Abstract
The significance of IgM on immunofluorescence in renal biopsy specimens remains unclear. This retrospective case study was conducted to define the clinical features, response to therapy and outcome of patients with Mesangioproliferative Glomerulonephritis (MGN) with diffuse IgM deposition. Of 1919 native renal biopsies performed over a ten-year period, 139 (7.2%) had light microscopic features of MGN and manifested IgM as the dominant immunoglobulin. When exclusion criteria (more than a trace of IgA or IgG, segmental IgM, evidence of SLE, vasculitis, FSGS or Alport's syndrome and pregnant patients) were applied, 60 patients (3.1%) remained. Follow-up data were available for 54 cases with a mean age of 26.5 years (range 1.7–63). Mean follow-up period was 7.4 years (range 4.7–22.2). Forty-one per cent presented with nephrotic syndrome (NS), 26% with asymptomatic proteinuria (>250mg/24hr), 18% with macroscopic hematuria and 15% with isolated microscopic hematuria. Twenty-one percent of patients were hypertensive at presentation. Creatinine was initially <120(mol/L in all but one patient. Only four patients (7.4%), all nephrotic, suffered a decline in renal function despite treatment; all 4 developed ESRF after a mean of 5.6 years (range 2–8.3). Two of these were subsequently re-biopsied and found to have FSGS. No patients with isolated microscopic / macroscopic hematuria or asymptomatic proteinuria suffered a decline in renal function. Protein excretion rate fell into the normal range in 63% of those receiving steroids, with 82% becoming steroid dependent. Of those treated with cyclosporine (48%) or cyclophosphamide (52%) only 9.5% and 14.5% respectively remained in prolonged remission after discontinuing treatment. It is concluded that MGN with IgM deposition carries a very favorable prognosis except in patients with NS who develop FSGS. However there is a high incidence of steroid dependence and resistance in the proteinuric group.
INTRODUCTION
The natural history of primary glomerular disease associated with IgM deposition remains undetermined. It was initially postulated as representing a distinct entity in 1978 with the description of a small series of patients with heavy proteinuria and apparent steroid resistance Citation[[1]]. Subsequent studies have both supported Citation[2-9] and refuted Citation[10-19] this assertion, with some authors Citation[[20]] postulating that IgM deposition plays an important role in the putative transformation of minimal change disease into focal sclerosing glomerulopathy. It has been suggested that IgM associated immune complexes are either formed in-situ or deposited in the mesangium, resulting in a local T-cell mediated inflammatory response, mesangial proliferation and glomerular injury Citation[[4]]. Others have maintained that, as a consequence of mesangial proliferation, monocytic clearance of IgM molecules is delayed and that their presence on immunofluorescence merely represents an immunological “epi-phenomenon” Citation[[21]]. Analysis of the literature is be-devilled by differences in definition and specifically by the inclusion of nephrotic patients with focal segmental glomerulosclerosis.
In order to obtain a clinically relevant viewpoint we rigidly defined “IgM nephropathy”, specifically excluding patients with normal glomeruli or focal segmental glomerulosclerosis by light microscopy and including paediatric patients. Our aim was to identify the clinico-pathological features of patients with primary glomerular disease and diffuse mesangial IgM deposition and to describe the natural history over a minimum of 4 years.
METHODS
Patients
Beaumont Hospital is a tertiary referral center and the site of the National Kidney Center. Children with nephrotic syndrome were biopsied if they had clinical features atypical of Minimal Change Disease including steroid resistance and dependence. Our policy is to biopsy patients with non-nephrotic proteinuria and those with persistent microscopic hematuria (after negative cystoscopy, I.V.P., and renal ultrasound). Between 1986 and 1993 inclusive, 1919 adequate native renal biopsies were analyzed, of which 139 (7.2%) manifested diffuse mesangial proliferation and IgM deposition. Sixty (3.1%) satisfied our criteria for IgM nephropathy, with adequate follow up data being available for 54. The response to steroid therapy, relapse rate and response to cyclophosphamide / cyclosporine therapy were evaluated where indicated. Patients were followed until the date of last follow-up, death or the development of end stage renal failure. Clinical data were obtained directly from the patients' charts. In cases where this was not possible, the patients were contacted by telephone.
