Abstract
In this study, highly porous collagen–HA scaffolds were prepared by solid–liquid phase separation method. Microstructure of the composites was characterized by SEM, TEM and XRD. The results show that collagen–HA scaffolds are porous with three‐dimension interconnected fiber microstructure, pore sizes are 50–150 µm, and HA particles are dispersed evenly among collagen fiber. Compared with pure collagen, the mechanical property of collagen–HA composite improves significantly. To gain further insight into cell growth throughout 3D scaffolds, the cell proliferation and attachment on the scaffold in vitro was investigated. The collagen–HA composite has good biocompatibility, and adding HA does not affect the histocompatibility of the scaffold materials. The porous collagen–HA composite is suitable as scaffold used for bone tissue engineering.
Introduction
As known, bone substitutes are often required to replace defected tissue due to disease, trauma, or surgery. The traditional treatment for bone defects include autografts and allografts, though there are some restrictions, such as donor site morbidity and donor shortage for autograft, immunologic response and risk of infecting disease for allografts. Metals, ceramics, synthetic and natural polymers are also used for bone repair, but they do not exhibit the physiological or mechanical characteristics of true bone.
Recent year, tissue engineering offers a promising new approach to bone repair (Griffith and Naughton, [Citation2002]; Langer and Vacanti, [Citation1993]). For bone tissue engineering, a porous scaffold is needed to guide cell attachment, growth, and tissue regeneration in 3‐dimensions (Agrawal and Ray, [Citation2001]; Burg et al., [Citation2000]; Temenoff and Mikos, [Citation2000]). The scaffolds may also serve as carriers for cells, growth factor and other biomolecular signals. In the ideal case, scaffold is replaced by regenerated host tissue. There are several basic requirements for scaffold materials. They should be biocompatible, degradable, mechanical integrity and osteoconductive (Crane et al., [Citation1995]).
Numerous biomaterials have been investigated as scaffolds for bone tissue engineering and bone repair, including natural and synthetic materials. Although collagen is a kind of widely used biomaterial (Yang and Zhang, [Citation2001]; Zhang et al., [Citation2002a]), the disadvantage of the highly porous collagen scaffolds is that its mechanical property is relatively weak, which limits it use for bone tissue engineering. Hydroxyapatite (HA) has been investigated as bone replacement for a long time since it has similar biocompatibility and osteoconductivity to bone (Friedman et al., [Citation1998]). But it is brittle and different to process into complex shapes. Natural bone is a 3‐dimensional composite made up of collagen fiber threading through crystalline bonelike apatite. To imitate the natural bone, we develop the porous collagen–HA composite as bone tissue engineering scaffold in this article.
In this study, highly porous collagen–HA scaffolds were prepared by solid–liquid phase separation method. Microstructure, mechanical property of the composites was characterized. To gain further insight into cell growth throughout 3D scaffolds, the cell proliferation and attachment on the scaffold in vitro was investigated.
Materials and Methods
Materials
Collagen was extracted from bovine tendon and was treated by method of enzyme digestion described in Zhang et al. ([Citation2002a]). The defoamed collagen solution was used in the following procedure for preparing collagen–HA scaffolds. Hydroxyapatite powder was obtained from Longma Chemical Co. Ltd. Xin Jin.
Preparation of Collagen–HA Scaffolds
Collagen–HA scaffolds were prepared by a phase‐separation technique. HA powder was added into collagen solution, and homogenized by a speed stirrer. Mixtures with different compositions (see ) were poured onto 100 mm culture dishes, then rapidly transferred into a refrigerator at − 30°C to solidify the mixture and induce solid–liquid phase separation. The solidified mixture was maintained at that temperature for 2 h, then lyophilized for 2 days.
Table 1. Collagen–HA Composite Compositions
Crosslinking
At room temperature, the dried scaffolds were immersed in a crosslinking bath with formaldehyde concentration of 0.3%, pH 8.4, for 2 h, then washed by distilled water and lyophilized.
Scanning Electron Micrograph (SEM)
SEM was carried out on HITACHI X‐650 SEM. The specimens were fixed, dehydrated in alcohol, freeze dried and coated with gold for examination. The morphology and porosity of the scaffolds was observed.
Transmission Electron Microscopy (TEM)
The osmium tetroxide fixed membranes were cut by an ultrathin slicer, stained with uranyl acetate and lead citrate. The treated specimens were observed by an H‐600 transmission electron microscope.
