Abstract
Following the successful renewal of its Cancer Center Support Grant (CCSG), leadership of the UCSF Helen Diller Comprehensive Cancer Center (HDFCCC) began a strategic planning process. The motivation was to think about where cancer research was going in the future; and with this vision to define a general scientific direction, mission, and priorities. HDFCCC Leadership began discussions about a new strategic plan in early 2018. From these meetings, the theme of ‘Cancer Research in 2030’ arose: that is, what will cancer research look like in 2030? This forward-looking focus was intended to encourage creativity unconfined by a particular institutional structure or grant mechanism. Focusing on the science paved the way for an innovative, actionable, and motivating strategic planning process. Here, we describe the three-phase process, and the various groups involved across the HDFCCC and UCSF. We present the unique framework based on a cells-to-society model and an individual experience perspective, which led to the development of a logic model and ongoing implementation of tactics and tracking progress. We believe that sharing this process and its results will be of value to cancer centers and cancer researchers across the network of NCI comprehensive cancer centers, and cancer research centers in general.
1. Introduction
National Cancer Institute (NCI)-designated comprehensive cancer centers are defined by the Cancer Center Support Grant (CCSG), an NIH P30 mechanism that requires competitive renewal every five to seven years. The guidelines (PA-21-321) state one of the intended goals of the grant is to support ‘strategic planning and evaluation that further the research agenda of the Center.’ Although there are no specifications for the format of an appropriate strategic plan, it is common for centers to align processes and deliverables with the five-year timeline of the CCSG, with standard elements, such as a Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis, a list of goals and metrics, and tactics listed to achieve the desired outputs.
In 2018, following the successful renewal of the CCSG, leadership of the University of California, San Francisco (UCSF) Helen Diller Comprehensive Cancer Center (HDFCCC) began a new strategic planning process. The HDFCCC first achieved comprehensive status in 1999. It is a matrix center of over 450 members (UCSF faculty with cancer-relevant research portfolios), $64.7M in NCI-funded research grants, and 1,275 publications in 2021. The HDFCCC CCSG directly supports seven research programs; seven shared resources; clinical trials infrastructure; senior leadership; administration; and offices for education and training, community engagement, and diversity, equity, inclusion, and accessibility. Other funding sources allow the HDFCCC to support transdisciplinary initiatives and pilot awards that bring members together across the seven CCSG research programs.
In considering a new strategic plan, HDFCCC Senior Leadership (the Director, Deputy Director, and nine Associate Directors) developed the theme of ‘Cancer Research in 2030:’ that is, what will cancer research look like at UCSF in 2030? This forward-looking focus was intended to encourage creativity unconfined by a particular institutional structure or five-year grant mechanism. Furthermore, the motivation for this strategic planning was not in reaction to a specific problem to solve, but rather to think about where cancer research was going in the near future, and with this vision to define an overall scientific direction, mission, and priorities. Focusing on the science and not the requirements of a funding cycle paved the way for an innovative, actionable, and motivating strategic planning process. We believe that sharing this process and its results will be of value to centers and researchers across the network of 54 NCI comprehensive cancer centers, as well as other research centers.
2. Process and approach
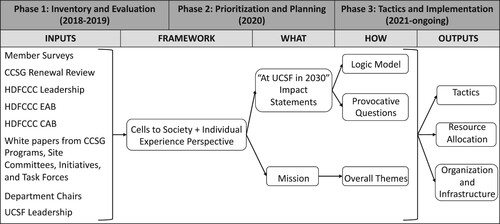
The HDFCCC Cancer Research in 2030 strategic planning process was divided into three phases (). Phase 1, Inventory and Evaluation, captures the logistics of gathering input from a variety of stakeholders and creating a cohesive set of recommendations. Phase 2, Prioritization and Planning, encompasses the development of an innovative framework that defines the Center’s philosophy for the next ten years. Phase 3, Tactics and Implementation, involves the creation of a logic model and actioned through new resource allocation and defined Provocative Questions. The process of each Phase is described below, and the resulting outputs detailed in the Results section.
Figure 1. Timeline and three phases of the Cancer Research in 2030 strategic plan. Each phase is detailed in the text. UCSF, University of California, San Francisco. HDFCCC, Helen Diller Family Comprehensive Cancer Center. CCSG, Cancer Center Support Grant. EAB, external advisory board. CAB, community advisory board.
