Abstract
Background: Continuous renal replacement therapy (CRRT) is increasingly used in managing acute renal failure (ARF) as it offers hemodynamic stability and significant solute clearance in this setting. However, it also requires anticoagulation. Traditionally, heparin has been the anticoagulant of choice but this increases hemorrhagic risk in already high-risk ARF patients. Regional citrate anticoagulation offsets this risk. However, it can be difficult to manipulate regional anticoagulation in CRRT. Moreover, citrate CRRT has been plagued by short optimal filter patency times. Methods: We designed a novel citrate-based anticoagulation schema for continuous venovenous hemofiltration (CVVHF). We implemented this schema prospectively in caring for 24 individuals admitted to the intensive care unit with ARF requiring CRRT. Each individual had a contraindication to systemic anticoagulation. We evaluated filter patency using Kaplan-Meier methodology, comparing the effect of this citrate-CVVHF system to historical, saline-flush control CVVHF systems. Results: 58 filters ran for a total of 2637.5 h. Average filter patency time was 45.4 ± 25.5 h. At 48 h, 70% of the CVVHF-citrate system filters remained patent compared to only 16% of historical control saline-flush systems (p = 0.0001). The average filtered urea nitrogen/blood urea nitrogen ratio was 0.84 ± 0.06 with an average urea clearance of 28.5 ± 4.1 mL/min for CVVHF-citrate-treated individuals. Only three patients experienced transient complications related to CVVHF-citrate with resolution of these complications within 24 h. Ultimately, 58.3% of the CVVHF-citrate-treated patients survived to ICU discharge. Conclusions: This novel CVVHF-citrate system achieved excellent clearance and dramatically improved filter patency compared to saline-flush systems. Moreover, it did so with minimal toxicity.
INTRODUCTION
Continuous renal replacement therapy (CRRT) is increasingly used in managing acute renal failure (ARF), primarily because it offers hemodynamic stability and better solute clearance than other dialytic modalities.Citation[[1]], Citation[[2]] The main disadvantage of CRRT however, is the need for anticoagulation. Heparin is the most frequently used anticoagulant for CRRT. Despite its utility, it places critically ill patients at risk for hemorrhagic complications.Citation[[3]] Indeed, up to 30% of all heparin-anticoagulated patients receiving CRRT experience hemorrhagic complications.Citation[[4]] Acutely ill patients also can have additional contraindications to heparin therapy, e.g., thrombocytopenia. To that end, various anticoagulation schema have been utilized in CRRT including low molecular weight heparin,Citation[[6]] other anticoagulants, e.g., hirudin,Citation[[7]] anti-platelet therapies,Citation[[8]] regional heparinization with protamine reversal,Citation[[9]] and no-anticoagulation schema.Citation[[10]] None of these have met with wide-ranging acceptance.
Citrate anticoagulation offsets the hemorrhagic risk of systemic anticoagulation by providing selective anticoagulation within the extracorporeal system. Citrate was first used as an anticoagulant in the early 1960s and has been a mainstay of anticoagulation with intermittent hemodialysis (IHD) for the last two decades. Citrate anticoagulation however for CRRT is relatively novel. Regional citrate anticoagulation was used successfully in continuous arteriovenous HD (CAVHD) and continuous arteriovenous hemodiafiltration (CAVHDF).Citation[[11]] However, improved blood pump technology has virtually eliminated the need for cannulating both an artery and a vein. As such, citrate CRRT can now be used for either continuous venovenous hemodiafiltration (CVVHDF) or continuous venovenous hemofiltration (CVVHF).
Citrate chelates calcium, thereby inducing anticoagulation within the circuit. However, ionized calcium levels return towards normal when blood from the extracorporeal circuit mixes with venous blood in the presence of a calcium infusion. After leaving the extracorporeal circuit, citrate is metabolized via the Krebs cycle to bicarbonate, raising serum bicarbonate levels and releasing the previously bound calcium.
Unfortunately, citrate anticoagulation can be complex and until recently, required special dialysate for continuous venovenous hemodialysis (CVVHD).Citation[[12]] Though somewhat effective in continuous venovenous hemofiltration (CVVHF), citrate-anticoagulated systems remained plagued by shortened filter patency.Citation[[13]] Moreover, citrate anticoagulation has possible risks in individuals with liver failure who may not be able to metabolize citrate, allowing it to accumulate, causing toxicity. We designed a novel method for using citrate anticoagulation and tested it in the intensive care unit (ICU) setting. We hypothesized that this methodology would prolong filter patency without significant complications.
