Abstract
Background. Distributing CRC screening through pharmacies, a highly accessible health service, may create opportunities for more equitable access to CRC screening. However, providing CRC screening in a new context introduces a substantial implementation challenge.
Methods. We conducted 23 semi-structured interviews with community pharmacists practicing in Washington state and North Carolina about distributing fecal immunochemical tests (FIT) to patients in the pharmacy. The Consolidated Framework for Implementation Research (CFIR) was used to guide analysis.
Results. Pharmacists believed that delivering FITs was highly compatible with their environment, workflow, and scope of practice. While knowledge about FIT eligibility criteria varied, pharmacists felt comfortable screening patients. They identified standardized eligibility criteria, patient-facing educational materials, and continuing education as essential design features. Pharmacists proposed adapting existing pharmacy electronic health record systems for patient reminders/prompts to facilitate FIT completion. While pharmacists felt confident that they could discuss test results with patients, they also expressed a need for stronger communication and care coordination with primary care providers.
Discussion. When designing a pharmacy-based CRC screening program, pharmacists desired programmatic procedures to fit their current knowledge and context. Findings indicate that if proper attention is given to multi-level factors, FIT delivery can be extended to pharmacies.
1. Background
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States (US) [Citation1]. Improving CRC screening rates in the US population would have a considerable public health impact. An increase of 20% in CRC screening rates using a variety of strategies is projected to reduce CRC incidence rates by 22% and CRC mortality rates by 33% [Citation2]. Despite the efficacy and availability of screening options, low screening rates persist, with only 69% of adults up-to-date with the United States Preventive Services Task Force (USPSTF) guideline-recommended testing in 2018 [Citation3]. Inequity in CRC screening rates is apparent across populations and geography due to the prevalence of CRC risk factors and differences in access to high-quality healthcare [Citation1].
A variety of CRC screening modalities are available and recommended by the USPSTF, ranging from stool-based tests to direct-visualization tests (e.g. colonoscopy) [Citation4]. Fecal immunochemical tests (FIT) are home-based tests that identify small amounts of blood in the stool. If results are positive, a follow-up colonoscopy is required to determine if the presence of blood is due to polyp growths or cancer in the colon and rectum. FITs have several advantages: they are inexpensive, easy to store and distribute, non-invasive, can be completed from home, and have the ability to reduce CRC mortality [Citation4,Citation5]. These attributes may be beneficial in communities with limited access to primary healthcare resources or endoscopy centers.
Community pharmacies, or pharmacies located outside of health systems and within communities including retail chain and independently owned pharmacies, may be promising venues to complement current CRC screening efforts. Most US residents (90%) live within five miles of a community pharmacy [Citation6]. Medicare beneficiaries visit pharmacies about twice as often as they do their primary care providers [Citation7]. Pharmacies may also be more convenient than primary care clinics for certain preventive services as they have longer operating hours, shorter wait times, and pharmacy staff can typically see patients without appointments. Since pharmacies are the most accessible healthcare setting in the US, they could meaningfully increase community capacity for CRC screening. Pharmacies are increasingly adopting preventive care services as part of their patient care. Pharmacy-based preventive services are feasible, acceptable, and can increase access to care [Citation8]. Primary care and preventive care services routinely offered at pharmacies include point-of-care testing (e.g. COVID testing), vaccinations, and tobacco cessation services. US state governments have broadened scope-of-practice by allowing pharmacists to provide vaccinations, prescribe medication, dispense and administer naloxone (for treating opioid overdose), and prescribe hormonal contraceptives [Citation9]. Pharmacists’ expanded scope of practice taken together with the accessibility of community pharmacies supports the notion that pharmacy-based screening could significantly and equitably improve CRC screening coverage.
Currently, little research has evaluated the implementation of pharmacy-based CRC screening services in the US [Citation10,Citation11]. The objective of our study was to explore the acceptability and feasibility of pharmacy-based CRC screening among community pharmacists and to describe pharmacists’ understanding of CRC screening. We aimed to identify programmatic features and processes recommended by pharmacists to inform the design of a pharmacy-based CRC screening program using FIT kits (a.k.a., PharmFIT™ program). Further, we aimed to provide an example of the use of qualitative methods to adapt an existing EBI into a new context while centering equity.
2. Methods
2.1. Study participants
This study was conducted by researchers at the Fred Hutchinson Cancer Center (Fred Hutch; Seattle, WA) and the University of North Carolina (UNC; Chapel Hill, NC), USA. From August 2019 to January 2020, we conducted semi-structured key informant interviews by telephone and Zoom (audio only) with pharmacists practicing in community pharmacies in Washington State (WA) and North Carolina (NC). Pharmacists were eligible to participate if they had an active pharmacy license and primarily practiced in community pharmacies. We used multiple methods for recruitment: (1) community-based sampling where study staff approached pharmacists at community pharmacies; (2) snowball sampling where recruited participants would refer colleagues; (3) online recruitment through state pharmacy association membership listservs, and (4) purposive sampling where study members would refer community pharmacist colleagues to participate in the study. A total of 38 pharmacists expressed initial interest in participating in the study. Of those, 23 pharmacists (61%) completed the key informant interview. Interviews were conducted until thematic saturation was determined to have been met by the interviewers. Participants received $50 in cash or gift cards as compensation for their time.