2.1. Phase 1: Inventory and evaluation
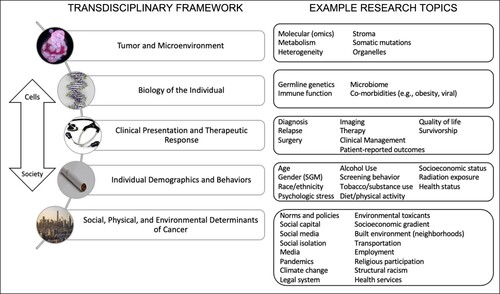
The UCSF Cancer Research in 2030 Strategic Planning process began in late 2018. HDFCCC leadership wanted to engage the entire HDFCCC membership in the process, in addition to taking into consideration feedback from UCSF leadership, the CCSG review, and HDFCCC advisory groups (the External Advisory Board, EAB, and Community Advisory Board, CAB). Therefore, Senior Leadership defined three broad groups of stakeholders from which it would be important to gather information and coalesce ideas (): (1) the ten extant CCSG Programs; (2) other research initiatives, not funded by the CCSG, some of which were defined during this planning process, but also including important aggregations of researchers such as (a) extant cancer site committees, which bring together clinical researchers to review and monitor clinical trials, and (b) developing initiatives in focus areas; and (3) thematic task forces. The thematic task forces were convened in brainstorming sessions as a new way of aggregating HDFCCC members, staff, and trainees outside of the formal CCSG program structure that covered the cancer continuum from basic discovery research to prevention to diagnosis and treatment and finally to the delivery of cancer care [Citation1]. Of note, the groups shown in and the inputs detailed in do not have mutually exclusive membership (i.e. a member of the Breast Oncology Program might be a member of the Breast Site Committee and also have participated on the Developing Cancer Cures Task Force).
Table 1. Contributing groups to the HDFCCC strategic plan. Groups 1 and 2 were extant groups; Group 3 was convened for the purposes of Phase 1 of the planning process and then disbanded.
From August 2018 to September 2019, the HDFCCC conducted membership-wide surveys, which informed the design of the task forces and subsequent meetings. Administration and leadership facilitated Center-wide brainstorming meetings for the groups in . Internal HDFCCC administration and faculty chosen by HDFCCC Leadership led these sessions, rather than a hired external consultant, in order to capitalize on the institutional knowledge and relationships these individuals have developed.
All groups in were asked to consider: (1) What will cancer research look like in 2030? and (2) What do we need to do scientifically to get there? Each group produced a brief (2-3-page) white paper following a template that outlined the current state of research, their predictions for 2030, what was needed to reach 2030 goals, and a summary of the themes that arose in discussion. In all, 349 HDFCCC members responded to surveys, and 214 participated in the thematic task forces, through the in-person meetings, email discussions, or contributing to the white paper draft.
HDFCCC Scientific Leadership and the HDFCCC EAB identified the common themes and priorities across all white papers, which were reflected in the overall framework document. In addition, the CAB’s own strategic planning preceded and informed the overall HDFCCC process with respect to community engagement and bidirectional partnership principles. The intent was to ensure all forms of current and anticipated cancer research would be represented in the strategic planning process. The scope was universal, but the focus was internal to what could be accomplished by UCSF and the HDFCCC.
2.2. Phase 2: Plan and prioritization
To coalesce the contents of the white papers and other inputs () into Center-wide mission, goals, research priorities, and provocative questions around which to provide institutional support, HDFCCC leadership, under advisement of the HDFCCC EAB, developed a (1) Transdisciplinary Framework and (2) Individual Experience Perspective Continuum to organize the common themes and priorities.
2.2.1. (1) Transdisciplinary framework
Transdisciplinary research is an approach that encourages researchers from multiple disciplines to tackle critical research problems by sharing a common framework and their disciplinary perspectives while being open to the opinions and contributions of others [Citation2–4]. Such frameworks, by definition, promote research questions and methodologies that are cross-disciplinary, based on team science, and translational aligning with a ‘cells to society’ ecosocial model [Citation5]. Importantly, this perspective also defines pathways, by which broader societal and environmental etiologic factors may be uncovered [Citation5,Citation6]. Furthermore, this framework ensures that understanding and addressing inequities in cancer is woven into all levels across the cancer continuum [Citation7]. shows the research topics along each step of the framework that were identified as research focus areas to move research forward to 2030. This framework does not prioritize one area over another; rather, it shows how all areas are interconnected and must be addressed with a transdisciplinary approach.