METHODS
Patient Population
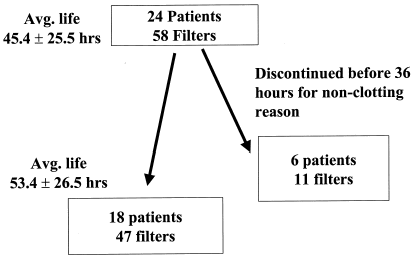
Twenty-four critically ill patients () consecutively admitted to the medical/surgical, cardiothoracic or burn ICUs at the University of Wisconsin hospitals during 1998 and 1999 were prospectively evaluated. Each patient required CRRT and received treatment with a new citrate anticoagulation system with CVVHF (CVVHF-citrate).
Table 1. Patient Demographics
All patients were 18 years of age or older. To be eligible for citrate CRRT, a patient had to have (1) acute renal failure of any etiology requiring CRRT; and (2) thrombocytopenia (platelet count < 50 000), or heparin-induced antibody production, or coagulopathy (an international normalized ratio > 1.5), or significant active bleeding (requirement for more than two units of packed red blood cells as a transfusion) within 24 h of initiation of renal replacement therapy.
System Design
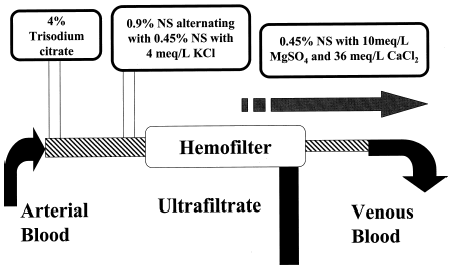
Vascular access was established with a double or triple lumen (Triflow®, Medcomp) dialysis catheter. If a double-lumen catheter was used then another port on a central line was used for infusion of the replacement fluids. A three-way stopcock was attached to the arterial outflow port for citrate infusion. 4% trisodium citrate (140 mmol citrate and 420 mmol sodium/liter, Baxter Corp® Deerfield, IL) was placed on a IVAC pump and infused through the three-way stopcock at a rate of 165 mL/h ().
Figure 1. Schematic of CVVHF-citrate circuit. Abbreviations used: NS = normal saline; L = liter; KCl = potassium chloride; MgSO4 = magnesium sulfate; CaCl2 = calcium chloride.
Post-filter ionized calcium (PF(Ca2+)i) levels were measured to assess adequacy of anticoagulation. The rate of citrate infusion was titrated to maintain an ionized PF(Ca2+)i ≤ 1.2 mg/dL (0.3 mmol/L). The Baxter BM11 system was used for the blood pump with initial blood flow rate of 125 mL/min. Blood was pumped through a Renaflo (Menntech, Minneapolis, MN) HF 1200 filter.
A single three-liter bag of 0.9% normal saline (NS) with potassium chloride (KCl) 4 meq/L was infused initially at 1000 mL/h pre-filter as replacement fluid. This was alternated with a three-liter bag of 0.45% NS with KCl at the same concentration.
The ultrafiltrate rate was set by attaching the tubing to an IVAC pump. This pump was set at 2000 mL/h, the maximum allowed by the pump. The system was flushed every four hours with 200 ml 0.45% NS to check the venous trap for clot formation. Net fluid removal was adjusted by increasing or decreasing the replacement fluids to achieve the desired net ultra-filtration.
A replenishment solution (0.45% NS with 36 meq/L of CaCl2 and 10 meq/L MgSO4) was infused in a third, separate port of the dialysis catheter at 175 mL/h. The rate was increased or decreased to maintain normal serum ionized calcium (S(Ca2+)i) levels.
Serum urea nitrogen (BUN) and ultrafiltrate urea nitrogen (FUN) levels were measured daily to calculate FUN/BUN ratios. The daily ultrafiltrate volume was also recorded. Urea clearances then were calculated by multiplying the ultrafiltrate urea level by the volume of ultrafiltrate.
Filters were initially maintained for 72 h with a standard filter and tubing change at that time. After demonstrating CVVHF-citrate safety and efficacy, we increased the mandatory filter change time to 120 h. Filters were discontinued prior to protocol change for (1) decreased FUN/BUN ratio; (2) clotting of filter or loss of ultrafiltration; (3) the patient no longer needed CRRT, or (4) the patient expired. PF(Ca2+)i and S(Ca2+)i, total serum calcium and magnesium levels were monitored every 8 h. The duration of each filter, daily electrolytes, post-filter and serum ionized calcium levels, FUN/BUN ratio, and reason for discontinuation were recorded.
Historical control data for non-heparinized, saline-flush CRRT systems (200 mL NS flush every hour) during the 12 months prior to starting CVVHF-citrate system were evaluated. These were used to establish average filter life for those exposed to saline only during treatment.
