Abstract
Tongxie Yaofang (TXYF) has therapeutic effect on the ulcerative colitis (UC) with liver depression and spleen deficiency syndrome. This study investigated the changes in the gut microbiota of UC rats treated with TXYF. Wistar rats were divided into three groups: Control, dinitrobenzene sulfonic acid (DNBS)-colitis, and DNBS + TXYF (n = 8 per group). UC model was constructed via intrarectally injection of DNBS (25 mg DNBS and 0.25 mL 50% ethanol); rats in DNBS + TXYF group were administered with TXYF (20.8 g/kg) for 21 days. Physiological parameters were measured. Five samples from each group were selected for metagenomic analysis. TXYF treatment significantly reduced disease activity index, colon mucosal damage index, and tumor necrosis factor-alpha level. TXYF treatment prevented DNBS-induced dysregulation of gut microbial communities. Compared with DNBS-colitis group, the abundance of Firmicutes increased significantly and that of Bacteroidetes and Proteobacteria decreased in DNBS + TXYF group. Pathways such as protein processing in the endoplasmic reticulum pathway was enriched in the DNBS-colitis group, whereas it was decreased in the DNBS + TXYF group. Thus, TXYF treatment could effectively restore the dysregulation of intestinal flora induced by UC.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the rectum and colon characterized by diarrhea, bloody stool, and abdominal pain, which has a negative impact on the quality of life (Rubin et al. Citation2019). UC is difficult to cure due to the course of the disease with repeated remission and recurrence (Hu et al. Citation2021). Although restorative proctocolectomy (RPC) is the standard surgical treatment for UC, postoperative complications occurring in patients with UC after RPC have been absolutely inevitable (Karjalainen et al. Citation2020). Thus, patients with UC are usually treated with drugs in clinical therapy; however, this therapy requires long-term adherence of patients (Lachaine et al. Citation2013). To date, several medicines, such as vedolizumab, azathioprine, adalimumab, and infliximab, have been used in the treatment of UC (Trigo-Vicente et al. Citation2018). However, these drugs may cause a series of serious adverse events (Guo et al. Citation2019). Recently, traditional Chinese medicines (TCMs) with natural properties and low side effects have been widely used in the treatment of UC, which have shown to be effective in the clinic (Ke et al. Citation2012).
According to the characteristics of common TCM syndromes, UC can be divided into several main types, including Qi deficiency in spleen and kidney syndrome, dampness and heat in large intestinal syndrome, liver depression and spleen deficiency syndrome, yin and blood deficiency syndrome, and blood stasis syndrome (Yue et al. Citation2010). TCM suggests that chronic intestinal inflammation is usually caused by liver Qi block as well as liver-spleen disharmony, which is essentially characterized by the impaired ability of liver and spleen to control digestion and transportation, leading to dysfunction in body fluid and energy metabolism. Thus, liver depression and spleen deficiency is the most common syndrome in TCM syndrome typing of UC (Wang et al. Citation2012).
Tongxie Yaofang (TXYF) is a famous Chinese herbal formula that is used to tonify the spleen, soften the liver, clear dampness, and treat diarrhea. TXYF was first recorded in the Ming Dynasty (1368-1644 AD) book of ‘Yi Fang Kao’. Up to now, it has long been used for the treatment of UC characterized by liver depression and spleen deficiency (Gong et al. Citation2018). Zhu et al. (Citation2010) indicated that TXYF could increase the level of interleukin-6 and nitric oxide in rats with inflammation, suggesting that it had remarkable anti-inflammatory effects. Additionally, Hou et al. (Citation2019) demonstrated that TXYF could effectively improve intestinal permeability and intestinal mucosal barrier function. Literature evidence indicates that the microbiome affects the susceptibility and resistance of humans to diseases, especially common inflammatory diseases (Booijink et al. Citation2007). Previous study has reported the application of metagenomics in the research of inflammation in the inflammatory bowel disease (IBD), suggesting that the gut microbial diversity serves vital role in the development of enteritis. Besides, research has revealed that TCMs exert their effects on diseases by regulating the balance of the intestinal microflora (Qiu et al. Citation2017). However, the effect of TXYF on the intestinal flora of patients with UC has not been elucidated.
In this study, UC rat models were established by using the dinitrobenzenesulfonate (DNBS)/ethyl alcohol clyster method. After establishing the model, the UC rat was gavaged with TXYF every day for 21 days. We aimed to investigate the ameliorative effects of TXYF on DNBS-induced experimental UC, and then explore the alteration in the composition and structure of colonic microbiota. Furthermore, it has been reported that endoplasmic reticulum stress (ERS) is associated with the pathogenesis of UC (Li et al. Citation2016). Thus, the ER-related pathways enriched by microbial genes were also identified.
