Abstract
Cyclosporine A (CsA) is one of the first line immunosuppressants employed in the management of solid organ transplantation and autoimmune diseases. The clinical utility of CsA is limited by the frequent occurrence of chronic nephrotoxicity, characterized by tubular atrophy, interstitial fibrosis and progressive renal impairment. The pathogenesis of CsA nephrotoxicity is still not well delineated. Recent evidences suggest that reactive oxygen species (ROS) play an important role in CsA nephrotoxicity. The present study was designed to demonstrate the role of oxidative stress, its relation to renal dysfunction and to investigate the effect of trimetazidine (TMZ), an anti-ischemic agent with free radical scavenging property, in CsA-induced nephrotoxicity. TMZ (2.5 mg/kg, p.o., twice a day) was administered 24 h before and 21 days concurrently with CsA (20 mg/kg, s.c.). Tissue lipid peroxidation was measured as thiobarbituric acid reacting substances (TBARS). Renal function was assessed by measuring the plasma and urine creatinine concentrations, blood and urine urea nitrogen levels and the creatinine and urea clearances. Renal morphological alterations were assessed by histopathological examination of Hematoxylin-Eosin, PAS and Masson's trichome stained sections of the kidneys. CsA (20 mg/kg, s.c) administration for 21 days produced elevated levels of TBARS and decreased renal function as assessed by increased plasma creatinine, BUN and decreased creatinine and urea clearances as compared to vehicle treated rats. The kidneys of CsA treated rats showed severe striped interstitial fibrosis, arteriolopathy, glomerular basement thickening, tubular vacuolization and hyaline casts. TMZ (2.5 mg/kg) markedly reduced elevated levels of TBARS, significantly attenuated renal dysfunction and the morphological changes in CsA treated rats. These results clearly demonstrate the pivotal role of reactive oxygen species and their relation to renal dysfunction and point to the therapeutic potential of an anti-ischemic agent, trimetazidine, in CsA-induced nephrotoxicity.
INTRODUCTION
Cyclosporine A (CsA) is a cyclic undecapeptide obtained from extracts of the soil fungus, Tolypocladium inflatum gams. It is a T-cell specific immunosuppressant that has been used following solid organ transplantation and in autoimmune diseases. However, nephrotoxicity remains the major limiting adverse effect of CsA use.Citation[[1]], Citation[[2]] CsA nephrotoxicity has been characterized by a 20–30% reduction in glomerular filtration rate (GFR) and up to a 40% reduction in renal blood flow (RBF)—resulting in an increase in the serum creatinine concentration (SCr), a decrease in creatinine clearance (CCr), and a reduction in both sodium and potassium excretion.Citation[[2]]
Different forms of CsA nephrotoxicity have been described. The acute form is dose-dependent and usually reversible and is characterized by a reduced GFR and RBF that appears to be related to afferent arteriolar vasoconstriction, possibly mediated by imbalance between renal vasoconstrictors (catecholamines, angiotensin II, endothelin, thromboxane, platelet-activating factor) and vasodilators (prostaglandins, nitric oxide).Citation[[3]] The chronic form of nephrotoxicity is characterized by structural changes in the kidney and appear as tubulointerstitial fibrosis of a striped pattern, arteriolopathy of the afferent arterioles, tubular atrophy and glomerulosclerosis.Citation[[4]]
The pathophysiology of CsA nephrotoxicity still remains unclear. Clinical and experimental studies have revealed that several factors may be involved. Recently oxidative stress has been proposed as a causative factor in CsA nephrotoxicity. It has been reported that free radicals in urine increase dramatically after CsA treatment.Citation[[5]] It has ben suggested that reactive oxygen species (ROS) could also be derived directly from CsA or during its metabolism by the cytochrome P450 (CYP450) system.Citation[[6]] In addition, CsA increases levels of superoxide () and hydrogen peroxide (H2O2) in vitro and in vivo.Citation[[7]], Citation[[8]], Citation[[9]] It is reported that CsA increased renal nerve activity resulting in vasoconstriction leading to renal ischemia.Citation[[10]] It has been demonstrated that CsA blocks mitochondrial calcium (Ca2+) release, inducing an increase in intracellular free calcium and that this that could account for the vasoconstrictive effect of CsA.Citation[[11]] Furthermore, CsA specifically inhibits mitochondrial energy production and decreases ATP levels, events characteristic of ischemia.Citation[[12]], Citation[[13]]
Trimetazidine (TMZ) (1-(2,3,4-trimethoxybenzyl) piperazine) is a novel anti-ischemic agent that exerts cytoprotection by counteracting the metabolic disorder at the level of ischemic cell, independently of any hemodynamic effect.Citation[[14]] TMZ limits intracellular acidosis, minimizes sodium and calcium accumulation, maintains intracellular ATP levels, and reduces creatinine phosphokinase release.Citation[[14]], Citation[[15]] This drug has been shown to protect cellular integrity by scavenging the ROS, inhibiting free radicals formation, and thus preventing the subsequent lipid peroxidation.Citation[[16]], Citation[[17]]
The present study was designed to demonstrate the role of CsA-induced oxidative stress, its relation to renal dysfunction and to investigate whether the cellular anti-ischemic agent, trimetazidine provides protection against cyclosporine A-induced nephrotoxicity in rats.