Statistical Methods
All analyses were performed with SAS statistical software (SAS Institute, Inc., Cary NC). Filter survival data were analyzed using the methodology of Kaplan and Meier. All data are presented as mean ± standard deviation unless otherwise stated. A p value ≤ 0.05 was considered significant.
RESULTS
Twenty-four individuals () who developed acute severe renal failure requiring CVVHF-citrate were studied. The average age for these individuals was 53.5 years (range 23–73 years). The average APACHE III score at day of ICU admission was 105 ± 25 (median 102). Fourteen patients (58.3%) survived to ICU discharge while ten died in the ICU (41.6%).
Filter Patency
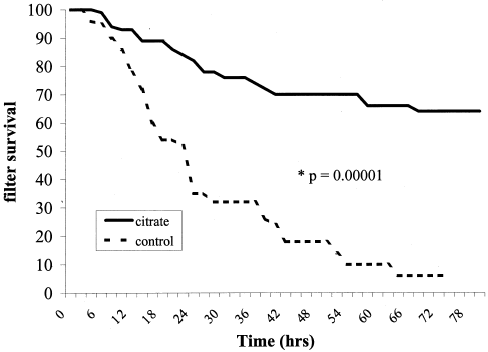
During the study time, 58 filters ran for a total of 2637.5 h. The average filter patency time was 45.4 ± 25.5 h (). After censoring data for patients who expired or no longer required CRRT before a 36-h threshold, there were 42 systems that ran for 2177.75 h. The average filter patency for this group was 53.4 ± 26.5 h. Filter patency for CVVHF-citrate filters was significantly longer than saline flush control filters. At 48 h, 70% of the CVVHF-citrate system filters remained patent versus 16% of the saline-only flush systems (p = 0.00001, ).
Adequacy
The FUN/BUN ratio was measured daily. The average daily ratio was 0.84 ± 0.06 with an average urea clearance of 28.5 ± 4.1 mL/min. The average PF(Ca2+)i was 1.52 ± 0.36 mg/dL. This declined to 1.28 ± 0.23 mg/dL during the final 3 months of the study period.
Complications
One patient developed hypercalcemia with laboratory evidence of citrate toxicity after liver transplantation, sepsis and multiple blood product transfusions. This necessitated discontinuation of the citrate system. This patient was converted to CVVHF with saline flushes only. There were no hemodynamic changes or arrhythmias noted. Following resolution of early graft dysfunction, the patient was able to return to CVVHF citrate on post-operative day 6 without further difficulties. The patient had no long-term adverse sequelae as a result of the citrate toxicity.
Two patients developed significant metabolic alkalosis with serum bicarbonate levels > 29 meq/dL and pH > 7.49. Both patients were receiving parenteral alimentation at the time they developed the significant metabolic alkalosis. The base excess was initially with treated with 1.0 M HCl and the parenteral nutrition was adjusted to remove excess sources of bicarbonate.
DISCUSSION
The expanding role of CRRT requires that a high-risk population be exposed to anticoagulation, often for prolonged periods of time. Before the advent of citrate CRRT, patients were maintained on heparin during CRRT. This placed patients at risk for clinically significant bleeding.Citation[[5]], Citation[[14]], Citation[[15]] The added morbidity and mortality associated with transfusion therapy in this setting compounded the problems affecting these patients.
Historically, at our institution, if a patient developed active bleeding during CRRT, the heparin infusion was discontinued and hourly saline flushes were instituted in to maintain filter patency. This methodology, while variably successful, is associated with decreased filter half-life compared to heparin systems.Citation[[14]] Consequently, there is no current standard for filter patency using saline-only flush protocols. Even the aggressive saline-alone flush protocols in our historical control group maintained filters for only 23.4 ± 14.85 h. Moreover, such attempts to maintain filter patency often came at the expense of net negative fluid balance.
Citrate decreases the risk of hemorrhage compared to heparin anticoagulation.Citation[[4]], Citation[[13]] Citrate anticoagulation offers the advantage of regional anticoagulation in CRRT and as such, is safe and efficacious. They may actually improve clearance in comparison to heparin CRRT systemsCitation[[5]], Citation[[16]] as a result of decreased micro-adhesion of activated platelets and red blood cells to the filter surfaceCitation[[17]] or from citrate-specific changes in clotting proteins.Citation[[18]] Our CVVHF-citrate system demonstrated good urea clearance comparable to other descriptions of urea clearance in citrate CRRT systems.Citation[[5]], Citation[[13]], Citation[[16]]
Citrate can increase the cost and complexity associated with CRRT. CVVHD-citrate is normally performed with a low-sodium dialysate to counterbalance the increased sodium load. The low bicarbonate and sodium concentrations require either a sophisticated pharmacy to prepare them on site or special order and shipment. Based on pricing by CAPS (Livonia, MI), it is possible to calculate sample costs for CVVHF-citrate. 4% trisodium citrate averaged $176.50 per day with replacement and replenishment fluid amounted to $62.13 per day. Interestingly, average daily costs for special order citrate-based dialysate were $285.50 per day. Thus, developing a CVVHF-citrate system as outlined above is potentially a cost-effective as well as a clinically efficacious measure.
