Abstract
Reactive oxygen species are generated during ischemia-reperfusion tissue injury. Oxidation of thymidine by hydroxyl radicals (HO˙) causes formation of 5,6-dihydroxy-5,6-dihydrothymidine (thymidine glycol). Thymidine glycol excreted in urine can be used as a biomarker of oxidative DNA damage. The aim of this study was to investigate the oxidative DNA damage in patients showing immediate allograft function after kidney transplantation, and to find out whether this damage correlates with glomerular and tubular lesions.
Time dependent changes in urinary excretion rates of thymidine glycol, but also of total protein, albumin, low molecular weight (α1-microglobulin, β2-microglobulin) and high molecular weight proteins (transferrin, IgG, α2-macroglobulin) were analyzed quantitatively and by polyacrylamide-gel electrophoresis in six patients. Urinary thymidine glycol was determined by a fluorimetric assay in combination with affinity chromatography and HPLC.
After kidney transplantation the urinary excretion rate of thymidine glycol increased gradually reaching a maximum within the first 48 hours (16.56 ± 11.3 nmol/mmol creatinine, ref. 4.3 ± 0.97). Severe proteinuria with an excretion rate of up to 7.2 g/mmol creatinine was observed and declined within the first 24 hours of allograft function (0.35 ± 0.26 g/mmol creatinine). The gel-electrophoretic pattern showed a nonselective glomerular and tubular proteinuria. The initial nonselective glomerular proteinuria disappeared within 48 hours, changing to a mild selective glomerular proteinuria. In this period (12–48 hours) higher levels of thymidine glycol excretion were observed, when compared to the initial posttransplant phase (13.66 ± 9.76 vs. 4.31 ± 3.61 nmol/mmol creatinine; p < 0.05).
An increased excretion of thymidine glycol is seen after kidney transplantation and is explained by the ischemia-reperfusion induced oxidative DNA damage in the kidney. In the second phase higher levels of excretion were observed parallel to the change from a nonselective to a selective glomerular and tubular proteinuria. An explanation may be sought in the repair process of DNA in the glomerular and tubular epithelial cells, appearing simultaneously with functional recovery.
INTRODUCTION
Global ischemia is a frequent and clinically important cause of renal injury. Especially after kidney transplantation ischemia-reperfusion induced injury of the kidney leads to acute renal failure in 30–40% of the recipients. The duration of ischemia is the most important prognostic factor for the development of acute renal failure. In clinical studies acute renal failure has proven to influence postoperative morbidity and renal allograft survival.Citation[[1]] For improvement of results after kidney transplantation it is most important to reduce the intensity of the ischemia-reperfusion injury. Current evidence suggests that fundamental injury during ischemia-reperfusion occurs in the tubular cells. However, even if immediate graft function takes place, morphological and functional changes are apparent in tubular and glomerular structures of the nephron.Citation[2-3]. Studies in models of acute renal failure have yielded convincing evidence that reactive oxygen species (i.e. free oxygen radicals such as O2˙-, H2O2 and HO˙) are involved in the pathogenesis of the kidney tissue injury during ischemia-reperfusion.Citation[4-6].
During reperfusion, oxygen availability in combination to the increased substrate concentration results in an increased generation of reactive oxygen species. Reactive oxygen species can attack various macromolecules, inducing the production of oxidized derivatives with functional or structural defects. Structural proteins, cell membranes, enzymes, lipids and DNA all classify as oxidizable substrates. Radical-dependent reactions on DNA chains can lead to characteristic modifications in all four DNA bases and to strand breaks. Especially, oxidation of thymidine by hydroxyl radicals (HO˙) in vivo causes the formation of 5,6-dihydroxy-5,6-dihydrothymidine (thymidine glycol). Thymidine glycol is excreted unaltered in the urine. After measurement of its excretion rate thymidine glycol can be used as a biomarker for oxidative DNA damage and/or as an indirect indicator of the severity of oxidative tissue injury Citation[7-10].
The aim of this study was to investigate whether an oxidative DNA damage occurs during ischemia-reperfusion in patients showing immediate allograft function, and whether the intensity of this damage correlates with glomerular or tubular dysfunction after kidney transplantation.