METHODS
Animals
Wistar rats (150–200 g) of either sex bred in central animal house of Punjab University, Chandigarh, India were used. The animals were housed under standard conditions of light and dark cycle, with free access to food (Hindustan Lever Products, Kolkata, India) and water. All the experimental procedures were carried out in accordance with the guidelines of the Animal Ethical committee, Punjab University, Chandigarh, India.
Drugs
CsA in powder form (Gift from Panacea Biotec Ltd., Lalru, India) was dissolved in olive oil so as to get a final concentration of 20 mg/mL. Trimetazidine dihydrochloride (Gift from Microlabs, Chennai, India) was dissolved in distilled water to give a final concentration of 2.5 mg/5 mL. All the solutions were made freshly at the beginning of each experiment.
Experimental Groups
Animals were distributed into three groups, each comprising 5 or 6 animals: (1) a control group received equivalent volume of vehicle for CsA i.e., olive oil, subcutaneously (s.c.) and distilled water perorally (p.o.) for 21 days; (2) a second group received CsA (20 mg/kg, s.c.) dissolved in olive oil for 21 days; and (3) a third group received a dose of TMZ (2.5 mg/kg, twice a day, p.o.) 24 h before administering CsA, and continued along with CsA for 21 days. The body weight of the animals was measured on day 0, 7, 14 and 21. Systolic blood pressure (SBP) was measured from the tail of the animals using blood pressure recorder (UGO Basile, Italy) on day 0 and 22 just before sacrificing the animal. The animals were placed in individual metabolic cages after the last dose of drug(s) to measure water intake and urine output for 24 h. On day 22, animals were sacrificed after anesthetizing with thiopentone sodium (25 mg/kg, i.p.) and blood was collected by the insertion of cannula into the abdominal aorta. The blood samples were centrifuged and plasma was collected.
Assessment of Renal Function
Renal function was assessed by colorimetric estimation of plasma and urine levels of creatinine and urea. Urea was measured as blood urea nitrogen (BUN). Creatinine and urine clearances as an index of GFR were calculated.
ESTIMATION OF RENAL LIPID PEROXIDES
Malondialdehyde (MDA), an indirect index of lipid peroxidation, was assayed in the form of thiobarbituric acid reacting substances (TBARS).Citation[[18]] The left kidney was isolated immediately after sacrificing the rat and placed in ice-cold saline. It was weighed and properly minced. To 0.2 mL of 10% (w/v) tissue homogenate prepared in 1.15% potassium chloride, 0.2 mL of 8.1% sodium lauryl sulphate, 1.5 mL of 20% acetic acid solution adjusted to pH 3.5 with sodium hydroxide and 1.5 mL of 0.8% of aqueous solution of thiobarbituric acid were added. The mixture was made up to 4.0 mL with distilled water, and heated at 95°C for 60 min. After cooling with tap water, 1.0 mL of distilled water and 5.0 mL of the mixture of n-butanol and pyridine (15 : 1, v/v) was added and centrifuged. The organic layer was taken out and its absorbance was measured at 532 nm. The TBARS were quantified using an extinction factor of 1.56 × 105 M−1/cm−1 and expressed as nmol of TBARS per mg protein. The tissue protein was estimated using Folin-Lowry method of protein assay.
