Abstract
The aim of this study was to determine whether treating concomitant depression improves quality of life and exercise tolerance in COPD patients. Out-patients with moderate to severe, stable COPD completed Hospital Anxiety-Depression (HAD) and General Health questionnaires. A psychiatrist interviewed those with high scores. In a randomised, double-blind fashion, 28 depressed COPD patients took a selective serotonin re-uptake inhibitor, Paroxetine 20 mg daily, or matched placebo for 6 weeks. Subsequently, all patients took un-blinded Paroxetine for 3 months. From these questionnaires, 35% of 135 patients had significant depression, but this was confirmed by psychiatric interview in only 21%. Throughout the study, there were no changes in laboratory lung function nor in home peak flow. Six weeks' treatment produced no significant differences between placebo and treatment group in either depression, quality of life scores or 6-minute walking distances, although overall improvements in depression, correlated with increases in walking distance. Three months of un-blinded treatment, significantly improved depression scores (self-complete HAD, Beck's Depression and psychiatrist-completed Montgomery-Asberg scores), walking distances (369 to 427 m, p = 0.0003) and St. George's Respiratory Questionnaire Total Scores (65 to 58, p = 0.033). Although self-complete questionnaires over-diagnose depression, the condition is nevertheless common in patients with moderately severe COPD. Six weeks of antidepressants is insufficient to improve either depression, quality of life or exercise tolerance. However, our study suggests that a longer course of treatment may be effective and that improvements in depression are associated with improvements in exercise tolerance. A larger, double blind study with a longer treatment period is indicated.
Introduction
The close relationship between psychological factors and both breathlessness and quality of life is well recognised in patients with chronic obstructive pulmonary disease (COPD). Burns and Howell's classic study Citation[[1]] reported that 52% of patients with severe chronic lung disease had depression, 22% had anxiety and 26% had hysteria. Subsequently, most studies have confirmed that COPD patients have a higher prevalence of mood disorder than healthy subjects Citation[2-6], although there are a few discordant views Citation[7-10]. Not withstanding, co-morbidity with depression has implications for the long-term management of patients with COPD. It is known that depression is more likely in patients with severe than with mild to moderate COPD, in those patients with the least reversibility of FEV1 and also in patients with severely impaired physical functioning Citation[[11]]. Depressed COPD patients have a poorer quality of lifeCitation[12&13] and more frequent hospital admissions, with a mean in-patient stay of 10 days compared with 2 days in non-depressed patients (p < 0.0001) Citation[[13]].
It has been recommended that pulmonary rehabilitation programmes incorporate social support groups Citation[[14]] and psychological support Citation[[15]]. However, few have studied the effect on dyspnoea and quality of life of treating the psychiatric problems of COPD patients without concomitant physical rehabilitation Citation[16-20]. In a small pilot study we have recently shown that group cognitive and behavioural psychotherapy produced significant and sustained improvements in exercise tolerance in ten anxious patients with moderately severe COPD Citation[[18]].
Although acknowledged to be important, there is a striking lack of data concerning the effects of treating depression on mood and physiological function in COPD patients. Studies involving patients with two concurrent conditions require complex monitoring of both conditions in order to detect potential interactions, such as whether a change in spirometry may influence mood. While early studies with tricyclic antidepressants failed to produce any improvement in dyspnoea Citation[19&20], the newer generation of antidepressants, selective serotonin uptake inhibitors (SSRI), have a potential advantage. They also improve panic disorder, probably by decreasing the CO2 sensitivity of the brain-stem respiratory control centres Citation[21&22]. COPD patients with greater degrees of CO2 sensitivity have more dyspnoea Citation[[23]]. In view of this and the postulated interactions between panic, dyspnoea and hyperventilation, SSRI inhibitors have been advocated for the management of breathless COPD patients, particularly those with either anxiety or depression. The aim of the present study was to establish whether SSRI's significantly improve exercise tolerance and quality of life in depressed COPD patients.