X‐ray Diffraction (XRD)
The X‐ray diffraction (XRD) spectra were obtained with D‐Max‐2500 powder X‐ray diffractometer at a scan speed of 8 deg/min. Cu Kα ratiation was used for the diffraction with a voltage of 40 kV and a current of 100 mA.
Mechanical Properties
The specimens were cut to 1 × 6 cm, and the thickness was measured with an electric digital caliper. Break strength of the scaffolds were tested by HE HOUNSFIELD with a drawing rate of 100 mm/min. Three specimens were tested for each sample.
In Vitro Mesenchymal Stem Cells Seeding
MSCs were isolated from the tibias and femurs of Wistar rats. 3 milliliters of bone marrow was aspirated into a syringe containing 100 units of heparin. Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum was added to the aspirate, and a cell pellet was produced by centrifugation. The pellet was resuspended in 5 milliliters of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum, and it was fractionated on a Percoll gradient (70 percent initial density). The upper 5 milliliters of the gradient was collected, and the number of nucleated cells was determined. The cells were plated at 10 × 106 cells per 100‐milliliter dish in low‐glucose of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum, and they were grown for 18 days at 37°C, in 5% carbon dioxide, with the medium changed every four days. After culturing for 3–4 generation, the cells were harvested and suspended in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum at density of 1 × 106 cells/ml for seeding scaffold (Ducy et al., [Citation2000]; Ishaug et al., [Citation1997]).
Before seeding, the specimens of CH1 and CH4 were cut into rectangular beams (10 × 5 × 3 mm), and 20 pieces of each kind of scaffold were put into 24‐well culture plate, and sterilized with γ‐irradiation. And then they were soaked with DMEM. 0.1 ml cell suspension (at 1 × 106 cells/ml) was added dropwise to the upper surface of each scaffold in each well. Those 24‐well culture plates were put into a humidified 5% CO2 incubator at 37°C. After 3 hours, 0.9 ml DMEM supplemented with 10% fetal calf serum, 100 IU/ml penicillin and 100 IU/ml streptomycin was added to each well. They were cultured at 37°C, in 5% carbon dioxide, 85% relative humidity again. The culture media was changed each other day.
SEM
For observing the growth of MSCs on the scaffolds, specimens of the cell‐seeded scaffolds were examined by SEM. After culture for 15 days, they were washed with phosphate‐buffered saline (PBS), and fixed with 2.5% glutaradehyde for 2 h at 4°C. After fixation, specimens were dehydrated through a series of graded alcohols and then critical point dried. The scaffolds were gold‐sputtered in vacuum and examined by HI‐TACHI X‐650 model SEM.
Cell Proliferating Assay
MSCs proliferation on both kinds of scaffolds was studied at 1, 4, and 7 days. The scaffolds were gently washed by PBS to remove unattached cells and remaining media. The adherent cells were removed from the scaffolds by incubation in 1 ml 0.25% trypsin for 5 min, and then they were thoroughly washed twice by 1 ml PBS. Cells were centrifuged, resuspended in fresh PBS, and counted.
Cell Attachment
For observing the cell attachment, MSCs were seeded into the both scaffolds at a density of 1 × 105 cells/cm2. The cells were allowed to attach to the scaffolds undisturbed for 2, 4, 6, and 8 hours, respectively. At each time interval, one specimen of each scaffold was rinsed with PBS and counted.
Results and Discussions
Microstructures of Porous Scaffolds
The microstructure of scaffold for bone tissue engineering influences the growth of osteoblast cultured on it. High porosity and permeability are both important for a tissue engineering scaffold. Porosity provides space not only for the cells to infiltrate the scaffold and attach but also for ECM formation. High permeability allows for the inflow of nutrients and the elution of metabolic waste and biodegradation by‐products. The scaffold with symmetrical and regularly arranged pore and 100 µm pore size will be the most suitable scaffold for osteoblast culturing (Agrawal and Ray, [Citation2001]).