Renal Histological Study
The right kidney was isolated immediately after sacrificing the animal and washed with ice cold saline. Then it was fixed in 10% neutral buffered formalin solution, embedded in paraffin and was used for histopathological examination. 5 µm thick sections were cut, deparaffinized, hydrated and stained with hematoxylin-eosin. Few sections were stained with periodic acid-schiff (PAS) and the remaining with Masson's trichome. A qualified pathologist examined the renal sections in all the treatment groups in a blinded fashion for apical blebbing, interstitial vacuolization, hyaline casts, glomerular changes, arteriolopathy and tubulointerstitial fibrosis. A minimum of 20 fields from each kidney slide were examined and assigned for severity of changes using scores on a scale of: − none, + mild, ++ moderate, +++ severe damage.
Statistical Analysis
The data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test for comparing means from different treatment groups. The data were expressed as mean ± SEM and a value of p < 0.05 was considered statistically significant.
RESULTS
Effect of Trimetazidine on Body Weight, Water Intake, Urine Output, and Systolic Blood Pressure in CsA Treated Rats
Chronic CsA treated rats failed to gain as much weight as those receiving vehicle with the difference achieving statistical significance. In trimetazidine treated group, there was a progressive and significant weight gain. CsA administration per se and in combination with trimetazidine had no significant effect on water intake, urine output and SBP ().
Table 1. Effect of Trimetazidine (TMZ) (2.5 mg/kg, p.o) on Body Weight, Water Intake, Urine Output, and Systolic Blood Pressure in CsA (20 mg/kg, s.c.) Treated Rats
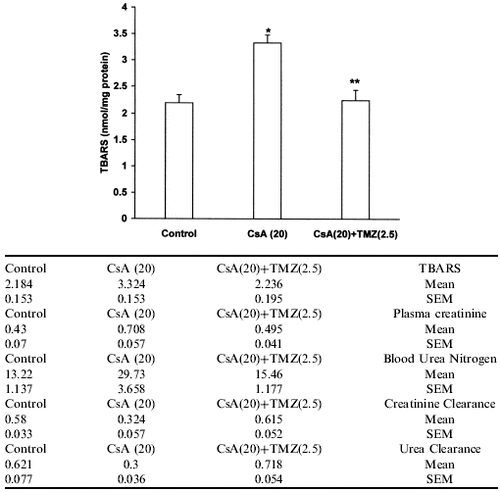
Effect of Trimetazidine on CsA-Induced Lipid Peroxidation
TBARS were elevated in kidneys of CsA treated rats as compared to vehicle treated rats. Concomitant treatment with trimetazidine significantly prevented the increase in TBARS level in CsA treated rats ().
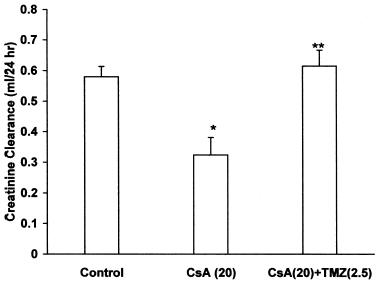
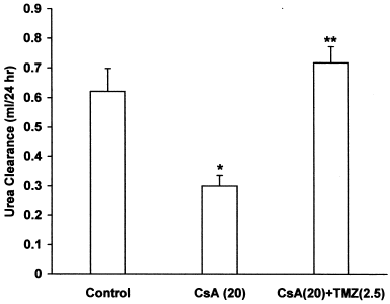
Effect of Trimetazidine on CsA-Induced Renal Dysfunction
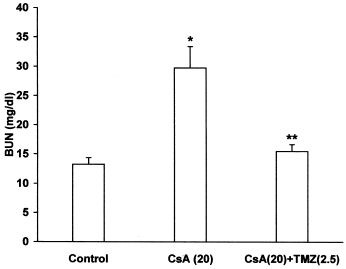
CsA per se caused a significant increase in plasma creatinine and BUN levels, while pretreatment with TMZ significantly prevented the elevated plasma creatinine () and BUN levels () in CsA treated rats. There was a significant decrease in creatinine and urea clearance in CsA treated rats. Concomitant treatment with TMZ markedly improved creatinine () and urea clearance () in CsA treated rats.
