Abstract
Aims: This study evaluates the effect of bilateral nephrectomy on the gastric emptying of a liquid meal. Methods: Male rats were submitted under anesthesia to cervical vessels cannulation and bilateral lumbar incision, followed or not by nephrectomy. Next day, they were gavage fed (1.5 mL) with phenol red (0.5 gmL−1) in 5% glucose solution and sacrificed 0, 10, 20, 30 or 45 min later. A blood sample was obtained for biochemical analysis while gastric dye retention was determined by spectrophotometry. Data (mean ± SEM) were compared by ANOVA and Student–Newman–Keuls tests. Results: Gastric emptying values from nephrectomy group at 10, 20, 30 and 45 min were lower (P<0.05) than those of sham-operated animals (22.0 ± 4.0 vs. 38.9 ± 6.1%, 34.1 ± 1.4 vs. 66.9 ± 1.3%, 45.5 ± 6.1 vs. 64.9 ± 5.4% and 59.7 ± 2.4 vs. 81.5 ± 4.0%, respectively). Mean arterial pressure, blood volume, serum osmolarity, urea, creatinine and potassium values were higher (P < 0.05) in nephrectomy group than in sham-operated animals (143.3 ± 2.7 vs. 100.5 ± 4.1 mmHg, 15.7 ± 0.9 vs. 8.9 ± 1.1 mL 100 g−1, 344.0 ± 10.8 vs. 299.4 ± 1.3 mOsm KgH2O−1, 344.0 ± 33.7 vs. 47.0 ± 2.8 mg dL−1, 3.6 ± 0.3 vs. 1.1 ± 0.1 mg dL−1, 6.4 ± 0.7 vs. 3.7 ± 0.2 mEq L−1, respectively). The plasmatic Na+ values did not change (139.3 ± 2.0 in sham-operation vs. 123.0 ± 7.5 mEq L−1 in nephrectomy). Conclusion: Acute loss of kidney function markedly delays the gastric emptying rates, which could be involved in gastrointestinal dysmotility complaints seen after renal failure.
INTRODUCTION
Since the development of dialysis, uremia is seldom the direct cause of death to renal failure patients. However, their quality of life is jeopardized by a large variety of gastrointestinal complaints.Citation[[1]] In spite of this, some aspects of their pathophysiological mechanisms, especially those related to gastrointestinal dysmotility are still not completely understood.Citation[[2]]
One aspect of this controversy comes from studies on gastric emptying. Some researchers have reported that patients on maintenance dialysis treatment have normal rates of gastric emptying for solid and liquid mealsCitation[[3]], Citation[[4]] whereas others have ascribed either delayed gastric emptying of solidsCitation[[5]], Citation[[6]] or even normal solid emptying with accelerated emptying of liquids.Citation[[7]] Besides, the presence of peritoneal dialysate may retard per se the emptying of a solid meal.Citation[[8]] A possible explanation for such conflicting results from human studies is due to the fact that they have included patients with important differences in age, sex, time interval after renal failure, dialysis schedule and presence or not of visceral autonomic neuropathy.
In the experimental setting, this subject is also not established yet. Raybould and co-workers (1994) observed that chronic uremic rats present a lower frequency of antral contractions with an increased gastric retention of solid but a normal gastric emptying of liquid.Citation[[9]] To the best of our knowledge there is only one report addressing the effects of acute loss of kidney function on gastrointestinal motility.Citation[[10]] In that study, Belangero and Collares (1998) compared the effects of bilateral nephrectomy on fractional gastric retention of different liquid meals—consisting of plain saline, sodium bicarbonate, glucose solution or commercial meal (Nefrodiet®). The authors showed that nephrectomized rats exhibited gastric retention values significantly higher in comparison to those of sham-operated animals.
Because acute renal failure also induces serum electrolytes, blood volume and hemodynamic abnormalities, which could interfere with gastrointestinal motor behavior,Citation[[11]] we decided to further study this subject.
MATERIAL AND METHODS
Male rats of Wistar strain (180–220 g of body weight, N = 50) were obtained from our institution's breeding station and housed under adequate conditions. All surgical procedures and animal treatments were carried out in accordance with the “Guide for the Care and Use of Laboratory Animals” (N.I.H., Bethesda, U.S.A.).
