Abstract
Phospholipase A2 (PLA2; EC 3.1.1.4) is a lipolytic enzyme that hydrolysis the ester bond in sn‐2 position of phospholipids. In this work, the PLA2 from hog pancreas was covalently coupled to porous glass. The properties of free and immobilized enzyme were also investigated and compared. The optimum pH and temperature were found as 8.5 and 50°C, respectively for both free and immobilized enzyme. The immobilized enzyme had good properties that potential for medical application is considerable. Its use in lowering plasma cholesterol concentrations in blood samples was also demonstrated.
Introduction
The phospholipase A2 (PLA2) superfamily consists of a broad range of enzymes defined by their ability to catalyze specifically the hydrolysis of the center (sn‐2) ester bond of substrate phospholipids (Arni and Ward, [Citation1996]; Chen and Chen, [Citation1998]; Madoery et al., [Citation1995]; Six and Dennis, [Citation2000]). It is a lipolytic enzyme that hydrolysis phosphoglycerids such as phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylserine (PS) at the acyl ester bond at the sn‐2 position into the corresponding lysophospholipid and fatty acid (Kaiser, [Citation1999]; Simonsson and Ahren, [Citation2000]; Six and Dennis, [Citation2000]).
The hydrolysis products of this reaction, free fatty acid and lysophospholipids have many important downstream roles and are derived from the activity of a diverse and growing superfamily of PLA2 enzymes. The lysophospholipid product is important in cell signalling, phospholipid remodelling and membrane perturbation. The fatty acids can be important as stores of energy, but more importantly arachidonic acid has also function as a second messenger and precursor of eicosanoids, which are potent mediators of inflammation and signal transduction (Balsinde et al., [Citation1999]; Capper and Marshall, [Citation2001]; Six and Dennis, [Citation2000]). Different forms of the enzyme exist, which are classified into a secretory (sPLA2) and a cytosolic (cPLA2) category. The secretory and cytosolic PLA2s are further subdivided into a number of subtypes depending on structure, mass, substrate specificity and Ca+ 2 dependency (Simonsson and Ahren, [Citation2000]; Six and Dennis, [Citation2000]). The secretory forms of PLA2 are enzymes of low molecular weight (14 kDa), which require calcium concentrations of milimolar levels for activity and preferentially hydrolyse arachidonic acid containing phosphatidylethanolamine. This group includes several types of sPLA2 such as those secreted in venoms, by the pancreas and by other mammalian cells. In contrast, the cytosolic forms of PLA2 are exclusively present inside the cell, are of high molecular weight, need only micromolar or submicromolar Ca+ 2 levels for their activation and show a preference for phosphatidylcholine with arachidonic acid in the sn‐2 position (Kaiser, [Citation1999]; Simonsson and Ahren, [Citation2000]; Yedgar et al., [Citation2000]).
Through its enzymatic activity, or by the generation of arachidonic acid and arachidonic acid metabolites, PLA2 is known to exert biological effects in several different cell systems: for example, as a digestive enzyme in intestinal juices, as protecting basal plasma membrane homeostasis in mast cell, as constituting the rate limiting event in biosynthesis of proinflammatory lipid mediators, and as participating in the enzyme cascades generating transduction signals in, for instance, macrophages, vascular smooth muscle cells, mast cells, and keratinocytes (Dennis, [Citation1994]; Simonsson and Ahren, [Citation2000]).
The PLA2s are widely distributed in nature and are present in most animal and human tissue and abundant in mammalian pancreas and in the venom of several snakes and bees (Arni and Ward, [Citation1996]; Chen and Chen, [Citation1998]). The hydrolytic reaction promoted by PLA2 has importance in biotechnology since the lysophospholipid properties. The use of lysolipids as food additives is well known. It is prevents unwanted colorization of food or contamination with microbial spore and plays a role in taste substances (Kim et al., [Citation2001]; Madoery et al., [Citation1995]). The new applications of these compounds were also reported in the pharmaceutical industry and in biomedicine (Madoery and Fidelio, [Citation2001]; Mukherjee, [Citation1990]; Tai and Cooper, Citation[1990]).