Figure 2. Effect of trimetazidine on plasma creatinine in CsA treated rats. Values expressed as mean ± SEM. *p < 0.05 as compared to control group, **p < 0.05 as compared to CsA group. (One-way ANOVA followed by Dunnett's test).
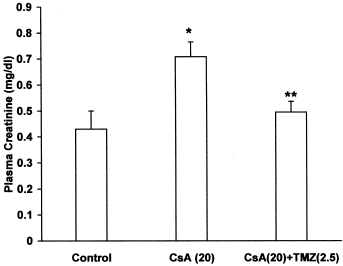
Figure 3. Effect of trimetazidine on blood urea nitrogen (BUN) in CsA treated rats. Values expressed as mean ± SEM. *p < 0.05 as compared to control group, **p < 0.05 as compared to CsA group. (One-way ANOVA followed by Dunnett's test).
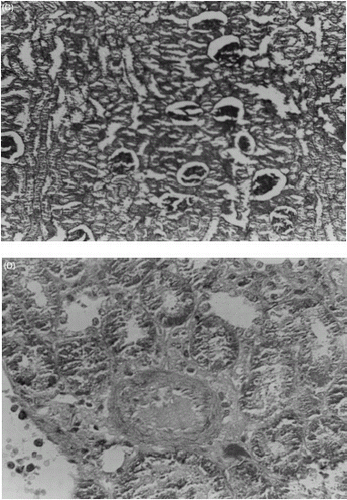
Effect of Trimetazidine on CsA-Induced Renal Morphological Changes
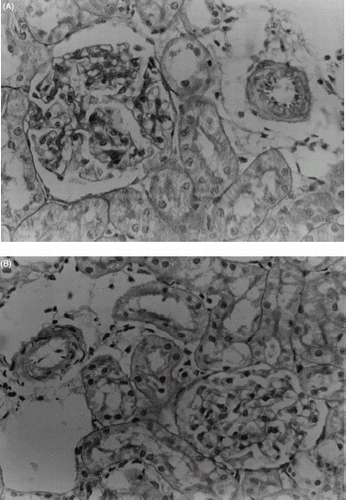
The renal morphological changes observed were scored and summarized in . The light microscopic findings of kidneys of control rats treated with olive oil for 21 days showed normal glomeruli, afferent arterioles, and tubule cells (A). By contrast, the kidneys of rats treated with CsA showed marked histological changes in the cortex and outer medulla. The sections showed severe apical blebbing, hyaline casts, and moderate interstitial vacuolization of the distal tubules and glomerular basement thickening. A marked tubulointerstitial fibrosis of stripped pattern in the cortex (B) and arteriolopathy with hyaline deposition within the tunica media of afferent arteriole and terminal portions of the interlobular arteries were also observed (C). Trimetazidine pretreatment in CsA treated rats showed normal glomeruli, afferent arterioles and tubules cells with mild epicel blebbing and hyaline casts (D).
Table 2. Effect of Trimetazidine (TMZ) (2.5 mg/kg, p.o) Administration on Morphological Changes as Assessed by Histopathological Examination of Kidney in CsA (20 mg/kg, s.c.) Treated Rats
Figure 6. Light microscopic observations of kidney sections of different treatment groups: (A) Renal cortex of a rat treated with vehicle (PAS, X500), (B) Interstitial fibrosis of stripped pattern and tubular atrophy in cortex of CsA treated rat (PAS, X125), (C) Arteriole with marked luminal narrowing and pronounced intimal thickening (Masson's trichome, X500), (D) Renal cortex of a rat treated with CsA (20 mg/kg) and TMZ (2.5 mg/kg) (PAS, X500).