Materials and methods
Preparation of TXYF
TXYF was prepared according to the description in a previous study (Lin et al. Citation2020). TXYF contained large head atractylodes rhizome (Rhizoma Atractylodis Macrocephalae), white peony root (Radix Paeoniae Alba), dried tangerine peel (Pericarpium Citri Reticulatae), and divaricate saposhnikovia root (Radix Saposhnikoviae) at a proportion of 2:2:1.5:1. The raw medicinal materials were soaked in a 10 × volume of cold distilled water for 30 min, followed by boiling for 30 min to extract the drug liquid. Next, the remaining residue was soaked in water at the ratio of 1:1.5, and then boiling these herbs for 20 min to collect the decoction. The two drug liquids were combined and heated on the electric stove to obtain a concentrated concoction. After disinfection, the samples were stored at 4°C for further use.
Construction and treatment of UC rat models
A total of 48 SPF Wistar rats (24 males and 24 females, 160–200 g) were obtained from Research Laboratory Animal Center of Gansu University of Chinese Medicine. All animal care and experimental procedures were performed according to the National Institute of Health Guides for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Gansu University of Chinese Medicine (2017-118). The rats were housed under standard temperature and humidity with free access to water and food for 1 week. Next, the rats were randomly divided into three groups: Control group, DNBS-colitis group, and DNBS + TXYF group. Eight rats were eventually included in each group because some died during the modeling process. The experimental design for DNBS and TXYF treatments is summarized in Figure (A). Except for the rats in the Control group, the limbs of all rats were shackled daily (at irregular intervals), and then they were restricted to free movement for 6 h. They were fed every other day to simulate irregular eating. After 1 week of constraint, all the rats were subjected to fasting for 24 h and anesthetized with 3% pentobarbital sodium (10 mL/kg). A 12-cm polypropylene tube (diameter, 2 mm) with a number of holes on the side was inserted into the distal colon at a distance of 8 cm from the anus, and the DNBS/ethanol solution (25 mg DNBS and 50% ethanol 0.25 mL; Sigma-Aldrich Inc, St Louis, MO, USA) was then injected into the enteric cavity by using rubber infusion tube and lien for several minutes. Next, approximately 0.4 mL of air was injected, the anus was squeezed, the tail of the rats was lifted and held upside down for 1 min to prevent backflow of the injected fluid, and it was ensured that the liquid made full contact with the colon. The rats were fed normally after awakening from anesthesia. After DNBS/ethanol solution enema modeling, rats were still restrained daily to restrict their free movement, and they ate food every other day for 2 weeks to obtain the UC rat model.
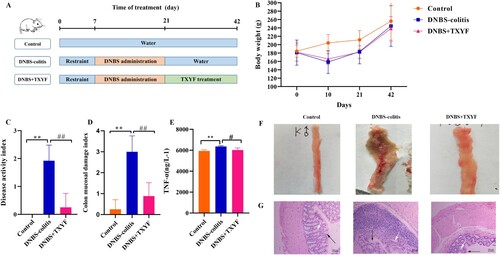
Figure 1. TXYF treatment could alleviate the symptoms of DNBS-induced ulcerative colitis (UC) in rats. (A) Experimental design of DNBS-induced UC and TXYF treatment. (B) Changes in body weights of rats. (C) Disease activity index (DAI) score. (D) Colon mucosal damage index (CMDI) score. (E) Tumor necrosis factor-α (TNF-α) level. (F) Microscopic view of the colon of the UC rats. (G) Hematoxylin-eosin (HE) staining of colon from each group. Black arrow represents mucosal architecture and white arrow represents goblet cells. All data are analyzed using ANOVA test and presented as the mean ± standard deviation (n = 8). **p-value < 0.01 compared with Control group, and ##p-value < 0.01, compared with the DNBS-colitis group .
After successfully establishing the UC model, the rats in the DNBS + TXYF group were administered TXYF crude medicine at a dose of 20.8 g·kg−1 per day, while the rats in the Control group and DNBS-colitis group were gavaged with equal volume of distilled water daily. After treatment for 21 days, the rats in each group were subjected to fasting for 12 h and anesthetized by intraperitoneal injection with 3% pentobarbital sodium (10 mL/kg). Blood samples were collected and centrifuged at 4000×g for 10 min at 4°C to separate serum sample. The level of TNF-α was determined using the commercially available enzyme-linked immunosorbent assay kit (Lianke, Hangzhou, China), following the instructions of the manufacturer. In brief, serum sample was added to the plate and treated by a color-developing agent. After color development at 37°C for 15 min in the dark, the reaction was terminated by adding 50 µL of stop solution to each well, and the absorbance of well was measured at 450 nm using a microplate reader (Bio-Rad iMark). TNF-α concentrations were calculated by the standard curves. Next, all the rats were sacrificed by cervical dislocation, and the entire colon was excised at a distance of 8 cm from anus. Colon length was recorded, and hematoxylin-eosin (HE) staining was performed using 10% phosphate buffered formalin fixed samples to detect Histopathology under a microscope (Leica, Wetzlar, Germany).
Evaluation of animal model
Observation of physiological indices
We observed water consumption, activity, coat color, stool traits, and bloody stools in rats daily. Additionally, the body weight of each rat was respectively monitored and recorded at the same time on the experiment days. Disease activity index (DAI) was evaluated (Porter et al. Citation1998) and calculated as the sum of scores assigned as follows: percentage of weight reduction (0: no change, 1: 1–5%, 2: 6–10%, 3: 11–15%, 4: > 15%), stool consistency (0: normal, 2: loose, 4: diarrhea), presence of fecal blood (0: normal, 2: positive occult blood test, 4: visible bleeding). This score was applied to evaluate the average of the scores for weight loss, stool consistency, and hemoccult.
