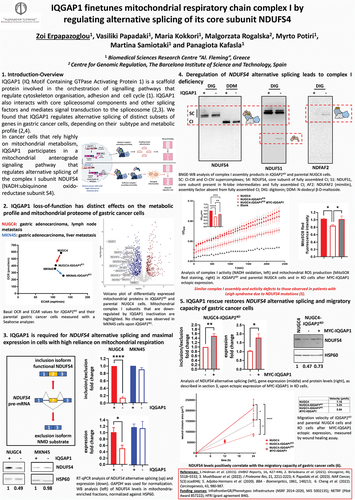
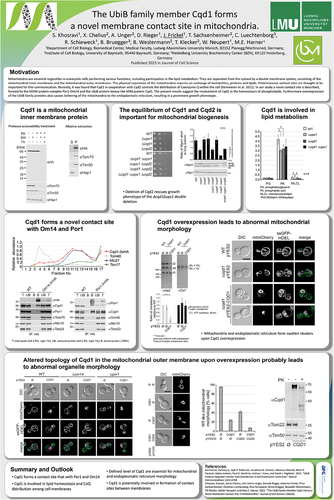
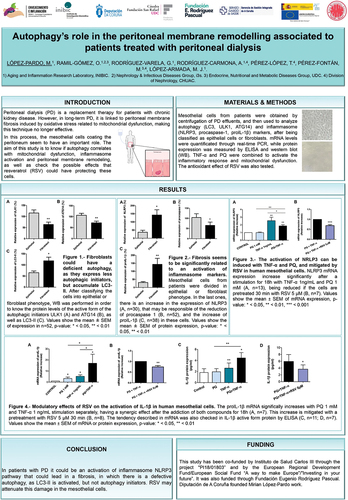
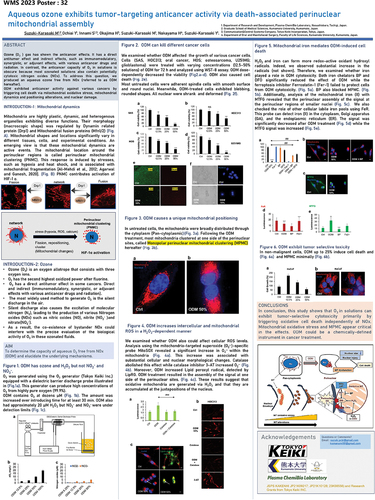
Vladimir Skulachev’s Strategic Impact on mitochondrial medicine: a tribute to his vision, discoveries, and legacy
Gogvadze, Vladimir
Division of Toxicology. Karolinska Institutet, Stockholm, Sweden
Looking back, it is impossible to imagine the development of the mitochondrial field without Prof. Vladimir Skulachev. This presentation is a tribute to this outstanding scientist who made an enormous contribution to the study of cell bioenergetics, primarily mitochondria, and outlined new strategies in medical mitochondriology. His wide-ranging interests included the mechanisms of biological oxidation, oxidative stress and antioxidants, investigation of processes of ageing and how to delay it, mitochondria-mediated modes of cell death. Since 2005, he headed a project aiming to create a geroprotective medications based on mitochondria-targeted antioxidants.
In 1969, Vladimir Skulachev, together with Efim Lieberman, obtained the first experimental proof of Mitchell’s chemiosmotic theory. Using lipophilic cations and anions, they showed that energised mitochondria can accumulate cations, while submitochondrial particles - anions. In addition to the importance of the results obtained, in this work, the term “protonophore” was first introduced into scientific literature, which is successfully used to this day. This publication was received with great interest by the scientific community and in recognition of its importance these ions were named “Skulachev ions” by the famous American biochemist David Green. For this work, in 1975 Vladimir Skulachev was awarded the State Prize of the USSR.
Of note the use of Skulachev ions was not only of fundamental, but also of practical importance. Exploiting of positively charged tetraphenylphosphonium, which is capable of accumulating in energised intact mitochondria, made it possible to direct compounds of interest to mitochondria. This finding opened a new page not only in mitochondrial investigations, but also in mitochondrial medicine. In 1999 Prof. Skulachev proposed a conception of the self-programmed death of an organism or Phenoptosis and put forward the hypothesis how to combat the process of ageing using antioxidants linked to positively charged Skulachev ion.
Vladimir Skulachev was a Full member of the Russian Academy of Natural Sciences, member of the European Academy and president of the club of its Russian branch, president of the All-Russian Biochemical Society, honorary president of the All-Russian Society of Biochemists and Molecular Biologists, full member of the Academy of Creativity; doctor honoris causa of Vilnius University, Member of the Academia Europaea.
Notes on contributor
Vladimir Gogvadze graduated in 1973 from the Tbilisi State University, USSR, in 1984 he obtained PhD in biology from the Institute of Biological Physics, Pushchino, Russia, and Doctor of Sciences degree from the Institute of Theoretical and Experimental Biophysics in 2002. Currently, he is an Associate Professor at Karolinska Institutet, Stockholm, Sweden. From 2011, he is a leading scientist at the Laboratory of Apoptosis Investigation, Faculty of Medicine, the Moscow State University, Russia. His scientific interests include mitochondria and their involvement in various modes of cell death, an interplay between different cell death modalities, oxidative stress.
The Vladimir Skulachev vision: advances in the development of mitochondria-targeted pharmaceuticals
Maxim Skulachev
Mitotech Pharma, Luxembourg
In the last 15 years of his life and scientific career, Vladimir Skulachev dedicated himself to his project on practical application of penetrating ions. This ambitious endeavour focuses on developing new pharmaceuticals based on mitochondria-targeted antioxidants of the SkQ class.
Our leading compound, SkQ1, is currently undergoing extensive development for different indications and in various pharmaceutical forms, including eye drop formulations (which have reached the third stage of clinical trials in the US), as well as oral and injectable formulations designed to tackle systemic indications such as Multiple Sclerosis and NASH.
In this presentation, I would like to share our recent findings from preclinical studies we completed using the latter formulations, where we successfully harnessed both the antioxidant and mild uncoupling properties of SkQ1 molecule.
Notes on contributor
Maxim Skulachev is the chief scientific officer of Mitotech, a UK based biotech company, which R&D programmes are focused on the development of mitochondrially targeted pharmaceuticals. Dr. Skulachev is also scientific supervisor of Mitolab company, Israel. It specialises in drug development contract research related to mitochondrial studies.
Cholesterol: why have mitochondrial biologists ignored this critical mitochondrial component for over a century?
Mikel Muñoz-Oreja, Abigail Sandoval, Ove Bruland, Diego Perez-Rodriguez, Uxoa Fernandez-Pelayo, Marina Villar-Fernandez, Amaia Lopez De Arbina, Ixiar Hernández, Haizea Hernández, Yohan Park, Itxaso Martí-Carrera, Mazahir T. Hasan, Matthew E. Gegg, Cecilie, Bredrup Per-Morten Knappskog, Gorka Gereñu, Kristin N. Varhaug, Laurence A. Bindoff, Antonella Spinazzola, Wan Hee Yoon and Ian J. Holt
Instituto de Investigación Sanitaria Biodonostia, Spain
As Michael Brown noted in his 1985 Nobel Lecture: Cholesterol is the most highly decorated small molecule in biology. However, cholesterol’s contribution to mitochondrial membranes has attracted little interest, as they are ‘cholesterol-poor organelles’ with 0.5-3% of the content found in the plasma membrane. And although high cholesterol has been linked to mitochondrial dysfunction, this merely implied that mitochondria have an aversion to cholesterol. Our first forays into this field came with the unexpected discovery that pathological mutant forms of the trans-mitochondrial membrane protein, ATAD3, completely reconfigure cellular cholesterol metabolism (Desai et al., 2017; Gunning et al., 2020).
Here, I will report the central role of cholesterol in the ATAD3 disease cascade, and crucially show that the molecular phenotypes stem from the mitochondrion’s absolute requirement for cholesterol.
References
Desai R, et al. 2017. ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism. Brain. 140:1595-1610.
Gunning AC, et al. 2020. Recurrent De Novo NAHR reciprocal duplications in the ATAD3 gene cluster cause a neurogenetic trait with perturbed cholesterol and mitochondrial metabolism. Am J Hum Genet. 106:272–279.
Brain organoids to model mitochondrial neurological diseases
Alessandro Prigione
Department of General Pediatrics, Neonatology, and Pediatric Cardiology, University Clinic Düsseldorf (UKD), Heinrich Heine University (HHU)
Energy metabolism is essential for providing the energy necessary to ensure proper cellular function. Mutations in genes regulating this process lead to inherited metabolic disorders that can particularly affect tissues with high energy demands like the brain. Among incurable inherited metabolic diseases, mitochondrial diseases represent a major therapeutic challenges, as that they can be caused by mutations in genes that are encoded by either the mitochondrial DNA (mtDNA) or the nuclear DNA (nDNA). Given the challenges associated with mtDNA engineering, there is a lack of effective model systems for screening and testing drugs.
In this talk, I will summarise our efforts in using patient-derived and engineered induced pluripotent stem cells (iPSCs) to study mitochondrial diseases. We focus primarily on Leigh syndrome, which is the most frequent and most severe mitochondrial disease affecting 1/40,000 newborns. We show that neuronal cultures and brain organoids derived from Leigh syndrome iPSCs can be used as model systems to investigate the neuropathological mechanisms and to carry out phenotypic compound screenings. Our data pave the way to the identification of disease-modifying therapies for currently incurable mitochondrial disorders.
Notes on contributor
Alessandro Prigione is a tenured Associate Professor of Pediatric Metabolic Medicine in the Department of General Pediatrics at Heinrich Heine University in Düsseldorf, Germany. His lab employs induced pluripotent stem cells (iPSCs) and derived neurons and brain organoids for disease modelling and drug discovery of rare mitochondrial neurological disorders. Dr. Prigione is the coordinator of the CureMILS EJPRD Consortium, a member of the scientific council of AFM Telethon, of the Scientific Committee of Mitocon and Cure Mito, and the current Editor-in-Chief of the journal Stem Cell Research.
The power of epigenetics: the patterns of mitoDNA methylation transmitted across generations
Marc-André Sirarda and Camila Bruna De Limab
aCentre de Recherche en Reproduction, Développement et Santé Intergénérationnelle (CRDSI); bFaculté des sciences de l’agriculture et de L’alimentation, Université Laval, Québec, Canada
The mammalian oocyte has unique mitochondria (mt) that are inherited exclusively from the female side and transmitted to the future embryo by passive segregation. The morphology and structures of the oocyte mt are associated with an inactive state although, numerous studies indicate that they play an important role in the cytoplasmic and nuclear maturation (meiosis) that precedes ovulation and fertilisation. Studies have shown that their morphology and functions can be altered by the mother’s diet or other metabolic environments Including in vitro culture resulting in the observation of mt dysfunction in the early embryo and potentially downstream effects associated with improper metabolic programming. The mt activities in the early embryo also influence several epigenetic processes such as DNA methylation and histone acetylation to name a few. We have used the bovine model as it is the most useful to compare with humans when we explore the conditions relate to in vitro fertilisation (IVF). To explore the epigenetic legacy, our laboratory has explored oocyte genomic (g) DNA and mtDNA methylation of the mt genome in association with mt gene expression and indirectly with genomic DNA programming of genes involved in the mt function (>1000). Our results indicate that contrarily to gDNA, mt DNA shows cytosine methylation outside of the CpG context and show a quite unique pattern in oocytes and early embryos compared to somatic tissues. This pattern is associated with oocyte quality and results in the modulation of mt specific gene expression. Indeed, the presence of methylation is associated with lower expression when we look at the average methylation for a specific gene. But we have also observed that some mtDNA seems to have much more methylation than others, supporting the hypothesis that the oocyte and early embryo may have a subpopulation of mt that are protected from being active by increased DNA methylation. In the mouse such observation has led scientist to believe that the methylation may act as a physical barrier against reactive oxygen species (ROS) while we believe that it may act through reduced expression and function of the electron chain complex. Microscopic observations also indicate two populations of mt in oocytes, often in different regions of the cell. In conclusion, our data indicate that oocyte mtDNA may carry metabolic information and display a new form of heteroplasmy based on DNA methylation patterns.
WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
Paul Hwang
National Heart, Lung and Blood Institute, USA
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a disorder characterised by various disabling symptoms including exercise intolerance. We report that overexpression of Wiskott-Aldrich Syndrome Protein Family Member 3 (WASF3), here identified in a 38-y-old woman suffering from long-standing fatigue and exercise intolerance, can disrupt mitochondrial respiratory supercomplex formation. Increased expression of WASF3 in transgenic mice decreased their treadmill running capacity and specific respiratory complexes.
Expanding on our findings in a single patient, skeletal muscle biopsy samples obtained from a cohort of patients with ME/CFS compared with healthy volunteers showed increased WASF3 protein levels associated with aberrant ER stress activation. Pharmacologic inhibition of ER stress decreased WASF3 and improved mitochondrial function in the cells of the patient with chronic fatigue, suggesting a therapeutic strategy for ME/CFS treatment.
Notes on contributor
Paul Hwang earned B.A. degrees in biochemistry and chemistry from the University of Kansas in 1985, after which he spent a year at the Swiss Federal Institute of Technology and University of Zurich as a Fulbright Scholar. He graduated from the Johns Hopkins University School of Medicine with an M.D. and Ph.D in 1993. He did his internship and residency in internal medicine at the UCSF School of Medicine in San Francisco, followed by a clinical fellowship in cardiology and postdoctoral research in molecular oncology at the Johns Hopkins University School of Medicine. Upon completion of his training in 2001, Dr. Hwang joined the NHLBI-NIH as an investigator and was tenured in 2011. He has been elected as member of the American Society for Clinical Investigation and fellow of the American College of Cardiology.
Defining the molecular nature of the mitochondrial permeability transition pore(s)
Paolo Bernardi
Department of Biomedical Sciences, University of Padova, Italy
Major progress has been made in defining the basis of the mitochondrial permeability transition, a Ca2+-dependent permeability increase of the inner membrane that has puzzled mitochondrial research for almost 70 years. Initially considered an artefact of limited biological interest by most, over the years the permeability transition has raised to the status of regulator of mitochondrial ion homoeostasis and of druggable effector mechanism of cell death. The permeability transition is mediated by opening of channel(s) modulated by matrix cyclophilin D, the permeability transition pore(s) (PTP). The field has received new impulse from the hypothesis that the PTP may originate from a Ca2+-dependent conformational change of F-ATP synthase and from the reevaluation of the long-standing hypothesis that it originates from the adenine nucleotide translocator.
I will discuss potential mechanisms for PTP formation from F-ATP synthase and the role of the permeability transition in pathophysiology.
Notes on contributor
Paolo Bernardi is a Professor and former Chair, Department of Biomedical Sciences of the University of Padova, where he also served as deputy Dean of the Medical Faculty. His studies on the role of mitochondria in disease pathogenesis contributed substantially to developing this field. His recent molecular definition of the permeability transition pore holds great promise for the treatment of degenerative diseases like muscular dystrophies through the development of mitochondrial drugs. He earned his M.D. at the University of Padova (Italy) and completed his education in Cellular and Molecular Biology as an NIH-Fogarty Fellow at the Whitehead Institute for Biomedical Research – M.I.T.
Protein transport across mitochondrial membranes: adapting mitochondrial gene expression
Peter Rehling
Department for Cellular Biochemistry, University Medical Center Goettingen
Max Planck Institute for Biophysical Chemistry, Fraunhofer Institute for Translational Medicine and Pharmacology – TNM
Mitochondrial proteins are predominantly encoded in the nucleus and post-translationally imported into the organelle. The translocase of the outer mitochondrial membrane (TOM complex) mediates protein transport across the outer membrane. Import across the inner membrane requires one of two translocases (TIM complexes). A subset of the mitochondrial proteome however is encoded by mitochondrial DNA. These proteins are co-translationally exported across the inner membrane by the OXA1L and assemble with newly imported proteins into membrane protein complexes of the oxidative phosphorylation system.
In order to maintain mitochondrial function, the assembly of the oxidative phosphorylation system complexes from imported and mitochondria-encoded subunits has to be tightly regulated to adapted to cellular requirements. However, malfunction of these regulatory processes are linked to human disorders. Yet, our understanding of mitochondrial gene expression and proteostasis are limited due to the lack of appropriate techniques to modulate and interfere with gene expression in mitochondria. Our recent analyses provide new strategies to target mitochondrial gene expression and address so far unresolved questions of mitochondrial biology.
Notes on contributor
Peter Rehling is chair of the Department for Cellular Biochemistry at the University Medical Center Goettingen. He is also a Research Fellow at the Max Planck Institute for Multidisciplinary Science in Goettingen. His research focuses on mitochondrial gene expression processes and proteostasis.
Mitochondrial presequence protein translocation
Nils Wiedemann
Institute of Biochemistry and Molecular Biology, ZBMZ, Faculty of Medicine, University of Freiburg, Germany
Virtually all of the ~1,000 different mitochondrial proteins are synthesised in the cytosol and must be imported into the organelle. Most of these mitochondrial precursor proteins contain an amino-terminal presequence, which forms a positively charged amphiphilic alpha-helix. The TIM23 translocase sorts these presequence proteins into the inner membrane or matrix. We mapped the interaction of the essential subunit Tim17 with presequence containing precursor proteins.
Tim17 contains conserved negative charged residues close to the intermembrane space side of the inner membrane, which are essential for presequence protein translocation along a distinct transmembrane cavity of the Tim17-bilayer interface.
Notes on contributor
Nils Wiedemann is a Professor (apl.) of Biochemistry and Molecular Biology, University of Freiburg, Germany. Prof. Wiedmann won the Young Investigator Award, German Society for Biochemistry and Molecular Biology (GBM) Frankfurt in the year 2007.
Mitochondrial monitoring in perioperative and critical care: recent advances & perspectives
Egbert Mik
Erasmus MC – University Medical Center Rotterdam, The Netherlands
Mitochondrial oxygen tension (mitoPO2) can be measured by oxygen-dependent delayed fluorescence of mitochondrial protoporphyrin IX (PpIX)(Mik et al. 2006). Dynamic measurement of mitoPO2 allows direct assessment of cellular respiration in vivo (Harms et al. 2013). Use of the technique in skin allows for non-invasive monitoring of mitochondrial oxygenation and respiration (Harms et al. 2015). Based on the protoporphyrin IX technology the first clinical monitor for Cellular Oxygen METabolism (COMET) has been developed (Ubbink et al. 2017). COMET has been used in various clinical studies in several institutions (Harms et al. 2023) and further clinical trials are ongoing.
The presentation will cover the development and evaluation of the technique. Preclinical results in both animals and man will be shown and the COMET monitor will be introduced.
References
Harms FA, et al. 2013 Sep. Cutaneous respirometry by dynamic measurement of mitochondrial oxygen tension for monitoring mitochondrial function in vivo. Mitochondrion. 13(5):507–514.
Harms FA, Bodmer SI, Raat NJ, Mik EG. 2015. Non-invasive monitoring of mitochondrial oxygenation and respiration in critical illness using a novel technique. Crit Care. 19(1):343.
Harms FA, et al. 2023 Feb 9. Monitoring of mitochondrial oxygen tension in the operating theatre: an observational study with the novel COMET® monitor. PLoS One. ;18(2):e0278561.
Mik EG, et al. 2006 Nov. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods. 3(11):939–495.
Ubbink R, et al. 2017 Dec. A monitor for Cellular Oxygen METabolism (COMET): monitoring tissue oxygenation at the mitochondrial level. J Clin Monit Comput. 31(6):1143–1150.
Notes on contributor
Bert Mik (1974) is anaesthesiologist in the Erasmus Medical Center in Rotterdam. He is head of the Laboratory of Experimental Anesthesiology in the Erasmus MC and is track- and block coordinator of the master Technical Medicine at the Delft University of Technology. He received his PhD degree at the University of Amsterdam where he developed optical methods to measure oxygen tension in tissues and cells. A number of his patented inventions have led to the development of a novel clinical monitor for measuring mitochondrial oxygen tension. Meanwhile this monitor is being evaluated in clinical studies in several centers in the Netherlands and abroad.
Interferometry light microscopy for quality control of isolated mitochondria for biotherapy
Christopher Ribesa, Kelly Aubertina, Marie Bergerb, Dmitry Ayolloa, Florence Gazeaua, Amanda K. A. Silvaa and Sabah Mozafaria
aUniversité Paris Cité, MSC Matière et Systèmes Complexes UMR7057, CNRS, Paris, France; bMyriade lab, Paris, France
Introduction: Mitochondrial dysfunction is associated with various degenerative, inflammatory, and metabolic disorders. Mitochondrial transplantation has emerged as a promising biotherapeutic approach, inspired by intercellular mitochondrial transfer mechanisms. However, the rapid and accurate measurement of isolated mitochondrial size and count remains challenging. Here, we investigated an interferometry-based method using Videodrop to determine mitochondrial concentration and size.
Materials & Methods: Human mesenchymal stromal cells (hMSCs) were used to isolate mitochondria, and their viability, structure, quality, and size were analysed using fluorescence microscopy, transmission electron microscopy (TEM), Western blotting and protein concentration measurements. Videodrop measurements were compared to these techniques whenever applicable.
Results: We found that Videodrop measurements correlated with mitochondrial protein concentration and TEM analyses for count and size, respectively. Our data demonstrate the potential of Videodrop for rapid and reliable characterisation of freshly isolated mitochondria.
Conclusion: This technology has promising applications in clinical settings, facilitating mitochondrial research and translation into therapeutic interventions.
References
Liu D, et al. 2021. Intercellular mitochondrial transfer as a means of tissue revitalisation. Signal Transduct Target Ther. 6:65. doi: 10.1038/s41392-020-00440-z.
Tan YL, et al. 2022. Mesenchymal stromal cell mitochondrial transfer as a cell rescue strategy in regenerative medicine: a review of evidence in preclinical models. Stem Cells Transl Med. 11:814–827.
Intercellular mitochondrial transfer: real-time monitoring and lineage cell tracing with novel reporter systems unveil roles in cancer innervation
Gregory Hoover, Olivia Curley, William Hixson, Angela Cioroch and Simon Grelet
Department of Biochemistry and Molecular Biology, College of Medicine, University of South Alabama, Mobile, AL, USA
Mitchell Cancer Institute, The University of South Alabama, Mobile, AL, USA
Introduction: The intercellular transfer of mitochondria is a burgeoning area of research in cell biology with profound implications for a range of pathological conditions, including metabolic disorders, cardiovascular diseases, neurodegenerative conditions, and cancer. Despite its significance, current investigative tools are insufficient for tracking mitochondrial transfers, leaving their biological mediators and biological impact poorly understood. To address this limitation, we engineered two groundbreaking genetic reporters designed for real-time tracking of mitochondrial transfers between cells and for the lineage tracing of recipient cells both in vitro and in vivo.
Materials & Methods: Our MitoREPORTER system utilises a tetracycline-transactivator for real-time visualisation of cell-cell mitochondria transfers. Meanwhile, the MitoTRACER strategy employs Cre recombinase technology to permanently mark recipient cells and their progeny, allowing lineage tracing of involved cells.
Results: We examined the role of nerve-cancer mitochondria transfers in the context of breast cancer innervation in vitro and in vivo. Our findings underscore the importance of these transfers as a key metabolic support mechanism within the tumour microenvironment, promoting the nerve-mediated breast cancer aggressivity.
Conclusion: MitoREPORTER and MitoTRACER offer powerful platforms for high-throughput analyses and lineage tracing studies and pave the way for future studies on the complex dynamics of intercellular mitochondrial transfers.
Funding
Supported by CCTS/NIH Partner Network Pilot Program [UL1TR003096]; Department of Biochemistry and Molecular Biology, Frederick P. Whiddon College of Medicine; Mitchell Endowment from the Frederick P. Whiddon College of Medicine; Patricia Cobb Rodgers Endowment from the Frederick P. Whiddon College of Medicine.
High sucrose diet triggers subunit I tyrosine 304 phosphorylation of cytochrome C oxidase, causing liver respiratory dysfunction in the cohen diabetic rat model
Tasnim Arrouma, Lucynda Phama, Taryn E. Raisanana, Junmei Wana, Rachel Laxb,c,d, Ann Saadab,c,d, Maik Hüttemanna* and Sarah Weksler-Zangenb,c,d*
aWayne State University, USA; bUniversity of Jerusalem, Israel; cHadassah Medical Center, Israel; dHadassah Academic College, Israel
Introduction: The mitochondrial oxidative phosphorylation process generates most of the cellular energy and free radicals in mammalian tissues. Both factors play a critical role in numerous human diseases that could be affected by reversible phosphorylation events that regulate the function and activity of the oxidative phosphorylation complexes.
Materials & Methods: In this study, we analyzed liver mitochondria of Cohen diabetes-sensitive (CDs) and Cohen diabetes-resistant (CDr) rats2, using blue native gel electrophoresis (BN-PAGE) in combination with mitochondrial activity measurements and a site-specific tyrosine phosphorylation implicated in inflammation, a known driver of diabetes pathology.
Results: We uncovered the presence of a specific inhibitory phosphorylation on tyrosine 304 of catalytic subunit I of dimeric cytochrome c oxidase (CcO, Complex IV). Driven by a high sucrose diet in both CDr and more pronounced in CDs rats, Y304 phosphorylation correlates with a decrease in CcO activity and respiratory dysfunction in rat liver tissue under hyperglycaemic conditions.
Conclusion: We propose that this specific phosphorylation, specifically seen in dimeric CcO, induced by high sucrose diet-induced inflammatory signalling, triggers enzymatic activity decline of complex IV dimers and the assembly of supercomplexes in liver tissue as a molecular mechanism underlying a (pre-) diabetic phenotype.
References
Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. 2008. Tumour necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 283:21134–21144.
Weksler-Zangen S, Yagil C, Zangen DH, Ornoy A, Jacob HJ, Yagil Y. 2001. The newly inbred cohen diabetic rat: a nonobese normolipidemic genetic model of diet-induced type 2 diabetes expressing sex differences. Diabetes. 50:2521–2529.
Mitochondrial transplantation therapy, the recent advances and perspective
James Mccully
Department of Cardiac Surgery, Harvard Medical School, Boston Children’s Hospital, Boston, USA
To ameliorate the effects of myocardial ischemia/reperfusion injury (IRI) we have utilised a novel therapeutic approach, mitochondrial transplantation, in which myocardial mitochondria damaged by ischemia/reperfusion injury are replaced or augmented with viable, respiration competent mitochondria obtained from the patient’s own body (McCully et al. 2009). The efficacy of mitochondrial transplantation has been demonstrated in in vitro, in vivo and clinical studies to rescue cells and significantly enhance functional recovery (Guariento et al. 2020; McCully et al. 2009; Masuzawa et al. 2013). Herein, we review the mechanisms of mitochondrial transplantation and discuss current and potential clinical applications.
Notes on contributors
James McCully is Associate Professor of Surgery in the Department of Cardiac Surgery at Boston Children’s Hospital and Harvard Medical School. Dr. McCully’s research has led to the development of the novel therapeutic intervention, mitochondrial transplantation, that delivers cell-free, functionally intact mitochondria directly to the target tissue to significantly rescue organ function. In 2014 Dr. McCully and Dr. Sitaram Emani successfully mitochondrial transplantation in the clinic, for the rescue of paediatric patients unable to recover from cardiogenic shock after ischemia-reperfusion injury. This first human study showed that mitochondrial transplantation was safe and was able to rescue injured heart muscle in children who prior to mitochondrial transplantation were unlikely to survive. These studies are now being expanded for treatment of stroke, genetic eye and muscle disease and organ preservation and transplantation in adults and children.
References
Doulamis IP, Nomoto RS, Tzani A, Hong X, Duignan T, Celik A, del Nido PJ, McCully JD. 2022 Dec 21. Transcriptomic and proteomic pathways of diabetic and non-diabetic mitochondrial transplantation. Sci Rep. 12(1):22101.
Guariento A, et al. 2020 Dec 1. Autologous mitochondrial transplantation for cardiogenic shock after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. S0022-5223(20)33142-1.
Masuzawa A, et al. 2013. Transplantation of autologously-derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Phys Heart Circ Physiol. 304:H966–H982.
McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. 2009. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Phys Heart Circ Phys. 296:94–105.
Repairing marginal kidneys with mitochondrial transplantation: a new powerful tissue engineering tool that will change the transplant landscape
Giuseppe Orlando
Wake Forest University, USA
Mitochondrial transplantation (MT) promises to revolutionise the science of organ preservation. In fact, due to the exceedingly high gap between the demand and the offer of transplantable organs, more and more “marginal” organs are being used nowadays. The common denominators of these organs are pronounced damage in association with an inferior functional reserve. As the utilisation of such organs is limited by a higher complication rate and an overall inferior outcome, strategies to repair and ultimately render these organs transplantable are urgently needed.
The overarching goal of the currently available organ preservation technologies is to repair (namely, “engineer”) such organs in order to maximise their functional reserve and render them transplantable. In this context, MT bears an immense potential and should be seen as a powerful tool to engineer and repair marginal organs.
Our group at Wake Forest has a large experience with the use of marginal organs, as well as with tissue and organ engineering. By harnessing transplant and regenerative medicine principles, we have recently conducted, in tandem with the lab at the University of Turin, a dual in vitro and ex vivo pilot trial to test MT in the setting of acute renal damage. For the in vitro experiments, human proximal tubular cells were damaged and then treated with mitochondria or placebo. For the ex vivo experiments, we developed a non-survival ex vivo porcine model mimicking the donation after cardiac death (DCD) renal transplantation scenario. One kidney was treated with mitochondria, while the mate organ received placebo, before being perfused at room temperature for 24H. Perfusate samples were collected at different time points and analysed with Raman spectroscopy. Biopsies taken at baseline and 24h were analysed with standard pathology, immunohistochemistry and RNA sequencing analysis.
Results were encouraging. In vitro, cells treated with MT showed higher proliferative capacity and ATP production, preservation of physiological polarisation of the organelles and lower toxicity and reactive oxygen species production. Ex vivo, kidneys treated with MT shed fewer molecular species, indicating stability. In these kidneys, pathology showed less damage while RNAseq analysis showed modulation of genes and pathways most consistent with mitochondrial biogenesis and energy metabolism and downregulation of genes involved in neutrophil recruitment, including IL1A, CXCL8, and PIK3R1.
Overall, our experience shows that MT mitigates acute tubular damage both in vitro and ex vivo. These findings are of immense interest to RT medicine and are being validated further.
Notes on contributor
Giuseppe Orlando, MD, PhD, Marie Curie Fellow, is a surgeon scientist at the Wake Forest School of Medicine in Winston Salem, North Carolina, US. He specialises in the transplantation, bioengineering and regeneration of the kidney and endocrine pancreas at Atrium Health Wake Forest Baptist Medical Center and the Wake Forest Institute for Regenerative Medicine. He received his MD, general surgery and PhD degrees from the University of Rome, Italy, and specialised in abdominal organ transplantation, transplant immunology, regenerative medicine and tissue engineering in Paris (France), Brussels (Belgium), Oxford (England) and Winston Salem. He is currently serving as counsellor of the Cell Transplant and Regenerative Medicine Society (CTRMS) and the International Pancreas and Islet Transplant Association (IPITA), as Chair of the Education Committees of both societies, and as Co-Chair of the Advisory Committee of the American Society of Transplantation (AST). He is also chairing the AST-Tissue Engineering and Regenerative Medicine International Society (TERMIS)-International Society of Cell and Gene Therapy (ISCT) cosponsored webinar series. The overarching goal of his scholarly activity is to bring the fields of transplant and regenerative medicine together to join forces and build their mutual future.