DISCUSSION
The introduction of cyclosporine A to the transplant community has revolutionized the field of organ transplantation, primarily due to the improved cadaveric graft survival rates with a corresponding decrease in the number and severity of rejection episodes. The most serious and limiting adverse effect associated with CsA use is nephrotoxicity.Citation[[1]]
In the present study, CsA treated animals showed a smaller increase in net weight gain than in control animals, despite normal food intake and lack of any signs of illness. The decrease in body weight gain in CsA treated animals has been seen in other studies.Citation[[19]], Citation[[20]] The high dose of CsA might have been a factor, but others have reported weight loss associated with lower doses of CsA in the rat.Citation[[21]] Moreover, weight loss has been reported in antioxidant deficient rats.Citation[[22]] The co-administration of trimetazidine along with CsA limited CsA-induced weight loss in our study, suggesting that oxidative stress might be an important factor involved in weight loss.
The exact mechanisms of CsA-induced nephrotoxicity are not known, but recent studies have shown that CsA-induced renal and cardiovascular toxicity may be due to enhanced oxidative stress.Citation[[23]], Citation[[24]], Citation[[25]] It has been reported that the binding of a 2-nitroimidazole, hypoxia marker pimonidazole in the kidney was increased nearly 3-fold by CsA, indicating marked tissue hypoxia.Citation[[26]] Moreover, free radicals in urine were increased dramatically after CsA treatment.Citation[[5]] It is also known that CsA increases renal nerve activityCitation[[27]] resulting in vasoconstriction in the kidney.Citation[[10]], Citation[[28]] It has been demonstrated that CsA blocks the mitochondrial Ca2+ release, inducing a drastic enhancement in intracellular free calcium that could account for CsA vasoconstrictive effect.Citation[[11]], Citation[[29]] Moreover, CsA affects mitochondrial function and cell energetics that specifically inhibits mitochondrial energy production and decreases ATP levels characteristic of ischemia in kidney in vitro and ex vivo.Citation[[12]], Citation[[13]] In addition, CsA increases ROS formation leading to reduction of oxidative phosphorylation and decreased mitochondrial ATP concentration.Citation[[30]] These alterations could theoretically lead to a classical ischemia-reperfusion injury involving free radicals. In addition, free radicals could be derived directly from CsA or during its metabolism by CYP450 system.Citation[[6]] Consistent with these possibilities, CsA increased lipid peroxidation in isolated liver microsomes.Citation[[31]] It has been demonstrated that CsA increases levels of superoxide () in endothelialCitation[[7]] and mesangial cells.Citation[[8]] A recent study has shown that CsA-induced local production of hydroxyl species, a highly active and detrimental radical play an important role in nephrotoxicity caused by CsA.Citation[[9]]
TBARS as measure of malondialdehyde, a stable lipid hydroperoxide, provides an index of the peroxidation of lipids by ROS in biological tissues.Citation[[18]] In the present study, CsA caused a marked production of TBARS in the kidney indicating a significant ROS generation. Pretreatment with trimetazidine prevented the CsA-induced rise in TBARS levels. Findings from in vitro and in vivo studies have demonstrated that trimetazidine limits sodium and calcium accumulation, and maintains intracellular ATP levels and reduces creatinine phosphokinase release.Citation[[14]], Citation[[15]] It also preserves mitochondrial function and energy metabolism in ischemic cells.Citation[[32]], Citation[[33]] It is recently reported that trimetazidine markedly improves resistance to hypoxic stress and ischemic-reperfusion injury in in vitro and ex vivo organs.Citation[[16]], Citation[[17]], Citation[[34]], Citation[[35]] This drug has also been shown to protect cellular integrity by scavenging the ROS, inhibiting free radicals formation, and subsequent lipid peroxidation in the red cells and ischemic heart.Citation[[16]], Citation[[17]] In consistence with these evidences, trimetazidine by its anti-ischemic and free radical scavenging effects might contribute to the decreased lipid peroxides in the kidneys of CsA treated rats. Our data suggests that TMZ through its scavenging effects on free radicals could inhibit lipid peroxidation in the kidney.