Sucrose preference test
Before the model was established, all the rats were fed 1% sucrose water for 48 h, and after 24 h of water interruption, the sucrose water intake of the rats was measured within 1 h. After constructing the UC model, the parameters were re-measured. The consumption of sucrose water in 1 h could reflect the reactivity of rats to reward and was a parameter of the pleasure behavior.
Observation of the colon tissue
Erosion, hyperemia, edema, inflammation, ulcer, and bleeding of intestinal mucosal tissues were observed. Furthermore, the colonic mucosal damage index (CMDI) was evaluated (Rees Citation1998), which is used to assess colon traits and ulcer numbers. The scoring criteria of CMDI were as follows: 0, no damage; 1, mild hyperemia, edema, smooth surface, and no erosion or ulcer; 2, hyperemia edema, mucous membrane rough granulation, and erosion or intestinal adhesion; 3, highly congestive edema, necrosis and ulceration on the surface, maximum diameter of ulceration < 1 cm, and thickening of intestinal wall or necrosis and inflammation on the surface; 4, highly congestive edema, mucosal necrosis and ulcer formation, maximum longitudinal diameter of ulcer > 1 cm, or full-wall intestinal necrosis, and death caused by toxic megacolon.
Metagenomic analysis
DNA extraction and sequencing
Five rats from each group were randomly selected for metagenomic analysis. In brief, total metagenomic DNA was extracted from the rat intestinal samples (5 rats per group) using DNA extraction kits based on the instructions provided by the manufacturer. Once extracted, DNA samples were disrupted by Covaris focused-ultrasonicator to approximately 350 bp and purified by QIA quick PCR Purification Kit (Qiagen), followed by PCR amplification. PCR products were purified using 2% agarose gel electrophoresis. Target fragments were recovered using a QIA quick Gel Extraction kit (Qiagen). The library was quantified by using Agilent 2100 Bioanaylzer and ABI StepOnePlus real-time PCR System. Furthermore, the library was sequenced using Illumina Hiseq 2000 platform and paired-end reads were generated. Next, raw reads were preprocessed to generate clean reads for further analyses.
Metagenomic de novo assembly and gene prediction
The samples were assembled using the assembly software IDBAUD (Peng et al. Citation2012). We used a series of different k-mer parameters to perform multiple assemblies for each sample, and SOAP2 (Li et al. Citation2009) was then used to compare each assembly result to evaluate the assembly effect. Finally, an optimal k-mer and its corresponding assembly result were selected by comprehensively considering the N50 and the ratio. Only contig > 500 bp were retained for further analysis. MetaGeneMark (version 2.10, http://exon.gatech.edu/GeneMark/metagenome/Prediction/) (Zhu, Lomsadze, et al. Citation2010) was utilized to predict the open reading frames, and then the predicted genes (the sequence similarity was > 90% and the alignment region was > 90% of the sequence length) from different samples were combined and clustered with CD-Hit (Fu et al. Citation2012) to remove redundant sequences.
Analysis of species abundance
MEGAN (version 4.6) (Huson et al. Citation2007) was used to process the Nr comparison results. This software re-combed the taxonomic content of a sample according to the NCBI taxonomy. The abundance of the same species in the sample was obtained by summing up the abundance of the genes. The results are shown in species bar chart, which could visually represent the composition and proportion of each sample species, as well as the changes of species.
Screening of differential species
Further, Wilcoxon rank-sum test (Matsouaka et al. Citation2018) was applied to conduct non-parametric tests on two independent samples. A p-value < 0.05 indicated the thresholds of the differentially expressed species. The common and unique information of the differential species was analyzed, which were represented in Venn diagram.
Screening of differential pathways
The differential pathways between two groups (DNBS + TXYF vs. Control, DNBS-colitis vs. Control, and DNBS + TXYF vs. DNBS-colitis) were identified by using the ReporterScore method. The p-value of KEGG Orthology (KO) was calculated by Reporterscore, and the corresponding Z-value of p-value was obtained by the inverse normal distribution. Z < −1.65 or > 1.65 indicated statistical significance.
Analysis of ER-related pathway
The ER is highly sensitive to changes in the environment inside and outside the cell. When the ER is seriously and continuously stimulated by pathogens, oxidative stress, physical and chemical factors, ischemia, and hypoxia, ER stress (ERS) is triggered and the unfolded protein response (UPR) is activated. Thus, ERS has an important role in the pathogenesis of UC. In the present study, the genes and their corresponding microbiota involved in the ER-related pathways were identified.
Statistical analysis
Each experiment was carried out in triplicate, and the data are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using SPSS software (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to estimate differences between different groups. Differential microbes between different groups were screened by using Wilcoxon rank-sum test. A p-value < 0.05 was considered statistically significant.