Material and Methods
Identification of Depressed COPD Patients
In order to identify suitable patients for further study, the Hospital Anxiety-Depression (HAD) Citation[[24]] and the 12-item General Heath (GHQ) Citation[[25]] questionnaires were administered to patients attending the weekly COPD clinics at Lewisham Hospital Chest Clinic. Although the questionnaires were explained, patients were given no assistance in completing them by either medical or nursing staff. All patients were in a stable clinical state with respect of their COPD at the time of study. Questionnaires were scored immediately—a score of 2 or more on the 12-item GHQ or a score of 10 or more on the HAD scale were taken as indicating a possible “case.” As many as possible of these patients were interviewed at this visit by a research psychiatrist (RH or SK) using a standard, structured interview technique. Each patient fulfilling the criteria for depression from the International Classification of Mental and Behavioural Disorders (ICD10) Citation[[26]] was invited to participate in the study. We continued to screen COPD patients until 28 with ICD10 criteria confirmed clinical depression were identified and were willing to enter into the study.
Subjects
The inclusion criteria were
Well-documented, stable, poorly reversible COPD with a change in FEV1 after bronchodilators of < 15% of predicted normal.
Either current or ex-smokers
Exercise tolerance limited by dyspnoea
Clinical depression, confirmed by standard psychiatrist interview using ICD10 criteria.
The exclusion criteria were
Previously diagnosed depression
Use of psychotropic drugs within 3 months of study
Significant co-morbidity limiting mobility, in particular cardiothoracic or locomotor problems.
Primary Outcome Measurements
Psychological and Quality-of-Life Questionnaires
On each study day patients completed self-administered HAD and Beck's Depression Inventory (BDI) Citation[[27]] questionnaires describing their symptoms in the preceding 6 weeks and then a psychiatrist completed their rating scale for the Montgomery Asberg Depression Score (MADRS) Citation[[28]]. In addition, patients completed a quality of life questionnaire specifically designed for COPD patients Citation[[29]]—the St. George's Respiratory Questionnaires (SGRQ). Patients were never shown their previous responses to questionnaires on subsequent visits and the researchers did not have access to the previous measurements of lung function and walking tests during the patients' visits.
Exercise Tolerance
On arrival in the clinic on each study day patients were given salbutamol 2.5 mg with ipratropium 0.5 mg via a nebuliser in order that subsequent lung function and walks were assessed when the patient was maximally and consistently bronchodilated. Forty minutes later, exercise tolerance was measured with a 6-minute walking test (6MWD) along indoor corridors. Patients were accompanied by one of the investigators who walked in front, encouraging the patients in a standardised fashion Citation[[30]] to walk as fast as they were able and to restart as soon as possible if they stopped. The subjects were discouraged from talking as they walked and asked to rest standing rather than sitting down during the test. On the first day patients also performed an initial “practice” 6MWD since there is a well-described learning effect with these tests Citation[[31]].
Secondary Outcome Measurements
Lung Function
The following lung function tests were performed to ensure that the COPD was in a stable state for the duration of the study; residual volume (RV) was measured in a computerised, constant volume, whole body plethysmograph, calibrated daily. The mean of 4–6 technically satisfactory measurements of RV was taken. FEV1, Forced Vital Capacity (FVC) and Slow Vital Capacity (SVC) were all measured with a turbine spirometer (Micromedical, Gillingham, Kent, UK) and the best of 3 technically satisfactory measurements was taken.
Diary Card Data
For the entire study patients kept diary cards, monitoring morning and evening peak expiratory flow (PEF), recording the best of 3 PEF measurements made 30 minutes after their morning and evening bronchodilator. They recorded their use of bronchodilators, nocturnal wakings due to dyspnoea, and also breathlessness and effect of breathlessness on quality of life on a 5-point scale ().
Protocol
Initial Double-Blind Placebo Controlled Phase of Study
Initially there was a 2-week run-in period. Then, patients received either Paroxetine 20 mg daily for 6 weeks or matched placebo in a randomised and double-blind fashion. The lung function tests, walks, and questionnaires were completed at baseline and after 6 weeks of blind treatment. In the event of intolerable side effects from the treatment, patients were offered a single-blind course of either Dothiepin 150 mg or Lofepramine 140 mg nocte.