Various studies have demonstrated that the increased lipid peroxidation accompanies loss of renal function in CsA-induced nephrotoxicity. We found that chronic CsA administration increased serum creatinine and urea, and decreased creatinine and urea clearance in rats. These alterations are mainly due to decreased GFR and RBF secondary to renal vasoconstriction and hypoperfusion of renal microcirculation, both at preglomerular and postglomerular sites caused by CsA.Citation[[28]], Citation[[36]] The mechanism by which CsA causes vasoconstriction remains unclear. Oxidative stress can promote the formation of a variety of vasoactive mediators that can affect renal function directly by causing renal vasoconstriction or decreasing the glomerular capillary ultrafiltration coefficient, and thus reduce GFR.Citation[[37]], Citation[[38]] Recent studies have provided evidences that CsA causes the increase in intracellular calcium that enhances the contractility of both mesangial cells and vascular smooth muscle cells leading to intrarenal vasoconstriction.Citation[[11]], Citation[[29]] In addition, this increased intracellular Ca2+ results in the release of vasoconstrictors such as Ang II,Citation[[28]] thromboxanes,Citation[[39]] and endothelin.Citation[[40]] The administration of trimetazidine along with CsA significantly improved creatinine and urea clearance, and decreased the elevated levels of serum creatinine and BUN. The improvement of renal function by TMZ may be attributed to interference with oxidative stress and checking intracellular calcium levels.
It is also known that ROS mediated peroxidation of lipids results in subcellular damage. In our study, the kidneys of CsA treated rats have shown characteristic morphological findings such as interstitial fibrosis and arteriolar hyalinosis. The vasoconstriction induced by CsA produces an ischemic local environment, which leads to a number of cellular changes, such as deterioration in membrane integrity. The marked histological changes were prominent in the outer cortex and medullary region of the kidney. Because of limited oxygen availability, these structures are particularly vulnerable to ischemia. The co-administration of trimetazidine along with CsA had shown mild vascular and glomerular changes, tubular vacuolization and limited marked histological changes in rats.
Thus this study provides convincing evidence for CsA nephrotoxicity in this rat model and indicates a relation between oxidative stress and renal dysfunction induced by CsA. We concluded that trimetazidine, an anti-ischemic agent attenuated oxidative stress mediated renal dysfunction and renal morphological changes by CsA in this rat model of chronic CsA-induced nephrotoxicity.
ACKNOWLEDGMENT
The authors would like to thank Ms. Saraswati Gupta for technical assistance.
REFERENCES
- Kahan B.D. Cyclosporine. New Engl. Med. 1989; 321: 1725–1737
- Weir M.R., Klasen D.K., Shen S.Y. Acute Effects of Cyclosporine on Renal Function in Healthy Humans. Transplant Proc. 1989; 21: 915–917
- Bennett W.M. Mechanisms of Acute and Chronic Nephrotoxicity from Immunosuppressive Drugs. Renal Fail. 1996; 18: 453–460
- Andoh T.F., Bennett W.M. Chronic Cyclosporine Nephrotoxicity. Curr. Opin. Nephrol. Hypertens 1996; 7: 260–270
- Knight J.A., Cheung A.K., Servilla K. Increased Urinary Excretion of Lipid Peroxide Levels in Renal Transplant Patients. Ann. Clin. Lab. Sci. 1996; 19: 238–243