2.2. Semi-structure interview guide and pharmacy environment survey
The semi-structured interview guide was informed by the Consolidated Framework for Implementation Research (CFIR). CFIR is an implementation science determinants framework that provides a pragmatic structure for approaching complex, interacting, multilevel constructs found in real-world settings [Citation12]. The 39 CFIR constructs were used by our team to systematically assess contextual, organizational, and individual factors that act as facilitators or barriers to the successful implementation of the PharmFIT™ program. Fred Hutch and UNC study staff (PS, DLA, RMF, CR, MW) conducted the audio-recorded interviews with participants. Interviewers were all research staff trained in qualitative interviewing and implementation science. Audio files were transcribed verbatim and timestamped by a professional transcription company [Citation13]. Interviews were an average 37 min in length.
The semi-structured interview guide was divided into three sections. In the first section, pharmacists were asked to describe their roles and responsibilities at the pharmacy and their pharmacy practice setting, such as the number and types of employees at the pharmacy, average number of prescriptions filled in a week, and types of insurances accepted at the pharmacy. We asked about their familiarity with stool-based testing for CRC and assessed knowledge of eligibility requirements for CRC screening using FIT. Participants were then provided with information about stool-based CRC testing before proceeding with the second part of the interview.
The second section of the interview gauged pharmacist’s interest in providing CRC screening at their pharmacy. We asked for their input on the pros and cons of delivering FITs through the pharmacy for eligible patients and inquired about potential facilitators and barriers to implementing this service in their pharmacy. This section also included interview questions about the acceptability and feasibility of FIT kit distribution, assessing patient eligibility, coordinating reminders for test completion, and communicating FIT results to patients and their providers. We also asked the pharmacists what type of resources (e.g. financial, staffing) they would need to successfully implement a FIT screening program in their pharmacy. In the third section, participants provided their sociodemographic characteristics which can be found in Table . At the end of each interview, the interviewer completed a debrief report summarizing the conversation and reporting any issues or feedback to be implemented in future interviews. The interview guide and debrief report can be accessed through Online Supplement (Appendix 1).
Table 1. Community pharmacist demographic characteristics.
To complement the pharmacy description, members of the research team also conducted a pharmacy site assessment survey in which they visited the pharmacies in-person and completed a standardized form or completed the form virtually at the time of the interview. Items on the form included hours of operation, type of pharmacy (e.g. independent, supermarket chain), services offered (e.g. blood pressure checks, flu shots) and structure of the pharmacy (e.g. waiting room, street parking). We also made note of the neighborhood features and surrounding areas where the pharmacy was located (e.g. in a shopping center, standalone building) and other details such as advertisements of services offered, layout of the waiting area, etc. Pharmacy characteristics can be found in Table . The pharmacy environment survey can be found in Online Supplement (Appendix 2).
Table 2. Pharmacy setting characteristics.
2.3. Qualitative data analysis
ATLAS.ti version 8 was used for data analysis and management. Transcripts were analyzed using content analysis guided by CFIR constructs from each of the five domains, and additional emergent codes were generated to capture important themes not described by CFIR [Citation14,Citation15]. The study codebook, which includes definitions of CFIR domains and constructs and illustrative examples, is found in Online Appendix 3. Coders (DLA, AI, PS) performed multiple rounds of consensus coding with two transcripts (one each from WA and NC), where the same transcript was reviewed and coded by all coders to ensure consistent code application. Following consensus of code application, the remaining 21 transcripts were independently coded by one member of the coding team (DLA, AI). After primary coding was completed, the transcripts were exchanged between coders to review the primary coding application, verify the primary coder’s assessment, or apply a disagreement code to the transcript. All disagreements were resolved through group discussion with the larger team, with disagreements resolved by consensus among the study principal investigators (AB, PS, SW). Code co-occurrence tables and queries were used to identify the CFIR constructs with the highest frequencies across all coded transcripts. Analyses included re-reading transcripts and creating matrices of CFIR constructs as they related to a pharmacists’ feedback on a proposed PharmFIT™ program. The COREQ checklist was used to report key components of qualitative research (Online Appendix 4).
3. Ethics approval and consent to participate
The Institutional Review Boards at the Fred Hutchinson Cancer Center (IRO#10229) and the University of North Carolina at Chapel Hill (IRB#18-1337) approved the study protocols. All methods were carried out in accordance with relevant guidelines and regulations at our institutions. We obtained verbal informed consent from all individuals who participated in interviews for this study.
