Abstract
Whether electromagnetic radiation (EMR) emitted from mobile phones is hazardous to human health is largely unknown. We investigated the effects of mobile phone radiation on critical organs in a rabbit model by exposing the animals to mobile phone radiation with sub-thermal specific absorption rate (SAR) of 1.0 and 0.7 W/kg for the head and the body, respectively, for 16 weeks (6 h/day, 6 days/week). There is no apparent change at the organ level. However, H&E staining showed that radiation-exposure significantly increased inflammatory cell infiltration in the liver and the lungs with a lesser degree of myocardial cell cytoplasmic vacuolation. In addition, results from γ-H2AX staining suggest that radiation can also cause DNA damage in the brain. Of note, no apparent activation of Caspase-3 in the organs examined. Our data altogether suggest that mobile phone radiation may be more hazardous to both the liver and the lungs, and less toxic to the brain and heart.
KEYWORDS:
1. Introduction
The number of mobile phone users has been increasing rapidly with approximately 7.68 billion by 2017 (Smith-Roe et al. Citation2020). Multiple studies suggest that mobile phone use may increase the risk of brain cancer and/or other diseases (Hardell et al. Citation2006; Muscat et al. Citation2006; Schüz et al. Citation2006; The interphone study group Citation2010; Duan et al. Citation2011; Levis et al. Citation2011; Morgan et al. Citation2015). However, most of the conclusions were based on either epidemiological studies or cultured cells. Results based on cultured cells suggest that cell phone use may involve DNA damage, ERK activation or repetitive DNA transcription (Diem et al. Citation2005; Friedman et al. Citation2007; Del Re et al. Citation2019).
More recently, rodents have been used as animal models to study the potential adverse effects of mobile phone radiation on human health (Ghoneim and Arafat Citation2016; Wyde et al. Citation2016). Elevated levels of oxidative stress and DNA damage have been reported in the rat brain when they were exposed to radiation (Alkis, Bilgin, et al. Citation2019). The radiation could also lead to changes in the levels of specific proteins or microRNAs in the rat brain (Dasdag et al. Citation2012; Dasdag et al. Citation2019). However, small animals are more different from humans in many aspects including different thicknesses of the skull which may affect radiation absorption. In this study, we used rabbit as a model system and real mobile phone communication to study whether mobile phone radiation has detrimental effects on health and which organs are more prone to the effects. We found that mobile phone radiation induces apparent lesions in the liver and the lungs with a lesser degree in brain, kidney, and stomach. Of note, DNA damage was observed in the brain tissues when the animals were exposed to cell phone radiation.
2. Materials and methods
2.1. Animals
New Zealand rabbits (3-month-old, 2–2.5 kg) were purchased from Hunan Taiping Biotechnology Co., Ltd. The rabbits were housed under standard conditions (23°C±1°C, 12-h light/dark cycle). All experiments were approved by the ethical committee of Central South University.
2.2. Experimental design
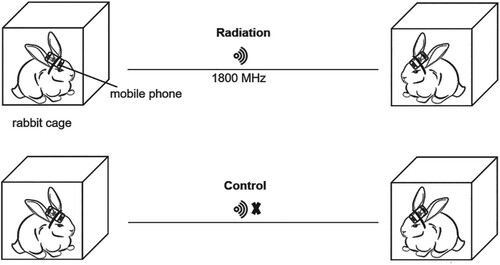
The rabbits were first housed for a week to adapt to the environment. For radiation treatment, 8 rabbits (3 females and 5 males) were exposed to mobile phones (GSM) radiofrequency radiation (RFR 1800 MHz) by fixing the mobile phones on the heads of the animals with straps to the neck, while 5 (2 females and 3 males) were not exposed to radiation to serve as controls (Figure ). The sub-thermal SARs for the head and the body were approximately 1.0 and 0.7 W/kg, respectively. Since these parameters were measured by the mobile phone manufacturer according to the National Standard of Communication (YD-T 16441-2007, China), these numbers only reflect the rough calculations of SARs in this research. The rabbits were paired and ‘talked' to each other during the period from 8:00 am to 8:00 pm. In the control group, mobile phones were not activated. The calls were made 3 times a day, 2 h each time, with 1-hour break between the calls; 6 consecutive days followed by one day break per week. The exposure time was determined based on previously publications (Nisbet et al. Citation2016; Bedir et al. Citation2018; Sharma et al. Citation2019). At the end of 16-week experiment, all rabbits were sacrificed and organs were isolated. The organ coefficient (organ weight/body weight) was estimated and histological assays were conducted.