Mitochondria organelle transplantation for neurological diseases and aging
Mark S. Kindy
Department of Pharmaceutical Sciences, Taneja College of Pharmacy, University of South Florida, USA; James A. Haley VA Hospital, Research Service, Tampa, USA
Mitochondria are subcellular self-autonomous organelles primarily responsible for the generation of energy and ATP synthesis. A decline in mitochondrial quality and activity has been associated with normal ageing and correlated with the development of a wide range of age-related diseases. Mitochondrial dysfunction, including decreased oxidative capacity and increased oxidative damage, is thought to substantially contribute to biological ageing. Mitochondrial targeting has been developed to study mitochondrial physiology and dysfunction and the interaction between mitochondria and other subcellular organelles and for treatment of a variety of diseases. Repair of damaged mitochondria is tricky, boosting biogenesis in cells with damaged mitochondria could be detrimental and anti-inflammatory/anti-oxidant drugs have not been successful. A novel advance that has recently been tested by researchers is the transplantation of fully functional mitochondria into defective/damaged cells. We have developing novel approaches in the isolation and application by using mitochondrial organelle transplantation (MOT™) to replace, repair and boost mitochondrial health both in vitro and in vivo. We have enhanced the isolation techniques and improved the quality, quantity and functionality through stimulation of biogenesis. We have expanded the drug delivery capabilities via nanostructures, hydrogels and artificial lipid membranes. In addition, using ICV, IV, IA and nasal delivery, we can deliver the mitochondria to anywhere in the body, efficiently. Using transgenic mice that express PhAMfloxed (photo-activatable mitochondria) mice or the MITO-tag mice, we have been able to show that mitochondria delivered by MOT™ are functional and help to prevent cell loss and restore function rather than only being anti-inflammatory or anti-oxidant in nature. Finally, using bioreactors, we have can provide GMP/GLP quality mitochondria for clinical trials. These studies demonstrate the viability and efficacy of MOT in the treatment of various mitochondrial disorders.
Notes on contributor
Mark S. Kindy received his Ph.D. in biochemistry from Boston University, did his post-doc at the Salk Institute, and was faculty at the University of Kentucky, Medical University of South Carolina and now at the University of South Florida as a Professor in the Taneja College of Pharmacy. He is also a Senior Research Career Scientist at the James A. Haley VA Medical Center. Dr. Kindy’s research has focused on the age, genetics, environment, inflammation and other factors in neurological and neurodegenerative disorders. He has published extensively in the field and has been funded by NIH, NSF, VA, AHA, among others. He is the Director of the Botanical Medicinal Research and Education Consortium at USF.
Targeting mitochondria based on mitochondrial drug delivery systems
Yuma Yamada
Hokkaido University, Japan
A number of mitochondrial drug delivery systems (DDS) have been reported during the past decade, but only a limited number of these are actually available for use in mitochondrial therapy. This is because these strategies face numerous problems including cell internalisation, size limitations, the physicochemical properties of the cargos, modification of a functional device, and the denaturation of the cargoes. We have succeeded in the development of a MITO-Porter, a nano DDS, that can be used to introduce macromolecular cargos into mitochondria via membrane fusion. This MITO-Porter can be used to deliver a wide variety of carrier-encapsulated molecules into mitochondria.
In this lecture, I will summarise the current state of mitochondrial DDS focusing on our research and especially show the research findings regarding cancer treatment targeting mitochondria. We investigated an innovative treatment strategy for resistant cancers by targeting mitochondria, the energy factories of cancer cells. Previous research achievements include studies using drug-resistant cancer cells, as well as observations of anti-tumour effects in tumour-bearing animal models. Furthermore, we also verified photodynamic therapy targeting cancer’ mitochondria. We demonstrated the efficacy of photodynamic therapy using cancer cells (in vitro) with a significantly lower dosage than existing drugs, and achieved promising results in the verification of cancer photodynamic therapy using tumour-bearing animal models (in vivo). In the future, we aim to advance research towards the development of academic-originated therapeutic drugs.
Notes on contributor
Yuma Yamada is a Professor in the Faculty of Pharmaceutical Sciences, Hokkaido University, Japan. His main research interest is the development of mitochondrial drug delivery system (DDS) towards innovative nanomedicine. He received the American Pharmacists Association’s 2022 Ebert Prize—the oldest and one of the most prestigious pharmacy awards in the US—further cements his standing in the field.
Metabolic effects of Cimicifuga racemosa extract on mitochondria and implications for the resistance against oxidative cell death and longevity
Carsten Clumsee
Institute for Pharmacology and Clinical Pharmacy, Biochemical-Pharmacological Center Marburg, University of Marburg, Germany; Center for Mind Brain and Behavior – CMBB, University of Marburg, Germany
Cimicifuga racemosa extract (CRE) is a well-established herbal medication to treat menopausal symptoms such as hot flashes and weight gain. In contrast to oestrogen replacement therapy or phytoestrogens, our findings suggest that CRE Ze 450 rather exerts direct effects on mitochondrial energy turnover through interference with components of the mitochondrial electron transport chain (ETC). To gain a comprehensive insight into the signalling effects of the extract on the mitochondrial proteome and metabolome, neuronal HT22 cells were treated with CRE Ze 450 and analysed by mass spectrometry. Real-time measurements of mitochondrial and glycolytic respiration were performed to detect acute effects of the Cimicifuga extract on the mitochondrial energy release. MitoPlates were used to understand how substrate utilisation and metabolic activity can be reprogrammed upon treatment.
We found that CRE Ze 450 inhibits glucose and glutamine utilisation in mitochondria leading to a suppressed mitochondrial-dependent biosynthetic activity. Cimicifuga racemosa extract decreases the flow of glucose- and glutamine-derived metabolic intermediates into the Tricarboxylic Acid (TCA) cycle, leading to reduced citrate production and de novo lipid biosynthesis. In models of oxidative stress, it was also shown that reprogramming of mitochondrial metabolism by CRE is largely dependent on glutamine depletion, as inhibition of glutaminolysis – but not the depletion of glucose entry into the TCA cycle, resulted in protection against ferroptosis in neuronal cells. In addition, these metabolic effects of CRE mediate anti-inflammatory effects in macrophages in vitro, and enhanced resilience against oxidative stress and longevity in C. elegans, in vivo. Our data indicate that the metabolic effects of CRE are due to restriction of important anaplerotic substrates required for TCA cycle-dependent biosynthesis. These observations provide both, new insight into the mechanism of CRE action on metabolic adaptations and also highlight its role for the resilience against age-related processes engaging impaired mitochondria and loss of antioxidative capacities.
Notes on contributor
Carsten Culmsee is Professor for Clinical Pharmacy and Vice Dean of the Faculty of Pharmacy at the University of Marburg, Germany, and Visiting Professor at the University of Zhengzhou, Zhengzhou, China. He graduated at the Faculty of Pharmacy, University of Marburg and received his Dr. rer. nat. degree in 1997. After a postdoctoral term at the Sanders Brown Research Center on Aging at the University of Kentucky, Lexington, USA (1999–2000) and a position as a group leader and lecturer at the University of Marburg (2000–2003) and the Centre of Drug Research, University of Munich, Germany (2003–2007) he returned in 2007 to the University of Marburg as a full professor for Clinical Pharmacy. His research focus is on the regulation of mitochondrial integrity and function in paradigms of programmed cell death, inflammatory processes and metabolic impairment contributing to neuronal dysfunction and death in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, in ischemic brain damage, and in psychiatric diseases. He investigates molecular and cellular mechanisms of metabolic and oxidative dysregulation that trigger disruption of the intracellular calcium homoeostasis, ER-stress, mitochondrial damage, bioenergetic failure and neuro-inflammatory responses. He reveals and validates neurobiological determinants and novel therapeutic targets in human diseases of the nervous system including therapeutic strategies with genetic and pharmacological approaches, including defined plant extracts. In addition to the experimental work in the laboratory he also leads a group of Clinical Pharmacy where he guides research in the clinic and in public pharmacies on contribution of pharmacists in drug safety and interprofessional patient care.
Translational insights from targeting mitochondria in rare diseases
David Brown
Stealth Biotherapeutics, USA
Dr. David A. Brown is Vice President of Mitochondrial Research at Stealth BioTherapeutics, and will give a lecture on “Translational insights from targeting mitochondria in rare diseases”. This talk will include an overview of Stealth’s clinical programmes to date, including updates on targeting rare mitochondrial diseases in several Phase 2/3 clinical trials, as well as emerging approaches to mitigate mitochondrial dysfunction in pathologies.
Stealth BioTherapeutics is a clinical-stage biotechnology company focused on the discovery, development, and commercialisation of novel therapies for diseases involving mitochondrial dysfunction. Dysfunctional mitochondria are centrally involved in a number of rare genetic diseases and many common age-related diseases, typically involving organ systems with high energy demands such as the eye, the neuromuscular system, the heart and the brain.
Mitochondrial dysfunctions in psoriatic mesenchymal stem cells
Mariangela Di Vincenzo, Anna Campanati, Ilaria Nunzi, Nada Dhaouadi, Annamaria Offidani, Monica Mattioli-Belmonte, Saverio Marchi and Monia Orciani
Marche Polytechnic University, Italy
Introduction: Psoriasis is a complex skin disease caused by multiple mechanisms that lead to imbalance of Th2/Th1-Th17. Recent studies highlighted that mesenchymal stem cells (MSCs) play a key role in psoriasis. Although oxidative stress is one of the main triggers of psoriasis, the involvement of mitochondria in the onset of psoriasis is still unclear, especially in MSCs.
Material & Methods: MSCs from skin biopsy of healthy control subjects (C-MSCs) and psoriatic patients (PSO-MSCs) were exposed to H2O2 and LPS to mimic the stressed environment of psoriasis. PSO-MSCs were also treated with adalimumab, an anti-TNF-alpha human monoclonal antibody.
Results: PSO-MSCs showed more elongated and interconnected mitochondria due to downregulation of the fission factor dynamin related protein-1 (DRP1), and a greater resistance to stress stimuli than C-MSCs mitochondria, which appeared more fragmented. This strong interconnection led to increased mitochondrial ROS production, calcium uptake and apoptosis compared to C-MSCs. Adalimumab restored mitochondrial morphology and physiology only at resting condition, whereas cells were unable to counteract additional inflammatory stimuli.
Conclusion: These results show as the onset of psoriasis is even more complex, with an involvement of mitochondria at stem level; this new hypothesis could provide initial clues for the theorisation of novel therapeutic approaches.
References
Diotallevi F, Di Vincenzo M, Martina E, et al. 2022. Mesenchymal stem cells and psoriasis: systematic review. Int J Mol Sci. 23:15080.
Marchi S, Guilbaud E, Tait SWG, et al. 2023. Mitochondrial control of inflammation. Nat Rev Immunol. 23:159–173.
Orciani M, Campanati A, Salvolini E, et al. 2011. The mesenchymal stem cell profile in psoriasis. Br J Dermatol. 165:585–592.
Cardiomyocyte MTFP1 loss induces heart failure fostered by innate immunity
Erminia Donnarummaa, Micheal Kohlhaasb, Elodie Vimonta, Marcio Campos-Ribeiroa, Etienne Kornobisc, Thibault Chazed, Mariette Matondod, Quentin Gianettoc, Christoph Maackb and Timothy Waia
aMitochondrial Biology Group, Institut Pasteur CNRS UMR 3691, Paris, France; bDepartment of Translational Research, Comprehensive Heart Failure Center (CHFC), Germany; cBiomics, C2RT, Institut Pasteur, Paris, France; dUTechS Ultrastructural Bio Imaging, Institut Pasteur, Paris, France
Mitochondria are paramount to the metabolism and survival of cardiomyocytes. Here we show that Mitochondrial Fission Process 1 (MTFP1) is an inner mitochondrial membrane (IMM) protein that is dispensable for mitochondrial division yet essential for cardiac structure and function. Cardiomyocytes specific knockout of MTFP1 (cMKO) resulted in adult-onset dilated cardiomyopathy accompanied by extensive mitochondrial and cardiac remodelling during the transition to heart failure (HF). Prior to the onset of disease, cMKO mitochondria displayed specific IMM defects: futile proton leak dependent upon the adenine nucleotide translocase and an increased sensitivity to the opening of the mitochondrial permeability transition pore, with which MTFP1 physically and genetically interacts. At the onset of disease, we observed transcriptional induction of innate immune signalling and remodelling of cardiac immune cells reminiscent of chronic cardiac inflammation. The whole-body deletion of STING in cMKO mice mitigated cardiomyopathy, whereas IFNAR1 ablation fully rescued HF and extended lifespan of mice. Rescuing cardiac function in these double KO mouse models did not rescue either proton leak or mitochondrial permeability, indicating that cardiac inflammation was maladaptive. Our data reveal new functions of MTFP1 in the control of bioenergetic efficiency and cell death sensitivity and define its importance in preventing pathogenic cardiac remodelling.
Mitochondrial cargo in extracellular vesicles promotes microglia-mediated proinflammatory response
Henrique Tavaresa, Margarida Beatriza, Claudia Deusa, Ricardo Casqueirob, Nuno Raimundob,c, Paulo Oliveiraa and Carla Lopesb
aCenter for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal; bMultidisciplinary Institute of Ageing, University of Coimbra, Coimbra, Portugal; cDepartment of Cellular and Molecular Physiology, Penn State College of Medicine, Hershey, PA, USA
Extracellular vesicles (EV) containing mitochondrial components may trigger biological consequences upon incorporation into recipient cells (Beatriz et al. 2023; Todkar et al. 2021). Our hypothesis is that an impairment of the mitochondrial-lysosomal axis regulates the extracellular release of mitochondria-derived vesicles (MDV), causing a downstream proinflammatory profile in recipient cells. We investigated if AntiOxCIN4, a novel mitochondria-targeted antioxidant (Deus et al. 2021), prevents the pro-inflammatory phenotype.
We utilised human dermal fibroblasts from control and sporadic Parkinson’s Disease (PD) patients as donor cells. Microglia, the recipient cells, were exposed to cell-free mitochondria, EVs, and EV-isolated DNA from AntiOxCIN4-treated and untreated fibroblasts to assess inflammatory responses.
Our findings revealed that inhibiting the mitochondrial-lysosomal axis resulted in an increase of EV secretion containing more mtDNA copies with elevated DNA oxidation. These EVs influenced microglia activation, promoting a proinflammatory profile (IL-1β, TNF-α, Arg1, and HMGB1). PD fibroblast-derived EVs with mitochondrial-lysosomal dysfunction triggered inflammation, partially mitigated by AntiOxCIN4 treatment. This effect was mediated by improved mitochondrial antioxidant defence, enhanced lysosomal function, and activation of the DNA damage response (ATM pathway) in cells, altering EV cargo.
In summary, dysfunctional mitochondrial-lysosomal axis influences EV function, potentially contributing to microglia activation in neurodegenerative diseases, connecting mitochondrial dysfunction, EV release, and immune responses.
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement MIA-Portugal No 857524 and the Comissão de Coordenação e Desenvolvimento Regional do Centro - CCDRC through the Centro2020 Programme.
References
Beatriz M, et al. 2023. Theranostics. 13(11):3707–3724.
Deus CM, et al. 2021. Redox Biol. 45:102037.
Todkar K, et al. 2021. Nat Commun. 12(1):1971.
Granulosa cell mitochondrial AKT signalling regulated ovarian folliculogenesis and whole body metabolism
Ping H. Wang
City of Hope National Medical Center, USA
Activating PI3K-AKT pathway triggers translocation of activated AKT into mitochondria. This project is to investigate the mechanistic roles of mitochondrial AKT in ovarian granulosa cells. To model ovarian insulin resistance in mitochondria, we have generated a novel transgenic mice line (ovdnAKT) with Cre-LoxP system to disrupt mitochondrial AKT signalling in granulosa cells by targeting a dominant negative AKT to mitochondria. The ovdnAKT mice had an increased number of early-stage ovarian follicles at preantral phase, but mature antral follicles were nearly absent. Menstruation cycles were irregular and prolonged in the ovdnAKT mice. In ovdnAKT ovary, single cell RNAseq cluster annotation confirmed an increased pool of preantral granulosa cells, decreased number of mature granulosa cells and atretic granulosa cells, and increased number of luteinising granulosa cells. Other ovarian cell types were not changed. Transcriptional analysis at preantral phase showed altered folliculogenesis-promoting and steroidogenesis gene expression in the ovdnAKT mice, and reversed the velocity of transcription during folliculogenesis. Body weight increased by 20% and fat mass/weight ratio by 83% in the ovdnAKT mice, indicating obesity and whole body metabolic change. These findings suggest impaired mitochondrial AKT signalling in granulosa cells could be a novel mechanistic link underlying the crosstalk of ovarian dysfunction and obesity.
Funding
Supported by National Institutes of Health [R01HL096987], Ko Family Foundation, City of Hope.
References
Deng W, Leu HB, Chen Y, Chen YH, Epperson CM, Juang C, et al. 2014. Protein kinase B (PKB/AKT1) formed signalling complexes with mitochondrial proteins and prevented glycolytic energy dysfunction in cultured cardiomyocytes during ischemia-reperfusion injury. Endocrinology. 155(5):1618–1628.
Lin H, Chen Y, Chen Y-H, Ta AP, Lee H-C, MacGregor GR, Vaziri ND, Wang PH. 2021. Tubular mitochondrial AKT1 activation during ischemia reperfusion injury and its critical role in predisposition to chronic kidney disease. Kidney Int. 99:870–884.
The influence of maternal obesity on offspring oocyte mitochondrial ultrastructure in primordial and activated follicles: interaction with the offspring diet
Inne Xhonneux, Waleed F. A. Marei and Jo L. M. R. Leroy
Gamete Research Centre, University of Antwerp, Belgium
Introduction: Maternal obesity induces oocyte mitochondrial dysfunction and ultrastructural damage, and predisposes offspring to metabolic disorders. Mitochondrial ultrastructural abnormalities were also reported in ovulated oocytes of offspring born to diet-induced obese mice. Whether such abnormalities already exist in dormant primordial follicles or develops during further follicular development remains unknown. Furthermore, the additive effect of an offspring obesogenic (OB) diet is also unknown. We examined effects and interaction of maternal and offspring OB diet on offspring oocyte mitochondrial ultrastructure in primordial, primary and secondary follicles.
Materials & Methods: Control (n=6) or OB (n=6, high-fat, high-sugar) diets were fed to female Swiss mice (7wk) and to their female offspring (7wk postweaning) in a 2x2 factorial design. Offspring (n=1/mother/group) ovarian sections were examined by transmission electron microscopy.
Results: In primordial and primary follicles, oocyte mitochondrial ultrastructure was unaffected by maternal obesity, but negatively affected by offspring diet. In secondary follicles, mitochondrial morphology was negatively influenced by maternal and offspring diet, with a major impact in OB-born control-fed offspring.
Conclusion: While not affecting primordial oocyte pools, maternal obesity might hamper the mitochondrial ability to handle increasing energy demands during follicular development leading to mitochondrial damage in growing oocytes, especially in control-fed offspring.
Funding
This research is supported by Research Foundation-Flanders [FWO, G038619N].
References
Elias-Lopez, et al. 2023. Mitochondrial dysfunction in the offspring of obese mothers and it’s transmission through damaged oocyte mitochondria: Integration of mechanisms. BBA- Mol Basis Dis. 1869:166802.
Wei, et al. 2023. The effect of maternal consumption of high-fat diet on ovarian development in offspring. Anim Reprod Sci. 255:107294.
Restoration of mitochondrial homoeostasis in embryos derived from metabolically-compromised oocytes using mitoquinone: insight from a bovine in vitro model
Waleed F. A. Marei and Jo L. M. R. Leroy
University of Antwerp, Belgium
Introduction: Mitochondrial oxidative stress plays a key role in the pathogenesis of reduced oocyte quality in obese women. Endogenous pro-survival mechanisms, including upregulated mitochondrial chaperones, fail to restore embryo cellular homoeostasis, leading to early embryo arrest. Our aim was to supplement Mitoquinone (MitoQ) as a mitochondria targeted antioxidant during in vitro culture (IVC) of embryos derived from metabolically-compromised oocytes, to re-establish mitochondrial homoeostasis and rescue embryo development and quality.
Materials & Methods: Bovine oocytes, from slaughterhouse material, were in vitro matured in media containing 1) high pathophysiological palmitic acid (PA) concentration (150µM; as in the follicular fluid of obese women), or 2) solvent (Control). Presumptive zygotes were cultured from day 1 to day 8 post-fertilisation in the presence or absence of MitoQ, in PA-free SOF media.
Results: Embryos derived from PA-exposed oocytes exhibited high fragmentation and low development rates to the blastocyst stage, high apoptotic rates, and increased expression of genes related to oxidative stress (CAT, SOD2, but not GPx); mitochondrial UPR (HSPE1 and HSPD1); and ER stress (ATF4, ATF6 and HSPA5). MitoQ completely alleviated these effects and rescued blastocyst development and quality.
Conclusions: For metabolically-compromised oocytes, MitoQ supplementation during in vitro embryo culture can rescue embryo development and quality.
References
Marei L. 2022. Adv Exp Med Biol. 1387:171–189.
Leroy, et al. 2022. Reprod Fertil Dev. 35:1–18.
Mismatch of mitochondrial and nuclear genomes leads to altered fitness
Marion Bonneau, Florencia Camus, Max Reuter and Kevin Fowler
UCL, UK
Introduction: Mitochondria enclose their own genome, as does the nucleus. The principal role of mitochondria is to produce energy for which interaction between the mitochondrial (mtDNA) and nuclear genomes (nuDNA) is indispensable[1]. Previous studies have shown that mismatch between mtDNA and nuDNA from different species lead to impaired mitochondrial function and altered fitness in hybrids[2]. What is less known is the extent of effects of population-specific mitochondrial genetic variance on life-history evolution.
Materials and methods: Here, we look at the consequences of different mtDNA and nuDNA combination on various phenotypic traits. Using a large diverse panel of Drosophila melanogaster with both coevolved and disrupted lines of mtDNA and nuDNA[3], we assess mitonuclear incompatibilities by studying changes in various metabolic and fitness traits including, fertility, development time and heat resistance. We then analysed the genomic sequence of key mitonuclear combinations, to pinpoint specific genes that could underlie mitonuclear communication and symbiotic evolution.
Results: When mitonuclear co-adaptation is disrupted, we observe trait alterations and gene miscommunication.
Conclusion: These results will help us better understand the communication between the nuclear and mitochondrial genomes and will shed some light on the role played by the mitochondrial genome in fitness traits.
Funding
Thank you to The Leverhulme Trust, UK for funding this work.
Thank you to The Genetics Society - Conference Grant Scheme B (Non-Genetics Society Meetings)- for funding my travels in order to assist to this conference.
References
Barreto FS, Watson ET, Lima TG, Willett CS, Edmands S, Li W, Burton RS. 2018. Genomic signatures of mitonuclear coevolution across populations of Tigriopus californicus. Nat Ecol Evol. 2:1250–1257.
Carnegie L, Reuter M, Fowler K, Lane N, Camus MF. 2021. Mother’s curse is pervasive across a large mitonuclear Drosophila panel. Evol Lett. 5:230–239. doi: 10.1002/evl3.221.
Lane N. 2005. Power, sex, suicide: mitochondria and the meaning of life. Oxford: Oxford University Press.
Oestrogen metabolism and mitochondrial function in the pathogenesis of idiopathic pulmonary arterial hypertension
Emanuel Guajardo-Correaa, Juan Francisco Silva-Agüeroa, Gerardo García-Rivasb, Mario Chionga, Mauricio Latorrec and Valentina Parraa
aUniversidad de Chile, Chile; bTecnológico de Monterrey, México; cUniversidad de O’Higgins, Chile
Introduction: Idiopathic pulmonary arterial hypertension (iPAH) is a chronic and incurable disease that mainly affects women. Elevated oestrogen (E2) plasma levels are a major risk factor for iPAH1. However, it is not known how E2 and its metabolism control the cancer-like reprogramming (higher proliferation and decreased mitochondrial metabolism) observed in pulmonary artery smooth muscle cells (hPASMC)2,3.
Methods: We used a systems biology approach to build a human genome-scale transcriptional regulatory network (TRN) to identify novel transcription factors (TF)-gene interactions and metabolic pathways (MP) affected in iPAH. The MP-integrated TRN was built using public iPAH patient gene expression and gene-metabolic databases. We also evaluated whether E2 or its metabolite, 4-Methoxyestradiol (4-ME), modulated the cancer-like reprogramming observed in hPASMC.
Results: The MP-integrated TRN showed an oestrogenic master pathway in iPAH due to oestrogen receptor alpha (ESR1)-mediated CYP1B1 upregulation of oestrogen and steroids metabolism. E2 and 4-ME both increased the proliferative G2/M phase and decreased mitochondrial potential, although E2 upregulated oxygen consumption. Using ESR-inhibitors, we determined that the oestrogenic proliferative effect was dependent on different receptors: ESR/E2 or GPER/4-ME, respectively.
Conclusion: To our knowledge, this is the first work showing that the phenotypic metabolic reprogramming in iPAH is controlled by different oestrogenic pathways.
Funding
This work was supported by grants ANID FONDECYT 1230195 (VP) and 1230194 (ML), FONDAP 15120011 (MC, VP) and 15090007 (ML), ANID ACT210004 (ML, VP) and Beca Doctorado Nacional ANID21191519 (EG-C).
References
Guajardo-Correa E, Silva-Agüero JF, Calle X, Chiong M, Henríquez M, García-Rivas G, Latorre M, Parra V. 2022. Oestrogen signalling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front Cell Dev Biol. 10:968373.
Pugh ME, Hemnes AR. 2010. Pulmonary hypertension in women. Expert Rev Cardiovasc Ther. 8:1549–1558.
White RJ. 2016. Oestrogen: friend or foe in pulmonary hypertension? Am J Respir Crit Care Med. 193:1084–1086.
Restoration of mitochondrial homoeostasis provides glaucoma neuroprotection
Arupratan Das
Indiana University, USA
Neurons have a substantial energy demand due to their constant requirement for neurotransmission and synaptic activity. The primary source of cellular energy, adenosine triphosphate (ATP), is generated by mitochondria. Dysfunctional mitochondria are a significant concern in central nervous system (CNS) disorders like glaucoma, amyotrophic lateral sclerosis (ALS), and Parkinson’s disease. Among these disorders, retinal ganglion cells (RGCs) of the optic nerve are especially susceptible to mitochondrial dysfunction. They require a continuous supply of ATP for firing action potentials along their lengthy, unmyelinated axons, even during resting periods.
Our research focuses on human stem cell-derived retinal ganglion cells (hRGCs), which have demonstrated efficiency in eliminating damaged mitochondria while concurrently producing healthy ones to maintain mitochondrial balance. However, hRGCs with a glaucomatous Optineurin mutation (E50K) exhibit a high rate of ATP production with fewer mitochondria compared to wild-type neurons. This imbalance leads to mitochondrial swelling and disrupts cellular homoeostasis.
Significantly, we’ve found that by enhancing mitochondrial biogenesis through the pharmacological inhibition of Tank binding kinase 1 (TBK1), we can restore energy equilibrium and mitigate mitochondrial swelling, thus providing neuroprotection against acute mitochondrial damage in glaucomatous hRGCs. This reveals a novel mechanism for neuroprotection. In our investigations, we conducted a small molecule screening and identified a novel pharmaceutical compound that promotes mitochondrial biogenesis and viability in glaucomatous E50K hRGCs.
Furthermore, this compound has demonstrated robust protection of retinal ganglion cells, axons in the optic nerve, and long-term preservation of vision, as measured by optokinetic response (OKR) and the visual cliff test in a mouse model of optic nerve crush (ONC) injury. Importantly, it exhibited no systemic toxicity in mice. As a result, our study unveils a groundbreaking neuroprotection mechanism by activating mitochondrial biogenesis, effective not only in human RGCs with genetic defects but also with optic neuropathies without genetic disorders but suffer disrupted mitochondrial balance.
Notes on contributors
In 2006, Dr. Arupratan Das received his M.Sc. in Biochemistry from Calcutta University, India and subsequently, in 2012, he completed his Ph.D. in Cell Biology under the mentorship of Rong Li at the Stowers Institute in Kansas City (KC), USA. Following the successful completion of his Ph.D., Dr. Das embarked on postdoctoral research, first with Clare Waterman at the National Institutes of Health (NIH), and later with Donald Zack at Johns Hopkins University. In November 2019, Dr. Das took a significant step in his career by establishing his own independent laboratory at the Indiana University School of Medicine (IUSM). In his research, Dr. Das employs a multidisciplinary approach, utilising stem cells, neurobiology, drug screening, and animal models to uncover the mechanisms of neurodegeneration at the single-cell level, with the ultimate goal of developing neuroprotection therapies for conditions such as glaucoma and amyotrophic lateral sclerosis (ALS).
Cardiac mitomed: from bench to bedside
Sang-Bing Ong
Centre for Cardiovascular Genomics & Medicine, The Chinese University of Hong Kong, HKSAR, China
Mitochondria@HeartHK
Cardiovascular disorders remain the leading cause of death and disability worldwide. The extent of cardiac cell death and left ventricular systolic function are the strongest predictors of morbidity and mortality following cardiac disorders. Despite optimal therapy, the morbidity and mortality of cardiovascular patients remain significantly high. On this background, there remains an urgent clinical need to discover novel therapies for reducing cardiac injury/death and preserving cardiac function so as to improve health outcomes for cardiovascular patients. In this regard, the viability of the heart and cardiac function are critically dependent on the ability of cardiac mitochondria to generate the energy required for optimal contractile function. Therefore, preventing mitochondrial dysfunction induced by cardiac disorders is an important therapeutic strategy for preserving cardiac viability and function. The mitochondria – energy-providing organelles in the cardiac cell, have been found to be at the convergence of multiple signalling pathways that dictate life or death in the heart. As opposed to conventional belief that the mitochondria are static organelles, mitochondria are actually dynamic whereby they change shapes (morphology), shift locations on the cytoskeletal tracks and are selectively degraded by a process known as mitophagy following damage/senescence. Recent efforts have focused on targeting the mitochondrial morphology to protect the heart against injury. We describe here the efforts of our team in genetically and pharmacologically manipulating the mitochondrial morphology using in vitro as well as in vivo models of acute myocardial infarction to achieve cardioprotection against ischemia-reperfusion injury.
Notes on contibutors
Sang Bing Ong has been conducting research studying the roles of cardiac mitochondrial morphology in cardioprotection for the past 15 years. He received his Bachelor’s degree in Malaysia, a Masters and a PhD in London UK followed by postdoctoral research in San Diego and Singapore. He was also a Hitachi Fellow in Hokkaido Japan and a Thailand MHESI’s Visiting Professor. He has held academic positions in Malaysia, Singapore and is now heading the Mitochondria@HeartHK laboratory at the Centre for Cardiovascular Genomics and Medicine at the Chinese University of Hong Kong.