Open Label Phase of Study
After discussions with psychiatric colleagues, it was decided that it was not ethical to withhold treatment for depression any longer than 6 weeks from patients with clinically significant depression, particularly since Paroxetine takes several weeks to produce its peak effect.
Accordingly, at 6 weeks, all patients continued taking Paroxetine 20 mg daily in an open fashion until all patients had received 3 months of active treatment. Thus, all patients received three months of active treatment by the end of the study. Patients were seen 2 weekly during both blinded and open label treatments to check for drug acceptability and also for compliance (with a tablet count). The Ethics Committee of Guys and Lewisham Hospitals approved the study and all patients gave written, informed consent to the study.
Analysis
The primary end-points of this study were the SGRQ quality of life scores, the walking distances and the depression scores that were used to confirm that the depression had been adequately treated. The Montgomery Asberg scores were considered the Gold Standard. The secondary end-points were the results of the lung function tests, used to confirm that there had been no change in the patients' physiological state during the conduct of the study.
The data were entered onto an Excel Spreadsheet. Means, standard deviations and 95% confidence intervals were calculated for the lung function measurements, the 6MWD, the questionnaire results and the diary card scores. Then, t tests were used in both parts of the study. The results from the last day of the treatment periods were compared with those from the last day of the run-in period. First, in the double-blind part of the study, unpaired t-tests compared changes found after active treatment with those found after placebo and then, in the open label part, paired t-tests compared results at baseline with those after 12 weeks of active antidepressant treatment. The diary card scores from the 2-week run-in period were compared with the last 2 weeks of the double-blind part of the study, as well as with the open study.
Bonferroni's corrections were applied as required in order to determine the statistical significance of the results. Bland Citation[[32]] has warned that the Bonferroni correction is highly conservative and may result in missing true significance and so we opted to apply it for 5 categories rather than for each individual measurement.
We have also calculated the effect of improved mood on exercise and quality of life, whether achieved by the active drug, placebo or by giving the extra attention to the patient during the conduct of the trial. The change in mood score before and after placebo and active drug was correlated with 6MWD and SGRQ scores.
Pearson's correlation coefficients were calculated to test for correlations between the different types of depression scores and also between the depression and anxiety scores with 6MWD and SGRQ scores.
Results
Prevalence of Mood Disorder
During the initial recruitment phase of this study 135 consecutive patients with moderate to severe COPD (FEV1≤ 60% predicted) were screened in our clinic. There were 72 males and 63 females with a mean age of 70 ± 10 (SD) years (range 48–90 years). All patients completed HAD questionnaires and 128 of them also completed GHQ questionnaires; 65% patients were identified as potential “psychiatric cases” from GHQ scores; 35% of patients completing the questionnaires demonstrated significant depression, 29% significant anxiety and 14% had both anxiety and depression on HAD scoring. A psychiatrist interviewed 74% of the potential cases and a diagnosis of mood disturbance was confirmed in 48% of those interviewed. Thus, the self-complete questionnaires over-diagnosed mood disorders in our COPD outpatients who completed the self-administered questionnaires. Of the original COPD patients surveyed, 21% were significantly depressed and 16% significantly anxious according to ICD10 criteria. Twenty-eight of these COPD patients with confirmed clinical depression agreed to partake in the study.
Patients and Treatment
There were 14 females and 14 males with a mean age of 66 ± 8 years (range 49–79 years) of whom 8 were current smokers and 20 ex-smokers. All had smoked for, at least, 20 pack years. Mean baseline FEV1 was 1.1 ± 0.6l (SD), mean FEV1 % predicted was 43 ± 16% and mean 6MWD was 369 ± 119 m. Mean baseline scores for depression were 12 ± 3 for HAD scores, 21 ± 7 for BDI and 23 ± 8 for MADRS questionnaires, while mean total St George's score was 65 ± 13. There were no significant differences at baseline in any of the measurements of lung function, exercise tolerance, mood scores and quality of life scores comparing patients taking active drug and those taking placebo.