Moreover, biomedical reactors with immobilized PLA2 and their application has been only attempted in the treatment of hypercholesterolemia to lower serum cholesterol and low‐density lipoproteins in plasma (Chen and Chen, [Citation1998]; Labeque et al., [Citation1993]; Shefer et al., [Citation1995a], [Citation1995b]; Shen and Cho, [Citation1995]). Low‐density lipoprotein (LDL) particle is the major cholesterol carrier in the plasma. Enzymatic modification of LDL particle with PLA2, by enzymatic hydrolysis of phospholipid on the particle surface, does not significantly change the basic structural features of the particle, including solubility. Several studies have established that the higher the total plasma cholesterol levels, the greater risk for coronary hearth disease. Drug therapy, diet control and direct removal of LDL from the blood can reduce plasma cholesterol level. Drug therapy may have side effects to limit its use. On the other hand, the sucsess of diet control depends heavily on continued and sometimes unplesant cooperation from patients. However, a promising and interesting route for treating high total plasma cholesterol and LDL‐cholesterol levels is to use an extracorporeal enzyme bioreactor which contains immobilized PLA2 to modify plasma LDL so that the modified LDL may be removed by an individual's own metabolic processes (Chen and Chen, [Citation1998]).
In this study, we have immobilized pancreatic phospholipase A2 on activated porous glass with a high fixation level. The macroporous structure of the support provides good diffusivity of LDL particles. Some properties of the immobilized enzyme were investigated and its use for lowering cholesterol concentration of serum was also demonstrated and compared with the free enzyme.
Materials and Methods
Materials
Phospholipase A2 (PLA2) from hog pancreas, 3‐sn‐phosphatidylcholine (lecithin) were obtained from Fluka Chemie AG (Switzerland). Glutaraldehyde and 3‐aminopropyltriethoxysilane (APTS) were obtained from Sigma Chem. Co. (St. Louis, USA). Porous glass beads were purchaced from Schott Glasswerke (Mainz, Germany). The cholesterol kit (cholesterol liquicolor) was purchased from Human Gessellschaft für Biochemica und Diagnostica GmbH (Germany). Other reagents were of analytical grades and purchased from Sigma Chem. Co. (St. Louis, USA) or E. Merck (Darmstad, Germany).
Methods
Immobilization of PLA2 on Porous Glass Beads
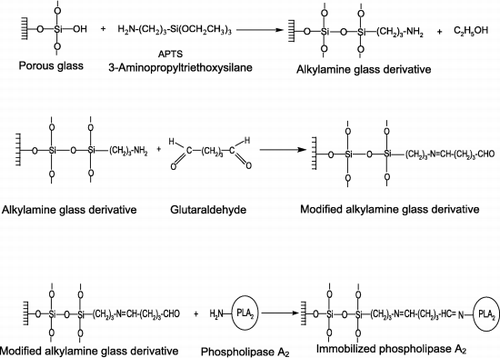
Porous glass beads were first activated by APTS. The silanization procedure was performed as described by Weethall ([Citation1993]). The ε‐amino groups of lysine on enzyme molecules were attached to the aminopropyl glass beads via glutaraldehyde (2.5%) ().
The beads (1 g) were first washed several times with distilled water and then suspended in 20 ml of 10% APTS solution. The pH of the medium was adjusted between pH 3.0–4.0 with concentrated HCl and then was agitated gently (150 rpm) at 75°C for 5 hours. The APTS activated glass beads were washed with distilled water for three times. The aminopropyl‐glass derivative was suspended in 25 ml of 2.5% glutaraldehyde solution (prepared in Tris–HCl buffer, 0.1 M, pH 8.0) and was agitated at 200 rpm at room temperature for one hour. The beads were separated and washed with the same buffer. They were added to 10 ml of PLA2, which prepared in Tris–HCl buffer with gentle shaking and incubated at 4°C overnight. The immobilized enzyme conjugate was separated by filtration and then in order to remove unbound proteins it was washed with the same buffer. The filtrate and washing solutions were collected and used for protein determination.