Definitions
| • IgM nephropathy: | = | Primary diffuse MGN with IgM being deposited diffusely in the mesangium as the sole or dominant immunoglobulin on immunofluorescence examination. The presence of thin glomerular basement membranes on electron microscopy did not exclude the patient from analysis. |
| • Nephrotic syndrome: | = | Proteinuria >3.5g/24 hrs in conjunction with edema and/or serum albumin <30g/dL. |
| • Asymptomatic proteinuria: | = | Proteinuria >250mg/24 hrs but not meeting the definition of nephrotic syndrome. |
| • Microscopic hematuria: | = | Dipstick positive for blood with >3 RBC/ hpf on three occasions. |
| • Hypertension: | = | The need for antihypertensive drug treatment. |
| • Renal impairment: | = | Serum creatinine >120umol/L. |
| • End-stage renal failure: | = | The need for permanent renal replacement therapy. |
| • Remission: | = | Reduction in proteinuria to <250mg/24 hrs. |
Exclusion Criteria
Evidence of systemic disease (systemic lupus erythematosus, vasculitis, Alport's syndrome, rheumatoid arthritis).
Patients under 18 months of age, in order to exclude patients with congenital nephrotic syndrome.
Pregnant patients.
More than a trace of IgA or IgG on immunofluorescence.
Focal segmental glomerulosclerosis or normal glomeruli on light microscopy.
Pathology
Renal biopsy specimens were examined by light, immunofluorescence, and electron microscopy. Tissue was stained with PAS and HE. Light microscopic grading of mesangial proliferation and assessment of electron microscopic changes were repeated in a blinded fashion; other histological features were graded according to the biopsy reports.
Mesangial proliferation was graded on a scale of zero to +3 as follows:
Zero: = Normal by light microscopy
+1: = Expansion of mesangial matrix only
+2: = Mesangial hypercellularity
+3: = Mesangial hypercellularity + expansion of mesangial matrix
Changes on electron microscopy of foot process effacement and / or electron-dense deposits were graded as follows:
0: = No deposits or foot process effacement
+1: = Non-specific electron-dense deposits or minor foot process effacement
+2: = Deposits characteristic of post-infectious glomerulonephritis or marked, diffuse foot process effacement
Tubular atrophy was graded as zero (absent), +1 (<50% of tubules) and +2 (>50% of tubules)
Vascular changes were graded as zero (absent), +1 (mild intimal thickening), +2 (moderate arteriosclerosis) and +3 (severe arteriosclerosis)
Focal global sclerosis was graded as zero (absent), +1 (<10% of glomeruli), +2 (10-20% of glomeruli) and +3 (>20% of glomeruli)
The inclusion of juxta-medullary glomeruli in the biopsy specimen was recorded, as was the degree of co-deposition of complement on immunofluorescence.
Statistical Anlaysis
For positively skewed data sets, logarithmic transformation was employed and the mean expressed as the geometric mean. Student's T-test and Chi2 test were applied where indicated using the SAS statistical package.
RESULTS
Clinical Features at Presentation
Mean age at presentation for all patients was 24.6 years (range 1.6–63.1), thirty-five (65%) of whom were male. Clinical features at the time of biopsy are summarized in . Forty-one percent of patients had nephrotic syndrome, 26% had asymptomatic proteinuria and the remainder presented with hematuria. There was a very low prevalence of renal insufficiency at presentation with serum creatinine under 120μmol/L in all but one patient. Overall, 21% of the patients were hypertensive at presentation, the vast majority of these belonging to the nephrotic or microscopic hematuria groups.
Table 1. Clinical features at the time of biopsy
Histological Features
Juxtamedullary glomeruli were included in 11 (50%) of the biopsies from nephrotic patients. Mesangial proliferation was graded as +1 in 16 (29.6%), +2 in 35 (64.8%) and +3 in 3 patients (5.6%). Intensity of IgM deposition was mild in 34 (65%), moderate in 16 (31%) and marked in 2 (4%). Thirty-eight patients (70.4%) had adequate electron photomicrographs. Of these, the majority had no or minor foot process effacement (97.3%) and no or non-specific electron-dense deposits (89.5%), although most of the nephrotic patients had received corticosteroids prior to biopsy. There were no significant differences between the four sub-groups with respect to the electron microscopic changes.
Table 2. Pathological features on renal biopsy
In patients with proteinuria, the protein excretion rate was found to correlate with the degree of tubular atrophy (p = 0.05) and intensity of IgM deposition (p < 0.01). There was no correlation between protein excretion rate or serum creatinine and degree of mesangial proliferation, co-deposition of complement, vascular changes or degree of focal global sclerosis. No patients had co-deposition of IgG or IgA. No patients had focal segmental sclerosis on light microscopy.