Four patients who had initially drawn active anti-depressant treatment developed significant side effects on Paroxetine—nausea and vomiting—and they finished the study taking Lofepramine 140 mg nocte in a single-blind fashion. One patient who had initially drawn placebo medication also developed nausea on the open Paroxetine period and continued in the single-blind arm of the study taking Dothiepin 150 mg daily. Two patients, one initially on active treatment and one on placebo, did not improve when on active Paroxetine, despite increasing the dose to 40 mg daily. Consequently, their treatment was changed to Dothiepin 150 mg daily for the second part of the study. All of these patients were assessed as part of the study and none of them developed side effects on their subsequent treatments.
Six patients were reviewed after completing 6 weeks of active treatment only. Of these, 3 patients were admitted to hospital with acute exacerbations of COPD, 1 patient felt very well and decided to stop his Paroxetine after 6 weeks. A further 2 patients were lost to follow up after 6 weeks active treatment despite claiming to feel better.
Primary End Points
Double-Blind Study
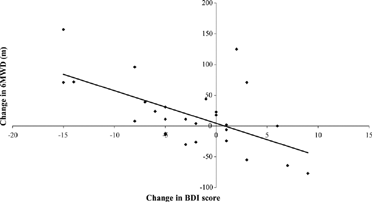
After 6 weeks, neither the patients on active anti-depressant nor those on placebo had clinically significant changes in 6MWD or SGRQ values (). On active anti-depressant all depression scores improved, particularly the MADR score. However, the changes did not reach statistical significance when compared the effects of placebo. In some patients placebo produced notable improvements in depression. When active treatment and placebo data were pooled, a highly significant correlation was found between reduction in depression score and improvement in exercise tolerance (n = 27, r = − 0.6012, p < 0.001) ().
Table 1 Clinic Data for COPD Patients Comparing the Difference Between Baseline and 6 Weeks for Placebo and Active Depressant.
Open Label Study
Significance levels have been calculated using the Bonferroni correction (). When the data from all 22 patients who completed three months of active antidepressant treatment is considered together, a significant improvement in 6MWD from 369 m to 427 m (p = 0.0003) was demonstrated (). During the 12 weeks of treatment, depression scores continued to improve until all reached highly statistically significant changes (). The baseline HAD (depression), BDI and MADRS scores of 12, 21 and 23 respectively fell significantly to 8, 12 and 9 (p < 0.0001) at the 12th week. In clinical practice, these scores are not generally associated with significant depression. HAD (anxiety), score also improved significantly by 3 months from 12 to 9 (p = 0.02). The total SGRQ quality of life score improved from 65 to 58 (p = 0.033). The main contributor to this improvement was the impact score, which improved from 51 to 41. The symptom score was unchanged at 74 and there was a small improvement in activity from 84 to 80, which failed to reach significance if the Bonferroni correction is applied (p = 0.215).
Table 2 Results comparing baseline with 3 months of open label antidepressant treatment (n = 23).
When active treatment and placebo data were pooled for double blind and open label antidepressant parts of the study, a highly significant correlation was found between improvement of mood and both exercise tolerance and SGRQ scores (n = 51). Improvement in BDI score was correlated with increased 6MWD (r = − 0.424, p < 0.01) whilst improvements in MADRS and in HAD anxiety scores were significantly correlated with improved symptom scores of the SGRQ (r = 0.3372, p < 0.02, and r = 0.279, p < 0.05, respectively).
Depression scores, whether self assessed or assessed by the psychiatrist, were highly significantly correlated; for HAD[depression] versus Beck r = 0.730, p < 0.0001, for HAD[depression] versus MADRS r = 0.570 p < 0.0001, for Beck versus MADRS r = 0.550 p < 0.0001).
Lung Function Tests and Diary Cards (Secondary End Points)
There were no significant changes in lung function tests nor in diary card scores at any time during both the double blind and the open label phases of the study whether patients were taking active antidepressants or placebo (Tables and ).