Recently, it was demonstrated that CVVHF-citrate could achieve excellent clearance and reduce system complexity.Citation[[13]] However, average filter patency with CVVHF-citrate was dramatically less than with CVVHD-citrate.Citation[[13]], Citation[[16]] We postulated that the variation in delivered citrate might contribute to decreased filter patency. Therefore, our CVVHF-citrate system attempted to regulate citrate anticoagulation while minimizing citrate exposure. By attaching the three-way stopcock to the arterial outflow, our system initiated citrate anticoagulation in the extracorporeal system at its most proximal site. This minimized the distance blood had to travel before anticoagulation. Such a structure to the extracorporeal circuit ultimately may be more to prevent filter clotting.Citation[[19]], Citation[[20]] Isolating the citrate infusion also allowed for easier control of anticoagulation. It is necessary in other systems to use either a different citrate concentration with replacement fluids or increase the rate of replacement fluids to change the degree of anticoagulation. The latter strategy, though quicker to implement, can potentially interfere with ultrafiltration goals. This is an important caveat to consider. Although our blood pumps restricted ultrafiltration to a set rate, recent data suggest that high ultrafiltration goals may have a significant beneficial impact on outcomes in CRRT.Citation[[21]]
Our system excluded magnesium from the replacement fluids. Typically, CVVHD or CVVHF-citrate systems contain magnesium in the dialysate or the replacement fluids. However, citrate will bind non-specifically to any divalent cation. Theoretically therefore, more citrate will be necessary to achieve the same level of anticoagulation in the presence of any fluid containing increased divalent cation concentrations. This further increases the risk for citrate toxicity. In addition, circulating magnesium-citrate complexes can increase arrhythmia risk by lowering serum ionized magnesium levels.
The decline in PF(Ca2+)i was paralleled by improved filter patency during the study period as practitioners and staff became more comfortable with the system. It could be argued that the saline flushes contributed to the exceptional filter patency. However, this likely represents a minimal contribution in light of the poor filter patency in the historical control group.
Finally, though there were a small number of individuals in this prospective trial, it was striking to note that 58% of CVVHF-citrate treated patients survived to ICU discharge. These were critically ill patients based on their average APACHE III scores. Their ICU outcomes raise the question as to whether CVVHF-citrate directly affected their survival. It is interesting to note that Hörl and associatesCitation[[22]] reported that citrate anticoagulation in IHD reduced neutropenia, chronic granulocyte degranulation and complement activation. Such an effect could be ongoing in CVVHF-citrate patients as well. Obviously, a more formal demonstration of this hypothesis and the testing of the same in a larger clinical trial is warranted.
In conclusion, we demonstrated that our CVVHF-citrate system achieved excellent clearance with dramatically better filter patency than previously described systems. Our system isolated magnesium replacement and thus required less citrate, reducing the likelihood of citrate toxicity. Since this system is CVVHF-based, it does not require the preparation of a specially formulated dialysate, making it simpler to implement and potentially, more cost-effective.
ACKNOWLEDGMENT
The authors greatly appreciate the interest, help, energy, and expertise of the University of Wisconsin-Madison dialysis and intensive care unit staff.