2.3. Immunohistochemistry and Hematoxylin and Eosin (H&E) staining
Tissues were fixed in 4% polyoxymethylene, dehydrated in graded alcohol solutions, cleared in xylene, and embedded in paraffin. Paraffin-embedded tissues were sectioned and stained with hematoxylin and eosin (Servicebio, China). Briefly, paraffin-embedded tissues were sectioned, deparaffinized and hydrated with xylene and graded-alcohol. For antigen retrieval, the sections were treated with 10 mM sodium citrate (pH 6.0) with 0.05% Tween-20 at 95°C for 20–30 min, 3% H2O2 in methanol for 5–10 min. The slides were blocked with 3% BSA for 50 min at room temperature followed by incubating in antibodies against γ-H2AX (Abcam, UK) or cleaved Caspase-3 (Servicebio, China) at 4°C overnight and biotinylated secondary antibodies for 1 h at room temperature. The tissues were stained with either 3, 3-diaminobenzidine (DAB) or hematoxylin and assessed under a light microscope.
2.4. Statistical analysis
All data were analyzed using SPSS 16.0 software, the organ weight and organ coefficient were expressed as the mean ± SD. The significance of differences was evaluated for independent sample t-test and Fisher’s exact two-tailed test. P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of mobile phone radiation on the organ coefficients
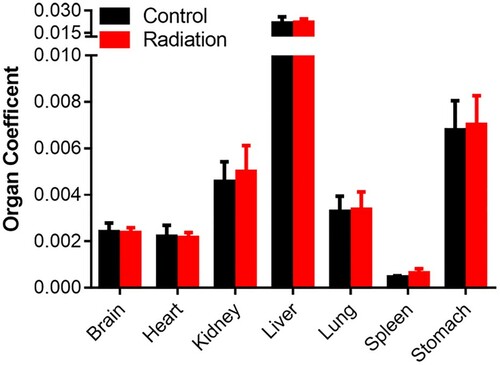
The organ coefficient is considered as a valuable indicator of health status and changes in organ coefficient usually precede morphological alterations. We compared the organ coefficients of the brain, heart, kidney, liver, lung, spleen, stomach, testis, uterus in radiation-exposed rabbits with that of the controls. As shown in Figure that exposure to the mobile phone radiation did not lead to overt change in the coefficients of all organs examined.
3.2. Effect of mobile phone radiation on the tissue levels
To further investigate the effect of mobile phone radiation on the animal at tissue levels, we first conducted H&E staining of several critical organs. It appears that the infiltration of inflammatory cells to the liver and the lungs increased significantly when the animals were exposed to radiation (Table and Figure (A)). Of note, the infiltrated inflammatory cells in the lungs were also aggregated into clusters (Figure (B)). The infiltration of inflammatory cells to the brain (1/8) and stomach (1/8) were also enhanced by exposure to the radiation with much lower frequencies than that in liver and lungs (Figure (C), and data not shown). However, there was no apparent difference between the control (1/5) and radiation-exposed groups (2/8) regarding infiltration of inflammatory cells in the kidney (Figure (D)). Interestingly, cytoplasmic maculation of myocardial cells was observed in radiation-exposed rabbits (Figure (E)). These data altogether suggest that mobile phone radiation could have detrimental effects on organ levels.
Figure 3. Histopathology analysis of rabbit tissues after 16 weeks-mobile phone radiation. Rabbit tissues were analyzed by H&E staining. Radiation, rabbits exposed to mobile phone radiation. Control, rabbits without exposure to mobile phone radiation. Normal/Lesion, normal or lesion tissues observed. Inflammatory cell infiltration (A, B, C, D) and cytoplasmic vacuolation (E) were indicated by black arrows. Representative results were shown. 200×, magnification.
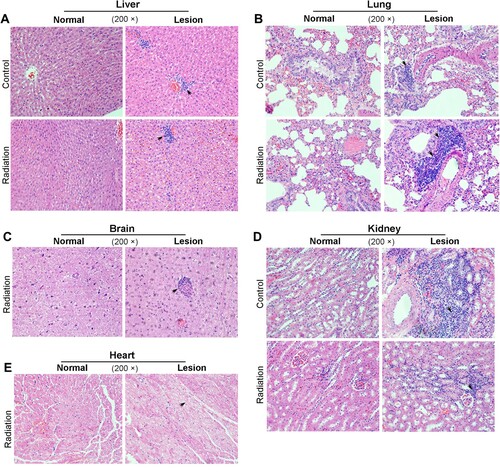
Table 1. Lesions of rabbits treated with radiation.