Personalised medicine in mitochondrial health and disease: myth and reality
Ciro Leonardo Pierri
Department of Pharmacy-Pharmaceutical Sciences, University of Bari, Italy
Introduction: Mitochondrial diseases (MDs) may result from mutations affecting nuclear or mitochondrial genes, encoding mitochondrial proteins, or non-protein-coding mitochondrial RNA. Despite the great variability of affected genes, in the most severe cases, a neuromuscular degenerative phenotype is observed, and no specific therapy exists for a complete recovery from the disease. The most used treatments are symptomatic and based on the administration of antioxidant cocktails, combined with antiepileptic/antipsychotic drugs and supportive therapy for multiorgan involvement. Unfortunately, antioxidant therapies have met limited success and it is still urgent to highlight new/real pharmacological targets and to design/identify highly selective/efficient drugs to rescue mitochondrial-impaired pathways, by employing omics approaches, crystallised protein structures, and the newly developed computational approaches for protein 3D-modelling and drug design.
Materials & Methods: In this context, very little attention was dedicated to proteins responsible for the crosstalk between mitochondria and cytoplasm such as mitochondrial transporters (i.e., SLC25A family members) and FAD/NADH dehydrogenases (i.e., AIF), playing a crucial role in mitochondrial function.
Results: Mitochondrial transporters, in particular, are responsible for the translocation of nucleotides, amino acids, organic acids, and other cofactors between the mitochondrial and cytosolic compartments, whereas AIF has the necessary biochemical/structural features to behave as a complete redox-exchange-system. Both, SLC25A family members and AIF are also involved in the regulation of mitochondrial apoptosis.
Conclusions: In this regard mitochondrial transporters of the SLC25A family and FAD/NADH dehydrogenases, appear to be able to efficiently modulate mitochondria/cytoplasm crosstalk, emerging as an interesting class of new possible pharmacological targets for MD treatments.
References
Todisco S, …, Pierri CL. 2023. Targeting mitochondrial impairment for the treatment of cardiovascular diseases: From hypertension to ischemia-reperfusion injury, searching for new pharmacological targets. Biochem Pharmacol.
Tragni V, …, Pierri CL. 2022. Personalised medicine in mitochondrial health and disease: molecular basis of therapeutic approaches based on nutritional supplements and their analogs. Molecules.
Trisolini L, …, Pierri CL. 2019. FAD/NADH dependent oxidoreductases: from different amino acid sequences to similar protein shapes for playing an ancient function. J Clin Med.
Internalisation of exogenous mitochondria for endothelial corneal dystrophy treatment
Patrick J. Rochette
Université Laval, CHUL, Canada
Fuchs endothelial corneal dystrophy (FECD) is a degenerative eye disease characterised by an accelerated loss of corneal endothelial cells (CEC). Since the function of these cells is to maintain the cornea in a state of deturgescence necessary for its transparency, the depletion of corneal endothelial cells ultimately causes corneal oedema and irreversible loss of vision. Corneal transplantation, for which the transplant supply is insufficient, is the only curative alternative for FECD. We have observed significant variability in the mitochondrial mass of FECD CECs, which led us to hypothesise that mitochondrial mass variability might play a key role in the chronology of events eventually leading to CEC death in FECD. We assessed mitochondrial health and functionality in FECD corneal endothelial explants, namely, intra-mitochondrial calcium, mitochondrial membrane potential, oxidation level and apoptosis. This has led us to describe a sequence of events leading to what we referred to as a mitochondrial burnout, and which goes as follow. FECD CECs initially compensate for endothelial cell losses by incorporating mitochondrial calcium to help generating more ATP, but this leads to increased oxidation. CECs then resist the sustained need for more ATP by increasing their mitochondrial mass, mitochondrial calcium and mitochondrial membrane potential. At this stage, CECs reach their maximum capacity and start to cope with irreversible oxidative damage, which leads to mitochondrial burnout. This burnout is accompanied by a dissipation of the membrane potential and a release of mitochondrial calcium, which in turn leads to cell death by apoptosis. When CECs die and are not replaced, the mitochondria of surviving cells must provide more energy to compensate, feeding the vicious cycle of mitochondrial burnout. We then tested whether incorporating healthy mitochondria into FECD cells would improve pathological molecular markers of the disease and reverse the vicious cycle of mitochondrial burnout.
We demonstrated that incorporation of exogenous mitochondria into FECD cells reduces oxidative stress, increases mitochondrial membrane potential, and reduces mitophagy. In addition, internalisation of exogenous mitochondria significantly reduces apoptosis (57% in FECD vs 12% in FECD with internalised mitochondria). Taken together, these results suggest that the internalisation of exogenous mitochondria reverses the vicious circle involved in FECD, thus revealing a much-needed novel treatment alternative for FECD.
Notes on contributor
Patrick J. Rochette is a senior researcher at the Research Centre of the CHU of Quebec – Laval University, in the area of regenerative medicine. He is also Full Professor and Director of Research in the Department of Ophthalmology of the Faculty of Medicine at Laval University. He specialised in photobiology and, more precisely, on the consequences of cell exposure to low-wavelength light rays (ultraviolet and blue) on cells. He was interested in mitochondria in relation to their involvement in the response to genotoxic stress from solar radiation. This expertise led him to investigate the involvement of this organelle in various ocular pathologies and to use it as an avenue of treatment.
Age-related changes in energy metabolism in Peripheral Mononuclear Blood Cells (PBMC) and the brains of cognitively healthy seniors
Gunter Peter Eckerta, Carmina Viktoria Silaidosa, Martina Reutzela; Fabian Dietera, Silke Maturab, Ulrich Pilatusb, Elke Hattingenb and Johannes Pantelb
aJustus-Liebig University Giessen, Germany; bUniversity Hospital, Goethe University, Frankfurt, Germany
Mitochondrial dysfunction is a hallmark of cellular senescence and many age-related neurodegenerative diseases. We therefore investigated the relationship between mitochondrial function in peripheral blood cells and cerebral energy metabolites in young and older sex-matched, physically and mentally healthy volunteers in a cross-sectional observational study involving 65 young and 65 older women and men. Cognitive health was evaluated using established psychometric methods. Peripheral blood mononuclear cells (PBMCs) were isolated. Mitochondrial respiratory complex activity, ATP, and citrate synthase activity (CS) were determined. Energy metabolites were quantified in brains using 1H - and 31P -magnetic resonance spectroscopic imaging (MRSI).
Complex IV activity (CIV) and ATP levels were reduced in PBMCs isolated from older participants. tNAA levels were reduced, Cr, and PCr levels were increased, and ATP levels were unchanged in the brains of older participants. Markers of energy metabolism in blood cells did not correlate with energy metabolites in the brain.
Age-related bioenergetic changes were detected in peripheral blood cells and the brains of healthy older people. However, mitochondrial function in peripheral blood cells do not reflect energy related metabolites in the brain. While ATP levels in PBMCs may be a valid marker for age-related mitochondrial dysfunction in humans, cerebral ATP remained constant.
Reference
Silaidos CV, …, Eckert GP. 2023 Jun 13. Age-related changes in energy metabolism in peripheral mononuclear blood cells (PBMCs) and the brains of cognitively healthy seniors. Geroscience. doi: 10.1007/s11357-023-00810-9.
Mito-DREADD: a new tool to increase mitochondrial activity and rescue cognitive alteration
Etienne Hebert-Chatelaina,b, Rebeca Martin-Jimeneza,b, Antonio Pagano-Zottolac,d, Genevieve Hamel-Cotea,b, Giovanni Marsicanoc,d and Luigi Bellocchioc,d
aUniversity of Moncton, Canada; bCanada Research Chair in Mitochondrial Signaling and Physiopathology; cINSERM U1215 NeuroCentre Magendie; dUniversity of Bordeaux, France
Most brain mitochondrial defects lead to cognitive impairment or neurodegenerative diseases. However, due to the lack of suitable tools, no direct link between acute mitochondrial activity and higher brain functions has been established so far. Heterotrimeric guanine nucleotide-binding (G) proteins are key players in brain metabolism and higher functions. Since G proteins can be found within mitochondria, we hypothesised that stimulation of specific G protein signalling within the organelle could modulate brain mitochondrial activity and possibly rescue behavioural defects associated to brain metabolic disorders. We developed mitoDREADD-Gs, a Galphas-linked recombinant designer receptor exclusively activated by designer drugs targeted to mitochondria, which can acutely increase mitochondrial metabolism in different types of cells both in vitro and ex vivo. Strikingly, in vivo activation of mitoDREADD-Gs expressed in specific brain circuits abolished cognitive impairments linked to mitochondrial alterations, including cannabinoid-induced amnesia and other memory impairment models. Our data show that mitoDREADD-Gs is a reliable tool to acutely increase mitochondrial activity. This will not only benefit our understanding of how mitochondria are involved in biological functions, but it will also provide new potential therapeutic concepts for the treatment of brain diseases associated to impaired cell metabolism.
References
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci. 104:5163–5168.
Chu CT. 2022. Mitochondria in neurodegeneration. Curr Opin Physiol. 100532.
Hebert-Chatelain E, et al. 2016. A cannabinoid link between mitochondria and memory. Nature. 539:555–559.
Oldham WM, Hamm HE. 2008. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 9:60–71.
Soria-Gomez E, et al. 2021. Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron 109:1513–1526.e11.
Incorporation of exogenous mitochondria amplifies ATP production and safeguards fibroblasts against oxidative stress
Yosif El-Darawisha,b, Takahiro Shibataa,b, Keiichi Sakakibaraa,b, Masae Takedaa,b, Junko Hayashia,b, Yoshiharu Nittaa,b, Yuma Yamadab, Masashi Suganumaa,b, Hisashi Otaa,b and Rick Tsaia
aLUCA Science Inc., Japan; bBiological Drug Develop. based DDS Tech., Fac. Pharmaceut. Sci., Hokkaido University, Japan
Introduction: Dysfunction of mitochondria may involve many metabolic/neurological disorders in addition to mitochondrial genetic disorders (1, 2). Yet, the challenge persists in sourcing viable mitochondria for therapeutic applications. We previously introduced our novel method for isolation of mitochondria (3), which ensures the structural and functional integrity of isolated mitochondria, even post freeze-thaw regimen. We hereby introduce the progress we have achieved in the development of mitochondria for therapeutic purposes.
Material & Methods: Isolated mitochondria (MRC-Q), obtained from HeLa cells and subsequently cryopreserved, underwent rigorous evaluation, encompassing assessments on integration into normal human dermal fibroblast (NHDF) recipient cells by both detection of mtDNA and fluorescent-imaging, and protective effect against oxidative stress by cell survival assay.
Results: NHDF cells incorporated fluorescent tagged MRC-Q, increased ATP production and enhanced resistance to oxidative stress. Protective effect of MRC-Q against oxidative stress was sustained even after removal of MRC-Q from culture. No adverse effects were observed in the recipient cells.
Conclusions: Storable mitochondria boosted intracellular level of ATP and prevented damage from oxidative stress in recipient cells. Further research is essential to determine the underlying mechanisms of action involving exogenous mitochondria. These results warrant new research paths and significant therapeutic potential for mitochondrial disorders.
References
Hosseinian S, et al. 2022. Prospects of mitochondrial transplantation in clinical medicine: Aspirations and challenges. Mitochondrion. 65:33–44. doi: 10.1016/j.mito.2022.04.006.
Liu Z, Sun Y, Qi Z, et al. 2022. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci 12:66. doi: 10.1186/s13578-022-00805-7.
WMS, Targeting Mitochondria. 2022. Berlin; Characterisation of frozen Mitochondria isolated by novel method. Yosif El-Darawish et al.
Platelet-derived mitochondria modulate the bioenergetic phenotype of human neutrophils
Marie-France N. Soucya,b, Jean-Luc Jougleuxa,b, Jacob L. Légera,b, Mathieu P. A. Héberta,b, Jérémie A. Doirona,b, Simon Lamarrea, Étienne Hébert-Chatelaina,b and Luc H. Boudreaua,b
aUniversité de Moncton, Canada; bCentre de Médecine de Précision du Nouveau-Brunswick, Canada
Introduction: Platelets are anucleated cells that release microvesicles (PMVs) upon activation. PMVs retain platelet cargo, including functional mitochondria1. PMVs actively participate in intercellular communication with immune cells, including neutrophils (PMN)2. However, mitochondrial content transfer from PMVs to PMN and its phenotypic consequences remains uninvestigated.
Materials & methods: PMVs and PMN were isolated from the blood of consenting donors. PMVs-PMN were first co-incubated to confirm the interaction (flow cytometry and confocal microscopy) and the bioenergetic phenotype modulation was determined by measuring cell viability (NETosis), mitochondrial respiration and ATP production. Inflammatory lipid mediators were measured by HPLC.
Conclusion: Platelet-derived mitochondria were found associated with PMN, consequently decreasing NETosis. PMVs induced both an increase in ATP production and the LEAK state in the recipient cell. To confirm the implication of platelet-derived mitochondria in the observed changes in the PMN’s phenotype, PMVs were subjected to freeze-thaw cycles. Dysfunctional mitochondria in PMVs did not affect the cellular respiration of PMN. Similar results were obtained when measuring mitochondrial reductase activity, in which only the fresh PMVs preparation increased the activity. Inflammatory lipid mediators were quantified, and we observed an increase in 12-lipoxygenase products, which is an enzyme found specifically in PMVs.
Funding
Supported by Canadian Institute of Health Research, New Brunswick Innovation Foundation, New Brunswick Health Research Foundation.
References
Boudreau LH, et al. 2014. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 124(14):2173–2183.
Léger JL, et al. 2022. Functional platelet-derived mitochondria induce the release of human neutrophil microvesicles. EMBO Rep. e54910.
Development of mitochondria-inspired lipopolyplexes for gene delivery
Ioannis Tsichlisa, Nikolaos Nazirisa, Antiopi Vardaxib, Stergios Pispasb and Costas Demetzosa
aSection of Pharmaceutical Technology, Department of Pharmacy, School of Health Sciences, National and Kapodistrian University of Athens, Panepistimioupolis Zografou, Athens, Greece; bTheoretical and Physical Chemistry Institute, National Hellenic Research Foundation, Athens, Greece
Introduction: Lipopolyplexes are hybrid complexes of cationic liposomes, polymers, and nucleic acids promising in gene therapy1. Mitochondria-inspired lipopolyplexes were developed as a novel nanoplatform to enhance the specificity and efficiency of gene delivery to mitochondria by mimicking their lipid composition.
Materials & Methods: The random copolymer P(DMAEMA-co-SMA) was synthesised by RAFT polymerisation. Polyplexes were prepared in suitable buffer by adding the solution of DNA to polymer at certain N/P ratios2. Moreover, a lipid nanoplatform inspired by the mitochondrial membrane was prepared, using POPE, DSPC, DOTAP, sphingomyelin, cholesterol lipids, through thin-film hydration method. Lipopolyplexes were developed by using a polyplex solution to hydrate the mitochondria-mimicking lipid film and the mixture was extruded through 400nm polycarbonate filters3. The physicochemical properties of the resulting nanosystems were measured in different media by dynamic and electrophoretic light scattering techniques. The effect of temperature on their physicochemical behaviour was studied.
Results: All the nanosystems showed superior colloidal stability over time. Notably, both polyplexes and lipopolyplexes were positively charged, indicating their potential for gene delivery. Moreover, they exhibited a mean hydrodynamic diameter of 160nm and 250nm respectively.
Conclusion: This study presents a mitochondrial-inspired nanosystem as hybrid lipopolyplexes for gene delivery and showed superior stability in different media.
References
Chrysostomou V, et al. 2022. Hydrophilic random cationic copolymers as polyplex-formation vectors for DNA. Materials (Basel). 15:2650.
Jerzykiewicz J, Czogalla A. 2022. Polyethyleneimine-based lipopolyplexes as carriers in anticancer gene therapies. Materials. 15:179.
Rezaee M, Oskuee RK, Nassirli H, Malaekeh-Nikouei B. 2016. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J Control Release. 236:1–14.
Mitochondrial encapsulation technology for mitochondrial transplantation therapy
Yonghui Wanga,b, Oliver Koivistoa,b, Chang Liu and Hongbo Zhanga,b
aPharmaceutical Sciences Laboratory, Åbo Akademi University, Finland; bTurku Bioscience Centre, University of Turku and Åbo Akademi University
In recent years, the therapy term “mitochondrial transplantation” has opened a new treatment horizon for mitochondria-related diseases. Findings derived from both preclinical investigations and clinical attempts demonstrated that this method is a new, innovative strategy for treating conditions associated with mitochondrial dysfunction. However, isolated mitochondria are easily disturbed by the external environment, which will affect their bioactivity. In addition, membrane potential and size make it difficult for target cells to phagocytosis.
Our team has developed a technique based on the biomineralization of mitochondria within nanoparticles. The nano-layer has adjustable molecular level pores, which prevent the macromolecular-like enzymes from entering, but allow the small molecules to pass. Therefore, this unique coating technology can guarantee the long-lasting maintenance of mitochondria bioactivity. Our preliminary results indicate that this technology can enable long-lasting mitochondria bioactivity at room temperature. It also contributes to a significantly higher intracellular delivery efficiency, with a 20-fold increase in uptake compared to pure mitochondria without coating. The good intercellular delivery efficiency and the possibility of using in vivo administration make our encapsulation technology fundamental for mitochondrial transplantation therapies.
References
Celik A, Orfany A, Dearling J, Del Nido PJ, McCully JD, Bakar-Ates F. 2023. Mitochondrial transplantation: effects on chemotherapy in prostate and ovarian cancer cells in vitro and in vivo. Biomed Pharmacother. 161:114524.
Zhang TG, Miao CY. 2023. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm Sin B. 13:1028–1035.
Liver regeneration: strategic role of mitochondria
Jiri Neuzila,b
aSchool of Pharmacy and Medical Science, Griffith University, Southport, Qld, Australia; bInstitute of Biotechnology, Czech Academy of Sciences, Prague, Czech Republic
Mitochondria are vital organelles that provide energy and are a hub of catabolic and anabolic reactions. We have recently shown that cancer cells with dysfunctional mitochondrial DNA import the organelles via horizontal mitochondrial transfer in order to restore respiration that, among other functions, is essential for de novo pyrimidine synthesis via its link with dihydroorotate dehydrogenase (DHODH). We reasoned that regeneration of liver, which growth back to its original size after partial hepatectomy of ~50% within 5-6 days in a mouse model, requires massive proliferation that needs to be supported by relevant processes, including DHODH-dependent de novo pyrimidine synthesis. Indeed, we found that blocking DHODH results in complete suppression of liver regeneration. Further, we observed that ammonia, under homoeostasis detoxified via the urea cycle in periportal regions, is utilised to form more glutamine, substrate of de novo pyrimidine synthesis, that is catalysed by glutamine synthetase in pericentral regions of the liver.
We conclude that liver regeneration is critically dependent on de novo pyrimidine synthesis and that its substrate formation is supported by a switch from ammonia detoxification to the use of this deleterious metabolite for anabolic reactions.
Notes on contributors
Jiri Neuzil focuses on the role of mitochondria in cancer and in their use as a target for cancer therapies, which resulted in development of mitochondrially targeted tamoxifen currently undergoing clinical testing. His major discovery has been the phenomenon of horizontal mitochondrial transfer in cancer and in non-cancerous settings. He has published about 250 peer-reviewed papers, his H-index is 65.
References
Bajzikova, et al. 2019. Cell Metab. 29:399–416.
Dong, et al. 2023. J Cell Biol. 222:e202211044.
Tan A, et al. 2015. Cell Metab. 21:81–94.
MNRR1/CHCHD2 and mitochondrial dysfunction in disease
Lawrence Grossman
Wayne State University, USA
MNRR1 (also called CHCHD2, PARK22, AAG10) is a bi-organellar regulator of cellular function that acts in both the mitochondria and the nucleus yet plays a different role in each. Mitochondrial MNRR1 binds to both cytochrome c oxidase and Bcl-xL to regulate the mitochondrial roles of energy metabolism and apoptosis, respectively. Nuclear MNRR1 can function as a transcriptional regulator of numerous genes to modulate the activation of stress-responsive genes including MNRR1 itself. We examined MNRR1 levels in several mitochondrial diseases including MELAS and also in preterm birth and found it to be reduced along with reduced mitochondrial respiration, reduced ATP production, and increased ROS levels. However, forced expression of MNRR1 reversed these phenotypic changes.
To investigate the therapeutic potential of increasing MNRR1 expression, we sought pharmacological activators by screening a library of FDA approved drugs and natural products and found compounds that increased MNRR1 expression. We focused on an anti-parasite drug, nitazoxanide, and found that its use in cultured cells was able to restore respiration in wild-type cells but not in cells lacking MNRR1. The mitochondrial disease we largely focused on was MELAS, caused in our model by the m.3243A→G mutation in tRNALeu(UUR). Using cybrid cells that contained 73% mutant mtDNA, we saw reduced levels of MNRR1 and could show that overexpressing it genetically increased oxygen consumption and ATP levels and reduced ROS. In addition, mtDNA levels and markers of mitophagy were increased. Strikingly, the proportion of wild-type mtDNA was increased. Importantly, pharmacological increase of MNRR1 with nitazoxanide could also shift heteroplasmy towards wild-type mtDNA, suggesting a potential treatment. In another example, we used a well characterised murine model of preterm birth caused by intra-amniotic lipopolysaccharide (LPS) injection.
We had previously shown that LPS treatment produces an inflammatory phenotype in a trophoblast cell line that includes reduced mitochondrial respiration and increased production of ROS and inflammatory cytokines such as TNF⍺. This murine model also showed reduction of MNRR1. In this inflammatory context birth was reduced by nearly two days post coitus but could be restored to normal, along with reduction in inflammatory cytokines, with use of nitazoxanide. In each model we investigated the mechanism for reduction of MNRR1 and found them to differ: in the case of trophoblast inflammation enhanced protein turnover took place whereas in MELAS reduced MNRR1 transcription took place.
Notes on contributors
Lawrence Grossman is the Henry L. Brasza Professor of Molecular Medicine and Genetics and Director of the Center for Molecular Medicine and Genetics; he is also Professor of Internal Medicine. He received an undergraduate degree in chemistry from the City College of New York and the PhD degree in genetics and biochemistry from the Albert Einstein College of Medicine, where he studied the replication and structure of yeast mitochondrial DNA with Julius Marmur. He then carried out postdoctoral research at the California Institute of Technology, working with Jerome Vinograd on mammalian mitochondrial DNA, where he was named a Fellow of the Jane Coffin Childs Memorial Fund for Medical Research. He has been a member of the Wayne State University community for more than 25 years. He serves on the editorial boards of Mitochondria and Cells and was previously Contributing Editor for Science. The Grossman lab works on mitochondrial genetics and function with a recent emphasis on regulation and dysregulation in disease and pharmacological regulation of gene expression towards restoration of normal phenotype.
Targeting mitochondrial activity in liver disease: barriers and perspectives
María Luz Martínez-Chantar
CIC bioGUNE, Spain
Mitochondrial dysfunction stands as a pivotal factor in the development and advancement of chronic liver diseases. This study endeavours to shed light on the central contributors, underlying causes, and outcomes associated with mitochondrial dysfunction in the context of liver health. In particular, our focus lies in investigating the potential therapeutic advantages stemming from the enhancement of mitochondrial activity through the modulation of genes related to the electron transport chain, mitochondrial metabolism, and cation modulators within the liver.
The results of this research yield compelling evidence, endorsing various genes as a promising therapeutic avenue. This approach not only mitigates liver damage but also promotes the process of liver regeneration.
Targeting MCJ/DnaJC15 to modulate mitochondrial respiration in disease
Mercedes Rincon
University of Colorado at Anschutz Medical Campus, USA
Mitochondria dysfunction is emerging as an underlying cause of a large number of diseases affecting liver, kidney, heart, brain and others. Impaired mitochondrial respiration is the main mechanism. However, restoring or increasing mitochondrial respiration has been a major challenge. We have developed a new strategy. We identified MCJ (also called DnaJC15) as a novel negative regulator of mitochondrial respiration. MCJ associates with Complex I of the ETC, and acts as a negative regulator of Complex I and mitochondrial respiration. MCJ deficiency results in increased mitochondrial respiration, but it has no effect on glycolysis and does not increase the production of reactive oxygen species (ROS). NAFLD (non-alcoholic fatty liver disease) is considered the 21st century epidemics (affects about 30% of the worldwide population), includes a wide spectrum of different pathological conditions and NASH (non-alcoholic steatohepatitis) is the pathological advanced form. No treatments for NASH have been FDA approved yet, despite the numerous trials testing new drugs. Using a number of mouse models of NASH, we have shown that increasing mitochondrial respiration by silencing MCJ expression in hepatocytes is a novel therapeutic strategy for treatment of NASH: increases the burning of lipids in hepatocytes, reduces liver steatosis and reduces liver fibrosis. We will present recent data for the development of the siRNA-based drug for humans, efficacy and safety (tox studies in non-human primates). We will also describe how loss of MCJ in cancer cells leads to increased mitochondrial respiration in chemoresistant cancer cells that enhances the activity of drug-efflux ABC transporters. We have developed MCJ peptide mimetics that reduce mitochondrial ATP production and overcome cancer chemoresistance when given in combination with standard chemotherapeutic drugs.
Notes on contributors
Mercedes Rincon, Ph.D. is a Professor in Department of Immunology and Microbiology at the University of Colorado at Anschutz Medical Campus (USA). She is an internationally recognised immunologist who has provided numerous contributions on the role of IL-6 in T cells in the areas of allergy/asthma, arthritis and influenza infection. In addition, Dr. Rincon identified MCJ/DnaJC15 as a negative regulator of mitochondria respiration and metabolic diseases. She has shown that therapeutic silencing MCJ in hepatocytes is a novel treatment for non-alcoholic fatty liver disease. Dr. Rincon is also investigating the use of MCJ as a target to improve T cell therapies. She has also developed therapeutic MCJ mimetics to inhibit mitochondrial respiration to overcome chemoresistance in breast and ovarian cancer. Her overall goal is to bring novel research findings from bench-to-bed and develop novel therapies for a broad range of diseases.
Mitochondrial stress as a central biological hub for spaceflight impact
Afshin Beheshtia,b
aBlue Marble Space Institute of Science, Seattle, WA, USA; bStanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, MA, USA
Determining the biological impact of spaceflight through novel approaches is essential to reduce the health risks to astronauts for long-term space missions. The current established health risks due to spaceflight are only reflecting known symptomatic and physiologic responses and do not reflect early onset of other potential diseases. There are many unknown variables which still need to be identified to fully understand the health impacts due to the environmental factors in space. Utilising a public omics data repository for space biology data (NASA GeneLab), a comprehensive multi-omics approach was implemented correlating transcriptomics, proteomics, metabolomics, and methylation analysis. We found that cells have stronger overall biological response than the tissues to spaceflight, with mitochondrial activity and innate immunity pathways being heavily impacted. NASA Twin Study results are consistent with a specific alteration in mitochondrial ATP production. In addition, when expanding this initial on other organisms (i.e. C. elegans, plants, etc.) we observe similar mitochondrial changes occurring during spaceflight. Our results indicate that the space environment can directly induce mitochondrial damage, with mitochondrial dysfunctions being a cause for chronic inflammation and both being involved in the development of metabolic disorders that cause changes in lipid metabolism. We also found biological changes occurring during spaceflight with cell cycle, circadian rhythm and olfactory activity pathways can also influence and be influenced by alterations on mitochondrial activity. In addition, from our earlier work, we demonstrated a circulating microRNA (miRNA) signature that is present and involved with the general increased health risks during spaceflight that impacts mitochondrial function directly.
From this work, we demonstrated that this miRNA signature impacted the overall biology and health with both the microgravity and space radiation components of the space environment. We showed that this miRNA signature can be an optimal biomarker for health risk and also has potential to be utilised as a countermeasure to mitigate the damage caused by the space environment by utilising a human 3D microvascular tissue model. By applying a novel self-delivery system to target three miRNAs (i.e. antagomirs) from our spaceflight miRNA signature impacting cardiovascular health risks, we were able to completely mitigate damage caused by exposure to simulated Galactic Cosmic Ray (GCR) irradiation. Here we further expand on the countermeasure experiments to uncover the specific novel biology involved with this countermeasure and in vivo experiments that demonstrates that these antagomirs rescue damage caused to certain organs due to both microgravity and space radiation. Specifically, the miRNAs rescued damage to the heart, immune suppression, and improved mitochondrial function that occurs during spaceflight in addition to other key biology. In addition, we have also observed with the 3D microvascular tissue model improved DNA double strand break repair machinery which can also contribute to improved recovery and protection against damage caused by space radiation.
This work expands on our previous work and further uncovers how a potential minimally invasive countermeasure can be used to mitigate space environment effects and that mitochondrial dysfunction is a key driver in biological response to spaceflight and potentially can lead to health risks.
Notes on contributors
Afshin Beheshti completed his PhD from Florida State University in physics and made a transition to cancer, systems biology, space biology, and radiation biology for his postdoctoral training. In 2014 he became an Assistant Professor at Tufts University School of Medicine/Tufts Medical Center where he continued his research as a systems biologist studying various aspects of cancer including microRNAs, ageing and cancer, cancer drug targets, and development of novel immunotherapy. In April 2017, Dr. Beheshti joined KBR, NASA Ames Research Center to be part of the GeneLab project assisting with developing the platform. Currently, Dr. Beheshti is now part of Blue Marble Space Institute of Science at NASA Ames Research Center and has his own grants and conducts research on multiple projects which include: how microRNAs and mitochondria will affect space biology and potential use for countermeasures to mitigate space radiation and microgravity; COVID-19 related research; cancer research; and impact of high altitude on human biology. Lastly, Dr. Beheshti currently also holds a Visiting Researcher appointment at Broad Institute of MIT and Harvard and is the Lead of a non-profit formed on March 2020 working on COVID-19 called COVID-19 International Research Team (COV-IRT, www.cov-irt.org). With COV-IRT, he has been working on many different subjects related COVID-19 research and has already produced several publications. Dr. Beheshti has been recognised for his work through multiple awards that include: International Space Station Research & Development Award for Compelling Results in Biology from American Astronaut Society/NASA, NASA Exceptional Scientific Achievement Medal, and One KBR Award.
What spaceflight and bed rest have in common: a proteomic point of view
Marta Murgia
University of Padova, Italy
Microgravity and ionising radiation, combined with confinement, sleep disruption and exposure to continuous ambient noise, challenge the health and wellbeing of astronauts during space missions. Skeletal muscle, which makes up around 40% of human body mass, undergoes extensive structural remodelling, resulting in atrophy and loss of force. Skeletal muscle deconditioning during spaceflight can only be partially attenuated by exercise countermeasures onboard.
We applied mass spectrometry-based proteomics to the analysis of muscle biopsies of two astronauts who spent six months on the International Space Station (ISS) and had very different levels of physical activity while on board. Comparing the effects of spaceflight on skeletal muscle to those of a ground-based models of unloading, bed rest, we found both common features and major differences.
Our analysis quantified over 70% of all proteins annotated to the mitochondrion from the astronauts’ muscle biopsies. In agreement with many studies, we measured in both astronauts a dramatic decrease in mitochondrial protein intensity during six months on the ISS. No comparable mitochondrial remodelling could be measured in the muscle fibres of subjects undergoing bed rest.