4. Results
4.1. Pharmacist demographics, community pharmacy characteristics, & qualitative themes
A total of 23 pharmacists were interviewed, 12 (52%) from North Carolina and 11 (48%) from Washington (Table ). About half of the pharmacists were female (52%), all were non-Hispanic (100%), and a majority identified as White (70%). Pharmacists reported an average of 4.5 years in practice (SD = 2.9). The pharmacists worked in a variety of pharmacy settings (Table ), including single location independent pharmacies (43%) and Federally Qualified Health Centers (FQHCs) (26%). All the pharmacies had a parking lot (100%), and most pharmacies had a waiting room or area (83%) and private exam room (65%). The majority of pharmacies advertised clinical services including blood pressure monitoring (78%), flu vaccination (78%), HPV vaccination (57%), tobacco cessation products and counseling (48%), and in-pharmacy urgent care clinics (26%).
The qualitative analysis resulted in themes that map onto four of the CFIR domains: intervention characteristics, outer setting, inner setting, and characteristics of individuals (Figure ). Qualitative Themes and the corresponding CFIR domains include: (1) PharmFITTM program design preferences (Intervention Characteristics and Outer Setting); (2) CRC screening compatibility in community pharmacies (Inner Setting); (3) Care coordination and internal workflow considerations and concerns (Outer Setting and Inner Setting); (4) Support for CRC screening and willingness to learn (Inner Setting and Characteristics of the Individual). Qualitative themes, corresponding CFIR domains and constructs, and additional illustrative quotes can be found in Table .
Figure 1. PharmFIT™ Intervention Features and Qualitative Themes Mapped onto CFIR Domains.
Notes: PharmFIT™ Intervention: Community pharmacy-based CRC screening intervention. CFIR: Consolidated Framework for Implementation Research. CRC: Colorectal Cancer. FIT kit: Fecal Immunochemical Testing kit. PCP: Primary Care Provider.
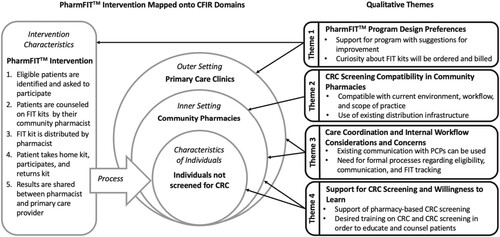
Table 3. Qualitative Themes, CFIR Domains and Constructs, and Illustrative Quotes for the Implementation of a Community Pharmacy-Based CRC Screening Intervention: PharmFIT™.
4.2. Theme 1: PharmFIT™ program design preferences
Pharmacists provided recommendations for PharmFIT™ Intervention Characteristics, specifically the Design Quality and Packaging of the program as well as an Outer Setting construct, External Policies and Incentives. Pharmacists overall endorsed the design of the PharmFIT™ program but made suggestions for components of the intervention that would increase pharmacy staff’s self-efficacy in distributing the kits and patient’s willingness to use the kits. Further, pharmacists expressed general curiosity and concern about how kits would be ordered and billed in their clinical setting.
Regarding Design Quality and Packaging, most pharmacists recommended including easy to understand instructions or informational pamphlets with each FIT kit that is dispensed from the pharmacy. Pharmacists viewed a lack of patient education about the importance of CRC screening and how to complete and return kits as a barrier to kit completion. Pharmacists wanted printed materials to refer to while counseling patients about FITs both for the patient and their benefit. To increase patient awareness and demand, pharmacists also stated that they would advertise FITs at the pharmacy similarly to other services such as vaccinations and blood pressure monitoring.
I guess advertisement. Especially if this is going to be a new thing. A lot of people won’t know about it. Advertising making it more accessible to patients. Having them know that this is option instead of having to make appointment with the doctor’s office you can just go to the pharmacy, get a kit, and get this test done. – Staff pharmacist at retail chain pharmacy in WA
I could see it depending on how it’s screened. If it’s purely patient pay, I think they’d be more comfortable with it, but if it’s something like having to bill their insurance, I think a lot of people just kind of get stressed out about it [insurance]. – Pharmacy manager at retail chain pharmacy in NC
4.3. Theme 2: CRC screening compatibility in community pharmacies
The second theme revolved around the CFIR domain of the Inner Setting, characterized as the community pharmacy itself where the pharmacists practiced. Pharmacists believed that FIT kit distribution was compatible with their environment, workflow, scope of practice, and values in providing healthcare to their communities, often drawing comparisons to established vaccination and point-of-care testing programs in which eligibility is regularly assessed prior to counseling or delivery of a patient care service. In fact, two pharmacists, one independent pharmacy and one FQHC pharmacy, shared that they were already distributing FIT kits at their pharmacies.