SUBJECTS AND METHODS
The protocol of the study was in accordance with the Declaration of Helsinki, and full, informed consent was obtained. Time dependent changes in proteinuria were quantified in 6 patients (47 ± 9 years, cold ischemia 13.7 ± 8.5 h; time for surgical anastomosis 35 ± 1 min) with immediate graft function after kidney transplantation. Clinical data of the patients are shown in . All kidneys were subjected to cold ischemia of more than 12 hours (range 12–22.5 h). Kidneys were preserved with the cold-storage solutions Eurocollins (1 kidney) or UW (University of Wisconsin; 5 kidneys). All patients were treated with a triple immunosupressive therapy consisting of prednisone (100 mg/d), azathioprine (100 mg/d) and cyclosporine. The plasma cyclosporine concentration was measured every day and overdose was excluded in all patients.
Table 1. Profiles of patients with kidney transplantation. Two cold storage solutions were used University of Wisconsin solution (UW) and Eurocollins solution (EC)
The urinary thymidine glycol and protein excretion rates were determined every hour after vascular anastomosis for 7 hours and every 24 hours thereafter for 10 days. Urinary samples stabilized by sodium azide were collected from a ureteral splint. Total protein, albumin, thymidine glycol (mmol/mmol creatinine), low molecular weight (α1-microglobulin, β2-microglobulin) and high molecular weight protein (transferrin, IgG, α2-macroglobulin) excretion rates were determined and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of urine samples was performed. Urinary excretion rates were expressed as creatinine ratio (g/mmol) in the urine samples Citation[[11]].
Urinary creatinine was determined by an automated Jaffe method (Boehringer, Mannheim, Germany) and total protein was measured by a biuret method (Boehringer, Mannheim, Germany). The molecular weight of urinary proteins was characterized by sensitive silver staining of gels after semi-automated microscale SDS-PAGE Citation[1-2] (Phast-system, Pharmacia, Uppsala, Sweden). Albumin, transferrin, IgG and α2-macroglobulin were determined using a nephelometric assay (Beckman, Munich, Germany). α1-microglobulin and α2-microglobulin were measured by a quantitative enzyme immunoassay (Elias, Freiburg, Germany). Thymidine glycol was isolated by affinity chromatography and high performance liquid chromatography (HPLC) and detected by a chemical assay in combination with fluorimetry Citation[13-14].
Data are given as a mean value ± SD. The probability of error for comparison of the measured values was calculated using the Wilcoxon signed rank test for paired data and the Mann-Whitney U test for unpaired data. The null hypothesis was rejected when p < 0.05. The curve fitting was performed using a polynomial function (median ± 95% confidence interval). The interdependence of the different variables was checked by means of a Pearson correlation analysis.
RESULTS
Urinary Thymidine Glycol
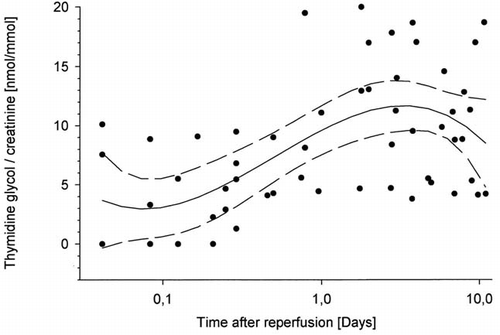
Immediately after kidney transplantation (up to 12 hours) the level of thymidine glycol excretion was within normal range (4.31 ± 3.61 nmol/mmol creatinine), when compared to the excretion rate in healthy subjects (4.30 ± 0.97 nmol/mmol creatinine). Thereafter it rose gradually and reached a maximum 48 hours after reperfusion (16.56 ± 11.3 nmol/mmol creatinine; ). In this second post transplant period (12–48 hours) urinary thymidine glycol excretion rate was significantly increased, when compared to the excretion immediatelly after reperfusion (13.66 ± 9.76 vs. 4.31 ± 3.61 nmol/mmol creatinine; p < 0.05) (). In a third phase (after 48 hours) persisting high levels of thymidine glycol excretion were observed (10.3 ± 5.1 nmol/mmol creatinine), which correlated with the duration of cold ischemia and anastomosis (r = 0.3, p < 0.001).
Table 2. Urinary excretion ratios of proteins (g/mmol creatinine) and of thymidine glycol (nmol/mmol creatinine) in 6 patients up to ten days after kidney transplantation
Figure 1. Time course of urinary thymidine glycol (TG)/creatinine excretion ratio (mmol/mmol) in 6 patients up to 10 days after renal transplantation (logarithmic scales for x-axis and linear for y-axis; curve fitting was performed using a polynomial function (median ± 95% confidence interval)).