My talk will explore the hypothesis that ionising radiation and other sources of oxidative stress onboard, but not inactivity per se, may be the primary hit of skeletal mitochondrial damage in space. Oxidative damage would be parallel and independent of the direct effects of unloading, explaining the limited efficacy of exercise countermeasures.
Notes on contributors
Marta Murgia works at the Dept. of Biomedical Sciences, University of Padova and at Max-Planck-Institute of Biochemistry, Germany. She applies mass spectrometry-based proteomics to single skeletal muscle fibres and is interested in every angle of skeletal muscle physiology and pathology. Her interest in proteomics is driven by the aim of understanding skeletal muscle fibre diversity and plasticity, which she had been studying as a postdoc in the laboratory of Stefano Schiaffino at University of Padova. Her interest for single cells stems from her PhD under the supervision of late Professor Tullio Pozzan, where she was embedded in a wonderfully stimulating environment focused on calcium signalling in mitochondria and other cellular compartments.
Gravitational and mechanical forces drive mitochondrial translation
Taisei Wakigawaa,b, Yusuke Kimuraa,b, Mari Mitob, Toshiya Tsubakia, Hironori Saitoa,b, Abdul Haseeb Khanc, Tohru Yamamorid, Tomokazu Yamazakie, Akira Higashibatae, Tatsuhisa Tsuboic, Taku Saitoa, Atsushi Higashitanif, Yuichi Shichinob and Shintaro Iwasakia,b
aThe University of Tokyo, Japan; bRIKEN Cluster for Pioneering Research, Japan; cTsinghua Shenzhen International Graduate School, China; dJapan Space Forum, Japan; eJapan Aerospace Exploration Agency, Japan; fTohoku University, Japan
Introduction: Life on Earth has evolved in a form suitable for the gravitational force of 1× g. Although the pivotal role of gravity in gene expression has been revealed by multiomics approaches in space-flown samples and astronauts, the molecular details of how mammalian cells harness gravity have remained unclear.
Materials & Methods: To investigate the translational landscape under microgravity, we conducted genome-wide ribosome profiling on mammalian cells and Caenorhabditis elegans cultured in the International Space Station. To address the regulatory mechanisms of mitochondrial translation, we employed MitoIP-Thor-Ribo-Seq, a ribosome profiling derivative tailored for mitochondrial translation in high resolution.
Results: We comprehensively surveyed the translational response to microgravity and found that mitochondrial translation is dramatically reduced by microgravity. In addition, we found that cell adhesion through laminin–integrin interaction, which is attenuated by microgravity, and the downstream FAK, RAC1, PAK1, BAD, Bcl-2 family proteins, and mtFAS relay the signals for mitochondrial protein synthesis. Mechanistically, the consumption of mitochondrial malonyl-CoA by activated mtFAS leads to the reduction of the malonylation of mitochondrial translation machinery, enhancing translation initiation and elongation rates.
Conclusions: Our work provides mechanistic insights into how cells convert gravitational and mechanical forces into translation in an energy-producing organelle.
Funding
Supported by by the JAXA Flight Control Team and Payload Flight Control Team. This project was supported by the Japan Society for the Promotion of Science (JSPS) (a Grant-in-Aid for Young Scientists [A], JP17H04998; a Challenging Research [Exploratory], JP19K22406), the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (a Grant-in-Aid for Transformative Research Areas [B] “Parametric Translation”, JP20H05784), the Japan Agency for Medical Research and Development (AMED) (AMED-CREST, 22gm1410001), Gushinkai Foundation, and RIKEN (“Biology of Intracellular Environments” and “Integrated life science research to challenge super aging society”).
References
da Silveira WA, et al. 2020. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell. 183:1185–1201.e20.
Wakigawa T, et al. 2023. Complexity and dynamics of in organello translation landscape assessed by high-resolution mitochondrial ribosome profiling. bioRxiv. doi: 10.1101/2023.07.19.549812.
Wakigawa T, et al. 2023. Gravitational and mechanical forces drive mitochondrial translation through the cell adhesion–FAK axis. bioRxiv. doi: 10.1101/2023.01.18.524628.
Examination of mitochondrial DNA variants and their association with brain structural measures and symptom severity in bipolar disorder
Ana Paula Mendes-Silvaa, Suyi Haob, Lucas Tanigutic, Kody Kennedyb, Mikaela Dimickb, Amanda Lisowaya, Clement Zaia,c, James Kennedya,c, Benjamin Goldsteinb,c and Vanessa Gonçalvesa,c
aPsychiatric Neurogenetics Section, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada; bCentre for Youth Bipolar Disorder, Child and Youth Psychiatry Division, Centre for Addiction and Mental Health, Toronto, Canada; cDepartment of Psychiatry, Faculty of Medicine, University of Toronto, ON, Canada
Introduction: Mitochondria are vital for cellular energy and play key roles in brain functions1. Dysfunctional mitochondria are linked to brain development issues and cognitive problems in mental disorders, including bipolar disorder (BD)2,3. This study aimed to identify mtDNA variants and gain insights into their potential role in the BD phenotype.
Materials & Methods: We examined the mitochondrial genome of 103 European-Caucasian adolescents (57 BD and 46 controls). The mtDNA-Server pipeline was used to identify mtDNA variants and their pathogenicity scores. Region-of-interest (ROI) analysis examined anterior cingulate cortex (ACC) and amygdala, assessing cortical thickness and volume. Linear regression was used to test associations between variants and relevant phenotypes, adjusting for age, sex, and intracranial volume as necessary. We applied a Bonferroni correction to account for multiple tests.
Results: Out of 1645 homoplasmic variants, 72 were common, of which 3 were classified as potentially harmful. The (MT-ND2):m.4917A>G pathogenicity score was associated with more severe BD symptoms. Regression analysis showed nominal associations between the (MT-ND1):m.4216T>C pathogenicity score and amygdala volume. No significant associations were found for ACC.
Conclusion: We identified mtDNA variants that may contribute to the pathogenesis of BD and its clinical manifestations. Further validation in larger samples is needed.
Funding
Supported by CAMH Discovery Fund and Womenmind.
References
Berk M, et al. 2011. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 35:804–817.
Bodenstein DF, et al. 2019. Mitochondrial DNA content and oxidation in bipolar disorder and its role across brain regions. NPJ Schizophr. 5:21.
Lord LD, Expert P, Huckins JF, Turkheimer FE. 2013. Cerebral energy metabolism and the brain’s functional network architecture: an integrative review. J Cereb Blood Flow Metab. 33:1347–1354.
Mitochondria and ROS signalling as key players in arrhythmogenic cardiomyopathy? A molecular screening-based answer
Reginald Philippea, Chiara Volania,b, Andrea Medicic; Elena Sommarivad, Giulio Pompiliod,e, Peter P. Pramstallera, Jakob Troppmairc and Alessandra Rossinia
aInstitute for Biomedicine, Eurac Research, Bolzano, Italy - Affiliated Institute of the University of Lübeck, Lübeck, Germany; bThe Cell Physiology MiLab, Department of Biosciences, Università degli Studi di Milano, Milano, Italy; cDaniel Swarovski Research Laboratory, Department of Visceral, Transplant and Thoracic Surgery, Medical University Innsbruck, Innsbruck, Austria; dUnit of Vascular Biology and Regenerative Medicine, Centro Cardiologico Monzino IRCCS, Milano, Italy; eHeart Rhythm Center, Centro Cardiologico Monzino IRCCS, Milano, Italy
Introduction: Arrhythmogenic cardiomyopathy (ACM) is a cardiac disease characterised by ventricular arrhythmia and a progressive replacement of myocardium by a fibro-fatty tissue. Our group, among others, showed that ACM Cardiac Stromal cells (CStCs) accumulate more lipids under adipogenic differentiation than control CStCs, which could be related to the disease pathophysiology1. Recent evidence also pointed towards a deregulation of mitochondrial activity and ROS signalling in ACM2,3. This work aimed to determine the activity of mitochondrial and ROS targeting compounds on ACM-CStCs lipid accumulation.
Material and Methods: Adipogenic differentiation of ACM-CStCs from one patient was triggered for seven days in presence of mitochondrial and ROS targeting compounds (MedChem Express). Lipid accumulation was determined using the neutral lipid staining dye BODIPY 493/503, cell number measured using Hoechst counterstaining, and images acquired using a high-content imaging system (ImageXpress, Molecular Devices).
Results: On 407 compounds tested, around 25% demonstrated a capacity to reduce lipid accumulation in ACM-CStCs in which 24 compounds strongly decreased lipid accumulation by at least 50%.
Conclusion: This result confirms the importance of mitochondrial and ROS signalling in the adipogenic differentiation of ACM-CStCs and interrogates on their potential targeting for the treatment of ACM tissue remodelling.
Funding
This research was funded by the Joint Project Südtirol - FWF (Italy-Austria)- for R.P.,A.M.,J.T. and A.R.
References
Sommariva, et al. 2016. doi: 10.1093/eurheartj/ehv579.
van Opbergen, et al. 2019. doi: 10.3389/fphys.2019.01496.
Volani, et al. 2022. doi: 10.1111/jcmm.17396.
The Mitochondrial Calcium Uniporter (MCU) is a molecular target for mobility by stimulating muscle performance and protecting chondrocytes during osteoarthritis
Flavien Bermonta, Aurelie Hermanta, Gerard Vinyesb, Nicolas Bonneta, Jerome N. Feigea; Marie-Noelle Horcajadaa and Umberto De Marchia
aNestlé Institute of Health Sciences - Nestlé Research, Switzerland; bNestlé Health Science, Switzerland
Introduction: We recently discovered that mitochondrial Ca2+ uptake declines during ageing and that genetic or nutritional activation of MCU boosts energy metabolism and performance in skeletal muscle1, 2. In parallel to clinical testing of a nutritional activator of MCU during exercise and aging3, we hypothesised that MCU may also contribute to the progression of osteoarthritis (OA), the degeneration of cartilage that causes articular pain via mechanical erosion of joints. In osteoarthritic patients, chondrocyte dysfunction is associated with impaired mitochondrial energy metabolism of chondrocytes. However, the role of mitochondria during OA progression and the biological effect of a mitochondria-targeted intervention remain poorly characterised.
Material & Methods and Results: By investigating mitochondrial and chondrocyte function in inflammatory-induced OA and in primary human OA chondrocytes with MCU knock-down, we found that OA impairs MCU-mediated mitochondrial activation, and that MCU causally regulates extracellular matrix secretion to support chondrocyte health. The natural polyphenolic MCU activators Quercetin and Oleuropein synergistically rescued mitochondrial and chondrocyte function in OA chondrocytes, but not in MCU-ablated cells.
Conclusions: We identify MCU as a multi-tissue molecular target for mobility and establish Quercetin and Oleuropein as nutrients that specifically target MCU to rescue chondrocyte and skeletal muscle function in ageing and OA.
References
Gheradi, et al. doi: 10.1101/2023.02.24.529830v1.
Weiser, et al. 2023. Redox Biol. 64:102759.
https://www.clinicaltrials.gov/study/NCT05350566 and https://www.clinicaltrials.gov/study/NCT05217433.
Mitochondrial pyruvate metabolism is disturbed in polymicrobial sepsis
Louise Nuyttens, Jolien Vandewalle and Claude Libert
Ghent University, Belgium
Introduction: Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. The WHO labelled sepsis as a global health priority due to high mortality rates and lack of curative treatments. Interestingly, increased blood lactate levels and mitochondrial dysfunction are a hallmark of sepsis pathophysiology. We aim to investigate the potential link between a disturbed mitochondrial pyruvate metabolism and increased lactate production as this is poorly understood.
Material and Methods: Mice were subjected to the cecal ligation and puncture (CLP) procedure to induce polymicrobial peritonitis. Liver mitochondria were isolated and the oxygen consumption rate (OCR) was measured via a seahorse assay. The liver mitochondria were also further processed for western blotting and enzyme activity assays.
Results: We obtained three important observations: 1) Liver mitochondria of septic mice showed reduced OCR after pyruvate administration which is not observed with TCA cycle substrates. 2) Liver mitochondria of septic mice showed reduced protein levels and activity of the mitochondrial pyruvate carrier (MPC), and, 3) showed a reduced pyruvate dehydrogenase (PDH) activity.
Conclusion: The mitochondrial pyruvate metabolism in CLP mice is disturbed at two main levels: 1) pyruvate import via the MPC and 2) the oxidative decarboxylation of pyruvate via PDH.
Funding
An FWO fundamental research grant to Louise Nuyttens supported this work.
References
Frezza C, Cipolat S, Scorrano L. 2007. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoco. 2(2):287–295.
Nedel W, Deutschendorf C, Cruz Portela LV. 2023. Sepsis -induced mitochondrial dysfunction: a narrative review. World J Crit Care Med. 12(3):139–152.
Vandewalle J, et al. 2021. Combined glucocorticoid resistance and hyperlactatemia contributes to lethal shock in sepsis. Cel Metabo. 33:1–14.
Mitochondrial DNA variants and microbiota: an experimental strategy to identify novel therapeutic targets in chronic inflammatory diseases
Misa Hirosea, Adina Hartmanna, Michael Olbricha, Axel Künstnera, Sven Künzelb, John F. Bainesb,c and Saleh M. Ibrahima,d
aLübeck Insitute of Experimental Dermatology, University of Lübeck, Germany; bMax Plank Institute for Evolutionary Biology, Germany; cInstitute of Expereimental Medicine, Kiel University, Germany; dCollege of Medicne, Khalifa University, UAE
We have previously demonstrated that mice carrying mtDNA natural variants, C57BL/6J-mtFVB/NJ (B6-mtFVB), exhibited (i) a partial protection from experimental skin inflammatory diseases compared to the wild-type C57BL/6J (B6) mice and (ii) a differential composition of gut microbiota. To confirm the causal effect of the gut microbiota on the disease, we generated germ-free B6-mtFVB and wild-type mice and evaluated their susceptibility to autoantibody-induced skin inflammation. Germ-free B6-mtFVB mice slightly but statisticallyy significantly showed milder disease severity compared with germ-free B6 mice (p = 0.0038), confirming that the disease susceptibility is determined by both gut microbiota and other host factors determined by differential host mtDNA genotype.
To further dissect these disease-contributing factors, shotgun metagenomic sequencing of caecum contents and untargeted metabolomics of liver samples from conventional B6-mtFVB and B6 mice were conducted. Subsequently, we identified N-acetyl-D-glucosamine as a candidate mediator that ameliorated disease severity in experimental skin inflammation by modulating CD4+ T cell function. Since B6-mtFVB mice show differential susceptibilities to a wide range of diseases models, this experimental approach is valuable to identify novel therapeutic targets not only for the skin inflammatory conditions but also for other chronic inflammatory diseases.
Enhanced activity of mitochondrial enzyme Fumarylacetoacetate Domain Containing Protein 1 (FAHD1) reduces cellular ROS levels in human bone osteosarcoma epithelial cells
Alexander K. H. Weiss, Anne Heberle, Elia Cappuccio and Tatjana Kuen
University of Innsbruck, Austria
The eukaryotic oxaloacetate decarboxylase FAH domain containing protein 1 (FAHD1) is involved in the regulation of the TCA cycle flux. While the downregulation/knockdown of FAHD1 in various cell types has already been well studied, this work aimed to pioneer the study of how increased catalytic activity of FAHD1 would influence the metabolism of human cells. We hypothesised, that upon the increase of FAHD1 activity mitochondrial levels of oxaloacetate would be depleted. This could eventually be followed by a reduction of the TCA cycle flux, accompanied by reduced ROS production.
In addition to the overexpression of human FAHD1 isoform 1 (hFAHD1.1), we generated stable U2OS cell lines that overexpress a catalytically enhanced, and a loss-of-function variant of hFAHD1.1 (T192S and K123A). Of interest, homologs to the T192S variant can be found in animals that display increased resistance to oxidative stress and cancer.
References
Heberle A., et al. Enhanced activity of mitochondrial enzyme FAHD1 reduces cellular ROS levels in human bone osteosarcoma epithelial cells. Manuscript submitted.
Seluanov A, et al. 2018. Mechanisms of cancer resistance in long-lived mammals. Nat Rev. Cancer. 18:433–441. doi: 10.1038/s41568-018-0004-9.
Weiss AKH, et al. 2020. Regulation of cellular senescence by eukaryotic members of the FAH superfamily - a role in calcium homoeostasis? Mech Ageing Dev. 190:111284. doi: 10.1016/j.mad.2020.111284.
Biological activity and antioxidant capacity of novel mitochondria-targeted estrogens
Geovanni Ruiz-Romeroa, Magdiel Orozco-Valdiviaa, Kanchan Chauhanb, Aldo Moreno-Ulloaa, Rafael Vazquez-Duhaltb and Carolina Alvarez-Delgadoa
aCentro de Investigación Científica y de Educación Superior de Ensenada, Mexico; bCentro de Nanociencias y Nanotecnología UNAM, Mexico
Oestrogens have beneficial effects on mitochondrial function, including improved energy and antioxidant metabolism. The aim of this work is to concentrate and potentiate these effects in mitochondria using novel mitochondria-targeted oestrogens and evaluate them as an alternative for the treatment of pathologies associated to mitochondrial dysfunction. These molecules were synthesised by coupling a triphenylphosphonium group to 17β-estradiol and ethinyl-estradiol, generating mitoE2 and mitoEE2, respectively (Patent pending MX/a/2023/006190). Cell viability of MFC-7 and CCD-1112Sk cell lines was evaluated after treatment with both molecules. Mitochondrial accumulation was determined by mass spectrometry. Superoxide dismutase activity was measured to assess the antioxidant potential of the compounds. MitoE2 and mitoEE2 preserved viability in both cell lines, however mitoE2 maintained cell viability over a broader concentration range. Both molecules showed increased levels of mitochondrial accumulation compared to estradiol and were more abundant than in the cytoplasm. After induction of oxidative stress, mitoE2 decreased the activity of MnSOD compared with estradiol, suggesting different mechanisms of action. The novel mitochondria-targeted oestrogens accumulate in mitochondria and, as such, could be exploited to treat pathologies associated with mitochondrial dysfunction. Our results suggest that mito-directed molecules have different biological and antioxidant effects than their oestrogen parent molecules.
Funding
Research Grant from the National Council of Humanities, Sciences and Technology (CONAHCYT), “Frontiers in Sciencie 2019” CF-6391. Internal Project 685-110 from the Scientific Research and Superior Education Center of Ensenada (CICESE). Scholarship by CONAHCYT, CVU 693063.
References
Guajardo-Correa E, et al. 2022. Oestrogen signalling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front Cell Dev Biol. 10:968373.
Russell OM, et al. 2020. Mitochondrial diseases: hope for the future. Cell. 181(1):168–188.
Zielonka J, et al. 2017. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 117(15):10043–10120.
Exploiting structural variations in highly conserved mitochondrial complex III to develop antifungal agents
Di Xiaa, Zhaohai Qinb, Lothar Essera, Fei Zhoua and Seyedmojtaba Seyedmousavic
aNational Cancer Institute, NIH, USA; bCollege of Science, China Agricultural University, China; cClinical Center, NIH, USA
Introduction: Respiratory Complex III or cyt bc1 are validated targets for antibiotics and fungicides. However, agents targeting cyt bc1 are often toxic to mammalian hosts, limiting their use in treating human infections. Curiously, some drugs such as the FDA-approved antimalarial drug atovaquone target specifically cyt bc1 of the parasite, posing little threat to its human host.
Materials & Methods: Using isolated mammalian mitochondrial and bacterial cyt bc1 complexes, we measured IC50 of various cyt bc1 inhibitors against the two complexes and determined their structures with bound inhibitors.
Results: Structural differences of binding of inhibitors to cyt bc1 complex of different organisms are revealed, exposing potential vulnerabilities to pharmacological exploitation. Using the cyt bc1 from the photosynthetic bacterium Rhodobacter sphaeroides (Rsbc1) and bovine mitochondria (Btbc1) as surrogates for fungal and mammalian enzymes, respectively, we identified pyramoxadone as a selective inhibitor of cyt bc1, with much greater affinity for Rsbc1. Importantly, pyramoxadone shows potent in vitro activity against some of the medically important and agricultural fungal pathogens, compared to currently available systemic fungicides, with low cytotoxicity in both cultured human cells and laboratory animals.
Conclusion: This study provides an example for exploiting structural differences in evolutionarily conserved enzymes to develop antifungal drugs.
Funding
Supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by a grant from the National Basic Research Science Foundation of China [2010CB126100] to ZQ.
References
Esser L, Elberry M, Zhou F, Yu CA, Yu L, Xia D. 2008. Inhibitor complexed structures of the cytochrome bc1 from the photosynthetic bacterium Rhodobacter sphaeroides at 2.40 Å resolution. J Biol Chem. 283:2846–2857.
Esser L, Yu CA, Xia D. 2014. Structural basis of resistance to anti-cytochrome bc1 complex inhibitors: implication for drug improvement. Curr Pharm Des. 20(5):704–724.
Jordan DB, Livingston RS, Bisaha JJ, Duncan KE, Pember SO, Picollelli MA, Schwartz RS, Sternberg JA, Tang XS. 1999. Mode of action of famoxadone. Pestic Sci. 55:105–118.
Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. 1997. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 277:60–66.
Mitochondrial gene expression is required for platelet function and blood clotting
Tara R. Richmana,b,c,d, Judith A. Ermera,b,c, Jessica Bakera,b,c,d, Stefan J. Siiraa,b,c, Benjamin T. Kilee, Matthew D. Lindenf, Oliver Rackhama,b,d,g,h and Aleksandra Filipovskaa,b,c,d
aHarry Perkins Institute of Medical Research, QEII Medical Centre, Nedlands, Western Australia, Australia; bARC Centre of Excellence in Synthetic Biology, QEII Medical Centre, Nedlands, Western Australia, Australia; cCentre for Medical Research, The University of Western Australia, QEII Medical Centre, Nedlands, Western Australia, Australia; dTelethon Kids Institute, Northern Entrance, Perth Children’s Hospital, Nedlands, Western Australia, Australia; eFaculty of Health and Medical Sciences, University of Adelaide, South Australia, Australia; fPathology and Laboratory Science, The University of Western Australia, Perth, Australia; gCurtin Medical School, Curtin University, Bentley, Western Australia, Australia; hCurtin Health Innovation Research Institute, Curtin University, Bentley, Western Australia, Australia
Introduction: Platelets are anucleate blood cells that contain mitochondria and regulate blood clotting in response to injury. Mitochondria contain their own gene expression machinery which relies on nuclear-encoded factors for the biogenesis of the oxidative phosphorylation (OXPHOS) system to produce energy, ions and metabolites required for thrombosis. Platelets provide a valuable model to understand the importance of mitochondrial gene expression and its autonomy from the nucleus in anucleate cells.
Materials and methods: We generated three different platelet-specific conditional knockout mouse models, each lacking a gene that is essential for mitochondrial gene expression at the level of RNA processing, stability and translation (Elac2Pf4∆/Pf4∆, Ptcd1Pf4∆/Pf4 and Mtif3Pf4∆/Pf4∆). We investigated how the loss of these RNA-binding proteins affected platelets and their precursor cells megakaryocytes through bleeding assays, FACS analyses of megakaryocyte and platelet function, cell staining and microscopy, and proteomics, transcriptomics and RNA-seq.
Conclusion: Loss of ELAC2, PTCD1 or MTIF3 leads to increased megakaryocyte ploidy, elevated circulating levels of reticulated platelets, thrombocytopenia and consequent extended bleeding time. Furthermore, impaired RNA metabolism also reduced agonist-induced platelet activation. Transcriptomic and proteomic analyses showed that mitochondrial gene expression and protein synthesis facilitate fibrinolysis, haemostasis and blood coagulation in response to injury.
Funding
Supported by fellowships and project grants from the National Health and Medical Research Council [GNT2017515, GNT1058442, GNT1045677, GNT1041582, GNT1023460, GNT 1005030, GNT1043978], the Australian Research Council [DP180101656], the Cancer Council of Western Australia. TR was a Raine/Bright Spark Foundation Research Fellow and is currently a CSIRO FSP Synthetic Biology Research Fellow. JB is supported by UWA Postgraduate Scholarships.
References
Siira SJ, et al. 2018. Concerted regulation of mitochondrial and nuclear non-coding RNAs by a dual-targeted RNase Z. EMBO Rep. 19(10):e46198.
Perks KL, et al. 2018. PTCD1 is required for 16S rRNA maturation complex stability and mitochondrial ribosome assembly. Cell Rep. 23(1):127–142.
Rudler DL, et al. 2019. Fidelity of translation initiation is required for coordinated respiratory complex assembly. Sci Adv. 5(12):eaay2118.
NME3 is a gatekeeper of DRP-1-dependent mitophagy
Zee-Fen Chang
National Taiwan University, Taiwan
Introduction: Selective removal of damaged mitochondria by mitophagy is critical for maintaining the quality of mitochondria after stress-induced injury. DRP1 is important for mitochondrial division and also the segregation of damaged domains of mitochondria in mitophagy. However, the regulation of DRP1 specific for mitophagy remains elusive. NME3, an NDP kinase, is located on the mitochondrial outer membrane. We found that NME3 interacts with DRP1 and is essential for DRP1-dependent mitophagy. The mechanistic investigation reveals the interplay of NME3 and MUL1 in the regulation of DRP1 for mitophagy.
Materials & Methods: Cells expressing mtKiema were incubated in a hypoxia chamber for mitophagy evaluation. Mice carrying an H135Q knock-in mutation disrupting active site histidine phosphorylation in the NME3 gene were generated for evaluation of ischemia/reperfusion-induced infarction and cerebellar function. Biochemical and image analyses were performed to understand the molecular mechanism for the regulation of DRP1 by NME3.
Conclusion: The role of NME3 in hypoxia-induced mitophagy is dependent on its active site phosphohistidine rather than its NDP kinase activity. NME3 is a gatekeeper for DRP1-mediated segregation of damaged domains of mitochondria by preventing DRP1 from inactivation by MUL1.
Funding
Supported by National Science and Technology Council, Taiwan NSCT 111-2320-B-002-088 and NSCT 111 −2326-B-002-022 to Z.F.C.
References
Chen CW, et al. 2019. Two separate functions of NME3 critical for cell survival underlie a neurodegenerative disorder. Proc Natl Acad Sci U S A. 116:566–574.
Konig T, Nolte H, Aaltonen MJ, Tatsuta T, Krols M, Stroh T, Langer T, McBride HM. 2021. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat Cell Biol. 23:1271–1286.
Onishi M, Yamano K, Sato M, Matsuda N, Okamoto, K. 2021. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40:e104705. doi: 10.15252/embj.2020104705.
Palikaras K, Lionaki E, Tavernarakis N. 2018. Mechanisms of mitophagy in cellular homoeostasis, physiology and pathology. Nat Cell Biol. 20:1013–1022.
Puri R, Cheng XT, Lin MY, Huang N, Sheng ZH. 2019. Mul1 restrains Parkin-mediated mitophagy in mature neurons by maintaining ER-mitochondrial contacts. Nat Commun. 10:3645. doi: 10.1038/s41467-019-11636-5.
Cryo-EM studies reveal the inactivation mechanism of ATP synthase leak channel and its contribution to mitochondrial permeability transition
Nelli Mnatsakanyana,b, Juliana Da Fonsecaa, Yangyu Wub, Daniel Morrisa and Amrendra Kumara
aPenn State University College of Medicine, USA; bYale University School of Medicine, USA
Introduction: Fluctuations in oxygen levels are detrimental to most organisms. While excessive oxygen causes oxidative stress, its deprivation (anoxia) leads to ATP depletion, activation of the mitochondrial permeability transition pore (mPTP), and cell death. The F1FO-ATP synthase was shown to play a crucial role in mPTP formation in mammals1, 2. Remarkably, the embryos of brine shrimp Artemia franciscana, tolerate anoxic conditions and lack the Ca2+-induced mPTP opening3. Here, we performed a comparative analysis of mammalian and Artemia ATP synthases to reveal the inactivation mechanism of mPTP.
Materials & Methods: We used fluorescent spectroscopy and electron microscopy to study the mPTP opening; single-particle cryo-electron microscopy (cryo-EM) and electrophysiology recordings for structural and functional analysis of the ATP synthase leak channel, respectively.
Results: Artemia ATP synthase demonstrated brief, Ca2+-insensitive channel openings after prolonged latencies in electrophysiology recordings when compared with the porcine heart ATP synthase. Cryo-EM structural analysis of the Artemia ATP synthase atomic model revealed the strikingly different structure of the membrane-embedded subunits, elucidating the inactivation mechanism of the ATP synthase leak channel and its role in mPTP inhibition.
Conclusions: Artemia ATP synthase has distinct amino acid composition and structure that prevents the mPTP activation and hypoxia-induced cell death in this organism.
Funding
Supported by NIH K01AG054734 and RF1AG072484 to NM.
References
Mnatsakanyan N, et al. 2019. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat Commun. 10:5823. PMID 31862883.
Menze MA, et al. 2005. Mitochondrial permeability transition in the crustacean Artemia franciscana: absence of a calcium-regulated pore in the face of profound calcium storage. Am J Physiol Regul Integr Comp Physiol. 289:R68–76. PMID 15718386.
Urbani A, et al. 2019. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat Commun. 10:4341. PMID 31554800.
ATFS-1 counteracts mitochondrial DNA damage by promoting repair over transcription
Chuanyang Daia, Chaichee Nga, Grace Ching Ching Hunga, Ina Kirmesa, Laetitia A. Hughesb,c,d, Yunguang Due, Christopher A. Brosnana, Arnaud Ahiera, Anne Hahna, Cole M. Haynese, Oliver Rackhamb,c,d,f, Aleksandra Filipovskab,c,d and Steven Zuryna
aClem Jones Centre for Ageing Dementia Research, Queensland Brain Institute, The University of Queensland; bHarry Perkins Institute of Medical Research; cARC Centre of Excellence in Synthetic Biology, QEII Medical Centre, The University of Western Australia; dTelethon Kids Institute, Northern Entrance, Perth Children’s Hospital; eDepartment of Molecular, Cell and Cancer Biology, UMass Chan Medical School; fCurtin Medical School and Curtin Health Innovation Research Institute, Curtin University
The ability to balance conflicting functional demands is critical for ensuring organismal survival. The transcription and repair of the mitochondrial genome (mtDNA) requires separate enzymatic activities that can sterically compete1, suggesting a life-long trade-off between these two processes. In Caenorhabditis elegans, we find that the bZIP transcription factor ATFS-1/Atf52,3 regulates this balance in favour of mtDNA repair by localising to mitochondria and interfering with the assembly of the mitochondrial pre-initiation transcription complex between HMG-5/TFAM and RPOM-1/mtRNAP. ATFS-1-mediated transcriptional inhibition decreases age-dependent mtDNA molecular damage through the DNA glycosylase NTH-1/NTH1, as well as the helicase TWNK-1/TWNK, resulting in an enhancement in the functional longevity of cells and protection against decline in animal behaviour caused by targeted and severe mtDNA damage. Together, our findings reveal that ATFS-1 acts as a molecular focal point for the control of balance between genome expression and maintenance in the mitochondria.