3.3. Effect of mobile phone radiation on DNA damage
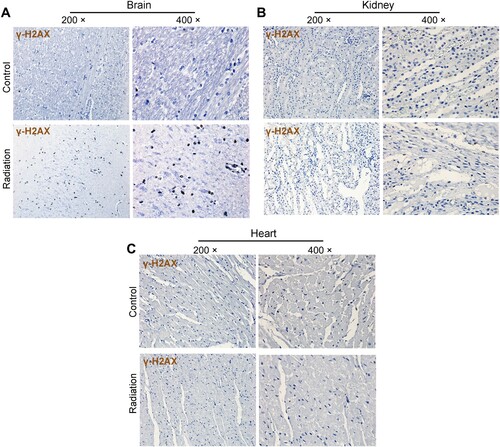
To determine if mobile phone radiation can lead to DNA damage. The levels of γ-H2AX, a marker of DNA double strand-break, were estimated in the brain, heart, liver, lung, kidney, and stomach. Positive IHC staining was seen in the brain (hippocampus) of one of the 8 radiation-exposed rabbits (1/8) but none in the control group (representative fields were shown in Figure (A)). The positivity of staining was not seen in any of the other organs tested (Figure (B and C)).
3.4. Effect of mobile phone radiation on cell apoptosis
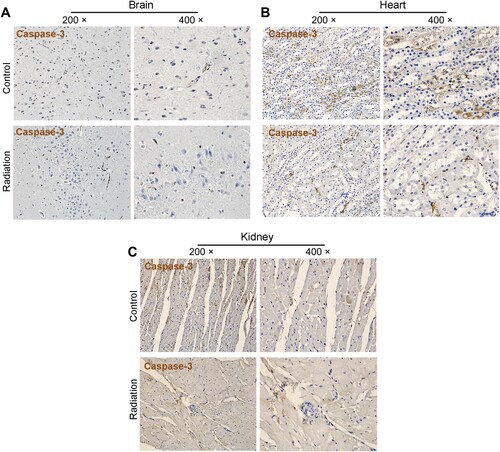
To determine if mobile phone radiation can enhance apoptosis, we examined the levels of cleaved caspase-3 in different tissues including the brain, heart, kidney. The results in Figure indicate that radiation exposure does not induce apoptosis in any of these organs.
4. Discussion
Mobile phones have provided great convenience for us but exposure to mobile phone-emitted radiation also increases potential risk to health. Therefore, the safety of mobile phone radiation has become a crucial issue. Most relevant researches have been based on epidemiological studies or cultured cells, while evidence from animal studies is preferred to draw more reliable conclusions. Most recent animal studies evaluated the genotoxicity of cell phone radiation to brain in rats and mice. Results from these studies suggest that cell phone radiation could lead to DNA damage (Sharma et al. Citation2019; Smith-Roe et al. Citation2020; Alkis, Akdag, et al. Citation2019; Alkis, Bilgin, et al. Citation2019) and these findings are in line with our results.
Previous epidemiological studies also linked mobile phone radiation with the occurrence of glioma (Wang et al. Citation2018). However, human skulls (7 mm) are much thicker than that of mice (0.43 mm) and rats (0.82 mm) (Chauveau et al. Citation2004; Lapchak et al. Citation2015). It is conceivable that the absorption of radiation would be affected by the thickness of the skulls and therefore larger animals such as rabbits would be more appropriate to examine the potential damage of the radiation to humans. In fact, large animals have been recently used to test potential effects of ultrasound on humans (Pelekanos et al. Citation2018). Therefore, we believe that our results using New Zealand rabbits as a model to study the effect of mobile phone radiation on brain tissues would be more reliable than rodent models. We noticed that exposure to mobile phone radiation caused inflammatory cell infiltration to the brain and brain DNA damage in one of the animals suggesting that mobile phone radiation is capable of penetrating the rabbit skull albeit less efficiently presumably due to its thickness. On the other hand, we have not observed apparent apoptotic change in the rabbit brain in comparison to the report that radiation may deregulate apoptosis in the rat brain (Dasdag et al. Citation2009), presumably also due to the difference of skull thickness in different models.
Interestingly, we found that more inflammatory cells infiltrated to the liver and the lungs when the animals were exposed to radiation and the infiltrated inflammatory cells in the lungs tend to cluster together. This suggests that the liver and lung tissues are more susceptible to radiation. Of note, the distance between an active cell phone and the liver/lung may be longer in humans than in rabbits. But mobile phones positioned around these human organs (e.g. in the pockets) may also cause damages to these organs in humans. It has been reported that radiation emitted from cellular phones also has potential to lead to cell damage in the testes (Alkis, Akdag, et al. Citation2019).