Thymidine glycol excretion rate after kidney transplantation correlated with the excretion rates of total protein (r = 0.35, p < 0.01), albumin (r = 0.34, p < 0.01), transferrin (r = 0.31, p < 0.05) and IgG (r = 0.30, p < 0.05).
Analysis of Urinary Proteins
One hour after kidney reperfusion a marked proteinuria was apparent (7.20 ± 0.63 g/mmol creatinine) and declined within the first 24–48 hours of graft function (0.35 ± 0.26 g/mmol creatinine; , ). Albumin excretion (5.83 ± 0.40 g/mmol creatinine), accounting for up to 70% of the total proteinuria, declined parallel to the total protein excretion ratio (r = 0.9). Excretion of high molecular weight proteins (transferrin 0.059 ± 0.13 g/mmol creatinine; IgG 0.151 ± 0.32 g/mmol creatinine; α2-macroglobulin 0.0024 ± 0.0053 g/mmol creatinine) was observed in all patients during the initial phase (up to 24 h) and subsequently disappeared in most of the patients (). Urinary excretion rates of albumin and high molecular proteins, such as IgG and transferrin, correlated positively during the observation period (r = 0.9). The urinary excretion ratio of low molecular weight proteins increased in all patients immediately after kidney transplantation (α1-microglobulin 0.082 ± 0.049 g/mmol creatinine, normal range < 0.012 g/mmol creatinine; β2-microglobulin 0.015 ± 0.0009 g/mmol creatinine, normal range < 0.003 g/mmol creatinine). Although the urinary excretion rate of these proteins decreased after 24 hours, normal values were not reached during the observation period (). Courses of α1-microglobulin and β2-microglobulin excretion rates were similar. The gel-electrophoretic pattern changed from a nonselective to a selective glomerular and tubular proteinuria within 48–72 hours. The tubular proteinuria persisted for 10 days, while the initial marked nonselective glomerular proteinuria disappeared after 48–72 hours.
Figure 2. Time course of urinary protein/creatinine excretion ratio (g/mmol) in 6 patients until 10 days after renal transplantation (logarithmic scales for x-axis and y-axis; curve fitting was performed using a polynomial function [median ± 95% confidence interval]).
![Figure 2. Time course of urinary protein/creatinine excretion ratio (g/mmol) in 6 patients until 10 days after renal transplantation (logarithmic scales for x-axis and y-axis; curve fitting was performed using a polynomial function [median ± 95% confidence interval]).](/cms/asset/105636d2-7a33-4599-b2bc-790a45420d4f/irnf_a_11378862_uf0002_b.gif)
No positive correlation was observed between duration of cold ischemia and the urinary excretion rates of total protein, albumin, transferrin, IgG, α2-macroglobulin, α1-microglobulin or β2-microglobulin.
DISCUSSION
Thymidine glycol is a product of oxidative DNA damage. Oxidation of thymidine by hydroxyl radical (HO˙) causes formation of thymidine glycol. This reaction is free radical specific and can be induced only by HO˙ in vivo. During the enzymatic repair process and degradation of the oxidative DNA damage thymidine glycol is released from the DNA chains. Without further catabolism thymidine glycol is excreted in urine and its excretion rate can be used as a biomarker of oxidative DNA damage Citation[[8]], Citation[[10]], Citation[[15]].
Ischemia-reperfusion is a cause of tissue injury in a wide variety of pathological conditions. In global ischemia metabolism is inadequate to sustain tissue viability. While reperfusion is necessary to reverse the progressing ischemic damage, it is also accompanied by its own injury component. Although the details of exact extra- and intracellular mechanisms are not yet completely understood, recent evidence implicates that reactive oxygen species, which are produced during ischemia-reperfusion, are mediators for at least part of this injury. Kidney transplantation, a predefined ischemia-reperfusion injury condition, includes a brief period of total ischemia during harvesting, followed by a prolonged period of cold ischemia and another brief period of ischemia-reperfusion during surgical vascular anastomosis. Injury may occur at each of these phases by different mechanisms.