After anesthesia with tribromoethanol, polyethylene catheters (PE 50) filled with heparin (500 U mL−1) were inserted into left jugular vein and right carotid artery of the rats. The distal end of each catheter was tunnelled subcutaneously to the back of the animals and its free end fixed by a silk ligature between the shoulders.
Next, the animals were submitted to a bilateral lumbar incision and randomly divided into control (sham operation, n = 24) and experimental (bilateral nephrectomy, n = 26) groups. In order to perform the nephrectomy, both kidneys were identified and each renal pediculus was blocked. Great care was taken to preserve the adrenal gland and its blood supply during the kidney exeresis. Sham operation consisted of bilateral kidney manipulation only. The rats were allowed to recover in individual standard raised mesh-bottom Bollman cages for 16 h in order to prevent coprophagy, being fasted with free access to water until 2 h before the experiment.
The methodology used to measure the gastric dye recovery is essentially the same described by Scarpignato and co-workers (1980). Firstly, the animals were gavage fed with 1.5 mL of a test meal (5% glucose solution and phenol red, 0.5-mg mL−1) and then anesthetized by iv. injection of pentobarbital at assigned time intervals, i.e., 0, 10, 20, 30 or 45 min after meal administration. After a laparotomy, the stomach was quickly clamped at pylorus and cardia ends and then removed. Next, the viscera was placed in 100 mL of NaOH solution (0.1 N), cut into small pieces and homogenized for 30 s. The suspension was allowed to settle for 30 min at room temperature and 10 mL of the resultant supernatant centrifuged for 10 min (2800 rpm). Proteins in 5 mL of the supernatant were precipitated with 0.5 mL of trichloroacetic acid (20% wt : vol) and centrifuged for 20 min (2800 rpm). Finally, 3 mL of this supernatant was added to 4 mL of NaOH solution (0.5 N). The sample absorbance was obtained by spectrophotometry (Jena, Germany) at a wavelength of 560 nm and expressed as optical density (O.D.). In each experiment, a standard dilution curve was made relating the concentration of phenol red to the optical density of NaOH solution (0.1 N). A linear coefficient of the dilution curve was determined and used to obtain the concentration (C) of the solution read at 560 nm and the amount of phenol red recovered from each viscera.Citation[[12]]
The gastric emptying for each rat was calculated according to the following formula:
Rats sacrificed immediately after test meal administration (t = 0 min) were used to establish the standard stomach values (100% phenol red in the stomach, n = 10).
The animals from control and experimental subsets (t = 20 min) were submitted 1 h before gavage to a continuous recording of the mean arterial blood pressure for 10 min. For this purpose, the carotid cannula was connected to a pressure transducer (P1000B) coupled to a polygraph (Mark IV Physiograph, Narco Byo-Systems, Houston, USA). After laparotomy, intracardiac blood samples were collected in order to assess some biochemical parameters. The Evans blue method was utilized to obtain the plasma volume and then to determine the blood volume (in mL 100 g of body weight−1) after hematocrit correction. The serum osmolarity values (in mOsm per KgH2O) were determined by cryoscopy. The Na+ and K+ plasma values (in mEq L−1) were determined by flame photometry. An automatic analyser (Stat Profile Plus 9, Nova Medical Corp) determined the urea (nitrogen excretion products) as well as creatinine plasma values (both in mg dL−1).
The results are expressed as mean ± SEM. Each control and experimental subsets consisted in 4 to 10 animals. The one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test was used to compare the differences between means from sham-operation and nephrectomy groups. The simple linear regression was used to correlate the fractional gastric retention and blood volume individual values. P values lower than 0.05 were considered significant.
RESULTS
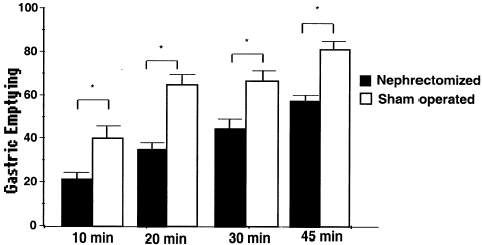
shows the gastric emptying patterns in sham-operated and nephrectomized groups. Bilateral nephrectomy delayed (P < 0.05) the gastric emptying rates of the liquid meal by 28.8 to 77.1%. The gastric emptying values in sham-operated animals were 38.9 ± 6.1% (n = 5), 66.9 ± 1.3% (n = 10), 64.9 ± 5.4% (n = 5) and 81.5 ± 4.0% (n = 4), respectively for 10, 20, 30 and 45 min. On the other hand, the gastric emptying values in nephrectomized animals were 22.0 ± 4.0% (n = 5), 34.1 ± 1.4% (n = 11), 45.5 ± 6.1% (n = 6) and 59.7 ± 2.4% (n = 4), respectively for 10, 20, 30 and 45 min.