References
Canugovi C, et al. 2010. The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair (Amst). 9:1080–1089. doi: 10.1016/j.dnarep.2010.07.009.
Fiorese CJ, et al. 2016. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 26:2037–2043. doi: 10.1016/j.cub.2016.06.002.
Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. 2012. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 337:587–590. https://doi.org:science.1223560
TPP-driven mitochondria-targeting compounds as agrochemicals
Zhaohai Qin
China Agricultural University, People’s Republic of China
Mitochondria are important organelles, and oxidative phosphorylation process taking place in mitochondria is one of the most important target of pesticides. As a highly lipophilic cation, triphenylphosphonium (TPP) salt has a high affinity for negatively charged mitochondria, which has attracted great attention and been successfully applied in the development of mitochondria-targeting drugs and probes. In recent years, our group has explored several pesticide candidates with excellent biological activities by conjugating TPP with pesticides acting on respiratory chain, which makes it possible for the development of TPP-driven mitochondria-targeting pesticides. Our studies have also shown that the action mechanism of these compounds may be complicated, including the damage to cell membrane caused by the surface effect of phosphonium salt, and the rapid destruction of electron transfer chain by ROS produced from the substrate-binding positions owing to the saturating inhibition of target proteins.
The rapid accumulation of TPP-conjugated cargos in mitochondria not only helps to improve their biological activity, but also favours to control the resistance generation of harmful organisms. Here we report the main progress in our lab in this field.
Funding
Supported by the National Natural Science Foundation of China [No. 21877125].
References
Liu XL, et al. 2022. Mitochondrion-targeted triphenylphosphonium-based kresoxim-methyl analogues: synthesis, fungicidal activity, and action mechanism approach. J Agric Food Chem. 70:13563−13573.
Wang JY, et al. 2022. Fungicidal action of the triphenylphosphonium-driven succinate dehydrogenase inhibitors is mediated by reactive oxygen species and suggests an effective resistance management strategy. J Agric Food Chem. 70:111–123.
Wang JY, et al. 2023. Discovery of triphenylphosphonium (TPP)-conjugated N-(1,1’-biphenyl) -2-yl aliphatic amides as excellent fungicidal candidates. Pest Manag Sci. doi: 10.1002/ps.7470.
Adjuvant therapy with molecular hydrogen improved platelet mitochondrial bioenergetics and coenzyme Q10 level in patients with non-alcoholic fatty liver disease
Zuzana Sumbalovaa, Jarmila Kucharskaa, Zuzana Rausovaa, Anna Gvozdjakovaa, Maria Zantovab, Branislav Kurac, Viliam Mojtob and Jan Slezakc
aFaculty of Medicine, Pharmacobiochemical Laboratory of 3rd Department of Internal Medicine, Comenius University in Bratislava, Slovakia; bFaculty of Medicine, 3rd Department of Internal Medicine, Comenius University in Bratislava, Slovakia; cCenter of Experimental Medicine, Institute for Heart Research, Slovak Academy of Sciences, Bratislava, Slovakia
Introduction: Non-alcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome. Mitochondrial dysfunction and oxidative stress play significant role in NAFLD progression. Molecular hydrogen (H2) recognised as a novel medical gas with antioxidant and anti-inflammatory effects can act as a stimulator of energy metabolism. We evaluated the effect of adjuvant therapy with hydrogen rich water (HRW) on platelet mitochondrial bioenergetics and endogenous coenzyme Q10 (CoQ10) levels of patients with NAFLD.
Methods & Materials: Patients with NAFLD received tablets producing HRW (H2 group) or placebo tablets (P group) for eight weeks. Platelet mitochondrial bioenergetics was assessed by high-resolution respirometry. The concentration of CoQ10-TOTAL was determined by HPLC, the parameter of oxidative stress TBARS was determined spectrophotometrically.
Results: In patients with NAFLD relatively higher CI-linked LEAK respiration, lower CI-linked OXPHOS and CII-linked ET capacity, and lower CoQ10 levels vs. control were recorded. Plasma TBARS concentration was increased in H2 group. After 8-week adjuvant therapy with HRW, deteriorated parameters of platelet mitochondrial bioenergetics improved, CoQ10 concentration in platelets increased, and plasma TBARS decreased.
Conclusions: Our results support the evidence that mitochondria are the primary target of H2 therapy. The application of H2 could be a new treatment strategy for mitochondrial disorders.
Funding
Supported by grants APVV-19-0317, VEGA 2/0063/18, 2/0092/222, 2/0148/22 and HRW Natural Products Inc.
References
Sumbalová Z, Kucharská J, Rausová Z, Gvozdjáková A, Szántová M, Kura B, Mojto V, Slezák J. 2023. The effect of adjuvant therapy with molecular hydrogen on endogenous coenzyme Q10 levels and platelet mitochondrial bioenergetics in patients with non-alcoholic fatty liver disease. Int J Mol Sci. 24(15):12477.
Effect of time-restricted feeding on mitochondria in skeletal muscle of aged mice
Andrea Del Campoa, Bastian Ramosa, Maria Jose Silvaa, Francisco Diaz-Castrob and Gonzalo Almarzaa
aPontificia Universidad Catolica de Chile, Chile; bINTA, Universidad de Chile, Chile
Introduction: Sarcopenia is the loss of muscle mass and function due to ageing. Herein, the role of mitochondrial dynamics in the muscle ageing process has not been fully elucidated. Studies show that time-restricted feeding (TRF) has positive effects on longevity, however its effect on skeletal muscle function and the mitochondrial network is controversial. Our study aims to determine the effect of TRF on mitochondrial morphology, and quality control in skeletal muscle of middle-aged mice.
Materials and Methods: C57BL/6 mice were divided into 6-month-old, 12-month-old, and 12-month-old undergoing TRF. At 10 months, the mice underwent a 2-month TRF protocol (12/12h) (bioethics protocol ID: 191226005). Soleus and gastrocnemius muscles were dissected. The effect of TRF model on muscle was evaluated by H&E staining, electron microscopy, and proteins related to mitochondrial quality control by Western blot.
Results: TRF causes a decrease in the length of the sarcomere in both muscles. Regarding mitochondrial populations, changes in mitochondrial morphology were observed depending on the composition of the muscle and the associated energy metabolism. Expression of CLPP and HSP60 was increased in both muscles.
Conclusion: TRF causes mitochondrial modifications, and a differential activation of mitochondrial quality control pathways dependent on muscle fibre type.
Funding
Supported by FONDECYT 1230428
References
Baker DJ, Betik AC, Krause DJ, Hepple RT. 2006. J Gerontol Ser A Biol Sci Med Sci. 61:675–84.
Williamson E, Moore DR. 2021. Front Nutr. 8:640621. doi: 10.3389/fnut.2021.640621.
Mitochondrial involvement in cardiac electrophysiological injury due to inhalation exposure to Electronic Nicotine Delivery Systems (ENDS)
Obada Abouassali, Bojjibabu Chidipi, Mengmeng Chang, Laurent Calcul and Sami Noujaim
Morsani College of Medicine, University of South Florida, USA
Introduction: ENDS use “e-liquids” to generate an inhalable aerosol containing nicotine and flavours (e-vapour). Flavoured ENDS are popular among teens who vape. Cardiac electrophysiological toxicity of vaping is uncertain. We tested if inhalation exposure to flavoured ENDS compromises the mitochondria, increases oxidative stress (OS), and leads to cardiac harm.
Materials and Methods: Gas chromatography/mass spectrometry (GC/MS), flow cytometry, microscopy, inhalation exposure, programmed electrical stimulation, tissue culture and a mouse model of mitochondrial catalase overexpression (MCAT) were used.
Results: HL-1 myocytes were exposed to e-vapour from 30 popular and differently flavoured e-liquids. Most e-liquids were toxic. We then quantified the concentrations of major flavouring carbonyls (cinnamaldehyde, vanillin, ethyl-vanillin, maltol, and ethyl-maltol) in the 30 e-liquids using GC/MS. Toxicity of the e-liquids in HL-1 cells correlated with their flavouring carbonyls concentration. OS was increased in HL-1 cells exposed to vanilla flavoured e-vapour but was blunted by N-acetylcysteine pretreatment. In mice, inhalation exposure to vanilla flavoured e-vapour increased inducible ventricular tachycardia duration in WT but not in MCAT mice versus controls. In electron microscopy, mitochondrial cristae thickness decreased in exposed WT, but not in exposed MCAT hearts versus controls.
Conclusion: Vaping negatively affects ventricular electrophysiology, partly via adverse mitochondrial remodelling, and increased OS.
References
D’Amario D, Migliaro S, Borovac JA, Vergallo R, Galli M, Restivo A, Bonini M, Romagnoli E, Leone AM, Crea F. 2019. Electronic cigarettes and cardiovascular risk: caution waiting for evidence. Eur Cardiol. 14:151–158.
Abouassali O, Chang M, Chidipi B, Martinez JL, Reiser M, Kanithi M, Soni R, McDonald TV, Herweg B, Saiz J, et al. 2021. In vitro and in vivo cardiac toxicity of flavoured electronic nicotine delivery systems. Am J Physiol Heart Circ Physiol. 20:H133–H143.
Wang TW, Gentzke AS, Neff LJ, Glidden EV, Jamal A, Park-Lee E, Ren C, Cullen KA, King BA, Hacker KA. 2021. Characteristics of e-cigarette use behaviours among US youth, 2020. JAMA Network Open. 4:e2111336.
Super-resolution imaging of voltages in the interior of individual, vital mitochondria
ChiaHung Lee, Douglas Wallace and Peter John Burke
UC Irvine, USA
We present super-resolution microscopy of isolated functional mitochondria, enabling real-time studies of structure and function (voltages) in response to pharmacological manipulation. Changes in mitochondrial membrane potential as a function of time and position can be imaged in different metabolic states (not possible in whole cells), created by the addition of substrates and inhibitors of the electron transport chain, enabled by the isolation of vital mitochondria.
By careful analysis of structure dyes and voltage dyes (lipophilic cations), we demonstrate that most of the fluorescent signal seen from voltage dyes is due to membrane bound dyes, and develop a model for the membrane potential dependence of the fluorescence contrast for the case of super-resolution imaging, and how it relates to membrane potential. This permits direct analysis of mitochondrial structure and function (voltage) of isolated, individual mitochondria as well as submitochondrial structures in the functional, intact state, a major advance in super-resolution studies of living organelles.
Funding
This work was supported in part by NIH grant 1 R01 CA259635-01A1 and 3 R01 CA243033-03S1A1, National Science Foundation (NSF) award #2153425, and Army Research Office through the ARO- (Contract Nos. W911NF-18-1-0076, W911NF2010103, and W911NF1910369).
References
Lee C, Wallace DC, Burke PJ. 2023. Super-resolution imaging of voltages in the interior of individual, vital mitochondria. ACS Nano doi: 10.1021/acsnano.3c02768.
ATP synthase c-Subunit upregulation causes mitochondrial dysfunction in SHANK3 mouse model of autism
Wajeha Hamoudi, Manish Kumar Tripathi and Haitham Amal
Hebrew University of Jerusalem, Israel
Introduction: A strong link between the SHANK3 gene mutations and autism spectrum disorder (ASD) has been established but its molecular mechanisms remain unknown1,2. Our preliminary proteomics study revealed an aberrant protein expression of ATP synthase c-subunit in the brain of the Shank3Δex4-22 mice. This aberrant expression affects ATP-synthase activity and may lead to mitochondrial dysfunction. Consequently, we aimed to investigate the role of this subunit and mitochondrial dysfunction in the Shank3 mice.
Methods: Global proteomics of the cortex in Shank3Δex4-22 and its wild-type (WT) mice were further analysed. Mitochondrial membrane potential (MMP) was assessed in the cortical neurons isolated from both groups using tetramethylrhodamine methyl ester (TMRM). Oxygen consumption rate (OCR) also was measured by Seahorse XF Assays. Shank3Δex4-22 mice were treated with HU-55, which modulates the ATP synthase leak, and the behaviour of these mice was investigated.
Results: The c-Subunit protein level was significantly increased in the cortex of Shank3Δex4-22 mice. Additionally, the cortical neurons of these mice exhibited increased mitochondrial proton leak and a reduction of MMP,indicating mitochondrial dysfunction. Treating the mutant mice with HU-55 attenuated autism-like behaviour.
Conclusions: These findings provide a new potential direction in the search for therapeutic interventions for ASD.
Funding
Supported by WH is a fellow of the Arian de Rothschild woman Doctoral Programme.
References
Durand CM, et al. 2007. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 39:25–27. doi: 10.1038/ng1933.
Phelan K, McDermid HE. 2012. The 22q13.3 Deletion syndrome (Phelan-McDermid syndrome). Mol Syndromol. 2:186–201. doi: 10.1159/000334260.
Mitochondrial transplantation therapy against ifosfamide induced toxicity on rat renal proximal tubular cells
Melika Mashhadi, Abdollah Arjmand and Jalal Pour Ahmad
School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Mitochondrial dysfunction is a basic mechanism leading to drug nephrotoxicity(1). Replacement of defective mitochondria with freshly isolated mitochondria is potentially the comprehensive tool to inhibit cytotoxicity induced by ifosfamide on renal proximal tubular cells (RPTCs)(2). We hypothesised that the exposure of freshly isolated mitochondria into RPTCs affected by ifosfamide might restore mitochondrial function and reduce the cytotoxicity. So, the aim of this study was to assess the protective effect of freshly isolated mitochondrial transplantation against ifosfamide-induced cytotoxicity in RPTCs.
Methods: Ifosfamide was incubated in the suspension of rat RPTCs in Earle’s solution incubated for 2h after addition. Fresh mitochondria were isolated from the rat kidney and diluted to the needed concentrations. The media containing suspended RPTCs was replaced with mitochondrial supplemented media, which was exposed to cells.
Results: Statistical analysis demonstrated that mitochondrial administration reduced cytotoxicity, lipid peroxidation, reactive oxygen species production, mitochondrial membrane potential collapse, lysosomal membrane damage, extracellular oxidised glutathione level, and caspase-3 activity induced by ifosfamide in rat RPTCs. Moreover, mitochondrial transplantation increased the intracellular reduced glutathione level in RPTCs affected by ifosfamide.
Conclusions: According to the current study, mitochondrial transplantation is a promising therapeutic method in xenobiotic-caused nephrotoxicity pending on successful complementary in vivo and clinical studies.
Refrences
Katrangi E, D’Souza G, Boddapati SV, Kulawiec M, Singh KK, Bigger B, et al. 2007. Xenogenic transfer of isolated murine mitochondria into human ρ 0 cells can improve respiratory function. 10(4):561–750.
McGuinness S, Ryan MJTiv. 1994. Mechanism of cisplatin nephrotoxicity in rat renal proximal tubule suspensions. 8(6):1203–1212.
Neural precursors derived from trisomy 21 IPSCS show a deficiency in mitophagy
Nunzia Mollo, Adriana Limone, Morena D’Ariano, Rosalba Natale, Maria Duraccio, Daniela Sarnataro, Simona Paladino, Gabriella De Vita, Anna Conti, Lucio Nitsch and Antonella Izzo
Department of Molecular Medicine and Medical Biotechnology, University Federico II, Naples, Italy
Introduction: Neural precursor cells (NPCs) obtained from trisomy 21 iPSCs manifest mitochondrial dysfunction, with accumulation of damaged mitochondria, very early during neuronal differentiation (1) suggesting a potential role in the alterations of neurogenesis observed in Down syndrome (DS). We assessed whether the mitochondrial quality control system is impaired in trisomic NPCs.
Materials & Methods: Isogenic trisomic and euploid iPSCs were differentiated into NPCs in monolayer cultures using the dual-SMAD inhibition protocol. Expression of fission/fusion machinery and mitophagy-related genes and proteins was assessed by qRT-PCR and Western Blot.
Results: DRP1 and MFN2, fission/fusion machinery genes, were upregulated and downregulated, respectively, in trisomic NPCs. In addition, the mitophagy PINK1/PARKIN-mediated mechanisms were altered. Indeed, increased expression of the kinase PINK1, the E3-ubiquitin ligase PARKIN and the mitophagic receptor OPTINEURIN was found, suggesting the recruitment of these proteins on damaged mitochondria. Autophagic flux was impaired as there was significant accumulation of autophagosomes (LC3II) after CCCP treatment, which was accompanied by a reduced expression of some lysosomal genes and proteins.
Conclusions: There is a mitophagy impairment in trisomic NPCs, which could contribute to the defect in neurogenesis and/or to neurodegeneration observed in DS. Mitophagy therefore represents a potential therapeutic target in DS.
Funding
Post-Doctoral Fellowship founded from The Jerome Lejeune Foundation of 130,000.00€ to the Department of Molecular Medicine and Medical Biotechnology, University Federico II, Naples, Italy in view of supporting the project of research managed by Dr. Nunzia Mollo and named “Defects in mitophagy and mitochondrial activity as key determinants of Alzheimer’s disease in Down Syndrome: novel therapeutic strategies”.
Reference
Mollo, et al. 2021 Jun 30. Human trisomic iPSCs from down syndrome fibroblasts manifest mitochondrial alterations early during neuronal differentiation. Biology (Basel). 10(7):609.
Aminolevulinate/iron exposure enhances mitochondrial health in DARS2 deficient fibroblasts
Jose Eduardo Abdenura,b, Wei-Lin Huanga, Tuany Eichwalda,b,c, Milad Gazanfaria, Alexander Stovera, Philip Schwartza and Alexandra Latinia,c
aCHOC Children’s Hospital of Orange, USA; bDepartment of Pediatrics, University of California Irvine, US; cDepartamento de Bioquímica, Universidade Federal de Santa Catarina, Brazil
Introduction: DARS2 deficiency is a mitochondrial disorder characterised by leukoencephalopathy, brainstem and spinal cord involvement, and lactate elevation. Considering that there is no cure for this metabolic disorder and that aminolevulinate plus iron (ALA/Fe) has shown to increase mitochondrial iron-containing proteins, the effect of ALA/Fe on energy and oxidant metabolisms in DARS2-deficient cells was investigated.
Material & Methods: Fibroblasts from two affected individuals and from a non-carrier asymptomatic sibling (control) were exposed to ALA/Fe (100mM/50mM) for 14 days and the energy metabolism and antioxidant status were assessed.
Results: DARS2-deficient cells showed reduced mitochondrial respiration and activity of complexes I and IV, with increased lactate. Reduced glutathione/oxidised glutathione ratio and catalase activity and increased reactive species production were also observed. ALA/Fe-exposure ameliorated these deficiencies and increased the non-compromised parameters at the basal condition, including superoxide dismutase and glutathione peroxidase activities. Mechanistically, the positive effects of ALA-Fe may be linked to Nrf-2 pathway activation, since the main downstream proteins, heme oxygenase-1 and NAD(P)H-quinone dehydrogenase-1, as well as the metabolite, bilirubin, were increased.
Conclusion: ALA/Fe exposure reestablished or attenuated the altered metabolism in DARS2-affected cells. Considering that ALA is an approved FDA compound, it is feasible to propose its off-label use in DARS2 deficiency.
Funding
Supported by CSO (16984019); Cure for LBSL(16984017).
References
Shimura M, Nozawa N, Ogawa-Tominaga M et al. 2019. Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci Rep. 9:10549.
Remor AP, da Silva RA, de Matos FJ, et al. 2019. Chronic Metabolic Derangement-induced cognitive deficits and neurotoxicity are associated with REST Inactivation. Mol Neurobiol. 56:1539–1557.
Van Der Knaap MS, Van Der Voorn P, Barkhof F, et al. 2003. A new leukoencephalopathy with brainstem and spinal cord involvement and high lactate. Ann Neurol. 53:252–258.
Inhibitors of fumarylacetoacetate domain containing protein 1 (FAHD1) induce cell death in basal BT-20 breast cancer cells in vitro
Andreas Andrica, Riccardo Giaquintaa, Tatjana Kuena, Hubert Gstachb and Alexander Weissa
aUniversity of Innsbruck, Austria; bUniversity of Vienna, Austria
The eukaryotic oxaloacetate decarboxylase FAH domain containing protein 1 (FAHD1) is involved in the regulation of the TCA cycle flux. Multi-omics data and text mining reports FAHD1 to be among metabolic genes that may serve as potential novel therapeutic targets in oestrogen-receptor negative breast tumours.
We reported how the mitochondrial enzyme FAHD1 regulates complex-II activity in breast cancer cells and why is indispensable for glutamine-dependent cells in vitro, such as basal BT-20 cells. Depletion of FAHD1 in BT-20 breast cancer cells eventually results in cell death, and we aim to mimic this effect by inhibition.
References
Dinesh KKB, et al. 2019. Prioritisation of metabolic genes as novel therapeutic targets in oestrogen-receptor negative breast tumours using multi-omics data and text mining. Oncotarget. 10(39). doi: 10.18632/oncotarget.26995.
Holzknecht M, et al. 2022. P. The mitochondrial enzyme FAHD1 regulates complex-II activity in breast cancer cells and is indispensable for basal BT-20 cells in vitro. FEBS Lett. 596. doi: 10.1002/1873-3468.14462.
Weiss AKH, et al. 2020. Regulation of cellular senescence by eukaryotic members of the FAH superfamily - A role in calcium homoeostasis? Mech Ageing Dev. 190:111284. doi: 10.1016/j.mad.2020.111284.
Weiss AKH, et al. 2021. Inhibitors of Fumarylacetoacetate Hydrolase Domain Containing Protein 1 (FAHD1). Molecules. 26(16):5009. doi: 10.3390/molecules26165009.
NAC regulatory effects on mitochondrial GSH, supercomplexes and ROS production alterations in experimental in chronic kidney diseases
Omar Emiliano Aparicio-Trejoa,b, Diana Nataly Meza-Estradaa, Belén Cuevas-Lópeza, Edilia Tapiaa, Juan Carlos León-Contrerasc, Alejandro Silva-Palaciosd and Laura Gabriela Sánchez-Lozadaa
aDepartment of Cardio-Renal Pathophysiology, Instituto Nacional de Cardiología, Mexico; bPharmacy Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City, Mexico; cExperimental Pathology Section, National Institute of Medical Sciences and Nutrition, Mexico City, Mexico; dDepartment of Cardiovascular Biomedicine, National Institute of Cardiology Ignacio Chávez, Mexico City, Mexico
Introduction: Mitochondrial dysfunction is key in chronic kidney disease (CKD) development, favouring kidney inflammation. However, the molecular mechanisms are not fully understood. It has suggested that supercomplexes formation is regulated by mitochondrial GSH levels which could be related to the supercomplexes lost, however their role in CKD has not been explored. The N-acetylcysteine (NAC) prevents the loss in mitochondrial GSH levels and supercomplexes lost in cardiac ischemia. However, if NAC could regulate the supercomplexes levels by mitochondrial GSH regulation in kidney has not been described.
Material & Methods: CKD was induced by 5/6 nephrectomy (NX5/6) in male Wistar rats. NAC was administrated intragastric (300 mg/Kg) 2 hours before the surgery and one dose daily. The groups were evolved for 10 days after surgery.
Results: 5/6NX decreased CI, CIII and OXPHOS, GPx and GR activity in renal mitochondria, reduced GSH levels and increased mitochondrial H2O2 production by supercomplexes downregulation, which correlated with the kidney damage markers increase. In contrast, NAC treatment reversed the mitochondrial alterations, by GSH and supercomplexes levels restoring in kidney, decreasing the CKD progression.
Conclusion: Our data suggest that the early decrease in GSH levels favours mitochondrial supercomplexes downregulation and ROS production increase, favouring CKD development.
References
Aparicio-Trejo OE, et al. 2019. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med. 130:379–396. doi: 10.1016/j.freeradbiomed.2018.11.005.
Avila-Rojas SH, et al. 2020. Alterations in mitochondrial homoeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem Toxicol. 145:111774. doi: 10.1016/j.fct.2020.111774.
Ramírez-Camacho I, et al. 2018. Cardioprotective strategies preserve the stability of respiratory chain supercomplexes and reduce oxidative stress in reperfused ischemic hearts. Free Radic Biol Med. 129:407–417. doi: 10.1016/j.freeradbiomed.2018.09.047.
Mitochondrial oxidative phosphorylation and dynamics in plasmodium berghei-infected mice treated with a novel compound purified from phyllanthus amarus
Opeyemi Babarinde, Oludele Olanlokun and Olufunso Olorunsogo
University of Ibadan, Nigeria, Nigeria
Introduction: Mitochondria occupy prominent positions in cell metabolism, however, infection by Plasmodium causes their dysfunction. Antimalarial orthodox drugs affect host oxidative phosphorylation and mitochondrial dynamics.
Materials & Methods: The influence of 1, 3, 16 trimethoxy-10 methyl-17-(pent-3-enyl)cyclopenta[α]phenanthrene (TMCP) purified from Phyllanthus amarus, on oxidative phosphorylation and mitochondrial dynamics in Plasmodium berghei-infected mice was investigated via the extent of expressions of genes that code for mitochondrial complexes, dynamics and mitophagy. Total RNA was isolated from grouped P. berghei-infected mice, treated with 10mg/kg TMCP, artesunate-amodiaquine (control) and infected control treated with vehicle. The PCR amplification for the respective genes were performed and amplified, while the amplicons were resolved on agarose gel and processed.
Conclusion: Plasmodium berghei infection decreased the expressions of mitochondrial complexes while TMCP increased them. The PGC-1α, prohibitins 1 and 2 were increased significantly by TMCP relative to control. Increase in DRP-1 mediates mitochondrial fission in the infected mice treated with TMCP while significant expressions of OPA1 and mitofusin1 in mice treated with TMCP elicit significant mitochondrial fusion. The TMCP initiated PINK 1-dependent mitophagy and FUNDC1 expression relative to control. The results indicate that TMCP prevents bioenergetic stress and modulate mitochondrial dynamics for effective mitophagy.
References
Hou HP, Zhang GP, Ma LN, Su P, Zhang ZX, Dai BQ, Ye ZG. 2020. Effects and mechanism of action of artemisinin on mitochondria of plasmodium berghei. Chin J Integr Med. 26(4):277–282.
Omotuyi OI, Nash O, Inyang OK, Ogidigo J, Enejoh O, Okpalefe O, Hamada T. 2018. Flavonoid-rich extract of Chromolaena odorata modulate circulating glp-1 in Wistar rats: computational evaluation of tgr5 involvement. 3 Biotech. 8(2):124.
Omotuyi OI, Nash O, Enejoh OA, Oribamise EI, Adelakun NS. 2020. Chromolaena odorata flavonoids attenuate experimental nephropathy: Involvement of pro-inflammatory genes downregulation. Toxicol Rep. 7:1421–1427.
Increased mitochondrial damage in salivary epithelial cells is associated to inflammation in Sjögren’s syndrome
María José Barreraa, Patricia Carvajalb, Francisco Sandovala, Víctor Bustamantea, Isabel Castrob, Daniela Jarab, Sergio Aguilerac, María José Yañeza, Félix Urrab, Sergio Gonzalezd, Claudio Molinaa and María Julieta Gonzalezb
aUniversidad San Sebastián, Chile; bFacultad de Medicina, Universidad de Chile, Chile; cDepartamento de Reumatología, Clínica INDISA, Chile; dFacultad de Medicina y Ciencias de la Salud, Universidad Mayor, Chile
Introduction: Sjögren’s syndrome (SS) is an autoimmune disorder characterised by salivary gland (SG) dysfunction. Emerging evidence indicates a potential link between SS and mitochondrial dysfunction. Here, we determined whether mitochondrial ultrastructural alterations in SS could be associated with glandular inflammation.
Materials & Methods: SGs from SS-patients and controls, and SS murine model (NOD.B10Sn-H2b/J mice) treated or not with tofacitinib anti-inflammatory were analysed. Mitochondrial ultrastructure was assessed using electron microscopy and protein levels of pattern recognition receptors (PRRs) were determined by Western blot. The presence of mtDNA in cytosol was determined by subcellular fractionation followed by real-time-PCR and immunofluorescence. Quantification of cGAMP, ATP and ROS levels was also determined.
Results: SGs epithelial cells of SS-mice presented mitochondrial ultrastructural alterations, consistent with observations in SS-patients. mtDNA was increased in cytosolic fractions of SG and epithelial cells cytoplasm of SS-patients. In SS-mice, tofacitinib improved mitochondrial architecture and ATP levels, while decreased levels of ROS, PRRs such as cGAS, and cGAMP.
Conclusion: In SS, SG epithelial cells release mtDNA, which is associated to increased expression of PRRs such as cGAS, possibly due to a damaged mitochondrial membrane integrity. Tofacitinib treatment suggests a plausible connection between dysfunctional mitochondria and SS-related inflammation.
Funding
Supported by Fondecyt Iniciación 11201058, Fondecyt Postdoctoral 3170023, Fondecyt 1210055, Fondecyt 1160015, Fondecyt 1230852.
References
Autoimmun Rev. 2021 Aug. 20(8):102867. doi: 10.1016/j.autrev.2021.102867.
Curr Protoc. 2022 Feb. 2(2):e372. doi: 10.1002/cpz1.372.
J Autoimmun. 2013 May. 42:7-18. doi: 10.1016/j.jaut.2013.02.001.
Mitochondrial specific peptides as novel therapeutics against sarcopenia
Yoonhwa Choia,c, Dong Heon Yib, Dongmin Kim Kima,c, Hyeong Woon Choea, Tae Yeon Kimb, Hyo Youl Moonb and Jaehoon Yua,c
aDepartment of Chemistry & Education, Seoul National University, Seoul, Korea, Republic of (South Korea); bDepartment of Physical Education, Seoul National University, Seoul, Korea, Republic of (South Korea); cCAMP Therapeutics, Seoul, Korea, Republic of (South Korea)
Sarcopenia is a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength by chronic diseases, ageing, and lack of exercise for a long period of time. One of the common characteristics of the disease includes alterations in mitochondrial function. Mitochondrial dysfunction in skeletal muscle cells is mainly derived from an increase of reactive oxygen species (ROS) inside of the organelle, resulting in muscle loss and a decrease in exercise capacity. We have been developing mitochondria-specific peptides, which can reduce ROS generation, correcting mitochondrial dysfunction. In this study, we try to reverse muscle dysfunction in two different pathogenic animal models for sarcopenia. One model is Drosophila model with phosphatidylserine synthase (Pss) mutation, which generates phenotypes of muscular dystrophy mutation. The other model is an old female mouse, where mitochondria in skeletal muscle are severely damaged in shapes and numbers. Treatment of the mitochondrial specific peptide to the Pss-mutated Drosophila model resulted in enhanced locomotive capacity of larva-stage fly as well as proper development of flight muscle in adult-stage fly. Treatment of the peptide to old mouse also showed enhanced exercise capacity with correcting mitochondrial morphology. Collectively, mitochondrial-targeted peptides may be to clinical trials for therapeutics against sarcopenia.
References
Campbell MD, Duan J, Samuelson AT, Gaffrey MJ, Merrihew GE, Egertson JD, … Marcinek DJ. 2019. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med. 134:268–281.
Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, … Blau HM. 2021. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. 371(6528):eabc8059.