Possible cellular sources of reactive oxygen species after kidney transplantation include the oxidant enzymes (xanthine oxidase, cyclooxygenase, lipooxygenase), the mitochondrial or microsomal electron transport chain and the phagocytes. During ischemia due to hypoxia and substrate lack, ATP is consumed and the intracellular calcium ([Ca2+]i) concentration increases. ATP catabolism supplies precursors (adenosine, inosin, hypoxanthine) and enhanced [Ca2+]i leads to an activation of xanthine oxidase during ischemia. Activation of the cytosolic phospholipase A2 also induced by the increased [Ca2+]i supplies the precursor (arachidonic acid) for the cyclooxygenase and the lipooxygenase pathways. The inhibition or uncoupling of the respiration chain occurring due to calcium overload and/or the ADP decrease during ischemia, leads after reperfusion (reoxygenation) to a production of superoxide anion at maximal rates in the mitochondria. Ischemic tissue is prone to the attack of granulocytes and macrophages, which also cause local generation of reactive oxygen species. Citation[[6]]
The aim of this study was to find out whether ischemia-reperfusion induced injury of the transplanted kidney is accompanied by an oxidative DNA damage. Furthermore, this study investigates whether the degree of oxidative DNA damage correlates with the structural and functional lesions of the glomerular and tubular epithelial cells, observed after reperfusion of the transplanted kidney. For this purpose thymidine glycol excretion was correlated with the course of proteinuria after kidney transplantation.
Only patients with immediate graft function could be included in this study since only in these patients is the analysis of both urinary proteins and thymidine glycol possible. These parameters respectively reflect the functional impairment of the glomerula or tubuli and the DNA damage during ischemia-reperfusion. In patients with acute renal failure after kidney transplantation (anuria), investigation of the kidney tissue injury would have required serial biopsies.
Despite rapid normalization of renal function, a functional impairment of the glomerular as well as of the tubular cells was obvious. Dysfunction of glomerular and tubular epithelia is a major cause of proteinuria. The functional impairment of the nephron was investigated by biochemical and electrophoretic characterization of excreted proteins. Citation[11-12], Citation[[16]] Whereas tubular lesions could be detected as an increased excretion of low molecular weight proteins, such as α1-microglobulin and β2-microglobulin, lesions of the glomerula were characterized by hyperalbuminuria and excretion of high molecular weight proteins, such as transferrin, IgG, and α2-macroglobulin.
Within 1 hour after kidney reperfusion a marked proteinuria was apparent and declined within the first 24 hours of graft function. This was in accordance with in vitro and in vivo kidney perfusion studies after cold storage and kidney transplantation Citation[[2]], Citation[[17]]. A highly increased excretion of albumin and high molecular weight proteins was observed in all patients during the initial phase (up to 24 h) and declined rapidly thereafter in most of the patients. The urinary excretion ratio of low molecular weight proteins was increased (up to 10-fold) in all patients. Although urinary excretion ratios of these proteins decreased after 24 hours, normal values were not reached during the observation period (10 days after transplantation). The gel-electrophoretic pattern revealed the change from a severe nonselective to a mild selective glomerular and tubular proteinuria within 48–72 hours (data not shown).
In addition to the biochemical analysis of excreted proteins, the determination of urinary thymidine glycol allowed direct estimation of the oxidative DNA damage, as the thymidine glycol production was correlated to the urinary excretion rate Citation[[8]], Citation[18-19]. Whereas this has previously been shown under steady state conditions, in this study the frequent determination of the thymidine glycol excretion rate allows the course of thymidine glycol production after kidney transplantation to be followed.
Analysis of thymidine glycol excretion revealed a strong increase after kidney transplantation, when compared to the excretion in healthy subjects. The level of excretion rose gradually and maximal rates were reached only 48 hours after reperfusion. Furthermore, thymidine glycol excretion correlated with the excretion rate of the high molecular proteins (total protein, albumin, transferrin and IgG). Thymidine glycol is derived in vivo only after repair or catabolism of oxidatively damaged DNA. Alternative sources such as the diet or bacterial flora can be excluded.Citation[[14]] Taking together these data clearly show that during ischemia-reperfusion production of reactive oxygen species occurs leading to an oxidative DNA damage with an increased thymidine glycol excretion after kidney transplantation.
Thymidine glycol is excreted through the kidney and less than 5% is found in the faeces. Therefore an accumulation of thymidine glycol in chronic kidney insufficiency is theoretically probable. An enhanced plasma concentration would lead to an increased excretion of the substance immediately after reperfusion. In our study the excretion of thymidine glycol rose gradually and it was within normal range in the initial period (up to 7 hours) after reperfusion. This time course shows that the increased excretion rates of thymidine glycol observed in our study are not caused by increased plasma concentrations prior to kidney transplantation.