Just thinking from that [vaccination] perspective … when we give vaccinations, we have to do similar processes. You know looking at age and comorbidities and history and things like that. So, I think that we definitely have a lot of that information readily available … and if we don’t, we are able to ask the patient … So, I think that [providing FIT kits] would be pretty feasible. – Floating staff pharmacist at retail chain pharmacies in NC
We could do free delivery on [the FIT kits]. Like [to] those retirement communities … we could even schedule with them and go out there for an hour or two and set up a booth at some point and generate some interest. And also, plan a time to get the kits to them, whether it be delivery or set up a time where … [community members] come pick up [their] packets … there’s different ways we could do it. But definitely pick up from the store, we could deliver the packets, we could set up a table or a booth at some of these retirement communities … I think those would all be viable options. – Independent pharmacy pharmacist and owner in NC
Pharmacists also stated that the available resources at the pharmacy (e.g. personnel, infrastructure) could aid in the implementation of a PharmFIT™ program. Most pharmacists mentioned that their pharmacy staff would not mind offering a new service such as CRC screening with FIT kits. While some pharmacists expressed that the implementation climate may create challenges to adopting and integrating a PharmFIT™ program at their pharmacy, such as changes to personnel time or pressure put on the pharmacy’s workflow to support the screening program, none of these concerns were perceived as insurmountable.
I really don’t think [the pharmacy staff] would mind [offering a new CRC screening service in the pharmacy] at all. The staff that we have now all kind of understand why we’re here and we’re at an independent pharmacy because we believe in patient care and access to healthcare. – Independent pharmacy manager and owner in NC
4.4. Theme 3: Care coordination and internal workflow considerations and concerns
The third theme bridges the Outer Setting and Inner Setting, focusing on the CFIR constructs of Cosmopolitanism and Networks and Communication. Responses varied among pharmacists when asked about how they would follow-up with patients and primary care providers if they needed to report abnormal FIT results. Most participants identified faxes and phone calls as the primary means of communicating results to providers, while some pharmacists insisted that a phone call would be the best way to communicate results due to the potentially distressing information of a positive FIT test.
You know, if they have a positive test result, a lot of people might be very concerned, and you can comfort them and let them know they need to follow up with the primary care doctor and just try to answer their questions to the best of my knowledge. – Independent pharmacy pharmacist and owner in WA
If it was a positive, I would probably call and speak with somebody [at the primary care provider’s office] to let them know. If it was negative, probably sending a fax [to the PCP office] would be just fine to add to their chart. – Clinical pharmacist at FQHC in WA
So, [if an abnormal result comes back and the patient has no primary care provider] there are local providers we work with. I could reach out to them and see if we wanted to setup a system where I refer them, if they wanted referrals for that [abnormal FIT results]. – Independent pharmacy manager and owner in NC
I think … working through what … that process look like and what is an efficient workflow to do [implement PharmFIT™]. And the follow-up and keeping track of which patients received those kits … What does that documentation and procedure look like potentially if the pharmacy were … the ones identifying or just providing those kits to patients? – FQHC pharmacy manager and pharmacist in NC
Pharmacy technicians, I think, will have a big role in this. They help us in identifying our patients as well as taking on the majority of the billing aspects of medications … I think interns and residents would also be able to focus on that clinical aspect of identifying patients and depending on their level of training, assessing and recommending, the [PharmFIT™] program to them. – Pharmacist manager at retail chain pharmacy in WA
4.5. Theme 4: Support for CRC screening and willingness to learn
The third theme focuses on the CFIR domains of Inner Setting or pharmacists’ access to knowledge and information about CRC screening and Characteristics of the Individual or their existing knowledge and beliefs about screening as it related to the PharmFIT™ program. Most pharmacists strongly endorsed the idea of distributing FIT kits in their pharmacy. They also expressed wanting education on how to appropriately assess patients CRC screening eligibility (including prior screening history and risk factors). Further, pharmacists acknowledged that not all staff in the pharmacy would be ready to add a new service to their workflow, thus they recommended training and information that emphasized the importance of CRC screening to pharmacy staff.
Pharmacists believed that increasing access to CRC screening was an important public health priority and they could easily see themselves participating in the PharmFIT™ program. Yet, further clarification about CRC screening in general was requested by many pharmacists. Most pharmacists perceived counseling on FIT kits as feasible if they are adequately trained on CRC screening modalities and guidelines. Pharmacists recommended also having access to reliable external support (e.g. online resources, physicians) in case questions arise during counseling.