Discussion
Prevalence
The prevalence of mood disturbance in our moderate to severe COPD patients was in keeping with that reported previously Citation[1-6]. Both self-complete HAD and GHQ scores over-estimated the prevalence of mood disturbance in COPD patients. In future studies, a higher cut-off point should probably be used (possibly a HAD score of 12) for the identification of mood disorder in this type of patient. Nevertheless, this study has demonstrated that about 21% of COPD patients attending an outpatient Chest Clinic in a deprived urban area in London have significant, previously undiagnosed depression.
Effect of Antidepressants
From the results of the lung function tests our patients were in a demonstrably stable state with regards to their COPD throughout the study. Consequently, improvements in exercise tolerance or quality of life cannot be attributed to improvements in airflow obstruction. We were only able to achieve a small treatment effect with the antidepressant tablets within the 6 weeks of the double blind part of the study and this was not statistically significant when compared with placebo. However, a clear correlation has been demonstrated between changes exercise tolerance and depression scores.
As witnessed by the reduction in self-complete HAD and BDI and also our gold standard, the psychiatrist-completed MADRS questionnaires scores, the trends towards improvements in anxiety and depression continued throughout the 3-month period of the open study until they were statistically significant. By the time the study was terminated, highly significant improvements in exercise tolerance were also recorded. Most aspects of quality of life tended to improve but, if the Bonferroni correction is employed, only the total SGRQ score reached statistical significance. However, as previously mentioned, there is some debate as to whether this correction is appropriate Citation[[32]]. Since COPD patients are able to perceive a change of 4 units in SGRQ as a change in quality of life Citation[[33]], this may be taken as clinically significant. Although the second part of the study was open label, this study suggested for the first time that alleviating depression and anxiety, either by response to active drugs, placebo or by the social interaction of participating in the study, was associated with improved exercise capacity and quality of life. Whether this was due solely to the anti-depressants needs to be verified with a further larger study in which the drugs are given for a longer period.
Previous studies with tricyclic antidepressants produced disappointing results. In the first Citation[[19]] neither depression, quality of life nor dyspnoea improved and the study was difficult to interpret due to its short duration, the short wash out period and the prominent anticholinergic side effects. The other larger, placebo-controlled study with nortryptyline Citation[[20]] successfully alleviated depression and anxiety in the majority of subjects and improved some aspects of quality of life and functional ability. The 9% improvement in Sickness Impact Profile compared favourably with that achieved by other medical interventions Citation[[34]] but it was not associated with reduced dyspnoea. Our results are in agreement with this.
Compared with tricyclic anti-depressants, SSRI drugs have proved useful anti-depressants with less side effects. They have also become first line treatment for panic disorder, Two pilot studies using Sertraline, in a total of only 9 patients, produced promising results. The drug was well tolerated and produced few side effects Citation[35&36]. In addition, it improved the activities of daily living and sense of general well being of 6 COPD patients, three of whom had some mood disturbance Citation[[35]]. In another case report, Sertraline also improved dyspnoea in three COPD patients with concomitant mood disturbance Citation[[37]]. More recently, Yohannes and colleagues attempted to do a similar trial to our own Citation[[13]]. They surveyed 137 outpatients with moderate to severe COPD and found 42% depressed according to the Geriatric Mental State scores. Only 14 of 57 depressed patients agreed to participating in a 6-month trial of antidepressants (fluoxetine). Disappointingly, only 7 subjects completed the study and, of these, only 3 responded adequately to treatment. Thus, it was impossible to evaluate the study.
Our patients were clinically anxious at the onset of the study. Reductions in HAD anxiety scores correlated with decreases in symptom scores of the SGRQ although HAD anxiety score did not significantly improve during the double-blind part of the study. This may have been partly because the alternative antidepressants used in 4 patients have less effect on anxiety than Paroxetine and partly because of the short course of treatment. Nevertheless, after 3 months of treatment, the anxiolytic effects of Paroxetine may have contributed to the increased exercise tolerance seen, since we have previously shown Citation[[18]] that a reduction in anxiety in COPD patients is associated with an improved exercise capacity. The patients were able to walk further without increasing their post-exertional dyspnoea scores. On the other hand, this effect was inadequate to improve our rather crude home dyspnoea scores and only reduced the total SGRQ score without significantly improving its component parts.