Results
TXYF attenuates DNBS-induced UC in Wistar rats
During the experiment, we first observed the activity state of the rats. In the Control group, the furs of the rats were smooth, clean, and shiny. Meanwhile, the rats were sensitive, active, and had normal diet and stool. During the experiment, the weight gradually increased without death. In the DNBS-colitis group, the rats gradually became unresponsive and depressed, and they showed weakened resistance as well as decreased activity and motivation under continuous restraint. We also found that the rats had dull coats, decreased appetite, loose stool, and bit each other when they were hungry. In addition, compared with the rats in the control group, the rats in the DNBS-colitis group had a significant decrease in sucrose preference (7.84 ± 0.04 mL vs. 11.06 ± 0.83 mL, p-value < 0.05), indicating that DNBS-induced colitis increased depression in rats. Furthermore, obvious bloody stool and perianal fouling appeared after DNBS administration. In the DNBS + TXYF group, the UC-related symptoms significantly improved; for instance, the activity of the rats gradually increased, they became more active, and their food intake increased. The cleanliness and smoothness of the fur was gradually restored, and the stools gradually formed. The perianal area was clean without dirt, and part of the stool returned to normal gray-brown granules.
Furthermore, the effect of TXYF on UC rats was evaluated by assessing body weight (Figure (B)), DAI score (Figure (C)), CMDI score (Figure (D)), TNF-α level (Figure (E)), photographs of colon (Figure (F)), and HE-stained images (Figure (G)). Compared with the Control group, a loss body weight, significant increased DAI score and CMDI score (p-value < 0.01), as well as increased TNF-α level were observed in rats from the DNBS-colitis group, indicating that the UC model was successfully established. Meanwhile, TXYF treatment significantly decreased the DAI score, CMDI score (p-value < 0.01), and TNF-α level (p-value < 0.05) (Tables and ).
Table 1. The DAI scores of rats in each group.
Table 2. CMDI scores of rats in each group.
As shown in Figure (F), the intestinal mucosa was smooth and the plica texture was clear, and no abnormalities were observed in the Control group. In the DNBS-colitis group, swelling of the intestine, thickening of the intestinal wall, edema, congestion and erosion of the intestinal mucosa, and obvious ulcers were observed. In addition, some intestines severely adhered to the surrounding organs and necrosis was observed. In the DNBS + TXYF group, most of the intestinal mucosal injuries remarkably improved, without adhesion and necrosis. Some ulcers were mildly hyperemia and edema, and several ulcer healing scars were visible.
Representative images for HE staining are displayed in Figure (G). In the Control group, the colon mucosa was intact with clear structure; the nuclear structure was clear; the goblet cell morphology was normal; and no obvious inflammatory cell infiltration and ulcer was observed. In the DNBS-colitis group, the colonic mucosa was incomplete (red arrow) and the morphology of some goblet cells was lost (green arrow). After TXYF treatment, the pathological changes improved, erosion and ulcers significantly reduced; moreover, the mucosal structure gradually became complete and new goblet cells were generated.
TXYF alters the composition of gut microbes
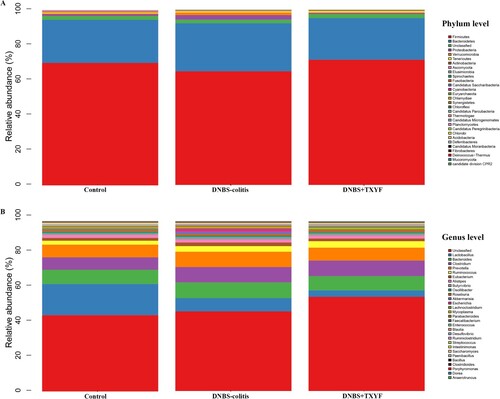
The clean reads were > 86% for all 15 samples, indicating that the reads were of high quality and the sequencing depth of microbiota gene analysis was good. The relative abundance of the gut microbiota was displayed using histogram. At the phylum level, all the groups were mainly composed of Firmicutes, Bacteroidetes, and Proteobacteria (Figure (A)). In brief, the abundance of Firmicutes in the DNBS-colitis group was lower than that in the Control group, while TXYF treatment returned the abundance of it to the normal level (Figure S1A). DNBS treatment induced an increase in the relative abundance of Bacteroidetes and Proteobacteria, whereas there was a decrease in the abundance of them after TXYF treatment (p-value < 0.001 for Proteobacteria, Figure S1B and S1C). At the genus level, Lactobacillus, Bacteroides, Clostridium, Prevotella, and Ruminococcus were the dominant bacteria genera in all the groups (Figure (B)). Specifically, the relative abundance of Lactobacillus was lower in the DNBS-colitis group than in the Control group, and a decreased trend was observed in the DNBS + TXYF group (Figure S1D). DNBS administration caused an increase in the abundance of Bacteroides and Prevotella, whereas TXYF treatment reversed the adverse trends of these two microbes and their abundances were similar to that observed in the control group (Figure S1E and S1F).