Mitochondrial Ca2+ and functional adaptations to nutritional signals: insights from caloric restriction
Julian David Cualcialpud Sernaa, Alicia Juliana Kowaltowskib, Anna Raffaelloa, Ana Claudia Bonassaa, Eloisa Vilas-Boasa, Andressa Godoy Amarala and Camille Caldeiraa
aUniversidade de Sao Paulo, Brazil; bUniversità di Padova
Caloric restriction (CR) increases life span and boosts resistance against various forms of injury. Previous data indicate that mitochondria from liver and brain of CR animals display reduced sensitivity to permeability transition (mPT), a critical pathophysiological event in cell damage1. Here, we characterised alterations in mitochondrial functionality after six months of CR in rat hearts and kidneys, both of which are protected by CR against Ca2+-mediated injuries. In heart, no CR-induced changes were observed in mitochondrial capacity to manage and store Ca2+, nor in bioenergetic and redox parameters. Interestingly, mitochondria exhibit higher NCLX levels and Na+-dependent Ca2+ efflux rates, which protect mitochondria against Ca2+ overload2. In kidney, CR promotes an increase in respiration and H2O2 release. Unexpectedly, sensitises mitochondria to mPT in a manner that can be reversed by antioxidants. Moreover, CR mitochondria displayed higher Ca2+ uptake rates, which correlate with MICU2 loss, a crucial modulator of the mitochondrial Ca2+ uniporter3. Similar alterations in MICU composition can be recapitulated in vitro (HeLa and HepG2) in response to insulin, growth factors, and nutrient deprivation.
Collectively, our findings highlight the organ-specific effects of CR and strongly suggest the remarkable plasticity of MICU proteins in the regulation of mitochondrial Ca2+ transport by nutrients.
Funding
Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP.
References
Bonora M, Giorgi C, Pinton P. 2022. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol. 23:266–285.
Giorgi C, Marchi S, Pinton P. 2018. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 19:713–730.
Luongo T, Lambert J, Gross P, et al. 2017. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homoeostasis and viability. Nature. 545:93–97.
Impact of mesenchymal stromal cell-derived mitochondria transplantation on RIG agonist-activated lung cells
Sebastián Castillo-Galana,b, Yessia Hidalgoa,b, Nicolas Georgesa,b, Felipe Grunenwalda,b and Jimena Cuencaa,b,c,d
aCentro de Investigación e Innovación Biomédica (CIIB), Universidad de los Andes, Santiago, Chile; bIMPACT, Center of Interventional Medicine for Precision and Advanced Cellular Therapy, Santiago, Chile; cCells for Cells, Santiago, Chile; dConsorcio REGENERO, The Chilean Consortium for Regenerative Medicine, Santiago, Chile
Introduction: Mitochondrial dysfunction is a common occurrence during viral respiratory diseases. Mitochondrial antiviral signalling protein (MAVS) plays a pivotal in the host defense against viral infections, and viruses have evolved various strategies to hijack this pathway (1). Transplantation of mitochondria represents a novel therapeutic approach for treating many diseases (2). In this study, we investigate the effects of mitochondria transplantation on lung epithelial cells activated with a synthetic RNA ligand.
Materials & Methods: Mitochondria and MAVS-enriched mitochondria were isolated from Mesenchymal Stromal Cells (MSC) (3). Using an in vitro model of lung epithelial cells activated with an RNA ligand, we assessed the impact of mitochondria transplantation on cell viability, ATP production, mitochondria membrane potential, and ROS. Additionally, functionally labelled mitochondria-rich fractions were intravenously transplanted into healthy mice to evaluate their biodistribution.
Results: Lung epithelial cells exposed to mitochondria-rich fractions isolated from MSC showed increased viability, recovered ATP levels and mitochondrial membrane potential, and decreased ROS. Labelled mitochondria were detected at varying intensity levels in all the studied organs.
Conclusion: Mitochondrial transplantation from MSCs successfully ameliorated mitochondrial and cellular dysfunction in RIG agonist-activated lung cells. Mitochondria-based therapy holds promise as a therapeutic approach for enhancing the treatment of viral respiratory diseases.
Funding
Supported by ANID-Fondecyt Regular N° 1211376, IMPACT #FB210024, Fondecyt Postdoctoral N° 323044.
References
Court AC, Le‐Gatt A, Luz‐Crawford P, et al. 2020. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 21.
Ren Z, Ding T, Zuo Z, et al. 2020. Regulation of MAVS expression and signalling function in the antiviral innate immune response. Front Immunol. 11:1030.
Velarde F, Ezquerra S, Delbruyere X, et al. 2022. Mesenchymal stem cell ‑ mediated transfer of mitochondria : mechanisms and functional impact. Cell Mol Life Sci.
Mitochondrial oxidative metabolism and glycolytic activity of leukaemic blasts as prognostic biomarkers predicting overall survival in acute myeloid Leukaemia patients
Claire Deganda, Quentin Foveza, Axel Chomya, Patrick Devosb, Laure Goursaudc, William Lainea, Tony Kaomad, Emeline Boete, Estelle Salande, Christian Rechere, Véronique De Masf, Ambrine Sahale, Enzo Bosettae, Céline Berthonc, François Vergezf, Christophe Roumierg, Claude Preudhommeg, Philippe Marchettia, Bruno Quesnelc, Jean-Emmanuel Sarrye and Jérôme Kluzaa
aUniversity of Lille, CANTHER, Oncolille, Leukaemia, France; bUniversity of Lille, ULR2694-METRICS, France; cHematology Department, CHU Lille, France; dBioinformatics Platform, Luxembourg Institute of Health, Luxembourg; eUniversity of Toulouse, Cancer Research Center, France; fHematology Department, CHU Toulouse, France; gBiology Pathology Genetics Department, CHU Lille, France
Introduction: The current AML patient classification ELN allows for prognostic stratification, which facilitates the proposal of therapeutic regimens. Although this classification has limitations in accurately predicting outcomes. Energy metabolism influences aggressiveness and treatment response in AML(1). Evaluating the bioenergetic activity of AML patient blasts could provide new predictive information on treatment response.
Methods: Using the XFe24 Seahorse, we determined different parameters of mitochondrial oxygen consumption rate and extracellular acidification rate (ECAR) of AML patient blasts at diagnosis(2). These measurements were collected from peripheral blood samples of 68 patients. Theses parameters were collected and have been evaluated as predictive biomarkers.
Results: We identified predictive parameters through a multistep statistical analysis to determine the relationship between each metabolic variable and overall survival (OS) using the Cox model. We found three groups based on spare respiratory capacity (SRC) and ECAR levels with different outcomes in terms of event-free survival (EFS) and OS. Patients with high SRC and ECAR blasts have prolonged EFS and OS compared to patients with decreased SRC values or high SRC but decreased ECAR values.
Conclusion: This study showed that mitochondrial and cellular energetic assessment represents new functional biomarkers and could help the clinicians to determine the prognosis.
References
Farge T. 2017. Chemotherapy-resistant human acute myeloid leukaemia cells are not enriched for leukaemic stem cells but require oxidative metabolism. Cancer Discov.
Fovez Q. 2021. Clinically relevant oxygraphic assay to assess mitochondrial energy metabolism in acute myeloid leukaemia patients. Cancers (Basel).
Isolated peripheral blood cells as a possible marker for mitochondrial dysfunction in patients with mild cognitive impairement
Fabian Dietera, Silke Maturab and Gunter Peter Eckerta
aJustus-Liebig-Universität Gießen, Germany; bUniversitätsklinikum Frankfurt - Goethe Universität, Germany
Introduction: Mitochondrial dysfunction is a hallmark of cellular senescence and many age-related neurodegenerative diseases1. We therefore investigated the relationship between mitochondrial function in peripheral blood cell energy metabolism in young, old and healthy individuals and individuals with mild cognitive impairment (MCI).
Material & Methods: Cognitive health was evaluated using established psychometric methods (MMSE, CERAD). PBMCs were isolated via density centrifugation. To identify possible differences between patient populations, we examined mitochondrial respiration, adenosine triphosphate (ATP) production, and citrate synthase activity (CS) in PBMCs.
Results: Comparing the groups with each other, it can be seen that young healthy subjects have a higher ATP level, increased complex activity of the respiratory chain and CS. Comparing MCI patients with healthy age-matched subjects, ATP levels are lower and endogenous respiration is significantly reduced, whereas CS activity is unchanged.
Conclusion: The measured bioenergetic values show that in the course of life the energy supply in PBMCs decreases. This seems to be the case more clearly in patients with MCI than in their peers. This could be an indication of mitochondrial dysfunction which could also affect cognitive status.
References
Haas RH. 2019. Mitochondrial dysfunction in ageing and diseases of ageing. Biology. 8(2):48.
CBT101, a creatine ester prodrug, secures energy supply and boosts mitochondrial dynamics in the 6OHDA rat model
Clemence Disdiera, Henri Benecha, Jacques Callebertb, Thomas Joudinauda and Aloïse Mabondzoa,c
aCERES BRAIN Therapeutics, Paris Brain Institute (ICM), Pitié-Salpêtrière Hospital, Paris, France; bInserm UMR-S 1144, Université Paris, Optimisation Thérapeutique en Neuropsychopharmacologie and Laboratoire de Biochimie et Biologie Moléculaire, Hôpital Lariboisière, Paris, France; cUniversité Paris Saclay, CEA, DMTS/SPI/Laboratoire d’Etude de l’Unité Neurovasculaire & Innovation Thérapeutique, Gif sur Yvette, France
Introduction: Creatine is promising against cerebral mitochondrial diseases as an energy buffer, antioxidant, antiapoptotic and antiexcitotoxic. However, oral creatine is ineffective due to poor brain and neuronal penetration. CBT101, a creatine prodrug1, is currently developed for the treatment of Creatine Transporter Deficiency Disease 2, 3. CBT101 has been proven to deliver creatine to neurons after intranasal dosing. The objective of this work is to demonstrate the therapeutic efficacy of CBT101 in a context of mitochondrial dysfunctions.
Methods: Striatal 6-hydroxydopamine lesioned rat were treated with CBT101 by the nose for a month. The amphetamine-induced turning test and the beam walking test was performed. Dopamine was quantified in the striatum. The transcriptional expression of key mitochondrial proteins was quantify by RT-qPCR in cortex.
Results: CBT101 improve behavioural test scores, increase dopamine levels in the striatum and increase transcriptional expression of fusion proteins such as OPA1, fission proteins DRP1 and FIS1 and some proteins implicated in the cristae structure in the cortex such as PHB1.
Conclusions: Our findings show that CBT101 given by the intranasal route over one month results in positive outcomes in this model of mitochondrial toxicity. By bringing creatine into neurons, CBT101 secure energy supply and boost mitochondrial protein expression.
Funding
Supported by CERES BRAIN THERAPEUTICS.
References
Mabondzo A, et al. 2023. Dodecyl creatine ester improves cognitive function and identifies key protein drivers including KIF1A and PLCB1 in a mouse model of creatine transporter deficiency. Front Mol Neurosci. doi: 10.3389/fnmol.2023.1118707.
Trotier-Faurion A, et al. 2015. Dodecyl creatine ester and lipid nanocapsule: a double strategy for the treatment of creatine transporter deficiency. Nanomedicine. 10(2):185–191. doi: 10.2217/nnm.13.205.
Ullio-Gamboa G, et al. 2019. Dodecyl creatine ester-loaded nanoemulsion as a promising therapy for creatine transporter deficiency. Nanomedicine. 14(12):1579–1593. doi: 10.2217/nnm-2019-0059.
Evaluation of CoQ10 and alpha-ketoglutarate effect on inflammatory and mitochondrial key genes expression in colitis
Karen Dubois-Camachoa,b, Sebastian Fuentes-Retamala, Marjorie De La Fuentec, Glauben Landskronc, Daniela Simiand, Camila Estayd, Hector Molinaa, Gonzalo Vásqueza, Daniela Paradab, Klaas N. Faberb, Marcela A. Hermosoa,b and Félix A. Urraa
aUniversidad de Chile, Chile; bUniversity Medical Center Groningen, The Netherlands; cUniversidad Finis Terrae, Chile; dHospital Clínico Universidad de Chile, Chile
Introduction: Ulcerative colitis (UC) is an inflammatory bowel disease related to mitochondrial dysfunction due to tricarboxylic acid cycle metabolite downregulation1, decreased electron chain transporter genes (ETC) with lower ETC complex activity2-3 in inflamed tissue. Alpha-ketoglutarate (α-KG) or CoenzymeQ10 (CoQ10) supplementation have antioxidant effects in in-vivo colitis models, although their role in mitochondrial adaptation and inflammation reversion in UC patients is unclear. We evaluate the effect of CoQ10 and cell-permeable α-KG derivative (DM-aKG) on inflammatory and mitochondrial markers in colonic inflamed biopsies from UC patients.
Materials & Methods: Inflamed colonic biopsies (UC-Mayo score 2-3/n=7) and healthy tissue (healthy controls-HC/n=4) were treated ex-vivo with CoQ10 10uM or DM-aKG 1mM (24 hours). Gene expression of mitochondria function/antioxidants-related genes (PGC1A, TFAM, and GPX1A) and cytokines (TNF, IL10) was evaluated by qPCR. Cytokines were measured by cytometric bead array from supernatants.
Results: PGC1A and TFAM transcripts were decreased (P<0.01, P<0.05) in UC versus HC basal biopsies. DM-aKG induced PGC1A, TFAM and GPX1A transcripts in UC versus HC (P<0.05). CoQ10 decreased TNF in supernatants (P<0.05) and tended to decrease IL6, increasing PGC1A transcript only in patients under 5ASA treatment.
Conclusion: CoQ10 might reduce inflammatory markers with PGC1A induction, mainly in UC patients 5ASA-treated.
Funding
Supported by Fondecyt 3210367, 11201322, 1220702, Anillo ACT210097.
References
Dubois-Camacho K, et al. 2019. Front Immunol. 10:2449. doi: 10.3389/fimmu.2019.02449.
Haberman Y, et al. 2019. Nat Commun. 10:38. doi: 10.1038/s41467-018-07841-3.
Ooi M, et al. 2011. Inflamm Res. 60:831–840. doi: 10.1007/s00011-011-0340-7.
Mitochondrial function characterisation of diabetes type 1B cases
Rana El Nahas, Basma Haris, Nicholas Van Panhuys, Khalid Hussain and Meritxell Espino Guarch
Sidra Medicine, Qatar
Introduction: Idiopathic diabetes type 1B (T1Db) lacks conventional pancreatic islet autoimmunity markers, resulting in permanent insulin deficiency and ketoacidosis susceptibility. We explore the potential link between T1Db and mitochondrial dysfunction, supported by mutations in the mitochondrial membrane protein KCNJ11 and mitochondrial DNA (mtDNA) in patients (1). We aim to identify mtDNA genetic variants and assess mitochondrial function in T1Db cases.
Materials and Methods: We collected samples from nine families, including T1Db patients, mothers, and healthy siblings. Intact peripheral blood mononuclear cells’ (PBMC) oxygen flux was measured using Oxygraph high-resolution respirometry (O2k, Oroboros). MtDNA sequencing involved long PCR amplification, purification, and Nextera Illumina library preparation (2).
Results: Seven cases exhibited a significant 20-40% decrease in uncoupled mitochondrial function, consistent even after normalising for mtDNA load and protein content. Notably, two families showed 0.99 allele frequency for T310C and T310G variants (present in <5% of the population by gnomAD). Additionally, two cases had novel frameshift variant, T13600TAAGC, in the ND5 gene. Variants with <10% heterogeneity affected key mitochondrial genes, including ND1, ND2, ND5, and CYTB, in other T1Db cases.
Conclusion: Findings validation enhances T1Db diagnosis accuracy in cases with mitochondrial dysfunction, potentially enabling the development of personalised treatments to restore mitochondrial function.
References
Abdel-Karim T, Haris B, Afyouni H, Mohammed S, Khalifa A, Al-Maadheed M, Zyoud M, Elawwa A, Al-Khalaf F, Petrovski G, et al. The epidemiology and genetic analysis of children with idiopathic type 1 diabetes in the state of Qatar.
Yao Y, Nishimura M, Murayama K, Kuranobu N, Tojo S, Beppu M, Ishige T, Itoga S, Tsuchida S, Mori M, et al. A simple method for sequencing the whole human mitochondrial genome directly from samples and its application to genetic testing.
JTV-519 directly modulates mitochondrial respiration
Moustafa Elkalaf, Pavla Staňková, René Endlicher, Zuzana Červinková and Otto Kučera
Faculty of Medicine in Hradec Kralove, Charles University, Czech Republic
Introduction: JTV-519 is a 1,4-benzothiazepine compound that prevents intracellular calcium leakage by binding to the ryanodine receptors (1). These properties suggested using JTV-519 as an antiarrhythmic cardioprotective agent (1) and recently, to reverse the effects of post-ischemic injury (2). The compound stabilises other ion channels as well, therefore, the side effects of JTV-519 are not well known (3). In this work, we test the effect of JTV-519 on mitochondrial metabolism and the bioenergetic profile of different types of cells.
Materials & Methods: Cellular respiration and the bioenergetic profile of HL-1, A549, and HepG2 cell lines and freshly isolated mouse hepatocytes were evaluated using an Agilent Seahorse XFe96 extracellular flux analyser (Agilent Technologies, USA). Mitochondrial respiration and calcium retention capacity were assessed by high-resolution respirometry (OROBOROS Oxygraph-2k, Austria).
Results: JTV-519 abolished the effect of protonophore uncoupler (FCCP) and minimised cellular maximal respiratory capacity. That inhibitory effect is restricted to NADH-linked respiration when pyruvate/malate were used as respiratory substrates. Succinate-stimulated respiration was not affected.
Conclusion: JTV-519 effects are not restricted to cardiac cells and directly interact with mitochondrial respiration limiting NADH-linked respiration in the uncoupled state (state 3).
Funding
This research was funded by a grant from the Ministry of Health of the Czech Republic [AZV NU21-01-00259].
References
Toischer K, et al. 2010 Mar. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol. 105(2):279–287.
Trinh MD, et al. 2022 Jun. Hypoxia-induced sarcoplasmic reticulum ca2+ leak is reversed by ryanodine receptor stabiliser JTV-519 in HL-1 cardiomyocytes. Anatol J Cardiol. 26(6):476–484.
Viswanathan MN, Page RL. 2009 Apr. Pharmacological therapy for atrial fibrillation: current options and new agents. Expert Opin Investig Drugs. 18(4):417–431.
IQGAP1 finetunes mitochondrial respiratory chain complex I by regulating alternative splicing of its core subunit NDUFS4
Zoi Erpapazogloua, Vasiliki Papadakia, Maria Kokkoria, Malgorzata Rogalskab, Myrto Potiria, Martina Samiotakia and Panagiota Kafaslaa
aBiomedical Sciences Research Centre “Al. Fleming”, Greece; bCentre for Genomic Regulation, The Barcelona Institute of Science and Technology, Spain
Introduction: IQGAP1 (IQ Motif Containing GTPase Activating Protein 1) is a scaffold protein involved in the orchestration of multiple signalling pathways. We recently showed that IQGAP1 regulates alternative splicing (AS) of cell cycle-related genes in gastric cancer cells1. IQGAP1 interacts with and mediates signal transduction to the spliceosome1,2.
Materials and Methods: We combined metabolic profiling, proteomic and RNA-seq analyses of IQGAP1-KO and parental gastric cancer cells. Mitochondrial respiratory chain complex I (CI) assembly and activity were analysed by Blue Native Gel Electrophoresis and NADH oxidation assays.
Results: IQGAP1 regulates AS of distinct gene subsets in gastric cancer cells depending on their metabolic profile. In cells that rely highly on mitochondrial metabolism, IQGAP1 regulates AS and expression of the CI core subunit NDUFS4 (NADH:Ubiquinone Oxidoreductase Subunit S4). In this case, IQGAP1-KO results in downregulation of NDUFS4 and other CI subunits, leading to CI assembly and activity defects like those observed in patients with Leigh syndrome3. Exogenous expression of IQGAP1 reverses these mitochondrial defects and restores the migratory capacity of IQGAP1-KO cells.
Conclusions: Our work describes a novel role of IQGAP1 in mitochondrial quality control and underlines the contribution of AS regulation in mitochondrial anterograde signalling.
Funding
Supported by InfrafrontierGR/Phenotypos Infrastructure, co-funded by Greece and the European Union (European Regional Development Fund) [NSRF 2014–2020, MIS 5002135]; Hellenic Foundation for Research & Innovation (HFRI) and the General Secretariat for Research and Tec.
References
Birladeanu A-M, Rogalska M, Potiri M, Papadaki V, Andreadou M, Kontoyiannis D.L, Lewis J.D, Erpapazoglou Z, Kafasla P. 2021. The scaffold protein IQGAP1 links heat-induced stress signals to alternative splicing regulation in gastric cancer cells. Oncogene. 40:5518–5532.
Breuer M.E, Willems P.H.G.M, Smeitink J.A.M, Koopman W.J.H, Nooteboom M. 2013. Cellular and animal models for mitochondrial complex I deficiency: A focus on the NDUFS4 subunit. IUBMB Life. 65:202–208.
Muehlbauer L.K, Wei T, Shishkova E, Coon J.J, Lambert PF. 2022. IQGAP1 and RNA splicing in the context of head and neck via phosphoproteomics. J Proteome Res. 21:2211–2223.
FC - ROS buster and the mitochondria rescuer
Agnieszka Fedoruk-Wyszomirska, Paweł Pawelczak, Dorota Gurda, Małgorzata Giel-Pietraszuk and Eliza Wyszko
Institute of Bioorganic Chemistry Polish Academy of Sciences, Poland
Introduction: Mitochondria are involved in cellular processes including energy production, metabolism, redox homoeostasis regulation, and apoptosis. Disruption of mitochondrial function results in reactive oxygen species (ROS) generation, and their accumulation leads to intracellular damages and cell death.1 Small-molecular compounds with antioxidant activities can mitigate the consequences of oxidative stress (OS) and prevent the development of some diseases2. FC is a small-molecular compound, a modified cytosine analogue.
In this study, we analysed the FC mitoprotective and antioxidative activity using 2D cell culture models: normal human fibroblasts (MRC-5) and immortalized human keratinocytes (HaCaT), and budding yeast Saccharomyces cerevisiae3, that has multiple features of higher eukaryotic models.
Material & Methods: Using flow cytometry, confocal microscopy, HPLC, western blot, qPCR, and the Oxygraph+ system we investigated the impact of FC on ROS generation and its effect on mitochondrial functions under stressful conditions.
Results: FC increases mitochondrial membrane potential (ΔΨm), modulates mtDNA/nDNA content and respiration level, and reduces ROS level after the stressor exposure. Moreover, FC modulates the expression level of genes associated with OS response, apoptosis, and cell survival.
Conclusion: FC significantly reduces the negative effects of ROS, modulates mitochondrial activity improves cell viability after oxidative stress induction making FC a potential free radicals scavenger.
Funding
This study was supported by the National Science Centre [grant no 2017/25/B/NZ7/02162].
References
Mehta J, Rayalam S, Wang X. 2018. Cytoprotective effects of natural compounds against oxidative stress. Antioxidants. 7:147.
Pawelczak P, Fedoruk-Wyszomirska A, Wyszko E. 2022. Antiaging effect of 4-N-furfurylcytosine in yeast model manifests through enhancement of mitochondrial activity and ROS reduction. Antioxidants. 11(5):850.
Zhang B, Pan C, Feng C, Yan C, Yu Y, Chen Z, Guo C, Wang X. 2022 Dec. Role of mitochondrial reactive oxygen species in homoeostasis regulation. Redox Rep. 27(1):45–52.
The UbiB family member Cqd1 forms a novel membrane contact site in mitochondria
Siavash Khosravia, Xenia Cheliusb, Ann-Katrin Ungerb, Daniela Riegera, Johanna Frickela, Timo Sachsenheimerc, Christian Luechtenborgc, Rico Schiewecka, Britta Brueggerc, Benedikt Westermannb, Till Kleckerb, Walter Neupertd and Max Harnera
aLMU Munich, Germany; bInstitute of Cell Biology, University of Bayreuth; cHeidelberg University Biochemistry Center (BZH); ddeceased
The use of Saccharomyces cerevisiae as a model organism to study eukaryotic cell functions has been used for decades. Like virtually all eukaryotic cells, they contain mitochondria as essential organelles performing various functions, including participation in the lipid metabolism. They are separated from the cytosol by a double membrane system, consisting of the mitochondrial inner membrane (MIM) and the mitochondrial outer membrane (MOM). This physical separation of the mitochondria requires an exchange of metabolites, proteins and lipids. Proteinaceous contact sites are thought to be important for this communication. Recently, it was found that Cqd1 in cooperation with Cqd2 controls the distribution of Coenzyme Q within the cell. In this study a novel contact site is described, formed by the MOM protein complex Por1-Om14 and the UbiB protein kinase-like MIM protein Cqd1. The present results suggest the involvement of Cqd1 in the homoeostasis of phospholipids. Moreover, we show that overexpression of the UbiB family proteins also causes tethering of the mitochondria to the endoplasmic reticulum which might explain previously reported rescue ERMES deletions through Cqd2 overexpression. Due to the conservation of the subunits of this contact site to higher eukaryotes, its identification in S. cerevisiae might provide avenues for further research.
Funding
Supported by Jung-Stiftung für Wissenschaft und Forschung; Friedrich-Baur-Stiftung;), LMUexcellent, Deutsche Forschungsgemeinschaft (DFG).
Characterization of platelet-derived mitochondria using the megakaryocyte cell line DAMI
Vanessa L. Gauvina,b, Jael Richarda,b, Étienne Hébert Chatelaina,c and Luc H. Boudreaua,b
aDepartment of Chemistry and Biochemistry, Université de Moncton, Moncton, New Brunswick, Canada; bNew Brunswick Centre for Precision Medicine, Moncton, New Brunswick, Canada; cDepartment of Biology, Université de Moncton, Moncton, New Brunswick, Canada
Introduction: Megakaryocytes are nucleated cells found in the bone marrow that release platelets, small anucleate cells, in the blood. Platelet production implicates the transfer of cytosolic biological content, including mitochondria, from the megakaryocyte to the circulating cell. Platelet activation can induce the release of extracellular mitochondria, which can interact with other immune cells. The specific effects of platelet-derived mitochondria on transcellular communication remains poorly understood, since fluorescent labelling of platelet mitochondria remains limited to conventional stains, such as MitoTrackers.
Material and Methods: DAMI cells were transfected with pcDNA3.1-mito-DSRed and differentiated with eltrombopag and phorbol myristate acetate. Mitochondrial fluorescence was evaluated by flow cytometry and confocal microscopy. Cellular respiration was determined by high-resolution respirometry. Mitochondrial content in platelets and platelet-derived microvesicles was determined by flow cytometry.
Results: We demonstrate that 95% of cells express fluorescent mitochondria. The cellular respiration and the release of platelets and platelet-derived microvesicles were not affected. However, only 20% of the platelets produced by differentiated cells expressed fluorescent mitochondria.
Conclusion: This study confirms that DAMI cells may not be a suitable model to investigate the role of mitochondria in transcellular communication. Further studies in other megakaryocyte cell lines are required to establish an efficient mitochondria-fluorescent platelet model.
Funding
Supported by Canadian Institutes of Health Research (CIHR).
References
Boudreau LH, Duchez A-C, Cloutier N, Soulet D, Martin N, Bollinger J, Paré A, Rousseau M, Naika GS, Lévesque T, et al. 2014. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 124(14):2173–2183. doi: 10.1182/blood-2014-05-573543.
Léger JL, Soucy M-FN, Veilleux V, Foulem RD, Robichaud GA, Surette ME, Allain EP, Boudreau LH. 2022. Functional platelet-derived mitochondria induce the release of human neutrophil microvesicles. EMBO Reports. 23(11):e54910. doi: 10.15252/embr.202254910.
SARS-CoV-2 ORF10 induces mitochondrial dysfunction
Jeffrey Allen Haltoma, Joseph W. Guarnieria, Nidia Sequeirad, Gabrielle A. Widjajaa, Urminder Singhb, Robert Schwartzc, Eve Syrkin Wurteleb and Douglas C. Wallacea
aChildren’s Hospital of Philadelphia, Philadelphia, PA, USA; bIowa State University, Ames, IA, USA; cWeill Cornell Medicine, New York, NY, USA; dNational Institutes of Health, Bethesda, Maryland, USA
Expression of SARS-CoV-2 ORF10 has been shown to induce mitophagy resulting in the degradation of the mitochondrial antiviral signalling protein (MAVS), antagonising an innate immune response. However, no one has yet to look into the broader effect of ORF10 expression on the host transcriptome. Utilising RNA-seq analysis, we determined relative expression levels of host genes in A549 and 293T-cells expressing either ORF10 or EGFP. In A549-cells, relative to EGFP, expression of ORF10 decreases expression of the mitochondria-encoded 12S-MT-RNR2 and 16S-MT-RNR1 and nine genes required for oxidative phosphorylation (OXHPOS), including 4 complex I subunits, 3 complex IV subunits, and 1 complex III and IV subunit. In 293T-cells, relative to EGFP, expression of ORF10 robustly downregulated several nuclear-encoded mitochondrial ribosomal proteins and complex I subunits NDUB7 (-3.1 l2fc) and NDUFS7 (-2.5 l2fc). Furthermore, pathway analysis using ToppGene Gene Ontology (GO), identified a downregulation in pathways associated with antiviral immune response in the ORF10 expressing cells. This alteration of mitochondrial transcripts would impair OXPHOS and decrease mitochondrial membrane potential, leading to elevated mitochondrial reactive species production. This indicates that ORF10 expression induces mitochondrial dysfunction that triggers mitophagy and the degradation of MAVS to abate the immune response.
References
pmid 35765167.
pmid 34845370.
Mitochondria-targeted CoQ10 therapy for acetaminophen liver injury
Mitsue Hibinoa, Masatoshi Maekia, Manabu Tokeshia, Hideyoshi Harashimab and Yuma Yamadab,c
aFaculty of Engineering, Hokkaido University, Japan; bFaculty of Pharmaceutical Sciences, Hokkaido University, Japan; cFusion Oriented REsearch for disruptive Science and Technology (FOREST) Program, Japan Science and Technology Agency (JST) Japan, Saitama, Japan
Introduction: Overdose of acetaminophen (APAP), an antipyretic analgesic, causes liver damage derived from oxidative stress in the mitochondria. Delivery of antioxidant molecules to mitochondria for APAP liver injury can be considered an effective therapeutic strategy. In this study, coenzyme Q10 (CoQ10), which has antioxidant properties, was packaged in MITO-Porter, a mitochondrial targeting lipid nanoparticle, to evaluate its therapeutic effect on APAP liver injury.
Materials & Methods: A MITO-Porter packaged CoQ10, which was named CoQ10-MITO-Porter, was prepared using a microfluidic device1. C57BL/6 J mice (male, 10 weeks old, 21±3 hr fasted) were injected intraperitoneally with 200 mg/kg of APAP, followed 1 hr later by PBS (-), CoQ10 suspension and CoQ10-MITO-Porter, respectively. 24 hours after APAP treatment, liver function tests and histological observations were performed.
Results: In serum ALT levels, a biomarker of liver function, the CoQ10-MITO-Porter group was significantly improved compared to the PBS(-) group and CoQ10 suspension group. Haematoxylin and eosin staining and terminal deoxynucleotidyl transferase dUTP nick end-labelling staining also confirmed the reduction of tissue injury by CoQ10-MITO-Porter administration.