It seems pretty easy and straightforward to implement, it doesn’t seem like it would be a significant burden to any of our staff. If they’re given the appropriate training, they should be able to handle it no problem. Again, being independent we’re always looking for ways that we can expand what we do and what we’re able to offer to the community to provide another level of healthcare service and resource. – Independent pharmacy pharmacist and owner in WA
I think if we had a guideline of the questions to ask, that’s really all we would need. Also, a way or a resource that we could use in case we did have questions that weren’t answered on any materials that we already had. – Independent pharmacy pharmacist and owner in NC
I think it would be nice to have some kind of protocol developed with either, a doctor’s office or, with you guys [the research team] that’s distributing the kit. The protocol being very specific about if the patient has these conditions, you wouldn't give it [FIT kit] to them; you would just refer them to the doctor’s office. – Staff pharmacist at retail chain pharmacy in WA
In terms of data, how many patients experience colorectal cancer and I’ll say fatalities may be a bit much, but I mean then there are still statistics that can be eye-opening to patients. I don’t want to use a scare tactic, by any means, but to provide them data I think is always helpful to see the importance of what we’re actually doing and trying to do. – Staff pharmacist at independent pharmacy in NC
5. Discussion
To our knowledge, this is the first qualitative study to assess pharmacists’ attitudes towards and perspectives of implementing a pharmacy-based CRC screening program in community pharmacies in the U.S. In this formative study, we described pharmacists’ understanding of CRC screening and their recommendations for designing and implementing the PharmFIT™ program. The qualitative findings can help prioritize implementation considerations to promote successful adaptation and integration of existing an EBI (CRC screening using FIT) into the community pharmacy setting. Three general implementation considerations include: (1) leveraging existing infrastructure in the pharmacy to support the distribution of FIT kits; (2) providing adequate CRC screening and FIT kit training for pharmacy staff; and (3) establishing communication systems between pharmacies and primary care providers to ensure appropriate care coordination for patients.
5.1. Leverage existing community pharmacy infrastructure
Most pharmacists in our study reported that the PharmFIT™ program was compatible with their existing environment, workflow, scope of practice, and values, often drawing comparisons to other services that are within the growing pharmacy scope of practice. Particularly, many pharmacists commented on the similarity of delivering FITs to vaccine provision at the pharmacy. Expanding pharmacy services to include vaccination has resulted in an increase in influenza and pneumococcal vaccination rates across the US [Citation16], and has been identified as an important mechanism to increase human papillomavirus vaccination [Citation17]. In fact, vaccinations have become so ingrained in pharmacy practice that pharmacies have become a crucial access point for COVID-19 vaccination and control of other infectious diseases in the US and globally [Citation18,Citation19].
Further, infrastructure that allowed for prior implementation and success of other preventive services, such as vaccinations, should be leveraged to implement CRC screening in the pharmacy setting. These existing infrastructures may include standards for written materials, advertising for clinical services, clinical databases, ordering and billing, tracking and reminder systems used for dispensing, and prescription distribution mechanism such as mail order or home delivery. While considering infrastructure already in place at a given pharmacy, the pharmacy personnel may necessarily need to tailor the PharmFIT™ program to their specific pharmacy setting.
5.2. Establish and maintain communication systems with primary care providers
Finally, CRC screening programs that have the greatest success take into account the stepwise cascade that occurs from the point of the initial screening examination to the timely receipt of any necessary diagnostic follow-up and treatment [Citation20]. As such, pharmacies that wish to implement a CRC screening program should prioritize establishing stronger ties with surrounding primary care clinics for patient care coordination. Many pharmacists were unsure what steps to take after being asked how they would handle abnormal FIT results that would require a follow-up colonoscopy. Unlike most other preventive patient care services provided by pharmacies, CRC screening requires a follow-up colonoscopy after an abnormal FIT result that can only be feasibly obtained in healthcare systems that have endoscopy centers. Thus, community pharmacies will need to develop stronger communication with primary care and with and referral networks to ensure appropriate patient care coordination.
Additionally, beyond establishing collaborative working relationships between pharmacies and primary care settings, our findings align with current literature that illustrates the care coordination between community pharmacists and primary care physicians often occurs through low-technology systems such as fax and phone calls and is at times difficult to establish [Citation21]. Ideally, follow-up care for a patient between a pharmacy and primary care clinic should be coordinated electronically. Since the enactment of the Health Information Technology for Economic and Clinical Health (HITECH) through the American Recovery and Reinvestment Act in 2009, significant gains have been made across healthcare settings in adopting electronic health record (EHR) systems [Citation22]. While most healthcare settings use EHRs, interoperability (i.e. the harmonious exchange of essential health information data between two electronic systems) has lagged significantly due to a lack of policy and financial incentives and industry collaboration [Citation22]. While progress towards EHRs inoperability is being made for pharmacies [Citation23], pharmacies interested in implementing a CRC screening program can still build professional connections with their primary care counterparts in their communities.