Problems with the Study
We document, in considerable detail, how the physiological state of our patients did not significantly fluctuate during this lengthy study. It was not possible to conduct a crossover study since, after a course of treatment, depression may or may not relapse. A further problem in our study was the side-effect profile of Paroxetine. Our results were more difficult to evaluate since 5 of our 28 patients had such unpleasant nausea that we were obliged to change their medication. In addition, the long interval to peak effect of the drug proved an unexpected problem. We had anticipated the peak effect to be at about 4 to 6 weeks, but at 6 weeks of standard, clinical doses, Paroxetine had only modestly improved depression scores. These continued to improve until the 12th week of treatment and it is possible that we had not reached peak effect even at 3 months. However, it was deemed unethical to withhold active treatment from depressed patients for longer than 6 weeks and so, at this point, we were obliged to continue the study in an open fashion. In so doing, the strength of our results and conclusions has inevitably been diminished. In retrospect, it would probably have been acceptable to continue the study since none of the placebo patients had worsening depression over the first 6 weeks; indeed some improved. Depression scores of two patients on active treatment had not improved significantly after 6 weeks. For the open study the dose of Paroxetine was initially increased to 40 mg daily. When this proved ineffective, treatment was changed to Dothiepin 150 mg daily.
Validation of Assessment Tools Used
In the treatment part of the study we were particularly anxious to have an accurate estimate of the patients' mental state for assessing the effects of the antidepressants and so we used three different questionnaires to monitor depression. Two were self-rating scores, the HAD score, used as in the first part of the study and the Beck Depression Scale, which has previously been shown to be repeatable and to correlate strongly with clinical diagnosis in a standardized interview Citation[[27]]. We also used the Montgomery Asberg scale, scored by a psychiatrist after a structured, standardized interview Citation[[28]], as our gold standard for depression rating. The results from the self-administered HAD and Beck questionnaires correlated strongly with the psychiatrist scores. Thus, these self-rated scales appear to be adequate for monitoring significant mood change in these patients and could be used in future studies without the time-consuming interviews with a psychiatrist.
As mentioned previously, the HAD score significantly overestimates depression. Similarly, the BDI contains 7 of 21 questions that relate to somatic symptoms, such as poor sleep or appetite, weight loss, tiredness, inability to work, tiredness and disinterestedness in sex. These are likely to be answered in the affirmative by patients with severe COPD and this would inflate the score.
We chose to use the SGRQ to monitor quality of life. It is repeatable and its activity score correlates with 6MWD Citation[[29]]. It is also sensitive to change in patients with COPD Citation[[13]]Citation[[29]]Citation[[33]] and reliable over time Citation[[33]]. Apart from the symptom scores, the other domains and the total SGRQ score all changed by more than 4 points (the threshold for clinical significance) following anti-depressant treatment. However, the questionnaire is long and the completion rate has been shown to be less good than that of the Chronic Respiratory Questionnaire Citation[[38]]. It is also onerous for the researcher to score.
In this, as in previous studies, the 6MWD has proved a reliable and reproducible measure of exercise tolerance in patients with COPD Citation[[18]]Citation[39&40]. As in our recent study on the effect of psychotherapy on anxious patients with COPD Citation[[18]], the 6MWD increased significantly as a consequence of the improvement in our patients' mood disturbance.
In conclusion, because of a number of problems in the conduct of the study, it should be regarded as a pilot study only. Six weeks of antidepressant treatment was insufficient to significantly ameliorate the depression in our group. However, depression significantly improved after 12 weeks of open-label anti-depressant. While this may not have been due to the active treatment, it was associated with worthwhile improvements in exercise capacity and also in quality of life. Depression is relatively common in patients with COPD. Its presence should not be missed, since its treatment is an important constituent of the overall management of these patients. SSRI drugs may be particularly useful since they also improve anxiety. Our findings indicate the importance of a holistic approach in the management of COPD patients. Further studies, are necessary to extend these results, using SSRI drugs with a much faster onset of action and fewer side-effects.