REFERENCES
- Clark W.R., Alaka K.J., Mueller B.A., Macias W.L. A Comparison of Metabolic Control by Continuous and Intermittent Therapies in Acute Renal Failure. J. Am. Soc. Nephrol. 1994; 4: 1413–1420
- van Bommel E., Bouvy N.D., So K.L., Zietse R., Vincent H.H., Bruining H.A., Weimar W. Acute Dialytic Support For the Critically Ill: Intermittent Hemodialysis Versus Continuous Arteriovenous Hemodiafiltration. Am. J. Nephrol. 1995; 15: 192–200
- van de Wetering J., Westendorp R.G., van der Hoeven J.G., Stolk B., Feuth J.D., Chang P.C. Heparin Use in Continuous Renal Replacement Procedures: The Struggle Between Filter Coagulation and Patient Hemorrhage. J. Am. Soc. Nephrol. 1996; 7: 145–150
- Ward D.M., Mehta R.L. Extracorporeal Management of Acute Renal Failure Patients at High Risk of Bleeding. Kidney Int. 1993; 43(Suppl. 41)S237–S244
- Mehta R.L. Anticoagulation During Continuous Renal Replacement Therapy. ASAIO J. 1994; 40: 931–935
- DePont A.C., Oudemans-vanStraaten H.M., Roozendaal K.J., Zandstra D.F. Nadroparin Versus Dalteparin Anticoagulation in High-Voluem, Continuous Venovenous Hemofiltration: A Double Blind Randomized Crossover Study. Crit. Care Medicine 2000; 28: 421–425
- Beholz S., Grubitzsch H., Bergmann B., Wollert H.G., Eckel L. Anticoagulation in Extracorporeal Circulation Using Recombinant Hirudin: A Case Report. Perfusion 2000; 15: 257–260
- Zusman R.M., Rubin R.H., Cato A.E., Cocchetto D.M., Crow J.W., Tolkoff-Rubin N. Hemodialysis Using Prostacyclin Instead of Heparin as the Sole Antithrombotic Agent. New Engl. J. Med. 1981; 304: 934–939
- Swartz R.D. Hemorrhage During High-Risk Hemodialysis Using Controlled Heparinization. Nephron 1981; 28: 65–69
- Tan H.K., Baldwin I., Bellomo R. Continuous Veno-Venous Hemofiltration Without Anticoagulation in High-Risk Patients. Intensive Care Med. 2000; 26: 162–165
- Mehta R.L., McDonald B.R., Aguilar M.M., Ward D.M. Regional Citrate Anticoagulation for Continuous Arteriovenous Hemodialysis in Critically Ill Patients. Kidney Int. 1990; 38: 976–981
- Palsson R., Nilesm J.L. Regional Citrate Anticoagulation in Continuous Venovenous Hemofiltration in Critically Ill Patients with a High Risk of Bleeding. Kidney Int. 1999; 55: 1991–1997
- Flanigan M.J., Von Brecht J., Freeman R.M., Lim V.S. Reducing the Hemorrhagic Complications of Hemodialysis: A Controlled Comparison of Low-Dose Heparin and Citrate Anticoagulation. Am. J. Kidney Dis. 1987; 9: 147–153
- Kaplan A.A., Longnecker R.E., Folkert V.W. Continuous Arteriovenous Hemofiltration: A Report of Six Months Experience. Ann. Intern. Med. 1984; 100: 358–367
- Cook D., Heyland D., Griffith L., Cook R., Marshall J., Pagliarello J. Risk Factors for Clinically Important Upper Gastrointestinal Bleeding in Patients Requiring Mechanical Ventilation. Canadian Critical Care Trials Group. Crit. Care Med. 1999; 27: 2812–2817
- Kutsogiannis D.J., Mayers I., Chin W.D., Gibney R.T. Regional Citrate Anticoagulation in Continuous Venovenous Hemodiafiltration. Am. J. Kidney Dis. 2000; 35: 802–811
- Mehta R.L., Pascual M.T., Zhuang S. for the ARF Collaborative Study Group: Citrate Anticoagulation (CIT) for Continuous Renal Replacement Therapy (CRRT). J. Amer. Soc. Nephrol. 2000; 11: 325A, abstract
- Stefanidis I., Hagel J., Frank D., Maurin N. Hemostatic Alteration During Continuous Venovenous Hemofiltration in Acute Renal Failure. Clin. Nephrol. 1996; 46: 199–205
- Davenport A. The Coagulation System in the Critically Ill Patient with Acute Renal Failure and the Effect of an Extracorporeal Circuit. Amer. J. Kidney Dis. 1997; 30(Suppl 4)S20–S27
- Cardigan R.A., McGloin H., Mackie I.J., Machin S.J., Singer M. Activation of the Tissue Factor Pathway Occurs During Continuous Venovenous Hemofiltration. Kidney Int. 1999; 55: 1568–1574
- Ronco C., Bellomo R., Homel P., Brendolan A., Dan M., Piccinni P., La Greca G. Effects of Different Doses in Continuous Veno–Venous Hemofiltration on Outcomes of Acute Renal Failure: A Prospective Randomized Trial. Lancet 2000; 356: 26–30
- Böhler J., Schollmeyer P., Dresseel B., Dobos G., Hörl W.H. Reduction of Granulocyte Activation During Hemodialysis with Regional Citrate Anticoagulation: Dissociation of Complement Activation and Neutropenia from Neutrophil Degranulation. J. Amer. Soc. Nephrol. 1996; 7: 234–241