Clinical Course
Mean follow up period from the time of presentation was 7.4 years (4.7–22.2). Four patients (7.4%) suffered a decline in renal function, all of whom were nephrotic at presentation. All of these progressed to end-stage renal failure after a mean of 5.6 years (range 2–8.3). In no other patient was there a rise in serum creatinine to above 120umol/L. There was a significant rise in serum creatinine compared to baseline in the nephrotic group, even after excluding patients progressing to ESRF, although many of the patients in this group entered their teens during the course of the study period (). Of the patients who progressed, two underwent repeat biopsy and in both of these, focal segmental glomerulosclerosis was seen on light microscopy. Juxtamedullary glomeruli were not present in either of their original biopsies. They were included in the final analysis as clinical decisions are made on the basis of the first biopsy. There was no significant correlation between risk of progression and baseline serum creatinine, degree of tubular atrophy, focal global sclerosis, vascular changes, intensity of IgM deposition, degree of mesangial proliferation or presence / absence of hypertension.
Table 3. Clinical features at the time of last follow up
Response to Treatment
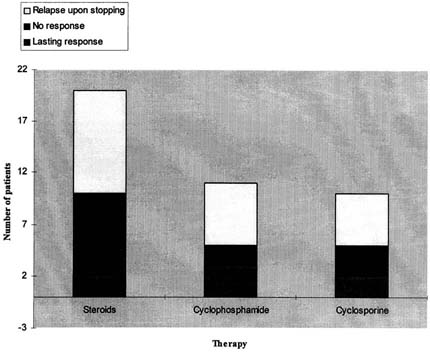
Only the patients in the nephrotic group received immunomodulatory therapy (). Twenty nephrotic patients (90.9%) received corticosteroids (2mg/kg), of whom 12 (63%) went into remission. Ten of these (82%) became steroid dependent with a mean relapse rate of 0.9 episodes per year. Eleven nephrotic patients received cyclophosphamide (50%) of whom 3 (27%) had a lasting response, 2 (18%) had no response and 6 (55%) relapsed upon stopping the therapy. Ten patients were treated with Cyclosporine A (45%) of whom 2 (20%) had a lasting response, 3 (30%) had no response and 5 (50%) relapsed upon stopping the therapy.
DISCUSSION
We have conducted a follow up study on 54 patients with the pathological picture of mesangioproliferative glomerulonephritis associated with diffuse IgM deposition on immunoflourescence. Follow-up was prolonged and few patients were lost. The literature in this area is confusing and contradictory, largely because of loose definitions. We specifically designed the study to strictly exclude patients with FSGS lesions in an effort to obtain a clearer picture of the natural history of the heterogeneous syndrome “IgM nephropathy”. We found that patients with this glomerular lesion presented with three clinical syndromes: nephrotic syndrome (41%), asymptomatic proteinuria (26%) or microscopic / macroscopic hematuria (33%). The non-nephrotic patients had an excellent long-term prognosis.
Since Border, Cohen and Glassock's description Citation[[1]], there have been 17 clinicopathological studies of “IgM nephropathy” Citation[[2]], Citation[6-20], Citation[[22]], with wide and varied definitions employed. Earlier studies included patients with normal glomeruli by light microscopy Citation[[1]], Citation[6-7], Citation[11-17], Citation[[19]], some included patients with focal segmental glomerulosclerosis Citation[[2]], Citation[[7]], Citation[[10]], Citation[[15]] and some excluded children. Initially, IgM deposition in nephrotic patients was found to confer steroid resistance and an adverse prognosis. Analogies have been drawn with MGN associated with IgA deposition Citation[[2]], an entity that now enjoys a unique clinico-pathological definition. The case mix in our study contrasts with those studies that included only a pediatric population Citation[11-14] in that we had a much smaller proportion of patients with nephrotic syndrome. O'Donoghue et al. Citation[[2]] performed a similar study to ours, with an equal number of patients and comparable clinical presentations. In common with our study, patients with low-grade proteinuria and hematuria had an excellent long-term renal prognosis. It is possible that these patients were recovering from an ill-defined post-infectious glomerular lesion. However, O'Donoghue's study reported an actuarial renal survival of 80% at five years and 64% at ten years. This contrasts sharply with the of 98% and 93% five- and ten-year renal survival in our study. This difference was due to the fact that patients with FSGS on the initial biopsy were included in O'Donoghue's study and it was in this group that patients suffered progressive renal insufficiency. Indeed, 18% of our nephrotic patients had an aggressive course with progression to ESRF. Two of these patients were subsequently found to have focal sclerosing lesions on repeat biopsy. It is probable that the other two with progressive renal disease also had FSGS, illustrating the problem of defining glomerular disease on the basis of initial biopsy findings.