References
- Burns B H, Howell J BL. Disproportionately severe breathlessness in chronic bronchitis. Q J Med 1969; 38:277–294. [PUBMED], [INFOTRIEVE]
- Agle D P, Baum G L, Chester E H, Wendt M. Multidisciplinary treatment of chronic pulmonary insufficiency. Psychological aspects of rehabilitation. Psychosom Med 1973; 35:41–45. [PUBMED], [INFOTRIEVE]
- Agle D P, Baum G L. Psychological aspects of chronic obstructive pulmonary disease. Med Clin North Am 1977; 61:749–758. [PUBMED], [INFOTRIEVE]
- Grant I, Heaton R K, McSweeny A J. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142:1470–1476. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Greenberg G D, Ryan J J, Bourlier P F. Psychological and neuro-psychological aspects of COPD. Psychosomatics 1985; 26:29–33. [PUBMED], [INFOTRIEVE]
- Yellowlees P M, Alpers J H, Bowden J J, Bryant G D, Ruffin R E. Psychiatric morbidity in patients with chronic obstructive pulmonary disease. Med J Aust 1987; 146:305–307. [PUBMED], [INFOTRIEVE]
- Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with COPD. Am J Psychiatry 1990; 147:200–201. [PUBMED], [INFOTRIEVE]
- Isoaho R, Keistinen T, Laippala P, Kivela S-L. Chronic obstructive pulmonary disease and symptoms related to depression in elderly persons. Psychol Rep 1995; 76:287–297. [PUBMED], [INFOTRIEVE]
- Engstrom C-P, Persson L-O, Larsson S, Ryden A, Sullivan M. Functional status and well being in chronic obstructive pulmonary disease with regard to clinical parameters and smoking: a descriptive and comparative study. Thorax 1996; 51:825–830. [PUBMED], [INFOTRIEVE]
- Van Eade L, Yzermans C J, Brouwer H J. Prevalence of depression in patients with chronic obstructive pulmonary disease: a systematic review. Thorax 1999; 54:688–692.
- van Manen J G, Bindels P J, Dekker F W, IJzermans C L, van der Zee J S, Schade W. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002; 57:412–416. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Yohannes A M, Roomi J, Baldwin R C, Connolly M J. Depression in elderly outpatients with chronic obstructive pulmonary disease. Age Ageing 1998; 27:155–160. [CSA]
- Yohannes A M, Baldwin R C, Connolly M J. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence and validation of BASDEC screening questionnaire. Int J Geriatr Psychiatry 2000; 15:1090–1096. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Muir J-F, Pierson D J. Pulmonary rehabilitation in chronic respiratory insufficiency. Thorax 1993; 48:854–862.
- Celli B R. Pulmonary rehabilitation in patients with COPD. Am J Respir Care Med 1995; 152:861–864. [CSA]
- Atkins B J, Kaplan R M, Timms R M, Reinsch S, Zofback K. Behavioral exercise programs in the management of chronic obstructive pulmonary disease. J Consult Clin Psychol 1984; 52:591–603. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sassi-Dambron D E, Eakin E G, Ries A L, Kaplan R M. Treatment of dyspnea in COPD: a controlled clinical trial of dyspnea management strategies. Chest 1995; 107:724–729. [PUBMED], [INFOTRIEVE]
- Eiser N M, Evans S, Jeffers A, Quirk F. Effects of psychotherapy on dyspnoea in moderately severe COPD: a pilot study. Eur Respir J 1997; 10:1581–1584. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Light R W, Merill E J, Despars J, Gordon G H, Mutalipassi L R. Doxepin treatment of depressed patients with chronic obstructive pulmonary disease. Arch Intern Med 1986; 146:1377–1380. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Borson S, McDonald G J, Gayle T, Deffebach M, Lakshminarayan S, VanTuinen C. Improvement in mood, physical symptoms and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics 1992; 33:190–201. [PUBMED], [INFOTRIEVE]
- Mueller R, Lundberg D, Breese G, Hedner J, Hedner T, Jonason J. The neuropharmacology of respiratory control. Pharmacology 1982; 34:255–285.