Screening of differential microbiota
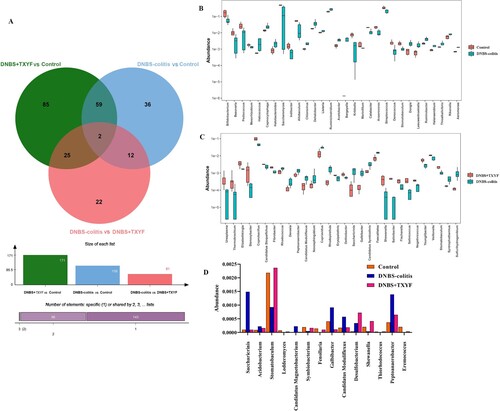
To identify the differential microbiota between groups, a differential analysis was performed at the genus level. A total of 109, 171, and 61 differential microbes were screened for DNBS-colitis vs. Control, DNBS + TXYF vs. Control, and DNBS-colitis vs. DNBS + TXYF, respectively (Figure (A)). Top 30 differential taxa in DNBS-colitis vs. Control or in DNBS-colitis vs. DNBS + TXYF are shown in Figure (B,C). We also observed that 14 overlapping microbes were obtained between DNBS-colitis vs. Control and DNBS-colitis vs. DNBS + TXYF. Among these, several microbes were more abundant in the DNBS-colitis group than in the DNBS + TXYF group, which included Saccharicrinis, Acidobacterium, Candidatus Magnetobacterium, Galbibacter, Candidatus Moduliflexus, and Peptoanaerobacter. In contrast, some microbes presented a marker downward trend after DNBS administration, while supplementation with TXYF restored the proportions of microbiota, such as Stomatobaculum, Symbiobacterium, and Shewanella, to the normal levels (Figure (D)). This finding suggests that these differential microbes may serve an important role in the treatment of UC with TXYF.
Figure 3. Identification of differential microbiota between the two groups at the genus level. (A) Venn diagram of the differential flora between different comparison groups. (B) Differential microbes in the Control group vs. DNBS-colitis group. (C) Differential microbes in the DNBS-colitis group vs. DNBS + TXYF group. (D) The relative abundance of differential microbes related to TXYF treatment. The x-axis represents the name of microbiota and the y-axis represents the relative abundance of microbiota.
TXYF affects the functional characterization of the colitis microbiome
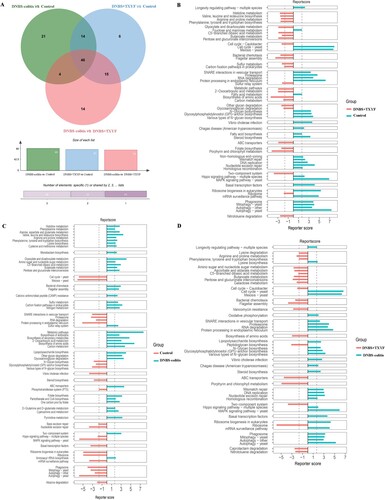
A total of 81, 85, and 79 differentially pathways were obtained for DNBS + TXYF vs. Control, Control vs. DNBS-colitis, and DNBS-colitis vs. DNBS + TXYF group, respectively (Figure (A)). As shown in Figure (B), several pathways were mainly enriched in the DNBS + TXYF group, such as flagellar assembly, biosynthesis of amino acids, and porphyrin and chlorophyll metabolism. In the Control group vs. DNBS-colitis group, pathways such as glyoxylate and dicarboxylate metabolism, metabolic pathways, and ABC transporters were primarily enriched in the DNBS-colitis group (Figure (C)). For DNBS + TXYF vs. DNBS-colitis group, flagellar assembly, peptidoglycan biosynthesis, and ABC transporters were mainly enriched in the DNBS + TXYF group. In contrast, RNA degradation, MAPK signaling pathway, and autophagy-yeast were mainly observed in the model group (Figure (D)).
Figure 4. Differential KEGG pathways between two groups. (A) Venn diagram of the differential pathways between different comparison groups. (B) DNBS + TXYF group vs. Control group. (C) Control group vs. DNBS-colitis group. (D) DNBS-colitis group vs. DNBS + TXYF group. Bar plots represent the reporter scores of each pathway in the two groups. A reporter score ≤ −1.65 indicates pathway enriched in the former group. A score > 1.65 indicates pathway enriched in the latter group.
Persistent and severe ERS induces autophagy in intestinal epithelial cells through UPR, further leading to inflammation (Ma et al. Citation2017). Meanwhile, excessive ERS also disrupts the intestinal mucosal barrier, ultimately resulting in UC (Yan et al. Citation2020). In this study, one ER-related pathway was identified from the overlapping pathways, namely, protein processing in the endoplasmic reticulum (map04141). The relative abundance of the protein processing in the endoplasmic reticulum pathway was mainly enriched in the DNBS-colitis group, whereas the abundance of this pathway decreased in the DNBS + TXYF or Control group. Thus, we speculated that bacteria associated with TXYF treatment may reverse the dysregulation of gene expression patterns induced by DNBS, especially for genes involved in this pathway. Despite that the expression levels of specific genes involved in this pathway were not validated, these results provide a favorable direction for further research.