Figure 1.Effect of bilateral nephrectomy (▪) or sham-operation (□) on the liquid gastric emptying rates in awake rats. The animals were studied 1 day after surgery and sacrificed at 0, 10, 20, 30 or 45 min after liquid meal (1.5 mL of 0.5 mg mL−1 phenol red in 5% glucose solution). Fractional dye recovery was obtained by spectrophotometry from the stomach of sham-operated and nephrectomy groups. Bars represent the gastric emptying (%) mean values while vertical lines means the standard error of mean. n, number of observations. *, P < 0.05 for sham-operation vs. nephrectomy values (ANOVA and Student–Newman–Keuls test).
presents the biochemical and hemodynamic parameters profile from sham-operated and nephrectomized groups. As one can see, the bilateral kidney exeresis induced a significant increase in the mean arterial pressure and plasma volume values, respectively from 100.5 ± 4.1 mmHg and 8.9 ± 1.1 mL 100 g−1 in sham-operated animals compared to 143.3 ± 2.7 mmHg and 15.7 ± 0.9 mL 100 g−1 in nephrectomy group. The serum osmolarity, urea, creatinine and potassium values of nephrectomized animals were also higher (P < 0.05) than those of sham-operated group (respectively 344.0 ± 10.8 vs. 299.4 ± 1.3 mOsm KgH2O−1, 344.0 ± 33.7 vs. 47.0 ± 2.8 mg dL−1, 3.6 ± 0.3 vs. 1.1 ± 0.1 mg dL−1, 6.4 ± 0.7 vs. 3.7 ± 0.2 mEq L−1). The plasma Na+ values did not change (139.3 ± 2.0 in sham-operation to 123.4 ± 7.5 mEq L−1 in nephrectomy, P > 0.05).
Table 1. Hemodynamic and Biochemical Parameters Profiles from Sham-Operated and Bilateral Nephrectomized Rats
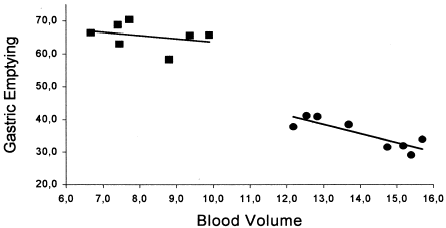
The relationship between each individual value of blood volume and its respective gastric emptying of a liquid meal in sham-operated and nephrectomized group is shown in . As one can see there is a strong (r2 = 0.73) correlation between the hypervolemic condition and the decreased dye emptying from the stomach of animals submitted to bilateral nephrectomy—as indicated by the linear regression equation y = 75.2−2.8x. On the other hand, there is no correlation between the blood volume values and gastric dye retention in sham-operated animals.
Figure 2. Correlations between gastric dye retention (%) and blood volume (mL/100 g) values obtained from sham-operated and nephrectomized animals. There is a strong correlation (r2 = 0.73) between gastric retention and blood volume in anephric animals (•—•) as indicated by the linear regression equation y = −2.8x + 75.2 while in sham-operated animals (▪—▪) there is no such correlation, according to the regression linear equation y = −1.0x + 73.6, r2 = 0.09.
DISCUSSION
The present study confirms and extends previous results of Belangero and Collares (1998) as it shows that bilateral nephrectomy induced a marked delay in the gastric emptying in awake rats. The decrease in gastric emptying of a liquid test meal we have observed was significant (28.8–77.1%) and lasted for at least 45 min after meal gavage. Our results also indicate that this phenomenon is present as early as 6 h after bilateral nephrectomy and increases as times passes by (Silva et al. unpublished results).