2.2.2. (2) Individual experience perspective
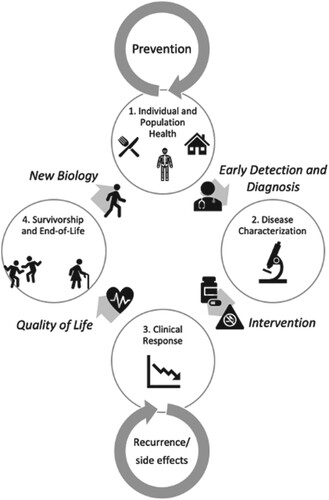
Although the focus of Cancer Research in 2030 strategic plan is on research, it is crucial to understand the impact of research on how an individual benefits from cancer research, prevention, and care not just in a clinical setting but also in the context of their community, social environment, and across the ecosocial spectrum. As an individual progresses through the continuum of disease prevention, detection and diagnosis, and treatment, their journey is affected by the social determinants of health encountered by this individual as a member of families, neighborhoods, towns, states, and nation within a social context. These considerations resulted in a ‘Individual Experience Perspective’ representing the continuum from individual and population health and disease prevention, to disease characterization, to clinical response, to survivorship and end of life ().
Figure 3. Individual Experience Perspective. 1. Individual and Population Health: An individual is living their life, with a certain genetic background, certain biology, in a certain place, and following certain behaviors. Some may be individual risk factors for cancer, some risks are a function of society and the environment, but all are factors that inform a person’s eventual risk. In this ‘pre-tumor’ phase, interventions focus on prevention, lifestyle behaviors, early detection, and improving determinants at a social level in order to allow individuals to have healthy lives in an equitable, fair and just environment. → Early Detection and Diagnosis→ 2. Disease Characterization: Some individuals may develop symptoms and be diagnosed with a tumor. As a patient, their tumor biology becomes the focus including both the characteristics of the tumor. The tumor’s microenvironment, and the interacting effects of the tumor and the broader characteristics of a patient’s biology (e.g. immune function, microbiome). → Intervention (Therapeutic and Non-therapeutic)→ 3. Clinical Response: Intervention(s) are implemented, targeting the tumor and patient biology. Layered on targeted therapies are non-therapeutic interventions (e.g. lifestyle behaviors, integrative medicine). Here, data on clinical response, resistance, and side-effects. are important to drive clinical decisions. Another factor here is the structural framework that supports patient compliance, access to clinical trials, and continuing care. → Quality of Life→ 4. Survivorship and End-of-life: The patient is on a quest to live a healthy life after cancer treatment, which may include symptom management, palliative care, monitoring/screening, changes in environment and behavior, and integrative medicine. These factors alter the individual biology as the patient re-enters the continuum cycle. This stage also includes accommodations and wellness measures to provide comfort and dignity to individuals at the end of their life. Inherent in discussions between patients and their caregivers is an understanding of the patient’s home and community framework that affect decision-making and adherence to interventions. → New Biology→ back to (1).
2.3. Phase 3: Tactics and implementation
2.3.1. HDFCCC mission statement and goals
By combining the Transdisciplinary Framework and the Individual Perspective structures, the HDFCCC defined goals and research priorities that have the highest impact and drives research forward in a way that directly affects patient care and cancer population health. At each stage of the Individual Perspective, research goals and priorities were identified from each level of the Framework. By defining goals at these levels, the HDFCCC was able to identify commonalities and define priority areas for inclusion in a logic model that identifies tactics to achieve goals and allows for the evaluation of progress in reducing the cancer burden in the HDFCCC catchment area. These goals and priorities were disseminated at leadership meetings, program meetings, online, and Center-wide Town Halls, allowing broad input and iteration as we completed the final document. HDFCCC leadership was advised throughout this process by its EAB and CAB, the latter of which also approved the final mission statement.