Conclusion: CoQ10-MITO-Porter showed therapeutic effects against acetaminophen liver injury2. An antioxidant therapy based on mitochondrial drug delivery systems have the potential to treat other mitochondria-related diseases.
Funding
Supported by This work was supported, in part, by a grant from the Special Education and Research Expenses of the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government (MEXT), JST FOREST [Grant no. JPMJFR203X], a grant from the KOSE C.
References
Hibino M, et al. 2019. J Pharm Sci.
Hibino M, et al. 2023. Sci Rep.
Detection and quantification of mitochondrial redox signals during stimulation of insulin secretion
Martin Jaburek, Pavla Pruchova, Eduardo Kloeppel, Hana Engstova and Petr Jezek
Institute of Physiology, Academy of Sciences, Czech Republic
Introduction: Redox signalling of mitochondrial origin drives a wide range of physiological responses and pathologies. However, measurements of the rates of production of superoxide and hydrogen peroxide in intact cells are sparse [1, 2].
Materials & Methods: We developed methods for the detection and quantification of H2O2 release during stimulation of insulin secretion in isolated pancreatic islets (PI) and insulinoma cells (INS-1E), utilising the Amplex Red/horseradish peroxidase assay.
Results: At low glucose, a constant rate of H2O2 release into the extracellular space within PI was ~15–20 pmol·min-1·10-6 PI-cells (considering 1500 cells per a single islet). The addition of either glucose or palmitic acid (PA) increased H2O2 release initially to ~85-190 pmol·min-1·10-6 PI-cells and saturated to 33±8 pmol·min-1·10-6 PI-cells. Similar results were observed in INS-1E cell suspensions. Both the PA-induced H2O2 release and PA-stimulated insulin secretion were inhibited with mitochondrially-targeted antioxidant SkQ, an inhibitor of fatty acid β-oxidation etomoxir, and upon catalase overexpression in INS1-E cells.
Conclusion: For the first time, we report and quantify the redox signals associated with secretagogue-stimulated insulin secretion. In addition, we demonstrate the participation of mitochondria in cellular redox signalling.
Funding
Supported by the National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104).
References
Plecitá L, et al. 2020. Diabetes 69:1341–1354.
Ježek J, et al. 2015. Antioxid Redox Signal. 23:958–972.
Mitochondrial threshold effect in longevity and diseases
Joshua David Jackson and Daniele Bano
DZNE, Germany
Aberrant mitochondrial oxidative phosphorylation (OXPHOS) contributes to a wide range of human disorders. Genetic lesions of the OXPHOS system can lead to clinical symptoms that vary in age of onset and severity. Similar lesions show extensive phenotypic pleiotropy, possibly due to the activation of compensatory mechanisms to buffer damage. A better understanding of this “mitochondrial threshold effect” may help to identify molecular processes that stimulate the survival of organisms carrying mitochondrial defects. Here we discuss these mechanisms in the context of Caenorhabditis elegans [1-3].
Firstly, we show that mutations that impair the OXPHOS system can promote C. elegans longevity through a variety of transcriptional programmes [1-3]. Key examples include the upregulation of components of the homotypic fusion and vacuole protein sorting (HOPS) complex [2], and the carboxylesterase cest-2.2 to stimulate lipid metabolism and fatty acid beta-oxidation [3]. Secondly, we show that overexpression of key components of these programmes can rewire metabolism in short-lived mutants to promote survival [3]. Finally, we show that some of these transcriptional programmes are conserved in mice and humans [2].
Together, we stress the importance of using Caenorhabditis elegans in the research of metabolic disease and neurodegeneration, and highlight potential targets for treatment of mitochondria-associated diseases.
References
Jackson J, Wischhof L, Scifo E, Pellizzer A, Wang Y, Piazzesi A, Gentile D, Siddig S, Stork M, Hopkins CE, et al. 2022. SGPL1 stimulates VPS39 recruitment to the mitochondria in MICU1 deficient cells. Mol Metab. 61:101503.
Piazzesi A, Papić D, Bertan F, Salomoni P, Nicotera P, Bano D. 2016. Replication- independent histone variant H3.3 controls animal lifespan through the regulation of pro- longevity transcriptional programmes. Cell Rep. 17(4):987–996.
Piazzesi A, Wang Y, Jackson J, Wischhof L, Zeisler-Diehl V, Scifo E, Oganezova I, Hoffmann T, Gómez Martín P, Bertan F, et al. 2022. CEST-2.2 overexpression alters lipid metabolism and extends longevity of mitochondrial mutants. EMBO Rep. 23(5):e52606.
Mitochondrial redox signals essential for insulin secretion
Petr Jezek, Martin Jaburek, Eduardo Kloppel, Pavla Pruchova, Hana Engstova and Andrea Dlaskova
Institute of Physiology ASCR, Czech Republic
Introduction: Fatty-acid-stimulated insulin secretion (FASIS) at low glucose has been questioned, similarly to stimulation of insulin secretion by branched-chain keto acids (BCKA-SIS).
Materials & Methods: Using AmplexUltraRed with HRP, we quantified the redox signal spreading up to the plasma-membrane as the H2O2 release to the exterior of INS-1E cells and pancreatic islets (PIs) isolated from wt and PNPLA8 knockout (KO) mice. PNPLA8 is a mitochondrial phospholipase A2, isoform γ.
Results: These redox signals ceased with mitochondrial antioxidant SkQ1, with etomoxir and upon catalase overexpression in INS-1E cells. Similarly, β-like oxidation of BCKAs provided redox signals with α-ketoisocaproate (KIC), α-ketoisovalerate, and α-ketomethylvalerate. Moreover KIC was able to shrink mt cristae similarly to glucose [1], indicating a redox based mechanism.
Conclusion: We now provide evidence that the redox signalling is essential for FASIS and BCKA-SIS. For FASIS, fatty acid (FA) β-oxidation forms a surplus superoxide/H2O2, which provides i) closure of ATP-sensitive K+-channels (KATP), together with elevated ATP [2]; and ii) activates mitochondrial (mt) redox-activated phospholipase iPLA2γ/PNPLA8. The latter cleaves FAs from mt phospholipids and supplies GPR40 metabotropic receptors, further amplifying insulin secretion [3]. Amplification is not present for BCKA-SIS.
Funding
Supported by the grant 21-01205S (GACR).
References
Dlasková A, et al. 2018. Biochim Biophys Acta. 1859:829–844.
Ježek J, et al. 2015. Antioxid Redox Signal. 23:958–972.
Plecitá L, et al. 2020. Diabetes. 69:1341–1354.
Mitochondrial-arginine theory of ageing
Alish oglu Kasumov, Ruslan Eldarovich Kasumov and Irina Viktorovna Kasumova
Research and Production Center «KORVET», Russian Federation
We assume and there are proofs that the electron transfers in the ETC, a cyclic low-amplitude swelling-shrinkage of mitochondria, and ATP synthesis are coupled (Kasumov et al. 2015).
Due to the fact that the synthesis of arginine decreases in the human body after 28 years, the deficiency of arginine and lysine, involved in energy transformation, both in the synthesis of ATP by ATP synthase in mitochondria and in the hydrolysis of ATP in muscles, leads not only to a lack of energy in the body, but also affects telomere length, hormonal regulation, epigenetic regulation of histones and other proteins. High glucose concentrations cause swelling of mitochondria (Alca´ntar-Ferna´ndez et al. 2019), which reduces the effect of hypoxia in cancer cells (Öğünç Keçeci and İncesu 2022) and causes glycation of protein lysine and arginine residues in the presence of ROS during ageing (Haus et al. 2007).
Mitochondrial dysfunction is caused by a decreases in the amount of water in the body and by a decreases in the frequency of a low-amplitude swelling-shrinkage cycle; by an increase in glucose concentration with age; by a decrease in physical activity (Kasumov et al. 2020), leading to an increase in the level of sugars. Due to mitochondrial dysfunction, protein glycation occurs with the increasing the concentration of sugars and ROS.
References
Alca´ntar-Ferna´ndez J, Gonza´lez-Maciel A, Reynoso-Robles R, Pe´rez Andrade ME, Herna´ndezVa´zquez AdJ, Vela´zquez-Arellano A, et al. 2019. High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans. PLoS ONE. 14(12):e0226652. doi: 10.1371/journal. pone.0226652.
Haus JM, Carrithers JA, Trappe SW, Trappe TA. 2007. Collagen, cross-linking, and advanced glycation end products in ageing human skeletal muscle, J Appl Physiol. 103, p. 2068-2076
Kasumov EA, Kasumov RE, Kasumova IV. 2015. A mechano-chemiosmotic model for the coupling of electron and proton transfer to ATP synthesis in energy-transforming membranes: a personal perspective. Photosynth Res. 123:1–22. doi: 10.1007/s11120-014-0043-3.
Kasumov EA, Kasumov RE, Kasumova IV. 2020. Mild depolarization of the inner mitochondrial membrane is a crucial component of the mechano-chemiosmotic mechanism of coupling. J Nov Physiother Phys Rehabil. 7:1, p. 33–35. doi: 10.17352/2455-5487.000075.
Öğünç Keçeci Y, İncesu Z. 2022. Mitochondrial oxidative phosphorylation became functional under aglycemic hypoxia conditions in A549 cells. Mol Biol Rep. doi: 10.1007/s11033-022-07400-6.
Mitochondrial BCL-2 digital protein expression analysis in HPV-positive liquid –based vaginocervical smears
Christina Karahaliou, Evangelos Tsiambas, George Agrogiannis, Andreas Lazaris and Nikolaos Kavantzas
First Department of Pathology, School of Medicine, National and Kapodistrian University of Athens, Greece
Introduction: B-cell lymphoma 2 (Bcl-2) (gene locus: 18q21.33) -located on the outer mitochondrial membrane- acts as an anti-apoptotic factor suppressing/blocking the apoptotic signal transduction. In the current study, we analysed Bcl-2 protein expression levels in a series of low and high squamous intraepithelial lesions (LSIL-HSIL) provided by Human Papilloma Virus (HPV) infected vaginocervical epithelia.
Materials and Methods: Using a liquid based cytology assay, twenty (n=20) vaginocervical cell specimens were obtained. All of the cases were HPV-positive according to the corresponding HPV-DNA tests. An immunocytochemistry assay was implemented. Digital image analysis was performed for evaluating objectively the corresponding immunostaining intensity levels.
Results: Bcl-2 high protein expression levels were observed in 11/20 (55%) cass, whereas the rest 9/20 (45%) demonstrated moderate to low expression levels. Among HSIL cases, 8/11 (72%) were overexpressed. The majority of LSIL cases (8/9-88%) demonstrated low to moderate expression (p=0.001). There was no statistical significance correlating high risk HPV types with the corresponding immunostained slides.
Conclusions: Bcl-2 over expression seems to be correlated with a progressive aggressiveness in squamous intraepithelial lesions (LSIL to HSIL). Bcl-2 is a critical anti-apoptotic factor and a target for specific targeted chemotherapeutic strategies for enhancing apoptotic rates in solid malignancies including cervical carcinoma.
References
Ayatollahi H, Sharifi N, Sadeghian MH, Alenabi A, Ghasemian-Moghadam HR. 2014. Immunohistochemical expression of apoptosis regulators in squamous cell carcinoma of the cervix and their association with human papillomavirus 16/18 subtypes. Balkan Med J. 31(3):202–207.
Nayak PK, Hussain N, Negi S, Agrawal S, Bagde N, Mitra S, Singh V. 2022. The immunohistochemical biomarker B-cell lymphoma-2 expression in malignant and premalignant lesions of the uterine cervix and its association with human papillomavirus infection. J Cancer Res Ther. 18(6):1485–1489.
Yumol J, Gabrielli B, Tayyar Y, McMillan NA, Idris A. 2020. Smart drug combinations for cervical cancer: dual targeting of Bcl-2 family of proteins and aurora kinases. Am J Cancer Res. 10(10):3406–3414.
A cardiolipin-specific peptide ameliorates obesity and NASH through mitochondrial function restoration
Dongmin Kima,b, Ji Hyeon Kangc, Taehyeon Jeongc, Yoe-Sik Baec and Jaehoon Yua,b
aDepartment of Chemistry and Education, Seoul National University, Korea, Republic of (South Korea); bCAMP Therapeutics; cDepartment of Biological sciences, Sungkyunkwan University, Korea, Republic of (South Korea)
Obesity is a disease characterised by the accumulation of fat in cells and an abnormal increase in weight. This fat accumulation in liver cells is the main cause of nonalcoholic fatty liver disease (NAFLD). Even though the disease does not have any pathological symptoms, NAFLD can progress to more severe forms of liver diseases, such as nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and cancer. Mitochondrial dysfunction is strongly associated with the development and progression of NAFLD, and the significant decline in mitochondrial function in fatty liver cells is closely related to the low expression of cardiolipin (CL), a specific phospholipid necessary for maintaining the structure and function of inner membrane of mitochondria (IMM). When CL is oxidised and pathogenically remodelled, protein complexes in IMM cannot carry out their roles properly, generating dysfunctional mitochondria and progressing NAFLD to NASH. We have been developing a CL-specific peptide, CMP3013, which can bind to normal or oxidised CLs in IMM, converting leaky to rigid IMM, correcting mitochondrial dysfunction. Treatment of this peptide to high-fat diet induced mouse showed a significant reduction of weight and inflammatory fatty liver damages in liver cells. Taken together, we have shown that the CL-specific peptide can be a plausible therapeutic against obesity related NASH.
References
Lee J, Hong SW, Kwon H, Park SE, Rhee EJ, Park CY, … Lee WY. 2018. Exendin-4 improves ER stress-induced lipid accumulation and regulates lipin-1 signalling in HepG2 cells. Cell Stress and Chaperones. 23:629–638.
Sarwar R, Pierce N, Koppe S. 2018. Obesity and nonalcoholic fatty liver disease: current perspectives. DiabetesMetab Syndr Obesity. 533–542.
Zhang J, Shi Y. 2022. In search of the holy grail: towards a unified hypothesis on mitochondrial dysfunction in age-related diseases. Cells. 11(12):1906.
CRISPR-Cas9 model of respiratory chain deficiency in vitro
Jana Krizova, Nikol Volfova, Hana Stufkova, Tereza Rakosnikova, Jiri Zeman, Marketa Tesarova and Hana Hansikova
Laboratory for Study of Mitochondrial Disorders, Department of Pediatrics and Inherited Metabolic Disorders, General University Hospital in Prague, First Faculty of Medicine, Charles University, Czech Republic
Introduction: In mammals, mitochondrial maturation is specific for transition from glycolysis to oxidative phosphorylation (OXPHOS). We have previously investigated the perinatal maturation of OXPHOS in rat liver and skeletal muscle1 and observed higher levels of coenzyme Q9 (CoQ9) in the postnatal period and that expression of Coq8a and other genes increased significantly postnatally.
Materials & Methods: HEK293 cell lines deficient in COQ8A, UQCRC2 and COX5A, generated by CRISPR-Cas9 system were analysed by respirometry, electron microscopy, measurements of OXPHOS, CoQ10 content and expression of some OXPHOS subunits.
Results: In COQ8A−/− HEK293 cells showed a reduction in CoQ10 biosynthesis to 17% of control. Respiration was absent in COX5A−/−, although the mitochondrial ultrastructure remained preserved. Complex I activity was decreased to 70%, unmeasureable complex IV activity in COX5A−/−. Western blot detection showed no specific pattern of OXPHOS subunit expression among cell lines, except mt-COI, COX2 and COX4 expression in COX5A−/− cells.
Conclusion: Based on these data, we hypothesise that COQ8A is not an essential regulator of CoQ biosynthesis and that even a reduction of CoQ10 to 17% of control does not have a major effect on cellular respiration and OXPHOS efficiency. COX5A deficiency shows dramatic phenotype in respiratory and complex IV analysis.
Funding
Supported by: VFN – General University Hospital – AZV MZ CR NU22-01-00499.
References
Krizova J, et al. 2021 May. Microarray and qPCR analysis of mitochondrial metabolism activation during prenatal and early postnatal development in rats and humans with emphasis on CoQ10 biosynthesis. Biology. 10(5):418. doi: 10.3390/biology10050418.
Inhibitors of fumarylacetoacetate domain containing protein 1 (FAHD1) induce premature cellular senescence in human endothelial fibroblasts in vitro
Tatjana Kuena, Riccardo Giaquintaa, Andreas Andrica, Hubert Gstachb and Alexander Weissa
aUniversity of Innsbruck, Austria; bUniversity of Vienna, Austria
FAH domain containing protein 1 (FAHD1) is involved as oxaloacetate decarboxylase in the regulation of the TCA cycle flux (Weiss et al. 2020). A lentiviral knockdown of FAHD1 can drive HUVEC cells into premature cellular senescence (Petit et al. 2017), and our working model refers to a mitochondrial dysfunction associated senescence-like phenotype (Etemad et al. 2019), mediated via a reduction of complex-II activity (Petit et al. 2017). We currently aim to mimic this effect by application of second generation FAHD1 inhibitios (Weiss et al. 2021).
References
Etemad S, Petit M, Weiss AKH, et al. 2019. Oxaloacetate decarboxylase FAHD1 – a new regulator of mitochondrial function and senescence. Mech Ageing Dev. 177:22–29. doi: 10.1016/j.mad.2018.07.007.
Petit M, et al. 2017. Depletion of oxaloacetate decarboxylase FAHD1 inhibits mitochondrial electron transport and induces cellular senescence in human endothelial cells. Exp Gerontol. 92:7–12. doi: 10.1016/j.exger.2017.03.004.
Weiss AKH, et al. 2020. Regulation of cellular senescence by eukaryotic members of the FAH superfamily - a role in calcium homoeostasis? Mech Ageing Dev. ;190:111284. doi: 10.1016/j.mad.2020.111284.
Weiss AKH, et al. 2021. Inhibitors of Fumarylacetoacetate Hydrolase Domain Containing Protein 1 (FAHD1). Molecules. 6(16):5009. doi: 10.3390/molecules2616500.
Cardiomyocyte-specific PCSK9 deficiency compromises mitochondrial bioenergetics and heart function
Marion Laudettea, Malin Lindboma, Muhammad Arifb, Mathieu Cinatoa, Mario Ruizc, Stephen Dorand, Azra Miljanovica, Mikael Rutberga, Linda Anderssona, Marcus Henricssona, Per-Olof Bergha, Entela Bollanoe, Marc Pilonc, Tuulia Hyötyläinenf, Matej Oresicg,h, Adil Mardinoglub,d, Malin Levina and Jan Borena,i
aDepartment of Molecular and Clinical Medicine/Wallenberg Laboratory, Institute of Medicine, the Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden; bScience for Life Laboratory, Royal Institute of Technology, Stockholm, Sweden; cDepartment of Chemistry and Molecular Biology, University of Gothenburg, Gothenburg, Sweden; dCentre for Host-Microbiome Interactions, Faculty of Dentistry, Oral & Craniofacial Sciences, King’s College London, London, UK; eCardiology, Sahlgrenska University Hospital, Gothenburg, Sweden; dSchool of Natural Sciences and Technology, Örebro University, Örebro, Sweden; gSchool of Medical Sciences, Örebro University, Örebro, Sweden; hTurku Bioscience Centre, University of Turku, Turku, Finland; iSahlgrenska University Hospital, Gothenburg, Sweden
PCSK9, which is expressed mainly in the liver and to a lesser extent in the heart, regulates cholesterol levels by directing low-density lipoprotein receptors to degradation. Studies to determine the role of PCSK9 in the heart are complicated by the close link between cardiac function and systemic lipid metabolism.
Mice with cardiomyocyte-specific deletion of Pcsk9 (hPcsk9–/–) had reduced contractile capacity, impaired cardiac function and left ventricular dilatation at 28 weeks of age and died prematurely. Transcriptomic analyses revealed alterations of signalling pathways linked to cardiomyopathy and energy metabolism in hearts from hPcsk9–/– mice versus wildtype littermates. In agreement, levels of genes and proteins involved in mitochondrial metabolism were reduced in hPcsk9–/– hearts. By using a Seahorse flux analyser, we showed that mitochondrial but not glycolytic function was impaired in cardiomyocytes from hPcsk9–/– mice. We further showed that assembly and activity of electron transport chain complexes were altered in isolated mitochondria from hPcsk9–/– mice. Circulating lipid levels were unchanged in hPcsk9–/– mice, but the lipid composition of mitochondrial membranes was altered.
PCSK9, despite its low expression in cardiomyocytes, plays a key role in cardiac metabolic function and PCSK9 deficiency is linked to cardiomyopathy, impaired heart function, and compromised energy production.
Long-term refrigerated storage of isolated mitochondria
Mina Lima, Da-Yoon Kima, Seong-Hoon Kima, Yunkeun Kanga, Minjeong Parka, Mi Jin Kima, Chang-Koo Yuna, Kyunghoon Minb and Yong-Soo Choia
aDepartment of Biotechnology, CHA University, Seongnam, South Korea; bDepartment of Rehabilitation Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, South Korea
Mitochondrial therapies (mitotherapy) are emerging as a promising milestone in the treatment of mitochondrial disorders. Currently, in clinical trials, freshly isolated mitochondria are used mainly as a single administration, but for long-lasting therapeutic effects, repeated administration is necessary. Therefore, in order to preserve large quantities of healthy isolated mitochondria with the same activity, cryopreservation with cryoprotectants is used, which has the problem of adding a washing step and reducing mitochondrial activity. In this study, we propose a method of preserving mitochondria in refrigerated conditions for several weeks without thawing and washing process. To validate our proposed method, isolated mitochondria were stored at 4 degrees for 4 weeks and changes in mitochondrial functions were identified. As a result, persevered mitochondria under our storage conditions showed identical or similar functional activities compared to fresh mitochondria in membrane potential levels, complex activity, citrate synthase activity, and intracellular delivery-based assays. The development of a storage solution that maintains mitochondrial activity not only allows for long-term refrigeration but also enables transplantation at an appropriate time for patients in need of mitochondrial transplantation and is expected to make a significant contribution to clinical treatment or the field of mitochondrial activity research.
References
Fu A. 2020. Curr Mol Pharmacol.13(1):41–49.
Kim MJ, et al. 2018. Sci. Rep. 8:3330
Yamada Y, et al. 2020. Int J Mol Sci. 21(17):6365
Autophagy’s role in the peritoneal membrane remodelling associated to patients treated with peritoneal dialysis
Mirian López-Pardoa, Olalla Ramil-Gómeza,b, Gonzalo Rodríguez-Varelaa, Ana Rodríguez-Carmonaa,c, Teresa Pérez-Lópezc, Miguel Pérez-Fontánb,c and María José López-Armadaa
aAging and Inflammation Research Laboratory, Biomedical Research Institute A Coruña (INIBIC), Spain; bEndocrine, Nutritional and Metabolic Diseases Group, University of A Coruña (UDC), Spain; cDivision of Nephrology, University Hospital A Coruña (CHUAC), Spain
Introduction: Peritoneal dialysis (PD) is a treatment for patients with chronic kidney disease. However, it is linked to peritoneal membrane fibrosis, induced by oxidative stress related to mitochondrial disfunction1,2, making this technique no longer effective3. The aim of this study is to know if autophagy correlates with mitochondrial dysfunction, inflammasome activation and peritoneal membrane remodelling .
Materials & Methods: Mesothelial cells from patients undergoing PD were used to analyse autophagy and inflammasome markers after being classified as epithelial cells or fibroblasts mRNA levels were quantificated through real time PCR, while ELISA and WB were performed for proteins. TNFα and PQ were combined to activate the inflammatory response and mitochondrial dysfunction. Antioxidant effect of resveratrol (RSV) was checked.
Results: Fibrosis seems to be significantly related to an activation of inflammasome markers (NLRP3, procasp1, proIL-1β), as well as LC3-II, but not autophagic initiators (ULK1 ATG-14). IL-1β and NLRP3 mRNA increase significantly after TNF-α and PQ stimulation, being reduced with RSV (p=0.005 and 0.0006). These results were also obtained with ELISA for IL-1β.
Conclusion: In patients with PD it could be an activation of inflammasome NLRP3 pathway that could lead in a fibrosis in which there is a defective autophagy. RSV may attenuate this damage.
Funding
This study has been co-funded by Instituto de Salud Carlos III through the project “PI18/01803” and by the European Regional Development Fund/European Social Fund “A way to make Europe”/”Investing in your future”. It was also funded through Fundación Euge.
References
Masola V, et al. 2022. Fibrosis of peritoneal membrane as target of new therapies in peritoneal dialysis. Int J Mol Sci. 23(9):4831.
Ramil Gómez O, et al. 2021. Mitochondrial dysfunction plays a relevant role in pathophysiology of peritoneal membrane damage induced by peritoneal dialysis. Antioxidants (Basel, Switzerland). 10(3):447.
Ramil Gómez O, et al. 2022. Involvement of mitochondrial dysfunction in the inflammatory response in human mesothelial cells from peritoneal dialysis effluent. Antioxidants (Basel, Switzerland). 11(11):2184.
Muscle-derived mesenchymal stem cells protect keratinocytes in an in vitro laminitis model. Possible role of mitochondrial transfer
Thierry Franck, Nazaré Storms, Justine Ceusters, Charlotte Sandersen, Geoffroy De La Rebiere, Ange Mouithys-Mickalad and Didier Serteyn
Université de Liège, Belgium
Introduction: Laminitis is a common and debilitating disease affecting feet of horses associated to ischemia and neutrophil inflammation (1, 2). In an in vitro laminitis model using keratinocytes submitted to anoxia-reoxygenation (A/R) combined with activated neutrophils, we studied the possible curative role of muscle derived mesenchymal stem cells (mdMSCs).
Material & methods: Laminitis-like model consists of a continuous cell line of keratinocytes HaCaT exposed to anoxia for 48 h in the presence of an activated neutrophils supernatant (ANS). Reoxygenation was performed by adding new media complemented with mdMSCs during 24h. Cell metabolism was evaluated using cell proliferation assay (MTS). Mitochondrial transfer from mdMSCs to HaCaT was evaluated using PK MitoRed as fluorescent probe.
Results: After A/R with ANS, HaCat showed a 50 % decrease of the metabolic activity compared to control HaCat (normoxia). The addition of mdMSCs for 24h allows to significantly increase the metabolic activity of HaCat from 70 % in normoxia and from 25 % after A/R. Microscopy imaging evidenced cell connections and mitochondrial transfer from mdMSCs to HaCat.
Conclusion: By their well-known anti-inflammatory potential (3) and ability to restore the metabolism of keratinocytes by mitochondrial transfer, mdMSCs constitute a promising future for cell therapy in laminitis.
Funding
This study was supported by Mitotransfert project (Walloon Region, Belgium).
References
de la Rebière de Pouyade G, Serteyn D. 2011 Jul. The role of activated neutrophils in the early stage of equine laminitis. Vet J. 189(1):27–33.
Franck T, Ceusters J, Graide H, Mouithys-Mickalad A, Serteyn D. 2021 Dec 10. Muscle derived mesenchymal stem cells inhibit the activity of the free and the Neutrophil Extracellular Trap (NET)-bond myeloperoxidase. Cells. 10(12):3486.
van Eps AW, Burns TA. 2019 Aug. Are there shared mechanisms in the pathophysiology of different clinical forms of laminitis and what are the implications for prevention and treatment? Vet Clin North Am Equine Pract. 35(2):379–398.
Decoding chronic fatigue syndrome - mitochondrial pathology in serum exposed bioengineered 3-D in vitro skeletal muscle tissues
Sheeza Mughala, Juan Manuel Fernández-Costaa and Javier Ramón-Azcóna,b
aInstitute of Bioengineering of Catalonia (IBEC), Spain; bICREA-Institució Catalana de Recerca i Estudis Avançats, Barcelona, Spain
Introduction: Chronic Fatigue Syndrome (CFS) or fibromyalgia, is a long-term debilitating condition that compromises a patient’s musculoskeletal system [1]. At present, there is no concrete understanding regarding disease progression, no identified biomarkers, and, therefore, no definitive diagnostic tests. Our research aims to understand the disease’s pathomechanism using bioengineered in vitro 3-D platforms.
Methodology: 3-D tissues were bioengineered by encapsulating human muscle satellite cells in Matrigel-Fibrin matrix on a PDMS support. To study the effect of CFS sera on muscle homoeostasis, mature tissues were treated with patient and healthy sera. Post-treatment, structural, contractile, and metabolic profiles of the tissues were analysed.
Conclusion: Preliminary comparative analyses of tissues treated with patient (CFS) and healthy sera after Electric Pulse Stimulatory (EPS) training indicated significantly weaker contractile strength of tissues treated with patient sera. These tissues also showed a larger myotube diameter compared to tissues treated with healthy sera. Quantitative Mitochondrial Morphometry indicates Stress-Induced Hyperfusion. Moreover, Seahorse Analyses revealed elevated OXPHOS and non-mitochondrial respiration. We hypothesise that the disease likely progresses from a Hypermetabolic state through a gradual accumulation of ROX until it reaches atrophy. These novel findings are the first to utilise static 3D bioengineered platforms to understand the CFS.
Funding
Supported by FPI MINECO Grant 2021-2025.
References
Salari N, Khodayari Y, Hosseinian-Far A. 2022. Global prevalence of chronic fatigue syndrome among long COVID-19 patients: A systematic review and meta-analysis. BioPsychoSocial Med. 16:21. doi: 10.1186/s13030-022-00250-5.
Studying mitochondrial dynamics in the locus coeruleus with in vivo acousto-optic two photon imaging in tauopathies
Theresa Niedermeier, Paul Feyen, Lars Paeger and Jochen Herms
Deutsches Zentrum für Neurodegenerative Erkrankungen, Germany
Ludwig-Maximilians University Munich, Germany
Introduction: The Locus coeruleus (LC) is a small nucleus in the brainstem which is of special interest in the context of neurodegenerative diseases such as Alzheimer’s disease (AD), where it is one of the first regions to show hyperphosphorylated ‘pretangle’ tau (1,2). While this region-specific vulnerability is not fully understood, mitochondria have been proposed to be linked to this pathology due to the bioenergetic needs of the tonically active LC neurons with their extensive unmyelinated axonal projections throughout the entire forebrain.
Methods: The study of mitochondria is often restricted to immunohisto- or cytochemical analysis, limiting conclusions about dynamics and progression over time. Here we present a novel in vivo two-photon imaging method utilising acousto-optics to study mitochondrial dynamics. Mice expressing GFP in the outer mitochondrial membrane confined to the LC and its axons in a Cre-dependent (3) manner were stereotactically injected with Cre-dependent AAVs carrying either human Tau-P301S-mKate2 or mKate2 and chronically imaged.
Conclusion: Our findings reveal a significant reduction in mitochondrial transport in the tauopathy model that stays consistent during chronic imaging. This highlights the importance of further investigations into the in vivo dynamics of mitochondria in tauopathies and other diseases.
References
Braak H, Thal DR, Ghebremedhin E, Del Tredici K. 2011 Nov. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. PMID: 22002422.
Fecher C, Trovò L, Müller SA, et al. 2019. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat Neurosci. 22:1731–1742. doi: 10.1038/s41593-019-0479-z.
Poe GR, Foote S, Eschenko O, et al. 2020. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. 21:644–659. doi: 10.1038/s41583-020-0360-9.