Activity Measurement of PLA2
The enzymatic assay used 3‐sn‐phosphatidylcholine (lecithin) as substrate. Lecithin emulsion was prepared as follows: 54 mg of sodiumdeoxycholate (2.6 mM) was dissolved in 50 ml of distilled water and then 1.25 g of lecithin (2.5%) was added. After stirring for 30 minutes at 4°C, the mixture was sonicated for 1 minute. The substrate emulsion was prepared daily. The enzymatic reaction was carried out in a vessel surrounded by a heating jacket through which water at 37°C was circulated. The mixture consists of 9.5 ml of 0.1 M NaCl, 12.5 ml of 0.01 M CaCl2 and 3 ml of lecithin emulsion. The mixture was stirred magnetically to ensure uniform temperature and material distribution in the reaction vessel and then 250 µl of free enzyme or 100 mg of immobilized enzyme was added. The free fatty acid that is produced in the enzymatic reaction was titrated 0.01 M NaOH using an automatic titration assembly (718 Stat Titrino Methrom, Ltd., Switzerland). Each mole of the subsrate was hydrolyzed by the enzyme to yield one mol of fatty acids that can be titrated against one equivalent NaOH. One unit of enzyme activity was defined as the amount of enzyme required to hydrolyze 1 µmol of substrate per minute (Chen and Chen, [Citation1998]; Kim et al., [Citation2001]).
Protein Determination
Protein concentrations were determined by the method of Lowry et al. ([Citation1951]) by using bovine serum albumin as standard. The amount of bound protein was calculated from the difference between the amount of protein introduced into the reaction mixture and the amount of protein in the filtrate and washing solutions after immobilization.
Cholesterol Measurement
Cholesterol concentrations were measured by using standard cholesterol kit; Cholesterol liquicolor. The cholesterol amounts were determined by using the formulas given below:
Effect of pH and Temperature on Activity
The effect of pH and temperature on activity of free and immobilized PLA2 were assayed in 50 mM phosphate buffer (pH 7.0–9.0 and temperature 30–70°C) by using the standard activity assay procedure mentioned above.
Operational Stability
The reaction of lecithin hydrolysis by the porous glass‐immobilized PLA2 was carried out for 6 hours at 37°C in a continuous stirred tank reactor, and followed by measurement of the activity of samples taken at regular time intervals, the relationship between operating time and the decrease in the enzyme activity was determined and also the half life (t½) of the biocatalyst was determined.
Hydrolysis of Cholesterol in the Reactor
A continuous stirred tank reactor was used for the hydrolysis of cholesterol by using free and immobilized PLA2. 3 ml of serum was mixed in the reactor for a few seconds and then an amount of 500 mg of immobilized PLA2 or 0.56 ml of free enzyme (0.8 mg dry enzyme/ml) solution with the same protein content (0.325 mg) was added. The reactor was maintained at 37°C and stirred continuously with magnetic stirrer (150 rpm). The samples were withdrawn after constant time of incubation and the amount of cholesterol was determined as mentioned above.
Results and Discussion
The immobilization of enzymes on inert and insoluble materials is currently a very active area of research because of their wide range of applications. Beside of this it offers several advantages over the use of soluble enzyme preparations including; easier separation of reaction products from the incubation mixture, the ability to recover and reuse of enzyme, stabilization of tertiary structure of enzyme and increased storage stability (Siso et al., [Citation1997]; Vaillant et al., [Citation2000]). Inorganic carriers employed in biotechnology are usually modified bifunctional organosilanes: in particular 3‐aminopropyltriethoxysilane. Such modified carriers, especially porous glasses having controlled pore dimensions are widely applied in biotechnology, predominantly for enzyme immobilization (Janowski and Fischer, [Citation1991]).
In this work, pancreatic phospholipase A2 (PLA2) was immobilized on porous glass beads by covalent coupling. After the silanization of the glass beads, enzyme was coupled to the carrier by crosslinking with glutaraldehyde. We have also searched the usability of the immobilized PLA2 for hypercholesterolemia treatment.
In previous works, phospholipases (PLA2) from several sources have been immobilized by different methods: PLA2 from cobra venom (Naja naja) was immobilized on the acrylic polymer Eupergit C (Madoery and Fidelio, [Citation2001]), on chitosan beads (Chen and Chen, [Citation1998]), on to agarose beads (Ferreira et al., [Citation1993]), from bee venom upon DEAE‐Sephadex (Madoery et al., [Citation1995]), from porcine pancreas in alginate–silicate sol gel matrix (Kim et al., [Citation2001]) and etc. The porous glass‐immobilized PLA2 retained 21% of original activity. The immobilization results are summarized in .