Scanning Electron Micrograph (SEM)
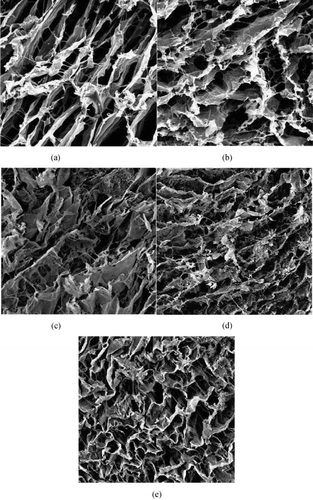
Various techniques, such as particulate leaching, liquid–liquid phase separation and other, have developed to fabricate highly porous scaffolds (Agrawal and Ray, [Citation2001]). In this work, porous collagen–HA composites were developed by phase separation method. Collagen/HA/solvent mixtures are frozen to induce solid–liquid separation, then frozen solvent crystal sublimes under vacuum condition to form low‐density porous scaffolds. SEM photograph of collagen–HA scaffolds show that collagen–HA scaffolds are porous with three‐dimension interconnected fiber microstructure, pore size are 50–150 µm ().
The pore size and structure of the scaffolds are controlled by the shape and quantity of frozen solvent crystal. The amount of solvent, which is changed with the concentration of solid, influences the size of frozen solvent crystal. So, when the concentration of collagen increases, the pore size decreases, there is more conglutination between pores. The concentration of solid is a key factor to control the pore size of scaffold, which is prepared by solid–liquid phase separation method.
With the proportion of HA in composites increasing, the pore size of the scaffold decreases, the pore wall become thicker, the structure becomes more irregular, and there appears honeycomb structure in the scaffold. HA particle, which is incorporated into the mixture, influences nucleation and growth the crystal. It results in such changing of microstructure of composite.
Transmission Electron Microscopy (TEM)
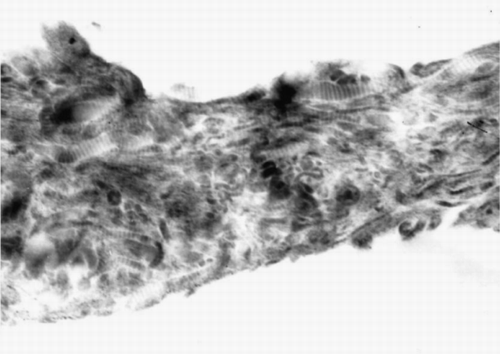
TEM photograph shows the ultrastructure of the collagen–HA composite (). In TEM photograph, HA appears as discrete, electron dense dot. Collagen molecule had a fibril structure of triple helices with 300 nm length. Lots of these collagen fibrils are bound together to form collagen fiber. In TEM, transverse stripe structure is typical structure of collagen fiber, which is caused by interlacedly paralleling of collagen fibrils (Pieper et al., [Citation1999]). TEM photograph of the collagen–HA composite shows that electron dense dot is dispersed evenly among parallel transverse stripe structures. It indicates that HA particles are wrapped and distributed evenly in continuous collagen phase, and the two phases have good compatibility and are combined tightly.
X‐ray Diffraction (XRD)
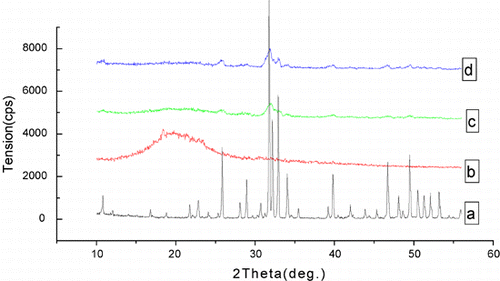
shows the XRD pattern of HA, collagen and collagen–HA composites. There are two strong characteristic peaks of HA at 2.8151 deg and 2.7185 deg. The pure collagen is amorphous without characteristic peaks. The XRD curve of collagen–HA composite shows the characteristic peaks of HA in the same position. With the proportion of HA in composites increasing, the intensity of characteristic peaks increases. It indicates that the crystallinity of HA in the composites is not affected by collagen.
Mechanical Properties
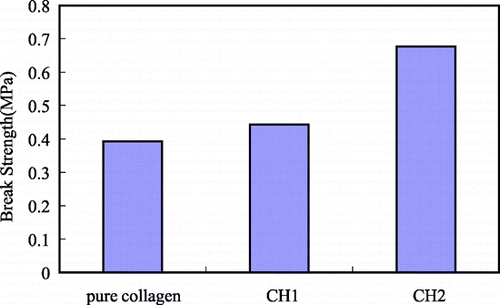
The scaffolds for bone tissue engineering should be mechanically stable to keep their structural integrity during the tissue regeneration. Compared with pure collagen (0.392 MPa), the break strength of porous collagen–HA composites increases significantly (0.422 MPa for CH1, 0.676 MPa for CH2). With the amount of HA incorporated into the collagen matrix increasing, the break strength increases.