The gradual increase of thymidine glycol excretion observed may be readily explained, taking into account that source of urinary thymidine glycol is the enzymatic repair process and/or the catabolism of the oxidative DNA damage, induced by reactive oxygen species during reperfusion. According to investigations on the influence of 12-O- tetradecanoyl-phorbol-13-acetate (PTA; a potent tumor promoter, which causes DNA damage in vivo) on SENCAR mice skin maximal formation of thymidine glycol occurs 6–8 hours after the action of PTA and its concentration on DNA chains remains high up to 24 hours decreasing thereafter, probably after activation of the DNA enzymatic repair system.Citation[[9]] The time course of thymidine glycol excretion observed in our study is in accordance with these findings.
The oxidative DNA damage with an increasing thymidine glycol excretion after kidney transplantation is paralleled to the ischemia-reperfusion induced glomerular dysfunction and the accompanying markedly increased proteinuria. These results implicate that reactive oxygen species may be involved in damage to renal capillaries, specifically to heparan sulfate proteoglycan and to the glomerular epithelia, which leads to proteinuria as a result of ischemia-reperfusion. Our data are in accordance with previous findings indicating an important role of reactive oxygen species in the pathophysiology of experimental glomerular disease. Citation[[20]]
Reactive oxygen species can mediate an injury to the ultrafiltration apparatus and induce proteinuria by following mechanisms: 1. Alteration of intraglomerular hemodynamics by excessive production of eicosanoids, in particular thromboxane A2 (TxA2), that has been associated with proteinuria.Citation[[21]] 2. Enhancement of susceptibility of the glomerular basement membrane to proteolytic degradation, which during reperfusion would be expected to cause damage to various components of the glomerular basement membrane, including type IV collagen, laminin, proteoglycans, and the sialoglycoproteins.Citation[[22]] 3. Influence on the biosynthetic profiles of proteoglycans (the prime regulators of charge-selectivity of the glomerular basement membrane). Even a mild exposure of the kidney to reactive oxygen species for a brief time is accompanied by drastic reduction in the de novo synthesis of proteoglycans.Citation[[23]] The enhanced proteolytic degradation of proteoglycans and the inhibition of their de novo synthesis induced by reactive oxygen species may be another explanation for the observed strong proteinuria in the initial phase after ishemia-reperfusion in kidney transplantation. This hypothesis is in accordance with previous studies, which show a protective effect of radical scavengers on the isolated perfused kidney - a partial ischemic system that is characterized by glomerular proteinuria and release of glomerular heparan sulfate Citation[[24]].
In the repair phase, which could be assigned to the second phase (12–48 h), the repair process or degradation of the oxidatively damaged DNA with an increasing urinary excretion of thymidine glycol was accompanied by a functional recovery of glomerular and tubular cells (). The increasing excretion of thymidine glycol is not only paralled by a decrease in proteinuria but also by the shift from a nonselective to a mild selective proteinuria which can be concluded from the gel electrophoretic pattern and from the decline of α2-macroglobulin and IgG excretion ratios (). An explanation may be sought in the ongoing repair process of DNA in the glomerular and tubular epithelial cells, appearing simultaneously with functional recovery. In the third phase (after 48 h) glomerular proteinuria was abolished in most of the patients. Tubular damage, however, seems to persist for a longer period since elevated excretion of α1- and β2-microglobulin still persists. An additional potential tubulotoxic effect of cyclosporine A cannot be excluded by the present data. According to previous studies on rats and isolated rat glomerula ciclosporine A was shown to induce lipidperoxidation and synthesis of H2O2 or O2˙- Citation[25-26]. Whether the elevated production of thymidine glycol in this late repair phase is associated with cyclosporine A remains to be clarified.
Antioxidants play a substantial role in attenuating injury caused by reactive oxygen species and are currently being tested in various clinical situations in humans, especially in transplantation medicine. In a prospective study Land et al. (1994) showed that supplementation of recombinant human SOD exerts a beneficial effect on acute and chronic outcome of kidney transplantation respectively by a reduction of the acute rejection episodes and an improvement of the actual 4-year graft survival.Citation[[27]] However the actual scavenger effect of antioxidants was not measured in these studies. Determination of the thymidine glycol excretion rate may prove a valuable noninvasive method for further clinical studies on the influence of antioxidants on ischemia-reperfusion induced kidney tissue injury and on prognosis of kidney transplantation. Whether analysis of the excretion rate of thymidine glycol will allow a prediction of the long term kidney function after transplantation has to be analyzed in future studies.