Table 1. Immobilization of PLA2 on Porous Glass Beads
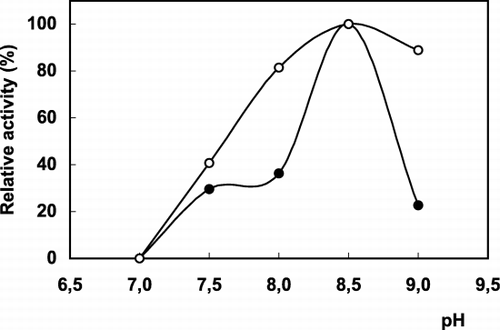
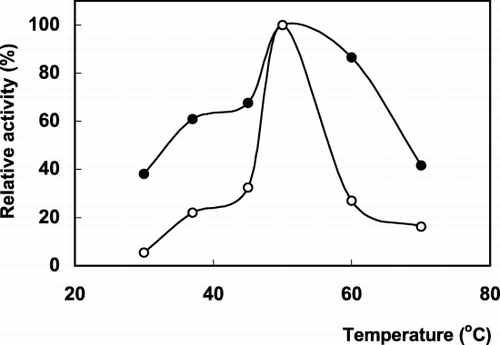
Some parameters affecting to the enzyme activity (pH and temperature) and operational stability were investigated. The activities of free and immobilized enzymes were determined at different pH values. The optimum pH values were determined from the graph of pH plotted against the percentage of relative activity (). As is seen from the , the optimum pH of the immobilized enzyme was pH 8.5. There is no shift from the pH optima of the free enzyme. The effect of temperature on the activity of the free and immobilized enzymes is shown in . The maximum activity was observed at 50°C for both forms of PLA2. However, the immobilized enzyme has a little broader temperature range than that of the free form. The immobilized PLA2 was less sensitive to temperature changes compared to soluble enzyme. It has been reported that immobilized enzyme molecules could be preserved from conformational alterations induced by pH and temperature changes by a protective effect of the support (Madoery et al., [Citation1995]).
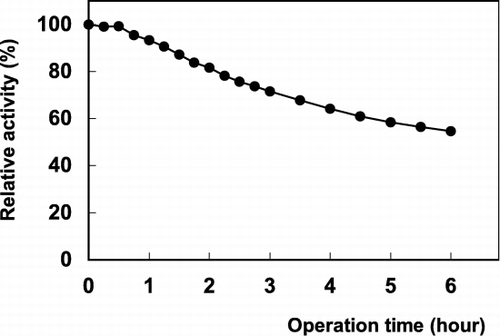
When comparing the catalysts intended for preparative or industrial use, characterization of their operational stabilities is very important. The operational stability of PLA2 for lecithin is very good. shows that the immobilized enzyme lost about nearly 40% of its activity within 6 h and the operational half life of the enzyme was calculated as 6.9 h by using the formula given as;where t is the operation time, kD is decay constant, Ao and A are the enzymatic activities at the beginning and t time.
Previous approaches utilizing immobilized enzyme therapeutically caused either degradation of a toxin or its conversion to a nontoxic substance. Here, the immobilized enzyme modifies an existing molecule in the blood so that the body's own metabolic process can more easily eliminate it. The process of LDL removal by PLA2 consists of two main steps: modification of LDL particles by enzymatic hydrolysis of the phospholipids on the surface of LDL to lysophosphatidylcholine and the enhanced uptake of the modified LDL by the liver.
To test the biomedical application of the immobilized PLA2, we have carried out preliminary in vitro bioreactor study for lowering plasma cholesterol concentration in hypercholesterolemic humans. In treated serum a significiant decrease in total cholesterol concentration was observed after 6 h treatment using a continuous stirred reactor containing immobilized PLA2. The initial serum cholesterol concentration was 5.6 mmol/l (216 mg/dl). After the enzyme treatment, the cholesterol concentration decreased 17.7% as 4.61 mmol/l (178 mg/dl). In control experiments, with unmodified porous glass beads there was no change of cholesterol concentration.
In conclusion, the proposed immobilization procedure is very simple to carry out. The immobilized enzyme also showed good properties that are very important parameters in applications. Further optimization of this covalent immobilization process should lead to an immobilized PLA2 system of great efficiency and useful for biotechnology processes or biomedical applications such as hypercholesterolemia treatment.