In dense composites, experimental and modeling studies show that the addition of higher elastic modulus glass or ceramic particles to a polymer matrix increases its modulus. In porous composites, pore structure and content affect the modulus in addition to the relative amount of glass and polymer (Zhang et al., [Citation2002b]). The following expression is used to account for elastic modulus of porous material, such as foam,where E0 is the modulus of the solid phase (without pores), P is the pore fraction and n is a constant that depends on the microstructure. For the composites in this study, E0 depends on the amount of HA incorporated into the collagen matrix. Compared with pure collagen, E0 of collagen–HA composites increases. SEM results show that the porosity of the collagen–HA composites (P) decreases with the proportion of HA in the composites increasing. Thus, according to Eq. 1, E of porous collagen–HA composites is higher than pure collagen, and increases with the amount of HA incorporated into the collagen matrix increasing. It is consistent with experiment results ().
Although mechanical property of porous collagen–HA composite is improved by addition of HA into the collagen matrix, its porosity decreases and its microstructure becomes irregular. That will affect the growing of osteoblast seeded on it, for the growing of osteoblast needs suitable space size and structure. Desired scaffold for bone tissue engineering should balance the mechanical property with the suitable microstructure.
In Vitro MSCs Seeding
The desired bone graft should has the similar aspects as natural bone, which as follows: 1) excellent histocompatibility, it will not elicit implant immunological response and anti host reaction after its implantation in human body; 2) osteoacusis, after the implantation of graft, the vascular and cells of host migrate into the graft, and with the degradation of graft, newborn bone formed and replace the scaffold. The final destination of artificial bone research is to form newborn bone in host and to replace the scaffold. So one of the most important objects in biomaterial science is to simulate the structure and function of natural tissue. And the key factors in the bone graft materials are biocompatibility, degradability, mechanical integrity, and osteoconductivity (Crane et al., [Citation1995]). The natural bone can be considered as a composite in which collagen is one of main natural extracellular matrices. The collagen material has great potential in the tissue engineering because of its low antigenicity and biodegradability (Zhang et al., [Citation1999]). The fibrous structure of the collagen is favorable to cell growth. We add HA into the scaffold for improving the materials' biomechanical property. The biocompatibility of biomaterials is closely related to cell behavior on contact with them and particular to cell adhesion to their surface. Through the comparison of cell growth in the specimens of CH1 and CH4 in vitro, we could find whether adding HA affect the histocompatibility of the scaffold materials.
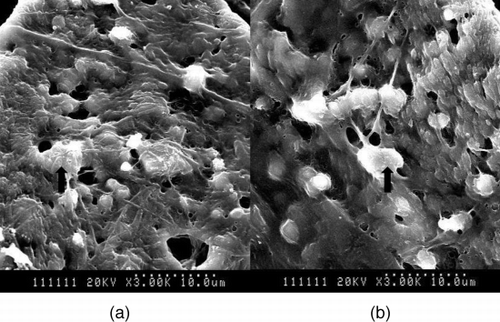
SEM
After implanted 15 days, the cells are dispersed among the scaffolds. There are much extracellular matrices (ECM) secreted by MSCs around the fiber of scaffolds. We could find many distinct differences in the density and the form of cells and ECM between CH1 and CH4 ().
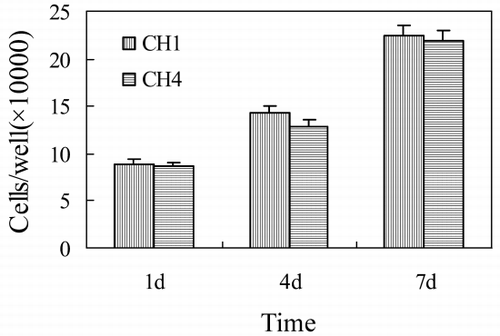
Cell Proliferation
MSCs proliferation on both scaffolds is studied after seeding for 1, 4, and 7 days (). We could not find any significantly different of the cells number between two kinds of scaffolds. Considering that cell proliferation is the basic of forming tissue, this result suggests that adding HA doesn't not affect the cell growth and the formation of new tissues. As it is known, collagen could promote cell–cell, cell–matrix interactions, and then accelerate the proliferation of cells (Young et al., [Citation1998]). In this study, we use collagen as scaffold, and use HA to improve its biomechanical property. We hope the scaffold could be excellent in both histocompatibility and biomechanics for bone graft.