However, this study was focused on describing pharmacist recommended programmatic features and processes to inform the design of a pharmacy-based CRC screening program. Less focus was given to care pathways to follow-up colonoscopy among those who receive an abnormal FIT result. Low follow-up colonoscopy rates after FIT continues to be a problem across screening initiatives [Citation24]. Therefore, beyond formalizing communication systems with primary care providers, pharmacies who implement PharmFIT™ may benefit from integrating services that have been shown to improve abnormal FIT kit follow-up such as patient navigation [Citation25]. Future research would benefit from understanding how services such as patient navigation could be achieved in the PharmFIT™ model to avoid widening existing colonoscopy follow-up inequities.
5.3. Provide adequate CRC screening training to community pharmacy staff
A lack of training and education on CRC screening was perceived as a highly modifiable barrier to the implementation of the PharmFIT™ program by pharmacists. For the most part, pharmacists are not trained to counsel patients and deliver CRC screening. They and other support personnel (e.g. intern pharmacists, pharmacy technicians) would require comprehensive training to effectively identity eligible individuals (including prior screening history and risk factors), communicate the importance of CRC screening, and counsel them on self-administered FITs. This finding is consistent with the literature on pharmacist-delivered tobacco cessation programs in which pharmacists’ lack of training and counseling self-efficacy were barriers to the successful implementation of pharmacist-delivered tobacco cessation program [Citation26,Citation27]. However, once feasibility concerns were raised and education was received, pharmacist-delivered smoking cessation programs have been able to produce cessation rates as high as 77% [Citation28,Citation29]. Although educational barriers were identified in our findings, the literature suggests that these barriers may be easily addressable [Citation30]. One clear solution to standardize pharmacy training in CRC screening would be through knowledge-, application-, or practice-based continuing pharmacy education (CPE) [Citation31]. As such, a strategy to increase wider adoption of pharmacy-based CRC screening programs would be through CPE programs provided by state and national pharmacy associations that also instruct pharmacists on CRC screening program implementation.
5.4. Contributions to the implementation science literature
This study provides an example of using qualitative methods to collect perspectives on delivering an existing EBI (i.e. CRC screening through FIT) through a new context (i.e. community pharmacies) while centering on equitable access to a cancer prevention service – adding to a fundamental gap in the screening implementation literature [Citation32,Citation33]. Our study investigated pharmacy’s organizational culture and staff support for implementing an adapted intervention (i.e. PharmFIT™), commonly reported barriers to successful implementation of EBIs, further illustrating how qualitative methods can be used to guide implementation [Citation34]. Further, our study adds to a much needed implementation science literature focused on equity-based intervention adaptation that acknowledges that the success of an intervention relies on a complex interaction among multi-level factors, not just on the population of interest [Citation32,Citation33].
5.5. Strengths and limitations
This study is the first of its kind to evaluate, in-depth, pharmacists’ perceptions of implementing CRC screening services in community pharmacies in the US. This study benefits from including pharmacists who practiced in diverse community pharmacy settings in Washington state and North Carolina. Further, the use of the Consolidated Framework for Implementation Research (CFIR) provides a universally accessible and adaptable approach for future pharmacy-based CRC screening qualitative research. The current study also has limitations to consider. First, the findings of this study may not be directly comparable to literature outside of the US nor generalizable to health systems outside of the US due to difference in how primary care and pharmacies are structured and financed [Citation35–37]. Further, this analysis focused on the perceptions of community pharmacists in the US, however, primary care and patient perspectives on the PharmFIT™ program can be found elsewhere [Citation38,Citation39]. Second, thematic interpretations that we identified could differ among other researchers. However, great care was taken to strengthen this study’s methodological approach by cognitively interviewing [Citation40] community pharmacists to ensure our interview guide was understandable as intended by our research team and involving researchers who are experts in pharmacy practice, CRC screening interventions, qualitative research methods, and health services research in all stages of the qualitative analysis. Third, we relied on a convenience sample of pharmacists who may not represent the general views of pharmacists practicing across the country. Future studies on pharmacists’ attitudes towards and perceptions of pharmacy-based CRC screening services would benefit from additional qualitative studies conducted in other regions of the US and quantitative research approaches such as national surveys. Another limitation of our study was the lack of inquiry about reimbursement or compensation structures to sustain the PharmFIT™ program at pharmacies after implementation. Future studies will need to address this sustainability challenge as financial compensation, especially as it aligns with population health metrics and goals for both primary care practices and pharmacies, is an important consideration for pharmacists in adopting and integrating new patient care services [Citation41].