2.3.2. Logic model
To bring the HDFCCC mission and strategic plan from an academic exercise into something that can have real-world impact on decision-making and resource allocation, we used a logic model approach to define activities and metrics which align with the identified research priorities. Logic models are a way to track activities against goals with a long-term perspective, recognizing that research projects and institutional change may take many years [Citation8]. Logic models combine an inventory of the resources available, the activities possible with these resources, the output of these activities individually and collectively, the measurable outcomes over a shorter time period, leading to the eventual desired impact [Citation9].
2.3.3. Provocative questions
Identifying a list of tactics and activities does not inspire sufficient motivation for members to align their individual research with center-wide goals. Therefore, we used the NCI Provocative Questions Initiative as a model to organize research priorities into tangible questions to which researchers can creatively respond with their current interests and expertise. The draft questions were shared with membership for input, and Senior Leadership finalized the four PQs, around which future fundraising and funding opportunities can be aligned.
3. Results
3.1. Cancer Research in 2030
Each White Paper included a set of predictions for what cancer research will look like in 2030 (examples from task forces are provided in ). These statements ranged from the very general, e.g. ‘At UCSF in 2030, the biologic, social, and environmental context of the patient (host) will be an integral component of the treatment paradigm,’ to the specific, ‘At UCSF in 2030, commercialization of cancer early detection with genomic tools (e.g. direct to consumer tests, ctDNA, liquid biopsy, imaging) will become more common and require better understanding of both positive and negative impacts.’
Table 2. Example predictions from the thematic task forces.
From these white papers, a set of seven HDFCCC-wide Impact Statements were created to capture the Center-wide predictions. These predictions now serve as the goals of the HDFCCC moving forward and are used within the logic model to drive activities.
The first four Impact Statements align with the steps along the Individual Experience Perspective cycle and incorporate research across the cells-to-society framework we developed to understand the mechanisms at each step, as will be clear in the logic model.
At UCSF in 2030, we will understand the biological, behavioral, environmental, and social determinants of risk and disease onset applied to prevention.
At UCSF in 2030, we will understand the tumor and the patient – by translating discovery, clinical, and population research – to inform appropriate risk stratification, prevention, screening, diagnosis, and interventions.
At UCSF in 2030, we will understand the intervention – by translating discovery, clinical, and population research – and health outcomes research will assure all people receive timely, affordable, and high-quality care, regardless of who they are, where they live, or where they get their care.
At UCSF in 2030, we will understand the changes in the tumor and the patient over time and following treatment – by translating discovery, clinical, and population research – to inform appropriate secondary, palliative, and symptom management interventions.
The next three Impact Statements ensure we are addressing the needs of the communities and populations we serve, that the next generation of researchers are trained to align with what we predict for 2030, and that we ensure a diverse and equitable healthcare workforce.
At UCSF in 2030, we will understand the inequities in our catchment area related to care, screening, training, leadership, and access to clinical trials that lead to increased morbidity and mortality in different populations, so that all patients have the same chance of preventing and surviving cancer.
At UCSF in 2030, multi-disciplinary training will shift from individual reward (e.g. fellowships, grants) to greater reward for active engagement in productive teams that are focused on major goals. Meaningful communication with other disciplines beyond medicine (e.g. toxicology, sociology, economics, political science) will be essential to reap the rewards of cross cutting discovery in other sciences. Cancer research training programs will emphasize new skills in team science and transdisciplinary approaches, engaging a diverse cohort of trainees both in the United States and globally.
At UCSF in 2030, we will enhance diversity, equity, inclusion, and accessibility (DEIA) in the research workforce, including trainees, faculty, and staff, Center leadership, and advisory boards. To accomplish this, HDFCCC uses an approach that is accountable, engages stakeholders, promotes institutional change that is individual-centered, and aims to provide increasing opportunities for all.
3.2. HDFCCC mission statement and goals
Standard strategic planning roadmaps refer to (1) Vision, (2) Key Aims, (3) Goals, (4) Strategies, and (5) Tactics, which are then ranked by ease of implementation and impact.
We believe our organization along the Translational Framework and Individual Experience Perspective is better aligned with how our investigators think about their work, collaborations, and impact on cancer research and care. The organization allows for immediate operationalization of scientific research because it is clear what infrastructure and resources are required and available to address priority areas defined in each step. This organization also allows the plan to be specific to UCSF, uniquely tailored to the research strengths, interests, and future directions of our members. This unique organization defines the refreshed HDFCCC Research Mission Statement: The UCSF Helen Diller Family Comprehensive Cancer Center (HDFCCC) seeks to drive scientific discovery and develop tailored interventions to improve cancer outcomes in the catchment area and beyond.