Discussion
UC is becoming more common worldwide, with a high incidence of persistent or recurring symptoms that affect the quality of patients’ life (Costello et al. Citation2019). Current therapies for UC include salicylic acid preparations, hormones, and immunosuppressants, but many patients do not have a response to these therapies or efficacy is not sustained (Moen et al. Citation2018). Therefore, natural herbs without side effects are commonly used for the treatment of UC. In this study, we studied the changes in intestinal microbiota of TXYF against UC in rat models. We observed that the colonic mucosa in the DNBS-colitis group was incomplete with extensive erosion and ulceration, while erosion and ulcer significantly reduced or disappeared after TXYF treatment. Meanwhile, the DAI and CMDI scores in DNBS + TXYF group were significantly lower compared with those in the DNBS-colitis group, suggesting TXYF had a good therapeutic effect on UC. Compared with the DNBS-colitis group, the relative abundance of Firmicutes in the DNBS + TXYF group increased, whereas that of Lactobacillus, Bacteroides, and Prevotella decreased. Pathway analysis indicated that the relative abundance of protein processing in endoplasmic reticulum pathway was mainly enriched in the DNBS-colitis group, which may serve a role in the pathogenesis of UC.
A complex interaction between intestinal microbiota and TCM therapy has been reported. TCM could improve the composition of gut microbiota, thus ameliorating its dysfunction and related pathological conditions (Xu et al. Citation2017). Similarly, Sang et al. (Citation2018) also found that TCM could improve intestinal flora structure by increasing probiotics and reducing pathogenic bacteria, which could prevent the occurrence and development of IBD. In this study, the abundance of Firmicutes increased after treatment with TXYF. A previous study demonstrated that a low abundance of Firmicutes might increase the risk of intestinal inflammation, but mice colonized with microbiota rich in Firmicutes showed relieved colonic inflammation and downregulated TH17 pathways (Natividad et al. Citation2015). In addition, Firmicutes exerted anti-inflammatory property and was considered beneficial bacteria (Eeckhaut et al. Citation2013; Dehghan et al. Citation2014). Thus, TXYF might play a therapeutic role by increasing the abundance of beneficial bacteria, such as Firmicutes.
Furthermore, we found that the abundance of Bacteroides reduced in the TXYF group. Bacteroidetes could induce colitis in a host genotype-specific manner in a rat model (Bloom et al. Citation2011). Basset et al. (Citation2004) also showed that enterotoxigenic Bacteroides fragilis was associated with IBD. However, a meta-analysis implied that low level of Bacteroides was related to IBD (Zhou and Zhi Citation2016), which was inconsistent with our findings. This inconsistency may be caused by the difference in the samples; thus, the detailed mechanism underlying this phenomenon still needs further elucidation. We also observed that Prevotella was relevant to UC, as its abundance was decreased in the TXYF-treated group. The results of previous studies indicated that the increase in Prevotella species at the mucosal sites was associated with local and systemic diseases, including periodontitis, metabolic disorders, and low-grade systemic inflammation. It has also been confirmed that Prevotella stimulated epithelial cells to produce inflammatory factors (including interleukin 6 and interleukin 8), and the Prevotella-mediated mucosal inflammation led to systemic transmission of inflammatory mediators, bacteria and bacterial products, which in turn affected the outcome of systemic diseases (Scher et al. Citation2013). Mouse model experiments confirmed that Prevotella could promote the clinical and inflammatory characteristics of human disease, indicating Prevotella might be a clinically important pathobiont that was involved in human disease by promoting chronic inflammation (Larsen Citation2017). Taken together, the TXYF might play important role in the treatment of UC by reducing the relative abundance of pro-inflammatory-related microflora, such as Prevotella. However, the corresponding pathways and potential mechanisms need further investigation using integrated genomic technology.
Accumulating evidence suggests that ERS pathway could affect the pathogenesis of UC. Cells that are highly secreted in the intestine, such as discoid cells and goblet cells, are particularly susceptible to ERS, and rely heavily on the response of the unfolded protein to maintain cellular viability and homeostasis (Asada et al. Citation2012). However, the effect of TXYF on ER-related pathways in patients with UC remained unclear. In this study, we found that genes enriched in protein processing in endoplasmic reticulum pathway were influenced by TXYF. Vinayaga-Pavan et al. (Citation2019) observed that differentially expressed genes involved in cell cycle control and protein processing in the ER were upregulated in patients with UC. Other study also reported that differential proteins between UC and normal tissues were enriched in immune response and protein processing in the ER (Schniers et al. Citation2019). Similarly, we also observed a high level of protein processing in the endoplasmic reticulum pathway in the DNBS-colitis samples. These findings suggested that protein processing in the endoplasmic reticulum pathway had an impact on the development of the UC. Although we identified that 357 microbe-related genes were involved in this pathway, their expression level was not verified. Thus, the specific roles of these genes in the pathogenesis and treatment of UC remain unclear. Further researches still need to be performed.