- Ahmed S.S., Napoli K.L., Strobel H.W. Oxygen Radical Formation During Cytochrome P450-Catalyzed Cyclosporine Metabolism in Rat and Human Liver Microsomes at Varying Hydrogen Ion Concentrations. Mol. Cell. Biochem. 1995; 151: 134–140
- Diederich D., Skopec J., Diederich A., Dai F.X. Cyclosporine Produces Endothelial Dysfunction by Increased Production of Superoxide. Hypertension 1994; 23: 957–961
- Perez de Lema G. Cyclosporine A-Induced Hydrogen Peroxide Synthesis by Cultured Human Mesangial Cells is Blocked by Exogenous Antioxidants. Life Sci. 1996; 62: 1745–1753
- Zhong Z., Connor H.D., Yin M., Moss N., Mason R.P., Bunzendahl H., Forman D.T., Thurman R.G. Dietary Glycine and Renal Denervation Prevents Cyclosporine A-Induced Hydroxyl Radical Production in Rat Kidney. Mol. Pharmacol. 1998; 56: 455–463
- Mehring N., Neumann K.H., Rahn K.H., Zidek W. Mechanisms of Cyclosporine A-Induced Vasoconstriction in the Isolated Perfused Rat Kidney. Nephron 1992; 60: 477–481
- Lo Russo A., Passaquin A.C., Andre P., Skutella M., Ruegg U.T. Effect of Cyclosporin A and Analogues on Cytosolic Calcium and Vasoconstriction: Possible Lack of Relationship to Immunosuppressive Activity. Br. J. Pharmacol. 1996; 118: 888–892
- Henke W., Nickel E., Jung K. Cyclosporine A Inhibits ATP Net Uptake of Rat Kidney Mitochondria. Biochem. Pharmacol. 1992; 43: 1021–1024
- Massicot F., Martin C., Ditertre-Catella H., Ellok-Achard S., Pham-Huy C., Thevenin M., Rucay P., Warnet J.M., Claude J.-R. Modulation of Energy Status and Cytotoxicity Induced by FK 506 and Cyclosporine A in a Renal Epithelial Cell Line. Arch. Toxicol. 1996; 71: 529–531
- Allibardi S., Chierchia S.L., Maegonato V. Effects of Trimetazidine on Metabolic and Functional Recovery of Post Ischemic Rat Hearts. Cardiovasc. Drugs Ther. 1998; 12: 543–549
- Salducci M.D., Chauvet-Monges A.M., Tillement J.P. Trimetazidine Reverses Calcium Accumulation and Impairment of Phosphorylation Induced by Cyclosporine A Isolated Rat Liver Mitochondria. J. Pharmacol. Expt. Ther. 1996; 277: 417–422
- Hauet T., Bauza G., Goujon J.M., Caritez J.C., Carretier M., Eugene M., Tillement J.P. Effect of Trimetazidine on Lipid Peroxidation and Phosphorous Metabolites During Cold Storage and Reperfusion of Isolated Rat Perfused Kidneys. J. Pharmacol. Expt. Ther. 1998; 285: 1061–1067
- Maridonneau-Parini I., Harpey C. Effect of Trimetazidine on Membrane Damage Induced by Oxygen Free Radicals in Human Red Cells. Brit. J. Clin. Pharmacol. 1985; 20: 148–151
- Ohkawa H., Oshishi N., Yagi K. Assay of Lipid Peroxidation in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979; 95: 331–358
- Wang C., Salahudeen A. Lipid Peroxidation Accompanies Cyclosporine Nephrotoxicity: Effects of Vitamin E. Kidney Int. 1995; 47: 927–934
- Clarke H., Ryan M.P. Cyclosporine A-Induced Alterations in Magnesium Homeostasis in the Rat. Life Sci. 1999; 64: 1295–1306
- Ferguson C.J., Ruhland C., Parry-Jones D.J. Low-Dose Cyclosporine Nephrotoxicity in the Rat. Nephrol. Dial. Transplant 1993; 8: 1258–1263
- Nath K.A., Salahudeen A.K. Induction of Renal Growth and Injury in Intact Rat Kidney by Dietary Deficiency of Antioxidants. J. Clin. Invest. 1990; 86: 1179–1192
- Suleymanlar G., Suleymanlar I., Shapiro J.I., Chan L. Possible Role Lipid Peroxidation in Cyclosporine Nephrotoxicity. Transplant. Proc. 1994; 26: 2888–2890