Biochemical and hemodynamic data found in the experimental animals demonstrate the acute loss of kidney function in nephrectomy group and these changes are quite similar to values reported by others.Citation[[13]], Citation[[14]]
Renal failure patients present several morphological and physiological abnormalities in the gastrointestinal tract.Citation[[15]] It has been reported that rats with renal failure (either acute or chronic) exhibit increased rates of gastric acid secretionCitation[[16]], Citation[[17]] and intestinal permeability to macromolecules.Citation[[18]], Citation[[19]] Because the marker (phenol red) is pH-sensitive, the measurements of actual gastric emptying could be based in conditions associated with higher acid secretion ratios. However, this would result in lower dye detection and not in an increased fractional gastric retention, as we verified. Thus, this effect appears to be related to gut motility rather than to changes in gastric acid secretion.
In rats submitted to chronic uremia, the gastric emptying of liquid is not significantly impaired.Citation[[8]] The discrepancy with our results can be explained by the striking different temporal patterns between these protocols. In chronic uremia, there are important morphological and functional abnormalities of the autonomic nervous systemCitation[[20]] that may interfere with the gastrointestinal motor behavior, while in acute azotemia possibly there is no time for such effects.
The gastric emptying delay after acute loss of kidney function could be the result of increased gastric relaxation, decreased antral contractility or enhanced pyloric and/or duodenal resistance. Since the test meal used in the present study was isotonic, it is unlikely that this effect is mediated through an enhanced intestinal inhibition of gastric emptying. It should be noticed that Ko and colleagues (1998) have shown an increased frequency of irregular gastric myoelectric activity in uremic patients.Citation[[21]]
Concerning the mechanisms involved in gastric emptying delay due to the loss of kidney function there are not much information available on the medical literature. It is conceivable that one of the several nitrogen compounds that accumulate in the extracellular fluid could inhibit the contractility of the gastrointestinal smooth muscle.Citation[[22]] Another possibility is that bilateral nephrectomy induces hemodynamic changes that could interfere with the gastrointestinal motor behavior. As a matter of fact, we have shown that acute hypervolemia, due to saline or blood infusion, increased the resistance offered by the gastroduodenal segment to the flow of liquid and delayed the gastric emptying of liquid in rats.Citation[[23]], Citation[[24]], Citation[[25]] This hypothesis is supported by the strong correlation (r2 = 0.73) between the hypervolemic condition and the increased gastric dye retention.
In summary, our results indicate that the gastric emptying of a liquid meal is markedly delayed after bilateral nephrectomy. The increased gastric retention may contribute to nausea, vomiting, epigastralgia, bloating and other gastrointestinal dysmotility complaints that are common in renal failure patients. Further studies are under way to outline the neurohumoral mechanisms involved in this phenomenon.
ACKNOWLEDGMENT
We are grateful to Mr. Haroldo Pinheiro for his helpful technical assistance and to Drs. Marcus R. Vale and Glauce S.B. Viana for providing access to laboratory facilities. This article is part of the requirements for a M.Sc. thesis in Pharmacology to be presented by A.P.T. Silva to the Department of Physiology and Pharmacology, Federal University of Ceará. Research scholarships and grants from CNPq and FUNCAP supported this work.