REFERENCES
- Merkus J W, Hoitsma A J, Koene R A. Detrimental effect of acute renal failure on the survival of renal allografts: influence of total ischaemia time and anastomosis time. Nephrol Dial Transplant 1991; 6: 881–886
- Kehrer G, Bretschneider H J. Postischemic diagnostic localization of tubular lesions. Klin Wochenschr 1990; 68: 223–236
- Bretschneider H J. Nierenprotektion. Klin Wochenschr 1988; 66: 817–827
- Baud L, Ardaillou R. Involvement of reactive oxygen species in kidney damage. Br Med Bull 1993; 49: 621–629
- Paller M S. Free radical-mediated postischemic injury in renal transplantation. Ren Fail 1992; 14: 257–260
- Greene E L, Paller M S. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab 1991; 17: 124–132
- Baleja J D, Buchko G W, Weinfeld M, Sykes B D. Characterization of gamma-radiation induced decomposition products of thymidine-containing dinucleoside monophosphates by nuclear magnetic resonance spectroscopy. J Biomol Struct Dyn 1993; 10: 747–762
- Simic M G. Urinary biomarkers and the rate of DNA damage in carcinogenesis and anticarcinogenesis. Mutat Res 1992; 267: 277–290
- Wei H, Frenkel K. In vivo formation of oxidized DNA bases in tumor promoter- treated mouse skin. Cancer Res 1991; 51: 4443–4449
- Ames B N. Endogenous DNA damage as related to cancer and aging. Mutat Res 1989; 214: 41–46
- Dyson E H, Will E J, Davison A M, O'Malley A H, Shepherd H T, Jones R G. Use of the urinary protein creatinine index to assess proteinuria in renal transplant patients. Nephrol Dial Transplant 1992; 7: 450–452
- Kierdorf H, Melzer H, Mann H, Sieberth H G. Modifikation of the silver staining of proteins in polyacrylamide gels for improved differentiation of urine proteins with phast-system and laser densitometry. Electrophoresis 1993; 14: 820–822
- Kocher K. Entwicklung eines analytischen Verbundverfahrens zur Bestimmung von Thymin- und Thymidinglykol im Urin als Biomarker für oxidative DNA-Schäden, Göttingen: Curvillier Verlag, 1995.
- Cathcart R, Schwiers E, Saul R L, Ames B N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci USA 1984; 81: 5633–5637
- Bergtold D S, Simic M G. Background levels of DNA damage. Free Radic Res Commun 1989; 6: 195–197
- Kirschstein M, Jensen R, Schroder R, Sack K. Proteinuria after renal transplantation: diagnosis with highly sensitive silver stain in sodium dodecylsulphate-polyacrylamide gradient gel electrophoresis. Klin Wochenschr 1991; 69: 847–852
- Ploeg R J, Vreugdenhil P, Goossens D, McAnulty J F, Southard J H, Belzer F O. Effect of pharmacologic agents on the function of the hypothermically preserved dog kidney during normothermic reperfusion. Surgery 1988; 103: 676–683
- Adelman R, Saul R L, Ames B N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci USA 1988; 85: 2706–2708
- Cao E H, Wang J J. Oxidative damage to DNA: levels of thymine glycol and thymidine glycol in neoplastic human urines. Carcinogenesis 1993; 14: 1359–1362
- Shah S V. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int 1989; 35: 1093–1106
- Lianos E A, Andres G A, Dunn M J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. J Clin Invest 1983; 72: 1439–1448
- Wolff S P, Dean R T. Fragmentation of protein by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J 1986; 234: 399–403
- Kashihara N, Dalecki T, Liu Z. Effect of reactive oxygen species on glomerular extracellular matrix proteoglycans. J Am Soc Nephrol 1990; 1: 527–527
- Tay M, Comper W D, Vassiliou P, Glasgow E F, Baker M S, Pratt L. The inhibitory action of oxygen radical scavengers on proteinuria and glomerular heparan sulphate loss in the isolated perfused kidney. Biochem Int 1990; 20: 767–778
- de Arriba G, Parra T, de Lema G Perez, Arribas I, Rodriguez-Puyol D, Rodriguez-Puyol M. Role of free radicals in cyclosporine A nephrotoxicity. Effects of vitamin E. J Am Soc Nephrol 1996; 7: 1839–1839
- Wang C, Salahudeen A K. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int 1995; 47: 927–934
- Land W, Schneeberger H, Schleibner S T, Illner W D, Abendroth D, Rutili G, Arfors K E, Messmer K. Beneficial effect of human recombinant superoxide dismutase on both acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 1994; 57: 211–217