This mission statement can further be captured in three major thematic areas:
Theme 1 (Innovative Discovery): Advance innovative basic, clinical, and population research, focused on unique characteristics of the individual, disease, population, and community.
Theme 2 (Effective Translation): Translate research to define risk, emphasize prevention, optimize diagnosis, tailor screening and treatment, and improve outcomes.
Theme 3 (Implementation and Dissemination): Reduce inequities in cancer awareness, prevention, early detection and diagnosis, care, treatment, and patient-centered outcomes, through data-driven science and community engagement.
3.3. Logic model
Each Impact Statement was analyzed against the output materials to identify specific activities (again, in each area of the cells-to-society framework and individual patient perspective) that are needed to meet the goals. These are organized into a logic model, which allows a clear picture for how activities lead to measurable outputs in alignment with the goals and HDFCCC mission. Each activity can be then assigned a champion and tracked, with updates presented at monthly Senior Leadership meetings and other HDFCCC events.
As one example, in the Impact Statement, ‘At UCSF in 2030, we will understand the tumor and the patient – by translating discovery, clinical, and population research – in order to inform appropriate risk stratification, prevention, screening, diagnosis, and interventions,’ one research priority is defined as, ‘A multi-dimensional, translational evaluation will be undertaken for every patient’s cancer, including a comprehensive tumor-omics profile (e.g. genomic, epigenomic, transcriptomic, metagenomic, metabolomic), an individualized assessment of the tumor immune microenvironment and microbiome, an evaluation of tumor heterogeneity and plasticity.’ To support these research priorities, activities will need to include developing research hubs or focus groups, redefining CCSG research program aims to include translational goals, and support research through pilot funding and grant support in tumor biology and host biology (). Senior leadership reviews progress along each goal at monthly meetings and identifies potential activities that can be developed to meet goals. This was especially important during the COVID-19 pandemic, when priorities drastically shifted. However, using the logic model, we could ensure that HDFCCC activities remained in alignment with our strategic plan. For example, the pandemic-related focus on telehealth and understanding burdens to screening help us achieve our Center-wide goals.
Figure 4. Snapshot of overall logic model focused on a single research priority (orange) in the area of a single impact statement, as described in the text.

HDFCCC administration then provides support to implement activities and leverage opportunities that may exist elsewhere on campus. Immediate actions included:
Established transdisciplinary Research Hubs that bring researchers together groups that don’t adhere to the strict definition of a CCSG Research Program, to tackle a priority area. For example, Hubs developed in survivorship and symptom management, integrative oncology, microbiome, cancer and cognition, and recruitment science. Each Hub is allocated $75,000 per year to develop an internal RFA for pilot funding or exploratory research.
Identified areas underrepresented by senior leadership, and adjusted Associate Director positions and job descriptions, including Associate Directors for Clinical Translation and Translational Laboratory Research in place of an Associate Director for Basic Research. Job descriptions were unified to include activities necessary for implementation of the strategic plan.
Created new and innovative Liaison leadership positions that had not previously existed in NCI Comprehensive Cancer Centers as far as we knew: each Research Program has a liaison that works with the HDFCCC Office of Education Training and a second one that works with the HDFCCC Office of Community Engagement.
Established new requirements on internal pilot funding opportunities to address a strategic priority.
Realigned HDFCCC membership into seven research Programs with new research aims. For example, a realignment of the Cancer Control Program defined an aim with an explicit focus on commercial interests as a vector of cancer causation (e.g. the tobacco and sugar industries).
3.4. Provocative questions
Finally, a set of four Provocative Questions were developed as described above. Currently, these are under consideration by Senior Leadership and will drive future initiatives and fundraising efforts.
PQ1: What are the unique independent and interactive contributions of structural, social, molecular, and genetic determinants of cancer among different demographic populations?
PQ2: How can we overcome intra-tumor heterogeneity (differences within a single tumor) to make cancer therapies work better?
PQ3: What are mechanisms of the biological, environmental, and social determinants of patient and tumor resistance to cancer immunotherapy?
PQ4: Does improved quality of life (management of pain, depression, fatigue, etc.) improve cancer mortality, and, if so, how?