6. Conclusion
Drivers of low CRC screening uptake in the United States are well known and have been known for the last decade [Citation42]. Previous research has firmly established the importance of increasing access to CRC screening modalities as an important determinant of their use among medically underserved populations. Pharmacies are underused for cancer prevention and control services and could be an effective healthcare setting to expand CRC screening services, thereby increasing community screening coverage. In our study, we obtained feedback from community pharmacists on ways to optimize implementation of pharmacy-based CRC screening services. As a result, we advise researchers and practitioners to consider three recommendations when designing and implementing a PharmFIT™ program: (1) leveraging existing pharmacy infrastructure to seamlessly offer CRC screening, (2) providing CRC screening training to community pharmacy staff, and (3) establishing and maintaining strong community pharmacy-primary care provider relationships to ensure patient care coordination. Overall, community pharmacists perceive CRC screening as compatible with their pharmacy setting, communicated some concerns about a need for training and care coordination, but overall supported CRC screening. Further our findings suggest that adapting EBI’s into new contexts through qualitative methods has the potential to acknowledge multi-level factors that influence intervention effects and can be used to center equity.
Abbreviations
| CRC | = | Colorectal cancer |
| FIT | = | fecal immunochemical tests |
| CFIR | = | Consolidated Framework for Implementation Research |
| EBI | = | evidence-based intervention |
| US | = | United States |
| USPSTF | = | United States Preventive Services Task Force |
| WA | = | Washington |
| NC | = | North Carolina |
Authors’ contributions
All authors contributed to the study conception and design. Study was conceptualized and lead by PDS, ATB, and SBW. Material preparation and data collection were performed by DLA, AAI, RMF, CLR, MW, RMC, RBI, DR, SBW, ATB, PDS. Data analyses were performed by DLA, AAI, PDS, ATB, SBW, ARW. The first draft of the manuscript was written by ARW, KM, and PDS and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Review Boards at the Fred Hutchinson Cancer Center (IRO#10229) and the University of North Carolina at Chapel Hill (IRB#18-1337) approved the study protocols.
Consent to publish
Not applicable, no identifiable data presented.
Availability of data and material
Data may be available upon reasonable request to the corresponding author.
Supplemental Material
Download MS Word (36.3 KB)Supplemental Material
Download MS Word (36.7 KB)Supplemental Material
Download MS Word (35.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020 May;70(3):145–164.
- Meester RGS, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015 Jul 1;121(13):2281–2285.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use – United States, 2018. MMWR Morb Mortal Wkly Rep. 2020 Mar 13;69(10):253–259.
- Lin JS, Perdue LA, Henrikson NB, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021 May 18;325(19):1978–1998.
- Bakhai S, Ahluwalia G, Nallapeta N, et al. Faecal immunochemical testing implementation to increase colorectal cancer screening in primary care. BMJ Open Qual. 2018 Oct 25;7(4):e000400.
- Berenbrok LA, Tang S, Gabriel N, et al. Access to community pharmacies: a nationwide geographic information systems cross-sectional analysis. J Am Pharm Assoc (2003). 2022 Jul 15;62(6):1816–1822.e2.
- Berenbrok LA, Gabriel N, Coley KC, et al. Evaluation of frequency of encounters with primary care physicians vs visits to community pharmacies among medicare beneficiaries. JAMA Netw Open. 2020 Jul 1;3(7):e209132.
- San-Juan-Rodriguez A, Newman TV, Hernandez I, et al. Impact of community pharmacist-provided preventive services on clinical, utilization, and economic outcomes: an umbrella review. Prev Med. 2018 Oct;115:145–155.
- Goode JV, Owen J, Page A, et al. Community-based pharmacy practice innovation and the role of the community-based pharmacist practitioner in the United States. Pharmacy (Basel). 2019 Aug 4;7(3):106. doi: 10.3390/pharmacy7030106
- Potter MB, Ackerson LM, Gomez V, et al. Effectiveness and reach of the FLU-FIT program in an integrated health care system: a multisite randomized trial. Am J Public Health. 2013 Jun;103(6):1128–1133.
- Holle LM, Levine J, Buckley T, et al. Pharmacist intervention in colorectal cancer screening initiative. J Am Pharm Assoc (2003). 2020 Mar 18;60(4):e109–e116.
- Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009 Aug 7;4:50.
- GMR Transcription. GMR Transcription [Internet]. [cited 2022 Nov 7]. Available from: https://www.gmrtranscription.com/.
- Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008 Apr;62(1):107–115.
- Breimaier HE, Heckemann B, Halfens RJG, et al. The consolidated framework for implementation research (CFIR): a useful theoretical framework for guiding and evaluating a guideline implementation process in a hospital-based nursing practice. BMC Nurs. 2015 Aug 12;14:43.
- Isenor JE, Edwards NT, Alia TA, et al. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine. 2016 Nov 11;34(47):5708–5723.
- Approved by the National Vaccine Advisory Committee on June 25, 2018. Strengthening the effectiveness of national: state, and local efforts to improve HPV vaccination coverage in the United States: recommendations from the national vaccine advisory committee. Public Health Rep. 2018 Aug 9;133(5):543–550.