Figure 2. PAS × 200. Biopsy from a patient presenting with hematuria and low-grade proteinuria. The glomerulus shows mesangial hypercellularity and mild endocapillary proliferation.
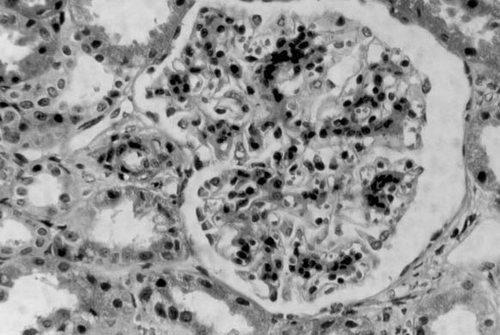
Figure 3. Direct Immunofluorescence for IgM × 200. Diffuse mesangial positivity with some capillary loop involvement in a patient presenting with hematuria and low-grade proteinuria.

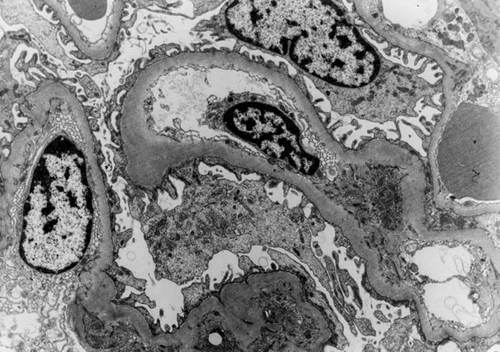
Figure 4. Electron Micrograph × 3000. Focal “hump-like” lesion (↑) and organizing sub-endothelial lesion (↑) from a patient presenting with hematuria and low-grade proteinuria.
Although not specifically designed to assess treatment response, our study supports the concept that nephrotic syndrome secondary to IgM nephropathy is associated with steroid dependence and reasonable but transient responses to cytotoxic agents and cyclosporine. This concept, however, has been challenged recently. In a case-control study performed by Al-Eisa et al. Citation[[12]] involving 90 children comparing immune-negative with IgM positive minimal change lesions, there was no difference in terms of steroid responsiveness and long-term prognosis between the two groups. It should be emphasized that the majority of the patients in this study had normal glomeruli by light microscopy and therefore would not come under our definition of IgM nephropathy. Pardo et al. Citation[[17]] reported another case-control study of 61 children with nephrosis, 13 of whom had mild mesangial proliferation. There was no difference with respect to response to therapy or outcome whether or not IgM deposition was present. These findings were expanded upon by the Southwest Paediatric Nephrology Study Group Citation[[13]] and in the International Study of Kidney Disease in Children Citation[[11]]. These authors concluded that it was the degree of mesangial hypercellularity rather than the intensity (or even the presence) of IgM on immunoflourescence that correlated closest with steroid responsiveness and outcome.
Thus, on the basis of these studies, limited to childhood nephrotics, there is currently debate as to whether IgM nephropathy is a distinct entity in this patient population or whether the immunoglobulin is an “immunological bystander”, trapped in damaged areas of the glomerulus. However, we believe that the inclusion of patients with minimal glomerular changes and FSGS in these studies distorts the picture, especially given emerging evidence that many cases of primary FSGS are in fact part of a systemic disease caused by a circulating factor Citation[23-24]. IgM Nephropathy, as designated in our study, provides a more meaningful basis on which to conduct further clinico-pathological studies. In addition, case control studies, with careful controlling for light microscopic appearance and age, are needed to elucidate whether IgM deposition in patients with MGN alters clinical outcome. Until these studies are done it is important and reassuring to know that a patient biopsied because of low-grade proteinuria or glomerular hematuria, and found to have IgM nephropathy, has an excellent long-term prognosis.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the assistance of Dr's Eileen Campbell, Orla Browne and Sean Leavey.