References
- Arni R. K., Ward R. J. Phospholipase A2—a structural review. Toxicon 1996; 34(8)827–841
- Balsinde J., Balboa M. A., Insel P. A., Dennis E. A. Regulation and inhibition of phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 1999; 39: 175–189
- Capper E. A., Marshall L. A. Mammalian phospholipases A2: mediators of inflammation, proliferation and apotopsis. Prog. Lipid Res. 2001; 40: 167–197
- Chen J. P., Chen J. Y. Preparation and characterization of immobilized phospholipase A2 on chitosan beads for lowering serum cholesterol concentration. J. Mol. Catal., B: Enzym. 1998; 5: 483–490
- Dennis E. A. Diversity of group types regulation and function of phospholipase A2. J. Biol. Chem. 1994; 269: 13057–13060
- Ferreira J. P., Sasisekharan R., Louie, Langer R. Influence of chemistry in immobilization of cobra venom phospholipase A2: implications as to mechanism. Biochemistry 1993; 32(32)8098–8102
- Janowski F., Fischer G. Aminopropylsilane treatment for the surface of porous glasses suitable for enzyme immobilization. J. Chem. Technol. Biotechnol. 1991; 51: 263–272
- Kaiser E. Phospholipase A2: its usefulness in laboratory diagnostics. Crit. Rev. Clin. Lab. Sci. 1999; 36(2)65–163
- Kim J., Lee C. S., Oh J., Kim B. G. Production of egg yolk lysolecithin with immobilized phospholipase A2. Enzyme Microb. Technol. 2001; 29: 587–592
- Labeque R., Mullan C. J.P., Efeerira J. P.M., Lees R. S., Langer R. Enzymatic modification of plasma low density lipoproteins in rabbits: a potential treatment for hypercholesterolemia. Proc. Natl. Acad. Sci. U. S. A. 1993; 90: 3476–3480
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193: 265–275
- Madoery R. R., Fidelio G. D. A simple method to obtain a covalent immobilized phospholipase A2. Bioorg. Med. Chem. Lett. 2001; 11: 1663–1664
- Madoery R. R., Gattone C. G., Fidelio G. Bioconversion of phospholipids by immobilized phospholipase A2. J. Biotechnol. 1995; 40: 145–153
- Mukherjee K. Phospholipase catalyzed reactions. Biocatalysis 1990; 3: 288–293
- Shefer S. D., Breslau J., Langer R. Computer simulation of low density lipoprotein removal in the presence of a bioreactor containing phospholipase A2. Biotechnol. Prog. 1995a; 1: 133–139
- Shefer S. D., Rosenberger V., Vahanian G., Wong W. T., Langer R. Implantable hollow fiber bioreactor as a potantial treatment for hypercholesterolemia: characterization of the catalytic unit. Biotechnol. Bioeng. 1995b; 48: 36–41
- Shen Z., Cho W. Highly efficient immobilization of phospholipase A2 and its biomedical applications. J. Lipid Res. 1995; 36(5)1147–1151
- Simonsson E., Ahren B. Phospholipase A2 and its potential regulation of islet function. Int. J. Pancreatol. 2000; 27(1)1–11
- Siso M. I.G., Lang E., Gomez B. J., Becerra M., Espinar F. O., Mendez J. B. Enzyme encapsulation on chitosan membranes. Process Biochem. 1997; 32: 211–216
- Six D. A., Dennis E. A. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta 2000; 1488: 1–19
- Tai L. K., Cooper E.. Use of Monoacylphosphoglicerids to Enhance the Corneal Penetration of Ophtalmic Drugs. CA, 114, 108919t. PCT Int. Appl. WO 90 11,070 (Cl.A61 K31/685), 04 Oct. 1990
- Vaillant F., Millan A., Millan P., Dornier M., Decloux M., Reynes M. Co‐immobilized pectinlyase and endocellulase on chitin and nylon supports. Process Biochem. 2000; 35: 989–996
- Weethall H. H. Preparation of immobilized proteins covalenty coupled through silane coupling agents to inorganic supports. Appl. Biochem. Biotechnol. 1993; 41: 157–188
- Yedgar S., Lichtenberg D., Schnitzer E. Inhibition of phospholipase A2 as a therapeutic target. Biochim. Biophys. Acta 2000; 1488: 182–187