4. Discussion
The HDFCCC Cancer Research in 2030 strategic planning process was designed to focus on research areas (Transdisciplinary Framework) and patient experience, rather than on solving a particular problem. The center-wide engagement of HDFCCC membership from across disciplines created a dynamic, creative environment. Extending the time frame to ten years, rather than five, allowed broader discussions and detached individuals from any preconceived notions about the immediate future and the next CCSG cycle: the process encouraged members to ‘think big’, outside of structural barriers and limited resources. Inherent also in this process, was a galvanizing, motivating structure for the membership that allowed them to think about how their own expertise in research, training and education, community engagement, and DEIA, might tie into the overall Center goals and prepare for the future. This mix of perspective in the brainstorming meetings and member surveys brought together ideas from fields that may not normally undertake such a process together and underscored the value of a comprehensive cancer center in breaking down silos and supporting transdisciplinary research.
Having internal HDFCCC administration moderate meetings and brainstorming sessions allowed groups to immediately dive into vibrant discussions, without needing to spend time providing context to an external moderator. The moderator could also table discussions that were focused on tactics and limitations. Importantly, the moderator can also provide continuity with HDFCCC Senior Leadership from planning to implementation, by overseeing the development of the logic model, tracking progress, and allocating resources to activities.
The use of a logic model also allows the focus to specific goals and milestones to be on the science, by focusing outputs and impact built on resources and tactics. The logic model is dynamic and iterative, in that new opportunities that arise in the future can be aligned with the overall impact. Further, the logic model does not depend on one specific resource or decision but focuses on the culmination of many activities and decisions towards a goal.
Acknowledgements
The authors would like to thank HDFCCC Senior Leadership for their involvement in the process: Rahul Aggarwal, MD; Emily Bergsland, MD; Ben Braun, MD, PhD; Charles Craik, PhD; Felix Feng, MD; Tung Nguyen, MD; Kim Rhoads, MD, MS, MPH; Kate Shumate, MPA; Charles (Chuck) Ryan, MD (now at the Prostate Cancer Foundation); Zena Werb, PhD (in memoriam). We also recognize the hard work of task force leaders Small, Hiatt, Braun, Werb, Matthew Cooperberg, MD, MPH, and Sabrina Ronen, PhD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- National Cancer Institute. Cancer Control Continuum 2020 [cited 2022 Sept 12]. Available from: https://cancercontrol.cancer.gov/about-dccps/about-cc/cancer-control-continuum.
- Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between the health and social sciences. Soc Sci Med. 1992;35(11):1343–1357. doi:10.1016/0277-9536(92)90038-r. PubMed PMID: 1462174.
- Stokols D, Hall KL, Taylor BK, et al. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008;35(2 Suppl):S77–S89. doi:10.1016/j.amepre.2008.05.002. PubMed PMID: 18619407.
- Ciesielski TH, Aldrich MC, Marsit CJ, et al. Transdisciplinary approaches enhance the production of translational knowledge. Transl Res. 2017;182:123–134. Epub 20161110. doi:10.1016/j.trsl.2016.11.002. PubMed PMID: 27893987; PMCID: PMC5362296.
- Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936–944. Epub 20120315. doi:10.2105/AJPH.2011.300544. PubMed PMID: 22420803; PMCID: PMC3484783.
- Hiatt RA, Breen N. The social determinants of cancer: a challenge for transdisciplinary science. Am J Prev Med. 2008;35(2 Suppl):S141–S150. doi:10.1016/j.amepre.2008.05.006. PubMed PMID: 18619394.
- Abrams DB. Applying transdisciplinary research strategies to understanding and eliminating health disparities. Health Educ Behav. 2006;33(4):515–531. doi:10.1177/1090198106287732. PubMed PMID: 16769758.
- Hiatt RA, Handley MA, Ling PM, et al. Origins of cancer disparities in young adults: logic models to guide research. Am J Prev Med. 2017;53(3S1):S95–S102. doi:10.1016/j.amepre.2017.05.022. PubMed PMID: 28818252.
- W.K. Kellogg Foundation. Logic Model Development Guide 2004. Available from: https://www.naccho.org/uploads/downloadable-resources/Programs/Public-Health-Infrastructure/KelloggLogicModelGuide_161122_162808.pdf.