We have also observed infiltrated inflammatory cells and cell vacuolation in some organs suggesting that the effect of mobile phone radiation on health could be multiplex. Of note, inflammatory cell infiltration is rare in most organs except the liver and the lungs, while the cytoplasmic vacuolation was restricted to the heart, and DNA damage was only detected in the brain. These observations are in line with that of the organ coefficients suggesting that mobile phone radiation does not cause extremely lesions at the organ levels. We only conducted the experiment for 16 weeks and the observed changes more likely to represent the effect of radiation at the early stages. Prolonged exposure to radiation might be needed to study the potential effect on carcinogenesis. On the other hand, the patterns of DNA damage and inflammatory cell infiltration differ dramatically suggesting that radiation-induced DNA damage and inflammatory cell infiltration could be two mutually exclusive events.
As a communication study, our results were not intended to be comprehensive. However, the findings from this research warrant future studies with larger animals and/or more prolonged exposure to the radiation; and with more animals for better statistics. It worth to point out that instead of using an artificial radiation field to mimic mobile phone communication we used mobile phone communication with sub-thermal SAR of 1.0 and 0.7 W/kg for the head and the body, respectively. Therefore, our results are likely to be more representative of the effect of mobile phone radiation on human health. In summary, by using a relatively larger animal model, which has not been used before, we demonstrated that mobile phone radiation could induce lesions in different organs, especially the liver and the lungs, and this provides a valuable reference for further evaluation of the safety of mobile phone radiation.
Acknowledgements
This study was funded by Hunan Province Bairen Program to S.Z.
Data availability statement
The data that support findings of this study are available from the corresponding author on reasonable request. The data are not publicly available at this time as the data also forms part of an ongoing study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Alkis ME, Akdag MZ, Dasdag S, Yegin K, Akpolat V. 2019. Single-strand DNA breaks and oxidative changes in rat testes exposed to radiofrequency radiation emitted from cellular phones. Biotechnol Biotechnol Equip. 33:1733–1740. doi: https://doi.org/10.1080/13102818.2019.1696702
- Alkis ME, Bilgin HM, Akpolat V, Dasdag S, Yegin K, Yavas MC, Akdag MZ. 2019. Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn Biol Med. 38:32–47. doi: https://doi.org/10.1080/15368378.2019.1567526
- Bedir R, Tumkaya L, Mercantepe T, Yilmaz A. 2018. Pathological findings observed in the kidneys of postnatal male rats exposed to the 2100 MHz electromagnetic field. Arch Med Res. 49:432–440. doi: https://doi.org/10.1016/j.arcmed.2018.12.010
- Chauveau N, Franceries X, Doyon B, Rigaud B, Morucci JP, Celsis P. 2004. Effects of skull thickness, anisotropy, and inhomogeneity on forward EEG/ERP computations using a spherical three-dimensional resistor mesh model. Hum Brain Mapp. 21:86–97. doi: https://doi.org/10.1002/hbm.10152
- Dasdag S, Akdag MZ, Kizil M, Kizil G, Cakir DU, Yokus B. 2012. Effect of 900 MHz radiofrequency radiation on beta amyloid protein, protein carbonyl and malondialdehyde in brain. Electromagn Biol Med. 31:67–74. doi: https://doi.org/10.3109/15368378.2011.624654
- Dasdag S, Akdag MZ, Ulukaya E, Uzunlar AK, Ocak AR. 2009. Effects of mobile phone exposure on apoptotic glial cells and status of oxidative stress in rat brain. Electromagn Biol Med. 28:342–354. doi: https://doi.org/10.3109/15368370903206556
- Dasdag S, Erdal ME, Erdal N, Tasdelen B, Kiziltug MT, Yegin K, Akdag MZ. 2019. 900 MHz radiofrequency radiation has potential to increase the expression of rno-miR-145-p in brain. J Int Dent Med Res. 12:1652–1658.