REFERENCES
- Kang J.Y. The Gastrointestinal Tract in Uraemia. Dig. Dis. Sci. 1993; 38: 257–268
- Etemad B. Gastrointestinal Complications of Renal Failure. Gastroenterol. Clin. N. Am. 1998; 27: 875–892
- Soffer E.E., Geva G., Helman C., Avni Y., Bar-Meir S. Gastric Emptying in Chronic Renal Failure Patients on Hemodialysis. J. Clin. Gastroenterol. 1987; 9: 651–653
- Wright R.A., Clemente R., Wathen R. Gastric Emptying in Patients with Chronic Renal Failure Receiving Hemodialysis. Arch. Int. Med. 1984; 144: 495–496
- Kao C.H., Hsu Y., Wang S.J. Delayed Gastric Emptying in Patients with Chronic Renal Failure. Nucl. Med. Commun. 1996; 17: 164–167
- McNamee P.T., Moore G.W., McGeown M.G., Doherty C.C., Collins B.J. Gastric Emptying in Chronic Renal Failure. Brit. Med. J. 1985; 291: 310–311
- Dumitrascu D.L., Barnet J., Kirsschner T., Weinbeck M. Antral Emptying of Semisolid Meal Measured by Real-time Ultrasonography in Chronic Renal Failure. Dig. Dis. Sci. 1975; 40: 636–644
- Brown-Cartwright D., Smith H.J., Feldman M. Gastric Emptying of an Indigestible Solid in Patients with End-Stage Renal Disease on Continuous Ambulatory Peritoneal Dialysis. Gastroenterol. 1988; 95: 46–51
- Raybould H.E., Plourde V., Zittel T., Bover J., Quintero E. Gastric Emptying of Solids but not Liquids is Decreased in Rats with Chronic Renal Failure. Dig. Dis. Sci. 1994; 39: 2301–2305
- Belangero V.M.S., Collares E.F. Gastric Emptying of Liquids in Rats with Acute Renal Insufficiency. Arq. Gastroenterol. 1998; 35: 278–282
- Malagelada J.R., Azpiroz F. Determinants of Gastric Emptying and Intestinal Transit. Handbook of Physiology: The gastrointestinal system, J.W. Wood. American Physiological Society, Bethesda 1989; Vol. I: 909–937, Section 6
- Scarpignato C., Capovilla T., Bertacini G. Action of Cerulein on Gastric Emptying of the Conscious Rat. Arch. Int. Pharmacodyn. 1980; 246: 286–293
- Gretz N., Meisinger E., Waldherr R., Strauch M. Acute Renal Failure After 5/6 Nephrectomy: Histological and Functional Changes. Contrib. Nephrol. 1988; 60: 56–63
- Ormrod D., Miller T. Experimental Uremia. Description of a Model Producing Varying Degrees of Stable Uremia. Nephron 1980; 26: 249–254
- Ryan J.C., Sleisenger M.H. Effects of Systemic and Extra-intestinal Disease on the Gut. Gastro-intestinal Disease, 5th ed., M.H. Sleisenger, F.S. Fordtran, Philadelphia, Saunders 1993; Vol.1: 219–220
- Quintero E., Guth P.H. Renal Failure Increases Gastric Mucosal Blood Flow and Acid Secretion in Rats. Am. J. Physiol. 1992; 263: G75–G80
- Kowalewski K., Secord D.C. Histamine-Stimulated Gastric Secretion in Rats with Chronic Renal Insufficiency. Digestion 1976; 14: 381–383
- Aviv A., Higashino H., Kobayashi T. The Intestinal Profile of Na+-K+-ATPase in Two Rat Models of Acute Renal Failure. J. Lab. Clin. Med. 1982; 100: 533–539
- Magnusson M., Magnusson K.-E., Denneberg T. Impaired Gut Barrier in Experimental Chronic Uremic Rats. Miner. Electrolyte. Metab. 1992; 18: 288–292
- Vita C., Messina C., Savica V., Bellinghieri G. Uremic Autonomic Neuropathy. J. Auton. N. Syst. 1990; 30: S179–S184
- Ko C.-W., Chang C.-S., Wu M.-J., Chen G.-H. Transient Impact of Hemodialysis on Gastric Activity of Uremic Patients. Dig. Dis. Sci. 1998; 43: 1159–1164
- Matsumoto A., Yamasaki M., Yonemura K., Tanaka I. Depression of Nerve Mediated Smooth Muscle Contractions In Vitro by Plasma of an Anephric Rabbit and Uremic Patients. Japan J. Physiol. 1981; 31: 947–956
- Capelo L.R., Cavalcante D.M., Leitão I.A., Filho G.C., Silva E.A.T. Modifications of Gastric Compliance Related to Changes of Extracellular Fluid Volume. Braz. J. Med. Biol. Res. 1983; 16: 73–76
- Xavier-Neto J., Santos A.A., Rola F.H. Acute Hypervolemia Increases Gastroduodenal Resistance to the Flow of Liquid in the Rat. Gut. 1990; 31: 1006–1010
- Gondim F.de A.A., Oliveira G.R., Graça J.R.V., Gondim R.B.M., Alencar H.M.P., Dantas R.P., Santos A.A., Rola F.H. Neural Mechanisms Involved in the Delay of Gastric Emptying of Liquid Elicited by Acute Blood Volume Expansion in Awake Rats. Neurogastroenterol. Motility 1999; 11: 93–99