- Wolf A., Clemann N., Frieauff W., Ryffel B., Cordier A. Role of Reactive Oxygen Formation in the Cyclosporin-A-Mediated Impairment of Renal Functions. Transplant. Proc. 1994; 26: 2902–2907
- Walker P.D., Lazzaro V.A., Duggin G.G., Horvat J.S., Tiller D.J. Evidence that Alteration in Renal Metabolism and Lipid Peroxidation May Contribute to Nephrotoxicity. Transplantation 1990; 50: 487–492
- Zhong Z., Arteel G.E., Connor H.D., Yin M., Frankenberg M.V., Stachlewitz R.F., Raleigh J.A., Mason R.P., Thurman R.G. Cyclosporin A Increases Hypoxia and Free Radical Production in Rat Kidneys: Prevention by Dietary Glycine. Am. J. Physiol. 1998; 275: F595–F604
- Moss N.G., Powell S.L., Falk S.L. Intravenous Cyclosporine Activates Afferent and Efferent Nerves and Causes Sodium Retention in Innervated Kidneys in Rats. Proc. Natl. Acad. Sci. USA 1985; 82: 8222–8226
- Murray B.M., Paller M.S., Ferris T.F. Effect of Cyclosporine Administration on Renal Hemodynamics in Conscious Rats. Kidney Int. 1985; 28: 767–774
- Avdonin P.V., Cottet-Maire F., Afanasjeva G.V., Loktionova S.A., Lhote P., Ruegg U.T. Cyclosporine A Up-Regulates Angiotensin II Receptors and Calcium Responses in Human Vascular Smooth Muscle Cells. Kidney Int. 1999; 55: 2407–2414
- Packer L. α-Lipoic Acid: A Metabolic Antioxidant which Regulates NF-κB Signal Transduction and Protects Against Oxidative Injury. Drug Metab. Rev. 1998; 30: 245–275
- Inselmann G., Hannemann J., Baumann K. Cyclosporine A-Induced Lipid Peroxidation and Influence on Glucose-6-Phosphatase in Rat Hepatic and Renal Microsomes. Res. Commun. Chem. Pathol. Pharmacol. 1990; 68: 189–203
- Guanieri C., Muscari C. Effect of Trimetazidine on Mitochondrial Function and Oxidative Damage During Reperfusion Ischemic Hypertrophied Rat Myocardium. Pharmacology 1997; 46: 324–331
- Kay L., Finelli C., Aussidat J. Improvement of the Long-Term Preservation of the Isolated Arrested Rat Heart by Trimetazidine: Effects on the Energy State and Mitochondrial Function. Am. J. Cardiol. 1995; 76: 45B–49B
- Hauet T., Goujon J.-M., Vandewalle A., Baumert H., Lacoste L., Tillement J.-P., Eugene M., Carretier M. Trimetazidine Reduces Renal Dysfunction by Limiting the Cold Ischemia-Reperfusion Injury in Autotransplanted Pig Kidneys. J. Am. Soc. Nephrol. 2000; 11: 138–148
- Ozden A., Aybek Z., Saydam N., Calli N., Saydam O., Dilzcan E., Guner G. Cytoprotective Effect of Trimetazidine on 75 min Warms Renal Ischemia-Reperfusion Injury in Rats. Eur. Surg. Res. 1998; 30: 227–234
- English J., Evan A., Houghton D.C., Bennett W.M. Cyclosporine-Induced Acute Renal Dysfunction in the Rat. Evidence of Arteriolar Vasoconstriction with Preservation of Tubular Function. Transplantation 1987; 44: 135–141
- Baud L., Ardaillou R. Involvement of Reactive Oxygen Species in Kidney Damage. Br. Med. Bull. 1993; 49: 621–629
- Galat J.A., Robinson A.V., Rhodes R.S. Oxygen Free Radical Mediated Renal Dysfunction. J. Surg. Res. 1989; 46: 520–527
- Perico N., Benigni A., Zoja C., Delaini F., Remuzzi G. Functional Significance of Exaggerated Renal Thromboxane A2 Synthesis Induced by Cyclosporin A. Am. J. Physiol. 1986; 251: F581–F587
- Kon V., Huntley T.E., Fogo A. Combined Antagonism of Endothelin A/B Receptors Links Endothelin to Vasoconstriction Whereas Angiotensin II Effects Fibrosis. Transplantation 1995; 60: 89–95