- Del Re B, Bersani F, Giorgi G. 2019. Effect of electromagnetic field exposure on the transcription of repetitive DNA elements in human cells. Electromagn Biol Med. 38:262–270. doi: https://doi.org/10.1080/15368378.2019.1669634
- Diem E, Schwarz C, Adlkofer F, Jahn O, Rüdiger H. 2005. Non-thermal DNA breakage by mobile-phone radiation (1800MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 583:178–183. doi: https://doi.org/10.1016/j.mrgentox.2005.03.006
- Duan Y, Zhang HZ, Bu RF. 2011. Correlation between cellular phone use and epithelial parotid gland malignancies. Int J Oral Maxillofac Surg. 40:966–972. doi: https://doi.org/10.1016/j.ijom.2011.03.007
- Friedman J, Kraus S, Hauptman Y, Schiff Y, Seger R. 2007. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J. 405:559–568. doi: https://doi.org/10.1042/BJ20061653
- Ghoneim FM, Arafat EA. 2016. Histological and histochemical study of the protective role of rosemary extract against harmful effect of cell phone electromagnetic radiation on the parotid glands. Acta Histochem. 118:478–485. doi: https://doi.org/10.1016/j.acthis.2016.04.010
- Hardell L, Carlberg M, Hansson Mild K. 2006. Pooled analysis of two case–control studies on use of cellular and cordless telephones and the risk for malignant brain tumours diagnosed in 1997–2003. Int Arch Occup Environ Health. 79:630–639. doi: https://doi.org/10.1007/s00420-006-0088-5
- Lapchak PA, Boitano PD, Butte PV, Fisher DJ, Hölscher T, Ley EJ, Nuño M, Voie AH, Rajput PS. 2015. Transcranial near-infrared laser transmission (nilt) profiles (800 nm): systematic comparison in four common research species. PLoS One. 10:e0127580. doi: https://doi.org/10.1371/journal.pone.0127580
- Levis AG, Minicuci N, Ricci P, Gennaro V, Garbisa S. 2011. Mobile phones and head tumours. The discrepancies in cause-effect relationships in the epidemiological studies – how do they arise? Environ Health. 10:59. doi: https://doi.org/10.1186/1476-069X-10-59
- Morgan LL, Miller AB, Sasco A, Davis DL. 2015. Mobile phone radiation causes brain tumors and should be classified as a probable human carcinogen (2A) (review). Int J Oncol. 46:1865–1871. doi: https://doi.org/10.3892/ijo.2015.2908
- Muscat JE, Hinsvark M, Malkin M. 2006. Mobile telephones and rates of brain cancer. Neuroepidemiology. 27:55–56. doi: https://doi.org/10.1159/000094381
- Nisbet HO, Akar A, Nisbet C, Gulbahar MY, Ozak A, Yardimci C, Comlekci S. 2016. Effects of electromagnetic field (1.8/0.9 GHz) exposure on growth plate in growing rats. Res Vet Sci. 104:24–29. doi: https://doi.org/10.1016/j.rvsc.2015.11.002
- Pelekanos M, Leinenga G, Odabaee M, Odabaee M, Saifzadeh S, Steck R, Götz J. 2018. Establishing sheep as an experimental species to validate ultrasound-mediated blood-brain barrier opening for potential therapeutic interventions. Theranostics. 8:2583–2602. doi: https://doi.org/10.7150/thno.22852
- Schüz J, Jacobsen R, Olsen JH, Boice Jr JD, McLaughlin JK, Johansen C. 2006. Cellular telephone use and cancer risk: update of a nationwide Danish cohort. J Natl Cancer Inst. 98:1707–1713. doi: https://doi.org/10.1093/jnci/djj464
- Sharma A, Sharma S, Shrivastava S, Singhal PK, Shukla S. 2019. Mobile phone-induced cognitive and neurochemical consequences. J Chem Neuroanat. 102:101684. doi: https://doi.org/10.1016/j.jchemneu.2019.101684
- Smith-Roe SL, Wyde ME, Stout MD, Winters JW, Hobbs CA, Shepard KG, Green AS, Kissling GE, Shockley KR, Tice RR, et al. 2020. Evaluation of the genotoxicity of mobile phone radiofrequency radiation in male and female rats and mice following subchronic exposure. Environ Mol Mutagen. 61:276–290. doi: https://doi.org/10.1002/em.22343
- The interphone study group. 2010. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 39:675–694. doi: https://doi.org/10.1093/ije/dyq079
- Wang P, Hou CX, Li YW, Zhou D. 2018. Wireless phone use and risk of adult glioma: evidence from a meta-analysis. World Neurosurg. 115:e629–e636. doi: https://doi.org/10.1016/j.wneu.2018.04.122
- Wyde ME, Horn TL, Capstick M, Ladbury J, Koepke G, Wilson P, Stout MD, Kuster N, Melnick R, Bucher JR, et al. 2016. Report of partial findings from the national toxicology program carcinogenesis studies of cell phone radiofrequency radiation in Hsd: Sprague Dawley ® SD rats (whole body exposures). doi:https://doi.org/10.1101/055699