- Czech M, Balcerzak M, Antczak A, et al. Flu vaccinations in pharmacies-a review of pharmacists fighting pandemics and infectious diseases. Int J Environ Res Public Health. 2020 Oct 29;17(21):7945. doi: 10.3390/ijerph17217945
- Hess K, Bach A, Won K, et al. Community pharmacists roles during the COVID-19 pandemic. J Pharm Pract. 2022 Jun;35(3):469–476.
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021 May 18;325(19):1965–1977.
- Renfro CP, Robinson JM, Turner K, et al. Care coordination and follow-up practices in the community pharmacy setting: a mixed methods study. J Am Pharm Assoc (2003). 2020 Jan 7;60(4):631–638.e2.
- Reisman M. EHRs: the challenge of making electronic data usable and interoperable. P T. 2017 Sep;42(9):572–575.
- Pharmacy Health Information Technology Collaborative. Pharmacy health information technology collaborative [Internet]. [cited 2022 Nov 15]. Available from: https://www.pharmacyhit.org/.
- Green BB, Baldwin L-M, West II, et al. Low rates of colonoscopy follow-up after a positive fecal immunochemical test in a medicaid health plan delivered mailed colorectal cancer screening program. J Prim Care Community Health. 2020 Jan-Dec;11:2150132720958525. doi: 10.1177/2150132720958525
- O’Leary MC, Reuland DS, Correa SY, et al. Uptake of colorectal cancer screening after mailed fecal immunochemical test (FIT) outreach in a newly eligible 45-49-year-old community health center population. Cancer Causes Control. 2023 Dec;34(Suppl. 1):125–133.
- Hudmon KS, Prokhorov AV, Corelli RL. Tobacco cessation counseling: pharmacists’ opinions and practices. Patient Educ Couns. 2006 Apr;61(1):152–160.
- Purcell JL, Farris KB, Aquilino ML. Feasibility of brief smoking cessation intervention in community pharmacies. J Am Pharm Assoc (2003). 2006 Oct;46(5):616–618.
- Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007 Jul;27(7):1040–1051.
- O’Reilly E, Frederick E, Palmer E. Models for pharmacist-delivered tobacco cessation services: a systematic review. J Am Pharm Assoc (2003). 2019 Jul 12;59(5):742–752.
- Hudmon KS, Kroon LA, Corelli RL, et al. Training future pharmacists at a minority educational institution: evaluation of the Rx for change tobacco cessation training program. Cancer Epidemiol Biomarkers Prev. 2004 Mar;13(3):477–481.
- Wheeler JS, Chisholm-Burns M. The benefit of continuing professional development for continuing pharmacy education. Am J Pharm Educ. 2018 Apr;82(3):6461.
- Movsisyan A, Arnold L, Evans R, et al. Adapting evidence-informed complex population health interventions for new contexts: a systematic review of guidance. Implement Sci. 2019 Dec 17;14(1):105.
- Brownson RC, Kumanyika SK, Kreuter MW, et al. Implementation science should give higher priority to health equity. Implement Sci. 2021 Mar 19;16(1):28.
- Bach-Mortensen AM, Lange BCL, Montgomery P. Barriers and facilitators to implementing evidence-based interventions among third sector organisations: a systematic review. Implement Sci. 2018 Jul 30;13(1):103.
- Vives N, Milà N, Binefa G, et al. Role of community pharmacies in a population-based colorectal cancer screening program. Prev Med. 2021 Apr;145:106420.
- Della Valle PG, Deandrea S, Battisti F, et al. The community pharmacy model for colorectal cancer screening: policy insights from a national programme. Res Social Adm Pharm. 2023 Dec;19(12):1595–1601.
- Lindsey L, Husband A, Nazar H, et al. Promoting the early detection of cancer: a systematic review of community pharmacy-based education and screening interventions. Cancer Epidemiol. 2015 Oct;39(5):673–681.
- Brenner AT, Rohweder CL, Wangen M, et al. Primary care provider perspectives on the role of community pharmacy in colorectal cancer screening: a qualitative study. BMC Health Serv Res. 2023 Aug 23;23(1):892.
- Ferrari RM, Atkins DL, Wangen M, et al. Patient perspectives on a proposed pharmacy-based colorectal cancer screening program. Transl Behav Med. 2023 Dec 15;13(12):909–918. doi: 10.1093/tbm/ibad057
- Beatty PC, Willis GB. Research synthesis: the practice of cognitive interviewing. Public Opin Q. 2007 Jun 5;71(2):287–311.
- Houle SKD, Grindrod KA, Chatterley T, et al. Paying pharmacists for patient care: a systematic review of remunerated pharmacy clinical care services. Can Pharm J (Ott). 2014 Jul;147(4):209–232.
- Post DM, Katz ML, Tatum C, et al. Determinants of colorectal cancer screening in primary care. J Cancer Educ. 2008;23(4):241–247.