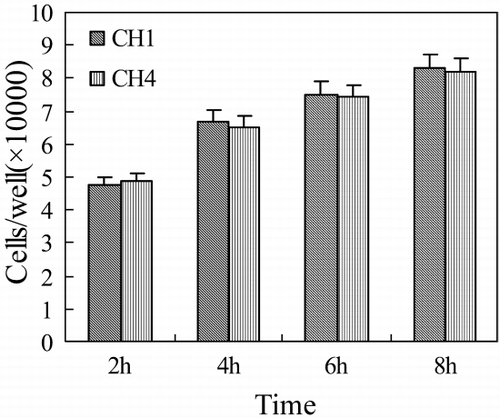
Cell Attachment
As a kind of natural component of human body, collagen has many polar groups on the surface of its fiber. Surface characteristics of materials, such as surface energy, play an essential part in cells adhesion on biomaterials. Polar groups could increase the water binding capability, and improve the cells to attach. Thus attachment and adhesion will influence the cell's capacity to proliferate and to differentiate itself on contact with the implant (Yang and Zhang, [Citation2001]). After the HA is added into the collagen scaffold, we find that the cell attachment capability doesn't change significantly ().
Conclusions
Porous collagen–HA composites were produced by phase separation techniques. Collagen–HA scaffolds are porous with three‐dimension interconnected fiber microstructure, pore size are 50–150 µm. HA particles are dispersed evenly among collagen fiber. Compared with pure collagen, the mechanical property of collagen–HA composite improves significantly. The collagen–HA composite has good biocompatibility, and adding HA does not affect the histocompatibility of the scaffold materials. So, The porous collagen–HA composite is suitable as scaffold used for bone tissue engineering.
Acknowledgment
This work is supported by the Emphasis Project of Tianjin Natural Science Foundation (003802511).
References
- Agrawal C. M., Ray R. B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001; 55: 141–150
- Burg K. J.L., Porter S., Kellam J. F. Biomateiral developments for bone tissue engineering. Biomaterials 2000; 21: 2347–2359
- Crane G. M., Ishaug S. L., Mikos A. G. Bone tissue engineering. Nat. Med. 1995; 1: 1322–1324
- Ducy P., Schinke T., Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science 2000; 289: 1501–1504
- Friedman C. D., Costantino P. D., Takagi S., et al. Bone source hydroxyapatite cement: a novel biomateiral for craniofacial skeletal tissue engineering and reconstruction. J. Biomed. Mater. Res. (Appl. Biomater.) 1998; 43: 428–432
- Griffith L. G., Naughton G. Tissue engineering—current challenges and expanding opportunities. Science 2002; 295: 1009–1014
- Ishaug S. L., Crane G. M., Mille M. J. Bone formation by three‐dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997; 36: 17–28
- Langer R., Vacanti J. P. Tissue engineering. Science 1993; 260: 920–926
- Pieper J. S., Oosterhof A., van Kuppevelt T. H., et al. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials 1999; 20: 847–858
- Temenoff J. S., Mikos A. G. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials 2000; 21: 2405–2412
- Yang H., Zhang Q. Preparation and characterization of collagen–GAGs bioactive matrices for tissue engineering. J. Mater. Sci. Technol. 2001; 17(5)495–500
- Young R. G., Bulter D. L., Weber W., et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 1998; 16(4)406–413
- Zhang Q. Q., Yao K. D., Liu L. R., et al. Evaluation of porous collagen membrane in guided tissue regeneration. Artif. Cells Blood Substit. Immobil. Biotechnol. 1999; 27(3)245–253
- Zhang L., Ma D., Zhang Q. The modification of scaffold material in building artificial dermis. Artif. Cells Blood Substit. Immobil. Biotechnol. 2002a; 30(4)319–332
- Zhang K., Ma Y., Francis L. F. Porous polymer/bioactive glass composites for soft‐to‐hard tissue interfaces. J. Biomed. Mater. Res. 2002b; 61: 551–563