- Lundberg D, Mueller R, Breese G. An evaluation of the mechanism by which serotoninergic activation depresses respiration. J Pharmacol Exp Ther 1980; 212:397–404. [PUBMED], [INFOTRIEVE]
- Matthews A W. The relationship between central carbon dioxide sensitivity and clinical features in patients with chronic airways obstruction. Q J Med 1977; 46:179–195. [PUBMED], [INFOTRIEVE]
- Zigmond A S, Snaith R P. The hospital anxiety-depression scale. Acta Psychiatr Scand 1983; 67:361–370. [PUBMED], [INFOTRIEVE]
- Goldberg D P, Hillier V F. A scaled version of the General Health Questionnaire. Psychol Med 1979; 9:139–145. [PUBMED], [INFOTRIEVE], [CSA]
- The ICD-10 Classification of Mental and Behaveoural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1993:119–141.
- Beck A T, Ward C H, Mendleson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571. [PUBMED], [INFOTRIEVE]
- Montgomery S A, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389. [PUBMED], [INFOTRIEVE]
- Jones P W, Quirk F H. A self complete measure of health status for chronic airflow limitation. Am Rev Respir Dis 1992; 145:1321–1327. [PUBMED], [INFOTRIEVE]
- Guyatt G H, Pugsley S O, Sullivan M J, Sullivan M J, Thompson P J, Berman L B, Jones N L, Fallen E L, Taylor D W. Effect of encouragement on walking test performance. Thorax 1984; 39:818–822. [PUBMED], [INFOTRIEVE]
- Eiser N, Willshire D, Dore C. Reliability, repeatability and sensitivity to change of externally and self-paced walking tests in COPD patients. Respir Med Apr., 2003; 97(4):407–414. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bland M. An Introduction to Medical Statistics. 3rd ed. Oxford Medical Publications, 2000:151.
- Jones P W, Lasserson D. Relationship between changes in St. George's questionnaire score and patients perception of treatment efficacy after one year of therapy with necrodomil sodium. Am J Respir Crit Care Med 1994; 149:A211.
- Bergner M, Bobbitt R A, Carter W B, Gilson B S. The sickness impact profile: development and final revision of a health status measure. Med Care 1981; 19:787–805. [PUBMED], [INFOTRIEVE]
- Papp L A, Weiss J R, Greenberg H E, Rifkin A, Scharf S M, Gorman J M, Klein D F. Sertraline for chronic obstructive pulmonary disease and co-morbid anxiety and mood disorders (letter). Am J Psychiatry 1995; 152:1531. [PUBMED], [INFOTRIEVE]
- Smoller J W, Pollack M H, Systrom D, Kradin R L. Sertraline effects on dyspnea in patients with obstructive airways disease. Psychosomatics 1998; 39:24–29. [PUBMED], [INFOTRIEVE], [CSA]
- Jones P W, Bosh T K. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med 1997; 155:1283–1289. [PUBMED], [INFOTRIEVE], [CSA]
- Harper R, Brazier J E, Waterhouse J C, Waters S J, Jones N MB, Howard P. Comparison of outcome measures for patients with chronic obstructive pulmonary disease (COPD) in an outpatient setting. Thorax 1997; 52:879–887. [PUBMED], [INFOTRIEVE]
- Mungall I PF, Hainsworth R. Assessment of respiratory function in patients with chronic obstructive pulmonary disease. Thorax 1979; 34:254–258. [PUBMED], [INFOTRIEVE]
- Eiser N, Denman W T, West C, Luce P. Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the “pink puffer”syndrome. Eur Respir J 1991; 4:926–931. [PUBMED], [INFOTRIEVE]