However, the limitations of this study should be mentioned. First, the differential microbes and pathways were obtained from the rat model. Previous study indicated that despite that many common genera were found in the human and murine intestine, they varied widely in abundance, with only 4% of bacterial genes sharing considerable similarity (Hugenholtz and de Vos Citation2018). Considering that rat intestinal microbiota cannot completely mimic the microenvironment of human flora, it may be not possible to extrapolate our results to human; hence clinical studies are necessary. Second, the expression levels of genes involved in the protein processing in endoplasmic reticulum pathway were not verified; therefore, further studies on genetic quantitative should be carried out. Finally, we only studied the therapeutic effects of TXYF from the perspective of microbial changes, and these preliminary findings need to be further explored in combination with other omics, such as transcriptomics and metabolomics.
Conclusions
In summary, TXYF treatment displayed significant protective effects against UC with the ‘liver depression and spleen deficiency’ syndrome, and TXYF maintained the balance of the intestinal microflora by increasing the abundance of beneficial bacteria and decreasing that of destructive bacteria. In addition, TXYF influenced the level of protein processing in endoplasmic reticulum pathway in UC. This study demonstrated that TXYF can improve intestinal ulcer symptoms by regulating intestinal microbiota, which supports the therapeutic value of TXYF in DNBS-induced UC.
Author contributions
Conception and design of the research: RS, XZ; acquisition of data: ML, LF, YL; analysis and interpretation of data: YW, RS; statistical analysis: QZ; obtaining funding: XZ, YZ; drafting the manuscript: YW, RS; conducting experiments: YZ, LF, YL; revision of manuscript for important intellectual content: XZ. All authors read and approved the final manuscript.
Supplemental Material
Download JPEG Image (401.1 KB)Acknowledgements
This work was supported by Key research and development projects in Ningxia Hui Autonomous Region (grant number 2022BEG02834).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics approval and consent to participate
All animal care and experimental procedures were performed according to the National Institute of Health Guides for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Gansu University of Chinese Medicine.
Data availability statement
The metagenomics sequencing data are uploaded to NCBI BioProject (https://www.ncbi.nlm.nih.gov/bioproject/) with the accession number PRJNA801003. The data that support the findings of pathological indices are available in 4TU.ResearchData at doi:10.4121/18319907 (https://figshare.com/s/09475f78c42ddbbc2d03).
Additional information
Funding
References
- Asada R, Saito A, Kawasaki N, Kanemoto S, Iwamoto H, Oki M, Miyagi H, Izumi S, Imaizumi K. 2012. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J Biol Chem. 287:8144–8153.
- Basset C, Holton J, Bazeos A, Vaira D, Bloom S. 2004. Are helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 49:1425–1432.
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne Jr WM, Allen PM, Stappenbeck TS. 2011. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 9:390–403.
- Booijink CC, Zoetendal EG, Kleerebezem M, De Vos WM. 2007. Microbial communities in the human small intestine: coupling diversity to metagenomics. Future Microbiol. 2:285–295.
- Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C. 2019. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 321:156–164.
- Dehghan P, Gargari BP, Jafar-Abadi MA, Aliasgharzadeh A. 2014. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int J Food Sci Nutr. 65:117–123.
- Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R. 2013. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 62:1745–1752.
- Fu L, Niu B, Zhu Z, Wu S, Li W. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 28:3150–3152.
- Gong S, Fan Y, Wang S, Han Q, Lv B, Xu Y, Chen X, He Y. 2018. Mucosa repair mechanisms of tong-Xie-Yao-fang mediated by CRH-R2 in murine, dextran sulfate sodium-induced colitis. World J Gastroenterol. 24:1766–1778.
- Guo C, Wu K, Liang X, Liang Y, Li R. 2019. Infliximab clinically treating ulcerative colitis: a systematic review and meta-analysis. Pharmacol Res. 148:104455.
- Hou Q, Huang Y, Zhu Z, Liao L, Chen X, Han Q, Liu F. 2019. Tong-Xie-Yao-Fang improves intestinal permeability in diarrhoea-predominant irritable bowel syndrome rats by inhibiting the NF-κB and notch signalling pathways. BMC Complement Altern Med. 19:337.
- Hu Y, Ye Z, Wu M, She Y, Li L, Xu Y, Qin K, Hu Z, Yang M, Lu F, et al. 2021. The communication between intestinal microbiota and ulcerative colitis: an exploration of pathogenesis, animal models, and potential therapeutic strategies. . Front Med (Lausanne). 8:766126.
- Hugenholtz F, de Vos WM. 2018. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 75:149–160.
- Huson DH, Auch AF, Qi J, Schuster SC. 2007. MEGAN analysis of metagenomic data. Genome Res. 17:377–386.
- Karjalainen E, Renkonen-Sinisalo L, Mustonen H, Färkkilä M, Lepistö A. 2020. Restorative proctocolectomy in ulcerative colitis: effect of preoperative immunomodulatory therapy on postoperative complications and pouch failure. Scand J Surg. 110:51–58.
- Ke F, Yadav PK, Ju LZ. 2012. Herbal medicine in the treatment of ulcerative colitis. Saudi J Gastroenterol. 18:3–10.
