6RA22712K
- Inui, T., Mandamparambil, R., Araki, T., Abbel, R., Koga, H., Nogi, M., & Suganuma, K. (2015). Laser-induced forward transfer of high-viscosity silver precursor ink for non-contact printed electronics. RSC Advances, 5(95), 77942–77947. doi:10.1039/C5RA14119B
- Jeong, S. W., & Kim, S. D. (2009). Aggregation and transport of copper oxide nanoparticles in porous media. Journal of Environmental Monitoring, 11(9), 1595–1600. doi:10.1039/b907658a
- Jillani, S., Jelani, M., Hassan, N. U., Ahmad, S., & Hafeez, M. (2018). Synthesis, characterization and biological studies of copper oxide nanostructures. Materials Research Express, 5(4), 045006. doi:10.1088/2053-1591/aab864
- Kalaitzis, A., Makrygianni, M., Theodorakos, I., Hatziapostolou, A., Melamed, S., Kabla, A., … Zergioti, I. (2019). Jetting dynamics of Newtonian and non-Newtonian fluids via laser-induced forward transfer: Experimental and simulation studies. Applied Surface Science, 465, 136–142. doi:10.1016/j.apsusc.2018.09.084
- Khaldari, I., Naghavi, M. R., & Motamedi, E. (2021). Synthesis of green and pure copper oxide nanoparticles using two plant resources: Via solid-state route and their phytotoxicity assessment. RSC Advances, 11(6), 3346–3353. doi:10.1039/d0ra09924d
- Koritsoglou, O., Theodorakos, I., Zacharatos, F., Makrygianni, M., Kariyapperuma, D., Price, R., … Zergioti, I. (2019). Copper micro-electrode fabrication using laser printing and laser sintering processes for on-chip antennas on flexible integrated circuits. Optical Materials Express, 9(7), 3046. doi:10.1364/OME.9.003046
- Magdassi, S., Grouchko, M., & Kamyshny, A. (2010). Copper nanoparticles for printed electronics: Routes towards achieving oxidation stability. Materials (Basel, Switzerland), 3(9), 4626–4638. doi:10.3390/ma3094626
- Magdassi, S., & Kamyshny, A. (2017). Nanomaterials for 2D and 3D printing (1st ed.). Weinheim: Wiley.
- Marín, L., Nanayakkara, C. E., Veyan, J.-F., Warot-Fonrose, B., Joulie, S., Estève, A., … Rossi, C. (2015). Enhancing the reactivity of Al/CuO nanolaminates by Cu incorporation at the interfaces. ACS Applied Materials & Interfaces, 7(22), 11713–11718. doi:10.1021/acsami.5b02653
- Nayak, L., Mohanty, S., Nayak, S. K., & Ramadoss, A. (2019). A review on inkjet printing of nanoparticle inks for flexible electronics. Journal of Materials Chemistry C, 7(29), 8771–8795. doi:10.1039/C9TC01630A
- Neikov, O. D., & Yefimov, N. A. (2019). Nanopowders: in Handbook of Non-Ferrous Metal Powders (2nd ed.). Elsevier Ltd.
- Ntasiou, P., Kaldeli Kerou, A., Karamanidou, T., Vlachou, A., Tziros, G. T., Tsouknidas, A., & Karaoglanidis, G. S. (2021). Synthesis and characterization of novel copper nanoparticles for the control of leaf spot and anthracnose diseases of olive. Nanomaterials, 11(7), 1667. doi:10.3390/nano11071667
- Piqué, A., & Chrisey, D. B. (2002). Direct-write technologies for rapid prototyping applications: Sensors, electronics and integrated power sources. San Diego: Academic Press.