REFERENCES
- Cohen A H, Border W A, Glassock R J. Nephrotic syndrome with Glomerular mesangial IgM deposits. Lab Invest 1978; 38: 610–619
- O'Donoghue D J, Lawler W, Hunt L P, Acheson E J, Mallick N P. IgM-associated primary diffuse mesangial proliferative glomerulonephritis: Natural history and prognostic indicators. Q J Med 1991; 79: 333–350
- Cavallo T, Johnson M P. Immunopathologic study of minimal change glomerular disease with mesangial IgM deposits. Nephron 1984; 27: 281–284
- del Moral R G, Gomez-Morales M, Cortes V, et al. Mononuclear cell subsets in IgM mesangial proliferative glomerulonephritis. Nephron 1993; 65: 215–221
- Hishiki T, Tomino Y, Inokuchi S, et al. Two adult cases of IgM-associated mesangial proliferative glomerulonephritis. Nippon Jinzo Gakkai Shi 1994; 36: 942–946
- Bhasin H K, Abuelo G, Nayak R F, Esparza A. Mesangial proliferative glomerulonephritis. Lab Invest 1978; 39: 21–29
- Hall C. Does IgM nephropathy exist?. Presented at Society of DGH Nephrologists. 21 Nov 1997
- Tejani A, Nicastri A D. Mesangial IgM nephropathy. Nephron 1983; 35: 1–5
- Saha H, Mustonen J, Pasternack A, Helin H. Clinical follow-up of 54 patients with IgM nephropathy. Am J Nephrol 1989; 9: 124–128
- Ji-Yun Y, Melvin T, Sibley R, Michael A F. No evidence for a specific role of IgM in mesangial proliferation of idiopathic nephrotic syndrome. Kidney Int 1984; 25: 100–106
- International Study of Kidney disease in children: Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. Kidney Int 1981; 20: 765–71
- Al-Eisa A, Carter J E, Lirenman D S, Magil A B. Childhood IgM Nephropathy: Comparison with Minimal change disease. Nephron 1996; 72: 37–43
- Southwest Pediatric Study Group: Childhood nephrotic syndrome associated with diffuse mesangial hypercellularity. Kidney Int 1983; 23: 87–94
- Lin J-T, Lin C-Y, Huang F-U, Lee P-P, Chen C-H, Hsu H-C. A clinicopathological study of idiopathic nephrotic syndrome in children. Nippon Jinzo Gakkai Shi 1995; 37: 442–450
- Hsu H C, Chen W Y, Lin G J, Chen L, Kao S-L, Huang C-C. Clinical and immunopathological study of mesangial IgM nephropathy: report of 41 cases. Histopathology 1984; 8: 435–446
- Vilches A R, Turner D R, Cameron J S, Ogg C S, Chantler C, Williams D G. Significance of mesangial IgM deposition in “minimal change nephrotic syndrome”. Lab Invest 1982; 46: 10–15
- Pardo V, Riesgo I, Zilleruelo G, Strauss J. The clinical significance of mesangial IgM deposits and mesangial hypercellularity in minimal change nephrotic syndrome. Am J Kidney Dis 1984; 3: 264–269
- Gonzalo A, Mampaso F, Gallego N, Querada C, Fierro C, Ortuno J. Clinical significance of IgM mesangial deposits in the nephrotic syndrome. Nephron 1985; 41: 246–249
- Looi L M, Wang F, Lam K L, Chua C T. Mesangial IgM in minimal change glomerular disease: A clinicopathological study in a Malaysian population. Pathology 1985; 17: 41–44
- Hirszel P, Yamase H T, Carney W R, et al. Mesangial proliferative glomerulonephritis with IgM deposits: clinicopathologic analysis and evidence for morphologic transition. Nephron 1984; 38: 100–108
- Michael A F, Nevins T E, Raij L, Keane W F, Scheinman J I. Macromolecular transport in the glomerulus: studies of the mesangium and epithelium in vivo and in vitro. Immunologic mechanisms of renal disease. Churchill, Livingstone, New york 1979; 167–213
- Kobayashi Y, Shigematsu H, Tgateno S, Hiki Y. Nephrotic syndrome with diffuse mesangial IgM deposits. Acta Pathol Jpn 1982; 32: 307–317
- Sharma M, Sharma R, McCarthy E T, Savin V J. “The FSGS factor”: enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol 1999; 10: 552–561
- Feld S M, Figueroa P, Savin V, et al. Plasmapheresis in the treatment of steroid-resistant focal segmental glomerulosclerosis in native kidneys. Am J Kidney Dis 1998; 32: 230–237