Molecular and cellular determinants of locus coeruleus-specific Tau pathology
Katharina Ochsa,b, Stephan Müllera,c, Stefan Lichtenthalera,c, Jochen Hermsa,b and Lars Paegera,b
aGerman Center for Neurodegenerative Diseases, Munich; bLudwig-Maximilians University Munich; cTechnical University of Munich
Introduction: The locus coeruleus (LC) is a small nucleus in the brainstem and the sole source of the neurotransmitter noradrenaline in the forebrain (1). In Alzheimer’s disease, it is among the first regions affected by Tau pathology and early neurodegeneration (2). However, the spatiotemporal development of Tau pathology as well as the underlying reasons for the selective vulnerability of LC neurons is still unclear. Given the high-energy demand of their unmyelinated long-ranging axons, tau-mediated mitochondrial impairment has been proposed as one important driver for the development of this neuropathological phenotype.
Materials & Methods: We performed stereotactic injections of Cre-dependent AAVs carrying either human Tau-P301S-mKate2 or mKate2 into the LC of mice expressing GFP in the outer mitochondrial membrane (OMM) specifically in LC neurons (3). We performed immunofluorescent analysis, 3D reconstructions as well as magnetic-activated cell sorting (MACS) of mitochondria for subsequent Mass Spectrometry analysis.
Conclusion: Our findings reveal a stable transduction of LC neurons and hyperphosphorylated Tau recapitulating human Tau pathology. Importantly, we observed the localisation of Tau with mitochondria. Proteomic analysis of isolated LC-mitochondria in human Tau-expressing mice revealed multiple alterations, pointing towards mitochondrial dysfunction and their involvement in the intriguing vulnerability of LC neurons.
References
Braak H, Thal DR, Ghebremedhin E, Del Tredici K. 2011 Nov. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. PMID: 22002422.
Fecher C, Trovò L, Müller SA, et al. 2019. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat Neurosci. 22:1731–1742. doi: 10.1038/s41593-019-0479-z.
Poe GR, Foote S, Eschenko O, et al. 2020. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. 21:644–659. doi: 10.1038/s41583-020-0360-9.
Ethyl acetate fraction of piptadeniastrum africanum (Hook. F.) modulates apoptosis in mice
Folake Olayinka Olojo, John Oludele Olanlokun, James Aanuoluwa Salemcity, Stella Ajedawun Ogundairo and Olufunso Olabode Olorunsogo
University of Ibadan, Ibadan, Nigeria
Introduction: Mitochondria synthesise ATP and initiate cell death through mitochondrial permeability transition (mPT) [1]. Plants contain compounds that can induce mPT pore opening and cell death to resist pathological conditions [2].
Materials & methods: Chloroform (CFPA), ethyl acetate (EFPA) and methanol (MFPA) fractions of Piptadeniastrum africanum obtained from methanol extract of PA were tested in vitro, on mPT [3]. They were later administered to mice at 25, 50, 100, 140 mg/kg for 14 days. The mPT and mitochondrial FoF1 (mATPase) activity were examined spectrophotometrically while caspases 9 and 3 were determined using ELISA kits.
Results: In vitro, EFPA maximally induced mPT pore opening (3.80, 5.60, 6.40, 8.10 and 8.90 folds) at 20, 60, 100, 140 and 180 µg/ml, respectively, enhanced mATPase activity (0.20±0.01, 0.35±0.10, 0.40±0.10, 0.45±0.20 and 5.20±0.80 µmole/Pi/mg/protein/min), relative to vehicle (0.05 µmole/Pi/mg/protein/min). In vivo, EFPA caused mPT induction of 2.50, 4.90 and 6.90 folds at 25, 50 and 100 mg/kg, respectively and activated caspase 9 (30%, 55% and 76%) and 3 (25%, 45% and 68%) activities versus control ((30%, 10%) while CFPA and MFPA had no activity.
Conclusion: The EFPA modulated apoptosis and may be useful for studies in drug development in diseases where apoptosis is down regulated
References
Bhola PD, Letai A. 2016. Mitochondria-Judges and Executioners of Cell Death Sentences. Mol Cell. 61(5):695–704.
Lapidus RG, Sokolove PM. 1993. Spermine inhibition of the permeability transition of isolated rat liver mitochondria: an investigation of mechanism. Arch Biochem Biophys. 306(1):246–253.
Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. 2012. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 12(10):1281–1305.
Opa1 is required for melanocyte stem cell maintenance
Akiko Omoria,b,c; Domenico Migliorinia, Masafumi Noguchid, Ritsuko Moritae, Naotada Ishiharac and Luca Scorranoa,b
aFondazione Ricerca Biomedica Avanzata VIMM, Italy; bDepartment of Biology, University of Padova, Italy; cGraduate School of Science, Department of Biological Sciences, Osaka University; dLaboratory of Pharmacology, School of Pharmaceutical Sciences, Wakayama Medical University; eGraduate School of Frontier Bioscience, Department of Biological Sciences, Osaka University
Introduction: Melanocytes produce melanin, which provides pigment to skin and protection against UV irradiation. In melanocyte biology, mouse models of hair greying processes can serve as useful systems to uncover mechanisms involved in ageing and the maintenance of stem cell function. A recent genome wide association study helped to reveal a direct link between changes in mitochondrial function and melanin production, pointing to a role for these organelles in melanocyte function and dysfunction (1, 2). Despite being a crucial regulator of mitochondrial and cellular homoeostasis (3), the roles of the inner mitochondrial membrane dynamin related GTPase Opa1 in the greying processes and hair follicle genesis are yet to be defined. Methods: we generated melanoblast-specific genetic deletion of Opa1 in mice and performed sequential stage of immunostaining tissue analysis.
Results: Opa1 is essential for continuously maintaining adequate levels of differentiated melanocytes during the hair follicle cycle. Indeed, Opa1 ablation in mouse melanocytes reduced differentiated and melanocyte stem cells during hair cycles and resulted in early hair greying, indicating a critical role for Opa1 in melanocyte stem cell survival.
Conclusion: we highlight the essential role of Opa1 in the melanocyte compartment and propose that Opa1 is a key player in melanocyte stem cells.
References
Kasahara A, et al. 2013. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signalling. Science. 342(6159):7.
Mort RL, et al. 2015. The melanocyte lineage in development and disease. Development. 142(4):620–632.
Sun Q, et al. 2023. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature. 616(7958):774–782.
Investigating NUBPL gene as a Parkinson’s risk factor in Drosophila melanogaster
Zehbanaz Javidbhai Panwala
De Montfort University, UK
The NUBPL gene plays a crucial role in mitochondrial complex I formation, essential for cellular energy production. Mutations in NUBPL result in mitochondrial complex I deficiency and associated diseases. Interestingly, mitochondrial complex I dysfunction is implicated in Parkinson’s disease (PD), suggesting a potential link between NUBPL and PD risk (Eis et al., 2020). To explore this connection, we conducted a study using wild-type and NUBPL mutant flies, examining NUBPL expression and mitochondrial phenotypes.
We investigated wild-type flies (w1118) and two mutant genotypes (NUBPLmut/+ and NUBPLmut/CyO) at ages 3 and 45 days. Our analysis included measuring NUBPL mRNA levels, assessing lifespan, ATP production, qPCR, mitochondrial respiration, and RNA sequencing. Notably, we observed a direct relationship between NUBPL levels and ATP generation. Surprisingly, mutant flies exhibited enhanced survival rates compared to controls, with adjustments in various biological and metabolic pathways.
In summary, our study unveils a significant association between NUBPL expression and ATP production, shedding light on its role in mitochondrial function. The unexpected increase in survival among NUBPL mutant flies suggests complex compensatory mechanisms. Further investigations will elucidate the genetic regulation by NUBPL mutation and delve into mitochondrial parameters, along with mechanisms underlying mitochondrial diseases their association to conditions like PD.
Funding
Supported by: 1st Supervisor: Nicoleta Moisoi, 2nd supervisor Neill Horley, De Montfort University, Leicester.
References
Eis PS, et al. 2020. Loss-of-function NUBPL mutation may link parkinson’s disease to recessive complex I deficiency. Front Neurol. 11. doi: 10.3389/fneur.2020.555961.
Role of mitochondrial phospholipase A2γ in oxidant-stimulated and norepinephrine-stimulated UCP1-dependent thermogenesis
Pavla Pruchova, Petr Jezek and Martin Jaburek
Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
Introduction: Mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) is a member of the enzyme family responsible for cleaving phospholipids at the sn-1 and sn-2 positions, releasing free fatty acids (FAs) and lysophospholipids. Uncoupling protein 1 (UCP1) is a mitochondrial carrier protein that facilitates FA-dependent proton translocation across the inner mitochondrial membrane, resulting in increased uncoupled respiration and heat generation.
Materials & Methods: We employed high-resolution respirometry to assess oxygen consumption in primary brown adipocytes and mitochondria isolated from brown adipose tissue of wild-type and iPLA2γ-ablated mice.
Results: Our findings reveal that oxidants activate iPLA2γ, leading to an elevation in mitochondrial respiration in brown adipose tissue mitochondria. The oxidant-induced increase in respiration was effectively inhibited by (i) R-bromoenol lactone (R-BEL), a selective inhibitor of iPLA2γ, and (ii) GDP, an inhibitor of UCP1. Notably, no effect of oxidant was observed in mitochondria isolated from mice lacking iPLA2γ. In addition, a norepinephrine-stimulated increase in respiration of isolated primary brown adipocytes was inhibited by R-BEL and was absent in adipocytes isolated from iPLA2γ-ablated mice.
Conclusion: Our preliminary results suggest that activating the redox-sensitive phospholipase iPLA2γ plays a critical role in oxidant and norepinephrine-stimulated UCP1-dependent proton transport, contributing to thermogenesis.
Funding
Supported by the National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104) – funded by the European Union – Next Generation EU.
References
Jabůrek M, Průchová P, Holendová B, Galkin A, Ježek P. 2021. Antioxidant synergy of mitochondrial phospholipase PNPLA8/iPLA2γ with fatty acid-conducting SLC25 gene family transporters. Antioxidants (Basel). 10:678.
Průchová P, Gotvaldová K, Smolková K, Alán L, Holendová B, Tauber J, Galkin A, Ježek P, Jabůrek M. 2022. Antioxidant role and cardiolipin remodelling by redox-activated mitochondrial Ca2+-independent phospholipase A2γ in the brain. Antioxidants (Basel). 11:198.
Orange juice carotenoid β-cryptoxanthin enhance mitochondrial activity in a cellular model of early Alzheimer’s disease
Alice Quentin, Franziska Schnellberger and Gunter Eckert
Justus-Liebig-Universität Gießen, Germany
Introduction: Carotenoids are naturally occurring pigments found mainly in fruits and vegetables that have antioxidant properties. β-cryptoxanthin contained in oranges and orange juice is one of the six major carotenoids found in human blood.1 Over the last several decades, an increasing number of studies have demonstrated a protective role of carotenoids in neurodegenerative disease.2 Since oxidative stress and apoptosis are critical parts of neurodegeneration in Alzheimer’s disease (AD), we tested the properties of β-cryptoxanthin on mitochondrial function in a cellular model of AD.
Methods: SH-SY5Y-APP695 cells were used as a model for an early stage of AD. Using these cells, we investigated adenosine triphosphate (ATP) production, mitochondrial respiration (OXPHOS), reactive oxygen species (ROS) and various genes of mitochondrial biogenesis resp. antioxidant system in cells treated with β-cryptoxanthin.
Results: Incubation with β-cryptoxanthin showed an increase in ATP levels. The OXPHOS remained unaffected. Measurement of ROS levels revealed decreased levels consistent with increased expression of SOD, CAT, GPx1, and PGC-1⍺.
Conclusion: Based on the increased ATP concentration, we postulate that incubation with β-cryptoxanthin leads to a higher energy availability in the cells. Together with the observed antioxidant effects, β-cryptoxanthin seems to be able to improve mitochondrial dysfunction in SH-SY5Y-APP695 cells.
References
Granado F, Olmedilla B, Blanco I, Rojas-Hidalgo E. 1996. Major fruit and vegetable contributors to the main serum carotenoids in the Spanish diet. Eur J Clin Nutr. 50(4):246.
Stahl W, Sies H. 2005. Bioactivity and protective effects of natural carotenoids. Biochimica et Biophysica Acta (BBA)-Mol Basis Dis. 1740(2):101–107.
Aqueous ozone exhibits tumour-targeting anticancer activity via death-associated perinuclear mitochondrial assembly
Manami Suzuki-Karasakia,b, Yushi Ochiaia, Shizuka Innamic, Hiroshi Okajimac, Miki Suzuki-Karasakia, Hideki Nakayamad and Yoshihiro Suzuki-Karasakia
aDepartment of Research and Development, Plasma ChemiBio Laboratory; bGraduate School of Medical Sciences, Kumamoto University; cCommunication&Control Systems Company, Tokyo Keiki Incorporation; dDepartment of Oral and Maxillofacial Surgery, Faculty of Life Sciences, Kumamoto University
Introduction: Ozone (O3) has been used to treat cancers for over a century. However, the cell injury pathway of O3 in solutions is obscure because it generally contains nitrogen oxides. To evaluate the anticancer activity of O3, we invented a new O3-generating system without nitrogen oxides. We explored the possible role of mitochondrial dynamics in the anticancer activity of the resulting aqueous O3 (ODM).
Materials & Methods: ODM was tested for its capacity to suppress cell growth, increase oxidative stress, and modulate mitochondrial morphology and subcellular distribution.
Results: ODM reduced the growth of various cancer cells, including radiation and drug-resistant cells. It increased caspase-independent cell death in most cases. Before cell death, mitochondria became fragmented, clustered, and gathered around one side of the damaged nucleus (Monopolar Perinuclear Mitochondrial Clustering, MPMC). It is caused by increasing mitochondrial reactive oxygen species via the production of H2O2. ODM also increases oxidative stress to nuclear and nucleolus. The remodelling blockade inhibited ODM-induced cell death. On the other hand, ODM did not injure non-malignant cells nor induce MPMC.
Conclusion: O3 exhibits potent tumour-targeting anticancer activity via MPMC.
Funding
Supported by 1. JSPS KAKENHI (JP21K09217); 2. JSPS KAKENHI (JP21K10128).
References
Suzuki-Karasaki M, et al. 2023. Ozone mediates the anticancer effect of air plasma by triggering oxidative cell death caused by H2O2 and iron. Eur J Cell Biol. 102:151346.
Mitochondrial BKCa channels in senescent vascular smooth muscle cells
Adam Szewczyk
Nencki Institute of Experimental Biology, Poland
Introduction: Large-conductance calcium-activated potassium channels (BKCa channels) play an important role in a negative feed-back loop on depolarisation-induced calcium ions influx and smooth muscle cells (SMCs) contraction. Herein, we confirm presence of its counterparts in the inner mitochondrial membrane (mitoBKCa channels). The mitoBKCa channels were shown to protect cardiomyocytes from the ischemia/reperfusion injury. Cardiac events are far more often in aged people, therefore question if those channels are present and active in senescent cells of cardiovascular system.
Materials & Methods: We induced senescence of human aorta-derived SMCs by H2O2 (stress-induced premature senescence). Then we described changes in mitochondrial network and expression level of the selected genes encoding proteins connected to mitochondrial function in senescent and control SMCs. Activity of mitoBKCa channels were measured with patch-clamp technique.
Results: During the study we detected no changes in the level of mRNA encoding α subunit of BKCa channels, despite lower level of this protein and no activity (measured with patch-clamp technique) of the mitoBKCa channels observed in senescent SMCs.
Conclusions: Putative role of the mitochondrial potassium channels in senescence induction needs further investigations.
Funding
This research was funded by the National Science Centre, grants nos. 2019/34/A/NZ1/00352 and OPUS UMO-2018/31/B/NZ3/02931.
References
Szabo I, Szewczyk A. 2023. Mitochondrial ion channels. Annu Rev Biophys. 52:229–254.
Inhibition of mitochondrial protein synthesis induces biosynthesis of oxidative phosphorylation complex V
Seungtae Lee, Jana Aref, Ulliana Savitskaya, Sakshi Runwal and Jan-Willem Taanman
UCL Queen Square Institute of Neurology, London, UK
Introduction: We studied the effects of pharmacological and genetic inhibition of mitochondrial protein synthesis on the expression of the OXPHOS complexes.
Material & Methods: Doxycycline and chloramphenicol were used to inhibit mitochondrial protein synthesis in A549 cell cultures. Fibroblast cultures from a patient with a mitochondrial translation deficiency were used as genetic inhibition model.
Results: Metabolic labelling experiments revealed that both antibiotics induced the synthesis of the two mtDNA-encoded subunits of Complex V (MTATP6 and MTATP8), whilst inhibiting synthesis of the other 11 mtDNA-encoded proteins [1]. Stimulation of MTATP6 and MTATP8 synthesis was only seen after prolonged treatment with antibiotics, short treatment resulted in an inhibition equal to the other mtDNA-encoded proteins. A similar induction of MTATP6 and MTATP8 synthesis was found with the patient culture. Immunoblot experiments demonstrated that levels of MTATP6 and MTATP8 remained stable, whereas levels of the other mtDNA-encoded proteins fell during treatment with the antibiotics and in patient cells. Levels and activities of Complexes I, III and IV decreased but of Complex V persisted.
Conclusion: Our observations suggest that the induction of MTATP6 and MTATP8 synthesis is part of a compensatory mechanism. We hypothesise that Complex V reverses its activity to preserve the membrane potential.
Funding
Supported by Royal Free Charity, Fund 42.
References
Dijk SN, Protasoni M, Elpidorou M, Kroon AM, Taanman J-W. 2020. Mitochondria as target to inhibit proliferation and induce apoptosis of cancer cells: the effects of doxycycline and gemcitabine. Sci Rep. 10:4363. doi: 10.1038/s41598-020-61381-9.
Pulsed faint electromagnetic field enhances mitochondrial electron transport chain and ameliorates depressive-like symptoms in mice with social defeat stress
Masaki Teranishi, Mikako Ito and Kinji Ohno
Neurogenetics, Nagoya University Graduate School of Medicine, Nagoya, Japan
Introduction: Mitochondrial dysfunction is implicated in depressive disorders (reviewed in 1, 2). We recently proved that extremely low-frequency pulses of faint electromagnetic field (ELF-EMF) of 10 µT induce mitophagy followed by mitochondrial biogenesis (3). We examined the effects of ELF-EMF on a mouse model of depression.
Material & Methods: Seven-week-old male C57BL/6N mice were subjected to chronic social defeat stress (CSDS) for 10 days. The ELF-EMF was applied thereafter for six weeks, and behavioural and biochemical features were analysed.
Results: After 10 days of CSDS, defeated mice showed depressive behaviours. Proteins in the electron transport chain (ETC) were variably decreased in the prefrontal cortex (PFC). ELF-EMF exposure improved the mouse behaviours; variably increased ETC proteins in the PFC; and upregulated the oxygen consumption ratio (OCR) in the cortex. The Sirt3-FoxO3a-SOD2 pathway, one of mitochondrial antioxidative pathways, was upregulated in the PFC.
Conclusions: ELF-EMF ameliorated a mouse model of depression. As the intensity of 10 µT is ~5 times less than that of the geomagnetic field on the earth’s surface and 100-times less than the upper limit of occupational exposure to the low-frequency electromagnetic field, ELF-EMF is expected to become a promising non-invasive therapeutic strategy for treating depressive disorders in human.
References
Bansal Y, Kuhad A. 2016. Mitochondrial dysfunction in depression. Curr Neuropharmacol. 14: 610–618.
Kim Y, Vadodaria KC, Lenkei Z, et al. 2019. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal. 31:275–317.
Toda T, Ito M, Takeda J, et al. 2022. Extremely low-frequency pulses of faint magnetic field induce mitophagy to rejuvenate mitochondria. Commun Biol. 5:453.
Mitophagy inhibition induce anti-leukaemic effects and sensitises acute myeloid leukaemia cells to venetoclax treatment
Romain Vazqueza, Rudy Birsena,b, Maria Lilia Cantero Aguilara, Zubaidan Tuerdia, Evelyne Laureta, Eric Grignanoa,b, Didier Bouscarya,b and Nicolas Chapuisa,b
aCochin Institute, Paris, France; bClinical hematology department, Cochin Hospital, Paris, France; cBiological hematology department, Cochin Hospital, Paris, France
Introduction: Acute myeloid leukaemia (AML) is a haematological malignancy highly challenging to treat. Several mitochondrial processes are affected during AML(1-3). Among them are mitochondrial dynamics and mitophagy. Despite their importance, little is known about their regulation in AML. Our work is focused on PINK1, a protein known for its central role in mitophagy regulation, and explores its potential for novel AML treatments(4).
Methods: PINK1 was silenced in AML cell lines OCI-AML2 and MV4-11 by using shRNA. Mitophagy was quantified by flow cytometry in cells expressing the mt-mKeima fluorescent protein. Mitochondrial mass was assessed with MitoTracker immunofluorescence staining, qPCR quantifying mtDNA/nDNA ratios.
Results: Silencing PINK1 resulted in increased mitochondrial mass and ROS. Mitophagy levels were decreased in PINK1-deficient cells, under normal and stress conditions (CCCP treatment). Functionally, PINK1 loss reduced cell proliferation. Notably, these cells were strongly sensitised to Venetoclax (a BCL-2 targeting drug used in AML treatment) as shown by much lower IC50. Taken together, our results show that silencing PINK1 prevents the removal of damaged mitochondria in AML cells.
Conclusion: We show that PINK1 is a critical regulator of mitophagy in AML cells. Furthermore, targeting this protein may be a promising therapeutic strategy especially by potentiating Venetoclax anti-leukaemic effects.
Funding
This work was partly funded by a grant [n°FDM202106013429] awarded by the “Fondation pour la Recherche Médicale”.
References
Glytsou C, et al. 2023. Mitophagy promotes resistance to BH3 mimetics in acute myeloid leukaemia. Cancer Discovery.
Harper JW, et al. 2018. Building and decoding ubiquitin chains for mitophagy. Nat Rev Mol Cell Biol.
Larrue C, et al. 2023. Mitochondrial fusion is a therapeutic vulnerability of acute myeloid leukaemia. Leukaemia.
Pei S, et al. 2018. AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell.
Impact of mesenchymal stem/stromal cells derived mitochondria on the progression of osteoarthritis in a collagenase induced in vivo murine model
Liliana Yantén-Fuentesa,b,c, Ana María Vega-Letterd, Cynthia Garcíaa,b, Eliana Lara-Barbaa,b, Felipe Bustamante-Barrientosa,b, Carolina Pradenasa,b, Yeimi Herrera-Lunaa,b, Maximiliano Barahonae,f, José Matasg, Rolando Vernalh,I, Alexander Ortloffj, Jorge Toledoc,f, Djouad Faridak and Patricia Luz-Crawforda,b
aCentro de Investigación e Innovación Biomédica, Universidad de los Andes, Santiago, Chile; bIMPACT, Center of Interventional Medicine for Precision and Advanced Cellular Therapy, Chile; cREDECA, Red de Equipamiento científico avanzado, Universidad de Chile, Santiago, Chile; dEscuela de Ingeniería Bioquímica, Pontificia Universidad Católica de Valparaiso, Valparaiso, Chile; eDepartamento de Ortopedia y Traumatología, Universidad de Chile, Santiago, Chile; fCICA, Centro de Investigación Clínica Aplicada, Universidad de Chile, Santiago, Chile; gDepartment of Orthopedic Surgery, Universidad de los Andes, Santiago, Chile; hDepartment of Conservative Dentistry, Faculty of Dentistry, University of Chile, Santiago, Chile; iPeriodontal Biology Laboratory, Faculty of Dentistry, University of Chile, Santiago, Chile; jDepartamento de Ciencias Veterinarias y Salud Pública, Facultad de Recursos Naturales, Universidad Católica de Temuco, Temuco, Chile; kIRMB, Université de Montpellier, INSERM, Montpellier, France
Introduction: Osteoarthritis (OA) causes chronic pain, stiffness, limited mobility, joint enlargement, and swelling. Current treatments provide palliative relief. Mesenchymal stem/stromal cells (MSCs) and their derivatives, including mitochondria, offer promise for various diseases, including OA (1). We evaluated the regenerative potential of MSCs-derived mitochondria (mito-MSCs) in an OA murine model, emphasising mitochondria as a valuable OA target due to their regenerative and metabolic impact (2).
Materials & Methods: OA was induced in mice, followed by intraarticular injection of isolated mitochondria derived from 1.000.000 (mito-MSCshigh) or 200.000 (mito-MSCslow) MSCs, and a control group injected with PBS. Mouse knee joints were collected for radiological analysis to measure bone mineral density and histological analysis to quantify a histological score, assessing their therapeutic potential on cartilage degradation (3).
Results: Radiological analysis revealed that Mito-MSCs and MSCs reduced bone mineral density. However, histological analysis showed that Mito-MSCs had a superior therapeutic effect, specifically in the medial and lateral tibia. Remarkably, mito-MSClow exhibited enhanced regenerative effects compared to the mito-MSCshigh group in the tibia.
Conclusion: Intra-articular mito-MSCs exhibit regenerative potential in mitigating OA-associated degenerative changes in a CIOA murine model. While the precise mechanisms require further investigation, these findings offer promising therapeutic prospects for osteoarthritis treatment using mito-MSCs.
Funding
Supported by: Impact FB 210024, Milenio ICN09_016, Fondecyt Inicio 11220549, Fondecyt Regular 1211353, Beca de Doctorado Nacional Folio 21220360.
References
Cosenza S, et al. 2017. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 7(1):16214.
Matas J, et al. 2019. Umbilical cord-derived Mesenchymal Stromal Cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 8(3):215–224.
Nunnari J, Suomalainen A. 2012. Mitochondria: in sickness and in health. Cell. 148(6):1145–1159.
Cardiolipin-specific peptides, designed to possess cell penetrating ability, rescue mitochondrial dysfunction
Gwangsu Shina, Soonsil Hyuna, Dongwoo Kima, Yoonhwa Choia,b, Kyu Hong Kimc, Dongmin Kimb, Soie Kwond, Seung Hee Yange, Kyeong-Ryoon Leef and Jaehoon Yua,b
aDepartment of Chemistry & Education, Seoul National University, Korea, Republic of (South Korea); bCAMP Therapeutics Co., Ltd., Seoul, Korea; cDepartment of Biomedical Sciences, Seoul National University Graduate School, Seoul, Korea; dDepartment of Internal Medicine, Seoul National University Hospital, Seoul, Korea; eKidney Research Institute, Seoul National University, Seoul, Korea; fLaboratory Animal Resource Center, Korea Research Institute of Bioscience and Biotechnology, Cheongju, Korea
Chemically important events in mitochondria occur in the inner membrane, where cardiolipin (CL), a specific phospholipid, causes curvature needed for functional proteins to function properly However, when abnormal CL remodelling occurs, it compromises this curvature and leads to mitochondrial malfunction. CMP001, an amphipathic dimeric α-helical peptide, penetrates into cells, binds to multiple CLs, and regenerates the mitochondrial inner membrane curvature. CMP001 reduces reactive oxygen species (ROS) and increases ATP production in mitochondria. The first target indication of CMP001 is acute kidney injury (AKI). AKI is derived from mal-function of the glomerulus and proximal epithelial cells that are lack of glycolysis to generate ATP. Mitochondrial dysfunction is central to the pathogenesis of AKI, regardless of whether I/R injury, sepsis or exposure to toxic reagents is the initiating insult. There is no specific therapeutic available so far. CMP001 attenuates nephrotoxicity induced by colistin in mouse model. CMP001 has the potential to be developed as a promising therapeutic for various diseases related to mitochondrial dysfunction.
References
Liu D, Shu G, Jin F, Qi J, Xu X, Du Y, … Du Y. 2020. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci Adv. 6(41):eabb7422.
The small GTPase RAP1 links the mitochondria-shaping protein OPA1 to angiogenesis inhibition
Margherita Zamberlana,b, Stéphanie Herkennec, Francesca Grisanb, Liliana Iannuccib, Giulietta Di Benedettob, Federica Vinellia,b, Konstantinos Lefkimmiatisb,d and Luca Scorranoa,b
aDepartment of Biology, University of Padova, Padova, Italy; bVeneto Institute of Molecular Medicine, Padova, Italy; cLaboratory of Molecular Angiogenesis, Giga-Research, Liège, Belgium; dDepartment of Molecular Medicine, University of Pavia, Pavia, Italy
Introduction: Changes in mitochondrial shape impact cell division, migration, activation, and differentiation across different tissues by inducing changes in gene expression profiles that appear cell type and context dependent. Signals linking mitochondrial morphology to gene expression rewiring are unclear1,3. Here we identify that loss of Optic Atrophy 1 (OPA1) inhibits angiogenesis by engaging the Ras-like GTPase RAP1.
Results: RNA sequencing of endothelial cells where OPA1 was downregulated uncovers a Ras-like GTPase RAP1, and its cyclic AMP (cAMP)-activated nucleotide exchange factor EPAC1 signature2. EPAC1 and RAP1 localise proximal to mitochondria and upon OPA1 silencing, perimitochondrial cAMP levels increase, EPAC1 is recruited on mitochondria and activated, leading to RAP1 activation that impinges on NFκB to blunt angiogenesis. Genetic or pharmacological EPAC1 and RAP1 inhibition curtails NFκB activation and restores angiogenesis in endothelial cells lacking OPA1.
Conclusion: Our results nominate EPAC and RAP1 as rheostats of OPA1 loss in angiogenesis.
References
Fazal L, et al. 2017. Multifunctional mitochondrial Epac1 controls myocardial cell death. Circ Res. 120(4):645–657.
Herkenne S, et al. 2020. Developmental and Tumour Angiogenesis Requires the MitochondriaShaping Protein Opa1. Cell Metab. 31(5):987–1003 e8
Kasahara A, et al. 2013. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signalling. Science. 342(6159):734–737.
Mitochondria transplantation improves cardiac function of donor hearts
Yin Hua Zhang
Seoul National University College of Medicine, Korea, Republic of (South Korea)
Mitochondria transplantation (MT) emerges as an effective therapeutic strategy for ischemic-related diseases but the roles in the donor hearts for transplant remain unidentified. Here, we aimed to investigate the effects of MT on mitochondrial and cardiac function of donor hearts. Incubation with MT from platelet (pl-MT) resulted in the internalisation of pl-MT and the enhancement of ATP production in primary cardiomyocytes. In addition, incubation of rat hearts with pl-MT ex vivo for 9 h clearly demonstrated pl-MT transfusion into the myocardium. Mitochondria isolated from the hearts incubated with pl-MT showed increased mitochondrial membrane potential and greater ATP synthase activity and citrate synthase activity. Importantly, the production of reactive oxygen species from cardiac mitochondria was not different with and without pl-MT incubation. Functionally, the heartbeat and the volume of coronary circulation perfusate were significantly increased in the Langendorff perfusion system and the viability of cardiomyocytes was increased from pl-MT hearts. The effects of mitochondria from human umbilical cord mesenchymal stem cells (MSC-MT) on donor hearts showed similar effects. Taken together, these results suggest that incubation with Pl-MT improves mitochondrial activity and maintains the cardiac function of rat hearts with prolonged preservation time.