- Piqué, A., Kim, H., Auyeung, R. C. Y., Beniam, I., & Breckenfeld, E. (2016). Laser-induced forward transfer (LIFT) of congruent voxels. Applied Surface Science., 374, 42–48. doi:10.1016/j.apsusc.2015.09.005
- Piqué, A., Kim, H., Auyeung, R. C. Y., & Smith, A. T. (2013). Laser forward transfer of functional materials for digital fabrication of microelectronics. Journal of Imaging Science and Technology, 57(4), 40404-1–40404-8. doi:10.2352/J.ImagingSci.Technol.2013.57.4.040404
- Rahman, M. K., Lu, Z., & Kwon, K. S. (2018). Green laser sintering of copper oxide (CuO) nano particle (NP) film to form Cu conductive lines. AIP Advances, 8(9), 1–13. doi:10.1063/1.5047562
- Rajan, K., Roppolo, I., Chiappone, A., Bocchini, S., Perrone, D., & Chiolerio, A. (2016). Silver nanoparticle ink technology: State of the art. Nanotechnology, Science and Applications, 9, 1–13. doi:10.2147/NSA.S68080
- Ren, G., Hu, D., Cheng, E. W. C., Vargas-Reus, M. A., Reip, P., & Allaker, R. P. (2009). Characterisation of copper oxide nanoparticles for antimicrobial applications. International Journal of Antimicrobial Agents, 33(6), 587–590. doi:10.1016/j.ijantimicag.2008.12.004
- Serra, P., Fernández-Pradas, J. M., & Sopeña, P. (2017). Laser-induced forward transfer of low viscosity inks. Applied Surface Science., 418, 530–535. doi:10.1016/j.apsusc.2016.11.179
- Serra, P., & Piqué, A. (2019). Laser-induced forward transfer: Fundamentals and applications. Advanced Materials Technologies, 4(1), 1–33. doi:10.1002/admt.201800099
- Sopeña, P., Fernández-Pradas, J. M., & Serra, P. (2019). Laser-induced forward transfer of conductive screen-printing inks. Applied Surface Science., 507(December), 145047. doi:10.1016/j.apsusc.2019.145047
- Tamuly, C., Saikia, I., Hazarika, M., & Das, M. R. (2014). Reduction of aromatic nitro compounds catalyzed by biogenic CuO nanoparticles. RSC Advances, 4(95), 53229–53236. doi:10.1039/C4RA10397A
- Theodorakos, I., Kalaitzis, A., Makrygianni, M., Hatziapostolou, A., Kabla, A., Melamed, S., … Zergioti, I. (2019). Laser-induced forward transfer of high viscous, non-Newtonian silver nanoparticle inks: Jet dynamics and temporal evolution of the printed droplet study. Advanced Engineering Materials, 21(10), 1–12. doi:10.1002/adem.201900605
- Varympopi, A., Dimopoulou, A., Theologidis, I., Karamanidou, T., Kaldeli Kerou, A., Vlachou, A., … Skandalis, N. (2020). Bactericides based on copper nanoparticles restrain growth of important plant pathogens. Pathogens (Basel, Switzerland), 9(12), 1024. doi:10.3390/pathogens9121024
- Yin, M., Wu, C.-K., Lou, Y., Burda, C., Koberstein, J. T., Zhu, Y., & O'Brien, S. (2005). Copper oxide nanocrystals. Journal of the American Chemical Society, 127(26), 9506–9511. doi:10.1021/ja050006u
- Zayyoun, N., Jaber, B., Laânab, L., Ntsoenzok, E., & Bekkari, R. (2016). Effect of solvent on the morphological and optical properties of CuO nanoparticles prepared by simple sol-gel process. Journal of Materials and Environmental Science, 7(5), 1791–1797.
- Zeng, M., & Zhang, Y. (2019). Colloidal nanoparticle inks for printing functional devices: Emerging trends and future prospects. Journal of Materials Chemistry A, 7(41), 23301–23336. doi:10.1039/C9TA07552F
- Zenou, M., & Grainger, L. (2018). Additive manufacturing of metallic materials. Elsevier Inc.