- Lachaine J, Yen L, Beauchemin C, Hodgkins P. 2013. Medication adherence and persistence in the treatment of Canadian ulcerative colitis patients: analyses with the RAMQ database. BMC Gastroenterol. 13:23.
- Larsen JM. 2017. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 151:363–374.
- Li N, Wang X, Jiang L, Zhang M, Li N, Wei Z, Zheng N, Zhao Y. 2016. Effects of endoplasmic reticulum stress on the expression of inflammatory cytokines in patients with ulcerative colitis. World J Gastroenterol. 22:2357–2365.
- Li R, Yu C, Li Y, Lam T-W, Yiu S-M, Kristiansen K, Wang J. 2009. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 25:1966–1967.
- Lin Y, Ding Y, Lü B, Liu N. 2020. Effect of Tongxie Yaofang decoction on colonic mucosal protein expression profiles in rats with visceral hypersensitivity. J Tradit Chin Med. 40:245–252.
- Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang Y, Tso P, Wu G, Wu Z. 2017. Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel disease pathogenesis: an update review. Front Immunol. 8:1271.
- Matsouaka RA, Singhal AB, Betensky RA. 2018. An optimal Wilcoxon–Mann–Whitney test of mortality and a continuous outcome. Stat Methods Med Res. 27:2384–2400.
- Moen AEF, Lindstrøm JC, Tannæs TM, Vatn S, Ricanek P, Vatn MH, Jahnsen J. 2018. The prevalence and transcriptional activity of the mucosal microbiota of ulcerative colitis patients. Sci Rep. 8:17278.
- Natividad JM, Pinto-Sanchez MI, Galipeau HJ, Jury J, Jordana M, Reinisch W, Collins SM, Bercik P, Surette MG, Allen-Vercoe E. 2015. Ecobiotherapy rich in firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm Bowel Dis. 21:1883–1893.
- Peng Y, Leung HC, Yiu S-M, Chin FY. 2012. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 28:1420–1428.
- Porter S, Howarth G, Butler R. 1998. An orally administered growth factor extract derived from bovine whey suppresses breath ethane in colitic rats. Scand J Gastroenterol. 33:967–974.
- Qiu J, Liu Z, Zhao P, Wang X, Li Y, Sui H, Owusu L, Guo H, Cai Z. 2017. Gut microbial diversity analysis using illumina sequencing for functional dyspepsia with liver depression-spleen deficiency syndrome and the interventional Xiaoyaosan in a rat model. World J Gastroenterol. 23:810–816.
- Rees V. 1998. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391.
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. 2019. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 114:384–413.
- Sang T, Guo C, Guo D, Wang X. 2018. Effect of traditional Chinese medicine in inhibiting obesity and inflammatory diseases by regulating gut microbiota. China J Chin Mater Med. 43:3235–3242.
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB. 2013. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2:e01202.
- Schniers A, Goll R, Pasing Y, Sørbye SW, Florholmen J, Hansen T. 2019. Ulcerative colitis: functional analysis of the in-depth proteome. Clin Proteomics. 16:4.
- Trigo-Vicente C, Gimeno-Ballester V, García-López S, López-Del Val A. 2018. Systematic review and network meta-analysis of treatment for moderate-to-severe ulcerative colitis. Int J Clin Pharm. 40:1411–1419.
- Vinayaga-Pavan M, Frampton M, Pontikos N, Levine AP, Smith PJ, Jonasson JG, Björnsson ES, Segal AW, Smith AM. 2019. Elevation in cell cycle and protein metabolism gene transcription in inactive colonic tissue from Icelandic patients with ulcerative colitis. Inflamm Bowel Dis. 25:317–327.
- Wang J, Wang T, Wu X, Zhao Y, Xue X, Wang Q. 2012. Weighting coefficients of symptoms and signs in the diagnosis of corresponding TCM syndrome elements of ulcerative colitis based on expert questionnaire investigation. J Chin Integr Med. 10:398–405.
- Xu J, Chen HB, Li SL. 2017. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med Res Rev. 37:1140–1185.
- Yan S, Yingchao L, Zhangliu W, Xianli R, Si L, Siyi N, Jihong Z. 2020. Effect of berberine from on apoptosis of intestinal epithelial cells in a mouse model of ulcerative colitis: role of endoplasmic reticulum stress. Evid Based Complement Alternat Med. 2020:3784671.
- Yue H, Wang T, Chen J, Yang L, Zhao D, Zhao Y, Wu X, Qu K, Yu L, Zhao F. 2010. Modern literature of common syndromes and syndrome factors of ulcerative colitis. J Beijing Univ Tradit Chin Med. 33:306–308.
- Zhou Y, Zhi F. 2016. Lower level of Bacteroides in the gut microbiota is associated with inflammatory bowel disease: a meta-analysis. BioMed Res Int. 2016:5828959.
- Zhu A, Fang B, Zhang Y, Wu X, Tian Z, Guo S, Li D. 2010. Anti-inflammation effect of Tongxie Yaofang. China Pharm. 21:592–594.
- Zhu W, Lomsadze A, Borodovsky M. 2010. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 38:e132.